Abstract
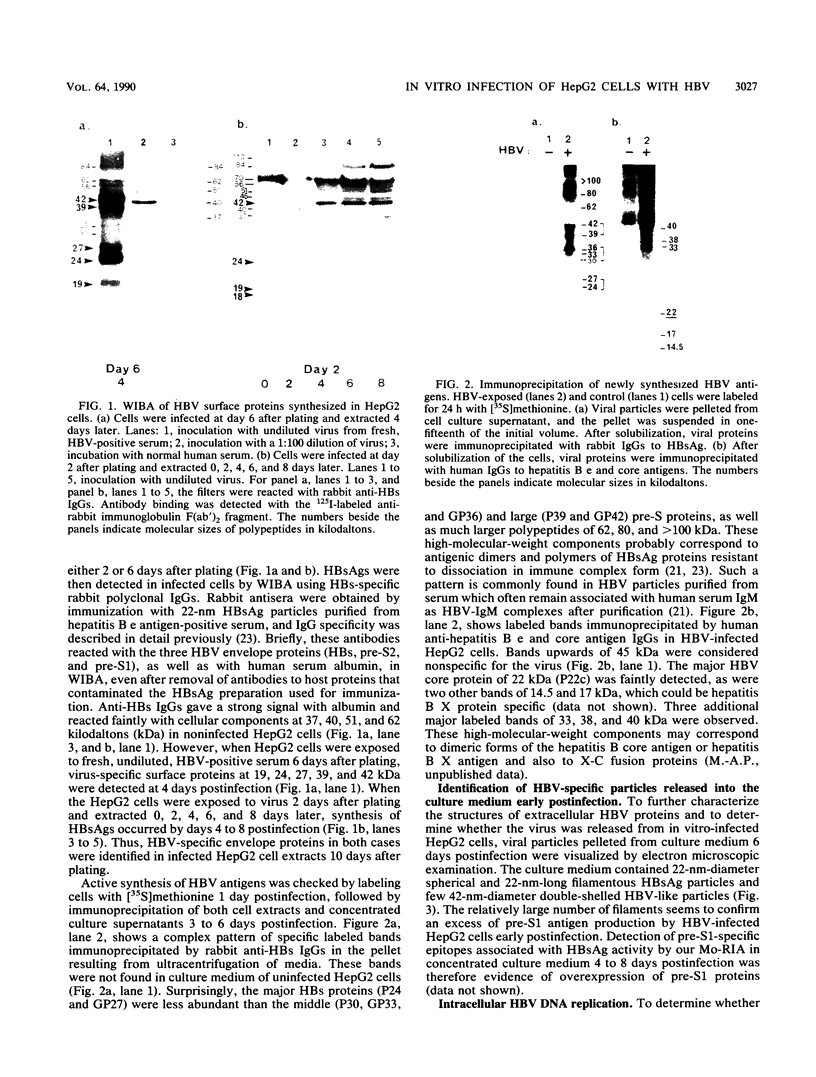
An in vitro system for production of hepatitis B virus (HBV) was established by infection of human hepatoma (HepG2) cells. HBV particles obtained from the serum of a chronic hepatitis B surface antigen (subtype ad) carrier were used to inoculate HepG2 cells. HBV envelope and core proteins were synthesized de novo by the infected cells and secreted into the medium 3 to 6 days postinfection. Viral covalently closed circular DNA, the putative template for viral RNA transcription, accumulated in the cells with increasing time postinfection. The HBV-infected HepG2 cells were maintained for several months (HepG2-BV cell line) and continued producing viral antigens. Both HBV DNA replicative intermediates and major HBV transcripts were identified in HepG2-BV cells. Complete HBV particles, which contain HBV DNA and DNA polymerase activity and express the three antigenic specificities of the envelope (hepatitis B surface antigen, pre-S2, and pre-S1), were released into the culture supernatant. Thus, successful in vitro infection of transformed human hepatocytes raising stable HBV-producing cells was achieved for the first time. This strongly suggests that HepG2 cells have a receptor(s) for virus attachment and penetration. Such a system represents a significant advance for the study of HBV-target cell interactions as the early events of HBV infection.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acs G., Sells M. A., Purcell R. H., Price P., Engle R., Shapiro M., Popper H. Hepatitis B virus produced by transfected Hep G2 cells causes hepatitis in chimpanzees. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4641–4644. doi: 10.1073/pnas.84.13.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aden D. P., Fogel A., Plotkin S., Damjanov I., Knowles B. B. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature. 1979 Dec 6;282(5739):615–616. doi: 10.1038/282615a0. [DOI] [PubMed] [Google Scholar]
- Alberti A., Pontisso P., Realdi G. Changes in hepatitis B virus DNA-polymerase activity in patients with chronic infection. J Med Virol. 1981;8(4):223–229. doi: 10.1002/jmv.1890080402. [DOI] [PubMed] [Google Scholar]
- Blum H. E., Haase A. T., Harris J. D., Walker D., Vyas G. N. Asymmetric replication of hepatitis B virus DNA in human liver: demonstration of cytoplasmic minus-strand DNA by blot analyses and in situ hybridization. Virology. 1984 Nov;139(1):87–96. doi: 10.1016/0042-6822(84)90332-5. [DOI] [PubMed] [Google Scholar]
- Feitelson M. A., Millman I., Halbherr T., Simmons H., Blumberg B. S. A newly identified hepatitis B type virus in tree squirrels. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2233–2237. doi: 10.1073/pnas.83.7.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler M. J., Monjardino J., Tsiquaye K. N., Zuckerman A. J., Thomas H. C. The mechanism of replication of hepatitis B virus: evidence of asymmetric replication of the two DNA strands. J Med Virol. 1984;13(1):83–91. doi: 10.1002/jmv.1890130110. [DOI] [PubMed] [Google Scholar]
- Gripon P., Diot C., Corlu A., Guguen-Guillouzo C. Regulation by dimethylsulfoxide, insulin, and corticosteroids of hepatitis B virus replication in a transfected human hepatoma cell line. J Med Virol. 1989 Jul;28(3):193–199. doi: 10.1002/jmv.1890280316. [DOI] [PubMed] [Google Scholar]
