Abstract
The tocopherol transfer protein (TTP1) is a member of the CRAL-TRIO family of lipid binding proteins that facilitates vitamin E transfer between membrane vesicles in vitro. In cultured hepatocytes, TTP enhances the secretion of tocopherol to the media; presumably, tocopherol transfer is at the basis of this biological activity. The mechanism underlying ligand transfer by TTP is presently unknown, and available tools for monitoring this activity suffer from complicated assay procedure and poor sensitivity. We report the characterization of a fluorescent vitamin E analog, R)-2,5,7,8-tetramethyl-chroman-2-[9-(7-nitro-benzo[1,2,5]oxadiazol-4-ylamino)-nonyl]-chroman-6-ol (NBD-TOH), as a sensitive and convenient probe for the ligand binding and transfer activities of TTP. Upon binding to TTP, NBD-TOH fluorescence is blue-shifted and its intensity is greatly enhanced. We used these properties to accurately determine the affinity of NBD-TOH to TTP. The analog binds to TTP reversibly and with high affinity (Kd=8.5 ± 6 nM). We determined the affinity of NBD-TOH to a TTP protein in which lysine 59 is replaced with a tryptophan. When occurring in humans, this heritable mutation causes the Ataxia with Vitamin E deficiency (AVED) disorder. We find that the affinity of NBD-TOH to this mutant TTP is greatly diminished (Kd = 71 ± 19 nM). NBD-TOH functioned as a sensitive fluorophore in fluorescent resonance energy transfer (FRET) experiments. Using the fluorescent lipids TRITC-DHPE or Marina Blue-DHPE as a donor or an acceptor for NBD-TOH fluorescence, we obtained high resolution kinetic data for tocopherol movement out of lipid bilayers, a key step in the TTP-facilitated ligand transfer reaction.
Keywords: affinity, fluorescence, kinetics, tocopherol, vitamin E, lipid transfer, TTP
Vitamin E is a lipophilic antioxidant, initially identified as a plant-derived factor that prevented fetal resorption in diet-restricted rats (1). The term “Vitamin E” refers to a class of structurally related molecules, all of which show potent radical trapping activity in vitro (2). The ability of vitamin E to protect cells against oxidative stress is thought to render it essential for membrane, protein, and DNA integrity. Vertebrates evolved efficient mechanisms for the selective retention of RRR- α-tocopherol (TOH) over other dietary tocols. This discrimination results from the combined effects of enzymatic degradation of other forms of the vitamin (3), and the high affinity of RRR-α-tocopherol to the hepatic tocopherol transfer protein (TTP; 4, 5). This 32 kDa protein is a member of the CRAL-TRIO family of proteins that bind small lipids such as phosphoinositides and retinoids (6). TTP was initially identified as a protein in liver cytosol that stimulates TOH transfer between membranes (7, 8). The transfer protein was subsequently shown to enhance TOH secretion into culture media when over-expressed in cultured hepatocytes (9-11). It is generally accepted that by virtue of its ability to facilitate the secretion of TOH from hepatocytes to circulating lipoproteins, TTP functions as a key regulator of vitamin E status.
The critical role of TTP in maintaining normal TOH levels is evident in patients with the syndrome Ataxia with Isolated Vitamin E Deficiency (AVED). This autosomal recessive disorder is characterized by low plasma levels of TOH, presumably giving rise to elevated oxidative stress that, in turn, lead to neurodegeneration (12). All known AVED patients bear mutations in the gene encoding TTP (13). The syndrome is thus thought to arise from structural defects in TTP that compromise its tocopherol transfer activity. Indeed, mice with targeted disruption of the TTP gene exhibit low plasma TOH levels, demonstrate signs of elevated oxidative stress, and eventually develop the neurological symptoms associated with AVED (14-17).
A number of mutations in TTP have been reported, most of which disrupt the primary structure of the protein (18-22). Six naturally occurring mutations that result in single amino acid substitutions in TTP are associated with the AVED pathology. Human carriers of the R59W, E141K, and R221W mutations develop an early-onset, severe form of the disease. Notably, mutations in homologous residues in other CRAL-TRIO proteins abolish their ligand transfer function (23, 24). In contrast, carriers of the three other missense mutations, occurring in non-conserved residues (H101Q, A120T, and R192H), develop a later-onset, milder form of the disease. We have shown previously that the latter class of mutations in TTP does not significantly impair the protein's ligand transfer activity, while those associated with the severe phenotype exhibit a ca. 3-fold impairment in the protein's in vitro transfer activity (25). Thus, the AVED mutants represent valuable tools for exploring the mechanism of TTP-facilitated tocopherol transfer in vitro, and secretion of the vitamin in vivo.
While facilitation of inter-membrane tocopherol transfer in vitro represents the “signature” activity of TTP, the molecular mechanisms underlying this activity are presently not clear. To date, thorough understanding of TTP's mechanism of action has been hampered by intrinsic limitations in the assays used to monitor the protein's transfer activity. Traditionally, the transfer of radioactively labeled TOH between two lipid vesicle populations was used to describe TTP activity (e.g. 5, 7, 8, 25, 26). This assay is limited by cumbersome physical manipulations, poor kinetic resolution, and low signal-to-noise. To overcome these experimental problems, we devised an assay that relies on the optical properties of a fluorescent TOH analog to monitor TTP activity. The availability of this novel experimental tool opens the door for detailed mechanistic studies of TTP action, and will greatly expand our understanding of this important regulatory protein.
