Abstract
A large range of neuroadaptations develop in response to chronic opioid exposure and these are thought to be more or less critical for expression of the major features of opioid addiction: tolerance, withdrawal and processes that may contribute to compulsive use and relapse. This review considers these adaptations at different levels of organization in the nervous system including tolerance at the μ-opioid receptor itself, cellular tolerance and withdrawal in opioid-sensitive neurons, systems tolerance and withdrawal in opioid-sensitive nerve networks, as well as synaptic plasticity in opioid sensitive nerve networks. Receptor tolerance appears to involve enhancement of mechanisms of receptor regulation, including desensitization and internalization. Adaptations causing cellular tolerance are more complex but several important processes have been identified including upregulation of cAMP/PKA and cAMP response element-binding signalling and perhaps the mitogen activated PK cascades in opioid sensitive neurons that might not only influence tolerance and withdrawal but also synaptic plasticity during cycles of intoxication and withdrawal. The potential complexity of network, or systems adaptations that interact with opioid-sensitive neurons is great but some candidate neuropeptide systems that interact with μ-opioid sensitive neurons may play a role in tolerance and withdrawal, as might activation of glial signalling. Implication of synaptic forms of learning such as long term potentiation and long term depression in opioid addiction is still in its infancy but this ultimately has the potential to identify specific synapses that contribute to compulsive use and relapse.
Keywords: morphine, opioid, opioid tolerance, opioid withdrawal, opioid addiction, desensitization, downregulation, neuroadaptation, synaptic plasticity
Introduction
Addiction to opioid drugs is a serious clinical and social problem. As with other addictive drugs, a substantial proportion of individuals who misuse opioids or use them clinically become addicted. Opioids are widely used in acute and chronic pain management, and some, for example oxycodone, are consistently among the most commonly prescribed drugs (Kuehn, 2007). Conservative estimates of patients prescribed long-term opioids who develop some sort of addictive disorder usually range from 2 to 6% (Fields, 2007), although some studies have reported higher rates (see Ballantyne and LaForge, 2007). Although accurate data on illicit users at large is more difficult to obtain, the proportion that becomes addicted is probably much higher than in a clinical setting and could be as high as 30% (Stafford et al., 2004). For heroin users in treatment programmes, relapse rates to first reuse are approximately 60% after 3 months and 75–85% after 12 months of cessation (Bradizza et al., 2006). The high incidence of addiction to opioids is not surprising because opioids strongly induce adaptations associated with all of the core features of addiction (DSMIV, 2000), including tolerance, withdrawal and associative processes that contribute to compulsive use and relapse.
Opioid tolerance is characterized by a reduced responsiveness to an opioid agonist such as morphine and is usually manifest by the need to use increasing doses to achieve the desired effect. Profound tolerance can develop during chronic opioid administration. In humans, experimental examples of long-term tolerance to hundreds of fold usual effective doses of morphine have been reported (Jaffe, 1985). Clinically, more than 10-fold dose escalations of opioid dose in chronic pain management are common (Buntin-Mushock et al., 2005) and illicit opioid addicts can consume daily doses tens to hundreds of fold higher than acutely effective doses (Stafford et al., 2004). The term tolerance is sometimes used rather loosely to refer either to very short or long-term loss of agonist efficacy. In experimental animals and isolated cells, ‘acute' tolerance can be observed rapidly (seconds to minutes) during the course of a single episode of opioid intoxication (Williams et al., 2001). This type of tolerance may be more closely related to processes of rapid μ-opioid receptor (MOPr) desensitization and internalization that should be distinguished from the more substantial tolerance that emerges after days to weeks of opioid administration. Tolerance results from adaptive mechanisms at the level of the drug target (MOPr), as well as at the cellular, synaptic and network levels, where adaptations due to homeostatic mechanisms tend to restore normal function in spite of the continued perturbations produced by opioid agonists. Associative or conditioned tolerance, where drug use is always paired with a distinctive environment, also plays an important role and is mediated by specific neural systems in behaving animals. Sensitization (reverse tolerance) to some behavioural outputs can develop, particularly in motivational neural systems (Kalivas and Duffy, 1987) and also in some cellular models (Hack et al., 2003; Ingram et al., 2007).
Abrupt cessation of chronic opioid use produces an intense but rarely life-threatening withdrawal syndrome in both humans and experimental animals. The signs and symptoms of withdrawal reach a peak intensity as occupancy of receptors by opioid agonists decline to a minimum and persist for a period from days to several weeks in humans. In this acute phase of withdrawal, the dysphoric, aversive nature of the withdrawal syndrome contributes to high rates of relapse. In experimental animals, this includes signs such as escape attempts (jumping), vocalization, hyperalgesia, ptosis, wet dog shakes, tachypnea and diarrhoea. Escape attempts and vocalization presumably indicate discomfort or aversiveness of withdrawal, which can be modelled using conditioned place aversion (see Williams et al., 2001). It is well established that different components of the global withdrawal response arise from different populations of neurons (see Williams et al., 2001). Some features of withdrawal may be more protracted and contribute to drug seeking and relapse. Because complex animal models are required to model uncontrolled use, drug seeking and relapse (Spanagel and Heilig, 2005), understanding of cellular and molecular mechanisms of these processes is less complete than for tolerance and withdrawal. Although anatomical substrates including the ventral tegmental area (VTA), shell of the nucleus accumbens and basolateral amygdala have been associated with different components of drug seeking and relapse (see Aston-Jones and Harris, 2004; Bossert et al., 2005; Harris and Aston-Jones, 2007), the cellular mechanisms responsible for these still remain largely unknown.
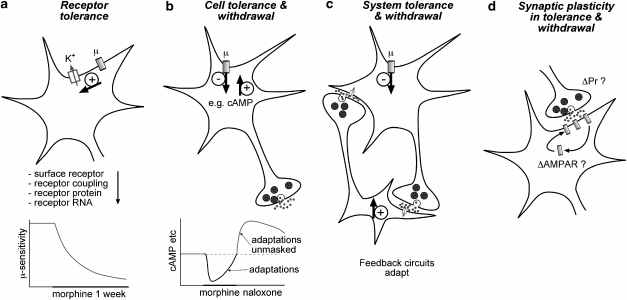
Disturbances of normal neural functions produced by opioids have been thought to initiate homeostatic processes leading to the development of opioid addiction for more than half a century (Himmelsbach, 1943). From the 1970s onwards, the general notion of homeostatic mechanisms of addiction developed into ‘opponent process' and more recently into ‘allostatic' models of adaptive processes (see Le Moal and Koob, 2007) Attempts to understand neuroadaptations related to opioid addiction have focussed on identifying mechanisms responsible for distinct features of addiction, particularly tolerance, the withdrawal syndrome, compulsive use and relapse. As outlined in Figure 1, ‘homeostatic' neuroadaptations develop at multiple levels of neural organization from the primary target of most opioid drugs, the MOPr (‘receptor tolerance', Figure 1a), homeostatic mechanisms in neurons that express MOPr (‘cellular tolerance', Figure 1b), excitability of neural and neuron-glial networks interacting with MOP-sensitive neurons (‘systems tolerance, Figure 1c). The potential for interaction of these adaptations with synaptic learning mechanisms is represented in Figure 1d (‘synaptic plasticity in tolerance and withdrawal'). This review considers adaptive processes at each of these levels of organization in terms of opioid tolerance, withdrawal and potential synaptic mechanisms of addiction. The types of adaptations outlined in Figure 1a–c involve mechanisms that are homeostatic in that they result simply from non-contingent, chronic opioid exposure and can in many cases be observed in isolated cells or neuronal cultures. Nonetheless, it should be noted that some of these homeostatic mechanisms, for example cAMP hypertrophy, can profoundly influence environment contingent aspects of addiction such as the capacity of synapses to strengthen or weaken motivational brain systems of behaving animals in the presence of environmental or interoceptive cues. Some downstream or circuit adaptations may persist after weeks of abstinence, and features of addiction related to relapse or hedonic dysregulation may persist for months to years (Koob and Le Moal, 2005). Some of these adaptations that are of a general structural nature, such as altered neuronal and synaptic architecture, dendritic morphology and axonal branching, may be driven by transcriptional effects of phospho-cAMP response element-binding (CREB) or other phosphorylation-transcriptional cascades including induction of Δ-fos-B (for example, Carlezon et al., 2005; Lu et al., 2006; Zachariou et al., 2006).
Figure 1.
Organization of opioid adaptations in the nervous system including: (a) receptor tolerance at the MOPr itself showing loss in the coupling of MOPr to a major cellular effector, the G-protein-regulated inwardly rectifying potassium channel, K+ channel. Several potential mechanisms could account for tolerance at this level of organization, but changes to coupling and perhaps surface expression appear to be most important. (b) Cellular tolerance and withdrawal in opioid-sensitive neurons is due to multiple adaptations to intracellular signalling cascades, but hypertrophy of cAMP signalling is the best established. (c) Systems feedback adaptations in opioid-sensitive nerve and neuroglial networks can develop and contribute to tolerance and withdrawal. (d) Synaptic plasticity and learning in opioid-sensitive nerve networks may involve changes in synaptic plasticity driven by changes in presynaptic release probability, which are well established at many opioid-sensitive GABAergic synapses, but more importantly, mechanisms resembling LTP and/or long-term depression probably involving AMPA receptor insertion in synapses may produce long-term changes in synaptic strength. It should be noted that adaptations outlined in (b) and (c) can strongly influence synaptic plasticity.