- Gripon P., Diot C., Thézé N., Fourel I., Loreal O., Brechot C., Guguen-Guillouzo C. Hepatitis B virus infection of adult human hepatocytes cultured in the presence of dimethyl sulfoxide. J Virol. 1988 Nov;62(11):4136–4143. doi: 10.1128/jvi.62.11.4136-4143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Isom I., Georgoff I., Salditt-Georgieff M., Darnell J. E., Jr Persistence of liver-specific messenger RNA in cultured hepatocytes: different regulatory events for different genes. J Cell Biol. 1987 Dec;105(6 Pt 2):2877–2885. doi: 10.1083/jcb.105.6.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Marion P. L., Oshiro L. S., Regnery D. C., Scullard G. H., Robinson W. S. A virus in Beechey ground squirrels that is related to hepatitis B virus of humans. Proc Natl Acad Sci U S A. 1980 May;77(5):2941–2945. doi: 10.1073/pnas.77.5.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. S., Aldrich C., Summers J., Taylor J. M. Asymmetric replication of duck hepatitis B virus DNA in liver cells: Free minus-strand DNA. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3997–4001. doi: 10.1073/pnas.79.13.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. S., Halpern M. S., England J. M., Seal G., Egan J., Coates L., Aldrich C., Summers J. Experimental transmission of duck hepatitis B virus. Virology. 1983 Dec;131(2):375–384. doi: 10.1016/0042-6822(83)90505-6. [DOI] [PubMed] [Google Scholar]
- Mason W. S., Seal G., Summers J. Virus of Pekin ducks with structural and biological relatedness to human hepatitis B virus. J Virol. 1980 Dec;36(3):829–836. doi: 10.1128/jvi.36.3.829-836.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. H., Robinson W. S. Hepatitis B virus DNA forms in nuclear and cytoplasmic fractions of infected human liver. Virology. 1984 Sep;137(2):390–399. doi: 10.1016/0042-6822(84)90231-9. [DOI] [PubMed] [Google Scholar]
- Ochiya T., Tsurimoto T., Ueda K., Okubo K., Shiozawa M., Matsubara K. An in vitro system for infection with hepatitis B virus that uses primary human fetal hepatocytes. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1875–1879. doi: 10.1073/pnas.86.6.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit M. A., Capel F., Riottot M. M., Dauguet C., Pillot J. Antigenic mapping of the surface proteins of infectious hepatitis B virus particles. J Gen Virol. 1987 Nov;68(Pt 11):2759–2767. doi: 10.1099/0022-1317-68-11-2759. [DOI] [PubMed] [Google Scholar]
- Petit M. A., Dubanchet S., Capel F. A monoclonal antibody specific for the hepatocyte receptor binding site on hepatitis B virus. Mol Immunol. 1989 Jun;26(6):531–537. doi: 10.1016/0161-5890(89)90004-7. [DOI] [PubMed] [Google Scholar]
- Petit M. A., Maillard P., Capel F., Pillot J. Immunochemical structure of the hepatitis B surface antigen vaccine--II. Analysis of antibody responses in human sera against the envelope proteins. Mol Immunol. 1986 May;23(5):511–523. doi: 10.1016/0161-5890(86)90114-8. [DOI] [PubMed] [Google Scholar]
