Abstract
Drs2p is a resident type 4 P-type ATPase (P4-ATPase) and potential phospholipid translocase of the trans-Golgi network (TGN) where it has been implicated in clathrin function. However, precise protein transport pathways requiring Drs2p and how it contributes to clathrin-coated vesicle budding remain unclear. Here we show a functional codependence between Drs2p and the AP-1 clathrin adaptor in protein sorting at the TGN and early endosomes of Saccharomyces cerevisiae. Genetic criteria indicate that Drs2p and AP-1 operate in the same pathway and that AP-1 requires Drs2p for function. In addition, we show that loss of AP-1 markedly increases Drs2p trafficking to the plasma membrane, but does not perturb retrieval of Drs2p from the early endosome back to the TGN. Thus AP-1 is required at the TGN to sort Drs2p out of the exocytic pathway, presumably for delivery to the early endosome. Moreover, a conditional allele that inactivates Drs2p phospholipid translocase (flippase) activity disrupts its own transport in this AP-1 pathway. Drs2p physically interacts with AP-1; however, AP-1 and clathrin are both recruited normally to the TGN in drs2Δ cells. These results imply that Drs2p acts independently of coat recruitment to facilitate AP-1/clathrin-coated vesicle budding from the TGN.
INTRODUCTION
Clathrin mediates protein transport between the plasma membrane, endosomes, and TGN through association with pathway-specific adaptors that link integral membrane cargo proteins to the clathrin lattice during vesicle formation. In mammalian cells, the ubiquitously expressed heterotetrameric AP-1A clathrin adaptor, composed of γ, β1, μ1A, and σ1-adaptins, appears to mediate both anterograde transport of proteins from the TGN to early endosomes, as well as the retrograde trip back to the TGN (Hinners and Tooze, 2003; Robinson, 2004). For its role in anterograde transport of lysosomal enzymes, AP-1A collaborates with monomeric adaptors called GGAs (Golgi localized, γ-ear–containing, Arf-binding proteins) to recruit the mannose-6-phosphate receptor into clathrin-coated vesicles (CCVs; Doray et al., 2002). The AP-1B complex, which differs from AP-1A by an alternate μ1B subunit, has a functionally distinct role in sorting cargo from the TGN to the basolateral membrane of polarized epithelial cells (Folsch et al., 1999). In budding yeast, there is evidence that AP-1 mediates early endosome to TGN retrograde transport (Valdivia et al., 2002; Foote and Nothwehr, 2006), but no anterograde role for AP-1 has yet been described. The yeast GGAs appear to function independently of AP-1 to mediate delivery of cargo from the TGN to the late endosome (Black and Pelham, 2000). Deletion of genes encoding AP-1 subunits or GGAs has little consequence to growth of yeast. However, the combined deletion of AP-1 and GGA causes a severe growth defect, suggesting that these two adaptors mediate parallel transport pathways and that yeast cannot tolerate loss of both pathways (Costaguta et al., 2001; Hirst et al., 2001).
The mechanism of AP-1/clathrin-coated vesicle (AP-1/CCV) formation is incompletely understood. A commonly held view is that ARF-dependent recruitment of AP-1 and clathrin to membranes induces localized assembly of clathrin triskelia into a lattice, and the intrinsic curvature of the growing clathrin lattice provides sufficient energy for the membrane deformation needed for vesicle budding. Consistent with this possibility is the observation that clathrin and AP-1 in cytosolic fractions can induce vesicle formation from liposomes in vitro, suggesting that AP-1/CCVs can be produced without assistance from integral membrane proteins (Zhu et al., 1999). However, it is not clear if CCVs bud in vitro from liposomes at a rate that could sustain protein trafficking pathways in vivo. Therefore, whether or not integral membrane proteins contribute to AP-1/CCV biogenesis in vivo remains an important unanswered question. In addition, it is possible that clathrin self-assembly alone does not provide enough energy to drive bending of biological membranes without assistance from accessory proteins (Nossal, 2001). The epsins, for example, are thought to facilitate membrane bending to assist clathrin in forming vesicles (Ford et al., 2002).
Phospholipid translocases, or flippases, have substantial potential to bend membranes by coupling a source of energy (ATP) to the unidirectional translocation of phospholipid across the bilayer. Flippase action can create an imbalance in phospholipid number between the two leaflets and cause bending of the membrane in the direction of lipid translocation (Graham, 2004). A large body of evidence indicates that P4-ATPases, which include the yeast Drs2, Dnf1, Dnf2, Dnf3, and Neo1 proteins, catalyze unidirectional phospholipid translocation from the extracellular leaflet of the plasma membrane, or the topologically equivalent lumenal leaflet of the Golgi and endosomes, to the cytosolic leaflet of these membranes (Tang et al., 1996; Gomes et al., 2000; Ujhazy et al., 2001; Pomorski et al., 2003; Natarajan et al., 2004; Saito et al., 2004; Alder-Baerens et al., 2006). Although a flippase activity has not been reconstituted with a purified P4-ATPase, we have shown that inactivation of a temperature-conditional mutant form of Drs2p (Drs2-ts) present in purified TGN membranes immediately disrupts phospholipid translocase activity measured with a fluorescent phospholipid derivative (Natarajan et al., 2004), supporting the view that Drs2p is a flippase. In these assays, Drs2p has a preferred substrate preference for phosphatidylserine (PS), but also appears to flip phosphatidylethanolamine (PE; Natarajan et al., 2004; Alder-Baerens et al., 2006). This Drs2-dependent flippase activity also appears to contribute to the asymmetric distribution of endogenous PS and PE to the cytosolic leaflet as drs2Δ mutants exhibit a loss of PS and PE asymmetry (Pomorski et al., 2003; Chen et al., 2006).
P4-ATPases also play essential roles in protein transport in the secretory and endocytic pathways (Chen et al., 1999; Hua et al., 2002; Hua and Graham, 2003; Pomorski et al., 2003; Sakane et al., 2006; Furuta et al., 2007). For example, inactivation of Drs2-ts in vivo rapidly blocks formation of a clathrin-dependent class of post-Golgi vesicles carrying exocytic cargo (Gall et al., 2002). These post-Golgi vesicles appear to be equivalent to one of the two classes of exocytic vesicles (the “higher density” class) first described by Harsay and Bretscher (1995). Formation of these exocytic vesicles is only mildly perturbed by deletion of AP-1 subunits (Gall et al., 2002); therefore, they likely differ from AP-1/CCVs proposed to mediate transport between the TGN and endosomes. Other data linking Drs2p to clathrin function include their colocalization to the TGN, a strong synthetic lethal relationship between drs2, arf1, and chc1 (clathrin heavy chain) alleles, and drs2 mutant phenotypes such as the accumulation of enlarged Golgi cisternae, a deficiency of CCVs, and the mislocalization of TGN resident proteins. These phenotypes are similar to what is observed in clathrin mutants (Chen et al., 1999).
These observations are consistent with a role for Drs2p in budding CCVs. However, because the relationship of Drs2p to well-known AP-1 and GGA clathrin adaptors has not been characterized, the precise role of Drs2p in the function of clathrin and its adaptors in the TGN–endosomal system is unclear. Clathrin mediates multiple transport pathways and so this information is essential to test if Drs2p contributes to vesicle biogenesis by helping recruit specific clathrin adaptor components to membranes or through a novel mechanism. Here we demonstrate a unique functional codependence between Drs2p and AP-1. Drs2p requires AP-1 for its normal trafficking itinerary, and the enzymatic activity of this integral membrane protein is essential for AP-1/clathrin function. This work provides the first example a clathrin accessory factor essential for AP-1 function that acts independently of coat recruitment, and also suggests that an ATP-powered pump provides a critical source of energy to drive AP-1/CCV formation from biological membranes.
