Abstract
Cold acclimation of plants involves extensive reprogramming of gene expression. In Arabidopsis (Arabidopsis thaliana), three cold-inducible transcriptional activators designated CBF1 to -3/DREB1a to -c have been shown to play an important regulatory role in this acclimation process. Similarly to Arabidopsis, boreal zone trees can increase their freezing tolerance (FT) in response to low temperature during the growing season. However, maximal FT of these trees requires short daylength-induced dormancy development followed by exposure to both low and freezing temperatures. To elucidate the molecular basis of FT in overwintering trees, we characterized the role of birch (Betula pendula) CBF transcription factors in the cold acclimation process. We identified four putative CBF orthologs in a birch expressed sequence tag collection designated BpCBF1 to -4. Ectopic expression of birch CBFs in Arabidopsis resulted in constitutive expression of endogenous CBF target genes and increased FT of nonacclimated transgenic plants. In addition, these plants showed stunted growth and delayed flowering, typical features for CBF-overexpressing plants. Expression analysis in birch showed that BpCBF1 to -4 are low temperature responsive but differentially regulated in dormant and growing plants, the expression being delayed in dormant tissues. Freeze-thaw treatment, simulating wintertime conditions in nature, resulted in strong induction of BpCBF genes during thawing, followed by induction of a CBF target gene, BpLTI36. These results suggest that in addition to their role in cold acclimation during the growing season, birch CBFs appear to contribute to control of winter hardiness in birch.
Plants native to temperate and boreal zones are characterized by their ability to tolerate freezing temperatures. Freezing tolerance (FT) is not a constant property but increases in response to various environmental cues preceding frost, such as low temperature (LT) and short daylength (SD) in woody perennials, and appears to involve extensive reprogramming of gene expression (Guy, 1990; Thomashow, 1999; Bassett et al., 2006; Welling and Palva, 2006; Yamaguchi-Shinozaki and Shinozaki, 2006). The best characterized genetic control of this cold acclimation process is executed by the CBF/DREB1 (for C-REPEAT BINDING FACTOR/DEHYDRATION RESPONSIVE ELEMENT BINDING) cold response pathway in Arabidopsis (Arabidopsis thaliana; Thomashow, 1999; Yamaguchi-Shinozaki and Shinozaki, 2006). CBF/DREB1 proteins belong to a transcription factor family that is characterized by the APETALA2/ETHYLENE RESPONSIVE FACTOR (AP2/ERF) DNA-binding domain. The AP2/ERF superfamily consists of 147 genes in Arabidopsis and is further divided into four families: AP2, ERF, RAV, and At4g13040, which was designated as soloist (Nakano et al., 2006). The ERF family is further divided into two major subfamilies, the ERF and CBF/DREB proteins (Sakuma et al., 2002). DREB proteins bind to the DRE/CRT/LTRE sequence (for DEHYDRATION RESPONSIVE ELEMENT/C-REPEAT/LOW TEMPERATURE RESPONSIVE ELEMENT), a cis-element containing the conserved CCGAC core sequence (Baker et al., 1994; Yamaguchi-Shinozaki and Shinozaki, 1994). Expression of the DREB2 genes is induced by drought and high salt but not by LT, whereas DREB1a to -c/CBF1 to -3 genes are responsive to LT but not to dehydration or salt stress. The DREB1d/CBF4 gene is not LT responsive (Haake et al., 2002). The DREB1/CBF proteins are characterized by conserved CBF signature sequences located immediately upstream (PKK/RPAGRxKFxETRHP) and downstream (DSAWR) of the AP2/ERF domain (Jaglo et al., 2001). Exposure to low, nonfreezing temperatures triggers the acclimation process in Arabidopsis, and within 15 min of LT exposure CBF1, CBF2, and CBF3 are induced (Stockinger et al., 1997; Gilmour et al., 1998; Liu et al., 1998; Kasuga et al., 1999; Medina et al., 1999). This is quickly followed by an up-regulation of a large number of cold-regulated (COR) genes whose products are thought to contribute to a plant's tolerance to abiotic stresses, like freezing and high salt (Fowler and Thomashow, 2002). About 12% of such COR genes encompassing more than 40 genes have been shown to belong to the “CBF regulon” (Fowler and Thomashow, 2002; Seki et al., 2002; Maruyama et al., 2004; Vogel et al., 2005). CBF regulon genes are typically characterized by the presence of the DRE/CRT/LTRE promoter element (Baker et al., 1994; Yamaguchi-Shinozaki and Shinozaki, 1994). Constitutive expression of CBF genes results in up-regulation of CBF regulon genes and increased nonacclimated FT, as demonstrated by overexpression studies (Jaglo-Ottosen et al., 1998; Kasuga et al., 1999; Gilmour et al., 2000, 2004; Haake et al., 2002) or in naturally existing cold-tolerant accessions of Arabidopsis (Hannah et al., 2006), suggesting that CBFs have an important role in cold acclimation.
In addition to Arabidopsis, the CBF/DREB1 transcription factor family is present in a wide array of plants, including plants that do cold acclimate, like Brassica napus (Jaglo et al., 2001) and barley (Hordeum vulgare; Choi et al., 2002), but they are also present in plants that do not cold acclimate, like tomato (Lycopersicon esculentum) and rice (Oryza sativa; Jaglo et al., 2001; Dubouzet et al., 2003; Zhang et al., 2004). Zhang et al. (2004) showed that the CBF regulon of tomato is smaller and less diverse than that of Arabidopsis, which may explain partly why tomato cannot cold acclimate. The other extreme in cold acclimation capacity is represented by boreal and temperate zone woody plants. Similarly to Arabidopsis, these trees can increase their FT in response to LT during the growing season (Puhakainen et al., 2004). However, maximal FT of these trees is achieved during seasonal acclimation, by a sequential process induced by SD and by low and freezing temperatures (Weiser, 1970; Welling and Palva, 2006). Boreal and temperate zone trees stop growth and enter dormancy as well as show a moderate increase in FT in response to SD in the fall. For example, a few weeks of SD exposure alone can increase FT of birch (Betula pendula) to approximately −20°C (Rinne et al., 1998, 1999; Welling et al., 2004). After encountering low, nonfreezing temperatures during late fall, FT of birch reaches temperatures below −40°C, but the maximal tolerance (below −70°C) is only achieved after the trees are exposed to freezing temperatures (Rinne et al., 1998; Welling et al., 2004). The deacclimation process of birch in the spring follows the increase in temperature, but the trees are also able to reacclimate upon a substantial temperature decrease (Welling et al., 2004). Despite the increasing number of reports characterizing the cold acclimation process of woody plants at the molecular level, the mechanism for the extreme hardiness during winter is still largely unknown. Our previous results indicated that the CBF pathway is also operational in trees (Puhakainen et al., 2004), and indeed, the presence of putative CBF orthologs was recently described in various woody plants, for example, in sweet cherry (Prunus avium; Kitashiba et al., 2004), poplar (Populus spp.; Benedict et al., 2006), Eucalyptus gunnii (El Kayal et al., 2006), grape (Vitis; Xiao et al., 2006), and Citrus (Champ et al., 2007). Similar to herbaceous species, woody plants show positive correlation between FT and CBF transcript accumulation (El Kayal et al., 2006; Champ et al., 2007). Benedict et al. (2006) demonstrated that constitutive expression of Arabidopsis CBF1 in poplar activates similar types of genes in poplar as it has been shown to activate in Arabidopsis. However, the CBF regulons were different between annual and perennial tissues (Benedict et al., 2006). In addition, although all four poplar CBF genes were up-regulated by LT in leaves, only two poplar CBFs were up-regulated by cold in perennial tissues (Benedict et al., 2006), suggesting that specific CBFs might have distinct roles in perennial and annual tissues.
Previous reports demonstrate extensive changes in transcript and protein accumulation in different tree species during the coldest winter months (Artlip et al., 1997; Rinne et al., 1998; Welling et al., 2004; Wisniewski et al., 2006), indicating that even the winter hardiness is regulated at the molecular level. One of the most extensively studied gene families contributing to cold tolerance of woody plants during overwintering encodes dehydrins. Several studies have demonstrated increased accumulation of dehydrin transcripts or proteins during the coldest months (Artlip et al., 1997; Rinne et al., 1998; Welling et al., 2004; Wisniewski et al., 2006). Recent studies demonstrate that similarly to Arabidopsis, cold-inducible dehydrin genes in woody plants contain CRT elements in their promoters (Puhakainen et al., 2004; Benedict et al., 2006; Wisniewski et al., 2006), suggesting that CBFs control their expression at LT. The birch dehydrin gene BpLTI36 is cold inducible and contains several CRT elements in its promoter. Reporter gene-promoter fusion analyses showed that Arabidopsis CBFs recognize the birch element, causing an induction of the reporter gene, suggesting that birch also has an operational CBF regulon (Puhakainen et al., 2004). In peach (Prunus persica), the LT-inducible dehydrin gene Ppdhn1 harbors several CRT promoter elements and is up-regulated during winter, whereas Ppdhn2 does not contain CRT regulatory elements and is solely drought inducible (Wisniewski et al., 2006), suggesting a role for CBFs in regulating LT-responsive genes during overwintering.