- Ruiz-Opazo N., Chakraborty P. R., Shafritz D. A. Evidence for supercoiled hepatitis B virus DNA in chimpanzee liver and serum Dane particles: possible implications in persistent HBV infection. Cell. 1982 May;29(1):129–136. doi: 10.1016/0092-8674(82)90097-6. [DOI] [PubMed] [Google Scholar]
- Sells M. A., Chen M. L., Acs G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1005–1009. doi: 10.1073/pnas.84.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Nambu S., Kojima T., Sasaki H. Replication of hepatitis B virus in culture systems with adult human hepatocytes. J Med Virol. 1986 Dec;20(4):313–327. doi: 10.1002/jmv.1890200404. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sprengel R., Kaleta E. F., Will H. Isolation and characterization of a hepatitis B virus endemic in herons. J Virol. 1988 Oct;62(10):3832–3839. doi: 10.1128/jvi.62.10.3832-3839.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., Mason W. S. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982 Jun;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- Summers J., Smolec J. M., Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4533–4537. doi: 10.1073/pnas.75.9.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureau C., Eichberg J. W., Hubbard G. B., Romet-Lemonne J. L., Essex M. A molecularly cloned hepatitis B virus produced in vitro is infectious in a chimpanzee. J Virol. 1988 Aug;62(8):3064–3067. doi: 10.1128/jvi.62.8.3064-3067.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureau C., Romet-Lemonne J. L., Mullins J. I., Essex M. Production of hepatitis B virus by a differentiated human hepatoma cell line after transfection with cloned circular HBV DNA. Cell. 1986 Oct 10;47(1):37–47. doi: 10.1016/0092-8674(86)90364-8. [DOI] [PubMed] [Google Scholar]
- Thézé N., Gripon P., Fourel I., Hantz O., Trepo C., Guguen-Guillouzo C. Maintenance of woodchuck hepatitis virus activity in woodchuck hepatocyte primary culture. J Gen Virol. 1987 Apr;68(Pt 4):1029–1039. doi: 10.1099/0022-1317-68-4-1029. [DOI] [PubMed] [Google Scholar]
- Tsurimoto T., Fujiyama A., Matsubara K. Stable expression and replication of hepatitis B virus genome in an integrated state in a human hepatoma cell line transfected with the cloned viral DNA. Proc Natl Acad Sci U S A. 1987 Jan;84(2):444–448. doi: 10.1073/pnas.84.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttleman J. S., Pourcel C., Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986 Nov 7;47(3):451–460. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]
- Tuttleman J. S., Pugh J. C., Summers J. W. In vitro experimental infection of primary duck hepatocyte cultures with duck hepatitis B virus. J Virol. 1986 Apr;58(1):17–25. doi: 10.1128/jvi.58.1.17-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will H., Reiser W., Weimer T., Pfaff E., Büscher M., Sprengel R., Cattaneo R., Schaller H. Replication strategy of human hepatitis B virus. J Virol. 1987 Mar;61(3):904–911. doi: 10.1128/jvi.61.3.904-911.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaginuma K., Shirakata Y., Kobayashi M., Koike K. Hepatitis B virus (HBV) particles are produced in a cell culture system by transient expression of transfected HBV DNA. Proc Natl Acad Sci U S A. 1987 May;84(9):2678–2682. doi: 10.1073/pnas.84.9.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]