MATERIALS AND METHODS
Media and Strains
Yeast strains were grown in standard rich medium (YPD) or synthetic defined (SD) minimal media. Calcoflour white (CW) sensitivity was tested on YPD 2% agar plates containing 100 μg/ml CW (Sigma-Aldrich, St. Louis, MO). Yeast strains used in this study were generated by standard genetic crosses, gene disruption, or integration methods as previously described and are listed in Supplementary Table 1. The yeast knockout collection and GFP collection were purchased from Invitrogen (Carlsbad, CA). To prevent accumulation in the endoplasmic reticulum, pGFP-DRS2 was cotransformed into yeast strains with a multicopy vector carrying CDC50 (pRS425-CDC50), encoding a chaperone for Drs2p. Strains carrying pChs3-GFP also carried a plasmid overexpressing the CHS7 chaperone (pCRP11). pGFP-drs2-12 (encoding GFP-Drs2-ts) was constructed by replacing the AgeI-ClaI fragment of pGFP-DRS2 with the AgeI-ClaI fragment from pRS313-drs2-12. To create pGFP-TLG1, genomic DNA was amplified with primers Tlg1-F (GTCAGTCGACAACAACAGTGAAGATCCGTTTC) and Tlg1-R (ACTGGTCGACTCAAGCAATGAATGCCAAAACTA), and the product was digested with SalI and subcloned into the SalI site of pRS416-GFP. All plasmids used in this study are listed in Supplementary Table S2.
Microscopy
To visualize green fluorescent protein (GFP) or DsRed-tagged proteins, cells were grown to early to mid-logarithmic phase, harvested, and resuspended in imaging buffer (10 mM Tris-HCl, pH 7.4, 2% glucose). Cells were mounted on glass slides and observed immediately at room temperature as previously described. Chitin staining was performed with cells incubated in 1 mg/ml CW in water for 5 min and washed three times before imaging. To inhibit endocytosis, midlog phase cells were collected and resuspended in SD medium containing 200 μM latrunculin A (Lat A). Brefeldin A (Sigma-Aldrich) was dissolved in ethanol at 10 mg/ml and used to treat cells at 100 μg/ml in imaging buffer.
Coimmunoprecipitation of AP-1 and Drs2p
For chemical cross-linking experiments, 50 OD600 units of each strain were incubated in 5 ml softening buffer (100 mM Tris-HCl, pH 9.4, 10 mM DTT) at 30°C for 10 min and then converted to spheroplasts by incubating in 1 ml spheroplast buffer (1 M sorbitol, 0.5% glucose, 10 mM KH2PO4, pH 7.0, 200 μg/ml Zymolase) at 30°C for 30 min. Spheroplasts were washed three times with cross-linking buffer (1 M sorbitol, 0.15 M NaCl, 0.1 M KH2PO4, pH 7.2), resuspended in cross-linking buffer supplemented with either 1 or 2 μM dithiobis(succinimidyl)propionate (DSP, 20 mg/ml stock in dimethylsulphoxide; Pierce, Rockford, IL) and incubated at room temperature for 30 min. Cells were then pelleted, resuspended in 1 ml ice-cold lysis buffer (20 mM Tris-HCl, pH 7.5, 1 mM EDTA, 150 mM NaCl, 1% CHAPS, 1× complete protease inhibitor cocktail lacking EDTA; Roche Diagnostics, Basel, Switzerland, and 1 mg/ml BSA), and incubated on ice for 15 min. The cell lysate was cleared by incubation with 30 μl protein G Sepharose (50% suspension, Amersham Biosciences, Piscataway, NJ) at 4°C for 30 min and centrifugation at 20,000 × g for 20 min. The supernatant was incubated with 9 μg monoclonal anti-hemagglutinin (HA-7, Sigma-Aldrich) or anti-Myc (9E10, Sigma-Aldrich) antibodies for 1.5 h at 4°C and subsequently with 30 μl protein G Sepharose for 1.5 h at 4°C. The protein G Sepharose beads were washed three times with washing buffer I (20 mM Tris-HCl, pH 7.5, 1 mM EDTA, 350 mM NaCl, 1% Tween-20, 1× complete protease inhibitor cocktail lacking EDTA) and twice with washing buffer II (20 mM Tris-HCl, pH 7.5, 1 mM EDTA, 150 mM NaCl, 1× complete protease inhibitor cocktail lacking EDTA). Bound material was eluted with SDS/urea sample buffer, separated by SDS-PAGE, and subjected to immunoblotting as previously described (Chen et al., 1999).
RESULTS
Drs2p is Required for AP-1 Function in TGN-early Endosome Pathways
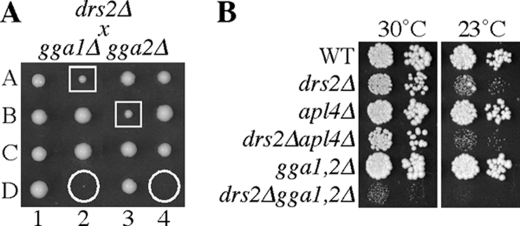
Prior studies implicated Drs2p in protein transport between the TGN and early endosome, suggesting a primary role for Drs2 in AP-1/clathrin function rather than the late endosome pathway mediated by GGAs (Chen et al., 1999). In budding yeast, simultaneous inactivation of both AP-1 and GGA function causes a severe synthetic growth defect (Costaguta et al., 2001; Hirst et al., 2001). If Drs2p is required for AP-1 function, a drs2 null allele (drs2Δ) should cause a strong synthetic growth defect when combined with null alleles of the two GGA genes (gga1Δ and gga2Δ), comparable to the poor growth of an apl4Δ (γ-adaptin) gga1Δ gga2Δ strain. In addition, deletion of an AP-1 subunit should have no additional consequence to a drs2Δ strain if AP-1 function is already lost in this strain. To test these predictions, we crossed drs2Δ with gga1Δ gga2Δ and apl4Δ and performed tetrad analyses. The drs2Δ gga1Δ gga2Δ triple mutant progeny were either inviable or grew extremely slowly. In addition, the drs2Δ gga2Δ double mutants grew slower than the single mutant or WT progeny (Figure 1, A and B). By contrast, the drs2Δ apl4Δ mutant exhibited a growth rate identical to the drs2Δ single mutant, even at 23°C, a semipermissive temperature for growth of cold-sensitive drs2Δ strains (Figure 1B). These results suggest that Drs2p acts in parallel with GGAs and functions in the same pathway as AP-1.
Figure 1.
Synthetic growth defect between drs2Δ and gga alleles suggests that Drs2 acts in AP-1 pathways. (A) Tetrads derived from a drs2Δ cross with gga1Δgga2Δ. Circles indicate drs2Δ gga1Δgga2Δ triple mutants, and boxes are drs2Δ gga2Δ double mutants (tetrads, 1–4; spores, A–D). The unmarked WT and single mutant progeny grow equally well. (B) Growth of drs2Δ apl4Δ (γ-adaptin) and drs2Δ gga1Δ gga2Δ strains. Tenfold serial dilutions of strains BY4742 (WT), ZHY615M2D (drs2Δ), BY4742 YPR029C (apl4Δ), KLY691 (drs2Δ apl4Δ), KLY751 (gga1Δ gga2Δ), and KLY741 (drs2Δ gga1Δ gga2Δ) were spotted onto YPD plates and grown at 30 and 23°C.
