Abstract
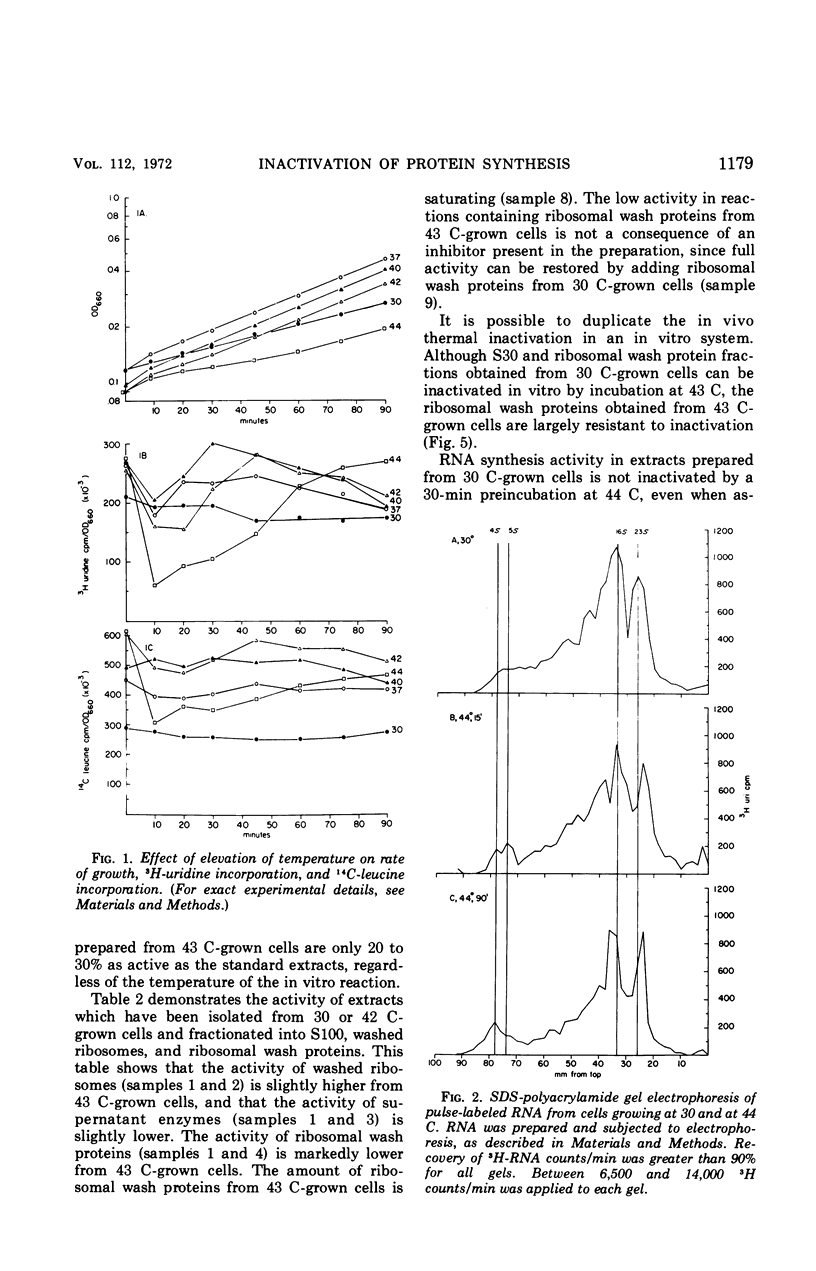
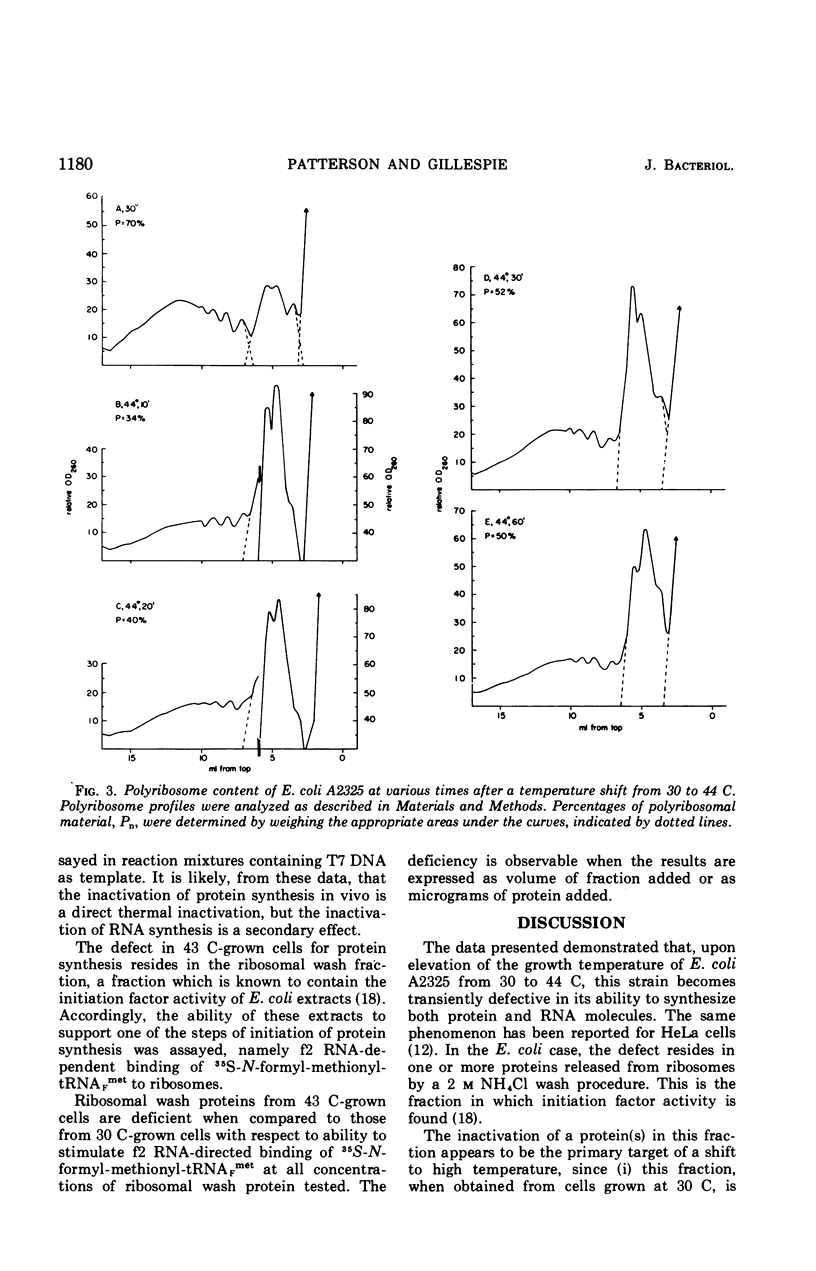
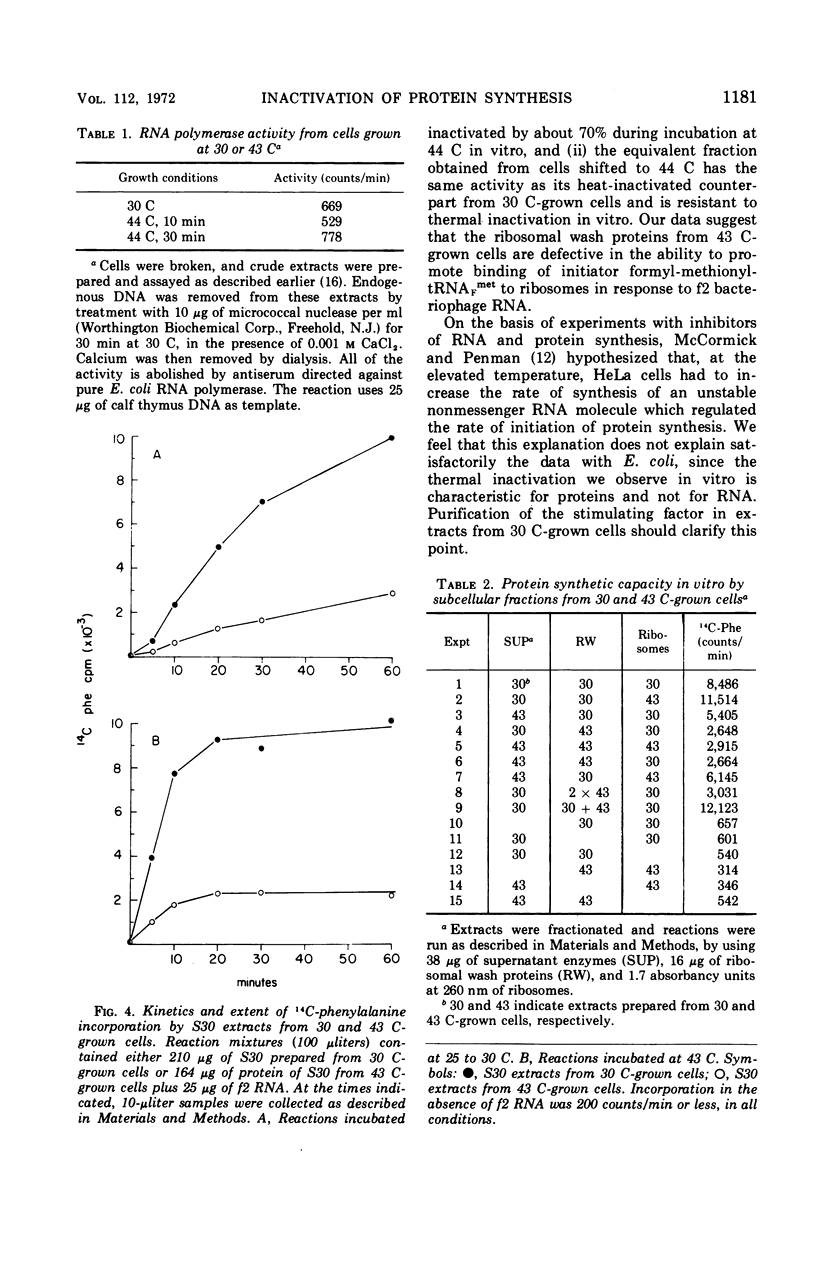
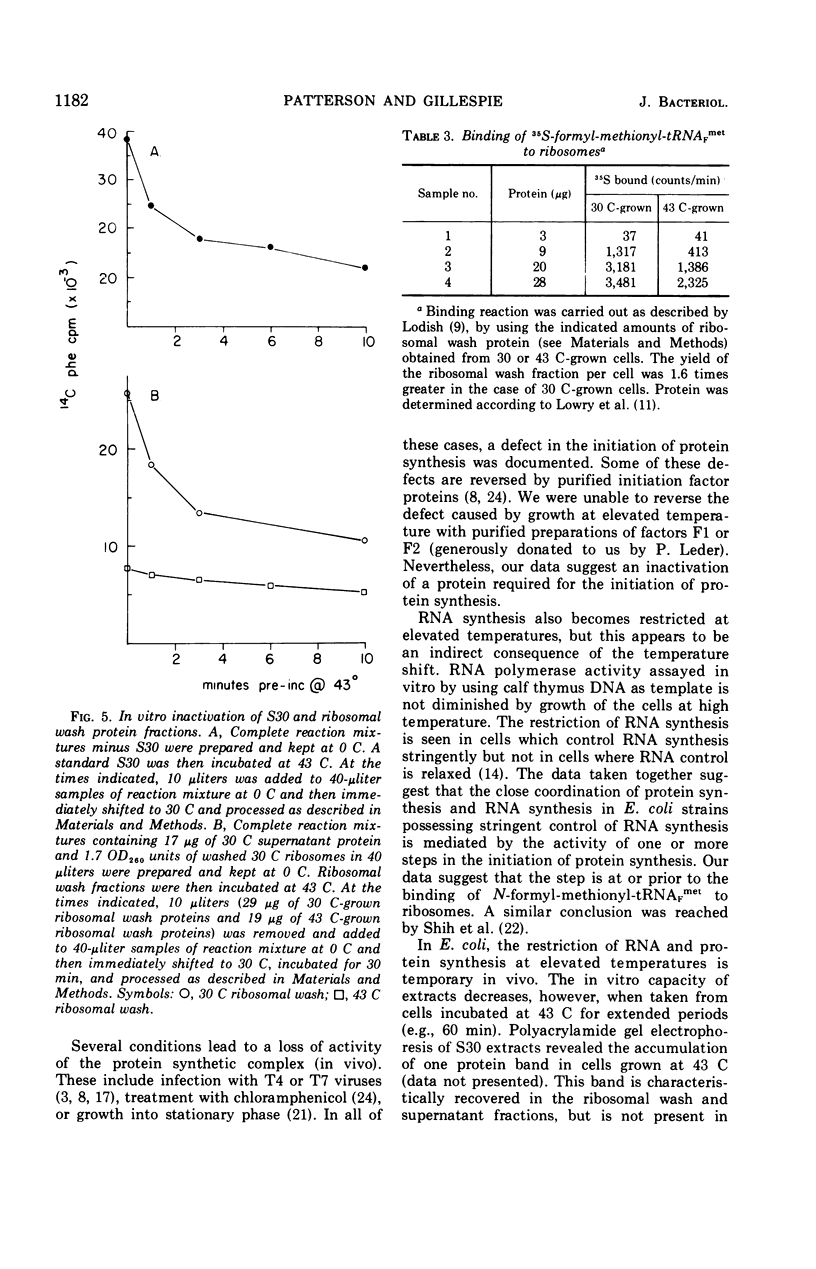
Upon a temperature shift from 30 C to 42 to 44 C, Escherichia coli experiences a transient slowdown in rate of optical density increase, uridine incorporation, and amino acid incorporation. The data show that the primary target for thermal inactivation is a molecule(s) required for the initiation of protein synthesis. The effect on ribonucleic acid synthesis is a secondary effect.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Boyd D., Nixon R., Gillespie S., Gillespie D. Screening of Escherichia coli temperature-sensitive mutants by pretreatment with glucose starvation. J Bacteriol. 1968 Mar;95(3):1040–1050. doi: 10.1128/jb.95.3.1040-1050.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube S. K., Rudland P. S. Control of translation by T4 phage: altered binding of disfavoured messengers. Nature. 1970 May 30;226(5248):820–823. doi: 10.1038/226820a0. [DOI] [PubMed] [Google Scholar]
