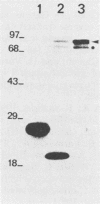
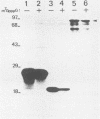
Abstract
In the human hepatitis B virus (HBV) genome, the 5' end of the polymerase coding sequence overlaps with the 3' end of the core protein coding sequence. Recent results obtained from genetic studies have suggested that translation of HBV polymerase initiates from the first ATG codon of the polymerase reading frame and is not a result of frameshift translation from the core protein reading frame, as in the case of retroviruses. By using in vitro-synthesized SP6 RNA transcripts, we now demonstrate that HBV core protein-specific mRNA can direct the synthesis of polymerase from the internal polymerase ATG codon in rabbit reticulocyte lysates and Xenopus oocytes. A related message with an additional 60 nucleotides at the 5' end (pre-core protein mRNA) was not as efficient as the core protein mRNA for translation of polymerase. Furthermore, translation of polymerase from the core protein mRNA was not inhibited by the cap analog m7GpppG. This result, together with the results described above, indicates that translation of HBV polymerase occurs in a novel, cap-independent manner.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang L. J., Pryciak P., Ganem D., Varmus H. E. Biosynthesis of the reverse transcriptase of hepatitis B viruses involves de novo translational initiation not ribosomal frameshifting. Nature. 1989 Jan 26;337(6205):364–368. doi: 10.1038/337364a0. [DOI] [PubMed] [Google Scholar]
- Enders G. H., Ganem D., Varmus H. Mapping the major transcripts of ground squirrel hepatitis virus: the presumptive template for reverse transcriptase is terminally redundant. Cell. 1985 Aug;42(1):297–308. doi: 10.1016/s0092-8674(85)80125-2. [DOI] [PubMed] [Google Scholar]
- Fajardo J. E., Shatkin A. J. Translation of bicistronic viral mRNA in transfected cells: regulation at the level of elongation. Proc Natl Acad Sci U S A. 1990 Jan;87(1):328–332. doi: 10.1073/pnas.87.1.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem D., Varmus H. E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Hickey E. D., Weber L. A., Baglioni C., Kim C. H., Sarma R. H. A relation between inhibition of protein synthesis and conformation of 5'-phosphorylated 7-methylguanosine derivatives. J Mol Biol. 1977 Jan 15;109(2):173–183. doi: 10.1016/s0022-2836(77)80027-2. [DOI] [PubMed] [Google Scholar]
- Jacks T., Varmus H. E. Expression of the Rous sarcoma virus pol gene by ribosomal frameshifting. Science. 1985 Dec 13;230(4731):1237–1242. doi: 10.1126/science.2416054. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien J. M., Petcu D. J., Aldrich C. E., Mason W. S. Initiation and termination of duck hepatitis B virus DNA synthesis during virus maturation. J Virol. 1987 Dec;61(12):3832–3840. doi: 10.1128/jvi.61.12.3832-3840.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerovitch K., Pelletier J., Sonenberg N. A cellular protein that binds to the 5'-noncoding region of poliovirus RNA: implications for internal translation initiation. Genes Dev. 1989 Jul;3(7):1026–1034. doi: 10.1101/gad.3.7.1026. [DOI] [PubMed] [Google Scholar]
- Ou J. H., Laub O., Rutter W. J. Hepatitis B virus gene function: the precore region targets the core antigen to cellular membranes and causes the secretion of the e antigen. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1578–1582. doi: 10.1073/pnas.83.6.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. H., Yeh C. T., Yen T. S. Transport of hepatitis B virus precore protein into the nucleus after cleavage of its signal peptide. J Virol. 1989 Dec;63(12):5238–5243. doi: 10.1128/jvi.63.12.5238-5243.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. H., Yen T. S., Wang Y. F., Kam W. K., Rutter W. J. Cloning and characterization of a human ribosomal protein gene with enhanced expression in fetal and neoplastic cells. Nucleic Acids Res. 1987 Nov 11;15(21):8919–8934. doi: 10.1093/nar/15.21.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziwill G., Tucker W., Schaller H. Mutational analysis of the hepatitis B virus P gene product: domain structure and RNase H activity. J Virol. 1990 Feb;64(2):613–620. doi: 10.1128/jvi.64.2.613-620.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roossinck M. J., Jameel S., Loukin S. H., Siddiqui A. Expression of hepatitis B viral core region in mammalian cells. Mol Cell Biol. 1986 May;6(5):1393–1400. doi: 10.1128/mcb.6.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhury S., Shih C. cis rescue of a mutated reverse transcriptase gene of human hepatitis B virus by creation of an internal ATG. J Virol. 1990 Mar;64(3):1063–1069. doi: 10.1128/jvi.64.3.1063-1069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicht H. J., Radziwill G., Schaller H. Synthesis and encapsidation of duck hepatitis B virus reverse transcriptase do not require formation of core-polymerase fusion proteins. Cell. 1989 Jan 13;56(1):85–92. doi: 10.1016/0092-8674(89)90986-0. [DOI] [PubMed] [Google Scholar]
- Standring D. N., Ou J. H., Masiarz F. R., Rutter W. J. A signal peptide encoded within the precore region of hepatitis B virus directs the secretion of a heterogeneous population of e antigens in Xenopus oocytes. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8405–8409. doi: 10.1073/pnas.85.22.8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring D. N., Ou J. H., Rutter W. J. Assembly of viral particles in Xenopus oocytes: pre-surface-antigens regulate secretion of the hepatitis B viral surface envelope particle. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9338–9342. doi: 10.1073/pnas.83.24.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumoto Y., Iwase R., Sekine M., Hata T., Ohshima Y. Pre-mRNA with a trimethylguanosine cap structure can be spliced efficiently in vitro. Biochem Biophys Res Commun. 1989 Aug 15;162(3):977–983. doi: 10.1016/0006-291x(89)90769-9. [DOI] [PubMed] [Google Scholar]
- Yaginuma K., Shirakata Y., Kobayashi M., Koike K. Hepatitis B virus (HBV) particles are produced in a cell culture system by transient expression of transfected HBV DNA. Proc Natl Acad Sci U S A. 1987 May;84(9):2678–2682. doi: 10.1073/pnas.84.9.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]