Abstract
We analyzed the ability of interferon (IFN)-γ knockout mice (GKO) to reject a colon carcinoma transduced with interleukin (IL)-12 genes (C26/IL-12). Although the absence of IFN-γ impaired the early response and reduced the time to tumor onset in GKO mice, the overall tumor take rate was similar to that of BALB/c mice. In GKO mice, C26/IL-12 tumors had a reduced number of infiltrating leukocytes, especially CD8 and natural killer cells. Analysis of the tumor site, draining nodes, and spleens of GKO mice revealed reduced expression of IFN- inducible protein 10 and monokine induced by γ-IFN. Despite these defects, GKO mice that rejected C26/IL-12 tumor, and mice that were primed in vivo with irradiated C26/IL-12 cells, showed the same cytotoxic T lymphocyte activity but higher production of granulocyte/macrophage colony–stimulating factor (GM-CSF) as compared with control BALB/c mice. Treatment with monoclonal antibodies against GM-CSF abrogated tumor regression in GKO but not in BALB/c mice. CD4 T lymphocytes, which proved unnecessary or suppressive during rejection of C26/IL-12 cells in BALB/c mice, were required for tumor rejection in GKO mice. CD4 T cell depletion was coupled with a decline in GM-CSF expression by lymphocytes infiltrating the tumors or in the draining nodes, and with the reduction and disappearance of granulocytes and CD8 T cells, respectively, in tumor nodules. These results suggest that GM-CSF can substitute for IFN-γ in maintaining the CD8–polymorphonuclear leukocyte cross-talk that is a hallmark of tumor rejection.
Keywords: interleukin 12, tumor immunity, knockout mice, interferon γ, CD4 T lymphocytes
Interleukin (IL)-12 is a heterodimeric cytokine produced by APCs, phagocytes, and granulocytes (1). Despite some in vitro direct effects of IL-12 on T and NK cells, where it acts as a growth factor, an enhancer of cytotoxicity, and activator of other cytokines, IL-12 activity in vivo is generally mediated by IFN-γ (1). IL-12 has been extensively tested for treatment of experimental tumors, and with few exceptions was found to cure or improve the survival of mice bearing a variety of tumors (2–4). The antitumor activity of IL-12 is largely mediated by the IFN-γ released at the tumor site through stimulation of macrophages, along with release of other cytokines such as TNF-α, upregulation of MHC expression on tumor cells, induction of IFN-inducible protein 10 (IP-10)1 by both tumor cells (4) and infiltrating T cells, and inhibition of angiogenesis (5, 6). Exogenous, systemic injection of IFN-γ cannot substitute for IL-12 in mediating responses of such magnitude (7), because in addition to a difference between the two cytokines in half-life, the IFN-γ receptor is ubiquitously expressed, whereas IL-12 receptors are expressed only on NK cells and activated lymphocytes (8). Accordingly, IL-12 exacerbates some autoimmune diseases characterized by local accumulation and activation of T cells (9).
rIL-12 induces elevated expression of IFN-γ in the absence of T cells but is ineffective in curing tumors injected into nude mice (7). Thus, IL-12–stimulated T cell functions or factors other than IFN-γ are needed for antitumor activity. Nonobese diabetic mice with a disrupted IFN-γ gene still develop diabetes, although the onset is delayed (10), and IL-12 appears to exert an effect that favors disease progression.
To investigate the antitumor activity of IL-12 in the absence of host-produced IFN-γ, IFN-γ knockout (GKO) mice were injected with C26 colon adenocarcinoma cells transduced or not with IL-12 genes. This tumor is virtually resistant to systemically given rIL-12 (11), but is rejected upon IL-12 gene transduction (12). Our results point to the critical role of CD4 T cells and GM-CSF for the IL-12–mediated tumor rejection in the absence of host-produced IFN-γ.
Materials and Methods
Tumors and Mice.
Colon adenocarcinoma cell line C26 was derived from BALB/c mice treated with N-nitroso-N-methylurethane (13). C26 cells were transduced with a retroviral vector bearing IL-12 genes as previously described (12) to obtain C26/ IL-12 cells. IL-12 concentration was determined by two-site sandwich ELISA using mAb 9A5 against p70 and the peroxidase-conjugated mAb 5C3-POD against p40 (provided by Dr. Luciano Adorini, Roche Milano Ricerche, Milano, Italy). Tumor cells were cultured in DMEM (GIBCO, Paisley, UK) supplemented with 5% FCS (GIBCO).
