Abstract
In mammals, basal currents through G protein-coupled inwardly rectifying K+ (GIRK) channels are repressed by Gαi/oGDP, and the channels are activated by direct binding of free Gβγ subunits released upon stimulation of Gαi/o-coupled receptors. However, essentially all information on G protein regulation of GIRK electrophysiology has been gained on the basis of coexpression studies in heterologous systems. A major advantage of the model organism, Arabidopsis thaliana, is the ease with which knockout mutants can be obtained. We evaluated plants harboring mutations in the sole Arabidopsis Gα (AtGPA1), Gβ (AGB1), and Regulator of G protein Signaling (AtRGS1) genes for impacts on ion channel regulation. In guard cells, where K+ fluxes are integral to cellular regulation of stomatal apertures, inhibition of inward K+ (Kin) currents and stomatal opening by the phytohormone abscisic acid (ABA) was equally impaired in Atgpa1 and agb1 single mutants and the Atgpa1 agb1 double mutant. AGB1 overexpressing lines maintained a wild-type phenotype. The Atrgs1 mutation did not affect Kin current magnitude or ABA sensitivity, but Kin voltage-activation kinetics were altered. Thus, Arabidopsis cells differ from mammalian cells in that they uniquely use the Gα subunit or regulation of the heterotrimer to mediate Kin channel modulation after ligand perception. In contrast, outwardly rectifying (Kout) currents were unaltered in the mutants, and ABA activation of slow anion currents was conditionally disrupted in conjunction with cytosolic pH clamp. Our studies highlight unique aspects of ion channel regulation by heterotrimeric G proteins and relate these aspects to stomatal aperture control, a key determinant of plant biomass acquisition and drought tolerance.
Keywords: stomata, heterotrimeric G protein complex, AGB1, GPA1, RGS1
G protein-coupled inwardly rectifying potassium or “GIRK” channels (also known as Kir3 channels) comprise important targets of heterotrimeric G protein regulation in mammals (1, 2). GIRK channels mediate signals from muscarinic, adrenergic, opioid, dopaminergic, and GABAB receptors (3). Basal activity of GIRK channels is repressed by their direct binding of Gαi/oGDP (4, 5) within a macromolecular complex that includes Gβγ (4–7). Upon activation of Gi/o-coupled G protein-coupled receptors (GPCRs), formation of the GTP-bound form of Gαi/o both alleviates Gα-mediated repression and releases βγ dimers that independently interact with the channel (7, 8). Gβ1-4γ binding strengthens GIRK interaction with phosphatidylinositol 4,5-bisphosphate (PtdInsP2), thereby promoting conformational changes that increase channel open time (3, 9–11). Conversely, Gαq-based activation of phospholipase C opposes GIRK activity via both depletion of PtdInsP2 and activation of PKC-based phosphorylation events (12).
Numerous studies analyzing GIRK activity in Xenopus oocytes and cultured mammalian cells have led to the beautifully intricate model described above. However, studies analyzing GIRK activity in the appropriate native cell context upon genetic depletion of Gβ subunits are lacking. Arabidopsis has single genes, AtGPA1 (henceforth referred to as GPA1) and AGB1, encoding canonical α and β subunits, two identified genes, AGG1 and AGG2, encoding γ subunits, and a single Regulator of G protein Signaling (RGS) gene, AtRGS1 (henceforth referred to as RGS1), encoding RGS1 which accelerates GTPase activity of GPA1 (13–15). The present study takes advantage of knockout mutants (13, 16) in the model plant system, Arabidopsis thaliana, to investigate G protein regulation of inward K+ (Kin) currents, outwardly rectifying K+ (Kout) currents, and slow anion currents in their native condition and to assess the roles of G protein-based pathways in cellular function.
Specialized guard cells residing in pairs in the leaf surface regulate the apertures of microscopic pores, “stomata,” through which plants both take up the CO2 required for photosynthesis and, inevitably, lose water vapor (17–20). Guard cell responses to the plant hormone abscisic acid (ABA) play a vital role in plant resistance to drought, a major cause of crop loss (18). ABA inhibits guard-cell Kin channels and activates Ca2+-permeable channels and anion channels through which anion efflux occurs (17–20). Osmotically driven guard cell inflation is thereby inhibited and guard cell deflation is promoted, resulting in inhibition of stomatal opening, promotion of stomatal closure, and reduced plant water loss. Guard-cell Kin currents share with GIRK channels the properties of inward rectification, activation by ATP, activation by PtdInsP2, and regulation by cellular redox status (9, 10, 20–23). We previously showed that genetic depletion of GPA1 relieves ABA inhibition of guard-cell Kin currents (24, 25). These studies led to a number of additional questions. (i) As in mammalian cells, do G protein subunits regulate basal levels of Kin current observed in the absence of agonist? (ii) Is loss of an inhibitory regulator, Gα, or gain of a stimulatory regulator, free Gβγ (as in mammals), responsible for the gpa1 phenotype? (iii) Do RGS proteins modulate K+ currents in plants as in mammalian cells? (iv) Is there G protein regulation of Kout channels? (v) Given our previous observations that plant Gα subunits also regulate slow anion channels (24, 25), do plant Gβ subunits participate in this regulation? (vi) What are the effects of altered expression of G protein components on integrated guard cell responses to ABA, as reflected in regulation of stomatal apertures?
Results
Basal Levels and ABA Inhibition of Kin Current Do Not Differ Among agb1 and gpa1 Single and gpa1 agb1 Double Mutants.
We applied patch-clamp whole-cell recording techniques to evaluate basal Kin current magnitude, i.e., in the absence of ABA application, in two independent agb1 mutants. Fig. 1 shows that basal (Control) K+ channel activity does not differ among the agb1 mutant lines and their isogenic wild type, Col-0. We next made a side-by-side comparison of Gα and Gβ single mutants and the double mutant derived from them. Because the Gβ mutants are in the Columbia (Col) ecotypic background, the Col gpa1 null mutant, gpa1-4, which has not been previously evaluated for its ion channel characteristics, was used for comparison instead of Ws (Wassilewskija)-based gpa1-1 or gpa1-2 (24, 25). None of the Gα or Gβ mutations affected basal Kin currents.
