Abstract
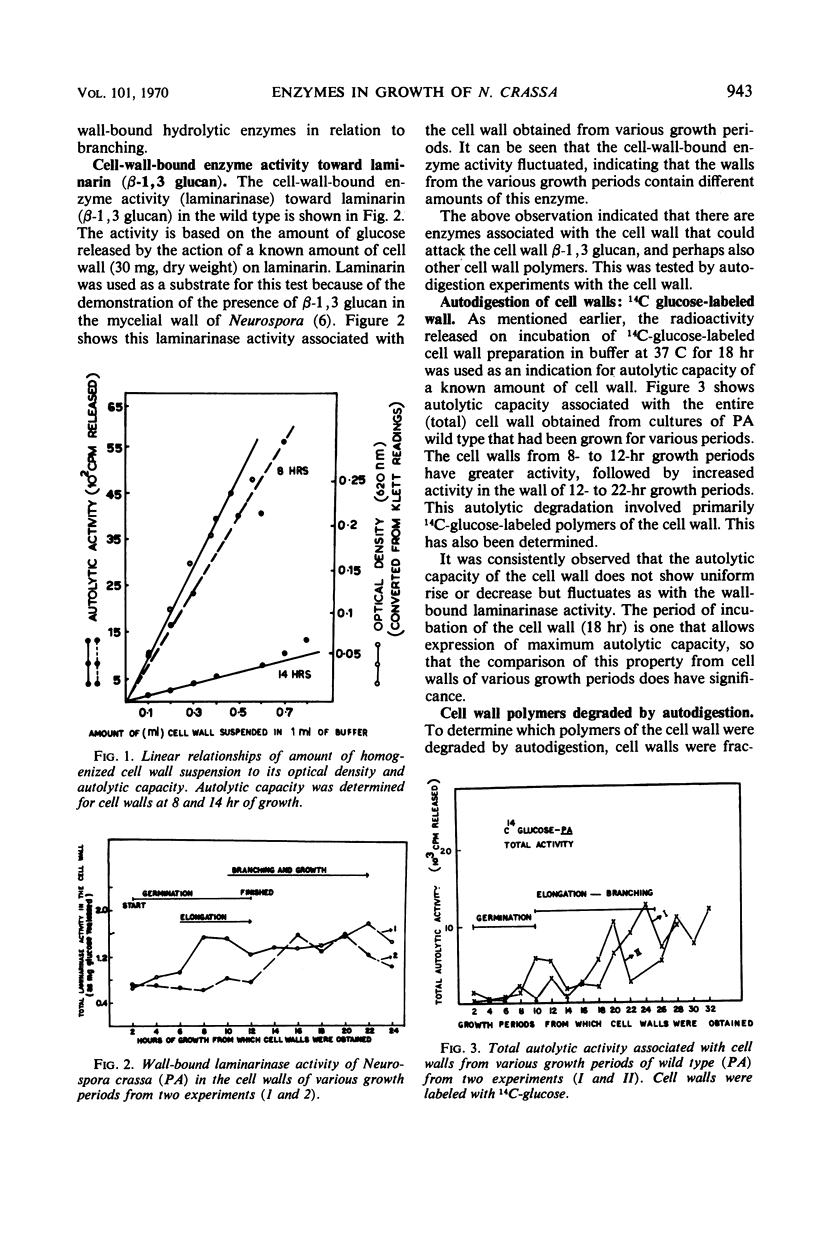
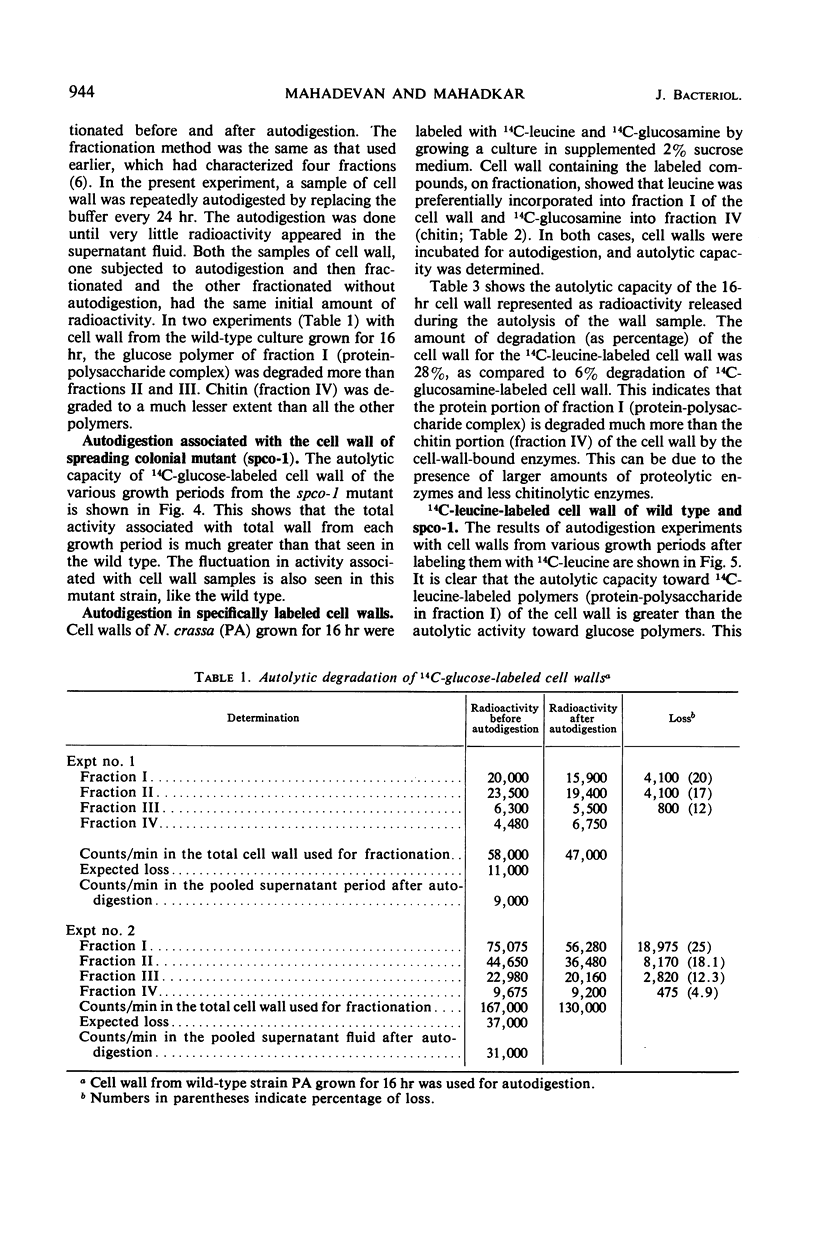
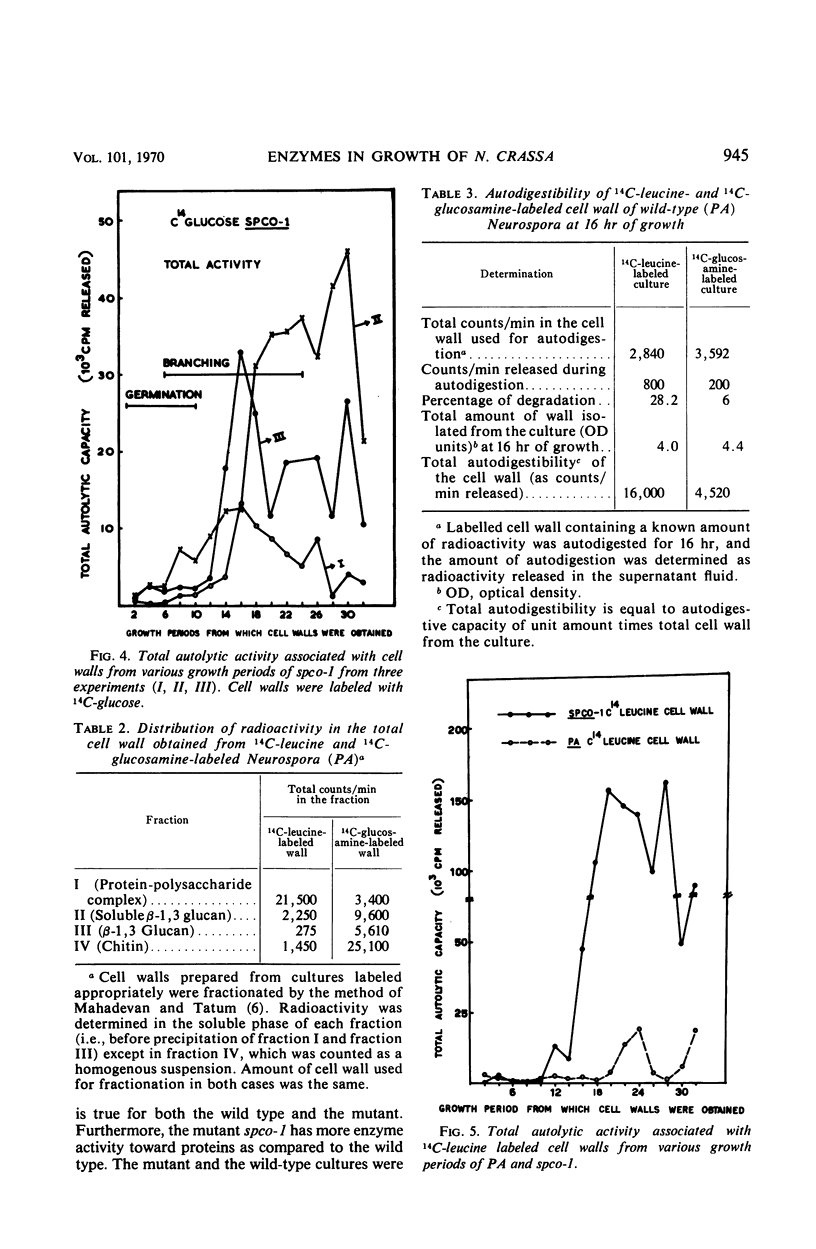
The cell wall of Neurospora crassa contains bound enzymes that can digest its structural polymers. These enzymes are not present at the same levels at all stages of growth. The levels of these autolytic enzymes vary and generally show some relationship to the process of branching. These enzymes were removed from the cell wall by β-mercaptoethanol extraction and were tested for activity against isolated cell wall fractions. Such studies, as well as autolytic studies, showed that enzymes acting on the protein portion of the cell wall (proteases) are more prominent than enzymes that act on the glucan portion (glucanases) of the cell wall. Comparative studies between the wild type and a spreading colonial mutant spco-1 showed that earlier and higher frequency of branching in spco-1 was correlated with a greater amount of