To elucidate the regulatory network controlling cold acclimation in birch, we isolated putative birch CBF orthologs and studied their expression both in actively growing trees in response to LT and in dormant plants in response to low and freezing temperatures. Sequence analysis of the isolated birch genes and functional studies in transgenic Arabidopsis suggest that they are indeed orthologs of Arabidopsis CBF genes participating in the regulation of cold acclimation. Real-time quantitative reverse transcription (RT)-PCR analysis on birch demonstrates that birch CBF genes are responsive to LT but differentially regulated in dormant and growing plants. Importantly, the responsiveness of the birch CBFs to freeze-thaw treatments indicates that, in addition to cold acclimation during the growing season, the CBFs are also involved in the regulation of cold tolerance during overwintering.
RESULTS
CBF Genes of Birch
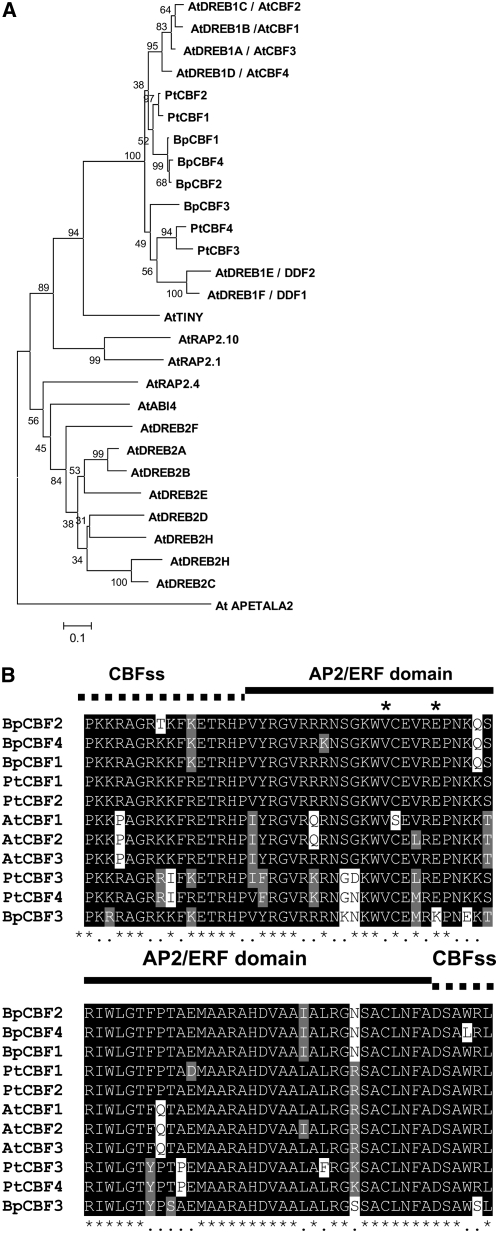
Using an iterative tBLASTn search against conserved AP2/ERF sequences from Arabidopsis, we found several clones encoding both AP2/ERF and flanking CBF signature domains in the birch EST database (Aalto and Palva, 2006). Further analysis revealed that they corresponded to four distinct genes designated BpCBF1, BpCBF2, BpCBF3, and BpCBF4 (for B. pendula CBF1, -2, -3, and -4, respectively). The BpCBF3 and BpCBF4 genes were represented by only one EST clone. BpCBF3 lacked the 5′ terminal end of the coding region, while the EST clone of BpCBF4 did not cover the whole 3′ terminus of the coding region. The full-length sequences encoded small proteins of 202 amino acids long. We made a phylogenetic analysis of the deduced protein sequences of the four birch CBF candidate genes with known Arabidopsis DREB family proteins and poplar CBF1 to -4 proteins, using the AP2 transcription factor as the out group. Poplar and birch CBF proteins were grouped together with Arabidopsis DREB1 proteins, distinct from the rest of the Arabidopsis DREB subfamily (Fig. 1A). PtCBF1 and -2, BpCBF1 and -2, and BpCBF4 were more closely related to Arabidopsis DREB1a to -d/CBF1 to -4 proteins, whereas PtCBF3 and -4 and BpCBF3 were more closely related to Arabidopsis DREB1e and -f (DDF) proteins.
Figure 1.
Comparison of CBF/DREB1 protein family members of Arabidopsis, poplar, and birch. A, A phylogenetic analysis of birch and poplar CBF proteins and the Arabidopsis DREB family of proteins. Analysis, based on minimum evolution, was performed with the full-length protein sequences using the AP2 transcription factor as an out group. Alignment was made using four birch CBF proteins: BpCBF1 (ABP98987), BpCBF2 (ABP98988), BpCBF3 (ABP98989), and BpCBF4 (FG124897); four poplar CBF proteins: PtCBF1 (JGI666968), PtCBF2 (JGI346104), PtCBF3 (JGI548519), and PtCBF4 (JGI63608); and the Arabidopsis DREB family of proteins, which includes four CBF/DREB1 proteins: AtDREB1b/AtCBF1 (At4g25490), AtDREB1c/AtCBF2 (At4g25470), AtDREB1a/AtCBF3 (At4g25480), and AtDREB1d/AtCBF4 (At5g51990), as well as AtDREB1F/DDF1 (At1g12610), AtDREB1E/DDF2 (At1g63030), AtTINY (At5g25810), AtRAP2.10 (At4g36900), AtRAP2.1 (At1g46768), AtABI4 (At2g40220), AtRAP2.4 (At1g78080), AtDREB2D (At1g75490), AtDREB2G (At5g18450), AtDREB2F (At3g57600), AtDREB2E (At2g38340), AtDREB2A (At5g05410), AtDREB2B (At3g11020), AtDREB2H (At2g40350), AtDREB2C (At2g40340), and AtAP2 (At4g36920). CBF proteins were initially aligned using ClustalW and used to conduct phylogenetic analysis using MEGA version 4.1 software (http://www.megasoftware.net/). The phylogenetic tree was constructed using the neighbor-joining method on 1,000 bootstrap replications. Bootstrap percentages are shown on the dendrogram branch points. B, Alignment of AP2 and flanking CBF signature sequences of Arabidopsis, poplar, and birch CBF transcription factors. CBF signature sequences (CBFss) are marked with a dotted line, and the AP2/ERF domain is marked with a solid line. The 14th and 19th amino acids of AP2/ERF domain are marked with asterisks.
Alignment of the deduced amino acid sequences of birch CBF1 to -4 with those of Arabidopsis CBF1 to -3 and Populus trichocarpa CBF1 to -4 demonstrated significant similarity in the AP2/ERF binding domain and the CBF signature sequences (Jaglo et al., 2001) between species (Fig. 1B). Sakuma et al. (2002) demonstrated that Val-14 with Glu-19 and Ala-14 together with Asp-19 in the AP2/ERF domain determine the binding specificity of DREB and ERF proteins, respectively. BpCBF1 and -2 and BpCBF4 carry both Val-14 and Glu-19, whereas BpCBF3 carries Lys-19. Similar to poplar, all birch CBFs utilize an Arg residue at the fourth position of the upstream CBF signature sequence instead of the Pro common to other dicots (Benedict et al., 2006). BpCBF3 also has a single amino acid substitution in the downstream CBF signature sequence, replacing an Arg residue with Ser, whereas BpCBF4 has Leu replaced with Trp (Fig. 1B). These results suggest that the birch CBFs share remarkable similarities at amino acid sequence levels with known CBF/DREB proteins and carry critical amino acids that are needed for binding to the CRT elements in the target genes. Alignments of full-length sequences also revealed the existence of additional regions outside the AP2/ERF domain and CBF signature sequences that were highly conserved between the Arabidopsis, poplar, and birch CBF proteins (Supplemental Fig. S1). In Southern-blot analysis, three to four fragments hybridizing to a BpCBF probe could be detected after cutting the DNA with restriction enzymes that do not cut within the four BpCBF genes, whereas when using EcoRI, which cuts BpCBF1 once, it was possible to detect five bands. The resulting fragments were rather large (most of them being more than 5 kb); hence, we cannot rule out the possibility that some of them might contain more than one CBF gene. These results indicate that birch CBF genes form a small gene family consisting of four to six members (Supplemental Fig. S2).