BALB/cnAnCr mice (Charles River, Calco, Italy) were maintained at the Istituto Nazionale Tumori under standard conditions according to Institutional guidelines. GKO mice (14) on a BALB/c background (BALB/c-Ifg<tm1 129>) were purchased from The Jackson Laboratory (Bar Harbor, ME). Homozygous mice were identified by PCR of tail-derived DNA using primers flanking the neo gene (direct: 5′-CAAGTGGCATAGATGTGGAAG-3′ and reverse: 5′-GGCAATACTCATGAATGCATCC-3′); the wild-type gene gave an amplified fragment of 340 bp, whereas the disrupted gene gave a 2,400-bp fragment. Homozygous mice were bred and maintained in isolators at the Charles River Animal Facility.
Tumorigenic activity of control and transduced C26 cells was assayed in mice injected subcutaneously in the left flank with 5 × 104 or 5 × 105 cells in 0.2 ml. Some mice were injected intraperitoneally over 2 d with 1 μg of rIL-12 (provided by Dr. Maurice Gately, Hoffmann-La Roche, Nutley, NJ) diluted in HBSS containing 100 μg/ml of mouse serum albumin. Control animals received HBSS only. Some mice were injected intraperitoneally weekly with 0.2 ml of HBSS containing 300 μg of anti-CD4 (GK1.5 hybridoma, L3T4) or anti-CD8 (53.6.72 hybridoma, Lyt2) mAbs obtained from American Type Culture Collection (ATCC, Rockville, MD). To neutralize the effect of host-produced GM-CSF, some mice were injected intraperitoneally, twice per wk, with 0.2 ml of HBSS containing 400 μg of a mixture of two rat anti–mouse GM-CSF mAbs (clones 22E9 and 31G6) obtained from ATCC with permission from Dr. J.A. Abrams (DNAX Research Institute, Palo Alto, CA).
Morphological Analysis and Immunocytochemistry.
Tumor fragments, tumor draining lymph nodes, and spleens were embedded in OCT compound (Miles Laboratories, Inc., Elkhart, IN), snap-frozen in liquid nitrogen, and stored at −80°C. Immunochemical analysis using the peroxidase-antiperoxidase (PAP) method was performed as previously described (15). In brief, 5-μm cryostat sections were fixed in acetone and immunostained with rat anti– mouse mAb against CD45 (M1/9.3.4.HL2 hybridoma, T200), CD8 (53.6.72 hybridoma, Lyt2), CD4 (GK1.5 hybridoma, L3T4), and Mac-3 (M37/84,6,34 hybridoma), all from ATCC; GR-1 (RB6-8C5 hybridoma), CD31/PECAM-1 (Mec 13.3 hybridoma), CD51/αv integrin (clone H9.2B8), and CD61/β3 integrin (clone 2C9.G2), all from PharMingen (San Diego, CA); and CD34 (clone 14.7 MEC), from Hycult Biotechnology, B.U., Uden, The Netherlands). Sections were preincubated with rabbit serum and sequentially incubated with optimal dilutions of primary antibodies, rabbit anti–rat IgG (Zymed Laboratories, Inc., San Francisco, CA) and rat PAP (Abbot Laboratories, North Chicago, IL). For immunostaining of NK cells, a rabbit anti-asialo GM1 serum (Wako, Osaka, Japan) was used in combination with a goat anti–rabbit and goat PAP (Sigma Chemical Co., St. Louis, MO). Each incubation step lasted 30 min and was followed by a 10-min wash in TBS. Sections were then incubated with 0.03% H2O2 and 0.06% 3,3′-diaminobenzidine (BDH Chemicals, Poole, England) for 2–5 min, washed in tap water, and counterstained with hematoxylin. The number of immunostained cells was determined by light microscopy at magnification ×400 in 5 fields on a 1-mm2 grid and is given as cells/mm2 (mean ± SD).
Reverse Transcriptase PCR.
Total cellular RNA was extracted by cesium chloride gradient, and first-strand cDNA was synthesized from 1 μg of RNA using Moloney murine leukemia virus reverse transcriptase (GIBCO BRL, Gaithersburg, MD) for 2 h at 42°C. A fraction of the cDNA was amplified by PCR with Taq DNA polymerase (Promega Corporation, Madison, WI) using primers specific for β-actin (Clontech Labs., Inc., Palo Alto, CA), monokine induced by γ-IFN (MIG); (direct, 5′-TCCGCTGTTCTTTTCCTTTTGG-3′; reverse, 5′-TTGAACGACGACGACTTTGGGG-3′), IP-10 (direct, 5′-GCGTTAACCTCCCCATCAGCACCATGAAC-3′; reverse, 5′-CCGCTCGAGGTGGCTTCTCTCCAGTTAAGGA-3′), and IFN-γ (direct, 5′-CCGAATTCTGAGACAATGAACGCTACAC-3′; reverse, 5′-GCTCGAGAATCAGCAGCGACTCCTTTTCCG-3′). PCR was carried out in a 50-μl vol (1 μM primers, 1.25 U Taq DNA polymerase, 1 mM MgCl2) for 25, 30, and 35 cycles (1 min denaturation at 94°C, 1.5 min annealing at 60°C, 2 min extension at 72°C) using a thermal cycler (Perkin-Elmer, Norwalk, CT). One-third of the PCR volume was then analyzed on a 1% agarose gel.