EXPERIMENTAL PROCEDURES
Synthesis of NBD-TOH
Details of the synthesis and initial chemical characterization of this analog were recently described (11, 27).
Protein expression and purification
TTP proteins were expressed in E. coli BL21 cells and purified essentially as previously described (25). Due to the low yield of most AVED variants of TTP, GST-fused proteins eluted from the affinity column were dialyzed against storage buffer (20mM Tris, pH 8.0, 150mM NaCl, 50% glycerol (v/v)), and used without further processing. All proteins were >90% pure as judged by SDS-PAGE. To overcome poor solubility, expression of TTP(R59W) was induced for 18-21 hours at 8°C. The presence of the amino-terminal GST tag did not affect TTP activity (data not shown). Prior to experiments, protein preparations were dialyzed out of storage buffer and into Buffer A (20mM Tris, pH 8.0, 150mM NaCl) to remove glycerol.
Preparation of lipid vesicles for FRET experiments
Sonicated unilamellar vesicles were prepared from egg-yolk phosphatidylcholine, NBD-TOH, Marina Blue-DHPE (or TRITC-DHPE, Molecular Probes), and butylated hydroxytoluene (molar ratio 98.2: 0.8: 0.5: 0.5) by sonication in SET buffer (0.25 M sucrose, 1 mM EDTA, 50 mM Tris, pH 8.0).
Fluorescence determinations
Measurements were performed on a Spex Fluorolog or Photon Technologies International QuantumMaster-4 in SET buffer. Ligands were added from concentrated methanol stocks, kept in the dark and under Argon atmosphere. For competition experiments, competitor was added to pre-loaded TTP·NBD-TOH in detergent-containing buffer (250 mM Sucrose, 50 mM Tris, 1 mM EDTA, 100 mM KCl, 0.1 mM Triton-X100, pH 7.5). The presence of detergent was needed in these experiments to provide the ligand with a thermodynamically favorable place in which to move; an aqueous buffer is insufficient for this purpose. Detergent alone does not remove the fluorophore once bound to TTP (data not shown). Kinetic data were acquired by mixing samples in a Hi-Tech SFA-20 stopped flow apparatus. Data were collected at 0.2 nm and 0.2 second intervals with 1 nm spectral resolution. Concentrations of mutant TTP proteins were equalized based on their number of active binding sites as measured by fluorescent titrations.
Determination of FRET distancess
Quantum yields of fluorescence were determined using known fluorophores . Reference fluorophores used for NBD-TOH, Marina Blue-DHPE, and TRITC-DHPE were Fluorescein, Quinine sulfate, and, 5-Carboxytetramethylrhodamine (5-CTMR), respectively (Molecular Probes). Förster radii and fluorophore distances were determined using the FRET calculator in Felix32 software (PTI, see ref. 28).
RESULTS AND DISCUSSION
Spectral properties of C9-NBD-TOH
We synthesized a novel fluorescent tocopherol analog, (R)-2,5,7,8-tetramethyl-chroman-2-[9-(7-nitro-benzo[1,2,5]oxadiazol-4-ylamino)-nonyl]-chroman-6-ol (NBD-TOH; 11), using a similar strategy to that employed for photoaffinity-labeled tocopherols 29, 30). Figure 1 depicts the chemical structure of this analog and its spectral characteristics. In methanol, NBD-TOH maximally absorbs at 466 nm, with peak fluorescence at 533 nm. Figure 2 shows the excitation and emission spectra of NBD-TOH in buffer and when bound to TTP. Upon association with TTP, ligand fluorescence is enhanced by ca. 50 fold, with a similar increase in excitation intensity (note different scales used for data obtained with and without TTP). These changes probably reflect the change in fluorophore microenvironment, from the polar buffer to TTP's binding pocket that is lined with hydrophobic residues (6, 31). This environmental sensitivity is also illustrated by a significant ‘blue-shift’ of NBD-TOH's excitation (from 474 to 466nm) and emission (from 547 to 526nm) maxima upon binding to TTP.
Figure 1. Chemical and spectral characteristics of NBD-TOH.
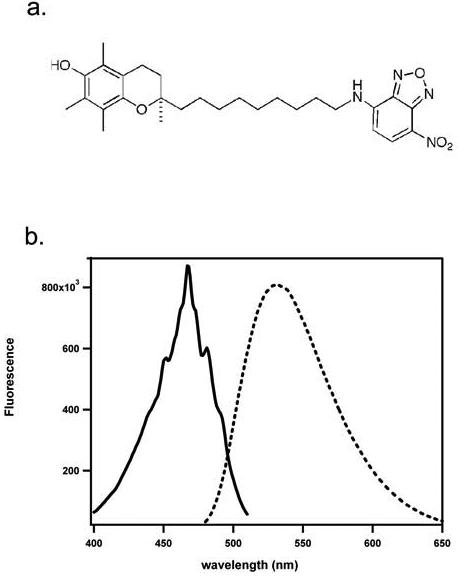
A) Structure of NBD-TOH. B) Excitation and emission spectra of NBD-TOH in methanol. Fluorescence excitation (solid line, emission monitored at 533 nm) and emission (dashed line, excitation at 466 nm) of 0.22 μM NBD-TOH in methanol were monitored. Readings were taken at 1nm and 1 second intervals. Spectral resolution = 1nm.