Birch CBFs Are Functional in Arabidopsis
The extensive structural similarity of birch CBFs to those of Arabidopsis suggested similar function. To test whether the birch CBF proteins are indeed orthologs of Arabidopsis CBF proteins, we generated transgenic Arabidopsis plants constitutively expressing BpCBF1 and BpCBF2 full-length cDNAs under the control of the cauliflower mosaic virus 35S promoter. Thirty-two and 18 independent transgenic lines expressing the BpCBF1 and BpCBF2 genes, respectively, were identified by kanamycin selection. These plants typically showed retarded growth compared with the wild-type and vector control plants and had shorter petioles and darker green leaves, as exemplified by the two independent lines expressing BpCBF1 and BpCBF2 (Supplemental Fig. S3). Most of the lines also showed delayed flowering, some of them flowering several weeks later than the wild type (data not shown). The functionality of both BpCBF1 and BpCBF2 in Arabidopsis was further demonstrated by up-regulation of the normally LT-responsive CBF target gene LTI78 (Jaglo-Ottosen et al., 1998) in nonacclimated transgenic plants (Supplemental Fig. S3). For more detailed functional studies, we selected two independent lines (9-3 and 9-17) that showed relatively high levels of expression of BpCBF1 and LTI78 genes (Supplemental Fig. S3) and yet were flowering and producing seeds in a reasonable time.
Overexpression of CBF genes results in induction of the CBF regulon and consequently an increase in plant FT (Gilmour et al., 2004). To confirm the functional similarity between birch and Arabidopsis CBF genes, we assessed the FT of the transgenic plants overexpressing the birch BpCBF1 gene and characterized the expression level of LTI78 and COR47, both known downstream target genes for CBF (Jaglo-Ottosen et al., 1998). FT was measured by the ion-leakage method. Under nonacclimating conditions, wild-type and vector control plants had LT50 values (defined as the temperature that causes 50% leakage of the ions) of −5°C and −5.5°C, respectively, whereas plants overexpressing BpCBF1 had LT50 of −12°C and plants overexpressing AtCBF3 had LT50 of −13°C (Supplemental Fig. S4). Northern-blot analysis indicated that under normal growth conditions, transcript levels of LTI78 and COR47 were low in the wild-type and vector control plants, whereas the genes were highly up-regulated in plants overexpressing either the Arabidopsis CBF3 gene (CBF3) or the birch BpCBF1 gene (lines 9-3 and 9-17; Supplemental Fig. S4).
CBF Gene Expression Differs in Growing and Dormant Tissues in Birch
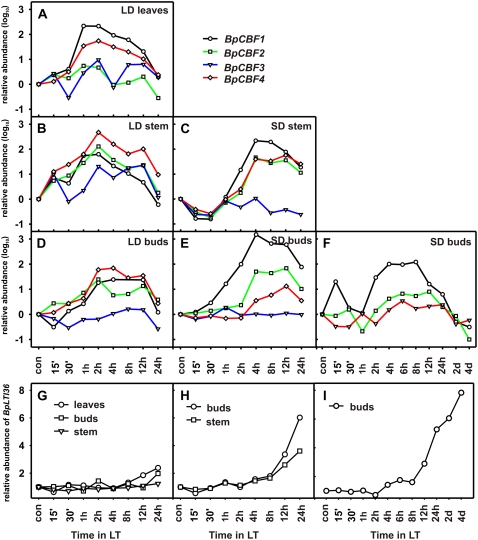
In Arabidopsis, CBF genes are rapidly but transiently induced in response to LT and in turn activate the expression of several genes that are thought to contribute to the increase in FT (Gilmour et al., 1998; Zarka et al., 2003). To explore the role of BpCBF genes in the regulation of FT in birch, we characterized transcript levels of birch BpCBF1 to -4 as well as the transcript level of a BpCBF target gene, BpLTI36, in response to LT both in growing plants under long-daylength (LD) conditions and in dormant plants under SD conditions with quantitative real-time RT-PCR. The four birch CBF genes were LT responsive, but the timing of expression was different in active and dormant tissues, so that under SD conditions transcript accumulation started later and lasted longer (Fig. 2). Under LD conditions, in growing birch, transcript levels of birch CBF genes started to accumulate after 15 or 30 min of exposure to LT, peaking between 1 and 2 h, and declining gradually after that (Fig. 2, A, B, and D). In dormant, SD-exposed plants, BpCBF transcripts started to accumulate in most cases at 1 or 2 h after the LT exposure, peaking between 4 and 12 h, and starting to decline after that. Except for BpCBF3 gene, transcript levels did not decline back to basic levels until after 2 d of LT exposure (Fig. 2, A–F). In buds that had been under SD conditions for 11 weeks prior to LT treatment, the timing of the CBF transcript accumulation was the same as after 6 weeks of SD treatment, but the maximum levels were lower (Fig. 2F). The maximal increase in transcript levels achieved varied tremendously between the genes. Of the four birch CBF genes, the increase in BpCBF1 transcript levels was several times higher in LD-grown leaves as well as in stems and buds under SD conditions compared with other BpCBF genes (Fig. 2, A, C, and E). Under LD conditions, the level of BpCBF4 transcript increased most in buds and stems, although the difference from other birch CBF genes was not as dramatic as that of BpCBF1 under SD (Fig. 2, B and D). The response of BpCBF2 to LT was strong in buds and stems both under SD and LD conditions. The level of BpCBF3 transcript was always much lower compared with that of other CBF genes, especially after 11 weeks of SD treatment, when the level was so low that it was not detected in real-time quantitative RT-PCR, except after the plants were exposed to freezing temperatures (Fig. 3). Transcript accumulation of the birch CBF target gene BpLTI36 was sequential to that of BpCBF genes, starting approximately 8 h after LT exposure in SD and LD conditions, and the transcript level continued to increase during the whole 24-h (Fig. 2, G and H) and 4-d (Fig. 2I) study periods. These results demonstrate that birch CBF genes respond rapidly to LT both in active and dormant tissues, suggesting that, in addition to being involved in cold acclimation during the growing season, CBFs could be involved in the regulation of winter hardiness in woody plants.
Figure 2.
Real-time quantitative RT-PCR analysis of BpCBF1 to -4 and BpLTI36 gene expression in growing and dormant birch in response to LT. Samples were collected from leaves (A), stems (B), and buds (D) of growing birch under LD conditions (22-h day, 18°C) or from stems (C) or buds of dormant birch grown under SD conditions (12-h day, 18°C) prior to LT treatment for 6 weeks (E) or 11 weeks (F). BpLTI36 was analyzed in corresponding samples under LD (G) or after 6 (H) or 11 (I) weeks under SD conditions. Samples were collected at 18°C (control) and after 15 or 30 min, 1, 2, 4, 6 (only after 11 weeks of SD), 8, 12, or 24 h, and 2 or 4 d (only after 11 weeks of SD) of LT (2°C) treatment in the corresponding daylength.
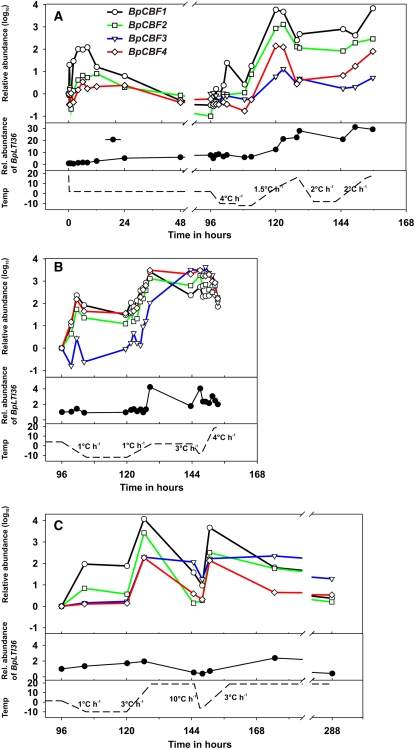
Figure 3.
Real-time quantitative RT-PCR analysis of BpCBF1 to -4 and BpLTI36 gene expression in dormant birch buds (A) and stems (B and C) in response to freeze-thaw treatment. A, Four-month-old birch seedlings were grown under SD conditions (12-h day, 20°C; lights were on from 6 am to 6 pm) for 6 weeks (B and C) or 11 weeks (A), after which they were exposed to LT (2°C [A and C] or 4°C [B]) under the same daylength for 4 d. After this, seedlings were exposed to different freeze-thaw temperatures indicated by the dashed line in the bottom panels. The rate of temperature change is indicated in numbers each time. Freezing treatment started at 9 am in each experiment, 3 h after the lights were switched on. Trees were sprayed with tap water after 1 h at below-zero temperatures to initiate extracellular freezing. Relative transcript abundance was compared with SD control samples before LT treatment (0-h time point; A) or with combined SD and 4-d LT treatment (96-h time point; B and C).