In Situ Hybridization.
The presence of cytokine mRNA was investigated by in situ hybridization using cDNA probes as previously described (15). MIG (361 bp), IP-10 (344 bp), and GM-CSF (368 bp) probes were prepared by PCR amplification of murine blast cDNA, using specific primers for MIG and IP-10 (see above) or primers for GM-CSF (Clontech Labs., Inc.). The products obtained were run on a 1% agarose gel and purified using the Qiaex II gel extraction kit (Qiagen, Hilden, Germany).
Cryostat sections were harvested on RNA-grade slides, air-dried, and fixed in 4% buffered paraformaldehyde for 10 min, and then were dehydrated in ethanol, sequentially rehydrated in PBS/ 50 mM MgCl2, washed in 200 mM Tris-HCl-glycine, acetylated in 2× SSC, 0.1 M triethanolamine, and 0.5% acetic anhydride (pH 8.0), washed in 2× SSC, and finally dehydrated in ethanol. Slides were then prehybridized for 10 min at 70°C with 2× SSC, 50% formamide, and 500 μg/ml salmon sperm DNA, and hybridized overnight at 42°C with 32P-labeled–specific cDNA probes (0.5 × 106 cpm/section), 2× SSC, 500 μg/ml yeast RNA, 5× Denhardt's solution, 10 mM dithiothreitol, and 10% dextran sulfate. Unbound and nonspecifically bound probes were removed by sequential washes in 2× SSC, 50% formamide, and 1× SSC, 50% formamide at 45°C, and in 0.1× SSC at room temperature. Slides were then dehydrated in ethanol, dipped in autoradiographic emulsion NTB-2 (Eastman Kodak Co., Rochester, NY), and exposed for 24–72 h at 4°C in a light-tight box, then developed in D19 (Eastman Kodak Co.), fixed in Rapid Fixer (Eastman Kodak Co.), and counterstained or not with hematoxylin. RNA specific binding was controlled by previous digestion with 100 μg/ml ribonuclease A and 10 U/ml ribonuclease T (Sigma Chemical Co., Poole, England). PstI-digested pUC9 plasmid fragments were used as negative controls. Cytosmears of LPS + IFN-γ–stimulated or PHA + ionomycin-stimulated splenocytes were used as positive controls in each experiment.
Cytokine Production.
In vitro cytokine production by total or purified T cells isolated from lymph nodes draining the site of tumor injection was induced by culturing lymphocytes (2 × 105/ well) in 96-well flat-bottomed plates precoated or not with 10 μg/ml of anti-CD3 mAb (145-2C11 hybridoma) at 37°C for 18 h. Supernatants were recovered and assayed for IFN-γ, GM-CSF, TNF-α, IL-4, and IL-10 production by specific ELISA (all from PharMingen). Cytokine levels were calculated using standard curves constructed using recombinant murine cytokines.
CD8 and CD4 purified lymphocytes were obtained by magnetic cell sorting. In brief, lymphocytes derived from draining lymph nodes were labeled with rat anti–mouse CD4 (L3T4)- or CD8 (Ly-2)–conjugated paramagnetic microbeads (MiniMacs; Miltenyi Biotec., Bergisch Gladbach, Germany) and separated by a magnetic field using a positive selection column.
Additional experiments were performed with the same splenocytes used for NK cell assay (see below). In those cases, splenocytes from mice treated with rIL-12 or untreated were cultured in vitro for an additional 18 h in the presence or absence of rIL-12 (10 ng/ml) before CD3 stimulation.
NK Cell Assay.
To measure NK cell activity in response to IL-12, BALB/c and GKO mice were injected intraperitoneally with rIL-12 (1 μg/mouse) or HBSS for 2 d, and at day 3 fresh splenocytes were tested for cytotoxic activity against 51Cr-labeled YAC cells in the presence of 500 U/ml of human rIL-2 (Chiron-Italia, Milan, Italy).
Mixed Lymphocyte Tumor Culture and Cell-mediated Cytotoxicity Assays.
Mixed lymphocyte tumor culture was performed in RPMI 1640 medium (BioWhittaker, Walkersville, MD) supplemented with 10% FCS (Hyclone, Logan, UT). Responder lymph node or spleen cells were stimulated with γ-irradiated (15,000 rad) C26 cells. Responders and stimulators were suspended to 2.5 × 105 and 2.5 × 104 cells/ml, respectively, and mixed in a total volume of 2 ml in 24-well plates (Costar, Cambridge, MA). Cultures were incubated in a humidified atmosphere of 5% CO2 in air. In cell-mediated cytotoxicity (CMC) assays, C26 cells were the specific target, YAC-1 cells were used as controls for NK cell–mediated lysis, and F1 spontaneously transformed fibroblasts (BALB/c) were the negative controls for C26 tumor-specific lysis. In some experiments, additional targets were syngeneic Con A–induced blast cells pulsed or not with the AH1 peptide, the immunodominant epitope of the C26-associated gp70 antigen (16). Tumor specific lysis was measured as previously described (11).