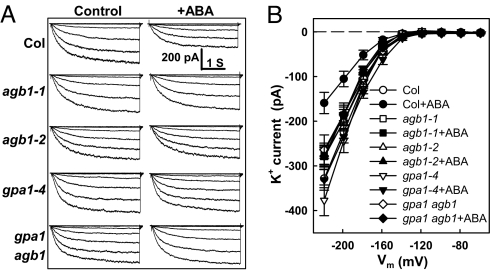
Fig. 1.
ABA sensitivity of guard cell Kin current regulation is abrogated in agb1-1, agb1-2, and gpa1-4 single mutants and in agb1-2 gpa1-4 double mutants. (A) Representative whole-cell recordings of Kin currents (Col, agb1-1, agb1-2, gpa1-4, gpa1 agb1 double mutant) with or without 50 μM ABA. Whole-cell currents were recorded from a holding potential of −79 mV with 3.9-s voltage steps from −219 to −59 mV in +20-mV increments, 10 min after achieving the whole-cell configuration. Time and current scales are shown in A. (B) Current–voltage (I–V) curves (mean ± SE) of time-activated whole-cell Kin currents. Time-activated currents were calculated by subtracting the instantaneous current at 20 ms from the average steady-state current between 3.55 and 3.87 s. n = 13, 15 cells for control and ABA treatment of Col; n = 11, 11 cells for agb1-1; n = 10, 12 cells for agb1-2. n = 15, 10 cells for gpa1-4; and n = 10, 10 cells for gpa1-4 agb1-2.
ABA inhibition of Kin current was attenuated in the agb1-1 and agb1-2 mutants. Identical attenuation was also seen in gpa1-4 and in the gpa1-4 agb1-2 double mutant (Fig. 1); i.e., no additive or synergistic effects were observed (Fig. 1).
ABA Inhibition of Kin Currents Is Unaltered in AGB1-Overexpression (OX) Lines.
If, as in mammalian cells, Gβ subunit release from the heterotrimer promotes K+ channel activity, then overexpression of AGB1 would be expected to stimulate Kin currents. We evaluated two independent lines in which AGB1 overexpression in a wild-type background was clearly observed by immunoblot analysis [supporting information (SI) Fig. S1]. However, the Kin currents of these lines showed wild-type sensitivity to inhibition by ABA, as did the control empty vector lines (Fig. 2). A wild-type phenotype was maintained even at subsaturating ABA concentrations which might be expected to more readily reveal any ABA-hypersensitive phenotype (Fig. S2). In addition, agb1-2 lines complemented with an AGB1 construct and exhibiting AGB1 overexpression showed wild-type ABA responses (Fig. S3), whereas gpa1-4 agb1-2 double mutants complemented with this construct retained ABA hyposensitivity (Fig. S3).
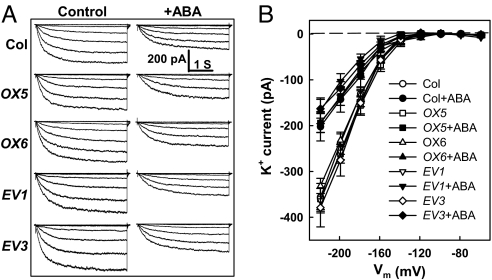
Fig. 2.
ABA inhibits Kin currents similarly in wild-type and AGB1 overexpressing lines. (A) Whole-cell recordings of Kin currents with or without 50 μM ABA from Col, two AGB1 overexpressing lines (OX5, OX6), and two empty vector lines (EV1, EV3) as controls for the OX lines. (B) I–V curves (mean ± SE) of time-activated whole-cell Kin currents as recorded in A. n = 7, 8 cells for control and ABA treatment of Col; n = 9, 10 cells for OX5; n = 13, 11 cells for OX6; n = 10, 10 cells for EV1; and n = 8, 9 cells for EV3.
rgs1 Null Mutation Does Not Affect K+ Current Amplitude or ABA Response but Affects Kinetics of Voltage Activation.
There are no previous studies on RGS regulation of plant ion channel activity or guard cell function. We found that RGS1 is expressed in guard cells (Fig. S4), but lack of RGS1 did not affect the magnitude of basal K+ current, its voltage dependency, or its inhibition by ABA (Fig. S5). However, accelerated kinetics of Kin current response after voltage activation were observed in the rgs1 mutants under control conditions (Fig. S5). This finding indicates that RGS1 is important in the dynamics of voltage-dependent activation of Kin current.
Gα and Gβ Subunit Interaction.
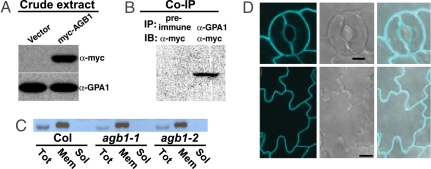
To confirm interaction between GPA1 and AGB1, a myc epitope-tagged AGB1 construct was transformed into Arabidopsis suspension cells by Agrobacterium-mediated transformation (Fig. 3A). Protein extracts were subjected to immunoprecipitation by using anti-GPA1 antibodies (26) (Fig. S6) or preimmune serum, and anti-myc antibody was used in subsequent immunoblot analysis. Fig. 3B shows the coimmunoprecipitation of AGB1 with GPA1.
Fig. 3.
