Abstract
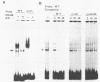
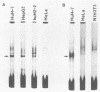
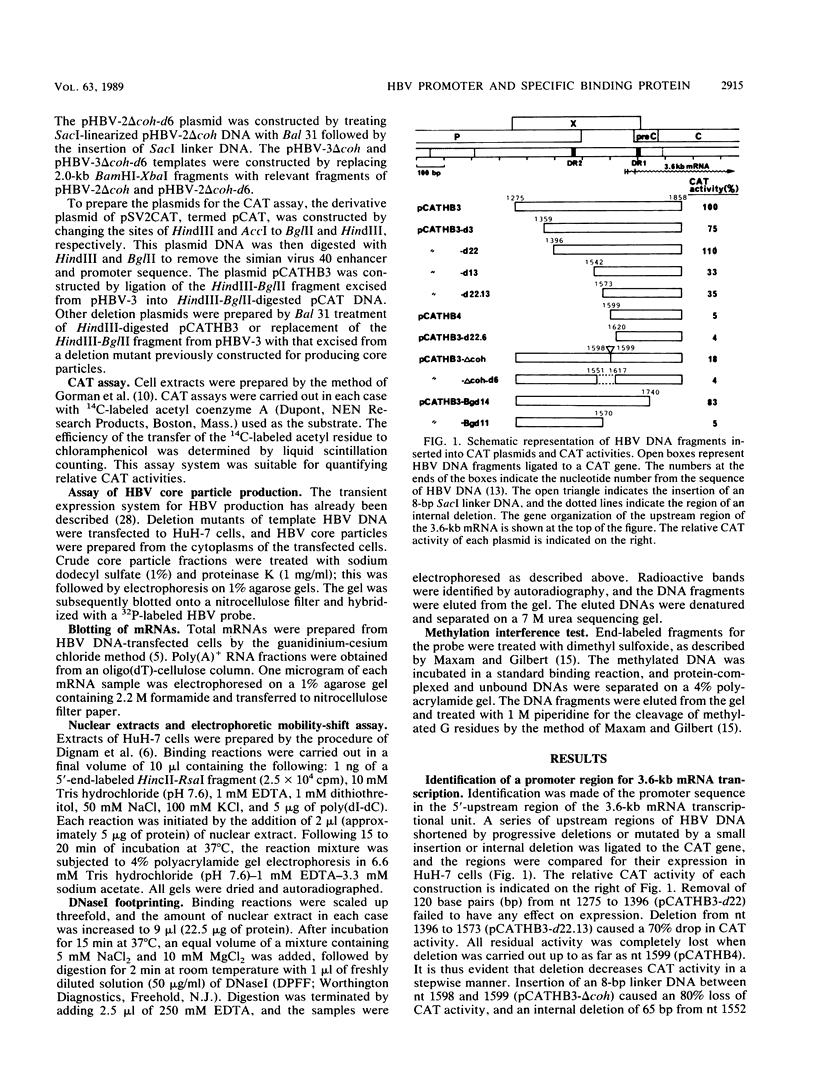
The promoter region for transcription of the 3.6-kilobase mRNA of hepatitis B virus was identified by the chloramphenicol acetyltransferase assay by using HuH-7 hepatoma cells and was found to function directly in virus production by way of the transient expression system of HBV. The 5'-upstream sequence from nucleotides 1573 to 1657 (the transcription start site) was indispensable for promoter function, while the AT-rich sequence (from nucleotides 1581 to 1604) containing a directly repeated sequence TGTT connecting the same flanking sequence PyAAAGAC (where Py is a pyrimidine) at both sides was an essential element within this promoter region. A specific cellular factor which interacted with the essential element was detected in the HuH-7 cell extract. A similar binding factor was also observed in HepG2 and huH2-2 hepatoma cells. This factor may thus be responsible for regulating 3.6-kilobase mRNA, pregenome RNA transcription, or both.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beasley R. P., Hwang L. Y., Lin C. C., Chien C. S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981 Nov 21;2(8256):1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Will H., Hernandez N., Schaller H. Signals regulating hepatitis B surface antigen transcription. Nature. 1983 Sep 22;305(5932):336–338. doi: 10.1038/305336a0. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Will H., Schaller H. Hepatitis B virus transcription in the infected liver. EMBO J. 1984 Sep;3(9):2191–2196. doi: 10.1002/j.1460-2075.1984.tb02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. M., Jeng K. S., Hu C. P., Lo S. J., Su T. S., Ting L. P., Chou C. K., Han S. H., Pfaff E., Salfeld J. Production of hepatitis B virus in vitro by transient expression of cloned HBV DNA in a hepatoma cell line. EMBO J. 1987 Mar;6(3):675–680. doi: 10.1002/j.1460-2075.1987.tb04807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders G. H., Ganem D., Varmus H. E. 5'-terminal sequences influence the segregation of ground squirrel hepatitis virus RNAs into polyribosomes and viral core particles. J Virol. 1987 Jan;61(1):35–41. doi: 10.1128/jvi.61.1.35-41.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders G. H., Ganem D., Varmus H. Mapping the major transcripts of ground squirrel hepatitis virus: the presumptive template for reverse transcriptase is terminally redundant. Cell. 1985 Aug;42(1):297–308. doi: 10.1016/s0092-8674(85)80125-2. [DOI] [PubMed] [Google Scholar]
- Galibert F., Chen T. N., Mandart E. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: comparison with the hepatitis B virus sequence. J Virol. 1982 Jan;41(1):51–65. doi: 10.1128/jvi.41.1.51-65.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heermann K. H., Goldmann U., Schwartz W., Seyffarth T., Baumgarten H., Gerlich W. H. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol. 1984 Nov;52(2):396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Koike K. Complete nucleotide sequence of hepatitis B virus DNA of subtype adr and its conserved gene organization. Gene. 1984 Oct;30(1-3):227–232. doi: 10.1016/0378-1119(84)90124-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Möröy T., Etiemble J., Trépo C., Tiollais P., Buendia M. A. Transcription of woodchuck hepatitis virus in the chronically infected liver. EMBO J. 1985 Jun;4(6):1507–1514. doi: 10.1002/j.1460-2075.1985.tb03810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabayashi H., Taketa K., Miyano K., Yamane T., Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982 Sep;42(9):3858–3863. [PubMed] [Google Scholar]