In Vitro Macrophage NO2 − Production.
Thioglycolate-elicited macrophages were washed from the peritoneal cavity and resuspended in complete medium. Adherent macrophage monolayers were obtained by plating cells in 24-well plates at 2 × 106 cells/well for 2 h at 37°C in 5% CO2. Nonadherent cells were removed and freshly prepared complete medium was added with the indicated experimental reagents (1 μg/ml LPS, 200 U/ml IFN-γ). Nitrite concentration in the medium was measured by a microplate assay method (17). In brief, 100-μl aliquots were removed from conditioned medium and incubated with an equal volume of Griess reagent (1% sulfanilamide, 0.1% naphthylethylene diamine dihydrochloride, 2.5% H3PO4) at room temperature for 10 min. Absorbance at 550 nm was determined in a microplate reader. NO2 − concentration was determined using sodium nitrite as a standard.
Results
Accelerated Tumor Take and Reduced Tumor Regression in GKO Mice Bearing the IL-12–producing C26 Carcinoma.
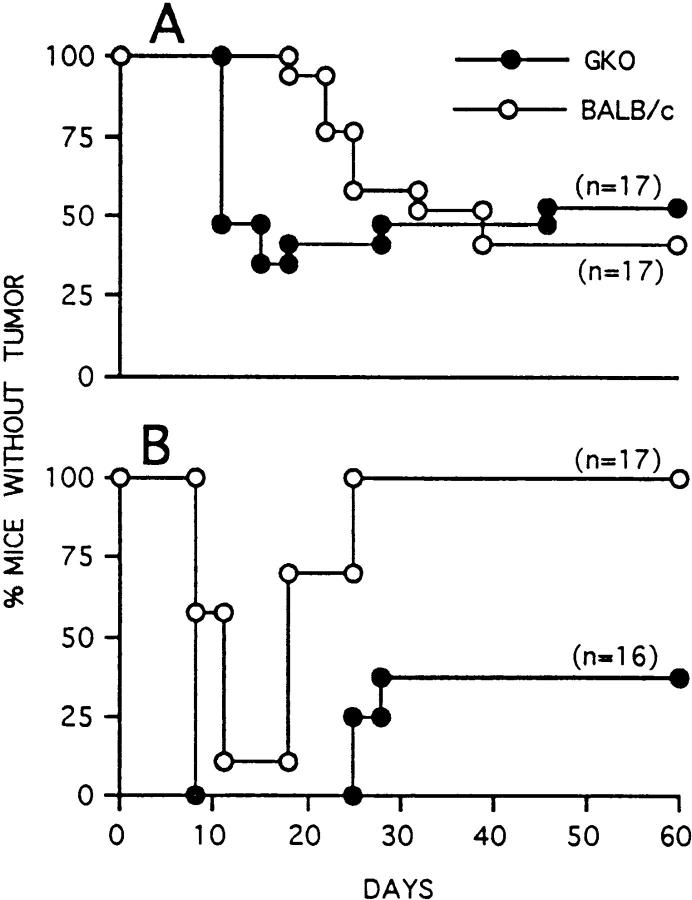
We previously reported that C26 cells transduced with IL-12 genes (C26/IL-12) showed reduced tumor take (<50%) and delayed onset in BALB/c mice injected at a dose of 5 × 104 cells (the LD100 for nontransduced C26), whereas a dose of 5 × 105 cells resulted in initial tumor take followed by regression in 80–100% of injected mice (12). In GKO mice injected with 5 × 104 cells, C26/IL-12 tumor onset was accelerated but tumors developed in only 40% of the mice (Fig. 1 A). At a dose of 5 × 105, C26/IL-12 cells formed tumors in all GKO mice with onset similar to that in BALB/c mice, but only a few GKO mice were able to reject the initial tumor (Fig. 1 B). An enhanced rate of C26/IL-12 tumor take was also observed in BALB/c mice treated with neutralizing mAb to IFN-γ (data not shown). These results indicate that the antitumor activity initiated by IL-12 released at the tumor site is only partially dependent on host-produced IFN-γ, although IFN-γ appears to be important in the early response to the tumor.
Figure 1.
Tumorigenicity and latency of C26/IL-12 cells injected into BALB/c (open circles) and GKO mice (closed circles) at doses of 5 × 104 (A) and 5 × 105 (B).
Unimpaired Memory and CTL Responses and Increased GM-CSF Production in GKO Mice.