AGB1 interacts with GPA1 but does not influence GPA1 expression levels. (A) Myc-AGB1 expression in Arabidopsis suspension cells transformed with 35S::myc epitope-tagged AGB1, detected by using anti-myc antibody. Immunoblot with anti-GPA1 antibody illustrates equal loading. (B) AGB1 coimmunoprecipitates with GPA1. Total protein extracts were coimmunoprecipitated (IP) by anti-GPA1 (α-GPA1) or by preimmune serum, then immunoblotted (IB) with the anti-myc (α-myc) antibody. (C) Immunoblot of GPA1 in Col and two agb1 mutants. GPA1 is expressed at a similar total level among Col wild-type and agb1 mutant lines, and membrane vs. soluble localization of GPA1 is not affected in agb1 mutants. Tot, total fraction; Mem, crude membrane fraction; Sol, soluble protein fraction. (D) Confocal laser scanning microscopy images of guard and epidermal cells showing membrane localization of GPA1-CFP in the agb1-2 mutant background. Shown are confocal (Left), differential interference contrast microscopy (Center), and merged (Right) images of guard cells (Upper) and epidermal cells (Lower). (Scale bars: Upper, 5 μm; Lower, 10 μm.)
One explanation for the similarity of the Kin phenotypes of agb1 and gpa1 plants would be that the agb1-2 mutation affects GPA1 expression. However, reverse-transcriptase PCR (RT-PCR) and quantitative reverse-transcriptase real-time PCR (Q-PCR) analyses indicate that mutations in agb1 have no effect on expression of GPA1, nor does the gpa1 null mutation affect AGB1 expression (Fig. S7). In addition, immunoblot analyses performed on total, crude membrane and soluble protein fractions from rosette leaves showed that the presence or absence of AGB1 did not affect either total GPA1 levels or GPA1 partitioning to the microsomal fraction (Fig. 3C). To confirm the uniform membrane-delimited localization of GPA1 in agb1-2 mutants, confocal laser scanning microscopy was performed on epidermes of agb1-2 seedlings expressing a GPA1-CFP fusion protein. GPA1-CFP fluorescence in both leaf epidermal cells and guard cells was observed at the plasma membrane (Fig. 3D); this fluorescence receded from the cell wall upon guard cell plasmolysis, indicating that the fluorescence was indeed associated with the membrane as opposed to the cell wall (data not shown).
Kout Currents Are Unaltered in Gβ Mutants, Whereas Anion Currents Show Conditional Alteration.
In plant cells, inward and outward K+ currents are mediated by molecularly distinct channel proteins (20, 27). Wild-type Col did not show ABA regulation of Kout currents (24, 28). In addition, Kout currents exhibited wild-type behavior in agb1 mutants, the gpa1-4 mutant, the gpa1 agb1 double mutant, and AGB1-OX lines (Fig. 4 A–D).
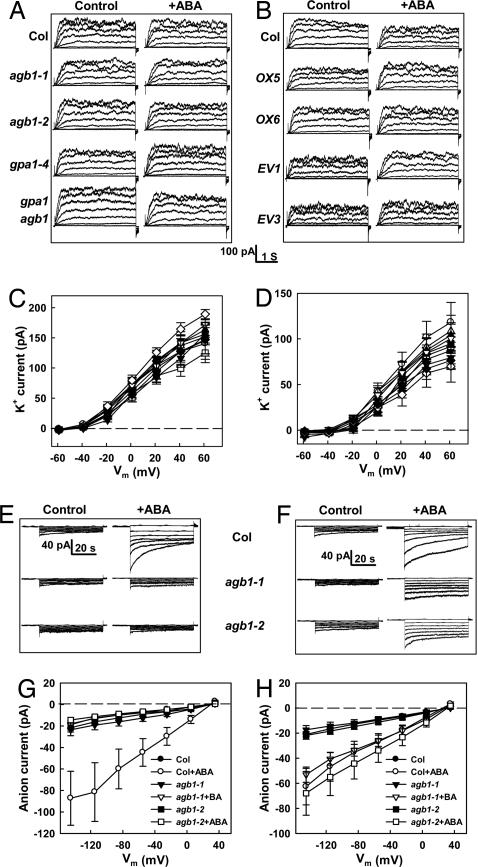
Fig. 4.
ABA does not affect Kout currents of either wild-type or G protein mutants and affects anion currents in a pH- and G protein-dependent manner. (A) Typical whole-cell recordings of Kout currents (Col, agb1-1, agb1-2, gpa1-4, gpa1 agb1 double mutant) with or without 50 μM ABA. Currents were recorded from a holding potential of −79 mV with 3.9-s voltage steps from −59 to 61 mV in +20-mV increments, 10 min after achieving the whole-cell configuration. Time and current scales are shown below B. (B) Typical whole-cell recordings of Kout currents from Col, AGB1 overexpression lines (OX5 and OX6) and pGWB42 empty vector control lines (EV1 and EV3) with and without 50 μM ABA treatments. (C) I–V curves (mean ± SE) of time-activated whole-cell Kout currents, calculated by subtracting the instantaneous current at 20 ms from the average steady-state current between 3.55 and 3.87 s. n = 13, 15 cells for control (○), ABA treatment of Col (●); n = 11, 11 cells for control (□), ABA treatment of agb1-1 (■); and n = 10, 12 cells for control (▵), ABA treatment of agb1-2 (▴). n = 15, 10 cells for control (▿), ABA treatment of gpa1-4 (▾); and n = 10, 10 cells for control (◇), ABA treatment of gpa1-4 agb1-2 (♦). (D) I–V curve (mean ± SE) of time-activated whole-cell Kout currents. n = 7, 8 cells for control (○) and ABA treatment of Col (●); n = 9, 10 for OX5 (□, ■); n = 13, 11 for OX6 (▵, ▴); n = 10, 10 for EV1 (▿, ▾); n = 8, 9 for EV3 (◇, ♦). (E) Typical whole-cell recordings of slow anion currents (Col, agb1-1, agb1-2) with or without 50 μM ABA under strong cytosolic pH buffering (10 mM Hepes-Tris). Whole-cell anion currents were recorded 12 min after achieving the whole-cell configuration. The holding potential was +30 mV and voltage steps were from −145 to +35 mV in +30-mV increments. (F) Typical whole-cell recordings of anion currents (Col, agb1-1, agb1-2) plus or minus 50 μM ABA under weak cytosolic pH buffering (0.1 mM Hepes-Tris). (G) I–V curves (mean ± SE) of steady-state whole-cell anion currents as recorded in E. Steady-state currents were acquired by subtracting the basal currents at a holding potential of +30 mV from the average currents between 42.5 and 50.0 s. n = 12, 13 cells for control, ABA treatment of Col; n = 12, 12 cells for agb1-1; and n = 13, 13 cells for agb1-2. (H) I–V curves (mean ± SE) of steady-state whole-cell anion currents as recorded in F. Steady-state currents were acquired as in G. n = 19, 23 cells for control, ABA treatment of Col; n = 10, 10 for agb1-1; and n = 17, 21 for agb1-2.