CBF Gene Expression in Birch in Response to Freeze-Thaw
Trees achieve their maximum FT during overwintering in response to freezing temperatures (Weiser, 1970). To study whether birch CBFs are also involved in this type of winter hardiness development, we mimicked the conditions during winter (Welling and Palva, 2006) by exposing dormant birch seedlings to various freeze-thaw cycles followed by measurement of the BpCBF transcript levels by real-time quantitative RT-PCR. In the first experiment, plants were first kept at 2°C for 4 d and then exposed to two subsequent freezing events, first to −12°C and then to −8°C, interrupted by thawing to 18°C. Transfer of plants to 2°C resulted in a transient increase in BpCBF1, -2, and -4 transcripts, returning to the basal level after approximately 48 h of LT exposure. The level of BpCBF3 transcript was below the detection limit (Figs. 2F and 3A). Expression of the target gene, BpLTI36, started to increase after 12 h of LT exposure and remained elevated during the 4 d at 2°C (Figs. 2I and 3A). During rapid freezing (4°C h−1), there was neither accumulation of any of the birch CBF transcripts nor any significant increase in the level of the BpLTI36 transcript. In contrast, thawing of the plants resulted in a very strong accumulation of all BpCBF genes. Similar to the response to combined SD and LT treatment (Fig. 2), transcript levels of BpCBF1 and BpCBF2 increased most in response to freeze-thaw, while the response of BpCBF4 and BpCBF3 was more modest (Fig. 3A). The level of the BpLTI36 transcript increased constantly during the temperature increase (Fig. 3A). Transcript levels of BpCBF genes started to decrease at elevated temperatures, but subsequent freezing prevented further decreases and the levels remained rather constant until the next thawing period, when transcript levels of both BpCBF and BpLTI36 genes increased further (Fig. 3A).
To rule out the possibility that the relatively rapid changes in temperatures would have only shifted the CBF transcript accumulation to the thawing phase, we decreased the rate of temperature changes (Fig. 3B). Dormant seedlings were first exposed to 4°C for 4 d, after which the temperature was decreased to −10°C by 1°C h−1. Plants were kept overnight at −10°C, after which the temperature was increased slowly to 2°C and kept at this temperature for 15 h. This was followed by a second brief freeze-thaw event. A slight increase in BpCBF transcript levels could be seen at the beginning of the freezing period. However, the most prominent increase in the BpCBF transcript level was not evident until during the thawing phase (note the logarithmic scale). The transcript level of BpCBF1 started to increase first, followed by BpCBF4, BpCBF2, and BpCBF3. The transcript levels of BpCBF genes remained high between the freezing periods at 2°C, and the second freezing event increased the levels only slightly (Fig. 3B). The BpLTI36 transcript level also increased during the thawing period, subsequent to those of BpCBFs. The level decreased somewhat between the freezing periods, and the second freeze-thaw cycle elevated the level only slightly (Fig. 3B).
Since the transcript levels of BpCBF genes remained rather stable at 2°C following freeze-thaw (Fig. 3B), in contrast to being transiently elevated as in response to LT only (Figs. 2 and 3A), we characterized the effect of deacclimation temperature on transcript levels of BpCBF and BpLTI36 genes. Plants acclimated for 4 d at 2°C were exposed to two subsequent freeze-thaw cycles, but in contrast to the experiment shown in Figure 3B, the temperature between the two freezing periods was increased to 18°C for 16 h. The level of all BpCBF genes and BpLTI36 transcript accumulated during the thawing phase, but the level of the transcripts also decreased rapidly in response to the elevated temperature during deacclimation. After 5 d of deacclimation, the transcript level of the BpLTI36 had also decreased to a very low level (Fig. 3C). Taken together, these results show that the birch cold-regulated genes BpCBF1 to -4 and BpLTI36 are responsive to freeze-thaw cycles, with the most prominent transcript accumulation taking place during the thawing phase.
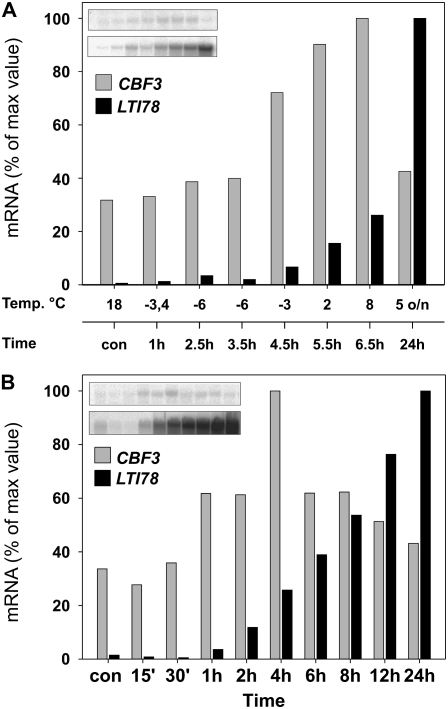
To explore whether the CBF transcript accumulation in response to freeze-thaw is specific to woody plants or was due to the dormant status of the plants, we characterized the effect of freeze-thaw on Arabidopsis CBF expression. The plants were frozen gradually to −6°C, initiating freezing after the plants had been at freezing temperatures for 1 h. Similar to birch, there was no accumulation of CBF3 in Arabidopsis during the freezing phase, but the transcript started to accumulate during the thawing period, after temperature was increased from −6°C to −3°C, and peaked at +8°C, 6 h after the beginning of the freezing experiment (Fig. 4A). This was 5 h later than if the plants were exposed to 2°C only (Fig. 4B). Up-regulation of the CBF target gene LTI78 was initiated 4.5 h after the beginning of freezing experiment, whereas following LT treatment, LTI78 transcripts started to accumulate after 1 h, indicating that freezing delayed the start of transcript accumulation. These results suggest that transcript accumulation during the thawing phase in freeze-thaw experiments could be a common feature between plant species.
Figure 4.
Effects of low and freezing temperatures on the expression response of Arabidopsis CBF3 and LTI78 genes. A, Expression response of CBF3 and LTI78 genes to freezing temperature in Arabidopsis. Three-week-old soil-grown (23°C, 12-h day; lights were on from 6 am to 6 pm) Arabidopsis plants were exposed to freezing temperatures by placing them directly in −2.6°C for 0.5 h, after which the temperature was decreased to −6°C at 1°C h−1. Temperature was kept at −6°C for 1 h, after which it was allowed to increase to 8°C during the next 3 h. Plants were kept at low positive temperature overnight. The experiment started at 8:30 am, and after 1 h at freezing temperature plants were sprayed with tap water to initiate extracellular freezing. The 1-h time sample was collected just before spraying. All of the leaves in the rosette were collected for RNA analysis. B, Expression response of CBF3 and LTI78 genes in Arabidopsis in response to LT. The experimental design was the same as in the freezing experiment, except that plants were exposed to LT (2°C, 12-h day) for the indicated times. LT treatment started at 9 am. The samples were analyzed with RNA gel-blot hybridization using a 32P-labeled cDNA probe for LTI78 and a gene-specific probe for CBF3. The histograms show normalized values for each gene after standardization to ribosomal signal intensities, presented as percentages of the highest value.
DISCUSSION
Birch CBF Genes Are Orthologs of Arabidopsis CBFs
CBF transcription factors have been extensively studied in Arabidopsis and shown to constitute an important element in the cold acclimation process. Although CBF/DREB1 genes are exclusively cold inducible, they are found both in species that can cold acclimate and in plants that do not tolerate freezing, like tomato (Jaglo et al., 2001). CBF transcription factors have also been described in trees (Kitashiba et al., 2004, Benedict et al., 2006, El Kayal et al., 2006; Xiao et al., 2006; Champ et al., 2007), whose cold acclimation capacity exceeds that of any other plant species (for review, see Welling and Palva, 2006). Earlier studies have suggested an important role for CBF transcription factors also in the regulation of cold acclimation in response to LT in woody plants during the growing season. However, temperate and boreal zone trees achieve their maximal cold tolerance during overwintering in response to cold and freezing temperatures. In this study, we have characterized birch CBF genes and demonstrated their induction in both growing and dormant plants in response to low and freezing temperatures, indicating that CBF genes are not only involved in cold acclimation during the growing season to survive episodic frosts but are likely to play a role in the regulation of overwintering of trees, being part of the sensitive mechanisms that trees utilize to acclimate to changing environmental conditions.
This study confirms and expands our earlier suggestion that birch has a functional CBF cold response pathway (Puhakainen et al., 2004). We identified several birch EST clones corresponding to four distinct putative CBF genes. Sequence comparison of these with known CBF genes and functional studies suggest that the identified BpCBF genes are orthologs of Arabidopsis CBF genes. In addition to the conserved AP2/ERF DNA-binding domain, each birch gene was also carrying the CBF signature sequences bracketing the AP2/ERF domain (Fig. 1B), a hallmark of CBF proteins (Jaglo et al., 2001; Nakano et al., 2006). BpCBF1, BpCBF2, and BpCBF4 were very similar in both their nucleotide and their amino acid sequences and were in the same group in the phylogenic tree (Fig. 1A), while the BpCBF3 gene showed some notable differences from the other birch CBF genes. Within the AP2/ERF binding domain, the most interesting difference in BpCBF3 was the Lys-19 instead of the commonly occurring Glu-19 in DREB proteins or Val-19 in ERF proteins (Fig. 1B). Although the 19th amino acid is not as critical as the 14th amino acid in determining the binding specificity of ERF and DREB proteins, according to Sakuma et al. (2002), BpCBF3 still might have some special binding properties compared with other birch CBF proteins. Since the function of the CBF signature sequence after the C terminus of the AP2/ERF domain (DSAWR) is not known, it remains to be elucidated how the replacement of last Arg with Ser in BpCBF3 or the replacement of Leu with Trp in BpCBF4 affects the function of the protein. In plant species whose genomes have been sequenced, it has been verified that CBF homologs are normally encoded by small gene families consisting of three to four members (Gilmour et al., 1998; Dubouzet et al., 2003; Benedict et al., 2006). Accordingly, Southern-blot analysis indicated that birch CBF genes are encoded by a small gene family of four to six members (Supplemental Fig. S2). This approximation could be rather fair, since among the 75,000 birch ESTs representing a large fraction of birch genes (Aalto and Palva, 2006), we identified only the four different CBFs presented in this work.
