Abstract
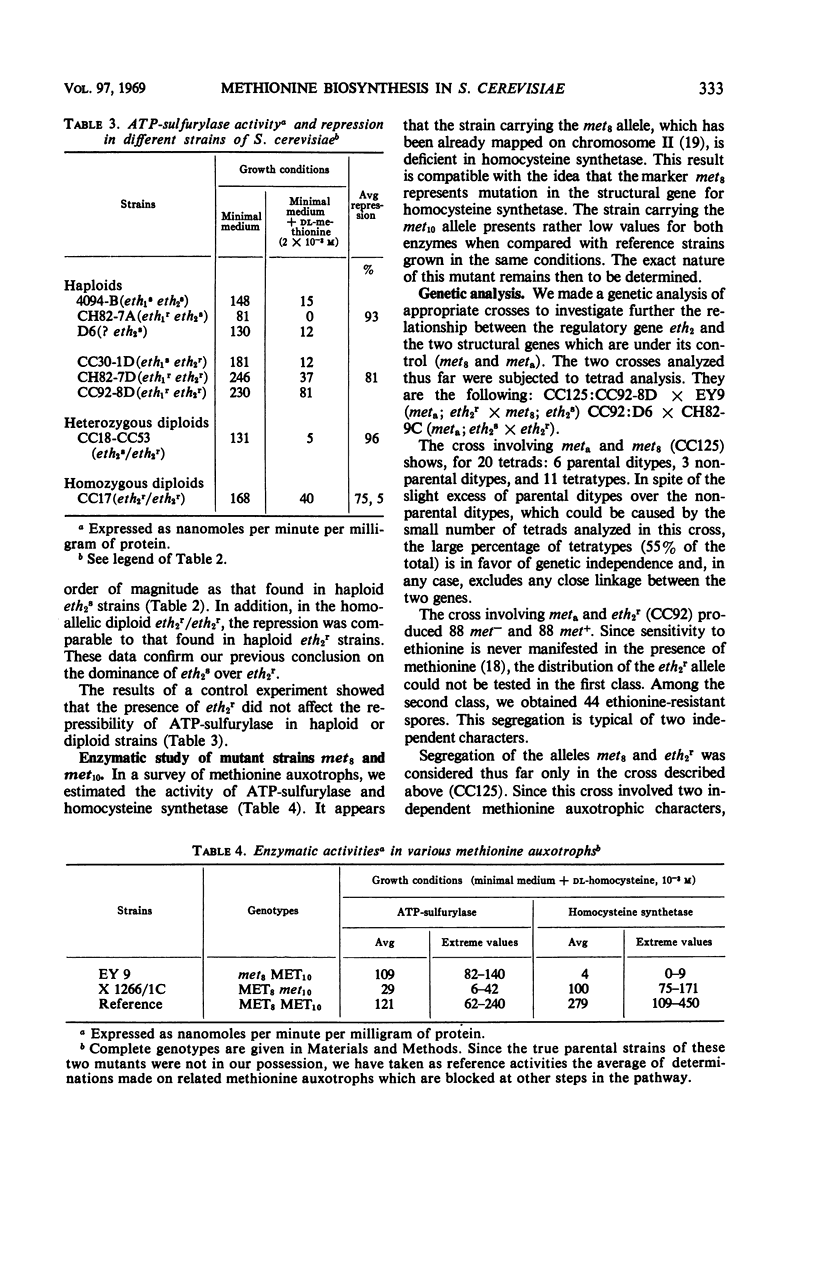
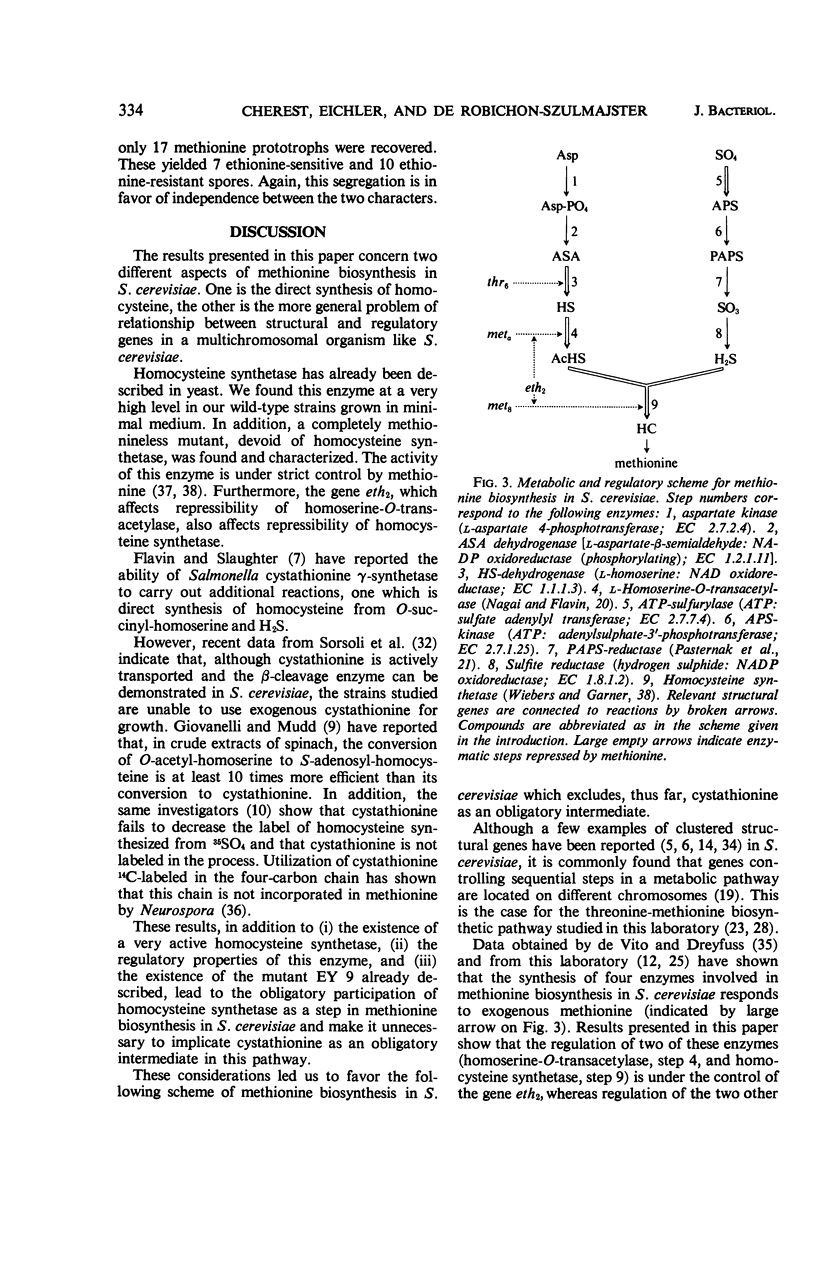
Methionine biosynthesis and regulation of four enzymatic steps involved in this pathway were studied in Saccharomyces cerevisiae, in relation to genes concerned with resistance to ethionine (eth1 and eth2). Data presented in this paper and others favor a scheme which excludes cystathionine as an obligatory intermediate. Kinetic data are presented for homocysteine synthetase [Km(O-acetyl-l-homoserine) = 7 × 10−3m; Ki (l-methionine) = 1.9 × 10−3m]. Enzymes catalyzing steps 3, 4, 5, and 9 were repressible by methionine. Enzyme 4 (homoserine-O-transacetylase) and enzyme 9 (homocysteine synthetase) were simultaneously derepressed in strains carrying the mutant allele eth2r. Studies on diploid strains confirmed the dominance of the eth2s allele over eth2r. Regulation of enzyme 3 (homoserine dehydrogenase) and enzyme 5 (adenosine triphosphate sulfurylase) is not modified by the allele eth2r. The other gene eth1 did not appear to participate in regulation of these four steps. Gene enzyme relationship was determined for three of the four steps studied (steps 3, 4, and 9). The structural genes concerned with the steps which are under the control of eth2 (met8: enzyme 9 and meta: enzyme 4) segregate independently, and are unlinked to eth2. These results are compatible with the idea that the gene eth2 is responsible for the synthesis of a pleiotropic methionine repressor and suggest the existence of at least two different methionine repressors in S. cerevisiae. Implications of these findings in general regulatory mechanisms have been discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK S., WRIGHT N. G. Aspartic beta-semialdehyde dehydrogenase and aspartic beta-semialdehyde. J Biol Chem. 1955 Mar;213(1):39–50. [PubMed] [Google Scholar]
- Cherest H., Robichon-Szulmajster H. Résistance a l'éthionine chez Saccharomyces cerevisiae. I. Etude génétique. Genetics. 1966 Oct;54(4):981–991. doi: 10.1093/genetics/54.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELAVIER-KLUTCHKO C., FLAVIN M. ENZYMATIC SYNTHESIS AND CLEAVAGE OF CYSTATHIONINE IN FUNGI AND BACTERIA. J Biol Chem. 1965 Jun;240:2537–2549. [PubMed] [Google Scholar]