Slow anion currents of guard cells are enhanced by ABA (29). gpa1 null mutants exhibited wild-type anion channel response to ABA in the absence of cytosolic pH clamp and loss of this response in the presence of a cytosolic pH clamp (24). This same interaction with cytosolic pH status was observed for the agb1 mutants (Fig. 4 E–H).
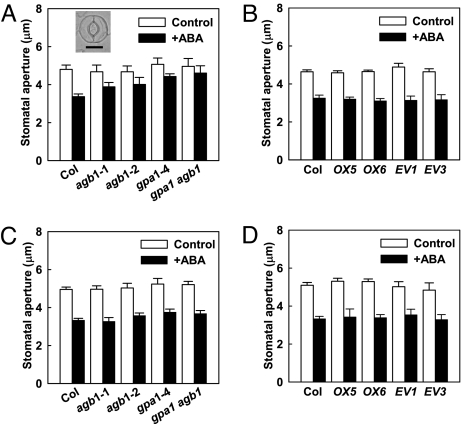
ABA-Inhibition of Stomatal Opening Is Altered in Gβ Mutants and in GαGβ Double Mutants.
To assess a correlation between ion channel regulation and cellular function, assays of stomatal responses were conducted. ABA inhibition of stomatal opening (Fig. 5A) was attenuated in the two agb1 mutants compared to wild type. Just as for ion channel regulation, the double gpa1 agb1 mutant phenotype was identical to that of the single mutants (Fig. 5A). In the AGB1-OX lines, ABA inhibition of stomatal opening was retained at wild-type levels (Fig. 5B and Fig. S3), consistent with the wild-type response of these lines for ABA modulation of Kin current. rgs1 null mutants also showed wild-type ABA inhibition of stomatal opening (Fig. S8), consistent with the wild-type response of these mutants with regard to steady-state K+ current magnitude after ABA application. All genotypes exhibited wild-type ABA induction of stomatal closure (Fig. 5 C and D).
Fig. 5.
ABA inhibition of stomatal opening is impaired in agb1 and gpa1 single and double mutants and is unaltered in AGB1-OX lines. (A) Reduced ABA (20 μM) inhibition of stomatal opening (mean ± SE) in agb1-1, agb1-2, gpa1-4, and gpa1-4 agb1-2 double mutants as compared to wild type. ABA significantly inhibited stomatal opening in Col (P ≤ 0.001) but had no significant effect in any of the other mutant lines (P > 0.05). Inset illustrates two Col guard cells defining an open stomatal pore. (Scale bar: 10 μm.) Data are mean ± SE from five independent replicates. In each replicate, >150 stomata were measured for each genotype × treatment combination. (B) Significant ABA (20 μM) inhibition of stomatal opening in AGB1-OX lines (OX5, OX6), empty vector controls (EV1, EV3), and wild type (Col). Mean ± SE from four replicates. (C) Significant ABA (20 μM) promotion of stomatal closure (mean ± SE) in agb1-1, agb1-2, gpa1-4, and gpa1-4 agb1-2 double mutants. Mean ± SE from four replicates. (D) Significant ABA (20 μM) promotion of stomatal closure in AGB1-OX lines (OX5, OX6) as compared to empty vector controls (EV1, EV3). Mean ± SE from three replicates.
Discussion
Regulation of Plant Kin Channels by G Protein Complex Components.
The plant hormone ABA inhibits Kin currents of wild-type guard cells (17) and null mutation of the Arabidopsis G protein α subunit gene, GPA1, results in loss of this response (24, 25). A primary goal of the present report was to determine the roles of Gα vs. Gβγ in this phenomenon and to determine whether there was evidence that the heterotrimeric state of the Arabidopsis G protein complex could play a regulatory role.
In mammalian systems, Gα, within the heterotrimeric complex, suppresses basal GIRK current levels in the absence of agonist, and upon agonist perception, freed Gβγ dimer acts to increase the open probability of GIRK channels (5, 7). Three key findings of our study are that, in Arabidopsis: (i) loss of either the Gα or Gβ subunits has no effect on basal (−ABA) K+ current; (ii) loss of Gα or Gβ or both causes identical hyposensitivity to ABA-inhibition of Kin currents and stomatal opening; and (iii) overexpression of the Gβ subunit has no effect on Kin current, either in the absence or in the presence of ABA. These observations lead to our first conclusion: the Gβγ dimer does not operate on K+ channels in the same manner in plant and mammalian cells. Specifically, the mammalian mechanism in which free Gβγ activates the channels is not supported by our AGB1 knockout mutant and overexpression data in Arabidopsis. The interpretation of the wild-type nature of the AGB1 overexpression phenotype is tempered by the possibility that active Gβγ is limited in plant cells, for example by a fixed pool of Gγ subunits. However, this is unlikely in that AGB1 overexpression confers clear scorable phenotypes (30, 31).