Overexpression of Arabidopsis CBF1 to -3 in transgenic Arabidopsis plants activates the expression of the target genes containing CRT promoter elements and enhances FT in the absence of LT stimulus (Jaglo-Ottosen et al., 1998; Liu et al., 1998; Kasuga et al., 1999; Gilmour et al., 2000; Haake et al., 2002). To explore the function of birch CBF genes, we used the same approach and expressed BpCBF1 and BpCBF2 in Arabidopsis under the control of the 35S promoter. The transgenic lines overexpressing birch CBF genes were characterized by small size and dark green color compared with control lines (Supplemental Fig. S3), a typical feature of plants overexpressing CBF genes (Liu et al., 1998; Kasuga et al., 1999; Haake et al., 2002; Gilmour et al., 2004). Ectopic expression of BpCBF1 in Arabidopsis resulted in increased FT of these transgenic lines under nonacclimating conditions (Supplemental Fig. S4). These lines also showed constitutive expression of the CBF target genes LTI78 and COR47 (Supplemental Fig. S4), implying that activation of the CBF regulon genes has resulted in the increased FT under nonacclimating conditions. This is in accordance with previous studies in Arabidopsis overexpressing Arabidopsis CBFs (Fowler and Thomashow, 2002; Seki et al., 2002) and with studies by Zhang et al. (2004) and Kitashiba et al. (2004), who showed that ectopic expression of functional CBF orthologs of tomato and sweet cherry, respectively, increased Arabidopsis stress tolerance. Taken together, the phenotypes of the transgenic Arabidopsis lines overexpressing birch CBF genes suggest that the studied birch genes indeed are orthologs of Arabidopsis CBF genes.
Differential CBF Expression in Growing and Dormant Plants
In Arabidopsis, CBF genes are rapidly but transiently up-regulated in response to low, nonfreezing temperatures (LT; Fowler and Thomashow, 2002). Similarly, LT stimulus induced rapid but transient expression of birch CBF genes in leaves, stems, and buds of growing birch as well as in stems and buds of dormant plants followed by down-regulation after 1 or 2 d (Fig. 2). Interestingly, a switch from active growth to dormancy or the SD stimulus seems to alter a plant's response to LT, causing a delay in LT-induced CBF expression under SD conditions (Fig. 2). Comparison of expression kinetics of CBF orthologs in various plant species shows induction of CBF genes in response to LT varying from 15 min in Arabidopsis to several days in grape (Xiao et al., 2006), demonstrating differential regulation of CBF genes between different species and under different conditions. In Eucalyptus leaves, SD did not alter the timing of CBF expression in response to LT but it did enhance the level of CBF expression (El Kayal et al., 2006). This is in accordance with our previous results showing that SD potentiates LT-induced expression of the CBF target gene BpLTI36 in birch leaves (Puhakainen et al., 2004). However, direct comparisons with our data here cannot be done, since in contrast to Puhakainen et al. (2004) and El Kayal et al. (2006), we measured CBF transcript levels in perennial tissues of plants that had been under SD for several weeks and were thus in a dormant state. Physiological changes under SD conditions are accompanied by extensive reprogramming of gene expression, causing, for example, alterations in hormonal responses and down-regulation of genes participating in cell division, expansion, and differentiation as well as in translation and protein turnover (Schrader et al., 2004; Bassett et al., 2006). Such alterations in the physiological state of the plant may explain the observed delays in BpCBF expression. Moreover, trees that are in a dormant state already possess a significant FT without being exposed to LT (Welling et al., 1997, 2002; Rinne et al., 1998). SD has been shown to modulate the expression of a number of CBF regulon genes, including genes for dehydrins or CBFs themselves (Schrader et al., 2004). It has been postulated that CBF paralogs (Novillo et al., 2004) or CBF target genes inhibit CBF expression (Guo et al., 2002), and it remains to be elucidated whether the observed delayed response in LT-induced CBF expression under SD conditions has resulted from such a negative feedback regulation.
CBF Genes Contribute to Overwintering in Trees
Trees achieve their highest FT during winter months in response to freezing temperatures (Weiser, 1970). Consequently, it was of special interest to explore whether CBF genes participate in this type of cold acclimation, as, for example, Wisniewski et al. (2006) showed that one of the dehydrin genes in peach harboring CRT promoter elements was up-regulated in winter during the coldest months. Due to the rapid turnover of CBF transcripts (Zarka et al., 2003), it would be very difficult to study CBF genes under field conditions; therefore, we performed a series of experiments in controlled conditions simulating the freeze-thaw conditions in winter (Welling et al., 2004). Transcript levels of birch CBF genes increased transiently after each freezing period, followed by a gradual increase in expression of the CBF target gene BpLTI36 (Fig. 3). However, transcripts did not start to accumulate until the thawing phase. This pattern of gene expression was evident also in two consecutive experiments with different freeze-thaw temperature regimes (Fig. 3, B and C). Freeze-thaw treatments resulted in a much more dramatic increase in BpCBF gene expression as well as in that of the CBF target gene BpLTI36 compared with LT treatment only (Figs. 2 and 3), suggesting that CBF proteins indeed participate in the third stage of acclimation of woody plants, induced by freezing temperatures (Weiser, 1970).
The reason for the delay in transcript accumulation during freeze-thaw could be that, during freezing, transcript accumulation is simply inhibited. If freezing is very slow, some increase in BpCBF transcript levels could be seen, but after the temperature was low enough, transcripts did not accumulate further (Fig. 3B). Release of this block by thaw implies that some factor in cells “memorizes” the experienced stress, and when conditions become less severe, allows cells to respond accordingly. Several physical or chemical factors might prevent transcript accumulation during freezing conditions. For example, the freezing temperature as such could inhibit cellular functions (i.e. enzymatic reactions). Another possibility is that during freezing, ice formation in the apoplast results in a substantial increase in the osmotic potential of the symplast (Améglio et al., 2001), likely to prevent most of the cellular functions. It has been postulated that Ca2+ functions as an important element in the induction of cold-regulated genes in response to LT (Knight et al., 1996). It is possible that during freezing, the first steps in the cold signaling cascades are activated; for example, Ca2+ is released to cytoplasm, but as the conditions become too severe, the rest of the signaling cascade can be activated only during the thawing phase, resulting in CBF transcript accumulation with increasing temperature (Fig. 3).
It has been known for some time that the ability of cold-hardy plants to resist deacclimation during transient warm spells and to reacclimate when cold temperatures return are pivotal for the winter survival of trees (Kalberer et al., 2006). The mechanism of this regulation, however, is largely unknown. We suggest that the remarkable postfreezing accumulation of CBFs and subsequently their target genes serves to secure adequate FT during overwintering, when trees very commonly can be exposed to temperatures between −30°C and +5°C on consecutive days (Welling et al., 2004). In growing plants, CBF genes are expressed transiently in response to LT, activating CBF target genes, such as BpLTI36, leading to cold acclimation (Supplemental Fig. S5). The level of CBF target genes remains high until the temperature increases again, leading to down-regulation of CBF target genes and deacclimation of the plants (Supplemental Fig. S5). During overwintering, due to the dormant state of the trees and to freezing temperatures that plants are exposed to, the regulation of CBF gene expression is different (Supplemental Fig. S5). Response to low, nonfreezing temperature is delayed in dormant plants but nevertheless leads to transient expression of CBF genes that, in turn, activate CBF target genes (Supplemental Fig. S5). It is important to note that dormant plants already are cold tolerant (Welling et al., 1997, 2002; Rinne et al., 1998), so exposure to freezing temperatures does not cause injury to the plants but functions rather as a booster, leading to the transient increase of BpCBF gene expression during the thaw following each freezing period. BpCBFs in turn activate transcript accumulation of the CBF target genes to a certain level defined by the temperature differential that plants are exposed to (Supplemental Fig. S5). A corresponding accumulation of proteins of the CBF regulon would, in turn, result in an increase in FT. The response of birch to different deacclimation temperatures suggests that, similar to Arabidopsis (Zarka et al., 2003), trees also require desensitization before they are able to respond to decreases in temperature by increasing CBF transcript levels. In addition, since expression of BpLTI36 and other CBF target genes is down-regulated only after a prolonged exposure to warm temperatures (Fig. 3, B and C; Supplemental Fig. S5), plants remain cold acclimated as long as warm spells are not too long and stay prepared for the next freezing event until freezing temperatures no longer exist during spring. Our results thus indicate that CBF genes not only participate in the regulation of FT in birch during the growing season or during autumn and early winter in response to LT and freezing temperatures, respectively, but are also involved in regulation during the deacclimation phase in spring, to help plants adjust their FT level according to the prevailing temperatures.