these enzymes bound to the cell walls. It is concluded from these observations that autolytic enzymes acting on the protein and glucan portion of the cell walls occur as wall-bound and participate in the process of branching in Neurospora.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCK T. D. Physiology of the conjugation process in the yeast Hansenula wingei. J Gen Microbiol. 1961 Nov;26:487–497. doi: 10.1099/00221287-26-3-487. [DOI] [PubMed] [Google Scholar]
- CONTI S. F., BROCK T. D. ELECTRON MICROSCOPY OF CELL FUSION IN CONJUGATING HANSENULA WINGEI. J Bacteriol. 1965 Aug;90:524–533. doi: 10.1128/jb.90.2.524-533.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE TERRA N., TATUM E. L. Colonial growth of Neurospora. Sorbose and enzymes alter the composition of the cell wall and induce morphological changes. Science. 1961 Oct 13;134(3485):1066–1068. doi: 10.1126/science.134.3485.1066. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Hitchins A. D., King W. L. Function and location of a "germination enzyme" in spores of Bacillus cereus. J Gen Microbiol. 1966 Aug;44(2):293–502. doi: 10.1099/00221287-44-2-293. [DOI] [PubMed] [Google Scholar]
- Mahadevan P. R., Tatum E. L. Localization of structural polymers in the cell wall of Neurospora crassa. J Cell Biol. 1967 Nov;35(2):295–302. doi: 10.1083/jcb.35.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan P. R., Tatum E. L. Relationship of the major constituents of the Neurospora crassa cell wall to wild-type and colonial morphology. J Bacteriol. 1965 Oct;90(4):1073–1081. doi: 10.1128/jb.90.4.1073-1081.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Pooley H. M., Thompson J. S. Autolytic enzyme system of Streptococcus faecalis. 3. Localization of the autolysin at the sites of cell wall synthesis. J Bacteriol. 1967 Nov;94(5):1525–1530. doi: 10.1128/jb.94.5.1525-1530.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