Coimmunoprecipitation of GPA1 and AGB1 from Arabidopsis cells (Fig. 3 A and B) supports previous observations of their interaction in heterologous systems (30, 32) as well as biochemical evidence for interaction in rice and pea (33, 34). Interaction between AGG1 or AGG2 and AGB1 is seen in yeast two-hybrid and in vitro binding assays and by FRET (15, 32). Collectively, these data support the notion that plant α, β, and γ subunits form heterotrimers. Given the existence of a heterotrimer, three hypotheses are consistent with the identical phenotypes of the agb1 and gpa1 null mutants: (i) the independent action of free Gα and free Gβγ subunits sum to mediate ABA-inhibition of the Kin channels, and loss of either Gα or Gβ is sufficient to cause ABA hyposensitivity; (ii) the Gα subunit mediates ABA inhibition of the Kin channels; and (iii) ABA regulation of the Kin channels is mediated by the G protein heterotrimer.
If the first hypothesis were correct, we might anticipate that the double gpa1 agb1 mutant would exhibit greater ABA hyposensitivity than gpa1 or agb1 single mutants, e.g., in stomatal opening assays, and that overexpression of AGB1 would result in ABA hypersensitivity, but these phenotypes were not observed (Figs. 1 and 2 and Fig. S2). Accordingly, we discuss the latter two hypotheses below.
In mammalian systems, Gβγ dimers are important for maintenance of appropriate signaling through Gαs, and thus Gα-dependent signaling (hypothesis ii) can also be disrupted in Gβ mutants, as we observed. In mammalian cells, Gβ knockdown also can affect Gα expression or targeting (35–37); however, we saw no evidence for these phenomena in Arabidopsis (Fig. 3 C and D and Fig. S7). Instead, AGB1 may have more direct regulatory effects on GPA1. For some mammalian Gαs, Gβγ may act as a lever to promote conformational change of the Gα subunit upon GPCR activation, thus promoting GDP release (38, 39) (but also see ref. 40). In addition, segments of Gβ as well as Gγ interact with GPCRs (41), and Gγs (and Gβ5) appear to play important roles in GPCR-Gα coupling specificity (41), and disruption of such coupling may be occurring in the Arabidopsis agb1 mutants.
In plants, data from previous patch-clamp experiments employing pharmacological G protein modulators implicated regulation of Kin channels by both cytosol-mediated and membrane-delimited G protein-based pathways (13, 42, 43). Indirect regulation of Arabidopsis Kin channels via a signaling cascade would parallel the indirect inhibition of GIRK channels by Gαq, via activation of PLC and PKC (12). Of interest in this regard are the observations that ABA elevates PLC activity in guard cells (44) and that the guard-cell Kin channels are activated by PtdInsP2 (23). PLC-mediated hydrolysis of PtdInsP2 may reduce availability of this Kin channel activator in the guard-cell plasma membrane. In guard cells, PLC and PLD appear to operate in the same ABA-signaling cascade (45), and phosphatidic acid (PA), the product of PLD activity, is another attractive candidate regulator: PA inhibits guard-cell Kin currents (45), and PLD is ABA-activated (45) and regulated by Gα in guard cells (46). In addition, one class of Shaker-like channels, the KCNQ channels, whose mutation is associated with genetic diseases including long QT syndrome and atrial arrhythmias (47), displays modulation via a Gs-dependent cascade, including activation by PtdInsP2 (47, 48). On a sequence homology basis, the guard cell Kin channels, despite evincing inward rectification, are similar to metazoan Shaker K+ channels (27).
Alternatively, the ABA hyposensitive phenotypes reported here may be accounted for by disruption of signaling via a nondissociated heterotrimer (hypothesis iii), as occurs in certain yeast and mammalian G protein signaling cascades (49–51). Heterotrimer-dependent signaling would be expected to be perturbed equally in agb1 single mutants, gpa1 single mutants, and gpa1 agb1 double mutants, i.e., the phenotypes we observe. Our data do not directly speak to the question of whether such a heterotrimer would contain GDP-GPA1 or GTP-GPA1: one FRET study on plant cells indicates that a mutant, constitutively active (GTP-bound) form of GPA1 can still exhibit FRET with AGB1, consistent with retention of a GTP-GPA1 subunit in a heterotrimer (32, 50, 52). Alternatively, ABA might stimulate activity of an as yet unidentified GDI or RGS protein (other than RGS1) and thus shift GDP-Gα into the heterotrimeric complex.
The basal state in animals is reduced Kin channel activity in the absence of agonist. In contrast, in Arabidopsis, the reduced activity level occurs when the agonist (ABA) is present. If the above scenario proves to be correct, Gα may inhibit GIRK-like current in a mechanistically similar manner in Arabidopsis cells and mammalian cells and this may have been the ancestral action of Gα on K+ channel activity. Consistent with the idea of mechanistic similarities, during regulation of mammalian GIRK channels by RGS, alteration in current kinetics but not steady-state current–voltage relationships is commonly observed (e.g., 6, 53–55), and this feature is also shown here for Arabidopsis Kin channels (Fig. S5).
Integrated Guard Cell Responses in G Protein Complex Mutants.
Kout channels of guard cells are Shaker-like channels that mediate K+ efflux during stomatal closure (20, 27). As reported previously (24, 28), we did not observe ABA activation of Kout channels in wild-type plants. Although G proteins regulate some mammalian Shaker-type channels (47, 48), we found no evidence for G protein involvement in modulation of either basal Kout currents or their ABA responsiveness (Fig. 4), consonant with our observation that wild-type ABA induction of stomatal closure occurs in the G protein mutants and transgenics (Fig. 5).