- Persing D. H., Varmus H. E., Ganem D. Inhibition of secretion of hepatitis B surface antigen by a related presurface polypeptide. Science. 1986 Dec 12;234(4782):1388–1391. doi: 10.1126/science.3787251. [DOI] [PubMed] [Google Scholar]
- Rall L. B., Standring D. N., Laub O., Rutter W. J. Transcription of hepatitis B virus by RNA polymerase II. Mol Cell Biol. 1983 Oct;3(10):1766–1773. doi: 10.1128/mcb.3.10.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger C., Ganem D., Varmus H. E. Biochemical and genetic evidence for the hepatitis B virus replication strategy. Science. 1986 Apr 25;232(4749):477–484. doi: 10.1126/science.3961490. [DOI] [PubMed] [Google Scholar]
- Seeger C., Ganem D., Varmus H. E. Nucleotide sequence of an infectious molecularly cloned genome of ground squirrel hepatitis virus. J Virol. 1984 Aug;51(2):367–375. doi: 10.1128/jvi.51.2.367-375.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells M. A., Chen M. L., Acs G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1005–1009. doi: 10.1073/pnas.84.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring D. N., Ou J. H., Rutter W. J. Assembly of viral particles in Xenopus oocytes: pre-surface-antigens regulate secretion of the hepatitis B viral surface envelope particle. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9338–9342. doi: 10.1073/pnas.83.24.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureau C., Romet-Lemonne J. L., Mullins J. I., Essex M. Production of hepatitis B virus by a differentiated human hepatoma cell line after transfection with cloned circular HBV DNA. Cell. 1986 Oct 10;47(1):37–47. doi: 10.1016/0092-8674(86)90364-8. [DOI] [PubMed] [Google Scholar]
- Treinin M., Laub O. Identification of a promoter element located upstream from the hepatitis B virus X gene. Mol Cell Biol. 1987 Jan;7(1):545–548. doi: 10.1128/mcb.7.1.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurimoto T., Fujiyama A., Matsubara K. Stable expression and replication of hepatitis B virus genome in an integrated state in a human hepatoma cell line transfected with the cloned viral DNA. Proc Natl Acad Sci U S A. 1987 Jan;84(2):444–448. doi: 10.1073/pnas.84.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will H., Reiser W., Weimer T., Pfaff E., Büscher M., Sprengel R., Cattaneo R., Schaller H. Replication strategy of human hepatitis B virus. J Virol. 1987 Mar;61(3):904–911. doi: 10.1128/jvi.61.3.904-911.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaginuma K., Kobayashi M., Yoshida E., Koike K. Hepatitis B virus integration in hepatocellular carcinoma DNA: duplication of cellular flanking sequences at the integration site. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4458–4462. doi: 10.1073/pnas.82.13.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaginuma K., Shirakata Y., Kobayashi M., Koike K. Hepatitis B virus (HBV) particles are produced in a cell culture system by transient expression of transfected HBV DNA. Proc Natl Acad Sci U S A. 1987 May;84(9):2678–2682. doi: 10.1073/pnas.84.9.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]