Postfreezing transcript accumulation correlates roughly with FT of the plants (Supplemental Fig. S5) and thus explains its adaptive value for plants. It is possible that freeze-induced genes such as dehydrins encode proteins that have similar protective functions in low and freezing temperatures, or that they are needed specifically in freeze-thaw conditions. For example, Decourteix et al. (2006) showed that the transcript level of the xylem Suc transporter gene JrSUT1, which shows annual variation in walnut (Juglans regia) tree, did not increase in response to freezing but rather to freeze-thaw treatment. The authors suggest that JrSUT1 participates in the repair of freeze-induced xylem vessel embolism by increasing Suc concentrations in xylem sap, which results in active refilling of embolized vessels through the creation of winter stem pressure. Another example is chestnut (Castanea sativa) small heat shock protein CsHSP17.5, which has been shown to function as a chaperone for cold-labile enzymes, protecting them during repeated freeze-thaw conditions in vitro (Lopez-Matas et al., 2004). Freeze-induced genes are also present in herbaceous species; for example, in barley, some of the dehydrin family members did not accumulate in response to LT but were only responsive to freeze-thaw treatment (Zhu et al., 2000), whereas in Arabidopsis, CBF transcripts accumulated in responses to LT but the expression level was higher after plants were exposed to freezing temperatures (Zarka et al., 2003). We also demonstrated that in Arabidopsis, transcripts of freeze-induced genes (e.g. CBF3 or LTI78) did not accumulate until the thawing phase (Fig. 4), verifying that this feature is shared by different plant species. Therefore, it seems that plants have both genes that are induced in response to low and freezing temperatures and genes that are induced in either condition. Extensive microarray analysis would be required to reveal the differential regulation of cold-induced genes in response to low and freezing temperatures but would no doubt bring new insights to the overwintering regulation of trees.
Taken together, our results bring new insights to the roles and regulation of CBF transcription factors, suggesting that, in addition to being important regulators in LT-induced cold acclimation in growing plants, CBFs are also part of the mechanism that enables very hardy plant species, boreal zone trees, to cope with repeated freeze-thaw periods during winter and to maintain adequate levels of FT. To elucidate the mechanism of transcript accumulation in freeze-thaw conditions is one of the future challenges in the field of cold acclimation studies, and once solved, it would help us understand the mechanism of the extreme FT in plants.
MATERIALS AND METHODS
Growth Conditions and Experimental Design
Seeds of birch (Betula pendula) were derived from trees grown in Kittilä, Finland (67° 40′N). They were germinated and grown in the greenhouse under LD (22.5-h day, 20 ± 2°C) with supplemental lightning (100 μmol m−2 s−1) in 6:2:1 peat:sand:vermiculite (Finnpeat M6; Kekkilä Oyj) with slow-release fertilizer (Osmocote; Scotts) for 4 months, at which time the seedlings were approximately 60 cm long. Some of the plants were then placed in SD conditions (12-h day; 20 ± 2°C, lights on from 6 am to 6 pm; when natural daylength exceeded this, black curtains were used to obtain the desired daylength) for 6 or 11 weeks. At this time, they were in an endodormant state, as measured by bud bursting of single-node cuttings (Rinne et al., 1998). LT and FT were done in a programmable walk-in Phytotron chamber. LT treatment started in each case at 9 am, which for SD-grown plants was 3 h after the lights were turned on. For RNA extraction, three fully grown uppermost leaves, 15-cm pieces of the uppermost part of the stem, and uppermost buds were collected from LD-grown plants, and corresponding stem and bud samples were collected from SD-grown plants. Samples were placed immediately in liquid N2, in which they were stored at −80°C until extraction. Samples were collected at 15 and 30 min and 1, 2, 4, 6 (only 11-week SD-treated plants), 8, 12, and 24 h after beginning the LT treatment.
For freezing experiments, dormant seedlings that had been grown under SD conditions for 6 or 11 weeks were first treated with LT (2°C or 4°C) for 4 d. After this, seedlings were exposed to freeze-thaw treatments of two freezing and thawing cycles. The program and the rate of temperature change for each experiment are indicated in the diagrams in Figure 3. In the first experiment, seedlings were frozen to −12°C and −8°C, letting the temperature increase shortly to 18°C between and at the end of the treatments (Fig. 3A). In the next experiment, seedlings were exposed to −12°C and −8°C, but letting the temperature increase only to 2°C for 16 h between the freezing treatments. The rates of freezing and thawing were also slower (Fig. 3B). In the third experiment, plants were frozen to −10°C and −6°C, and the temperature was increased to 19°C for 15 h between the freezing treatments. After the second freeze cycle, plants were allowed to deacclimate at 19°C for 5 d (Fig. 3C). In each experiment, extracellular freezing was initiated by spraying the seedlings with tap water after 1 h at subzero temperature. Bud and stem samples for RNA extractions were collected and stored in the same way as after the LT treatment at the time points indicated in Figure 3. Special care was taken not to thaw samples before they were set in liquid N2.
Arabidopsis (Arabidopsis thaliana) plants were derived from ecotype Columbia, except AtCBF3-overexpressing plants, which were in the ecotype Wassilewskija background. Seeds were germinated on Murashige and Skoog medium (Sigma-Aldrich) plates with kanamycin (50 μg/L) selection for transgenic plants, and after 1 week seedlings were transferred to soil. Plants were grown in 1:1 peat:vermiculite (Finnpeat B2; Kekkilä Oyj) with a 12-h light period (lights were on from 6 am to 6 pm) at 23°C. Three- to 5-week-old plants were used for experiments.
For LT treatment, 3-week-old wild-type Arabidopsis plants were transferred to a walk-in Phytotron chamber at 2°C under a 12-h daylength. The leaf samples for RNA-blot analysis were collected at 15 and 30 min and 1, 2, 4, 6, 8, and 24 h after the beginning of the LT treatment. In freeze-thaw treatments, 3-week-old soil-grown Arabidopsis plants were exposed to freezing temperatures by placing them directly in −2.6°C for 0.5 h, after which the temperature was decreased to −6°C at 1°C h−1. Temperature was kept at −6°C for 1 h, after which it was allowed to increase to 8°C during the next 3 h. Plants were kept at 5°C overnight. The experiment was started at 8:30 am, and after 1 h at freezing temperature, plants were sprayed with tap water to initiate extracellular freezing. The 1-h time sample (Fig. 4) was collected just before spraying, and the next sample was collected at 12 noon, when the temperature had decreased to −6°C, after which samples were collected at 1-h intervals until 4 pm, when temperature had increased to 8°C, and then at 9 am the next morning. The control samples were collected before transferring the plants to LT treatment. The leaf samples from both experiments were closed in Eppendorf tubes, immediately frozen in liquid N2, and kept at −80°C until RNA extraction.
Generation of Transgenic Plants
PCR was used to clone a 609-bp full-length fragment for birch BpCBF1 and BpCBF2 from birch EST library clones using the primers 5′-GGTACCATGGATGTTTTCTCTCAATAT-3′ for BpCBF1 and 5′-GGTACCATGGATGTTTTCTCCCAATAT-3′ for BpCBF2 and 5′-GCGGCCGCTCAAATTGAGTAACTCCACA-3′ for both genes. The full-length PCR products were run on a 1% agarose gel and purified with a gel purification kit (Qiagen), after which they were digested with KpnI and NotI and cloned into the corresponding sites of the binary vector pCP60. pCP60 is derived from pBIN19 containing the 35S promoter of cauliflower mosaic virus, multiple cloning sites, and NOS (Kariola et al., 2005). The sense orientation of the 35S promoter directing the expression of full-length birch CBF genes was verified by restriction analysis and by sequencing. Arabidopsis transformation was performed as described previously (Clough and Bent, 1998). Transgenic progeny lines were selected on Murashige and Skoog plates containing kanamycin. The empty vector pCP60 was used to generate transgenic control plants in a similar manner. T3 and T4 generations of transformed lines were used in all experiments.
Assessment of FT
To determine the FT of the Arabidopsis wild type, vector control, and transgenic lines harboring Arabidopsis CBF3 or birch BpCBF1, 3-week-old soil-grown plants were harvested without roots, and electrolyte leakage of the leaves was measured as described by Kariola et al. (2006). Three plants were used for each freezing temperature in each experiment. The LT50 values shown are averages (±se) of the three independent experiments and denote the temperature causing 50% of the electrolytes to leak out.
Southern-Blot Analysis
For Southern-blot hybridizations, genomic DNA from birch was extracted as described (Lodhi et al., 1994). Ten micrograms of DNA was digested with restriction enzymes that did not cleave within BpCBF coding sequences (HindIII, NcoI, and EcoRV) or that cleaved BpCBF1 once (EcoRI). Digested DNA was separated on a 0.9% (w/v) agarose gel by standard procedures and transferred to a nylon membrane by capillary transfer. The hybridizations were done in the same way as for northern blots (see below), using full-length BpCBF1 gene as a probe. Due to extensive sequence similarity between the birch CBF genes, it is likely that BpCBF1 cross-hybridizes with all BpCBF genes.