ABA activation of ion channels other than Kout channels may be more central to ABA promotion of stomatal closure. Anion loss through ABA-activated slow anion channels (29) decreases anion content in the guard cell and promotes membrane depolarization which drives K+ efflux, resulting in water efflux, guard cell deflation, and stomatal closure. Our previous research indicated that Gα-dependent and pHi-dependent cascades provide redundant pathways for ABA-activation of anion channels in guard cells (24). We similarly observed a wild-type activation of anion channels by ABA in the agb1-1 and agb1-2 mutants and, as for gpa1 mutants, this ABA-activation was eliminated in guard cells subjected to cytosolic pH clamp (Fig. 4 E–H). These results further support the conclusion that ABA-related guard cell phenotypes of Gβ mutants recapitulate those of Gα mutants. Intriguingly, for other ABA-related processes (seed germination, root growth, seedling gene expression), gpa1 and agb1 mutants exhibit ABA hypersensitivity (56), suggesting unexplored richness in the mechanisms of hormonal signaling through plant heterotrimeric G proteins.
Materials and Methods
Plant Material and Growth Conditions.
All transgenics were in the Col accession of Arabidopsis thaliana. An ethyl methanesulfonate-generated mutant line (agb1-1), transfer (T)-DNA insertional mutant lines (agb1-2, gpa1-4, rgs1-1, rgs1-2), and gpa1-4 agb1-2 double-mutant lines have been described (16, 26, 31).
To generate AGB1-overexpressing lines (AGB1-OX), AGB1 cDNA was cloned into the vector pGWB1:35S:YFP; corresponding empty vector lines (EV) were also isolated as controls. The GPA1-CFP construct was as described in ref. 26. Constructs were transformed into plants by Agrobacterium-mediated transformation. The myc epitope-tagged AGB1 was cloned into Gateway plant destination vector pGWB21 and transformed into Arabidopsis suspension cells by Agrobacterium-mediated transformation.
For electrophysiological and physiological assays, all lines were grown under 0.120 mmol m−2 s−1 light (8 h/16 h day/night cycle) with ≈80% relative humidity, and 22°C/20°C day/night temperatures. For each experiment, wild-type Col plants were grown and assessed simultaneously with the mutant and/or transgenic lines.
Patch-Clamp Analyses.
Guard cell protoplast isolation and K+ current recording were performed as described (24, 25), with minor modifications (see Fig. 1 legend and SI Text). Anion current recording was performed according to Pei et al. (29) and Wang et al. (24) with some modifications (see Fig. 4 legend and SI Text). Data were compared by using the Student t test. Results with P ≤ 0.05 were considered statistically significant.
GPA1 Coimmunoprecipitation and Immunoblot Analysis.
Total protein extracts of Arabidopsis suspension cells were coimmunoprecipitated by anti-GPA1 antibodies or by preimmune serum as described (26). Protein lysates from young fully expanded rosette leaves of 4- to 5-week-old plants were prepared as described (25). Proteins from total, microsomal, and soluble fractions were separated on 10% SDS-polyacrylamide gels, electroblotted onto nitrocellulose membrane, and immunoblotted (56) (SI Text).
Confocal Microscopy.
Confocal imaging was performed by using an inverted Zeiss LSM510 Confocal microscope with a Plan-Neofluar 40×/1.3 oil differential interference contrast microscopy objective. For CFP, an excitation wavelength of 458 nm was used, and fluorescence was detected by using a 475–525-nm band-pass filter. Postacquisition image processing was done with the LSM 5 Image Browser (Zeiss) and Adobe Photoshop.
Stomatal Bioassay.
Stomatal aperture bioassays were conducted as described (24), with minor modifications (see SI Text). Values are means ± SE from at least three independent replicates, with at least 150 stomatal apertures measured per each replicate. Stomatal aperture measurements (Fig. 5) were performed blind.
Supplementary Material
Acknowledgments.
We thank Ms. Zhixin Zhao for assistance in preparation of plant material, Dr. Sona Pandey (Penn State University) for providing cDNA from guard cell and mesophyll cell protoplasts, and Drs. Jirong Huang and Jiansheng Liang for assistance with protein interaction experiments. This work was supported by National Science Foundation Grants MCB-0209694 (to S.M.A.) and MCB-0209711 (to A.M.J.), U.S. Department of Agriculture Grant 2006-35100-17254 (to S.M.A.), National Institutes of Health Grant GM65989-01 (to A.M.J.), and Department of Energy Grant DE-FG02-05ER15671 (to A.M.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800980105/DCSupplemental.
References
- 1.Sadja R, Alagem N, Reuveny E. Gating of GIRK channels: Details of an intricate, membrane-delimited signaling complex. Neuron. 2003;39:9–12. doi: 10.1016/s0896-6273(03)00402-1. [DOI] [PubMed] [Google Scholar]
- 2.Dascal N. Ion-channel regulation by G proteins. Trends Endocrinol Metab. 2001;12:391–398. doi: 10.1016/s1043-2760(01)00475-1. [DOI] [PubMed] [Google Scholar]
- 3.Mark MD, Herlitze S. G-protein mediated gating of inward-rectifier K+ channels. Eur J Biochem. 2000;267:5830–5836. doi: 10.1046/j.1432-1327.2000.01670.x. [DOI] [PubMed] [Google Scholar]
- 4.Schreibmayer W, et al. Inhibition of an inwardly rectifying K+ channel by G-protein α-subunits. Nature. 1996;380:624–627. doi: 10.1038/380624a0. [DOI] [PubMed] [Google Scholar]
- 5.Peleg S, Varon D, Ivanina T, Dessauer CW, Dascal N. Gαi controls the gating of the G protein-activated K+ channel, GIRK. Neuron. 2002;33:87–99. doi: 10.1016/s0896-6273(01)00567-0. [DOI] [PubMed] [Google Scholar]