- DEVITO P. C., DREYFUSS J. METABOLIC REGULATION OF ADENOSINE TRIPHOSPHATE SULFURYLASE IN YEAST. J Bacteriol. 1964 Nov;88:1341–1348. doi: 10.1128/jb.88.5.1341-1348.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robichon-Szulmajster Régulation du fonctionnement de deux chaines de biosynthèse chez saccharomyces cerevisiae: thréonine-méthionine et isoleucine-valine. Bull Soc Chim Biol (Paris) 1967 Dec 18;49(11):1431–1462. [PubMed] [Google Scholar]
- Fink G. R. A cluster of genes controlling three enzymes in histidine biosynthesis in Saccharomyces cerevisiae. Genetics. 1966 Mar;53(3):445–459. doi: 10.1093/genetics/53.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavin M., Slaughter C. Enzymatic synthesis of homocysteine or methionine directly from O-succinyl-homoserine. Biochim Biophys Acta. 1967 Mar 15;132(2):400–405. doi: 10.1016/0005-2744(67)90158-1. [DOI] [PubMed] [Google Scholar]
- GALZY P., SLONIMSKI P. P. Evolution de la constitution enzymatique de la levure cultivée sur acide lactique ou sur glucose comme seule source de carbone. C R Hebd Seances Acad Sci. 1957 Dec 23;245(26):2556–2558. [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H. Enzymatic synthesis of cystathionine by extracts of spinach, requiring O-acetylhomoserine or O-succinylhomoserine. Biochem Biophys Res Commun. 1966 Nov 11;25(3):366–371. doi: 10.1016/0006-291x(66)90787-x. [DOI] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H. Synthesis of homocysteine and cysteine by enzyme extracts of spinach. Biochem Biophys Res Commun. 1967 Apr 20;27(2):150–156. doi: 10.1016/s0006-291x(67)80054-8. [DOI] [PubMed] [Google Scholar]