RNA Gel-Blot Analysis
Total RNA from the buds, stems, and leaves of birch was extracted according to Chang et al. (1993). Total RNA was extracted from Arabidopsis leaves with RNA extraction buffer (200 mm Tris-HCl, pH 8.8, 400 mm LiCl, 25 mm EDTA, and 1% [w/v] SDS), extracted with 25:24:1 phenol:chloroform:isoamyl alcohol, followed by extraction with 24:1 chloroform:isoamyl alcohol. RNA was precipitated overnight at −20°C with 0.278 m LiCl and 70% ethanol (Kingston, 1997). The resulting pellet was dissolved in water. Northern-blot hybridizations of Arabidopsis RNA were done as described earlier (Welling et al., 2002), except that the hybridizations and washes were done at 65°C. Full-length probes for BpCBF1, BpCBF2, LTI78, and COR47 were generated with PCR using cDNA as a template. For AtCBF3, a gene-specific probe was generated using the primer pair 5′-TGACGACGTATCGTTATGGAG-3′ and 5′-GAATCAATTTAATTTACACTCG-3′ and DNA as a template. PCR products were run with 1% agarose gels and purified with a gel purification kit (Qiagen). Probes were labeled with [α-32P]dCTP using the labeling kit Ready-to-Go (Amersham Pharmacia Biotech).
Transcript Profiling
The response of different birch CBF genes to changing temperatures was studied with quantitative real-time RT-PCR analysis. Total RNA was DNase treated (Turbo DNA-free Kit; Ambion), after which the cDNA synthesis was performed with SuperScript III (Invitrogen) reverse transcriptase from 1 to 2 μg of total RNA and 83 μm oligo(dT)20 primer according to the manufacturer's instructions at 48°C to 50°C for 16 h. The cDNA product was purified with the QIAquick PCR purification kit (Qiagen). Gene-specific primers were designed for each birch CBF gene. For BpCBF1, forward: 5′-TCGAGGAACCCGAAGAA, reverse: 5′-AGACCCACTTCCCGGAA-3′; for BpCBF2, forward: 5′-GGAGTGAGGAGGAGGAACTCT-3′, reverse: 5′-GTCGGAAATGTCCCCAA-3′; for BpCBF3, forward: 5′-GCGGCTTTGGCTCTTAGA-3′, reverse: 5′-GCAGCCCTCTGTATCTCACCT-3′; for BpCBF4, forward: 5′-CGTGGGACTCGGAGACC-3′, reverse: 5′-CTTCTTCGGATTCCTCGAG-3′; for BpLTI36, forward: 5′-CCTAGTTACGAGTACGAGACCG-3′, reverse: 5′-GAGATTGATCAGACACCTTGACTT-3′; and for birch actin, forward: 5′-TGGTCAAGGCTGGGTTTGC-3′, reverse: 5′-CTGACCCATCCCAACCATGA-3′. The specificity of the primer pairs for each gene was tested with PCR using genomic DNA as a template before running quantitative real-time RT-PCR.
Quantitative real-time RT-PCR was conducted using 100 ng of cDNA as a template, LightCycler 480 SYBR Green I Master Mix (Roche Diagnostics), and 5 pmol of each primer per reaction. The PCR cycles for LightCycler 480 (Roche Diagnostics) were as follows: initial denaturation at 95°C for 10 min; denaturation at 95°C for 15 s; and primer annealing and extension at 60°C for 1 min. The birch actin gene was used as a reference gene. Three technical repeats were used for each gene and sample pair. The average threshold cycle (Ct) value of the actin gene was subtracted from the corresponding Ct value of each gene to obtain the normalized ΔCt value. The efficiency of the each reaction was defined here as 100%, thus allowing the calculation of relative expression levels compared with the control with the formula 2−ΔCt (user bulletin no. 2; Applied Biosystems).
Statistical Analysis
A nonparametric Kruskal-Wallis test was used to test the significant differences in FT between the different lines. A critical difference value of 0.05 was used with post-hoc comparison. χ2 = 11,053, and degrees of freedom = 4.
Sequence data from this article can be found in the GenBank/EMBL data libraries under the accession numbers as follows: BpCBF1 (EF530204), BpCBF2 (EF530205), BpCBF3 (EF530206), and BpCBF4 (FG124897).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Sequence alignment of CBF/DREB1 protein family members of Arabidopsis, poplar, and birch.
Supplemental Figure S2. Southern-blot analysis of birch CBF genes.
Supplemental Figure S3. Characteristics of transgenic Arabidopsis plants overexpressing birch CBF genes.
Supplemental Figure S4. FT and expression analysis of transgenic Arabidopsis plants overexpressing BpCBF1.
Supplemental Figure S5. Model of the regulation of BpCBF and its target gene expression in response to LT during the growing season and in freeze-thaw conditions during overwintering.
Supplementary Material
Acknowledgments
Leena Laakso and Anna Pentinena are thanked for excellent technical assistance. We also thank Anita Hegedus for the pCP60 plasmid and Prof. Michael Thomashow for the CBF3-overexpressing Arabidopsis line. Dr. Jorma Vahala and Dr. Mikael Brosché are acknowledged for helping with the phylogenetic tree construction and quantitative real-time RT-PCR analysis, and Elina Helenius is thanked for designing the primers for the CBF3, COR47, and LTI78 probes.
This work was supported by the Academy of Finland (project nos. 79776, 202886, 1213509, and 1115280, Finnish Center of Excellence Programs 2000–2005 and 2006–2011) and Biocentrum Helsinki.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: E. Tapio Palva (tapio.palva@helsinki.fi).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Aalto MK, Palva ET (2006) Control of growth and cold acclimation in silver birch. In THH Chen, M Uemura, S Fujikawa, eds, Cold Hardiness in Plants. Molecular Genetics, Cell Biology and Physiology. CABI Publishing, Oxfordshire, UK, pp 153–166
- Améglio T, Ewers FW, Cochard H, Martignac M, Vandame M, Bodet C, Cruiziat P (2001) Winter stem xylem pressure in walnut trees: effects of carbohydrates, cooling and freezing. Tree Physiol 21 387–394 [DOI] [PubMed] [Google Scholar]
- Artlip TS, Callahan AM, Basset CL, Wisniewski ME (1997) Seasonal expression of a dehydrin gene in sibling deciduous and evergreen genotypes of peach (Prunus persica [L.] Batsch). Plant Mol Biol 33 61–70 [DOI] [PubMed] [Google Scholar]
- Baker SS, Wilhelm KS, Thomashow MF (1994) The 5′-region of Arabidopsis thaliana cor15a has cis-acting elements that confer cold-, drought- and ABA-regulated gene expression. Plant Mol Biol 24 701–713 [DOI] [PubMed] [Google Scholar]
- Bassett CL, Wisniewski ME, Artlip TS, Norelli JL, Renaut J, Farrell RE Jr (2006) Global analysis of genes regulated by low temperature and photoperiod in peach bark. J Am Soc Hortic Sci 131 551–563 [Google Scholar]
- Benedict C, Skinner JS, Meng R, Chang Y, Bhalerao R, Huner NPA, Finn CE, Chen THH, Hurry V (2006) The CBF1-dependent low temperature signalling pathway, regulon, and increase in freeze tolerance are conserved in Populus spp. Plant Cell Environ 29 1259–1272 [DOI] [PubMed] [Google Scholar]
- Champ KI, Febres VJ, Moore GA (2007) The role of CBF transcriptional activators in two Citrus species (poncirus and citrus) with contrasting levels of freezing tolerance. Physiol Plant 129 529–541 [Google Scholar]
- Chang S, Puryear J, Cairney C (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11 113–116 [Google Scholar]
- Choi DW, Rodriguez EM, Close TJ (2002) Barley Cbf3 gene identification, expression pattern, and map location. Plant Physiol 129 1781–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Decourteix M, Alves G, Brunel N, Améglio T, Guilliot A, Lemoine R, Pétel G, Soulaiman S (2006) JrSUT1, a putative xylem sucrose transporter, could mediate sucrose influx into xylem parenchyma cells and be up-regulated by freeze-thaw cycles over the autumn-winter period in walnut tree (Juglans regia L.). Plant Cell Environ 29 36–47 [DOI] [PubMed] [Google Scholar]
- Dubouzet JG, Sakuma Y, Ito Y, Kasuga M, Dubouzet EG, Miura S, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J 33 751–763 [DOI] [PubMed] [Google Scholar]
- El Kayal W, Navarro M, Marque G, Keller G, Marque C, Teulieres C (2006) Expression profile of CBF-like transcriptional factor genes from Eucalyptus in response to cold. J Exp Bot 57 2455–2469 [DOI] [PubMed] [Google Scholar]
- Fowler S, Thomashow MF (2002) Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 14 1675–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour SJ, Fowler SG, Thomashow MF (2004) Arabidopsis transcriptional activators CBF1, CBF2, and CBF3 have matching functional activities. Plant Mol Biol 54 767–781 [DOI] [PubMed] [Google Scholar]
- Gilmour SJ, Sebolt AM, Salazar MP, Everard JD, Thomashow MF (2000) Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol 124 1854–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour SJ, Zarka DG, Stockinger EJ, Salazar MP, Houghton JM, Thomashow MF (1998) Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J 16 433–442 [DOI] [PubMed] [Google Scholar]