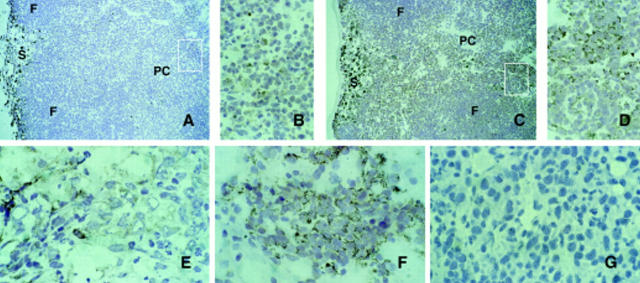
BALB/c mice that did not develop tumors were susceptible to subsequent challenge with parental C26 cells, whereas mice that remained tumor-free after rejecting incipient C26/IL-12 tumors were resistant to challenge (Table 1). These results are consistent with our previous suggestion that debulking of an incipient tumor allows a prolonged exposure of tumor-associated antigens to host T cells, whereas an immune response rapid enough to inhibit initial tumor take might avoid T cell activation (18). Indeed, all GKO mice that lacked the early response but still rejected C26/IL-12 cells were resistant to C26 challenge (Table 1). Furthermore, splenocytes from resistant GKO mice were cytotoxic against C26 but not against F1 transformed fibroblasts (Table 1). We previously showed that the TCRVβ repertoire of CTL recognizing C26 is restricted mainly to Vβ6 (19); immunohistochemical analysis of the site of tumor challenge revealed positive staining for both CD8 and Vβ6 in most infiltrating lymphocytes (Fig. 2). Together, these results suggest that an IL-12–producing tumor can be rejected despite the lack of IFN-γ through a mechanism that clearly involves T cell activation since memory T cells and CTLs were detected both in vivo and in vitro.
Table 1.
Tumorigenicity of C26/IL-12 and Induction of both Memory Response and CMC to Parental C26 Cells in BALB/c and GKO Mice
| Recipients | No. of cells injected | Primary response | Secondary response* | CMC on targets‡ (% lysis of target) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C26 | F1 | |||||||||||||
| BALB/c | 5 × 104 | 10/17§ | 6/6§ | E/T | ||||||||||
| 5 × 105 | 0/17 | 1/9 | 50 | 40.7 | 12.6 | |||||||||
| 25 | 36.9 | 7.5 | ||||||||||||
| 12 | 22.4 | 5.1 | ||||||||||||
| GKO | 5 × 104 | 8/17 | 0/7 | E/T | ||||||||||
| 5 × 105 | 11/16 | 0/2 | 50 | 76.3 | 15.3 | |||||||||
| 25 | 58.8 | 10.1 | ||||||||||||
| 12 | 40.3 | 6.3 | ||||||||||||
Mice that were tumor-free at day 60 were challenged subcutaneously with 105 C26 cells.
Tumor-free mice, which were not challenged, were killed and their splenocytes were tested for CMC activity in vitro. Splenocytes were restimulated in vitro with irradiated C26 cells for 5 d before CMC assay.
No. of mice with tumor/no. of mice injected.
Figure 2.
Immunostaining of lymphocytes infiltrating the site of C26 challenge. Tumor sections from BALB/c (A) and GKO mice (B and C) that had already rejected C26/IL-12 cells were immunostained for TCR Vβ6 (A and B) and for CD8 T cells (C). The distribution of TCR Vβ6+ around a neural structure (B) is similar to that of CD8+ lymphocytes (C). Frozen sections were immunostained using the PAP method and counterstained with hematoxylin. Original magnification: ×300.