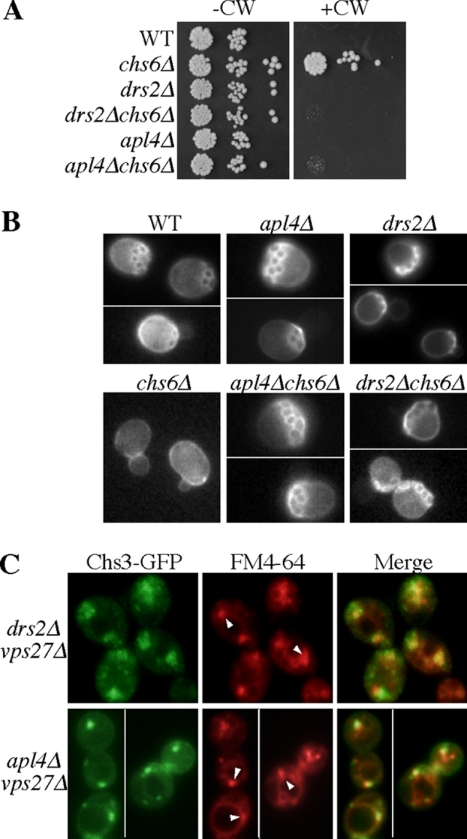
A well-defined role for AP-1 in yeast is in the trafficking of chitin synthase III (Chs3p) between the early endosomes and TGN (Valdivia et al., 2002), and so we tested if Drs2p is required in this pathway. Chs3p deposits a ring of chitin around emerging buds that remains as a scar after the bud is released. Most Chs3p is retained intracellularly by AP-1–dependent transport between the TGN and early endosome, but a portion is diverted to the plasma membrane in a cell cycle–regulated process that requires the Chs5p and Chs6p coat complex (Trautwein et al., 2006; Wang et al., 2006). Thus, chs6Δ cells retain nearly all Chs3p intracellularly and have reduced chitin incorporation at the bud site. This makes chs6Δ cells resistant to the toxic effects of CW, a chitin-binding fluorescent compound (Figure 2A). In the chs6Δ background, deletion of any AP-1 subunit disrupts intracellular Chs3p retention and allows cell surface transport of Chs3p in constitutive exocytic vesicles, restoring the appearance of chitin rings and CW sensitivity to an AP-1 chs6Δ double mutant (Valdivia et al., 2002). Like the chs6Δ apl4Δ strain, the chs6Δ drs2Δ double mutant failed to grow on the medium containing CW (Figure 2A) and formed chitin rings indistinguishable from those in the WT and drs2Δ cells (Figure 2B).
Figure 2.
Drs2p is required for AP-1–dependent retrograde transport of Chs3p. (A) Loss of Drs2p or AP-1 restores CW sensitivity to chs6Δ cells. Tenfold serial dilutions of strains BY4742 (WT), ZHY615M2D (drs2Δ), BY4742 YPR029C (apl4Δ), BY4742 YJL099W (chs6Δ), KLY022 (drs2Δ chs6Δ), and KLY492 (apl4Δ chs6Δ) were spotted onto rich medium containing 100 μg/ml CW and incubated at 30°C. (B) Loss of Drs2p or AP-1 restores chitin-rich bud scars to chs6Δ cells. Cells (same as in A) were stained with CW and visualized by fluorescence microscopy. (C) Loss of Drs2p or AP-1 causes missorting of Chs3-GFP to the prevacuolar compartment (arrowheads) of vps27Δ cells. Cells (KLY782 and KLY711) expressing Chs3-GFP were stained with FM4-64 to mark the prevacuolar compartment as previously described (Liu et al., 2007).
As a cargo of the AP-1-mediated early endosome to TGN retrograde pathway, Chs3p normally avoids transport to the late endosome, but transits this compartment frequently in AP-1 mutants (Valdivia et al., 2002). This perturbation is revealed in a class E vps AP-1 double mutant (apl4Δ vps27Δ), where Chs3p is trapped in enlarged endosomal compartments adjacent to the vacuole (class E compartment) that also accumulate the endocytic tracer FM4-64. As in apl4Δ vps27Δ cells, Chs3p accumulates in the class E compartment of drs2Δ vps27Δ cells (Figure 2C). The class E compartment in drs2Δ vps27Δ cells has a larger and more diffuse morphology than in vps27Δ or apl4Δ vps27Δ cells. However, the drs2Δ vps27Δ and vps27Δ cells exhibit a similar defect in the kinetics of FM4-64 delivery to the vacuole, and so the drs2Δ mutation, although altering the class E compartment morphology, does not suppress the vps27Δ FM4-64 trafficking defect. In summary, the data shown in Figure 2 indicates that loss of Drs2p disrupts Chs3p trafficking in a manner similar to loss of AP-1.
Missorting of Drs2p to the Plasma Membrane of AP-1 Mutants
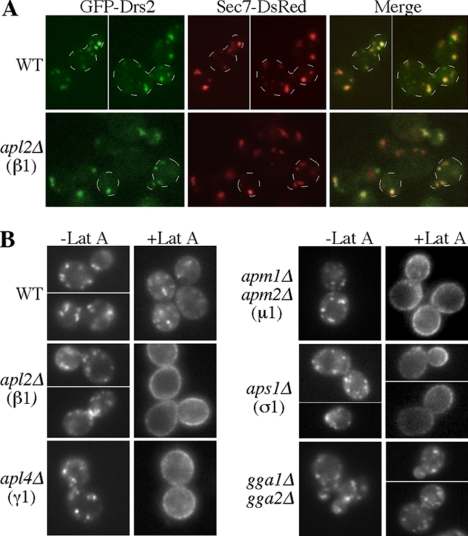
Our previous work suggested that Drs2 primarily traffics between the TGN and early endosome in order to maintain its TGN localization (Liu et al., 2007). To test if Drs2p localization requires AP-1, we first examined the distribution of GFP-Drs2p relative to the TGN marker Sec7-RFP in WT and AP-1–deficient cells (Figure 3A). GFP-Drs2p localized to several discrete puncta in WT cells, and this pattern was not altered in cells lacking AP-1 (apl2Δ). Furthermore, 81% of Drs2p-GFP puncta were coincident with Sec7-DsRed puncta in WT cells, whereas 82% of Drs2p-GFP puncta colocalized with Sec7-DsRed puncta in apl2Δ cells. Therefore, the steady-state localization of GFP-Drs2p to the TGN is not noticeably perturbed by AP-1 disruption.
Figure 3.
Loss of AP-1 perturbs Drs2p trafficking but not its steady-state TGN localization. (A) Colocalization of GFP-Drs2 and Sec7-DsRed at the TGN of WT (KLY1101) and AP-1–deficient (apl2Δ) cells (KLY351). Some of the cells are outlined for clarity. (B) Disruption of AP-1 subunit genes causes missorting of GFP-Drs2p to the plasma membrane, where accumulation was induced by blocking endocytosis with latrunculin A (Lat A) at room temperature for 30 min. WT (BY4741) and AP-1 mutant cells (BY4741 derivatives) were treated with Lat A and imaged as previously described (Liu et al., 2007).
We then addressed whether deletion of AP-1 has an effect on trafficking of GFP-Drs2p. Acute inhibition of endocytosis by treating cells with Lat A, an inhibitor of actin assembly that blocks endocytosis in yeast, causes a slow accumulation of GFP-Drs2p on the plasma membrane over the course of about 3 h. This indicates a relatively rare incorporation of Drs2p into transport vesicles targeted to the plasma membrane. In addition, Drs2p contains multiple endocytosis signals, and so any Drs2p molecules that are transported to the plasma membrane are rapidly retrieved through endocytosis (Liu et al., 2007). If GFP-Drs2p requires AP-1 for TGN to early endosome transport, we would expect an increased frequency of Drs2p transport to the plasma membrane in an AP-1 mutant. To test this, we treated WT and AP-1 mutant strains expressing GFP-Drs2p with Lat A to inhibit endocytosis and imaged the cells over time (Figure 3B). In WT cells, GFP-Drs2p was primarily retained intracellularly in small puncta after 30 min of Lat A treatment. In stark contrast, deletion of the AP-1 β subunit (apl2Δ), γ subunit (apl4Δ), or σ subunit (aps1Δ) caused accumulation of nearly all GFP-Drs2p on the plasma membrane within 30 min of blocking endocytosis with Lat A.
Cell surface accumulation of GFP-Drs2p was less pronounced in apm1Δ and nearly absent in apm2Δ cells treated with Lat A. These mutants harbor deletions of the partially redundant AP-1 μ1 subunits (unpublished observations). However, deletion of both APM1 and APM2 leads to plasma membrane accumulation comparable to deletion of other AP-1 subunits (apm1Δ apm2Δ; Figure 3B). These data indicate that Drs2p trafficking primarily relies on Apm1p, although Apm2p can partially contribute. The gga1Δ gga2Δ strain was also treated with Lat A, and these cells slowly accumulated GFP-Drs2p on the plasma membrane, similarly to WT cells (Figure 3B). We conclude that AP-1 is required, and GGAs dispensable, for efficient exclusion of Drs2p from exocytic vesicles targeted to the plasma membrane. This likely reflects a role for AP-1 in transporting Drs2p from the TGN to early endosomes; however, see Discussion for an alternative model.