Opioid tolerance
Partial loss of receptor function through the mechanisms summarized in Figure 1a contribute to tolerance development. Most of the actions of MOPrs have been reported to be completely dependent on activation of the pertussis toxin-sensitive G-protein subfamilies, Gi and Go (Connor and Christie, 1999), so that tolerance has usually been measured in cellular systems as a loss of coupling to activation of G-protein -regulated inwardly rectifying potassium channels, inhibition of voltage-gated calcium channels, inhibition of adenylate cyclase (AC; Connor et al., 2004) or stimulation of mitogen-activated protein kinase (MAPK) pathways. It should be noted that a range of other cellular effectors of MOPrs have been identified (Williams et al., 2001) that may also mediate tolerance. Mechanisms downstream of β-arrestin2 (also known as arrestin 3; Arr3) recruitment to the MOPr could also play a role (Shenoy and Lefkowitz, 2003). Tolerance-producing adaptations that simply blunt MOPr activation are likely to be largely passive in terms of contribution to the most obvious signs of opioid withdrawal and other features of addiction because endogenous opioid-MOPr signalling systems appear to exhibit low or subtle basal activity in most circumstances, so simply reducing sensitivity of the MOPr does not produce withdrawal rebound on cessation of drug use. This is evidenced by the observation that administration of MOPr antagonists to normal animals or humans do not produce profound effects on ongoing behaviour, although some aversive effects have been reported (for example, Burgdorf et al., 2001). More subtle aspects of opioid dependence influenced by endogenous opioid—MOPr interactions, perhaps ‘hedonic set-points' (Koob and Le Moal, 2005), might be influenced by impaired MOPr signalling, but direct evidence for this is still limited.
Receptor tolerance in opioid sensitive neurons
In Figure 1a, ‘receptor tolerance' refers to partial loss of capacity of the MOPr to signal to intracellular effectors over time. Mechanisms involving either decreased cell surface expression of MOPr and/or reduced coupling efficacy of receptors remaining on the cell surface both contribute to this. Much research on opioid receptor tolerance has focussed on reduced coupling of MOPr to its effectors, because the general consensus of studies to date is that there is little or no change in mRNA or because MOPr protein expression develops with chronic opioid, particularly morphine, treatment (Stafford et al., 2001; Williams et al., 2001; Patel et al., 2002; Johnson et al., 2005). For the limited cases where uncoupling of MOPr to cellular effectors has been examined in functioning neurons, intense chronic morphine treatment regimes have resulted in only a two- to threefold reduction in potency of opioid agonists (for example, Williams et al., 2001; Bagley et al., 2005a). It seems unlikely that these reductions in coupling can fully account for behavioural tolerance, which is at least an order of magnitude greater (see Figures 1b and c). Directly relating MOPr tolerance in a particular neuron to behavioural tolerance is also complicated because the extent of uncoupling of MOPrs from G-protein activation varies greatly among different types of neurons after chronic opioids (Sim-Selley et al., 2000). As discussed below, interaction of diverse signalling systems with MOPr coupling in different neurons demands that mechanisms be determined for each type of neuron of interest. Numerous processes that can modulate MOPr coupling have been reported (see Johnson et al., 2005), but some mechanisms examined in heterologous expression systems such as Xenopus laevis oocytes (for example, Celver et al., 2001) are difficult to relate to functioning mammalian neurons because the stoichiometry of pivotal signalling cascades and rates of desensitization and internalization in model systems can differ vastly from real neurons. Where the experiments have been done, this review therefore focusses on adaptations at each level of organization in neurons.
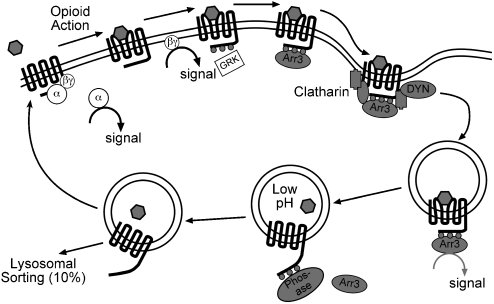
Rapid mechanisms of receptor desensitization and internalization have been studied extensively and appear to be involved in both acute and long-term opioid tolerance (Whistler and von Zastrow, 1998; Whistler et al., 1999; Bohn et al., 2000; He et al., 2002; Koch et al., 2005). As outlined in Figure 2, the process of MOPr regulation is widely assumed to resemble that of the more thoroughly studied β2-adrenergic receptor (Connor et al., 2004; Gainetdinov et al., 2004). According to this scheme, agonist activation induces MOPr phosphorylation by G-protein-coupled receptor kinase 2 increasing the affinity of interaction with Arr3. The binding of Arr3 uncouples the receptor from G-protein signalling (that is, desensitizes the receptor) and initiates the process of receptor sequestration and internalization through an Arr3- and dynamin-dependent mechanism (Bohn et al., 2000; Connor et al., 2004; Gainetdinov et al., 2004). This sequence of events has been validated for much of the process of MOPr regulation (see Connor et al., 2004), but the importance of these mechanisms for the loss of receptor function (acute tolerance or desensitization) or development of long-term tolerance are still uncertain. Knockout of a key protein for MOPr internalization, Arr3 has provided the most compelling evidence to date that Arr3 and, by implication, desensitization and/or internalization mechanisms are involved in both acute and long-term opioid tolerance (Bohn et al., 2000). Both loss of behavioural antinociceptive sensitivity to morphine, interpreted as acute tolerance, and development of long-term tolerance were profoundly blunted in Arr3 knockout mice (Bohn et al., 2000). However, the precise mechanisms by which desensitization and internalization of MOPr occur and how these influence tolerance remain controversial, particularly in native neurons, so the interpretation that Arr3-mediated internalization somehow causes tolerance must be considered tentative.
Figure 2.
Scheme of opioid receptor activation and internalization based on general model developed for the β-adrenergic receptor. Growing evidence suggests that receptor tolerance involves enhancement or acceleration of these processes. Although opioid receptor activation, desensitization and internalization are well established, the precise mechanisms are not clearly known. Desensitization of the receptor certainly precedes internalization, but it is not known whether this is dependent of GRK association and phosphorylation. It is probably not dependent on Arr3 binding because desensitization proceeds in the Arr3 knockout mice. The major initial signalling steps, that is, release of G-protein α and βγ subunit, are attenuated by the enhanced desensitization or internalization, as is signalling downstream from the MOPr-Arr3 complex. Abbreviations: Arr3, arrestin3; GRK, G protein coupled receptor kinase; DYN, dynamin. Adapted from Connor et al., 2004.
One complication is that desensitization and internalization are often considered as a single phenomenon. Operationally, any process that reduces MOPr sensitivity over a relatively rapid time course can be described as desensitization whether or not it involves internalization of receptors. However, functional desensitization of MOPr is distinct from and precedes internalization when measured over appropriate time scales. If desensitization is measured over the time scales that include internalization (minutes to hours), then measurements are necessarily a composite of both rapid desensitization and internalization (see Connor et al., 2004). Importantly, MOPrs desensitize robustly in cultured locus coeruleus (LC) neurons when internalization is blocked using concanavalin-A (Arttamangkul et al., 2006) or when Arr3 is knocked out in cultured sensory neurons (Dang and Christie, 2006; Walwyn et al., 2007). The failure of Arr3 deletion to prevent desensitization also raises the possibility that consequences of knocking out Arr3 independent of MOPr desensitization and internalization cause the observed blunting of acute and long-term tolerance in behaving animals. Walwyn et al. (2007) have proposed that Arr3 deletion leads to constitutive activation of MOPr by preventing Arr3-dependent targeting and internalization of constitutively active receptors by c-Src tyrosine kinase, but it is also possible that developmental adaptations to the gene deletion are important.
Another complication is that the opioid agonist, morphine, most widely used clinically and in studies of tolerance has relatively low efficacy for G-protein activation and desensitization but a disproportionately very low efficacy for producing MOPr internalization when compared with other efficacious agonists such as D-ala-methionine ekephalin glyol (DAMGO), methadone or sufentanyl (Keith et al., 1996; Whistler et al., 1999; Borgland et al., 2003). This difference may produce differences in rate or extent of tolerance development, but the evidence in experimental animals is limited (Bailey and Connor, 2005). Morphine produces little or no internalization in most neurons (Trafton et al., 2000; He et al., 2002) and many heterologous expression systems but has some efficacy in others (for example, Borgland et al., 2003) or in some neuronal compartments, for example, dendrites of striatal neurons (Haberstock-Debic et al., 2003), presumably because mechanisms for internalization are inherently more efficient in those cells/compartments.
The mechanisms responsible for the very poor efficacy of morphine to produce internalization are yet to be established. Morphine stimulates phosphorylation of MOPrs less effectively than strongly internalizing agonists such as DAMGO (see Connor et al., 2004), but it can induce internalization if the efficacy of the signalling system is increased, for example, by overexpression of G-protein coupled receptor kinase (GRK) (Whistler and von Zastrow, 1998). However, it is not known whether distinct sites on MOPrs are phosphorylated by morphine versus internalizing agonists. In at least one heterologous expression system (HEK293 cells), DAMGO but not morphine acting on MOPrs can stimulate Phospholipase D2, which might promote internalization (Koch et al., 2003). Morphine might also interact differently from other agonists with a range of signalling mechanisms potentially involved in internalization (but perhaps only desensitization), including PKC isoforms (Bailey et al., 2006) phosphoinositide-3 kinases, GRK, calmodulin kinases and MAPK cascades (Johnson et al., 2005).