- 6.Saitoh O, Kubo Y, Miyatani Y, Asano T, Nakata H. RGS8 accelerates G-protein-mediated modulation of K+ currents. Nature. 1997;390:525–529. doi: 10.1038/37385. [DOI] [PubMed] [Google Scholar]
- 7.Riven I, Iwanir S, Reuveny E. GIRK channel activation involves a local rearrangement of a preformed G protein channel complex. Neuron. 2006;51:561–573. doi: 10.1016/j.neuron.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Rishal I, Porozov Y, Yakubovich D, Varon D, Dascal N. Gβγ-dependent and Gβγ-independent basal activity of G protein-activated K+ channels. J Biol Chem. 2005;280:16685–16694. doi: 10.1074/jbc.M412196200. [DOI] [PubMed] [Google Scholar]
- 9.Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 10.Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE. 2001;2001:RE19. doi: 10.1126/stke.2001.111.re19. [DOI] [PubMed] [Google Scholar]
- 11.Lei Q, et al. Activation and inhibition of G protein-coupled inwardly rectifying potassium (Kir3) channels by G protein βγ subunits. Proc Natl Acad Sci USA. 2000;97:9771–9776. doi: 10.1073/pnas.97.17.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breitwieser GE. GIRK channels: Hierarchy of control. Focus on “PKC-delta sensitizes Kir3.1/3.2 channels to changes in membrane phospholipid levels after M3 receptor activation in HEK-293 cells”. Am J Physiol. 2005;289:C509–C511. doi: 10.1152/ajpcell.00237.2005. [DOI] [PubMed] [Google Scholar]
- 13.Jones AM, Assmann SM. Plants: The latest model system for G-protein research. EMBO Rep. 2004;5:572–578. doi: 10.1038/sj.embor.7400174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temple BR, Jones A. The plant heterotrimeric G-protein complex. Annu Rev Plant Biol. 2007;58:249–266. doi: 10.1146/annurev.arplant.58.032806.103827. [DOI] [PubMed] [Google Scholar]
- 15.Trusov Y, et al. Heterotrimeric G protein γ subunits provide functional selectivity in Gβγ dimer signaling in Arabidopsis. Plant Cell. 2007;19:1235–1250. doi: 10.1105/tpc.107.050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perfus-Barbeoch L, Jones AM, Assmann SM. Plant heterotrimeric G protein function: Insights from Arabidopsis and rice mutants. Curr Opin Plant Biol. 2004;7:719–731. doi: 10.1016/j.pbi.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Blatt MR. Cellular signaling and volume control in stomatal movements in plants. Annu Rev Cell Dev Biol. 2000;16:221–241. doi: 10.1146/annurev.cellbio.16.1.221. [DOI] [PubMed] [Google Scholar]
- 18.Schroeder JI, Kwak JM, Allen GJ. Guard cell abscisic acid signalling and engineering of drought hardiness in plants. Nature. 2001;410:327–330. doi: 10.1038/35066500. [DOI] [PubMed] [Google Scholar]
- 19.Hetherington AM. Guard cell signaling. Cell. 2001;107:711–714. doi: 10.1016/s0092-8674(01)00606-7. [DOI] [PubMed] [Google Scholar]
- 20.Pandey S, Zhang W, Assmann SM. Roles of ion channels and transporters in guard cell signal transduction. FEBS Lett. 2007;581:2325–2336. doi: 10.1016/j.febslet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Wu WH, Assmann SM. Is ATP required for K+ channel activation in Vicia guard cells? Plant Physiol. 1995;107:101–109. doi: 10.1104/pp.107.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeidner G, Sadja R, Reuveny E. Redox-dependent gating of G protein-coupled inwardly rectifying K+ channels. J Biol Chem. 2001;276:35564–35570. doi: 10.1074/jbc.M105189200. [DOI] [PubMed] [Google Scholar]
- 23.Liu K, Li L, Luan S. An essential function of phosphatidylinositol phosphates in activation of plant Shaker-type K+ channels. Plant J. 2005;42:433–443. doi: 10.1111/j.1365-313X.2005.02384.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang XQ, Ullah H, Jones AM, Assmann SM. G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science. 2001;292:2070–2072. doi: 10.1126/science.1059046. [DOI] [PubMed] [Google Scholar]
- 25.Coursol S, et al. Sphingolipid signalling in Arabidopsis guard cells involves heterotrimeric G proteins. Nature. 2003;423:651–654. doi: 10.1038/nature01643. [DOI] [PubMed] [Google Scholar]
- 26.Chen JG, et al. A seven-transmembrane RGS protein that modulates plant cell proliferation. Science. 2003;301:1728–1731. doi: 10.1126/science.1087790. [DOI] [PubMed] [Google Scholar]
- 27.Pilot G, Pratelli R, Gaymard F, Meyer Y, Sentenac H. Five-group distribution of the Shaker-like K+ channel family in higher plants. J Mol Evol. 2003;56:418–434. doi: 10.1007/s00239-002-2413-2. [DOI] [PubMed] [Google Scholar]
- 28.Becker D, et al. Regulation of the ABA-sensitive Arabidopsis potassium channel gene GORK in response to water stress. FEBS Lett. 2003;554:119–126. doi: 10.1016/s0014-5793(03)01118-9. [DOI] [PubMed] [Google Scholar]
- 29.Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI. Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell. 1997;9:409–423. doi: 10.1105/tpc.9.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen JG, et al. RACK1 mediates multiple hormone responsiveness and developmental processes in Arabidopsis. J Exp Bot. 2006;57:2697–2708. doi: 10.1093/jxb/erl035. [DOI] [PubMed] [Google Scholar]
- 31.Ullah H, et al. The β-subunit of the Arabidopsis G protein negatively regulates auxin-induced cell division and affects multiple developmental processes. Plant Cell. 2003;15:393–409. doi: 10.1105/tpc.006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adjobo-Hermans MJW, Goedhart J, Gadella TW., Jr Plant G protein heterotrimers require dual lipidation motifs of Gα and Gβ and do not dissociate upon activation. J Cell Sci. 2006;119:5087–5097. doi: 10.1242/jcs.03284. [DOI] [PubMed] [Google Scholar]
- 33.Kato C, et al. Characterization of heterotrimeric G protein complexes in rice plasma membrane. Plant J. 2004;38:320–331. doi: 10.1111/j.1365-313X.2004.02046.x. [DOI] [PubMed] [Google Scholar]