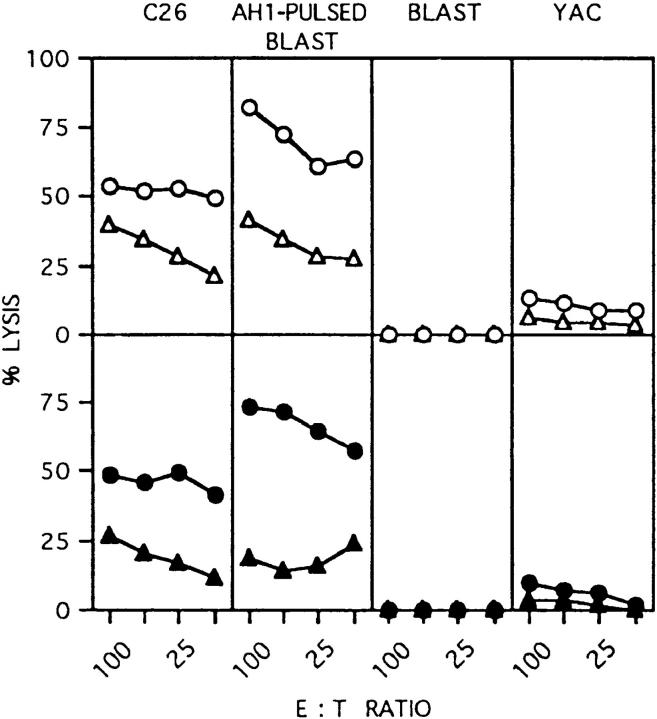
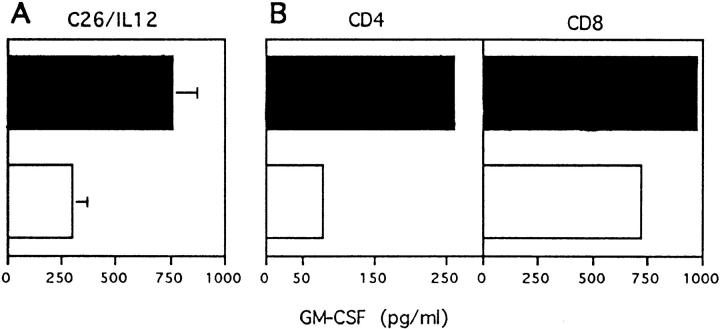
We compared C26 and C26/IL-12 cells for CTL induction in BALB/c and GKO mice injected in the footpad with irradiated cells followed by restimulation of lymphocytes from the draining popliteal nodes with C26 cells in vitro. C26/IL-12 primed mice of both strains for CTL induction with similar efficacy, whereas C26 cells induced a slightly reduced CTL activity in GKO mice (Fig. 3). Analysis of cytokine release by primed T cells using the same lymphocytes stimulated with mAb to CD3 coated on plastic revealed threefold higher levels of GM-CSF produced by lymphocytes from GKO mice than by lymphocytes from BALB/c mice primed with C26/IL-12 (Fig. 4 A). Analysis of CD4 and CD8 T lymphocytes sorted with magnetic beads before CD3 stimulation showed that CD8 T cells produced the higher absolute amount of GM-CSF, whereas the relative contribution of CD4 lymphocytes to the difference in GM-CSF production between BALB/c and GKO mice was greater (Fig. 4 B). IL-4 and IL-10 were undetectable, whereas TNF-α levels were unchanged in both BALB/c and GKO lymphocytes (data not shown).
Figure 3.
CTL activity after in vivo priming of BALB/c (open symbols) and GKO mice (closed symbols) with irradiated C26 (triangle) or C26/IL-12 cells (circle). Target cells are indicated on top of the figure.
Figure 4.
CD3-stimulated GM-CSF production by BALB/c (white bars) or GKO (black bars) lymphocytes from popliteal lymph nodes draining the site of C26/IL-12 injection (A). Mean ± SD of three independent experiments. Release of GM-CSF upon CD3 stimulation by purified CD4 and CD8 lymphocytes (B).
NK Cytotoxicity and Macrophage NO2 − Production in Response to IL-12 Stimulation in GKO Mice.
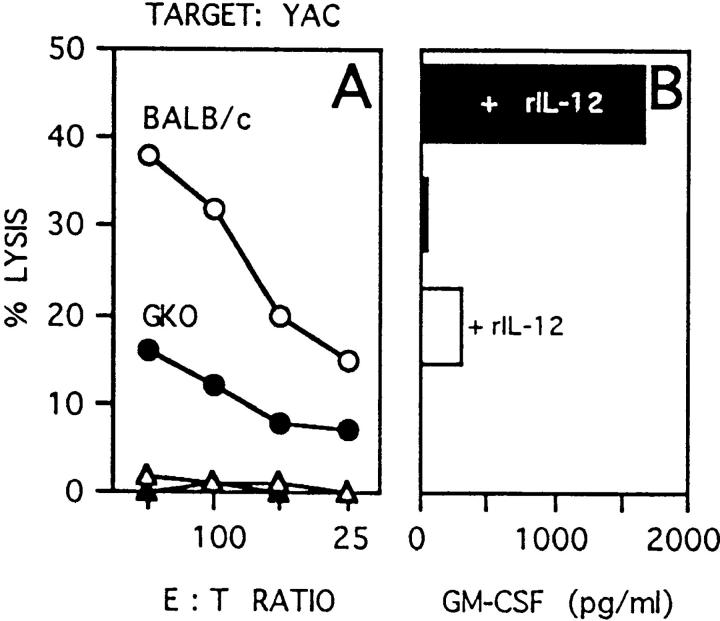
To analyze the nature of the early defective response in GKO mice, NK activity in response to rIL-12 was tested in mice injected or not with rIL-12 (1 μg/mouse per day) for 2 d; at day 3, fresh splenocytes were tested for cytotoxic activity against YAC cells. NK cytotoxicity was indeed reduced, but not abrogated, in GKO mice injected with rIL-12 (Fig. 5 A) although splenocytes from these mice showed increased GM-CSF production upon CD3 stimulation (Fig. 5 B).
