Abstract
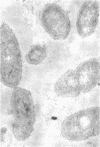
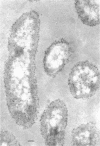
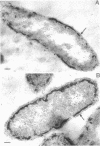
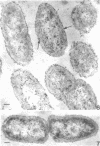
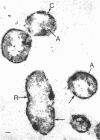
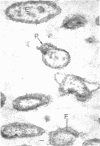
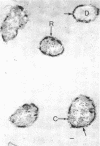
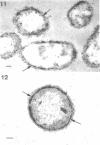
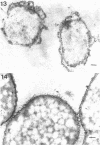
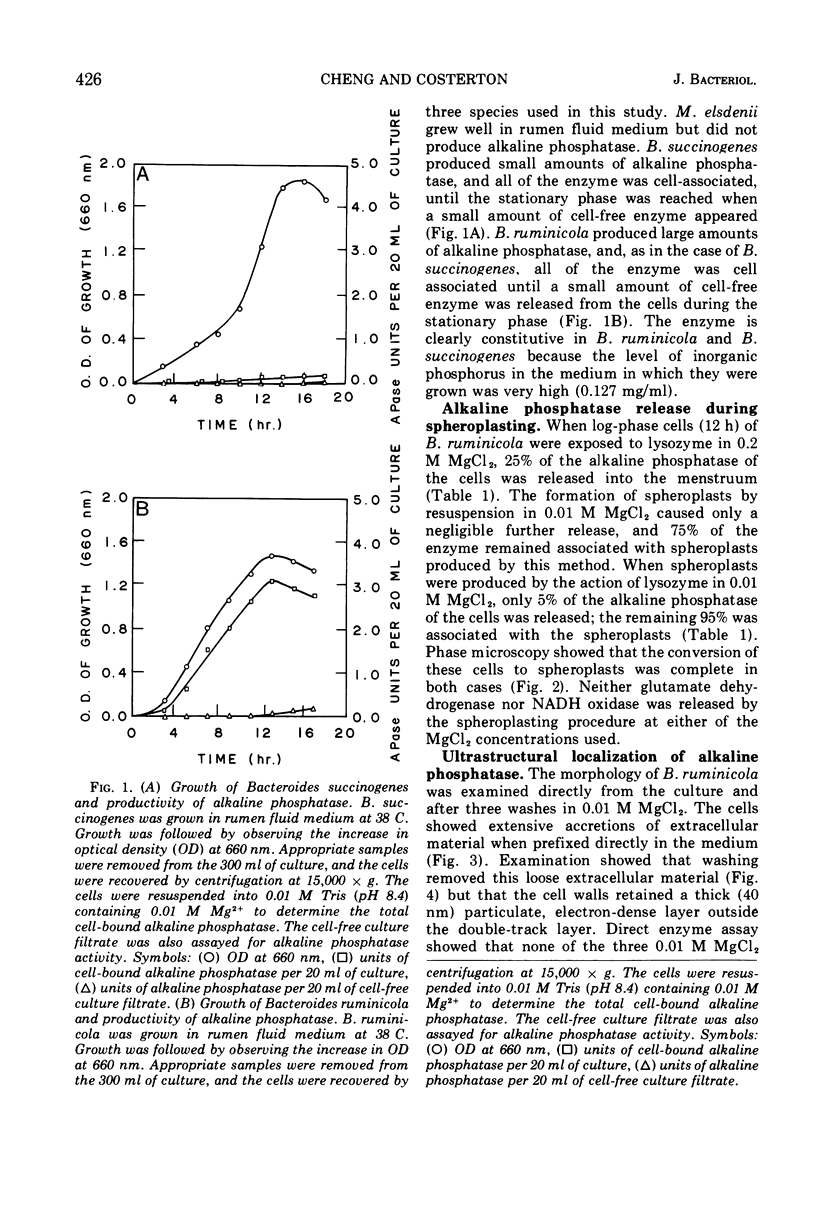
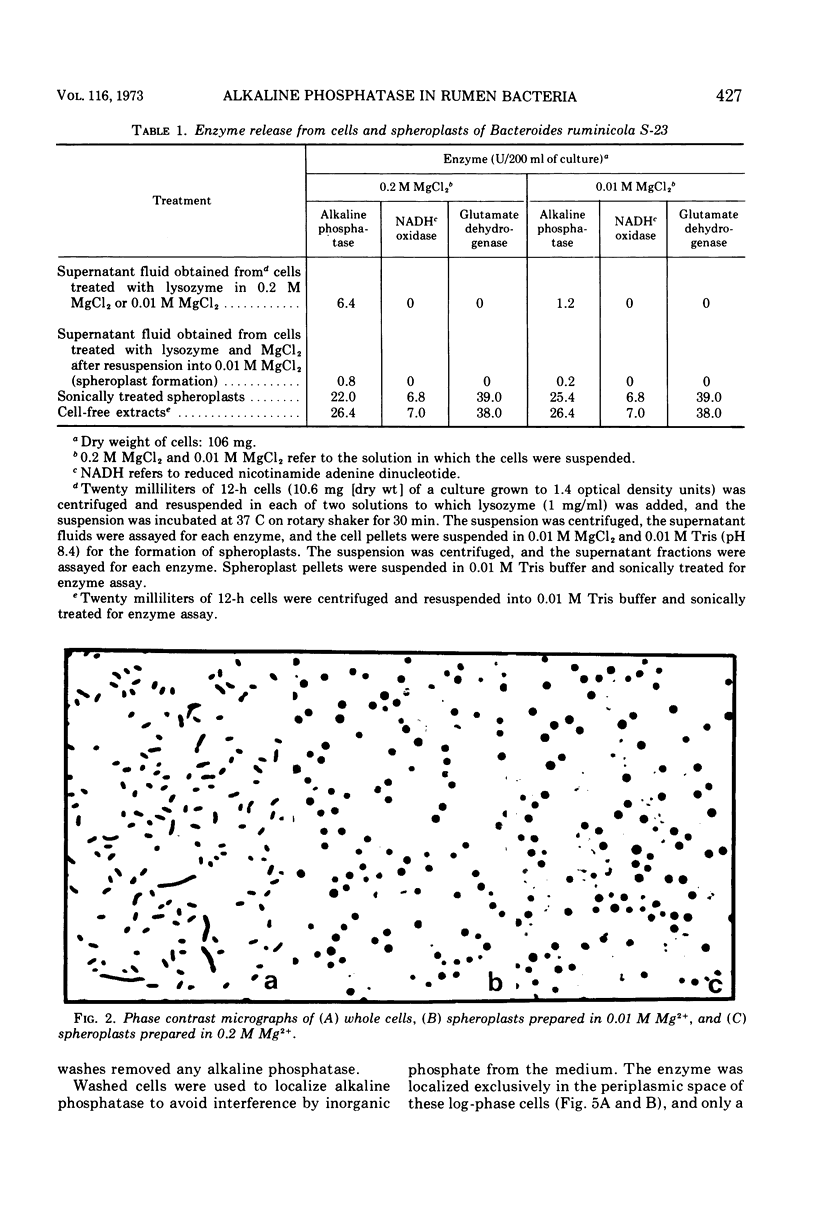
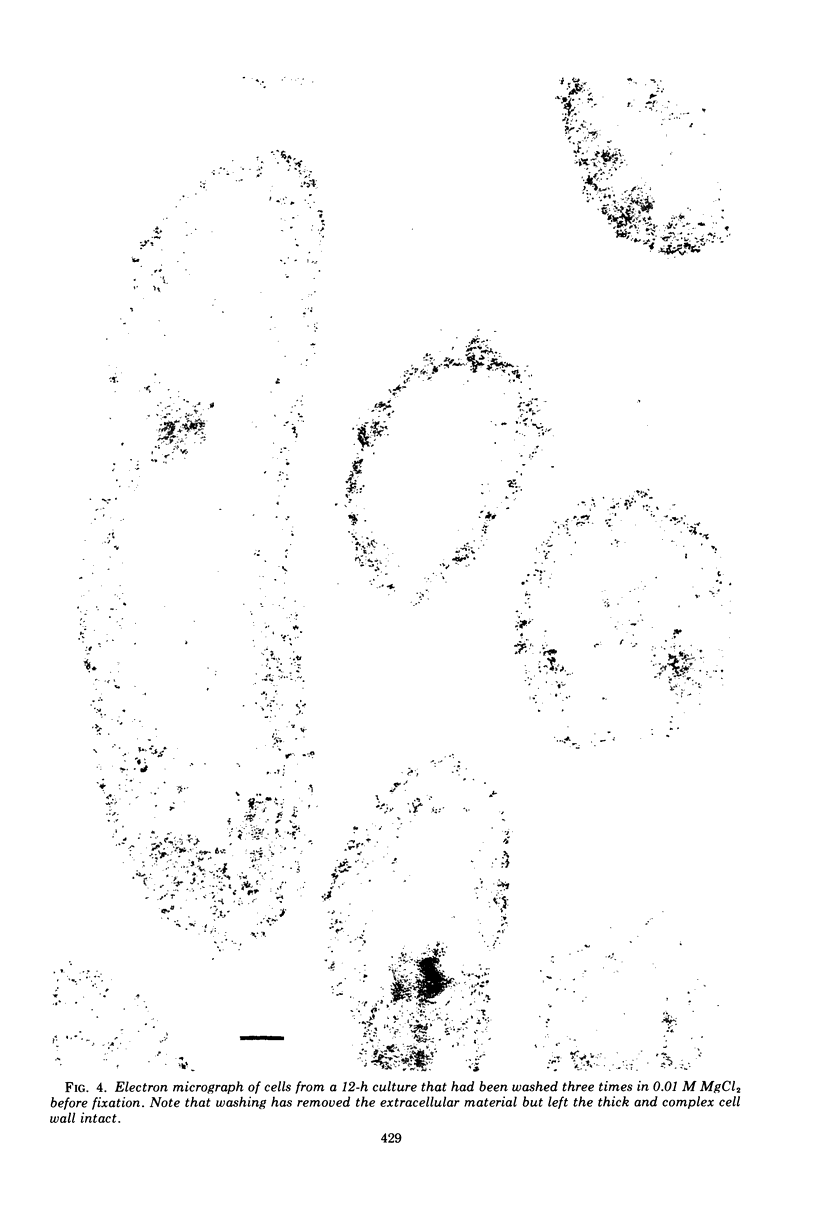
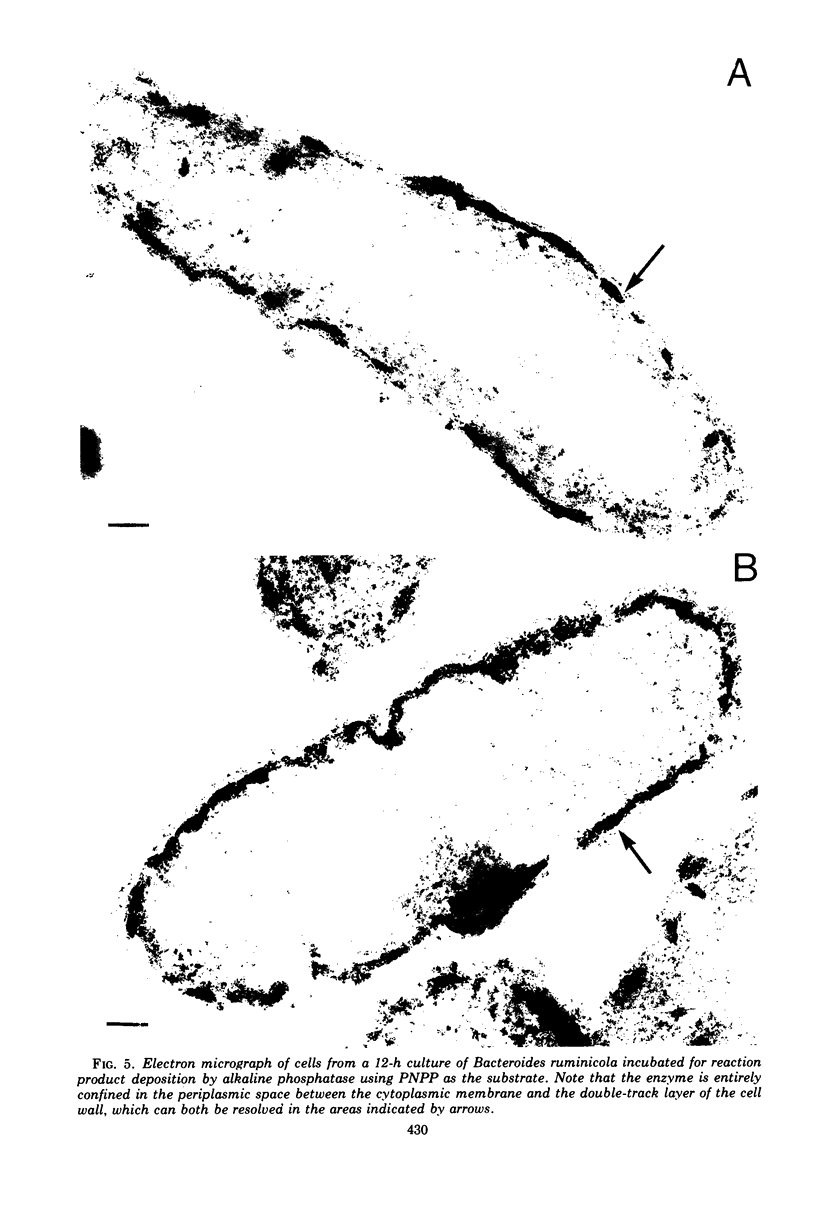
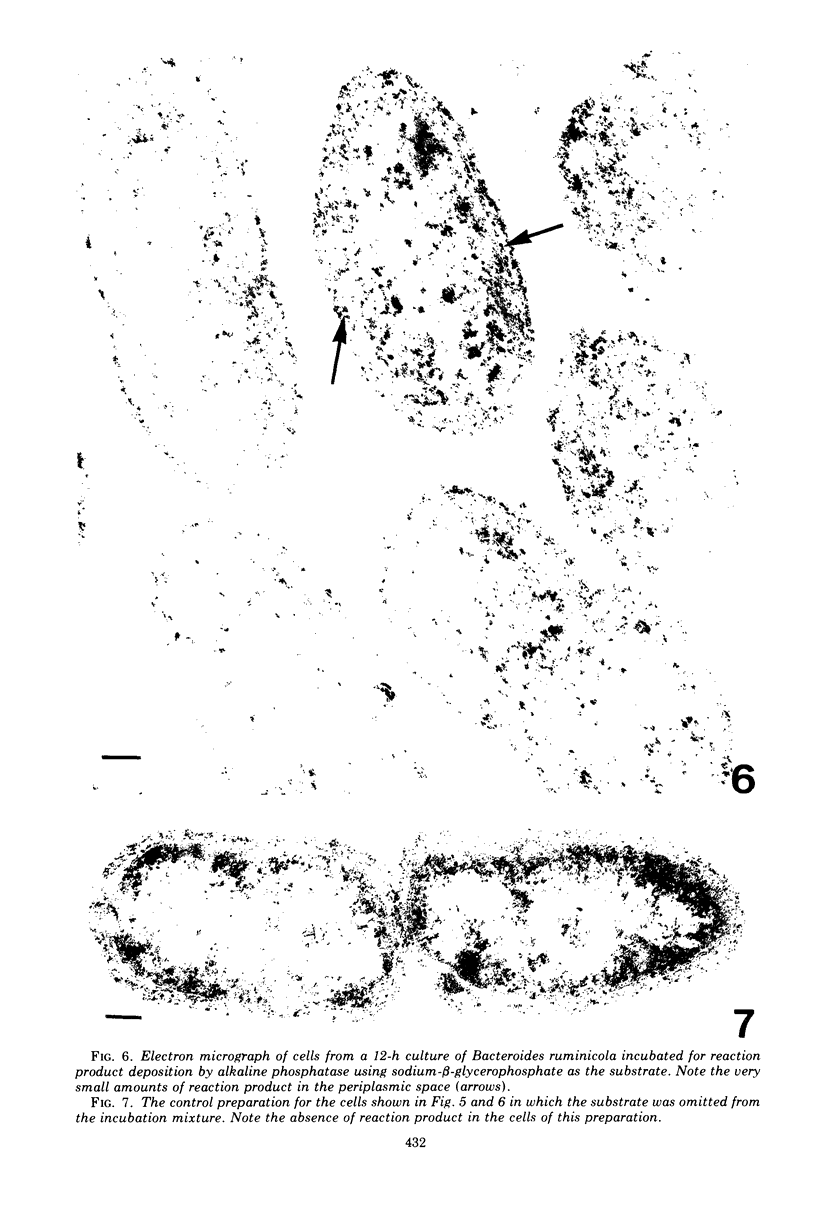
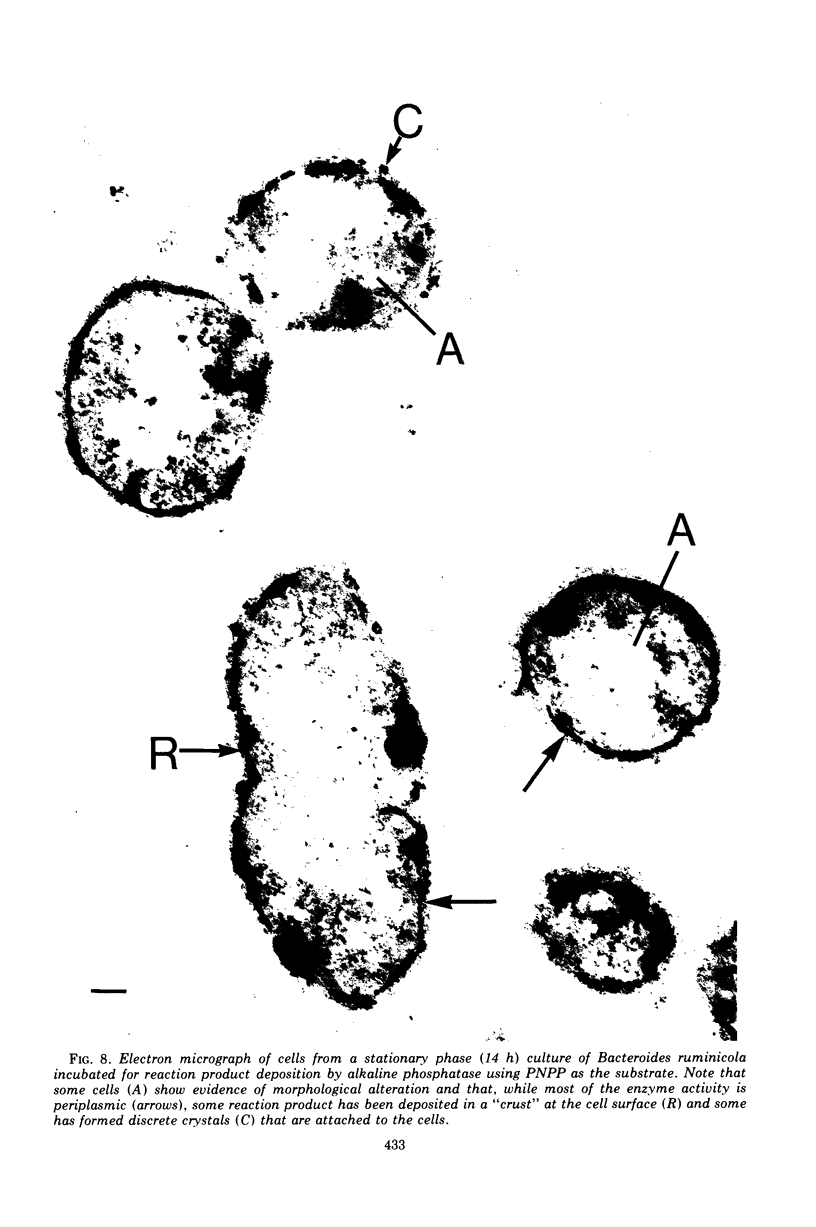
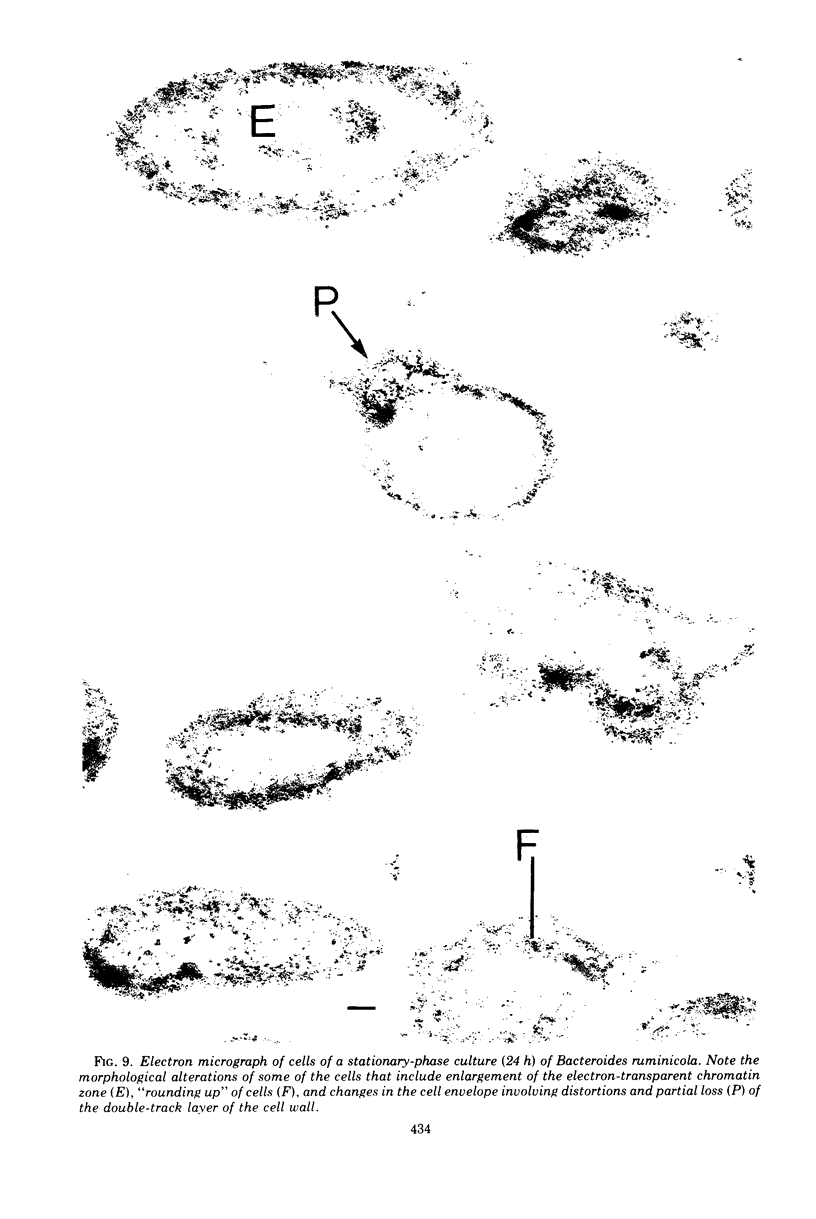
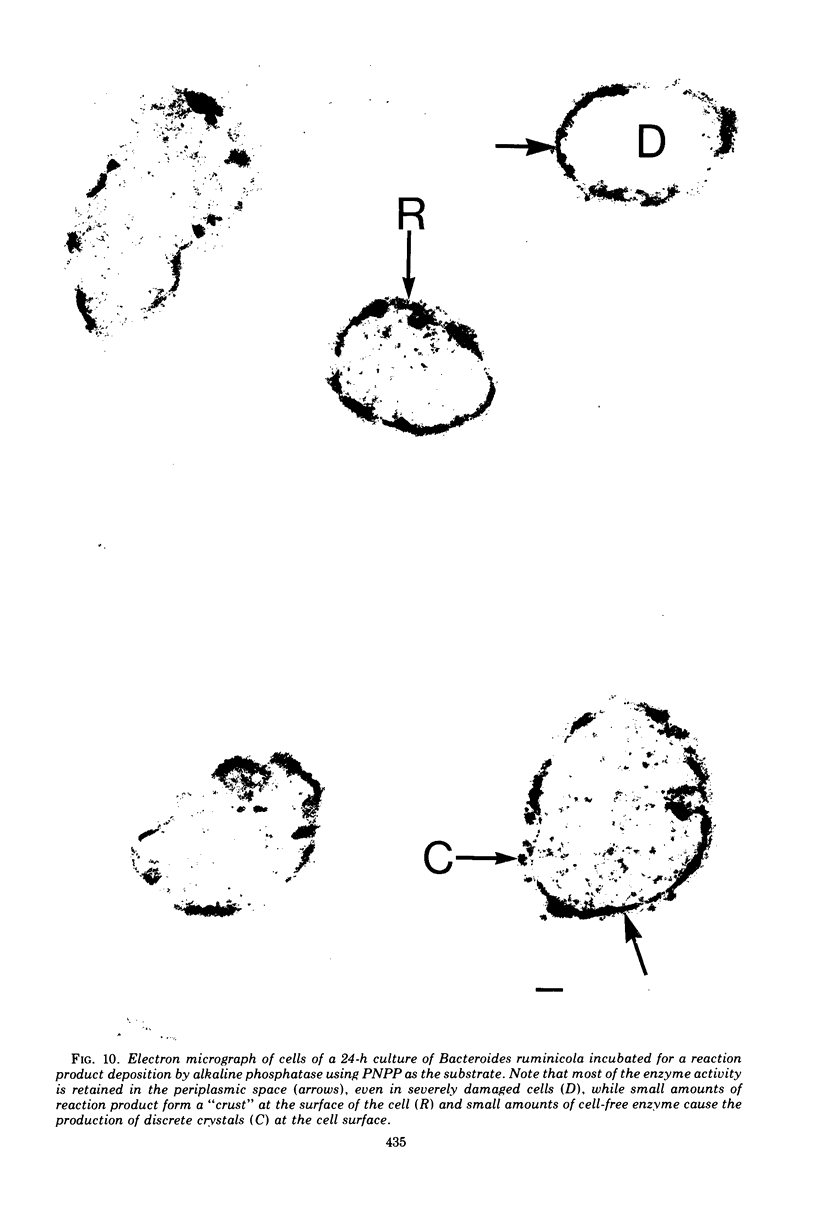
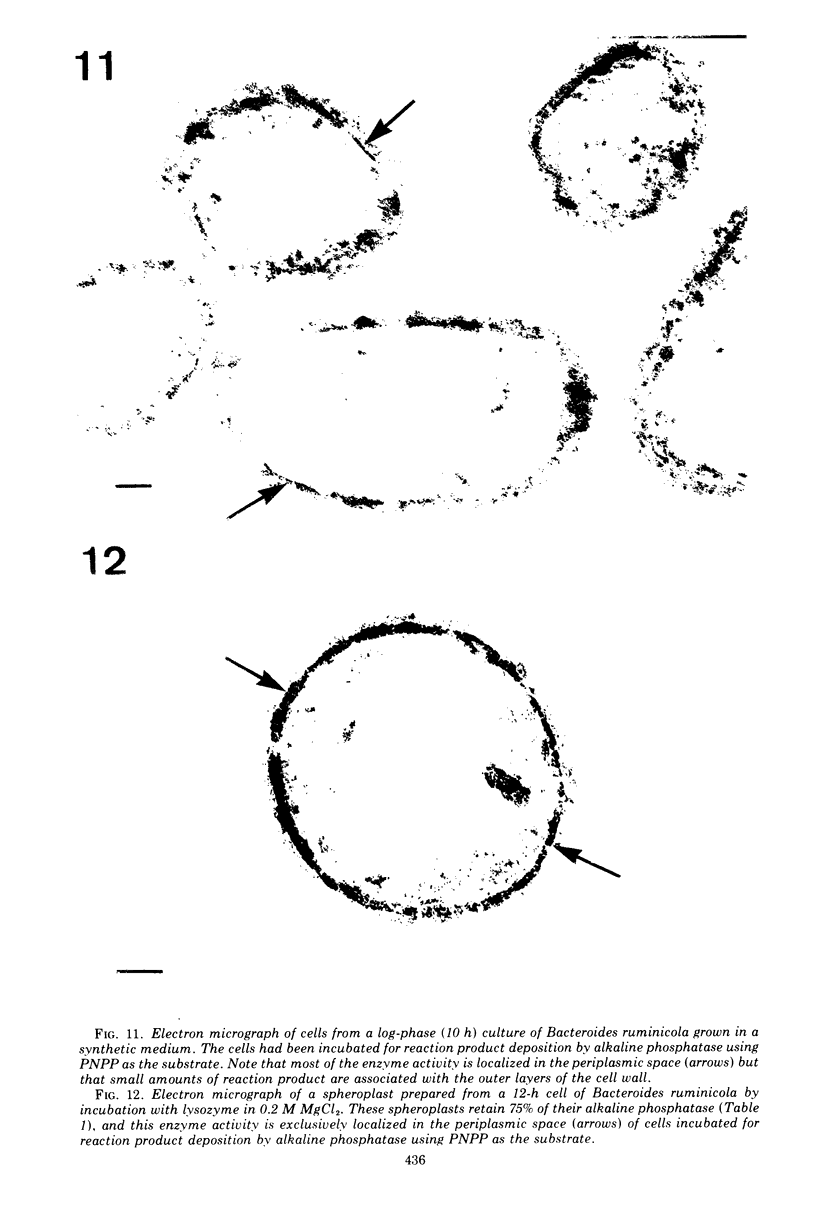
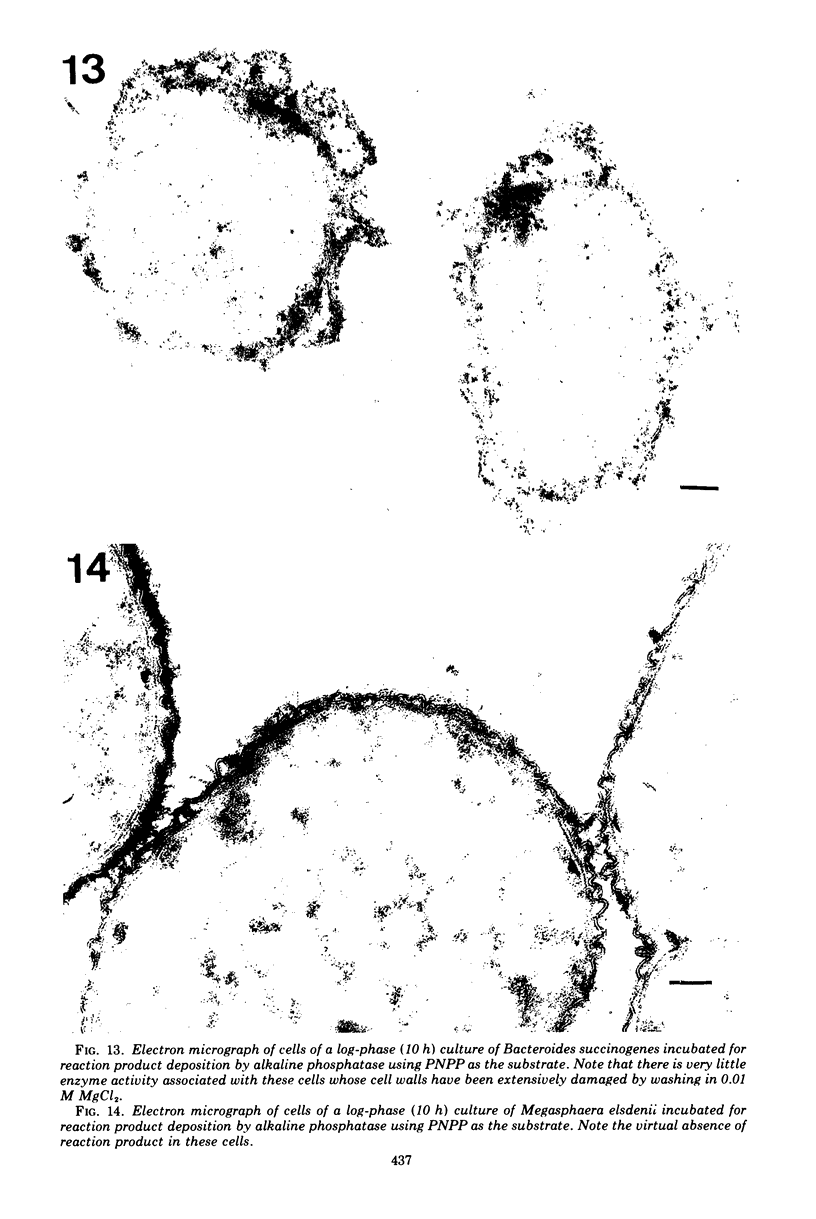
Of the three species (Bacteroides ruminicola, B. succinogenes, and Megasphaera elsdenii) of anaerobic gram-negative rumen bacteria studied, only B. ruminicola produced significant amounts of alkaline phosphatase. This enzyme, which is constitutive, showed a greater affinity for p-nitrophenylphosphate than for sodium-β-glycerophosphate