Although chronic treatment of animals with morphine does not reduce MOPr density, agonists that efficaciously drive internalization can produce MOPr downregulation. Chronic treatment with high doses of etorphine produces moderate downregulation of MOPrs probably because a small proportion of MOPrs are targeted to lysosomes and degraded during each internalization cycle driven by etorphine but not by morphine (Stafford et al., 2001; Patel et al., 2002). It should also be noted that alternatively spliced MOPrs, which vary in the C-terminal domains that associate with trafficking proteins, can be cycled at different rates (Koch et al., 1998). Tissue-specific expression of splice variants could therefore differentially affect tolerance development in different neurons or cellular compartments.
Considering the very weak efficacy of morphine to produce internalization, the effects of chronic opioid exposure on MOPr desensitization might be more important than internalization for functional uncoupling of MOPr. Desensitization consistently precedes internalization when both are measured in the same cell type using electrophysiological methods (which can resolve desensitization on the second-to-minute scale), coupled with MOPr immunohistochemistry in AtT20 cells (for example, Borgland et al., 2003) or with fluorescent peptide ligands in cultured LC neurons (Arttamangkul et al., 2006). Unfortunately, the molecular mechanisms of desensitization in neurons (rather than internalization) are still unclear. Although widely assumed to require association of GRK or binding of Arr3 (but see above that desensitization is not blocked in Arr3 knockouts) to MOPr, this has not been directly established in neurons, so other phosphorylation events or protein–protein interactions could be just as important. MOPr desensitization is a low-efficiency process compared with activation of G-proteins, regardless of agonist (for example, Borgland et al., 2003). Morphine, with lower intrinsic efficacy than DAMGO for G-protein activation and desensitization (Borgland et al., 2003), produces almost no desensitization in some neurons (for example, LC neurons; Blanchet and Luscher, 2002; also see Dang and Williams, 2005), whereas in others, substantial desensitization can be observed (for example, in AtT20 cells; Borgland et al., 2003) or can be induced by stimulation of other signalling cascades such as activation of PKC in LC neurons (Bailey et al., 2004). Differential activity of signalling cascades that modulate MOPr desensitization in different neurons might be responsible for differences in MOPr uncoupling reported after chronic opioid treatment of animals (Sim-Selley et al., 2000).
Although desensitization and internalization are considered important for tolerance development, interpretations of the role of internalization mechanisms in tolerance from different studies, particularly following chronic morphine treatment, are difficult to reconcile. For example, the loss of morphine tolerance in Arr3 knockout mice implies that internalization mechanisms are necessary for tolerance, but the opposite interpretation was suggested by the studies of He et al. (2002). In these studies, chronic intrathecal morphine administration in rats was associated with little or no internalization of MOPr but produced profound tolerance. Tolerance was reversed by co-administration of a sub-therapeutic dose of DAMGO, which strongly stimulates internalization. This was interpreted to suggest that chronic morphine treatment produces an accumulation of inactive MOPrs on the surface membrane because internalization and recycling are (assumed to be) required to produce re-activated receptors following phosphorylation and desensitization, and that interaction of DAMGO with MOPr oligomers would drive internalization. However, there is no direct evidence to show that internalization is required for recovery of MOPr function, and no evidence has been found for the proposition to the extent that it has been examined directly in native neurons (Bailey et al., 2003). Furthermore, in other studies of heterologous expression systems (HEK293 cells), recycling agonists promoted cellular tolerance and potential withdrawal (Koch et al., 2005). Nonetheless, the basis of the observation that very low doses of a recycling agonist such as DAMGO can blunt tolerance development warrants further investigation because it could provide a rational basis for the clinical rotation of different opioids to overcome tolerance. However, limited clinical studies to date have not supported rescue from tolerance by this mechanism (Mercadante et al., 2004).
Desensitization and/or internalization mechanisms may have an important role in long-term tolerance development. Early studies in isolated tissues, model cells and neurons have all found a loss of agonist efficacy to stimulate MOPr following chronic morphine treatment (see Williams et al., 2001). The loss of function was homologous in LC neurons, that is, sensitivity of other receptors such as the α-2 adrenoceptor to couple with the same effector mechanisms was not impaired. This tolerance was interpreted as a specific reduction in coupling capacity of MOPr to signal through Gi/o-dependent mechanisms because a reduction of surface receptors of approximately 80% would be required to account for the observed loss of coupling efficacy (Christie et al., 1987), although the consensus of many studies is that chronic morphine treatment does not substantially affect MOPr-binding density (Williams et al., 2001). More recent studies have begun to explain the specificity of the homologous reduction in MOPr coupling after chronic morphine. Dang and Williams (2004, 2005) observed that the sensitivity of MOPrs was unaffected by chronic morphine treatment when probed in LC neurons without previous exposure to high concentrations of agonist (earlier studies using sharp electrodes almost invariably first tested a very high concentration of agonist to establish viability of a neuron before proceeding to any other experiments). Importantly, initial sensitivity of the MOPr was normal, but desensitization was more profound and recovered much more slowly after chronic morphine, even after very brief (1–2 min) applications of agonist. This adaptation may be quite persistent because Ingram et al. (2007) reported similar findings in periaqueductal grey (PAG) neurons more than 24 h after morphine withdrawal. These studies suggest that enhanced desensitization may be an important component of receptor tolerance. One potential mechanism to enhance desensitization of MOPr include adaptations to PKC signalling, which, when activated in LC neurons, has been shown to enhance the efficacy of MOPr desensitization (see Bailey et al., 2004, 2006; Bailey and Connor, 2005). Many other signalling cascades could be both involved in efficacy of desensitization and upregulated after chronic morphine, perhaps through GRK (Whistler and von Zastrow, 1998), RGS proteins such as RGS4 (Gold et al., 2003), Arr3 (Bohn et al., 2000), MAPK (Eitan et al., 2003) or other downstream mechanisms.
Desensitization and internalization may also be regulated by other G-protein coupled receptors that can interact more or less directly with MOPr. Chronic morphine treatment induces emergence of the δ-opioid receptor to the surface membrane in some neurons (for example, Hack et al., 2005). δ-Opioid receptors can potentially form heterodimers with MOPrs. There is also some evidence that interactions at the two pharmacophores of other G-protein coupled receptor heterodimers can strongly influence their internalization (Jordan et al., 2001). Morphine tolerance development is impaired in δ-opioid receptor knockouts and, intriguingly, tolerance and dependence development is blunted by bifunctional MOPr agonist/δ-opioid receptor antagonist molecules designed to interact preferentially with heterodimers (Daniels et al., 2005), which may influence MOPr internalization. Development of novel ligands that regulate internalization and/or desenstization of MOPr may therefore prove useful for limiting tolerance development at the level of receptor uncoupling.
In summary, enhancement of homologous desensitization appears important for the development of morphine tolerance at the receptor level, but the potential role of internalization remains very unclear. MOPrs desensitize effectively in the absence of Arr3 interaction or internalization, but it needs to be established whether this is enhanced by chronic morphine in Arr3 knockouts. The failure of Arr3 knockout mice to develop tolerance to morphine, although we have observed tolerance using more intense, continuous morphine treatment (BCH Chieng and MJC, unpublished observation), may suggest that other Arr3-dependent signalling mechanisms such as recruitment of cSrc to the plasma membrane are responsible (Shenoy and Lefkowitz, 2003). It should also be noted that other signals among the myriad downstream cellular adaptations produced by chronic morphine in opioid-sensitive neurons could also be responsible.
Cellular tolerance and withdrawal in opioid-sensitive neurons
Mechanisms such as those outlined in Figure 1b may contribute substantially to behavioural tolerance. Using cAMP signalling as an example in Figure 1b, cellular adaptations in opioid-sensitive neurons can potentially drive both tolerance and opioid-withdrawal behaviour. As shown, chronic, excessive stimulation or inhibition of a signal downstream of MOPr activation can lead to homeostatic adjustment of the signalling system in the face of continued activation of MOPr, so tolerance results if the downstream signal directly controls neural excitability, as cAMP does in some nerve terminals (for example, see Ingram et al., 1998). Removal of the inhibitory stimulus on the hypertrophied cAMP signalling system during withdrawal can then lead to overshoot in downstream signalling mechanisms and neural excitation. Overshoot or rebound activity may drive initial steps of opioid withdrawal, but effects may also be more persistent, leading to long-term signalling aberrations, synaptic strengthening or weakening, or structural reorganization of neural arborizations. A single cellular adaptation could thereby contribute to both tolerance and withdrawal.