Missorting of Drs2p to the plasma membrane of AP-1 null cells could be an indirect consequence of chronically perturbing protein transport between the early endosomes and TGN. To distinguish chronic versus acute effects of AP-1 inactivation on Drs2p trafficking, an apl2 temperature-sensitive (ts) mutant was shifted to the nonpermissive temperature (37°C) to inactivate AP-1 just before Lat A addition. The apl2-ts strain accumulated GFP-Drs2 on the plasma membrane within 30 min at 37°C, whereas Lat A–treated cells maintained at the permissive temperature (24°C) or WT cells shifted to 37°C retained significant internal punctate fluorescence (Figure 4A). For other potential anterograde AP-1 cargos tested, a minor fraction of Chs3-GFP but no Kex2-GFP or GFP-Tlg1 accumulated on the plasma membrane of Lat A–treated apl4Δ cells (Figure 4B and unpublished observations). Thus, missorting of Drs2p to the plasma membrane is a specific and immediate consequence of AP-1 inactivation.
Figure 4.
GFP-Drs2p missorting to the plasma membrane is an immediate and specific consequence of AP-1 deficiency. (A) GFP-Drs2p is missorted to the plasma membrane of an apl2-ts (β1-adaptin) strain preincubated 30 min at the nonpermissive temperature (37°C) before Lat A addition. Partial plasma membrane accumulation of GFP-Drs2p was also observed at the permissive temperature suggesting a partial loss of AP-1 function at 24°C. Lat A incubations were for 30 min at the indicated temperature. (B) Localization of GFP-Drs2 in rcy1Δ and snx4Δ cells treated with Lat A for 30 min at 30°C. Localization of GFP-Tlg1 (C) and Chs3-GFP (D) in WT and AP-1–deficient (apl4Δ) cells treated with Lat A as in B.
To rule out the possibility that missorting of GFP-Drs2 to the plasma membrane is a secondary consequence of inactivating AP-1 retrograde function, we examined the trafficking of GFP-Drs2p in two other mutants that display a defect in the retrograde pathway. The Snx4-Snx41-Snx42 sorting nexin complex and the F-box protein Rcy1p function in early endosome to TGN retrograde transport (Wiederkehr et al., 2000; Hettema et al., 2003). However, disruption of RCY1 only slightly increased GFP-Drs2 accumulation on the plasma membrane upon addition of Lat A, whereas disruption of SNX4 has no apparent impact on Drs2p trafficking (Figure 4C). Therefore, wholesale missorting of GFP-Drs2p to the plasma membrane in AP-1–deficient cells is not a secondary consequence of disrupting early endosome to TGN retrograde transport.
The observation that AP-1 disruption rerouted Drs2p to the plasma membrane without substantially perturbing its steady-state localization to the TGN, suggested that endocytosis and early endosome to TGN transport of GFP-Drs2 was unaffected. To test if AP-1 disruption perturbs retrograde transport of Drs2p similarly to Chs3p, localization of GFP-Drs2p was monitored in apl4Δ vps27Δ cells. In contrast to Chs3-GFP (shown in Figure 2), GFP-Drs2p did not accumulate in the class E compartment marked with FM4-64 (Figure 5A). In addition, GFP-Drs2 was efficiently endocytosed from the plasma membrane and transported to the TGN in AP-1–deficient cells. To show this, the apl2Δ strain expressing GFP-Drs2 and Sec7-DsRed was treated with Lat A to accumulate GFP-Drs2 on the plasma membrane. After washing out the Lat A to lift the endocytosis block, GFP-Drs2 efficiently returned to Sec7-DsRed–marked TGN cisternae (Figure 5B). In summary, these data indicate that Drs2p requires AP-1 at the TGN for exclusion from exocytic vesicles. However, Drs2p can be retrieved from early endosomes and transported to the TGN in the absence of AP-1.
Figure 5.
Drs2p does not require AP-1 for early endosome-to-TGN retrograde transport. (A) An apl4 Δvps27Δ strain (KLY711) expressing GFP-Drs2 was stained with FM4-64 to mark the class E compartment and imaged. Arrowheads are within the vacuole region and point to the class E compartments. (B) An apl2Δ strain (KLY351) expressing GFP-Drs2 and Sec7-DsRed was treated with Lat A for 1 h to completely accumulate GFP-Drs2p on the plasma membrane. The cells were washed twice, resuspended in fresh medium without Lat A (Lat A washout) and incubated for 1 h at 30°C before imaging.
Drs2p Activity Drives Its Own Transport in the AP-1 Pathway
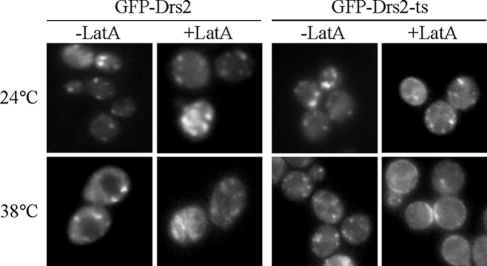
Drs2p is the only cargo thus far characterized in yeast that depends solely on AP-1 in what appears to be a TGN-to-endosome anterograde pathway. To determine if Drs2p activity is required for the function of AP-1 in this pathway, we examined the trafficking of a GFP-tagged Drs2 temperature-sensitive protein (Drs2-ts) at the permissive (24°C) and nonpermissive (38°C) temperatures. Because Drs2-ts is the only source of Drs2p in these cells, we could determine if inactivation of Drs2p ATPase and flippase activities at high temperature disrupts its own trafficking in the AP-1–dependent anterograde direction. GFP-Drs2 and GFP-Drs2-ts were expressed in a strain deficient for multiple P4-ATPases (drs2Δ dnf1Δ dnf2Δ dnf3Δ), where Drs2p is required to support viability (Hua et al., 2002), and we confirmed that the temperature-sensitive growth phenotype caused by the drs2-ts allele was not perturbed by the GFP moiety (unpublished observation). WT GFP-Drs2 maintained a punctate distribution at both temperatures in the presence or absence of Lat A (Figure 6). This indicates that the Dnf1, Dnf2, and Dnf3 P4-ATPases do not significantly contribute to the AP-1–dependent trafficking of Drs2p. In contrast, Lat A treatment caused accumulation of GFP-Drs2-ts on the plasma membrane of the cells shifted to 38°C, but not the cells maintained at permissive temperature (Figure 6). Therefore, the enzymatic activity of Drs2p is required to drive its own exclusion from exocytic vesicles in a pathway that also requires AP-1.
Figure 6.
Acute inactivation of Drs2p enzymatic activity causes its missorting to the plasma membrane. Strains KLY921 and KLY931, deficient for Dnf P4-ATPases and expressing GFP-Drs2 or GFP-Drs2-ts (pGFP-drs2-12) as the sole source of Drs2p, were incubated at the permissive (24°C) or nonpermissive (38°C) temperature for 1 h before a 30-min incubation with or without Lat A at the same temperature. Plasma membrane fluorescence is only observed with Lat A–treated cells expressing GFP-Drs2-ts at 38°C.
Drs2p Associates With AP-1 but does Not Recruit AP-1/Clathrin to Membranes
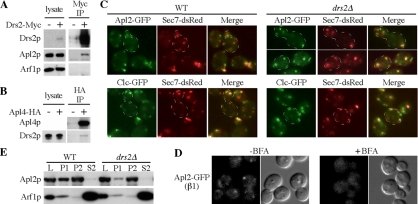
The functional codependence of AP-1 and Drs2p suggested that these proteins might interact. To test this, cells expressing functionally Myc-tagged Drs2p were treated with a cross-linking agent before lysis and immunoprecipitation of Drs2-Myc. After disruption of cross-links by reduction, the immunoprecipitates were probed by immunoblotting with antibodies to Arf1p and Apl2p, the AP-1β subunit. Apl2p, but not Arf1p, coimmunoprecipitated with Drs2p-Myc and Apl2p could not be detected in control immunoprecipitates from cells lacking the Myc tag on Drs2p (Figure 7A). In addition, cross-linking followed by immunoprecipitation of HA-tagged Apl4p, the AP-1 γ subunit, specifically isolated endogenous Drs2p (Figure 7B). However, only a small percentage (<0.5%) of Drs2p coimmunoprecipitated with AP-1 and so this complex is likely to be transient in vivo.