- Gillespie S., Gillespie D. Ribonucleic acid-deoxyribonucleic acid hybridization in aqueous solutions and in solutions containing formamide. Biochem J. 1971 Nov;125(2):481–487. doi: 10.1042/bj1250481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson G. N., Sinsheimer R. L. Lysis of Escherichia coli with a neutral detergent. Biochim Biophys Acta. 1967 Dec 19;149(2):476–488. doi: 10.1016/0005-2787(67)90175-x. [DOI] [PubMed] [Google Scholar]
- Jacobson A., Gillespie D. Genetic complementation between Escherichia coli RNA polymerase mutants. Biochem Biophys Res Commun. 1971 Sep;44(5):1030–1040. doi: 10.1016/s0006-291x(71)80189-4. [DOI] [PubMed] [Google Scholar]
- Klem E. B., Hsu W. T., Weiss S. B. The selective inhibition of protein initiation by T4 phage-induced factors. Proc Natl Acad Sci U S A. 1970 Oct;67(2):696–701. doi: 10.1073/pnas.67.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lodish H. F. Bacteriophage f2 RNA: control of translation and gene order. Nature. 1968 Oct 26;220(5165):345–350. doi: 10.1038/220345a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Secondary structure of bacteriophage f2 ribonucleic acid and the initiation of in vitro protein biosynthesis. J Mol Biol. 1970 Jun 28;50(3):689–702. doi: 10.1016/0022-2836(70)90093-8. [DOI] [PubMed] [Google Scholar]
- McCormick W., Penman S. Regulation of protein synthesis in HeLa cells: translation at elevated temperatures. J Mol Biol. 1969 Jan;39(2):315–333. doi: 10.1016/0022-2836(69)90320-9. [DOI] [PubMed] [Google Scholar]
- Müller-Hill B., Crapo L., Gilbert W. Mutants that make more lac repressor. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1259–1264. doi: 10.1073/pnas.59.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson D., Gillespie D. Deductive analysis of a protein-synthesis mutant of Escherichia coli. Biochem Genet. 1973 Feb;8(2):205–230. doi: 10.1007/BF00485547. [DOI] [PubMed] [Google Scholar]
- Patterson D., Gillespie D. Stringent response of RNA synthesis in E. coli produced by a temperature shift-up. Biochem Biophys Res Commun. 1971 Oct 15;45(2):476–482. doi: 10.1016/0006-291x(71)90843-6. [DOI] [PubMed] [Google Scholar]