Figure 2. Spectral changes of NBD-TOH upon binding to TTP.
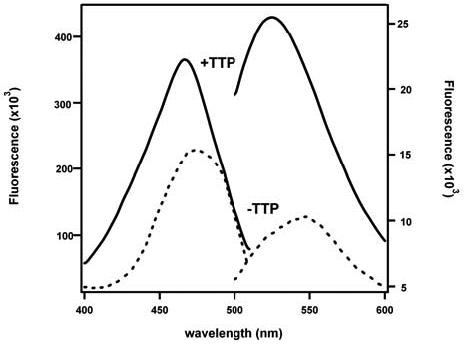
A) Fluorescence excitation and emission spectra of 0.22 μM NBD-TOH in Buffer A were recorded before (dotted line) or after (solid line) the addition of 5μM TTP. Note different scales for the free (right y-axis) and TTP-bound (left y-axis) fluorophores.
Affinity of NBD-TOH to TTP
We utilized the TTP-induced change in NBD-TOH fluorescence as a reporter for the occupancy state of the protein's binding pocket. The three-dimensional structure of TTP demonstrates that, in the absence of tocopherol, the protein is in the un-liganded (‘open’) conformation (6). It is therefore appropriate to treat recombinant TTP as a true apoprotein in ligand binding experiments. Titrations of TTP with NBD-TOH yielded incremental and saturable increases in NBD fluorescence, as shown in Figure 3. The fluorescence enhancement is specific to TTP, since it is not observed upon addition of NBD-TOH to buffer, or to a control protein (Figure 3A). The titration data fit well to a simple bimolecular association model (32), from which we extracted a reproducible value for the affinity of TTP for NBD-TOH (Kd = 8.5 ± 6.3 nM; n = 8). Interestingly, the observed affinity of TTP for NBD-TOH is higher than that previously reported for unmodified TOH utilizing chromatographic separation methods (ca. 30 nM; 4, 25). We observe that the addition of unlabeled tocopherol reverses the enhancement in NBD-TOH fluorescence, while another small neutral lipid, cholesterol, does not (Figure 3B). These observations indicate that upon binding to TTP, NBD-TOH occupies the protein's natural ligand binding pocket. Taken together, these data indicate that NBD-TOH closely mimics native tocopherol with regards to specific binding to TTP, and is therefore a suitable tool for probing TTP-ligand interactions.
Figure 3. Affinity and specificity of NBD-TOH to TTP.
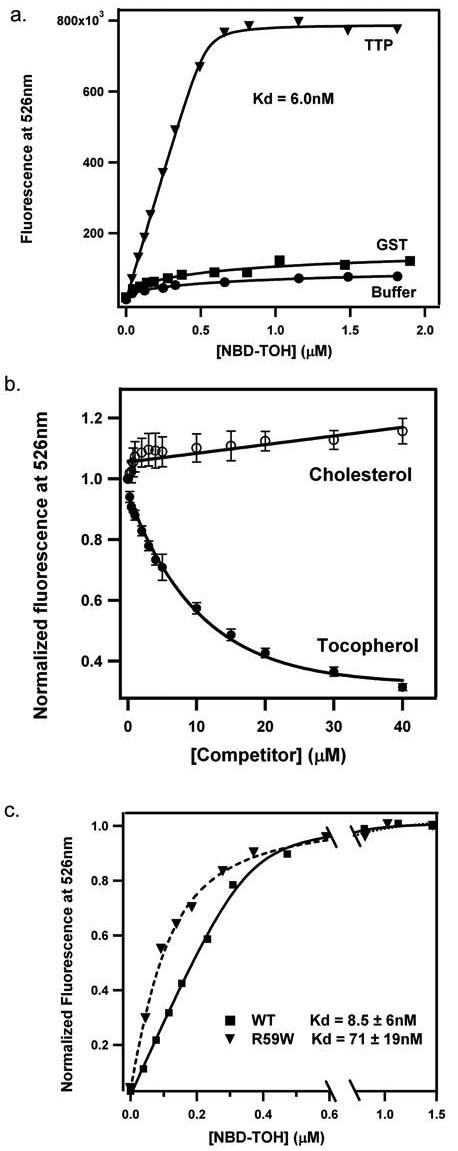
A) NBD-TOH was titrated into SET buffer lacking or containing 1 μM of the indicated protein. Fluorescence was excited at 466 nm, and intensity at 526 nm was recorded. Solid lines represent fit of the data to a simple association model. B) Increasing amounts of the indicated unlabeled competitor were added to pre-loaded TTP·NBD-TOH, and fluorescence at 526 nm was recorded. Analyses of NBD-TOH displacement revealed an apparent affinity of ca. 50 nM between TTP and native tocopheol. C) Titration data are shown for 1 μM wild-type or R59W TTP proteins. Average values and standard deviations were obtained from > 7 titrations, utilizing at least 3 independent protein preparations.