Identification of which adaptations to intracellular signalling are relevant for tolerance, withdrawal and addiction at the cellular and behavioural level is a daunting challenge. It requires identification of adaptations that can drive cellular excitation or synaptic plasticity in opioid-sensitive neurons that are related to identifiable behavioural outputs including those involved in compulsive use of opioids. Both the cAMP-PKA (through Gi/o) and MAPK (at least through Gβγ subunits) cascades are of interest because they lie more or less directly downstream from MOPr activation. Adaptation to other kinase cascades may be more or less directly involved in tolerance and withdrawal at the cellular level, including PKC (Bailey et al., 2006) and many other signalling systems (Bailey and Connor, 2005; Johnson et al., 2005). Because the evidence for these cascades is less complete, they will not be discussed at length here. Mechanisms of cellular tolerance in opioid-sensitive neurons are probably not only limited to signalling cascades directly downstream of MOPr activation, but could also develop as a general cellular homeostatic response to depression of neural excitation by opioid agonists. In general, altered transmembrane chloride gradients, calcium homeostasis and synaptic structure and strength have been observed within neurons following persistent experimental manipulation of neural excitability (Rich and Wenner, 2007). It should also be noted that cellular adaptations in opioid-sensitive neurons may differ between experimental models that use continuous administration (for example, morphine pellets) versus intermittent dosing (for example, Inoue et al., 2003).
The AC–cAMP–PKA cascade
Chronic activation of G-protein coupled receptors of the Gi/o class that couple with inhibition of AC activity and reduce cAMP concentrations (for example, all opioid receptors, dopamine D2 and cannabinoid CB1 receptors) generally produce adaptive superactivation of AC (Watts and Neve, 2005). Depressed cAMP concentrations return to normal during continued exposure to opioids in part because AC is superactivated, which may be related in part to regulation of AC by Gβγ subunits (see Williams et al., 2001; Watts and Neve, 2005). Simplistically, downstream effectors of cAMP therefore develop tolerance following chronic exposure. However, persistent exposure to opioids differentially regulates isozymes of AC through distinct mechanisms (Watts and Neve, 2005; Schallmach et al., 2006). This is unsurprising because each of the nine isoforms exhibits distinct modulation by G-proteins (including βγ and different α-subunits) as well as the phosphorylation state of PKA and its interacting proteins (Wong and Scott, 2004; Watts and Neve, 2005; Schallmach et al., 2006; Chakrabarti and Gintzler, 2007). Adaptations to cAMP signalling are also complicated by a range of protein signalling complexes such as A-kinase anchoring proteins that structurally localize different kinases, phosphatases, phosphodiesterases and other signalling molecules to distinct cellular compartments, which almost certainly varies among cell types and cellular compartments (Wong and Scott, 2004).
AC–cAMP-related mechanisms might also feed back on the initial process of MOPr signalling to qualitatively modify transduction. Early studies suggested that MOPrs could interact with Gs rather than Gi in cultured dorsal root ganglion explants and proposed that chronic morphine treatment shifted the balance of interaction to stimulatory, Gs-mediated effects (for example, Shen and Crain, 1990). Whereas most direct studies of MOPr coupling provided no support for a Gs interaction (see Williams et al., 2001), more recent work has suggested that chronic morphine reduces the phosphorylation of Gs and that dephosphorylated Gs can interact with MOPr (Chakrabarti and Gintzler, 2007) in heterologous expression systems and spinal cord tissue. This finding warrants further investigation of mechanism of altered MOPr transduction mechanisms. Comparatively, signalling of the β-adrenergic receptor can shift from Gs to Gi coupling when phosphorylated by PKA (Zamah et al., 2002), emphasizing the need to examine similar mechanisms for MOPr signalling. Other potential mechanisms whereby adaptations to cAMP signalling may feed back on MOPr function also require further study in functioning neurons. These include the possibilities that constitutive MOPr activity may develop after chronic morphine treatment in some neurons or heterologous systems (Sadee et al., 2005; Walwyn et al., 2007) or that guanine nucleotide exchange factors activated by cAMP (EPACs) that can modulate G-protein signalling efficacy (see Wong and Scott, 2004).
The complexity of adaptations in the AC–PKA cascade in response to chronic opioids could vary greatly among opioid-sensitive neurons under different opioid treatment conditions, necessitating identification of adaptations and their influence on neural excitability in types of neurons relevant for tolerance, withdrawal and addiction. The most complete studies to date have focussed on adaptations in the LC and PAG that may be related to somatic and aversive signs of withdrawal, as well as the nucleus accumbens and VTA, which are more related to compulsive use of opioids.
Although there is some evidence that adaptations in the noradrenergic LC play an important role in opioid withdrawal behaviour, nearly complete ablation of the nucleus does not prevent the expression of withdrawal behaviour (Caille et al., 1999; see Christie et al., 1997), suggesting possible involvement in more subtle signs of withdrawal. There is good evidence that ventral noradrenergic neurons projecting to the bed nucleus of the stria terminalis are more important then the LC for withdrawal-induced aversion (Delfs et al., 2000), but the connection between opioid-induced adaptations in these neurons and withdrawal is still unknown. MOPr activation produces Gβγ subunit gating of G-protein-regulated inwardly rectifying potassium channels that directly inhibits the activity of action potentials in LC neurons (Williams et al., 2001). Despite concerns about the functional significance of the LC for withdrawal behaviour, there is growing evidence at the cellular level that LC neurons exhibit withdrawal hyperexcitiation in vitro (Ivanov and Aston-Jones, 2001; Han et al., 2006), and this is associated with regulation of the CREB (Han et al., 2006). Transcriptional control through phosphorylation state of CREB, which affects protein synthesis of a large number of neurotransmitter receptors, AC isoforms and other signalling proteins may influence neural excitation (Carlezon et al., 2005). In vivo, there is a profound excitation of action potential activity beyond the normal range for LC neurons (hyperexcitation), but the rebound is more modest in vitro (Ivanov and Aston-Jones, 2001; Han et al., 2006), suggesting that much of the activity in vivo is driven by afferents to LC. Using viral vectors to specifically enhance or blunt CREB expression in LC neurons, Han et al. (2006) demonstrated that enhancement of CREB expression strengthened withdrawal behaviours and vice versa. In parallel brain slice experiments, up- or downregulation of CREB had comparable effects on action potential rates of LC neuron in the presence of forskolin, suggesting there may be a direct link between excitability and withdrawal behaviour regulated by CREB. CREB may have a more widespread role in opioid addiction because phospho-CREB is elevated during opioid withdrawal in several brain regions implicated in addiction (Shaw-Lutchman et al., 2002) and is involved in excitability (Dong et al., 2006).
cAMP-related adaptations have been identified in PAG neurons that are linked to ion channel mechanisms that could drive withdrawal behaviour. A range of microinjection studies have implicated the PAG in mediation of opioid-withdrawal signs (see Williams et al., 2001). Acutely, MOPr agonists inhibit a subpopulation of neurons through activation of G-protein-regulated inwardly rectifying potassium channels as well as producing presynaptic inhibition (Williams et al., 2001). The major action of MOPr agonists is thought to be disinhibition of projection neurons via direct inhibition of GABAergic cell bodies and synapses (Williams et al., 2001). In brain slices containing PAG cell bodies, opioid withdrawal in vitro produces hyperexcitation of opioid-sensitive neurons during opioid withdrawal, using electrophysiological methods that avoid disruption of the intracellular milieu (Chieng and Christie, 1996; Bagley et al., 2005b). In this case, elevation of cAMP–PKA signalling during withdrawal induces opening an MOPr-sensitive (MOPr activation inhibits the channel) cation channel that produces hyperexcitation (Chieng and Christie, 1996; Bagley et al., 2005b; Ingram et al., 2007). Intriguingly, the withdrawal-activated cation channel is blocked by specific inhibitors of the neuronal GABA transporter type 1 (Bagley et al., 2005b). The properties of this ‘channel' current do not appear to be associated with changes in GABA flux through the electrogenic transporter, suggesting that the PKA-mediated effect is due to channel-like behaviour of GABA transporter type 1, similar to channel behaviour of other neurotransmitter transporters (for example, Ingram et al., 2002). Because GABA transporter type 1 is expressed exclusively by GABAergic neurons in most brain regions (see Bagley et al., 2005b), these findings suggest that the primary locus of opioid withdrawal in PAG is in opioid-sensitive GABAergic neurons. Indeed, approaches using c-fos as a marker of neural excitation during withdrawal have shown that activation in PAG is strongly enriched in GABAergic neurons (Chieng et al., 2005; Hacker et al., 2006). It will be of interest to determine whether the same or similar mechanisms are involved in cellular opioid withdrawal in other GABAergic neurons involved in opioid withdrawal and dependence. GABAergic neurons such as the medium spiny neurons of nucleus accumbens and dorsal striatum are known to be involved in addiction to opioids and are readily amenable to such investigation. The relatively sparse GABAergic neurons of the VTA have been difficult to investigate in vitro but are a potentially important site of such adaptations.