and was shown to be located exclusively in the periplasmic space of log-phase cells. Small amounts of this enzyme were released from these cells in stationary-phase cultures, but washing in 0.01 M MgCl2 and the production of spheroplasts by using lysozyme in 0.01 M MgCl2 did not release significant amounts of the enzyme. Exposure to 0.2 M MgCl2 did not release significant amounts of the periplasmic alkaline phosphatase of the cell, and when these cells were spheroplasted with lysozyme in 0.2 M MgCl2 only 25% of the enzyme was released. Spheroplasts were formed spontaneously in aging cultures of B. ruminicola, but even these cells retained most of their periplasmic alkaline phosphatase. It was concluded that the alkaline phosphatase of B. ruminicola is firmly bound to a structural component within the periplasmic area of the cell wall and that the enzyme is released in large amounts only when the cells break down. The behavior of alkaline phosphatase in this bacterium contrasts with that of conventional periplasmic enzymes of aerobic bacteria, which are released upon conversion into spheroplasts by lysozyme and ethylenediaminetetraacetic acid and by other types of cell wall damage. All three species of bacteria studied here, as well as bacteria found in mixed populations in the rumen, have thick, complex layers external to the double-track layer of their cell walls. In addition, B. ruminicola produces a loose extracellular material.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRYANT M. P. Bacterial species of the rumen. Bacteriol Rev. 1959 Sep;23(3):125–153. doi: 10.1128/br.23.3.125-153.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P., SMALL N., BOUMA C., CHU H. Bacteroides ruminicola n. sp. and Succinimonas amylolytica; the new genus and species; species of succinic acid-producing anaerobic bacteria of the bovine rumen. J Bacteriol. 1958 Jul;76(1):15–23. doi: 10.1128/jb.76.1.15-23.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman R. W., Heppel L. A. On the localization of alkaline phosphatase and cyclic phosphodiesterase in Escherichia coli. Biochemistry. 1968 Jul;7(7):2554–2562. doi: 10.1021/bi00847a016. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., White D. C., Bryant M. P., Doetsch R. N. Specificity of the heme requirement for growth of Bacteroides ruminicola. J Bacteriol. 1965 Dec;90(6):1645–1654. doi: 10.1128/jb.90.6.1645-1654.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Day D. F., Costerton J. W., Ingram J. M. Alkaline phosphatase subunits in the culture filtrate of Pseudomonas aeruginosa. Can J Biochem. 1972 Mar;50(3):268–276. doi: 10.1139/o72-038. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Alkaline phosphatase localization and spheroplast formation of Pseudomonas aeruginosa. Can J Microbiol. 1970 Dec;16(12):1319–1324. doi: 10.1139/m70-218. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Interactions of alkaline phosphatase and the cell wall of Pseudomonas aeruginosa. J Bacteriol. 1971 Jul;107(1):325–336. doi: 10.1128/jb.107.1.325-336.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Release of alkaline phosphatase from cells of Pseudomonas aeruginosa by manipulation of cation concentration and of pH. J Bacteriol. 1970 Nov;104(2):748–753. doi: 10.1128/jb.104.2.748-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J. Spheroplast formation by an anaerobic gram-negative bacterium Bacteroides ruminicola. Can J Microbiol. 1973 May;19(5):667–669. doi: 10.1139/m73-108. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Forsberg C., Matula T. I., Buckmire F. L., MacLeod R. A. Nutrition and metabolism of marine bacteria. XVI. Formation of protoplasts, spheroplasts, and related forms from a gram-negative marine bacterium. J Bacteriol. 1967 Nov;94(5):1764–1777. doi: 10.1128/jb.94.5.1764-1777.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W. Relationship of a wall-associated enzyme with specific layers of the cell wall of a gram-negative bacterium. J Bacteriol. 1973 Jun;114(3):1281–1293. doi: 10.1128/jb.114.3.1281-1293.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W. The structure and function of the cell envelope of gram-negative bacteria. Rev Can Biol. 1970 Sep;29(3):299–316. [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Hofstad T., Kristoffersen T., Selvig K. A. Electron microscopy of endotoxic lipopolysaccharide from Bacteroides, Fusobacterium and sphaerophorus. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(3):413–419. doi: 10.1111/j.1699-0463.1972.tb00054.x. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., SECHAUD J., RYTER A. Electron microscopical studies of phage multiplication. IV. The establishment of the DNA pool of vegetative phage and the maturation of phage particles. Virology. 1959 Aug;8:478–498. doi: 10.1016/0042-6822(59)90050-9. [DOI] [PubMed] [Google Scholar]
- Kushnarev V. M., Smirnova T. A. Electron microscopy of alkaline phosphatase of Escherichia coli. Can J Microbiol. 1966 Aug;12(4):605–607. doi: 10.1139/m66-086. [DOI] [PubMed] [Google Scholar]
- Lindsay S. S., Wheeler B., Sanderson K. E., Costerton J. W., Cheng K. J. The release of alkaline phosphatase and of lipopolysaccharide during the growth of rough and smooth strains of Salmonella typhimurium. Can J Microbiol. 1973 Mar;19(3):335–343. doi: 10.1139/m73-056. [DOI] [PubMed] [Google Scholar]
- Lopes J., Gottfried S., Rothfield L. Leakage of periplasmic enzymes by mutants of Escherichia coli and Salmonella typhimurium: isolation of "periplasmic leaky" mutants. J Bacteriol. 1972 Feb;109(2):520–525. doi: 10.1128/jb.109.2.520-525.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLEOD R. A., ONOFREY E. Nutrition and metabolism of marine bacteria. III. The relation of sodium and potassium to growth. J Cell Physiol. 1957 Dec;50(3):389–401. doi: 10.1002/jcp.1030500305. [DOI] [PubMed] [Google Scholar]
- MALAMY M., HORECKER B. L. The localization of alkaline phosphatase in E. coli K12. Biochem Biophys Res Commun. 1961 Jun 2;5:104–108. doi: 10.1016/0006-291x(61)90020-1. [DOI] [PubMed] [Google Scholar]
- MacAlister T. J., Costerton J. W., Thompson L., Thompson J., Ingram J. M. Distribution of alkaline phosphatase within the periplasmic space of gram-negative bacteria. J Bacteriol. 1972 Sep;111(3):827–832. doi: 10.1128/jb.111.3.827-832.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaretten W., Morgan C., Rosenkranz H. S., Rose H. M. Effect of hydroxyurea on virus development. I. Electron microscopic study of the effect on the development of bacteriophage T4. J Bacteriol. 1966 Feb;91(2):823–833. doi: 10.1128/jb.91.2.823-833.