We employed NBD-TOH titrations to measure the affinity of a mutated TTP molecule, in which Arg59 is replaced with a tryptophan. This single amino acid substitution was found in several human patients that display low plasma tocopherol levels, and early-onset, severe form of the AVED disorder (18). Fluorescent titrations of the TTP(R59W) protein with NBD-TOH revealed a significantly weaker association (Kd = 71 ± 19 nM; n = 8) than that observed with the wild type protein. These observations confirm previous reports that demonstrated a ca. 5-fold lower affinity of this mutant to tocopherol, as compared to wild-type TTP (25). Moreover, these data illustrate the utility of NBD-TOH for the detection of subtle perturbations to TTP's ligand binding pocket.
NBD-TOH as a probe for the ligand transfer activity of TTP
The ‘signature’ activity of TTP, that was used to purify the native protein, is the facilitation of tocopherol transfer between lipid bilayers (33). Traditionally, this activity was measured by monitoring the protein-dependent movement of radioactively-labeled tocopherol from sonicated vesicles to mitochondria or microsomes (e.g. 5, 7, 26). To understand the molecular mechanisms that underlie TTP-mediated ligand transfer, we wished to ‘dissect’ this activity into its component reactions. Specifically, we are interested in monitoring the TTP-facilitated step of ligand dissociation from the bilayer. Toward this end, we incorporated NBD-TOH into sonicated vesicles that contain an additional fluorophore covalently attached to a lipid (Table 1). If the appropriate conditions for fluorescence energy transfer are met, fluorescence energy from the donor fluorophore will be transferred to the acceptor (28). We first investigated the ability of NBD-TOH (fluorescence maximum = 533 nm) to serve as an energy donor to the fluorescent lipid TRITC-DHPE (absorbance maximum = 540 nm). We observed that the fluorescence of NBD-TOH is greatly quenched in vesicles that contain TRITC-DHPE, compared to its fluorescence in vesicles lacking a fluorescent lipid (Figure 4A) indicating that energy transfer indeed occurs. Further demonstration of energy transfer from NBD to TRITC was obtained by monitoring the NBD-dependent increase in TRITC emission. Figure 4B shows emission spectra (excitation = 466 nm) obtained during the titration of TRITC-containing liposomes with NBD-TOH. Addition of the vitamin E analog to these liposomes enhanced energy transfer from NBD to TRITC, as evident from the dose-dependent increase in fluorescence at 575 nm. The addition of TTP to liposomes that contain both NBD-TOH and TRITC-DHPE abolishes the interaction between the fluorophores (Figure 4C). We observed that TTP induces an increase in NBD fluorescence (526 nm), accompanied by loss of TRITC emission (575 nm). Thus we were able to directly monitor the isolated step of the transfer reaction in which the vitamin dissociates from the bilayer.
Table I.
Spectral characteristics and fluorescence resonance energy transfer (FRET) parameters of probes used in this study
| ε (M−1 cm−1)a | maximal absorbance (nm) |
maximal fluorescence (nm) |
QYb | R0(Å)c | rDA(Å)d | Ee | |
|---|---|---|---|---|---|---|---|
| Marina Blue-DHPE | 18,000 | 365 | 464 | 0.269 | |||
| NBD-tocopherol | 17,602 | 466 | 533 | 0.093 | |||
| TRITC-DHPE | 93,000 | 540 | 575 | 0.454 | |||
| Marina Blue-DHPE • NBD-tocopherol | 35.2 | 30.5 | 0.701 | ||||
| NBD-tocopherol • TRITC-DHPE | 37.7 | 37.3 | 0.52 | ||||
Molar extinction coefficient in ethanol
Quantum yield of fluorescence
Förster distance
Distance between donor and acceptor
Efficiency of FRET
Figure 4. Fluorescence energy transfer from NBD-TOH to TRITC-DHPE.
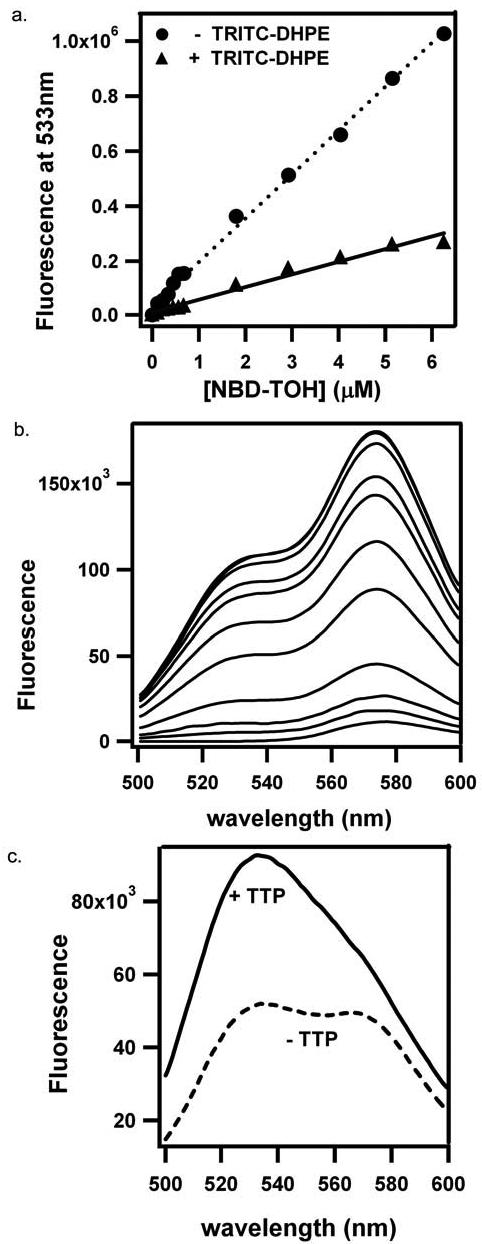
A) Fluorescence emission at 533 nm (excitation = 466 nm) was monitored upon titration with NBD-TOH to liposomes lacking or containing TRITC-DHPE in SET buffer. B) Fluorescence emission (excitation = 466 nm) was monitored upon titration of liposomes containing 100 μM phosphatidylcholine / 0.1 μM TRITC-DHPE with NBD-TOH in SET buffer. C) Fluorescence emission (excitation = 466 nm) of liposomes containing both TRITC-DHPE and NBD-TOH was monitored in SET buffer, before (dotted line) or after (solid line) the addition of 10 μM TTP.