Figure 7.
Drs2p interacts with AP-1 but is not required for AP-1 or clathrin recruitment to Golgi membranes. Coimmunoprecipitation of AP-1 and Drs2p. Cells expressing epitope tagged Drs2p (A), Apl4p (B), or control cells without the tag were treated with chemical cross-linker before lysis and immunoprecipitation (IP) with tag antibodies. 0.5% of the initial lysate was loaded in the “lysate” lanes, and the indicated proteins were detected by immunoblot. (C) WT (KLY791, KLY792) and drs2Δ (KLY361, KLY363) strains expressing the indicated fusion proteins were grown at 30°C to midlog phase, shifted to 15°C for 2 h (a nonpermissive growth temperature for drs2Δ), and imaged by fluorescence microscopy. The same results were obtained with cells maintained at 30°C (not shown). (D) Cells expressing GFP-tagged Apl2p (KLY551) were grown at 30°C, treated with or without brefeldin A (BFA), in imaging buffer for 30 min and imaged. (E) Lysates (L) from WT and drs2Δ cells grown at 30°C were prepared as previously described (Chuang and Schekman, 1996) and centrifuged sequentially at 13,000 × g for 20 min (P1 pellet) and 100,000 × g for 1 h (P2 pellet and S2 supernatant). Each fraction was then immunoblotted for Apl2p and Arf1p.
To determine if Drs2p is required to recruit AP-1 and clathrin onto TGN–endosomal membranes, we compared the localization of AP-1β-GFP (Apl2-GFP) to the TGN marker Sec7-RFP in WT and drs2Δ cells. Both strains showed a pattern of Apl2-GFP spots typical of the yeast TGN and early endosomal compartments with 51% of Apl2-GFP puncta coincident with Sec7-DsRed puncta in WT cells and 73% of Apl2-GFP puncta colocalized with Sec7-DsRed puncta in drs2Δ (Figure 7C). The increased association of Apl2-GFP with Sec7-positive membranes is likely caused by normal recruitment of AP-1 to the TGN, combined with a defect in the budding of AP-1/clathrin-coated vesicles. Localization of GFP tagged clathrin light chain (Clc1-GFP), Gga2 and AP-3 were also examined in these strains, and their membrane association was unaffected (Figure 7C and data not shown). As a control, cells expressing AP-1-GFP were treated with brefeldin A, an inhibitor of ARF guanine nucleotide exchange factors (Peyroche et al., 1996), and the punctate pattern was dispersed (Figure 7D). Membrane association of ARF and AP-1 was also examined in WT and drs2Δ mutant cells by differential centrifugation. Cell lysates (L) were sequentially centrifuged at 10,000 × g and 130,000 × g to collect P1 and P2 pellets, respectively, and 130,000 × g supernatants (S2), which were probed for AP-1 (Apl2p) and ARF. No significant loss from the membrane fractions (P1 and P2) or increase in the cytosolic (S2) fraction of AP-1 or ARF was detected for the drs2Δ strain relative to WT (Figure 7E). Thus, Drs2p is not required for recruitment of AP-1 or clathrin to membranes, even though AP-1/clathrin pathways are disrupted by drs2Δ.
DISCUSSION
The results of this work provide a number of unique observations with significant mechanistic implications for clathrin-mediated protein transport from the TGN and early endosomes. We find that an integral membrane P-type ATPase called Drs2p is essential for AP-1/clathrin function in vivo. The strong genetic interaction between drs2 and gga1/gga2 alleles, the complete lack of a genetic interaction between drs2 and AP-1 alleles, and the phenotypic similarities between drs2 and AP-1 mutants strongly suggest that AP-1 function is lost in drs2 cells. In fact, Drs2p and AP-1 exhibit a novel functional codependency as we show that AP-1 is required at the TGN to prevent Drs2p transport to the plasma membrane. In addition, Drs2 flippase activity is required to drive its own trafficking in the AP-1 pathway that excludes Drs2p from exocytic vesicles. Importantly, we also show that Drs2p is not required for recruitment of AP-1 and clathrin to the TGN, thus providing the first example of an essential AP-1/clathrin accessory protein that acts independently of coat recruitment.
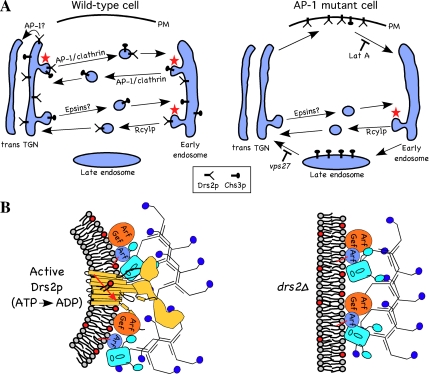
AP-1 was initially found to localize to the TGN of mammalian cells and was assumed to mediate protein transport from the TGN to the endosomal system. However, the finding that mutations of AP-1 subunits in mice and yeast appeared to cause trafficking defects in endosome to TGN retrograde transport raised questions as to whether AP-1 plays a primary role in retrograde or anterograde transport (reviewed in Hinners and Tooze, 2003). Our results show that AP-1 is required at the TGN to sort Drs2p out of the exocytic pathway. The simplest interpretation of these data are that Drs2p is packaged into AP-1/CCVs at the TGN for delivery to the early endosome (Figure 8A, WT cell). In the absence of AP-1, Drs2 is rerouted to the plasma membrane where it can be trapped by blocking endocytosis with Lat A (Figure 8A, AP-1 mutant cell). Mislocalization of Drs2p to the plasma membrane is an immediate consequence of inactivating a temperature-conditional AP-1 mutant allele (apl2-ts). It is also a specific consequence as other TGN resident proteins are not mislocalized to the plasma membrane in AP-1 mutants. In contrast to Chs3p, Drs2p does not depend solely on AP-1 for early endosome-to-TGN retrograde transport. Cdc50p, a Drs2p binding partner and chaperone, accumulates in the early endosomes of an rcy1Δ mutant (Furuta et al., 2007). Therefore, Drs2p is likely retrieved back to the TGN primarily through the Rcy1p (AP-1-indepedent) pathway (Figure 8A). However, these observations do not rule out a minor role for AP-1 in Drs2p-Cdc50p retrieval from the early endosome.
Figure 8.
Models for the codependency of Drs2p and AP-1 in vesicle-mediated protein transport. (A) AP-1 and clathrin are required for anterograde transport of Drs2p from the TGN to the early endosome. Alternatively, it is possible that AP-1 mediates retrieval of Drs2 from the TGN to earlier Golgi cisternae (pathway marked AP-1?). Anterograde transport of Chs3p occurs, for the most part, independently of AP-1 and perhaps requires the Golgi epsins (Ent3p and Ent5p). AP-1 mutant cells treated with Lat A accumulate all of Drs2p and a fraction of Chs3p on the plasma membrane. Retrograde transport of Drs2p from the early endosome back to the TGN does not depend on AP-1, but appears to use the Rcy1p pathway. In contrast, Chs3p depends solely on AP-1 for early endosome to TGN retrograde transport. AP-1 mutants missort most of Chs3p, but no Drs2p, to the late endosome where it can be trapped behind a vps27 block. Pathways that require Drs2p activity for vesicle formation are indicated with a red star. (B) In the absence of Drs2p (drs2Δ), AP-1 and clathrin are recruited to the membrane, but vesicles are not produced because the coat components cannot induce sufficient curvature in the membrane. (C) Enzymatically active Drs2p (yellow), modeled with a similar structure as SERCA1(Toyoshima et al., 2000), is required for budding AP-1/clathrin-coated vesicles. Interaction of Drs2p with Arf guanine nucleotide exchange factors (Arf Gef; Chantalat et al., 2004) and AP-1 should concentrate activated Arf-GTP, AP-1 and clathrin at sites of lipid translocation where the clathrin coat can efficiently mold the curved membrane into a vesicle.