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Nisonson I., Tannenbaum M., Neu H. C. Surface localization of Escherichia coli 5'-nucleotidase by electron microscopy. J Bacteriol. 1969 Nov;100(2):1083–1090. doi: 10.1128/jb.100.2.1083-1090.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shands J. W. Localization of somatic antigen on gram-negative bacteria using ferritin antibody conjugates. Ann N Y Acad Sci. 1966 Jun 30;133(2):292–298. doi: 10.1111/j.1749-6632.1966.tb52372.x. [DOI] [PubMed] [Google Scholar]
- Shilo M. Lysis of blue-green algae by myxobacter. J Bacteriol. 1970 Oct;104(1):453–461. doi: 10.1128/jb.104.1.453-461.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. P., Cheng K. J., Costerton J. W., Idziak E. S., Ingram J. M. Sensitivity of normal and mutant strains of Escherichia coli to actinomycin-D. Can J Microbiol. 1972 Jun;18(6):909–915. doi: 10.1139/m72-139. [DOI] [PubMed] [Google Scholar]
- Wetzel B. K., Spicer S. S., Dvorak H. F., Heppel L. A. Cytochemical localization of certain phosphatases in Escherichia coli. J Bacteriol. 1970 Oct;104(1):529–542. doi: 10.1128/jb.104.1.529-542.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]