In addition to persistent cAMP-related actions in cell bodies, adaptations that could drive withdrawal behaviours have also been found at synapses in several brain regions associated with opioid withdrawal and addiction, including PAG, VTA and nucleus accumbens (see Williams et al., 2001). In rat PAG, opioid withdrawal is associated with a substantial increase in GABAergic (but not glutamatergic) synaptic neurotransmission, which is dependent on activation of PKA (Ingram et al., 1998; Hack et al., 2003). This is due to an increased presynaptic GABA release probability during withdrawal (opioids acutely inhibit GABA synapses). In VTA, MOPr agonists directly inhibit excitability of GABAergic cell bodies and synapses (Johnson and North, 1992), but the organization of opioid actions among specific subsets of VTA neurons is complex (Ford et al., 2006; Margolis et al., 2006), suggesting a need to study these actions in appropriate functional groups of GABAergic cells and synapses. In both VTA (Bonci and Williams, 1997) and nucleus accumbens (Chieng and Williams, 1998), GABAergic release probability during withdrawal or sensitivity to stimulation of AC by forskolin are enhanced. Similar findings have also been reported in the MOPr-sensitive rostral ventromedial medulla (Ma and Pan, 2006). This phenomenon might therefore be quite widespread among opioid-sensitive GABAergic synapses and, because of its rapid onset (seconds to minutes), is probably due to the effects of PKA phosphorylation on GABAergic vesicle dynamics. In VTA, some cAMP-dependent effects of opioid withdrawal on synaptic transmission can persist for at least 1 week after withdrawal (Bonci and Williams, 1996). These longer-term effects may be mediated by persistent changes to cAMP–PKA signalling or could be further downstream, perhaps via CREB, which itself can influence synaptic strength and plasticity (Dong et al., 2006; Hyman et al., 2006). It should be noted that, in VTA, nucleus accumbens and mouse but not in rat PAG, enhancement of GABAergic transmission during opioid withdrawal is blunted by extracellular elevation of adenosine concentrations that almost certainly result from elevation of intracellular cAMP followed by transport of adenosine (or cAMP) to the extracellular space to act on inhibitory A1 adenosine receptors (Williams et al., 2001; Hack et al., 2003).
Other MOPr-activated cascades: MAPKs and phosphoinositide-3 kinases
Mitogen-activated PK cascades, particularly extracellular-signal-regulated kinases (ERKs), are stimulated (phosphorylated) following MOPr activation (Li and Chang, 1996) by morphine and other opioid agonists (for example, Trapaidze et al., 2000). In heterologous expression systems, ERKs are activated by release of Gβγ subunits from MOPr in a Ras-dependent manner (Li and Chang, 1996), but other mechanisms of activation, for example, via Arr3 recruitment of c-Src to the plasma membrane or on endocytic vesicles (Shenoy and Lefkowitz, 2003) are presumably also stimulated by MOPrs. ERK activation by opioids could have important roles in opioid tolerance, withdrawal, and addiction because it has been shown to be important for (unresolved) desensitization/internalization of MOPr in heterologous expression systems (Chinese hamster ovary cells; Polakiewicz et al., 1998) and is involved in synaptic plasticity (see Sweatt, 2004). In vivo, the evidence for ERK activation by MOPrs is less convincing. Although ERK1/2 were phosphorylated in opioid-sensitive brain regions following acute morphine treatment, this generally (with the exception of LC neurons) occurred in cells adjacent to opioid-sensitive neurons (Eitan et al., 2003). Unfortunately, the role of ERK activation in opioid tolerance, withdrawal and addiction has been less thoroughly explored than in models of cocaine addiction (see Lu et al., 2006), where there is growing evidence for an important role in the persistence of a number of aspects of addiction. Other kinase cascades are activated by release of Gβγ subunits from MOPr including phosphoinositide-3 kinases (see Law et al., 2000; Bailey and Connor, 2005; Bailey et al., 2006), which could stimulate the PKC activity that is thought to play a role in the efficacy of MOPr desensitization.
In summary, it is intriguing that the major signalling cascades, particularly AC–cAMP–PKA–CREB and MAPK that adapt to non-contingent, chronic opioid exposure and contribute to cellular tolerance, and withdrawal in both cell bodies and nerve terminals, are also intimately involved in synaptic plasticity. These non-contingent adaptations could therefore have a profound influence on other features of addiction that involve learning and memory in motivational systems. Because of the complexities of the elements of these signalling cascades (see above), better anatomical methods (for example, to map elevated cAMP during withdrawal in specific cell populations) are needed to develop a global understanding of cellular withdrawal mechanisms beyond the small number of candidate neuronal groups studied to date. Some progress in this direction has been made indirectly using a transgenic mouse expressing an optical marker, CRE-LacZ (Shaw-Lutchman et al., 2002).
Tolerance, withdrawal and addiction in neural systems
Systems tolerance and withdrawal is represented in Figure 1c. Homeostatic adaptations t neural firing, synaptic strength and neurochemical balance have been shown to develop throughout neural and neuron-glial networks simply as a result of depressed or enhanced electrical activity (Rich and Wenner, 2007). Therefore changes in neural excitation produced by chronic opioid exposure in one component of a neural network can indirectly produce homeostatic adaptations to excitability of other neurons and synapses throughout the network. For example, although MOPr agonists do not directly act on dopaminergic neurons in VTA, a number of adaptations develop in these neurons that may be important for addiction (for example, Russo et al., 2007). Similar to cellular adaptations in opioid-sensitive neurons, network adaptations contribute to tolerance, withdrawal and long-term features of addiction, but finding the relevant adaptations is no less daunting a task than for cellular adaptations in opioid-sensitive neurons.
Quite specific antagonistic or compensatory neural mechanisms have been postulated for opioid-sensitive networks, sometimes based conceptually on the co-localization peptide containing neural systems in close apposition to endogenous MOPr signalling systems. These have been proposed to function as ‘anti-opioid' systems, that is, networks or neurochemical systems that are functionally antagonistic to endogenous MOPr signalling systems. It is an appealing idea that neural systems expressing receptors closely related to MOPr are organized in a functionally antagonistic manner to MOPr, including the κ-opioid- or NOPr and their endogenous peptide ligands (dynorphins and nociceptin/OFQ, respectively). There is some evidence that κ-opioid-receptor signalling systems are organized in neural systems in a functionally antagonistic manner to MOPr systems (Pan, 1998; Shippenberg et al., 2007), although this is not invariant, with some overlap of κ-opioid-receptor and MOPr in pain transmission systems (Marinelli et al., 2002). Although this idea has been most fully developed for the κ-opioid-receptor and dynorphin signalling in forebrain motivational systems during development of psychostimulant addiction, there is evidence for a similar role in opioid addiction (Shippenberg et al., 2007). Likewise, ORL1-receptor knockouts, ORL1-receptor antagonists (Ueda et al., 2000) and Nociceptin/OFq knockouts (Chung et al., 2006) suggest the ORL1-receptor-nociceptin/OFQ systems may function as anti-opioids during development of tolerance and dependence (Ueda et al., 2000). There is also evidence from studies using neurokinin 1-receptor antagonists and neurokinin 1-knockout mice that ‘anti-opioid' neurokinin 1 signalling contributes to opioid tolerance and perhaps hyperalgesia associated with withdrawal in nociception control systems (for example, King et al., 2005). Other potential antagonistic neural systems include those that express neuropeptide FF and cholecystokinin (Waldhoer et al., 2004).
Changes in glial function represent another potential form of systems adaptation beyond neural networks. Study of contribution of glial function to opioid addiction is still relatively recent, but there is growing evidence that activation of astrocytes (and perhaps microglia) in response to chronic morphine treatment (Song and Zhao, 2001; Narita et al., 2004) contributes to tolerance and withdrawal (Watkins et al., 2005) and can be suppressed by inhibitors of glial activation. There is good evidence that glial activation influences neural excitability and synaptic plasticity and that these may play an important role in opioid tolerance and/or dependence (Watkins et al., 2005). The mechanisms responsible for glial activation during chronic opioid exposure or their influence on opioid-sensitive neurons and pathways are still poorly understood, but the ameliorative effects of glial inhibitors on tolerance and withdrawal in animal studies suggest a potential avenue for therapeutic development. This is also a potentially important area of research because activated glia are a rich source of cytokines that can profoundly influence neural activity and synaptic plasticity. For example, brain-derived neurotrophic factor contributes to cAMP–CREB activation in LC neurons (Akbarian et al., 2002). The source of brain-derived neurotrophic factor has not been identified, but it could be derived from activated glia. Conversely, glial cell-derived neurotrophic factor in VTA has a suppressive effect on drug-induced reward, and chronic morphine reduces glial cell-derived neurotrophic factor (Messer et al., 2000).
Synaptic plasticity
Addictive drugs, including opioids, profoundly influence synaptic plasticity that underlies learning and memory in neural systems important for the development of addiction (recently reviewed by Hyman et al., 2006; Kauer and Malenka, 2007). Recent studies have focussed on two processes thought to be pivotal for memory consolidation, long-term potentiation (LTP) and long-term depression. As outlined in Figure 1d, in most cases, these processes are thought to involve subtype-specific changes to synthesis (for example, Mameli et al., 2007), insertion, removal and stabilization and consolidation of glutamate (usually AMPA) receptor subunits at synapses (Derkach et al., 2007). Understanding how aberrations to the processes of synaptic learning ultimately contribute to long-term features of addiction in specific neural systems associated with components of addiction such as VTA, nucleus accumbens and other components of basal forebrain and extended amygdala is a major challenge that is just beginning to yield insights.