In a similar manner, we used NBD-TOH as a FRET energy acceptor from a fluorescent lipid. When incorporated into liposomes, Marina Blue-DHPE maximally emits at 464 nm, close to the maximal excitation wavelength of NBD-TOH (466 nm). Titration of liposomes that contain Marina Blue-DHPE with NBD-TOH results in a dose-dependent decrease in Marina Blue fluorescence (Figure 5A). This demonstrates that NBD-TOH serves as a FRET acceptor that efficiently quenches Marina Blue fluorescence. Upon the addition of TTP to liposomes that contain both Marina Blue and NBD-TOH, the tocopherol analog is removed from the bilayer, thereby releasing FRET and restoring Marina Blue fluorescence, as shown in Figure 5B. To our knowledge, this is the first demonstration of TTP's activity in facilitating the dissociation step of the tocopherol transfer reaction. Table I summarizes the steady-state FRET parameters for both fluorophore pairs.
Figure 5. Energy transfer from Marina Blue-DHPE to NBD-TOH.
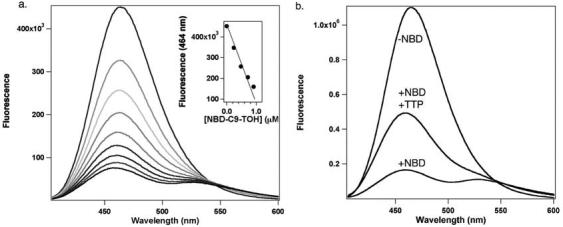
A) Quenching of Marina Blue fluorescence by NBD-TOH. Emission spectra (excitation = 367nm) were collected upon the addition of 0, 0.24, 0.48, 0.71, 0.95, 1.12, 1.42, 1.66, and 1.89 μM NBD-TOH (upper to lower spectra, respectively) to liposomes containing 100μM phosphatidylcholine / 0.1 μM Marina Blue-DHPE in SET buffer. Inset: Linearity of Marina Blue fluorescence quenching by NBD-TOH. Fluorescence intensity at 464nm was plotted as a function of NBD-TOH concentration; data were fit to a line function. B) Release of FRET upon binding of NBD-TOH by TTP. Fluorescence emission was monitored before (upper trace) and after (lower trace) the addition of 1.2 μM NBD-TOH. Following the addition of 5μM TTP, Marina-Blue fluorescence is restored (middle trace). Excitation: 367 nm; spectral resolution = ± 7 nm.
Kinetic resolution of TTP's ligand transfer activity
Fluorescence energy transfer between NBD-TOH and a fluorescent lipid provides a convenient tool for mechanistic studies of the ligand transfer reaction. The FRET assays described above can serve to monitor the ‘departure' of tocopherol from the bilayer into TTP's binding pocket. Figure 6A shows representative data from stopped-flow experiments in which the time-dependent change in FRET between NBD-TOH and TRITC-DHPE (lower panel) or Marina Blue-DHPE (upper panel) are monitored upon mixing the vesicles with TTP. We did not observe any change in fluorescence in the presence of buffer or a control protein, suggesting that the spontaneous movement of NBD-TOH out of the vesicles occurs at an exceedingly slow rate. The addition of TTP to these liposomes, however, results in a time-dependent decrease in FRET between NBD and either TRITC-DHPE or Marina Blue DHPE (ΔF > 25%). We attribute these fluorescence changes to the removal of tocopherol from the bilayer into the binding pocket of TTP. The kinetics of tocopherol dissociation from membranes are best fit by a biphasic model, composed of a single exponential process accompanied by a slower linear phase (Figure 6). We interpret the major, exponential component (70% of the fluorescence change) to reflect tocopherol removal from the bilayer. The minor, linear component of the fluorescence change probably reflects bleaching of the fluorophore that is TTP-bound. This suggests that NBD-TOH is more protected from photo-bleaching when in the lipid bilayer than when bound to TTP. Another possibility is that TTP-bound NBD-TOH partitions between the binding pocket and the aqueous buffer, where it is less fluorescent and more susceptible to light-induced degradation.
Figure 6. TTP-induced tocopherol release from membrane bilayers.