Figure 5.
NK-mediated cytotoxicity of YAC cells (A) and splenocyte GM-CSF production (B) after in vivo stimulation with rIL-12. Splenocytes were freshly isolated from BALB/c (white bars) and GKO (black bars) mice treated twice with 1 μg of rIL-12, and GM-CSF production was measured by ELISA after 18 h incubation in wells coated with mAb to CD3.
To test GKO macrophage function, NO2 − production was measured in thioglycolate-elicited macrophages treated with LPS in the presence or absence of IFN-γ (Table 2). Although GKO macrophages in the absence of exogenous IFN-γ were less responsive than their BALB/c counterparts to LPS, they promptly recovered the ability to produce NO2 − after addition of IFN-γ. Consistent with this result, injection of mice with rIL-12 primed BALB/c (20), but not GKO macrophages, against the subsequent exposure to LPS (Table 2). Thus the absence of IFN-γ impairs the NO response of macrophages, and IL-12 does not restore this response.
Table 2.
Nitrite Production by Macrophages from BALB/c and GKO Mice
| Treatment | NO2 − (nmol/2 × 106 cells ± SD) | |||||
|---|---|---|---|---|---|---|
| In vivo | In vitro | BALB/c | GKO | |||
| – | – | 0.7 ± 0.3 | 0.5 ± 0.3 | |||
| – | LPS | 49 ± 6 | 25 ± 6 | |||
| – | LPS + IFN-γ | 54 ± 14 | 69 ± 16 | |||
| rIL-12 | LPS | 79 ± 18 | 28 ± 6 | |||
Immunohistology of Leukocytes Infiltrating the C26/IL-12 Tumor.
Reduced NK activity in the presence of an intact and robust CTL response might explain the accelerated onset of C26/IL-12 tumor formation when 5 × 104 cells are injected, but not the reduced rejection of the same cells injected at a dose of 5 × 105. To gain some insight into the tumor-associated events occurring in vivo, the entire site of tumor injection, the incipient tumors when present, and the draining lymph nodes were dissected at different time points and analyzed by immunocytochemistry. Immunostaining of infiltrating leukocytes indicated a profound reduction in lymphocyte number, mainly CD8, and in NK cells, but an increased number of macrophages and granulocytes in the C26/IL-12 tumors from GKO mice (Table 3). This effect was clearly due to the lack of IFN-γ, since treatment of BALB/c mice with mAb to IFN-γ produced the same effect (Table 3). Thus, in GKO mice, NK cells and CTLs are induced but apparently cannot infiltrate the C26/IL-12 tumors.
Table 3.
Immunocytochemical Characterization of Leukocytes Infiltrating C26/IL-12 Colon Carcinoma Injected into BALB/c, GKO, or Anti–IFN-γ–treated BALB/c Mice
| Days | CD45 | CD4 | CD8 | MAC-3 | GR-1 | AsialoGM1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BALB/c | 3* | 450 | 15 | 73 | 131 | 161 | 48 | |||||||
| 5 | 948 ± 64‡ | 103 ± 4 | 225 ± 42 | 316 ± 58 | 115 ± 18 | 108 ± 6 | ||||||||
| 7 | 997 ± 71 | 180 ± 45 | 336 ± 43 | 193 ± 15 | 69 ± 21 | 196 ± 51 | ||||||||
| 10 | 892 ± 84 | 99 ± 22 | 214 ± 42 | 282 ± 54 | 139 ± 24 | 146 ± 15 | ||||||||
| GKO | 3 | 211 ± 23 | 7 ± 5 | 1 ± 2 | 92 ± 14 | 129 ± 8 | 3 ± 3 | |||||||
| 5 | 365 ± 17 | 49 ± 17 | 29 ± 8 | 159 ± 27 | 109 ± 12 | 11 ± 5 | ||||||||
| 7 | 691 ± 43 | 67 ± 5 | 12 ± 2 | 311 ± 21 | 359 ± 92 | 7 ± 5 | ||||||||
| 10 | 911 ± 25 | 112 ± 22 | 19 ± 3 | 445 ± 19 | 241 ± 54 | 17 ± 9 | ||||||||
| BALB/c + anti–IFN-γ | 3* | 165 | – | – | 130 | 41 | 2 | |||||||
| 7 | 682 ± 68 | 46 ± 11 | 5 ± 6 | 395 ± 17 | 201 ± 13 | 21 ± 3 |
One mouse killed for the analysis; all other data are from two mice for each time point.
Mean number of cells/mm2 ± SD positive for immunostaining.
Tumor-associated and Systemic Expression of IP-10 and MIG.