Aside from investigation of the acute actions of opioids on synapses, adaptations following chronic opioids to presynaptic transmitter release probability together with virtually all of the signalling systems described above can influence LTP and long-term depression (Malenka and Bear, 2004; Hyman et al., 2006; Kauer and Malenka, 2007). For example, after chronic, intermittent morphine treatment, LTP at excitatory synapses onto CA1 neurons in hippocampus was greatly inhibited (MOPrs act directly on GABAergic interneurons in this region; Williams et al., 2001), an effect that could be reversed by acute morphine treatment (Pu et al., 2002). This appeared to be due to elevated cAMP signalling during withdrawal, because it could be blocked by PKA inhibitors. The status of intoxication or withdrawal could therefore be expected to have very different influences on consolidation of environmental or interoceptive cues associated with opioid addiction.
Even acute administration of opioids produces powerful adaptations to synaptic plasticity in systems important for opioid addiction. In the VTA, a single dose of morphine or other abused drugs such as cocaine in vivo induced enhancement of the ratio AMPA/NMDA receptor contribution to excitatory synapses onto dopaminergic neurons in vitro, which was interpreted as strengthening of AMPA receptor-mediated neurotransmission (but quantal synaptic amplitudes were not measured, so the interpretation is tentative) equivalent to LTP (Ungless et al., 2001; Saal et al., 2003). For cocaine, this plasticity is dependent on the hypothalamic peptide, orexin (Borgland et al., 2006). The persistence of this adaptation during chronic morphine administration is not yet fully understood, but orexin does influence morphine dependence (Georgescu et al., 2003). Conversely, single exposure to morphine, either in vivo or in vitro, inhibited LTP induction at GABAergic synapses onto VTA dopaminergic neurons (Nugent et al., 2007) for up to 24 h. The mechanism of LTP at this synapse is heterosynaptic, depending on NMDA receptor-mediated generation of NO to activate guanylate cyclase. These adaptations, if they persist during repeated morphine exposure, may act in concert to disturb the responses of VTA dopaminergic neurons to ongoing synaptic activity for at least 24 h following a single dose of opioid agonist. It will also be important to examine plasticity following both non-contingent and self-administered opioids, because these have been shown to have different effects on the activity of GABAergic projection neurons in VTA in vivo (Steffensen et al., 2006).
Concluding remarks
Much anatomical and behavioural research on neuroadaptations in opioid addictions focusses specifically on motivational or appetitive aspects of the disorders that drive compulsive drug seeking and relapse in forebrain motivational and emotional learning systems. Indeed, many of the pharmacological interventions contemplated for addiction, in general, focus on these systems (for example, Heidbreder, 2005). The cue-related nature of these disorders tends to implicate mechanisms of synaptic plasticity and learning mechanisms. As discussed above, study of the direct involvement of synaptic plasticity in development of opioid addiction is very much in its infancy. Nonetheless, a relatively good understanding of the non-contingent mechanisms of receptor and cellular adaptations to chronic opioid exposure is emerging and therapeutic developments may follow. These adaptations often differ both qualitatively and quantitatively in distinct populations of opioid-sensitive neurons and systems of neurons. Opioid receptor tolerance certainly appears to involve mechanisms of adaptation to desensitization and internalization. This has prompted development of opioid ligands, which either do not appear to engage the Arr3-internalization machinery at all (Groer et al., 2007) or regulate the MOPr in novel ways and produce less tolerance and dependence presumably because they can interact with heterodimers (Waldhoer et al., 2005). Such developments may provide benefits for long-term opioid therapy. Another long-term benefit of this approach may be reduction in cellular tolerance, which, by way of dysregulation of signalling systems such as cAMP, CREB and MAPK pathways, could have profound impacts not only on withdrawal, but also on synaptic plasticity involved in cue-related aspects of opioid addiction. Systems-level adaptations are complex and still poorly understood but could provide important therapeutic opportunities if the suggestion that astroglial activation plays a role in opioid tolerance and withdrawal, and perhaps synaptic plasticity through cytokine production, proves to be important in opioid addiction.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (Program Grant 351446, Fellowship 253799).
Abbreviations
- AC
adenylate cyclase
- Arr3
arrestin 3 (also β-arrestin2)
- DAMGO
D-ala-methionine ekephalin glyol
- ERK
extracellular signal-regulated kinase
- GRK
G-protein coupled receptor kinase
- GPCR
G-protein coupled receptor
- LC
locus coeruleus
- LTP
long-term potentiation
- MAPK
mitogen-activated protein kinases
- MOPr
μ-opioid receptor
- PAG
periaqueductal grey
- VTA
ventral tegmental area
Conflict of interest
The authors state no conflict of interest.
References
- Akbarian S, Rios M, Liu RJ, Gold SJ, Fong HF, Zeiler S, et al. Brain-derived neurotrophic factor is essential for opiate-induced plasticity of noradrenergic neurons. J Neurosci. 2002;22:4153–4162. doi: 10.1523/JNEUROSCI.22-10-04153.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S, Torrecilla M, Kobayashi K, Okano H, Williams JT. Separation of mu-opioid receptor desensitization and internalization: endogenous receptors in primary neuronal cultures. J Neurosci. 2006;26:4118–4125. doi: 10.1523/JNEUROSCI.0303-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacol. 2004;47 Suppl 1:167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Bagley EE, Chieng BC, Christie MJ, Connor M. Opioid tolerance in periaqueductal gray neurons isolated from mice chronically treated with morphine. Br J Pharmacol. 2005a;146:68–76. doi: 10.1038/sj.bjp.0706315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley EE, Gerke MB, Vaughan CW, Hack SP, Christie MJ. GABA transporter currents activated by protein kinase A excite midbrain neurons during opioid withdrawal. Neuron. 2005b;45:433–445. doi: 10.1016/j.neuron.2004.12.049. [DOI] [PubMed] [Google Scholar]
- Bailey CP, Connor M. Opioids: cellular mechanisms of tolerance and physical dependence. Current Op Pharmacol. 2005;5:60–68. doi: 10.1016/j.coph.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Bailey CP, Couch D, Johnson E, Griffiths K, Kelly E, Henderson G. Mu-opioid receptor desensitization in mature rat neurons: lack of interaction between DAMGO and morphine. J Neurosci. 2003;23:10515–10520. doi: 10.1523/JNEUROSCI.23-33-10515.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Kelly E, Henderson G. Protein kinase C activation enhances morphine-induced rapid desensitization of mu-opioid receptors in mature rat locus ceruleus neurons. Mol Pharmacol. 2004;66:1592–1598. doi: 10.1124/mol.104.004747. [DOI] [PubMed] [Google Scholar]
- Bailey CP, Smith FL, Kelly E, Dewey WL, Henderson G. How important is protein kinase C in mu-opioid receptor desensitization and morphine tolerance. Trends Pharmacol Sci. 2006;27:558–565. doi: 10.1016/j.tips.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Ballantyne JC, LaForge KS. Opioid dependence and addiction during opioid treatment of chronic pain. Pain. 2007;129:235–255. doi: 10.1016/j.pain.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Blanchet C, Luscher C. Desensitization of mu-opioid receptor-evoked potassium currents: initiation at the receptor, expression at the effector. Proc Natl Acad Sci USA. 2002;99:4674–4679. doi: 10.1073/pnas.072075399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin F-T, Lefkowitz RJ, Caron MG. μ-Opioid receptor desensitization by β-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- Bonci A, Williams JT. A common mechanism mediates long-term changes in synaptic transmission after chronic cocaine and morphine. Neuron. 1996;16:631–639. doi: 10.1016/s0896-6273(00)80082-3. [DOI] [PubMed] [Google Scholar]
- Bonci A, Williams JT. Increased probability of GABA release during withdrawal from morphine. J Neurosci. 1997;17:796–803. doi: 10.1523/JNEUROSCI.17-02-00796.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Connor M, Osborne PB, Furness JB, Christie MJ. Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. J Biol Chem. 2003;278:18776–18784. doi: 10.1074/jbc.M300525200. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Bradizza CM, Stasiewicz PR, Paas ND. Relapse to alcohol and drug use among individuals diagnosed with co-occurring mental health and substance use disorders: a review. Clin Psychol Rev. 2006;26:162–178. doi: 10.1016/j.cpr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Buntin-Mushock C, Phillip L, Moriyama K, Palmer PP. Age-dependent opioid escalation in chronic pain patients. Anesth Analg. 2005;100:1740–1745. doi: 10.1213/01.ANE.0000152191.29311.9B. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J, Shippenberg TS. Evaluation of rat ultrasonic vocalizations as predictors of the conditioned aversive effects of drugs. Psychopharmacol. 2001;155:35–42. doi: 10.1007/s002130100685. [DOI] [PubMed] [Google Scholar]