- JOHNSTON J. R., MORTIMER R. K. Use of snail digestive juice in isolation of yeast spore tetrads. J Bacteriol. 1959 Aug;78:292–292. doi: 10.1128/jb.78.2.292-292.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M., Tomkins G. M. The enzymic synthesis of L-cysteine in Escherichia coli and Salmonella typhimurium. J Biol Chem. 1966 Nov 10;241(21):4955–4965. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacroute F. Regulation of pyrimidine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1968 Mar;95(3):824–832. doi: 10.1128/jb.95.3.824-832.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAW G. A. Ability of S-methyl-L-cysteine to annul the inhibition of yeast growth by L-ethionine and by S-ethyl-L-cysteine. J Gen Microbiol. 1961 Jul;25:441–449. doi: 10.1099/00221287-25-3-441. [DOI] [PubMed] [Google Scholar]
- McClary D. O., Nulty W. L., Miller G. R. EFFECT OF POTASSIUM VERSUS SODIUM IN THE SPORULATION OF SACCHAROMYCES. J Bacteriol. 1959 Sep;78(3):362–368. doi: 10.1128/jb.78.3.362-368.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Genetic mapping in Saccharomyces. Genetics. 1966 Jan;53(1):165–173. doi: 10.1093/genetics/53.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai S., Flavin M. Acetylhomoserine. An intermediate in the fungal biosynthesis of methionine. J Biol Chem. 1967 Sep 10;242(17):3884–3895. [PubMed] [Google Scholar]
- PASTERNAK C. A., ELLIS R. J., JONES-MORTIMER M. C., CRICHTON C. E. THE CONTROL OF SULPHATE REDUCTION IN BACTERIA. Biochem J. 1965 Jul;96:270–275. doi: 10.1042/bj0960270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Separation of the two enzymatic phases in active sulfate synthesis. J Biol Chem. 1958 Sep;233(3):681–685. [PubMed] [Google Scholar]
- ROWBURY R. J., WOODS D. D. O-SUCCINYLHOMOSERINE AS AN INTERMEDIATE IN THE SYNTHESIS OF CYSTATHIONINE BY ESCHERICHIA COLI. J Gen Microbiol. 1964 Sep;36:341–358. doi: 10.1099/00221287-36-3-341. [DOI] [PubMed] [Google Scholar]
- Robichon-Szulmajster H., Cherest H. Regulation of homoserine O-transacetylase, first step in methionine biosyntheis in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1967 Jul 21;28(2):256–262. doi: 10.1016/0006-291x(67)90438-x. [DOI] [PubMed] [Google Scholar]