- Guo Y, Xiong L, Ishitani M, Zhu JK (2002) An Arabidopsis mutation in translation elongation factor 2 causes superinduction of CBF/DREB1 transcription factor genes but blocks the induction of their downstream targets under low temperatures. Proc Natl Acad Sci USA 99 7786–7791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy CL (1990) Cold acclimation and freezing stress tolerance: role of protein metabolism. Annu Rev Plant Physiol Plant Mol Biol 41 187–223 [Google Scholar]
- Haake V, Cook D, Riechmann JL, Pineda O, Thomashow MF, Zhang JZ (2002) Transcription factor CBF4 is a regulator of drought adaptation in Arabidopsis. Plant Physiol 130 639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah MA, Wiese D, Freund S, Fiehn O, Heyer AG, Hincha DK (2006) Natural genetic variation of freezing tolerance in Arabidopsis. Plant Physiol 142 98–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglo KR, Kleff S, Amundsen KL, Zhang X, Haake V, Zhang JZ, Deits T, Thomashow MF (2001) Components of the Arabidopsis C-repeat/dehydration-responsive element binding factor cold-response pathway are conserved in Brassica napus and other plant species. Plant Physiol 127 910–917 [PMC free article] [PubMed] [Google Scholar]
- Jaglo-Ottosen KR, Gilmour SJ, Zarka DG, Schabenberger O, Thomashow MF (1998) Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 280 104–106 [DOI] [PubMed] [Google Scholar]
- Kalberer SR, Wisniewski M, Arora R (2006) Deacclimation and reacclimation of cold-hardy plants: current understanding and emerging concepts. Plant Sci 171 3–16 [Google Scholar]
- Kariola T, Brader G, Helenius E, Li J, Heino P, Palva ET (2006) EARLY RESPONSIVE TO DEHYDRATION 15, a negative regulator of abscisic acid responses in Arabidopsis. Plant Physiol 142 1559–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariola T, Brader G, Li J, Palva ET (2005) Chlorophyllase 1, a damage control enzyme, affects the balance between defense pathways in plants. Plant Cell 17 282–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1999) Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol 17 287–291 [DOI] [PubMed] [Google Scholar]
- Kingston RF (1997) Precipitation and analysis of RNA. In FM Ausubel, R Brent, RE Kingston, DD Moore, JG Seidman, JA Smith, K Struhl, eds, Current Protocols in Molecular Biology. John Wiley and Sons, New York, pp 4.3.1–4.3.4
- Kitashiba H, Ishizaka T, Isuzugawa K, Nishimura K, Suzuki T (2004) Expression of a sweet cherry DREB1/CBF ortholog in Arabidopsis confers salt and freezing tolerance. J Plant Physiol 161 1171–1176 [DOI] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR (1996) Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10 1391–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodhi MA, Ye GN, Weeden NF, Reisch BI (1994) A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Plant Mol Biol Rep 12 6–13 [Google Scholar]
- Lopez-Matas MA, Nuñez P, Soto A, Allona I, Casado R, Collada C, Guevara MA, Aragoncillo C, Gomez L (2004) Protein cryoprotective activity of a cytosolic small heat shock protein that accumulates constitutively in chestnut stems and is up-regulated by low and high temperatures. Plant Physiol 134 1708–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K, Sakuma Y, Kasuga M, Ito Y, Seki M, Goda H, Shimada Y, Yoshida S, Shinozaki K, Yamaguchi-Shinozaki K (2004) Identification of cold-inducible downstream genes of the Arabidopsis DREB1A/CBF3 transcriptional factor using two microarray systems. Plant J 38 982–993 [DOI] [PubMed] [Google Scholar]
- Medina J, Bargues M, Terol J, Pérez-Alonso M, Salinas J (1999) The Arabidopsis CBF gene family is composed of three genes encoding AP2 domain-containing proteins whose expression is regulated by low temperature but not by abscisic acid or dehydration. Plant Physiol 119 463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Suzuki K, Fujimura T, Shinshi H (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140 411–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novillo F, Alonso JM, Ecker JR, Salinas J (2004) CBF2/DREB1C is a negative regulator of CBF1/DREB1B and CBF3/DREB1A expression and plays a central role in stress tolerance in Arabidopsis. Proc Natl Acad Sci USA 101 3985–3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhakainen T, Li C, Boije-Malm M, Kangasjarvi J, Heino P, Palva ET (2004) Short-day potentiation of low temperature-induced gene expression of a C-repeat-binding factor-controlled gene during cold acclimation in silver birch. Plant Physiol 136 4299–4307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne P, Welling A, Kaikuranta P (1998) Onset of freezing tolerance in birch (Betula pubescens Ehrh.) involves LEA proteins and osmoregulation and is impaired in an ABA-deficient genotype. Plant Cell Environ 21 601–611 [Google Scholar]
- Rinne PLH, Kaikuranta PLM, van der Plas LHW, van der Schoot C (1999) Dehydrins in cold-acclimated apices of birch (Betula pubescens Ehrh.): production, localization and potential role in rescuing enzyme function during dehydration. Planta 209 377–388 [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K (2002) DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem Biophys Res Commun 290 998–1009 [DOI] [PubMed] [Google Scholar]
- Schrader J, Moyle R, Bhalerao R, Hertzberg M, Lundeberg J, Nilsson P, Bhalerao RP (2004) Cambial meristem dormancy in trees involves extensive remodelling of the transcriptome. Plant J 40 173–187 [DOI] [PubMed] [Google Scholar]
- Seki M, Narusaka M, Ishida J, Nanjo T, Fujita M, Oono Y, Kamiya A, Nakajima M, Enju A, Sakurai T, et al (2002) Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J 31 279–292 [DOI] [PubMed] [Google Scholar]
- Stockinger EJ, Gilmour SJ, Thomashow MF (1997) Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA 94 1035–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50 571–599 [DOI] [PubMed] [Google Scholar]
- Vogel JT, Zarka DG, Van Buskirk HA, Fowler SG, Thomashow MF (2005) Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J 41 195–211 [DOI] [PubMed] [Google Scholar]
- Weiser CJ (1970) Cold resistance and injury in woody plants. Science 169 1269–1278 [DOI] [PubMed] [Google Scholar]
- Welling A, Kaikuranta P, Rinne P (1997) Photoperiodic induction of dormancy and freezing tolerance in Betula pubescens: involvement of ABA and dehydrins. Physiol Plant 100 119–125 [Google Scholar]
- Welling A, Moritz T, Palva ET, Junttila O (2002) Independent activation of cold acclimation by low temperature and short photoperiod in hybrid aspen. Plant Physiol 129 1633–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welling A, Palva ET (2006) Molecular control of cold acclimation in trees. Physiol Plant 127 167–181 [Google Scholar]
- Welling A, Rinne P, Vihera-Aarnio A, Kontunen-Soppela S, Heino P, Palva ET (2004) Photoperiod and temperature differentially regulate the expression of two dehydrin genes during overwintering of birch (Betula pubescens Ehrh.). J Exp Bot 55 507–516 [DOI] [PubMed] [Google Scholar]
- Wisniewski ME, Bassett CL, Renaut J, Farrell R Jr, Tworkoski T, Artlip TS (2006) Differential regulation of two dehydrin genes from peach (Prunus persica) by photoperiod, low temperature and water deficit. Tree Physiol 26 575–584 [DOI] [PubMed] [Google Scholar]
- Xiao H, Siddiqua M, Braybrook S, Nassuth A (2006) Three grape CBF/DREB1 genes respond to low temperature, drought and abscisic acid. Plant Cell Environ 29 1410–1421 [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K (1994) A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6 251–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57 781–803 [DOI] [PubMed] [Google Scholar]
- Zarka DG, Vogel JT, Cook D, Thomashow MF (2003) Cold induction of Arabidopsis CBF genes involves multiple ICE (inducer of CBF expression) promoter elements and cold-regulatory circuit that is desensitized by low temperature. Plant Physiol 133 910–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Fowler SG, Cheng H, Lou Y, Rhee SY, Stockinger EJ, Thomashow MF (2004) Freezing-sensitive tomato has a functional CBF cold response pathway, but a CBF regulon that differs from that of freezing-tolerant Arabidopsis. Plant J 39 905–919 [DOI] [PubMed] [Google Scholar]
- Zhu B, Choi DW, Fenton R, Close TJ (2000) Expression of the barley dehydrin multigene family and the development of freezing tolerance. Mol Gen Genet 264 145–153 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.