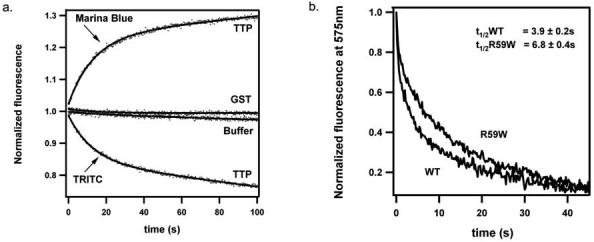
A) Time-dependent changes in FRET between NBD-TOH and TRITC-DHPE (lower curve) or Marina-Blue-DHPE (upper curve), upon mixing with TTP. Five μM TTP (or 30μM GST as a control protein) were mixed with sonicated vesicles containing 25μM phosphatydilcholine, 0.2μM NBD-TOH, and 0.125 μM fluorescent lipid (TRITC-DHPE or Marina-Blue-DHPE). FRET from Marina Blue-DHPE to NBD-TOH was followed by monitoring the recovery of Marina-Blue fluorescence (excitation: 365 nm; emission: 464 nm). FRET from NBD-TOH to TRITC-DHPE was followed by monitoring TRITC emission (575 nm) while exciting NBD-TOH (466 nm). Raw fluorescence values were normalized to the value of fluorescence change under each condition. Raw data (dots) were fitted to a function summing an exponential and a linear term (solid line).
B) Impaired tocopherol transfer activity of TTP(R59W). Liposomes containing 25 μM phosphatydilcholine, 0.125 μM TRITC-DHPE, and 0.2 μM NBD-TOH were mixed with 30 μM active TTP (wild-type or R59W), and the fluorescence changes recorded as described in panel A.
Notably, the observed rate of the tocopherol release reaction is identical in both reporter systems (9.4 ± 0.6 seconds for 50% release; n = 6). This observation further supports the notion that both systems report on the same rate-limiting step, i.e. the dissociation of NBD-tocopherol from the bilayer.
We have previously shown that several TTP variants associated with the AVED pathology are impaired in facilitating vesicle-to-vesicle tocopherol transfer in vitro (25). We asked whether we could detect analogous differences in the kinetics of the isolated partial reaction (tocopherol dissociation from the bilayer). Figure 6B shows that the addition of the wild type TTP to vesicles that contain both NBD-TOH and TRITC-DHPE results in tocopherol release (evidenced by a time-dependent decrease in TRITC emission) with a half-life of 3.9 ± 0.2 seconds (n = 9). The TTP(R59W) mutant stimulates tocopherol dissociation at a rate that is ca. 2-fold slower (t1/2 = 6.8 ± 0.4 seconds; n = 5). Apparently, the R59W mutant is defective in both its affinity for tocopherol (Figure 3C), and its ability to catalyze tocopherol removal from the membrane.
SUMMARY
Taken together, the results reported in this study demonstrate that NBD-TOH is a useful tool with which to assess ligand binding and transfer by TTP. The environmental sensitivity of this tocopherol analog enables the accurate determination of the affinity that governs ligand binding. The ability of NBD-TOH to function as a FRET partner with TRITC-DHPE or Marina Blue-DHPE enables real-time monitoring of the initial step in ligand transfer, namely dissociation of the ligand from the lipid bilayer. We utilized these properties of NBD-TOH to determine the affinity and catalytic capacity of a TTP variant that is associated with a severe form of the AVED disorder in humans, the R59W mutant. Using NBD-TOH as a molecular probe we found that TTP(R59W) is impaired both in its affinity for the ligand, and in its ability to catalyze removal of the ligand from lipid bilayers. In future studies we will utilize this reagent to determine the molecular mechanism by which TTP catalyzes the transfer reaction, and to characterize the biophysical parameters that contribute to efficient tocopherol release from membranes by the transfer protein.
TOC graphic
ACKNOWLEDGEMENTS
We are grateful to Noa Noy for expert help in the development and implementation of the fluorescence assays.
Footnotes
ABBREVIATIONS
NBD-TOH, (R)-2,5,7,8-tetramethyl-chroman-2-[9-(7-nitro-benzo[1,2,5]oxadiazol-4-ylamino)-nonyl]-chroman-6-ol; TTP, alpha tocopherol transfer protein; AVED, ataxia with vitamin E deficiency; TOH, RRR-α-tocopherol; GST, glutathione-S-transferase; IPTG, isopropyl-β-D-thiogalactopyranoside; DTT, dithiothreitol; EDTA, ethylenediaminetetraacetic acid, PMSF, phenylmethylsulfonyl fluoride; TRIS, tris(hydroxymethyl)aminomethane; FPLC, fast performance liquid chromatography; ABC, ATP-binding cassette transporter; Marina Blue-DHPE, Marina Blue 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine; TRITC-DHPE, N-(6-tetramethylrhodaminethiocarbamoyl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt; PC, phosphatidylcholine; IgG, immunoglobulin G.