- Robichon-Szulmajster H., Cherest H. Résistance a l'éthionine chez Saccharomyces cerevisiae. II. Etude physiologique. Genetics. 1966 Oct;54(4):993–1006. doi: 10.1093/genetics/54.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichon-Szulmajster H., Magee P. T. The regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. I. Threonine deaminase. Eur J Biochem. 1968 Feb;3(4):492–501. doi: 10.1111/j.1432-1033.1967.tb19558.x. [DOI] [PubMed] [Google Scholar]
- Robichon-Szulmajster H., Surdin Y., Mortimer R. K. Genetic and biochemical studies of genes controlling the synthesis of threonine and methionine in Saccharomyces. Genetics. 1966 Mar;53(3):609–619. doi: 10.1093/genetics/53.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorsoli W. A., Buettner M., Parks L. W. Cystathionine metabolism in methionine auxotrophic and wild-type strains of Saccharomyces cerevisiae. J Bacteriol. 1968 Mar;95(3):1024–1029. doi: 10.1128/jb.95.3.1024-1029.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surdin Y., Sly W., Sire J., Bordes A. M., Robichon-Szulmajster H. Propriétés et contrôle génétique du système d'accumulation des acides aminés chez Saccharomyces cerevisiae. Biochim Biophys Acta. 1965 Oct 18;107(3):546–566. [PubMed] [Google Scholar]
- Tingle M., Herman A., Halvorson H. O. Characterization and mapping of histidine genes in Saccharomyces lactis. Genetics. 1968 Mar;58(3):361–371. doi: 10.1093/genetics/58.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIEBERS J. L., GARNER H. R. Metabolic relationship between cystathionine and methionine in Neurospora. J Bacteriol. 1960 Jul;80:51–60. doi: 10.1128/jb.80.1.51-60.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebers J. L., Garner H. R. Acyl derivatives of homoserine as substrates for homocysteine synthesis in Neurospora crassa, yeast, and Escherichia coli. J Biol Chem. 1967 Dec 10;242(23):5644–5649. [PubMed] [Google Scholar]
- Wiebers J. L., Garner H. R. Homocysteine and cysteine synthetases of Neurospora crassa. Purification, properties, and feedback control of activity. J Biol Chem. 1967 Jan 10;242(1):12–23. [PubMed] [Google Scholar]