- Caille S, Espejo EF, Reneric JP, Cador M, Koob GF, Stinus L. Total neurochemical lesion of noradrenergic neurons of the locus ceruleus does not alter either naloxone-precipitated or spontaneous opiate withdrawal nor does it influence ability of clonidine to reverse opiate withdrawal. J Pharmacol Exptl Ther. 1999;290:881–892. [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Celver JP, Lowe J, Kovoor A, Gurevich VV, Chavkin C. Threonine 180 is required for G-protein-coupled receptor kinase 3- and beta-arrestin 2-mediated desensitization of the mu-opioid receptor in Xenopus oocytes. J Biol Chem. 2001;276:4894–4900. doi: 10.1074/jbc.M007437200. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Gintzler AR. Phosphorylation of Galphas influences its association with the micro-opioid receptor and is modulated by long-term morphine exposure. Mol Pharmacol. 2007;72:753–760. doi: 10.1124/mol.107.036145. [DOI] [PubMed] [Google Scholar]
- Chieng B, Christie MJ. Local opioid withdrawal in rat single periaqueductal gray neurons in vitro. J Neurosci. 1996;16:7128–7136. doi: 10.1523/JNEUROSCI.16-22-07128.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng B, Williams JT. Increased opioid inhibition of GABA release in nucleus accumbens during morphine withdrawal. J Neurosci. 1998;18:7033–7039. doi: 10.1523/JNEUROSCI.18-17-07033.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng BC, Hallberg C, Nyberg FJ, Christie MJ. Enhanced c-Fos in periaqueductal grey GABAergic neurons during opioid withdrawal. Neuroreport. 2005;16:1279–1283. doi: 10.1097/01.wnr.0000175246.35837.5c. [DOI] [PubMed] [Google Scholar]
- Christie MJ, Williams JT, North RA. Cellular mechanisms of opioid tolerance: studies in single brain neurons. Mol Pharmacol. 1987;32:633–638. [PubMed] [Google Scholar]
- Christie MJ, Williams JT, Osborne PB, Bellchambers CE. Where is the locus in opioid withdrawal. Trends Pharmacol Sci. 1997;18:134–140. doi: 10.1016/s0165-6147(97)01045-6. [DOI] [PubMed] [Google Scholar]
- Chung S, Pohl S, Zeng J, Civelli O, Reinscheid RK. Endogenous orphanin FQ/nociceptin is involved in the development of morphine tolerance. J Pharmacol Exptl Ther. 2006;318:62–267. doi: 10.1124/jpet.106.103960. [DOI] [PubMed] [Google Scholar]
- Connor M, Christie MJ. Opioid receptor signalling mechanisms. Clin Exptl Pharmacol Physiol. 1999;26:493–499. doi: 10.1046/j.1440-1681.1999.03049.x. [DOI] [PubMed] [Google Scholar]
- Connor M, Osborne PB, Christie MJ. Mu-opioid receptor desensitization: is morphine different. Br J of Pharmacol. 2004;143:685–696. doi: 10.1038/sj.bjp.0705938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Christie MJ.Beta-arrestin2 independent regulation of mu opioid receptor in locus coeruleus neurons Soc Neurosci Abstr 2006. 426.11P; available at
- Dang VC, Williams JT. Chronic morphine treatment reduces recovery from opioid desensitization. J Neurosci. 2004;24:7699–7706. doi: 10.1523/JNEUROSCI.2499-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Williams JT. Morphine-induced mu-opioid receptor desensitization. Mol Pharmacol. 2005;68:1127–1132. doi: 10.1124/mol.105.013185. [DOI] [PubMed] [Google Scholar]
- Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci USA. 2005;102:19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nature Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- Dong Y, Green T, Saal D, Marie H, Neve R, Nestler EJ, et al. CREB modulates excitability of nucleus accumbens neurons. Nature Neurosci. 2006;9:475–477. doi: 10.1038/nn1661. [DOI] [PubMed] [Google Scholar]
- DSMIV Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR 2000American Psychiatric Association: Washington, DC; 4th edn text revision. [Google Scholar]
- Eitan S, Bryant CD, Saliminejad N, Yang YC, Vojdani E, Keith D, Jr, et al. Brain region-specific mechanisms for acute morphine-induced mitogen-activated protein kinase modulation and distinct patterns of activation during analgesic tolerance and locomotor sensitization. J Neurosci. 2003;23:8360–8369. doi: 10.1523/JNEUROSCI.23-23-08360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL. Should we be reluctant to prescribe opioids for chronic non-malignant pain. Pain. 2007;129:233–234. doi: 10.1016/j.pain.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci. 2006;26:2788–2797. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, et al. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold SJ, Han MH, Herman AE, Ni YG, Pudiak CM, Aghajanian GK, et al. Regulation of RGS proteins by chronic morphine in rat locus coeruleus. Eur J Neurosci. 2003;17:971–980. doi: 10.1046/j.1460-9568.2003.02529.x. [DOI] [PubMed] [Google Scholar]
- Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, et al. An opioid agonist that does not induce μ-opioid receptor-arrestin interactions or receptor internalization. Mol Pharmacol. 2007;71:549–557. doi: 10.1124/mol.106.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstock-Debic H, Wein M, Barrot M, Colago EE, Rahman Z, Neve RL, et al. Morphine acutely regulates opioid receptor trafficking selectively in dendrites of nucleus accumbens neurons. J Neurosci. 2003;23:4324–4332. doi: 10.1523/JNEUROSCI.23-10-04324.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack SP, Bagley EE, Chieng BC, Christie MJ. Induction of delta-opioid receptor function in the midbrain after chronic morphine treatment. J Neurosci. 2005;25:3192–3198. doi: 10.1523/JNEUROSCI.4585-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack SP, Vaughan CW, Christie MJ. Modulation of GABA release during morphine withdrawal in midbrain neurons in vitro. Neuropharmacol. 2003;45:575–584. doi: 10.1016/s0028-3908(03)00205-3. [DOI] [PubMed] [Google Scholar]
- Hacker J, Pedersen NP, Chieng BC, Keay KA, Christie MJ. Enhanced Fos expression in glutamic acid decarboxylase immunoreactive neurons of the mouse periaqueductal grey during opioid withdrawal. Neurosci. 2006;137:1389–1396. doi: 10.1016/j.neuroscience.2005.10.052. [DOI] [PubMed] [Google Scholar]
- Han MH, Bolanos CA, Green TA, Olson VG, Neve RL, Liu RJ, et al. Role of cAMP response element-binding protein in the rat locus ceruleus: regulation of neuronal activity and opiate withdrawal behaviors. J Neurosci. 2006;26:4624–4629. doi: 10.1523/JNEUROSCI.4701-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Activation in extended amygdala corresponds to altered hedonic processing during protracted morphine withdrawal. Behav Brain Res. 2007;176:251–258. doi: 10.1016/j.bbr.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Fong J, von Zastrow M, Whistler JL. Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell. 2002;108:271–282. doi: 10.1016/s0092-8674(02)00613-x. [DOI] [PubMed] [Google Scholar]
- Heidbreder C. Novel pharmacotherapeutic targets for the management of drug addiction. Eur J Pharmacol. 2005;526:101–112. doi: 10.1016/j.ejphar.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Himmelsbach CK. With reference to physical dependence. Federation Proc. 1943;2:201–203. [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Fossum EN, Morgan MM. Behavioral and electrophysiological evidence for opioid tolerance in adolescent rats. Neuropsychopharmacol. 2007;32:600–606. doi: 10.1038/sj.npp.1301139. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Prasad BM, Amara SG. Dopamine transporter-mediated conductances increase excitability of midbrain dopamine neurons. Nature Neurosci. 2002;5:971–978. doi: 10.1038/nn920. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Vaughan CW, Bagley EE, Connor M, Christie MJ. Enhanced opioid efficacy in opioid dependence is caused by an altered signal transduction pathway. J Neurosci. 1998;18:10269–10276. doi: 10.1523/JNEUROSCI.18-24-10269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Mishina M, Ueda H. Locus-specific rescue of GluRepsilon1 NMDA receptors in mutant mice identifies the brain regions important for morphine tolerance and dependence. J Neurosci. 2003;23:6529–6536. doi: 10.1523/JNEUROSCI.23-16-06529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, Aston-Jones G. Local opiate withdrawal in locus coeruleus neurons in vitro. J Neurophysiol. 2001;85:2388–2397. doi: 10.1152/jn.2001.85.6.2388. [DOI] [PubMed] [Google Scholar]
- Jaffe JH.Drug addiction and drug abuse Goodman and Gilman's The Pharmacological Basis of Therapeutics 1985Macmillan: New York; 532–581.In: Goodman Gilman A, Goodman LS, Rall TW, Murad F (eds).7th edn. [Google Scholar]
- Johnson EE, Christie MJ, Connor M. The role of opioid receptor phosphorylation and trafficking in adaptations to persistent opioid treatment. Neurosignals. 2005;14:290–302. doi: 10.1159/000093044. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BA, Trapaidze N, Gomes I, Nivarthi R, Devi LA. Oligomerization of opioid receptors with beta 2-adrenergic receptors: a role in trafficking and mitogen-activated protein kinase activation. Proc Natl Acad Sci USA. 2001;98:343–348. doi: 10.1073/pnas.011384898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Sensitization to repeated morphine injection in the rat: possible involvement of A10 dopamine neurons. J Pharmacol Exptl Ther. 1987;241:204–212. [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nature Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, et al. Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem. 1996;271:19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- King T, Gardell LR, Wang R, Vardanyan A, Ossipov MH, Malan TP, Jr, et al. Role of NK-1 neurotransmission in opioid-induced hyperalgesia. Pain. 2005;116:276–288. doi: 10.1016/j.pain.2005.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch T, Brandenburg LO, Schulz S, Liang Y, Klein J, Hollt V. ADP-ribosylation factor-dependent phospholipase D2 activation is required for agonist-induced mu-opioid receptor endocytosis. J Biol Chem. 2003;278:9979–9985. doi: 10.1074/jbc.M206709200. [DOI] [PubMed] [Google Scholar]