REFERENCES
- 1.Evans HM, Bishop KS. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science. 1922;56:650–651. doi: 10.1126/science.56.1458.650. [DOI] [PubMed] [Google Scholar]
- 2.Burton GW, Cheeseman KH, Doba T, Ingold KU, Slater TF. Vitamin E as an antioxidant in vitro and in vivo. Ciba Found Symp. 1983;101:4–18. doi: 10.1002/9780470720820.ch2. [DOI] [PubMed] [Google Scholar]
- 3.Sontag TJ, Parker RS. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J Biol Chem. 2002;277:25290–6. doi: 10.1074/jbc.M201466200. [DOI] [PubMed] [Google Scholar]
- 4.Panagabko C, Morley S, Neely S, Lei H, Manor D, Atkinson J. Expression and refolding of recombinant human alpha-tocopherol transfer protein capable of specific alpha-tocopherol binding. Protein Expr Purif. 2002;24:395–403. doi: 10.1006/prep.2001.1576. [DOI] [PubMed] [Google Scholar]
- 5.Hosomi A, Arita M, Sato Y, Kiyose C, Ueda T, Igarashi O, Arai H, Inoue K. Affinity for alpha-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 1997;409:105–8. doi: 10.1016/s0014-5793(97)00499-7. [DOI] [PubMed] [Google Scholar]
- 6.Meier R, Tomizaki T, Schulze-Briese C, Baumann U, Stocker A. The molecular basis of vitamin E retention: structure of human alpha-tocopherol transfer protein. J Mol Biol. 2003;331:725–34. doi: 10.1016/s0022-2836(03)00724-1. [DOI] [PubMed] [Google Scholar]
- 7.Murphy DJ, Mavis RD. Membrane transfer of alpha-tocopherol. Influence of soluble alpha- tocopherol-binding factors from the liver, lung, heart, and brain of the rat. J Biol Chem. 1981;256:10464–8. [PubMed] [Google Scholar]
- 8.Verdon CP, Blumberg JB. An assay for the alpha-tocopherol binding protein mediated transfer of vitamin E between membranes. Anal Biochem. 1988;169:109–20. doi: 10.1016/0003-2697(88)90261-8. [DOI] [PubMed] [Google Scholar]
- 9.Arita M, Nomura K, Arai H, Inoue K. alpha-tocopherol transfer protein stimulates the secretion of alpha- tocopherol from a cultured liver cell line through a brefeldin A- insensitive pathway. Proc Natl Acad Sci U S A. 1997;94:12437–41. doi: 10.1073/pnas.94.23.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horiguchi M, Arita M, Kaempf-Rotzoll DE, Tsujimoto M, Inoue K, Arai H. pH-dependent translocation of alpha-tocopherol transfer protein (alpha-TTP) between hepatic cytosol and late endosomes. Genes Cells. 2003;8:789–800. doi: 10.1046/j.1365-2443.2003.00676.x. [DOI] [PubMed] [Google Scholar]
- 11.Qian J, Morley S, Wilson K, Nava P, Atkinson J, Manor D. Intracellular trafficking of vitamin E in hepatocytes: the role of tocopherol transfer protein. J Lipid Res. 2005;46:2072–82. doi: 10.1194/jlr.M500143-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Traber MG, Arai H. Molecular mechanisms of vitamin E transport. Annu Rev Nutr. 1999;19:343–55. doi: 10.1146/annurev.nutr.19.1.343. [DOI] [PubMed] [Google Scholar]
- 13.Ouahchi K, Arita M, Kayden H, Hentati F, Ben Hamida M, Sokol R, Arai H, Inoue K, Mandel JL, Koenig M. Ataxia with isolated vitamin E deficiency is caused by mutations in the alpha-tocopherol transfer protein. Nat Genet. 1995;9:141–5. doi: 10.1038/ng0295-141. [DOI] [PubMed] [Google Scholar]
- 14.Terasawa Y, Ladha Z, Leonard SW, Morrow JD, Newland D, Sanan D, Packer L, Traber MG, Farese RV., Jr. Increased atherosclerosis in hyperlipidemic mice deficient in alpha - tocopherol transfer protein and vitamin E. Proc Natl Acad Sci U S A. 2000;97:13830–4. doi: 10.1073/pnas.240462697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jishage K, Arita M, Igarashi K, Iwata T, Watanabe M, Ogawa M, Ueda O, Kamada N, Inoue K, Arai H, Suzuki H. Alpha-tocopherol transfer protein is important for the normal development of placental labyrinthine trophoblasts in mice. J Biol Chem. 2001;276:1669–72. doi: 10.1074/jbc.C000676200. [DOI] [PubMed] [Google Scholar]
- 16.Leonard SW, Terasawa Y, Farese RV, Jr., Traber MG. Incorporation of deuterated RRR- or all-rac-alpha-tocopherol in plasma and tissues of alpha-tocopherol transfer protein--null mice. Am J Clin Nutr. 2002;75:555–60. doi: 10.1093/ajcn/75.3.555. [DOI] [PubMed] [Google Scholar]