- Patterson D., Weinstein M., Marshall S., Gillespie D. A new RNA synthesis mutant of E. coli. Biochem Genet. 1971 Dec;5(6):563–578. doi: 10.1007/BF00485674. [DOI] [PubMed] [Google Scholar]
- Pollack Y., Groner Y., Aviv(Greenshpan) H., Revel M. Role of initiation factor B (F3) in the preferential translation of T4 late messenger RNA in T4 infected E. Coli. FEBS Lett. 1970 Aug 17;9(4):218–221. doi: 10.1016/0014-5793(70)80359-3. [DOI] [PubMed] [Google Scholar]
- Revel M., Herzberg M., Becarevic A., Gros F. Role of protein factor in the functional binding of ribosomes to natural messenger RNA. J Mol Biol. 1968 Apr 14;33(1):231–249. doi: 10.1016/0022-2836(68)90291-x. [DOI] [PubMed] [Google Scholar]
- Ron E. Z., Davis B. D. Growth rate of Escherichia coli at elevated temperatures: limitation by methionine. J Bacteriol. 1971 Aug;107(2):391–396. doi: 10.1128/jb.107.2.391-396.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron E. Z., Shani M. Growth rate of Escherichia coli at elevated temperatures: reversible inhibition of homoserine trans-succinylase. J Bacteriol. 1971 Aug;107(2):397–400. doi: 10.1128/jb.107.2.397-400.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheps R., Wax R., Revel M. Reactivation in vitro of inactive ribosomes from stationary phase Escherichia coli. Biochim Biophys Acta. 1971 Feb 25;232(1):140–150. doi: 10.1016/0005-2787(71)90498-9. [DOI] [PubMed] [Google Scholar]
- Shih A. Y., Eisenstadt J., Lengyel P. On the relation between ribonucleic acid synthesis and peptide chain initiation in E. coli. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1599–1605. doi: 10.1073/pnas.56.5.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. C. A simple method for extraction of RNA from E. coli utilizing diethyl pyrocarbonate. Anal Biochem. 1970 Feb;33(2):459–463. doi: 10.1016/0003-2697(70)90316-7. [DOI] [PubMed] [Google Scholar]
- Young R. M., Nakada D. Defective ribosomes in chloramphenicol-treated Escherichia coli. J Mol Biol. 1971 May 14;57(3):457–473. doi: 10.1016/0022-2836(71)90103-3. [DOI] [PubMed] [Google Scholar]