The yeast TGN appears to be a transient organelle with a half-life of only 1–2 min before it is consumed into many types of vesicles that are targeted to either the plasma membrane, early endosomes, late endosomes, the vacuole, or potentially earlier Golgi cisternae (Losev et al., 2006; Matsuura-Tokita et al., 2006). Thus, TGN residents rely on an active trafficking mechanism to maintain their TGN localization. As an alternative model that is consistent with our data, it is possible that AP-1/CCVs containing Drs2p bud from the TGN and are targeted directly to an earlier Golgi cisternae to facilitate the maturation of trans cisternae to TGN compartments (Figure 8A, pathway designated “AP-1?”). An intra-Golgi retrograde pathway would be a novel role for AP-1/clathrin and further work is needed to distinguish between these two models. Regardless of the vesicle destination, the current studies, combined with previously published data (Valdivia et al., 2002; Foote and Nothwehr, 2006), support a direct role for AP-1 in selecting cargo for inclusion into CCVs at the TGN (Drs2p) and early endosome (Chs3p and Ste13p). Most Chs3p is rerouted to the late endosome of AP-1 null cells, where it can be trapped behind a vps27Δ block (Figure 8A, AP-1 mutant cell). However, Chs3p is also weakly mislocalized to the plasma membrane of AP-1 null cells, and so this cargo can likely use either AP-1 or another adaptor, such as Ent3/5p, for anterograde transport (this work and Copic et al., 2007).
Several lines of evidence indicate that Drs2p plays an essential and direct role in bidirectional protein transport between the TGN and early endosome. DRS2 mutant alleles exhibit genetic interactions with mutations in a number of protein transport factors operating in the late secretory and early endocytic pathways. We originally linked Drs2p to clathrin-mediated transport through the recovery of several drs2 alleles in an arf1 synthetic lethal screen and the observation that drs2 is also synthetically lethal with clathrin heavy-chain temperature-sensitive alleles but not with mutations in COPI or COPII subunits (Chen et al., 1999). Subsequently, genetic interactions were discovered between Drs2p and ARF guanine nucleotide exchange factors (Gea1p and Gea2p), an ARF guanine nucleotide activating protein (Gcs1p), GGAs, Ypt31p (a rab protein), TRAPPII subunits, and a phosphatidylinositol 4-kinase (Pik1p; Chantalat et al., 2004; Sciorra et al., 2005; Sakane et al., 2006). In addition, the Drs2-Cdc50 complex physically interacts with Gea2p, AP-1, and Rcy1p, providing a direct link to ARF/AP-1/clathrin pathways as well as the Rcy1-dependent early endosome-to-TGN recycling pathway (this work and Chantalat et al., 2004; Furuta et al., 2007).
Consistent with these genetic and physical interactions, drs2 mutants exhibit protein transport defects specific to the TGN and early endosomal system (the red stars in Figure 8A indicate pathways that require Drs2p). As shown here, AP-1/clathrin-dependent pathways associated with the TGN and early endosome are abrogated in cells deficient for Drs2p. In addition, Drs2p is required for the early endosome to TGN retrograde transport of the SNARE protein Snc1 (Hua et al., 2002; Furuta et al., 2007), which is independent of AP-1 (our unpublished observations) but requires Rcy1p (Galan et al., 2001). Cdc50p, presumably in a complex with Drs2p, accumulates in the early endosomes of rcy1 mutants (Furuta et al., 2007). Therefore, Drs2 activity likely drives its own retrieval back to the TGN in the Rcy1p pathway. Because the majority of Drs2p localizes to the TGN, inactivation Drs2p activity initially causes its missorting to the plasma membrane rather than accumulation in the early endosome. The precise role of the F-box protein Rcy1 is unclear, although it functions with the Ypt31/32 rab pair, Gcs1p (Arf-GAP) and a COPI subcomplex in this recycling pathway (Chen et al., 2005; Robinson et al., 2006; Furuta et al., 2007).
In addition to the AP-1 and Rcy1 pathways, we previously described a role for Drs2p and clathrin in the formation of one class of post-Golgi exocytic vesicles (Gall et al., 2002). AP-1 mutations partially perturb formation of these exocytic vesicles, although it was not clear at the time whether the small defect observed reflected a primary or secondary consequence of AP-1 disruption. On the basis of the current studies, we suggest that AP-1 mutations influence the exocytic vesicles indirectly by altering the trafficking patterns of Drs2p (and perhaps other TGN residents as well). Drs2p is required to form the dense exocytic vesicles and AP-1 retrograde vesicles, but does not appear to be significantly incorporated into these vesicles. This is consistent with a role for Drs2p in priming the membrane of the TGN and early endosome to produce a structure that coats can more efficiently mold into a vesicle.
P4-ATPases in yeast (Drs2p, Dnf1p, Dnf2p, Dnf3p, and Neo1p) have a general function in vesicle-mediated protein transport in the Golgi–endosomal system (Chen et al., 1999; Hua et al., 2002; Hua and Graham, 2003). Although some vesicle classes budding from the TGN and early endosomes primarily require Drs2p (e.g., AP-1, Rcy1, and the dense exocytic vesicles), other pathways are less discriminate for their P4-ATPase requirement. For example, AP-3–dependent protein transport from the TGN to the vacuole requires either Drs2p or Dnf1p, such that a severe defect in this pathway is only observed in the drs2Δ dnf1Δ double mutant (Hua et al., 2002). Likewise, transport of carboxypeptidase Y from the TGN to late endosomes, perhaps carried in GGA/clathrin-coated vesicles, is kinetically delayed in the drs2Δ dnf1Δ double mutant (Hua et al., 2002). Importantly, not all vesicles budding from the Golgi require Drs2p. The “lighter density” class of exocytic vesicles appears to form normally in drs2Δ cells, and cargos normally secreted in the dense vesicles, such as invertase, get rerouted into the light exocytic vesicles (Chen et al., 1999; Gall et al., 2002; Hua et al., 2002). In fact, the rate of protein transport from the ER to the Golgi and subsequently to the cell surface in drs2Δ or drs2Δ dnf1Δ, or dnf1,2,3Δ cells is similar to that of WT cells (Chen et al., 1999; Gall et al., 2002; Hua et al., 2002). These data also indicate that COPI and COPII function in the early secretory pathway is unperturbed by loss of Drs2 and Dnf P4-ATPases. However, conditional alleles of the essential P4-ATPase NEO1 cause transport defects that are similar to COPI mutants and also perturb ARL-dependent transport from endosomes (Hua and Graham, 2003; Wicky et al., 2004). The proposed phospholipid flippase activity for P4-ATPases is best supported by studies showing a temperature-conditional loss of flippase activity in purified TGN membranes carrying a temperature conditional form of Drs2p (Drs2-ts; Natarajan et al., 2004). Inactivation of Drs2-ts in vivo causes an immediate defect in the budding of dense exocytic vesicles (Gall et al., 2002), and rerouting of Drs2-ts to the plasma membrane, implying a defect in budding AP-1/CCVs from the TGN (this work). Therefore, Drs2p flippase activity appears to drive formation of vesicles from the TGN.
Two models have been proposed to explain how the phospholipid flippase activity of Drs2p contributes to vesicle biogenesis (Chen et al., 1999; Gall et al., 2002; Graham, 2004). One possibility is that Drs2p recruits coat proteins to the membrane through protein–protein interactions, and/or by increasing the concentration of specific phospholipids on the cytosolic leaflet. However, our results rule out this possibility by showing that membrane association of ARF, AP-1, and clathrin does not require Drs2p (Figure 8B, right), in spite of the Drs2p requirement for AP-1 function. Therefore, our data argue that membrane recruitment of ARF, AP-1, and clathrin is insufficient to bud AP-1/CCVs in the absence of Drs2p. Even though epsins have been implicated in membrane deformation and AP-1 function (Ford et al., 2002; Hirst et al., 2003; Mills et al., 2003; Costaguta et al., 2006), the yeast Golgi epsins (Ent3p and Ent5p) also fail to support AP-1/CCV function in the absence of Drs2p.