- Koch T, Schulz S, Schroder H, Wolf R, Raulf E, Hollt V. Carboxyl-terminal splicing of the rat μ opioid receptor modulates agonist-mediated internalization and receptor resensitization. J Biol Chem. 1998;273:13652–13657. doi: 10.1074/jbc.273.22.13652. [DOI] [PubMed] [Google Scholar]
- Koch T, Widera A, Bartzsch K, Schulz S, Brandenburg LO, Wundrack N, et al. Receptor endocytosis counteracts the development of opioid tolerance. Mol Pharmacol. 2005;67:280–287. doi: 10.1124/mol.104.004994. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side' of drug addiction. Nature Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Kuehn BM. Prescription drug abuse rises globally. JAMA. 2007;297:1306. doi: 10.1001/jama.297.12.1306. [DOI] [PubMed] [Google Scholar]
- Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- Le Moal M, Koob GF. Drug addiction: pathways to the disease and pathophysiological perspectives. Eur Neuropsychopharmacol. 2007;17:377–393. doi: 10.1016/j.euroneuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Li LY, Chang KJ. The stimulatory effect of opioids on mitogen-activated protein kinase in Chinese hamster ovary cells transfected to express mu-opioid receptors. Mol Pharmacol. 1996;50:599–602. [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Ma J, Pan ZZ. Contribution of brainstem GABA(A) synaptic transmission to morphine analgesic tolerance. Pain. 2006;122:163–173. doi: 10.1016/j.pain.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mameli M, Balland B, Lujan R, Luscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci USA. 2006;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercadante S, Villari P, Ferrera P, Casuccio A. Addition of a second opioid may improve opioid response in cancer pain: preliminary data. Suppor Care Cancer. 2004;12:762–766. doi: 10.1007/s00520-004-0650-1. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Vaughan CW, Schnell SA, Wessendorf MW, Christie MJ. Rostral ventromedial medulla neurons that project to the spinal cord express multiple opioid receptor phenotypes. J Neurosci. 2002;22:10847–10855. doi: 10.1523/JNEUROSCI.22-24-10847.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer CJ, Eisch AJ, Carlezon WA, Jr, Whisler K, Shen L, Wolf DH, et al. Role for GDNF in biochemical and behavioral adaptations to drugs of abuse. Neuron. 2000;26:247–257. doi: 10.1016/s0896-6273(00)81154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Suzuki M, Narita M, Yajima Y, Suzuki R, Shioda S, et al. Neuronal protein kinase C gamma-dependent proliferation and hypertrophy of spinal cord astrocytes following repeated in vivo administration of morphine. Eur J Neurosci. 2004;19:479–484. doi: 10.1111/j.0953-816x.2003.03119.x. [DOI] [PubMed] [Google Scholar]
- Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446:1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- Pan ZZ. mu-Opposing actions of the kappa-opioid receptor. Trends Pharmacol Sci. 1998;19:94–99. doi: 10.1016/s0165-6147(98)01169-9. [DOI] [PubMed] [Google Scholar]
- Patel MB, Patel CN, Rajashekara V, Yoburn BC. Opioid agonists differentially regulate mu-opioid receptors and trafficking proteins in vivo. Mol Pharmacol. 2002;62:1464–1470. doi: 10.1124/mol.62.6.1464. [DOI] [PubMed] [Google Scholar]
- Polakiewicz RD, Schieferl SM, Dorner LF, Kansra V, Comb MJ. A mitogen-activated protein kinase pathway is required for μ-opioid receptor desensitization. J Biol Chem. 1998;273:12402–12406. doi: 10.1074/jbc.273.20.12402. [DOI] [PubMed] [Google Scholar]
- Pu L, Bao GB, Xu NJ, Ma L, Pei G. Hippocampal long-term potentiation is reduced by chronic opiate treatment and can be restored by re-exposure to opiates. J Neurosci. 2002;22:1914–1921. doi: 10.1523/JNEUROSCI.22-05-01914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich MM, Wenner P. Sensing and expressing homeostatic synaptic plasticity. Trends Neurosci. 2007;30:119–125. doi: 10.1016/j.tins.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Bolanos CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, et al. IRS2–Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nature Neurosci. 2007;10:93–99. doi: 10.1038/nn1812. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sadee W, Wang D, Bilsky EJ. Basal opioid receptor activity, neutral antagonists, and therapeutic opportunities. Life Sci. 2005;76:1427–1437. doi: 10.1016/j.lfs.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Schallmach E, Steiner D, Vogel Z. Adenylyl cyclase type II activity is regulated by two different mechanisms: implications for acute and chronic opioid exposure. Neuropharmacol. 2006;50:998–1005. doi: 10.1016/j.neuropharm.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Shaw-Lutchman TZ, Barrot M, Wallace T, Gilden L, Zachariou V, Impey S, et al. Regional and cellular mapping of cAMP response element-mediated transcription during naltrexone-precipitated morphine withdrawal. J Neurosci. 2002;22:3663–3672. doi: 10.1523/JNEUROSCI.22-09-03663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KF, Crain SM. Cholera toxin-A subunit blocks opioid excitatory effects on sensory neuron action potentials indicating mediation by Gs-linked opioid receptors. Brain Res. 1990;525:225–231. doi: 10.1016/0006-8993(90)90868-c. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. Multifaceted roles of beta-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochem J. 2003;375:503–515. doi: 10.1042/BJ20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Selley DE, Vogt LJ, Childers SR, Martin TJ. Chronic heroin self-administration desensitizes mu opioid receptor-activated G-proteins in specific regions of rat brain. J Neurosci. 2000;20:4555–4562. doi: 10.1523/JNEUROSCI.20-12-04555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39:281–286. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Heilig M. Addiction and its brain science. Addiction. 2005;100:1813–1822. doi: 10.1111/j.1360-0443.2005.01260.x. [DOI] [PubMed] [Google Scholar]
- Stafford J, Degenhardt E, Black R, Bruno R, Buckingham K, Fetherston J, et al. Australian Drug trends 2004 2004. Findings from the Illicit Drug Reporting System (IDRS) National Drug and Alcohol Centre (NDARC) Monograph No. 55
- Stafford K, Gomes AB, Shen J, Yoburn BC. μ-Opioid receptor downregulation contributes to opioid tolerance in vivo. Pharmacol Biochem Behav. 2001;69:233–237. doi: 10.1016/s0091-3057(01)00525-1. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Stobbs SH, Colago EE, Lee RS, Koob GF, Gallegos RA, et al. Contingent and non-contingent effects of heroin on mu-opioid receptor-containing ventral tegmental area GABA neurons. Exptl Neurol. 2006;202:139–151. doi: 10.1016/j.expneurol.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Trafton JA, Abbadie C, Marek K, Basbaum AI. Postsynaptic signaling via the [mu]-opioid receptor: responses of dorsal horn neurons to exogenous opioids and noxious stimulation. J Neurosci. 2000;20:8578–8584. doi: 10.1523/JNEUROSCI.20-23-08578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapaidze N, Gomes I, Cvejic S, Bansinath M, Devi LA. Opioid receptor endocytosis and activation of MAP kinase pathway. Brain Res Mol Brain Res. 2000;76:220–228. doi: 10.1016/s0169-328x(00)00002-4. [DOI] [PubMed] [Google Scholar]
- Ueda H, Inoue M, Takeshima H, Iwasawa Y. Enhanced spinal nociceptin receptor expression develops morphine tolerance and dependence. J Neurosci. 2000;20:7640–7647. doi: 10.1523/JNEUROSCI.20-20-07640.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- Waldhoer M, Fong J, Jones RM, Lunzer MM, Sharma SK, Kostenis E, et al. A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc Natl Acad Sci USA. 2005;102:9050–9055. doi: 10.1073/pnas.0501112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walwyn W, Evans CJ, Hales TG. Beta-arrestin2 and c-Src regulate the constitutive activity and recycling of μ opioid receptors in dorsal root ganglion neurons. J Neurosci. 2007;27:5092–5104. doi: 10.1523/JNEUROSCI.1157-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28:661–669. doi: 10.1016/j.tins.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Watts VJ, Neve KA. Sensitization of adenylate cyclase by Galpha i/o-coupled receptors. Pharmacol Ther. 2005;106:405–421. doi: 10.1016/j.pharmthera.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M. Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron. 1999;23:737–746. doi: 10.1016/s0896-6273(01)80032-5. [DOI] [PubMed] [Google Scholar]
- Whistler JL, von Zastrow M. Morphine-activated opioid receptors elude desensitization by beta-arrestin. Proc Natl Acad Sci USA. 1998;95:9914–9919. doi: 10.1073/pnas.95.17.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nature Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, et al. An essential role for DeltaFosB in the nucleus accumbens in morphine action. Nature Neurosci. 2006;9:205–211. doi: 10.1038/nn1636. [DOI] [PubMed] [Google Scholar]
- Zamah AM, Delahunty M, Luttrell LM, Lefkowitz RJ. Protein kinase A-mediated phosphorylation of the beta 2-adrenergic receptor regulates its coupling to Gs and Gi. Demonstration in a reconstituted system. J Biol Chem. 2002;277:31249–31256. doi: 10.1074/jbc.M202753200. [DOI] [PubMed] [Google Scholar]