- 34.Misra S, Wu Y, Venkataraman G, Sopory SK, Tuteja N. Heterotrimeric G-protein complex and G-protein-coupled receptor from a legume (Pisum sativum): Role in salinity and heat stress and cross-talk with phospholipase C. Plant J. 2007;51:656–669. doi: 10.1111/j.1365-313X.2007.03169.x. [DOI] [PubMed] [Google Scholar]
- 35.Fishburn CS, Pollitt SK, Bourne HR. Localization of a peripheral membrane protein: Gβγ targets GαZ. Proc Natl Acad Sci USA. 2000;97:1085–1090. doi: 10.1073/pnas.97.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evanko DS, Thiyagarajan MM, Siderovski DP, Wedegaertner PB. Gβγ isoforms selectively rescue plasma membrane localization and palmitoylation of mutant Gαs and Gαq. J Biol Sci. 2001;276:23945–23953. doi: 10.1074/jbc.M101154200. [DOI] [PubMed] [Google Scholar]
- 37.Krumins AM, Gilman AG. Targeted knockdown of G protein subunits selectively prevents receptor-mediated modulation of effectors and reveals complex changes in non-targeted signaling proteins. J Biol Chem. 2006;281:10250–10262. doi: 10.1074/jbc.M511551200. [DOI] [PubMed] [Google Scholar]
- 38.Iiri T, Farfel Z, Bourne HR. G-protein diseases furnish a model for the turn-on switch. Nature. 1998;394:35–38. doi: 10.1038/27831. [DOI] [PubMed] [Google Scholar]
- 39.Rondard P, et al. Mutant G protein α subunit activated by Gβγ: A model for receptor activation? Proc Natl Acad Sci USA. 2001;98:6150–6155. doi: 10.1073/pnas.101136198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majumdar S, Ramachandran S, Cerione RA. Perturbing the linker regions of the α-subunit of transducin: A new class of constitutively active GTP-binding proteins. J Biol Chem. 2004;279:40137–40145. doi: 10.1074/jbc.M405420200. [DOI] [PubMed] [Google Scholar]
- 41.Cabrera-Vera TM, et al. Insights into G protein structure, function, and regulation. Endocr Rev. 2003;24:765–781. doi: 10.1210/er.2000-0026. [DOI] [PubMed] [Google Scholar]
- 42.Wu WH, Assmann SM. A membrane-delimited pathway of G-protein regulation of the guard-cell inward K+ channel. Proc Natl Acad Sci USA. 1994;91:6310–6314. doi: 10.1073/pnas.91.14.6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wegner LH, de Boer AH. Two inward K+ channels in the xylem parenchyma cells of barley roots are regulated by G-protein modulators through a membrane-delimited pathway. Planta. 1997;203:506–516. [Google Scholar]
- 44.Lee Y, et al. Abscisic acid-induced phosphoinositide turnover in guard cell protoplasts of Vicia faba. Plant Physiol. 1996;110:987–996. doi: 10.1104/pp.110.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacob T, Ritchie S, Assmann SM, Gilroy S. Abscisic acid signal transduction in guard cells is mediated by phospholipase D activity. Proc Natl Acad Sci USA. 1999;96:12192–12197. doi: 10.1073/pnas.96.21.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mishra G, Zhang W, Deng F, Zhao J, Wang X. A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science. 2006;312:264–266. doi: 10.1126/science.1123769. [DOI] [PubMed] [Google Scholar]
- 47.Loussouarn G, et al. Phosphatidylinositol-4,5-bisphosphate, PIP2, controls KCNQ1/KCNE1 voltage-gated potassium channels: A functional homology between voltage-gated and inward rectifier K+ channels. EMBO J. 2003;22:5412–5421. doi: 10.1093/emboj/cdg526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sampson KJ, et al. Adrenergic regulation of a key cardiac potassium channel can contribute to atrial fibrillation: Evidence from an IKs transgenic mouse. J Physiol. 2008;586:627–637. doi: 10.1113/jphysiol.2007.141333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levitzki A, Klein S. G-protein subunit dissociation is not an integral part of G-protein action. Chembiochem. 2002;3:815–818. doi: 10.1002/1439-7633(20020902)3:9<815::AID-CBIC815>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 50.Bünemann M, Frank M, Lohse MJ. Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc Natl Acad Sci USA. 2003;100:16077–16082. doi: 10.1073/pnas.2536719100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Digby GJ, Lober RM, Sethi PR, Lambert NA. Some G protein heterotrimers physically dissociate in living cells. Proc Natl Acad Sci USA. 2006;103:17789–17794. doi: 10.1073/pnas.0607116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnston CA, et al. GTPase acceleration as the rate-limiting step in Arabidopsis G protein-coupled sugar signaling. Proc Natl Acad Sci USA. 2007;104:17317–17322. doi: 10.1073/pnas.0704751104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herlitze S, Ruppersberg JP, Mark MD. New roles for RGS2, 5 and 8 on the ratio-dependent modulation of recombinant GIRK channels expressed in Xenopus oocytes. J Physiol. 1999;517(Pt 2):341–352. doi: 10.1111/j.1469-7793.1999.0341t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doupnik CA, Davidson N, Lester HA, Kofuji P. RGS proteins reconstitute the rapid gating kinetics of Gβγ-activated inwardly rectifying K+ channels. Proc Natl Acad Sci USA. 1997;94:10461–10466. doi: 10.1073/pnas.94.19.10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeong SW, Ikeda SR. Differential regulation of G protein-gated inwardly rectifying K+ channel kinetics by distinct domains of RGS8. J Physiol. 2001;535:335–347. doi: 10.1111/j.1469-7793.2001.00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pandey S, Chen JG, Jones AM, Assmann SM. G-protein complex mutants are hypersensitive to abscisic acid regulation of germination and postgermination development. Plant Physiol. 2006;141:243–256. doi: 10.1104/pp.106.079038. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.