The model best supported by our data is that ATP-dependent transbilayer movement of lipids catalyzed by Drs2p imparts curvature to the membrane that is required to facilitate vesicle budding (Figure 8B, left). The bilayer-couple hypothesis predicts that an increase in surface area of one leaflet relative to the other will spontaneously induce membrane curvature (Sheetz and Singer, 1974). As little as a 1–2% difference in surface area between leaflets of a bilayer can have a substantial impact on membrane shape (Lim et al., 2002). Consistent with this hypothesis, bilayer surface area asymmetry induced by insertion of additional phospholipid in the extracellular leaflet of the plasma membrane and their subsequent flip to the cytosolic leaflet, catalyzed by the aminophospholipid translocase, leads to dramatic changes in the shape of red blood cells (Seigneuret and Devaux, 1984; Daleke and Huestis, 1985). Translocase-mediated influx of phospholipid to the cytosolic leaflet of the plasma membrane also stimulates endocytosis (Farge et al., 1999). By translocating phospholipid from the lumenal leaflet to the cytosolic leaflet of the TGN or early endosomes, Drs2p would increase the surface area of the cytosolic leaflet at the expense of the lumenal leaflet and bend the membrane toward the cytosol. This could provide a critical priming step to produce a membrane substrate that coat proteins can more efficiently mold into a vesicle. We propose that Drs2p imparts curvature to the membrane through a bilayer-couple mechanism that is subsequently captured and molded by AP-1/clathrin to produce vesicles.
Supplementary Material
ACKNOWLEDGMENTS
We thank Benjamin Glick (University of Chicago), Gregory Payne (University of California, Los Angeles), Susan Wente (Vanderbilt University), Scott Emr (Cornell University), Randy Schekman (University of California, Berkeley), and Hugh Pelham (Medical Research Council, Cambridge, United Kingdom) for providing plasmids, strains, and antibodies. We also thank Sophie Chen and J. Court Reese for assistance with plasmid and strain construction. This work was supported by Grant GM62367 from the National Institutes of Health to T.R.G.
Abbreviations used:
- ARF
ADP-ribosylation factor
- CCV
clathrin-coated vesicles
- CW
calcofluor white
- GEF
guanine nucleotide exchange factor
- GGA
Golgi-localized
- γ-ear–containing
ARF-binding protein
- P4-ATPase
type IV P-type ATPase
- PE
phosphatidylethanolamine
- PS
phosphatidylserine
- PVC
prevacuolar compartment.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-01-0025) on May 28, 2008.
REFERENCES
- Alder-Baerens N., Lisman Q., Luong L., Pomorski T., Holthuis J. C. Loss of P4 ATPases Drs2p and Dnf3p disrupts aminophospholipid transport and asymmetry in yeast post-Golgi secretory vesicles. Mol. Biol. Cell. 2006;17:1632–1642. doi: 10.1091/mbc.E05-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M. W., Pelham H. R. A selective transport route from Golgi to late endosomes that requires the yeast GGA proteins. J. Cell Biol. 2000;151:587–600. doi: 10.1083/jcb.151.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantalat S., Park S. K., Hua Z., Liu K., Gobin R., Peyroche A., Rambourg A., Graham T. R., Jackson C. L. The Arf activator Gea2p and the P-type ATPase Drs2p interact at the Golgi in Saccharomyces cerevisiae. J. Cell Sci. 2004;117:711–722. doi: 10.1242/jcs.00896. [DOI] [PubMed] [Google Scholar]
- Chen C. Y., Ingram M. F., Rosal P. H., Graham T. R. Role for Drs2p, a P-type ATPase and potential aminophospholipid translocase, in yeast late Golgi function. J. Cell Biol. 1999;147:1223–1236. doi: 10.1083/jcb.147.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Wang J., Muthusamy B. P., Liu K., Zare S., Andersen R. J., Graham T. R. Roles for the Drs2p-Cdc50p complex in protein transport and phosphatidylserine asymmetry of the yeast plasma membrane. Traffic. 2006;7:1503–1517. doi: 10.1111/j.1600-0854.2006.00485.x. [DOI] [PubMed] [Google Scholar]
- Chen S. H., Chen S., Tokarev A. A., Liu F., Jedd G., Segev N. Ypt31/32 GTPases and their novel F-box effector protein Rcy1 regulate protein recycling. Mol. Biol. Cell. 2005;16:178–192. doi: 10.1091/mbc.E04-03-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copic A., Starr T. L., Schekman R. Ent3p and Ent5p exhibit cargo-specific functions in trafficking proteins between the trans-Golgi network and the endosomes in yeast. Mol. Biol. Cell. 2007;18:1803–1815. doi: 10.1091/mbc.E06-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costaguta G., Duncan M. C., Fernandez G. E., Huang G. H., Payne G. S. Distinct roles for TGN/endosome epsin-like adaptors Ent3p and Ent5p. Mol. Biol. Cell. 2006;17:3907–3920. doi: 10.1091/mbc.E06-05-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costaguta G., Stefan C. J., Bensen E. S., Emr S. D., Payne G. S. Yeast Gga coat proteins function with clathrin in Golgi to endosome transport. Mol. Biol. Cell. 2001;12:1885–1896. doi: 10.1091/mbc.12.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daleke D. L., Huestis W. H. Incorporation and translocation of aminophospholipids in human erythrocytes. Biochemistry. 1985;24:5406–5416. doi: 10.1021/bi00341a019. [DOI] [PubMed] [Google Scholar]
- Doray B., Ghosh P., Griffith J., Geuze H. J., Kornfeld S. Cooperation of GGAs and AP-1 in packaging MPRs at the trans-Golgi network. Science. 2002;297:1700–1703. doi: 10.1126/science.1075327. [DOI] [PubMed] [Google Scholar]
- Farge E., Ojcius D. M., Subtil A., Dautry-Varsat A. Enhancement of endocytosis due to aminophospholipid transport across the plasma membrane of living cells. Am. J. Physiol. 1999;276:C725–C733. doi: 10.1152/ajpcell.1999.276.3.C725. [DOI] [PubMed] [Google Scholar]
- Folsch H., Ohno H., Bonifacino J. S., Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- Foote C., Nothwehr S. F. The clathrin adaptor complex 1 directly binds to a sorting signal in Ste13p to reduce the rate of its trafficking to the late endosome of yeast. J. Cell Biol. 2006;173:615–626. doi: 10.1083/jcb.200510161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford M. G., Mills I. G., Peter B. J., Vallis Y., Praefcke G. J., Evans P. R., McMahon H. T. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- Furuta N., Fujimura-Kamada K., Saito K., Yamamoto T., Tanaka K. Endocytic recycling in yeast is regulated by putative phospholipid translocases and the Ypt31p/32p-Rcy1p pathway. Mol. Biol. Cell. 2007;18:295–312. doi: 10.1091/mbc.E06-05-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan J. M., Wiederkehr A., Seol J. H., Haguenauer-Tsapis R., Deshaies R. J., Riezman H., Peter M. Skp1p and the F-box protein Rcy1p form a non-SCF complex involved in recycling of the SNARE Snc1p in yeast. Mol. Cell. Biol. 2001;21:3105–3117. doi: 10.1128/MCB.21.9.3105-3117.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall W. E., Geething N. C., Hua Z., Ingram M. F., Liu K., Chen S. I., Graham T. R. Drs2p-dependent formation of exocytic clathrin-coated vesicles in vivo. Curr. Biol. 2002;12:1623–1627. doi: 10.1016/s0960-9822(02)01148-x. [DOI] [PubMed] [Google Scholar]
- Gomes E., Jakobsen M. K., Axelsen K. B., Geisler M., Palmgren M. G. Chilling tolerance in Arabidopsis involves ALA1, a member of a new family of putative aminophospholipid translocases. Plant Cell. 2000;12:2441–2454. [PMC free article] [PubMed] [Google Scholar]