- 17.Yokota T, Igarashi K, Uchihara T, Jishage K, Tomita H, Inaba A, Li Y, Arita M, Suzuki H, Mizusawa H, Arai H. Delayed-onset ataxia in mice lacking alpha -tocopherol transfer protein: model for neuronal degeneration caused by chronic oxidative stress. Proc Natl Acad Sci U S A. 2001;98:15185–90. doi: 10.1073/pnas.261456098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavalier L, Ouahchi K, Kayden HJ, Di Donato S, Reutenauer L, Mandel JL, Koenig M. Ataxia with isolated vitamin E deficiency: heterogeneity of mutations and phenotypic variability in a large number of families. Am J Hum Genet. 1998;62:301–10. doi: 10.1086/301699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hentati A, Deng HX, Hung WY, Nayer M, Ahmed MS, He X, Tim R, Stumpf DA, Siddique T, Ahmed Human alpha-tocopherol transfer protein: gene structure and mutations in familial vitamin E deficiency. Ann Neurol. 1996;39:295–300. doi: 10.1002/ana.410390305. [DOI] [PubMed] [Google Scholar]
- 20.Yokota T, Shiojiri T, Gotoda T, Arai H. Retinitis pigmentosa and ataxia caused by a mutation in the gene for the alpha-tocopherol-transfer protein. N Engl J Med. 1996;335:1770–1. doi: 10.1056/NEJM199612053352315. [DOI] [PubMed] [Google Scholar]
- 21.Yokota T, Shiojiri T, Gotoda T, Arita M, Arai H, Ohga T, Kanda T, Suzuki J, Imai T, Matsumoto H, Harino S, Kiyosawa M, Mizusawa H, Inoue K. Friedreich-like ataxia with retinitis pigmentosa caused by the His101Gln mutation of the alpha-tocopherol transfer protein gene. Ann Neurol. 1997;41:826–32. doi: 10.1002/ana.410410621. [DOI] [PubMed] [Google Scholar]
- 22.Yokota T, Uchihara T, Kumagai J, Shiojiri T, Pang JJ, Arita M, Arai H, Hayashi M, Kiyosawa M, Okeda R, Mizusawa H. Postmortem study of ataxia with retinitis pigmentosa by mutation of the alpha-tocopherol transfer protein gene. J Neurol Neurosurg Psychiatry. 2000;68:521–5. doi: 10.1136/jnnp.68.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golovleva I, Bhattacharya S, Wu Z, Shaw N, Yang Y, Andrabi K, West KA, Burstedt MS, Forsman K, Holmgren G, Sandgren O, Noy N, Qin J, Crabb JW. Disease-causing mutations in the cellular retinaldehyde binding protein tighten and abolish ligand interactions. J. Biol. Chem. 2003;278:12397–12402. doi: 10.1074/jbc.M207300200. [DOI] [PubMed] [Google Scholar]
- 24.Phillips SE, Sha B, Topalof L, Xie Z, Alb JG, Klenchin VA, Swigart P, Cockcroft S, Martin TF, Luo M, Bankaitis VA. Yeast Sec14p deficient in phosphatidylinositol transfer activity is functional in vivo. Mol Cell. 1999;4:187–97. doi: 10.1016/s1097-2765(00)80366-4. [DOI] [PubMed] [Google Scholar]
- 25.Morley S, Panagabko C, Shineman D, Mani B, Stocker A, Atkinson J, Manor D. Molecular determinants of heritable vitamin E deficiency. Biochemistry. 2004;43:4143–9. doi: 10.1021/bi0363073. [DOI] [PubMed] [Google Scholar]
- 26.Mowri H, Nakagawa Y, Inoue K, Nojima S. Enhancement of the transfer of alpha-tocopherol between liposomes and mitochondria by rat-liver protein(s) Eur J Biochem. 1981;117:537–42. doi: 10.1111/j.1432-1033.1981.tb06370.x. [DOI] [PubMed] [Google Scholar]
- 27.Nava P, Cecchini M, Chirico S, Gordon H, Morley S, Manor D, Atkinson J. Preparation Of Fluorescent Tocopherols For Use In Protein Binding and Localization With The a-Tocopherol Transfer Protein (a-TTP) 2005 doi: 10.1016/j.bmc.2006.01.053. submitted for publication. [DOI] [PubMed] [Google Scholar]
- 28.Lackowicz JR. Principles of Fluorescence Spectroscopy. Plenum Press; New York: 1983. [Google Scholar]
- 29.Lei H, Marks V, Pasquale T, Atkinson JK. Synthesis of photoaffinity label analogues of alpha-tocopherol. Bioorg Med Chem Lett. 1998;8:3453–8. doi: 10.1016/s0960-894x(98)00655-6. [DOI] [PubMed] [Google Scholar]
- 30.Lei H, Atkinson J. Synthesis of phytyl- and chroman-derivatized photoaffinity labels based on alpha-tocopherol. J Org Chem. 2000;65:2560–7. doi: 10.1021/jo000029l. [DOI] [PubMed] [Google Scholar]
- 31.Min KC, Kovall RA, Hendrickson WA. Crystal structure of human alpha-tocopherol transfer protein bound to its ligand: implications for ataxia with vitamin E deficiency. Proc Natl Acad Sci U S A. 2003;100:14713–8. doi: 10.1073/pnas.2136684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manor D. Measurement of GTPase.effector affinities. Methods Enzymol. 2000;325:139–49. doi: 10.1016/s0076-6879(00)25438-4. [DOI] [PubMed] [Google Scholar]
- 33.Sato Y, Hagiwara K, Arai H, Inoue K. Purification and characterization of the alpha-tocopherol transfer protein from rat liver. FEBS Lett. 1991;288:41–5. doi: 10.1016/0014-5793(91)80999-j. [DOI] [PubMed] [Google Scholar]