- Graham T. R. Flippases and vesicle-mediated protein transport. Trends Cell Biol. 2004;14:670–677. doi: 10.1016/j.tcb.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Harsay E., Bretscher A. Parallel secretory pathways to the cell surface in yeast. J. Cell Biol. 1995;131:297–310. doi: 10.1083/jcb.131.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema E. H., Lewis M. J., Black M. W., Pelham H. R. Retromer and the sorting nexins Snx4/41/42 mediate distinct retrieval pathways from yeast endosomes. EMBO J. 2003;22:548–557. doi: 10.1093/emboj/cdg062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinners I., Tooze S. A. Changing directions: clathrin-mediated transport between the Golgi and endosomes. J. Cell Sci. 2003;116:763–771. doi: 10.1242/jcs.00270. [DOI] [PubMed] [Google Scholar]
- Hirst J., Lindsay M. R., Robinson M. S. GGAs: roles of the different domains and comparison with AP-1 and clathrin. Mol. Biol. Cell. 2001;12:3573–3588. doi: 10.1091/mbc.12.11.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J., Motley A., Harasaki K., Peak Chew S. Y., Robinson M. S. EpsinR: an ENTH domain-containing protein that interacts with AP-1. Mol. Biol. Cell. 2003;14:625–641. doi: 10.1091/mbc.E02-09-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Z., Fatheddin P., Graham T. R. An essential subfamily of Drs2p-related P-type ATPases is required for protein trafficking between Golgi complex and endosomal/vacuolar system. Mol. Biol. Cell. 2002;13:3162–3177. doi: 10.1091/mbc.E02-03-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Z., Graham T. R. Requirement for Neo1p in retrograde transport from the Golgi complex to the endoplasmic reticulum. Mol. Biol. Cell. 2003;14:4971–4983. doi: 10.1091/mbc.E03-07-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H. W. G., Wortis M., Mukhopadhyay R. Stomatocyte-discocyte-echinocyte sequence of the human red blood cell: evidence for the bilayer- couple hypothesis from membrane mechanics. Proc. Natl. Acad. Sci. USA. 2002;99:16766–16769. doi: 10.1073/pnas.202617299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Hua Z., Nepute J. A., Graham T. R. Yeast P4-ATPases Drs2p and Dnf1p are essential cargos of the NPFXD/Sla1p endocytic pathway. Mol. Biol. Cell. 2007;18:487–500. doi: 10.1091/mbc.E06-07-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losev E., Reinke C. A., Jellen J., Strongin D. E., Bevis B. J., Glick B. S. Golgi maturation visualized in living yeast. Nature. 2006;441:1002–1006. doi: 10.1038/nature04717. [DOI] [PubMed] [Google Scholar]
- Matsuura-Tokita K., Takeuchi M., Ichihara A., Mikuriya K., Nakano A. Live imaging of yeast Golgi cisternal maturation. Nature. 2006;441:1007–1010. doi: 10.1038/nature04737. [DOI] [PubMed] [Google Scholar]
- Mills I. G., Praefcke G. J., Vallis Y., Peter B. J., Olesen L. E., Gallop J. L., Butler P. J., Evans P. R., McMahon H. T. EpsinR: an AP1/clathrin interacting protein involved in vesicle trafficking. J. Cell Biol. 2003;160:213–222. doi: 10.1083/jcb.200208023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan P., Wang J., Hua Z., Graham T. R. Drs2p-coupled aminophospholipid translocase activity in yeast Golgi membranes and relationship to in vivo function. Proc. Natl. Acad. Sci. USA. 2004;101:10614–10619. doi: 10.1073/pnas.0404146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal R. Energetics of clathrin basket assembly. Traffic. 2001;2:138–147. doi: 10.1034/j.1600-0854.2001.020208.x. [DOI] [PubMed] [Google Scholar]
- Peyroche A., Paris S., Jackson C. L. Nucleotide exchange on ARF mediated by yeast Gea1 protein. Nature. 1996;384:479–481. doi: 10.1038/384479a0. [DOI] [PubMed] [Google Scholar]
- Pomorski T., Lombardi R., Riezman H., Devaux P. F., van Meer G., Holthuis J. C. Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol. Biol. Cell. 2003;14:1240–1254. doi: 10.1091/mbc.E02-08-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M., et al. The Gcs1 Arf-GAP mediates Snc1,2 v-SNARE retrieval to the Golgi in yeast. Mol. Biol. Cell. 2006;17:1845–1858. doi: 10.1091/mbc.E05-09-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. S. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Saito K., Fujimura-Kamada K., Furuta N., Kato U., Umeda M., Tanaka K. Cdc50p, a protein required for polarized growth, associates with the Drs2p P-type ATPase implicated in phospholipid translocation in Saccharomyces cerevisiae. Mol. Biol. Cell. 2004;15:3418–3432. doi: 10.1091/mbc.E03-11-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane H., Yamamoto T., Tanaka K. The functional relationship between the Cdc50p-Drs2p putative aminophospholipid translocase and the Arf GAP Gcs1p in vesicle formation in the retrieval pathway from yeast early endosomes to the TGN. Cell Struct. Funct. 2006;31:87–108. doi: 10.1247/csf.06021. [DOI] [PubMed] [Google Scholar]
- Sciorra V. A., Audhya A., Parsons A. B., Segev N., Boone C., Emr S. D. Synthetic genetic array analysis of the PtdIns 4-kinase Pik1p identifies components in a Golgi-specific Ypt31/rab-GTPase signaling pathway. Mol. Biol. Cell. 2005;16:776–793. doi: 10.1091/mbc.E04-08-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneuret M., Devaux P. F. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc. Natl. Acad. Sci. USA. 1984;81:3751–3755. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Singer S. J. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc. Natl. Acad. Sci. USA. 1974;71:4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Halleck M. S., Schlegel R. A., Williamson P. A subfamily of P-type ATPases with aminophospholipid transporting activity. Science. 1996;272:1495–1497. doi: 10.1126/science.272.5267.1495. [DOI] [PubMed] [Google Scholar]
- Trautwein M., Schindler C., Gauss R., Dengjel J., Hartmann E., Spang A. Arf1p, Chs5p and the ChAPs are required for export of specialized cargo from the Golgi. EMBO J. 2006;25:943–954. doi: 10.1038/sj.emboj.7601007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujhazy P., Ortiz D., Misra S., Li S., Moseley J., Jones H., Arias I. M. Familial intrahepatic cholestasis 1, studies of localization and function. Hepatology. 2001;34:768–775. doi: 10.1053/jhep.2001.27663. [DOI] [PubMed] [Google Scholar]
- Valdivia R. H., Baggott D., Chuang J. S., Schekman R. W. The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev. Cell. 2002;2:283–294. doi: 10.1016/s1534-5807(02)00127-2. [DOI] [PubMed] [Google Scholar]
- Wang C. W., Hamamoto S., Orci L., Schekman R. Exomer: A coat complex for transport of select membrane proteins from the trans-Golgi network to the plasma membrane in yeast. J. Cell Biol. 2006;174:973–983. doi: 10.1083/jcb.200605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicky S., Schwarz H., Singer-Kruger B. Molecular interactions of yeast Neo1p, an essential member of the Drs2 family of aminophospholipid translocases, and its role in membrane trafficking within the endomembrane system. Mol. Cell. Biol. 2004;24:7402–7418. doi: 10.1128/MCB.24.17.7402-7418.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederkehr A., Avaro S., Prescianotto-Baschong C., Haguenauer-Tsapis R., Riezman H. The F-box protein Rcy1p is involved in endocytic membrane traffic and recycling out of an early endosome in Saccharomyces cerevisiae. J. Cell Biol. 2000;149:397–410. doi: 10.1083/jcb.149.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Drake M. T., Kornfeld S. ADP-ribosylation factor 1 dependent clathrin-coat assembly on synthetic liposomes. Proc. Natl. Acad. Sci. USA. 1999;96:5013–5018. doi: 10.1073/pnas.96.9.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.