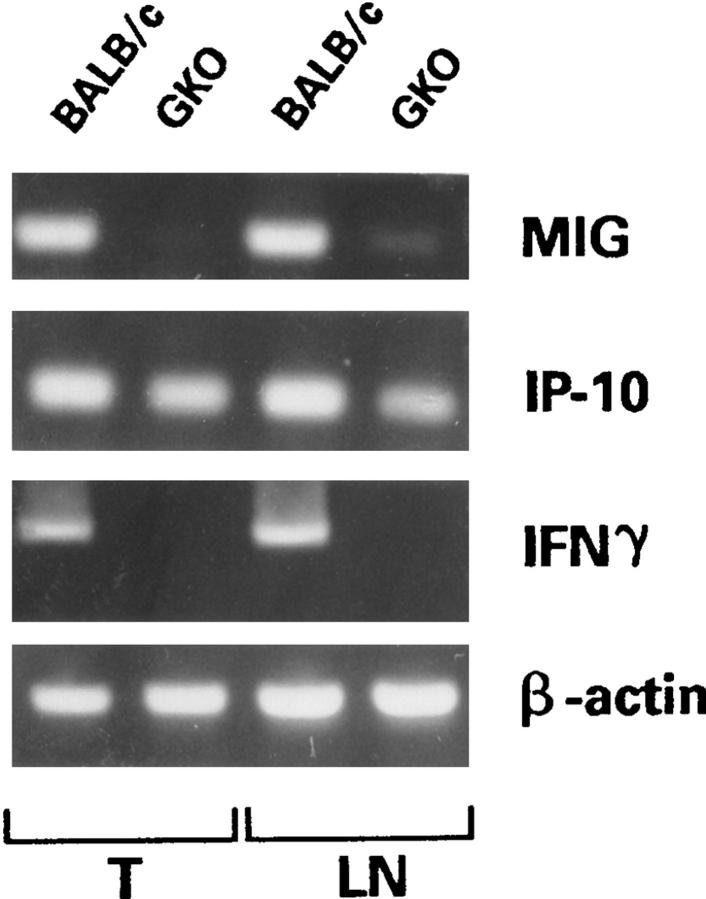
An antiangiogenic activity has been described elsewhere for IL-12 (21), which is mediated through IFN-γ and its induced chemokines IP-10 and MIG. PCR analysis of RNA extracted from C26/IL-12 tumors and draining nodes for expression of IP-10 and MIG revealed a slight reduction in IP-10 levels and undetectable MIG expression in both tumors and nodes of GKO mice (Fig. 6). In situ hybridization confirmed these results and revealed induction of IP-10 and MIG expression in BALB/c mice injected with C26/IL-12 cells (Fig. 7). The effect of IL-12 released by C26/IL-12 tumor cells on IP-10 and MIG was systemic since upregulation of both molecules was detected in spleen sections from BALB/c but not GKO mice (data not shown).
Figure 6.
MIG and IP-10 expression as detected by reverse transcriptase PCR in C26/IL-12 tumors (T) and draining lymph nodes (LN) from BALB/c and GKO mice. β-actin was used to control cDNA quality, and IFN-γ primers were used to confirm the lack of IFN-γ expression in GKO mice.
Figure 7.
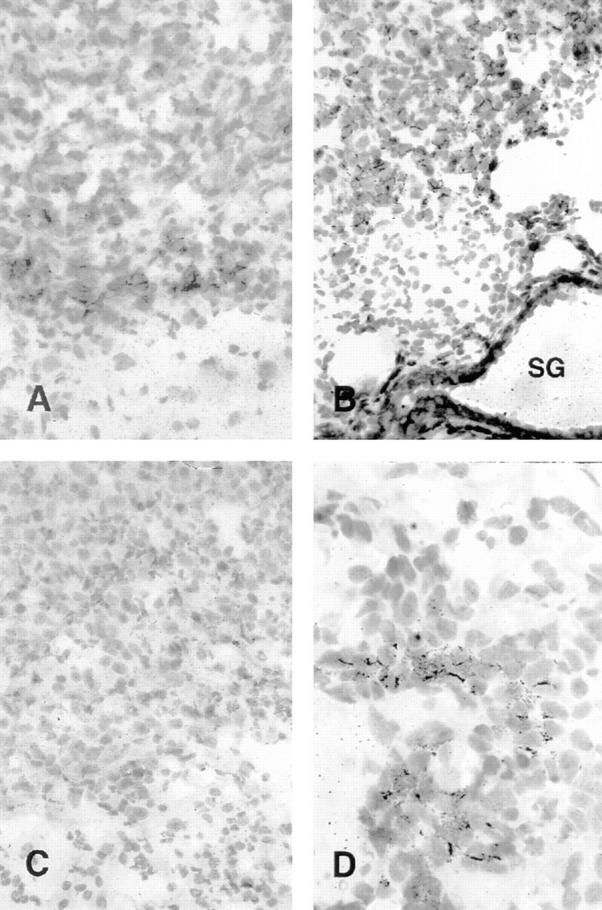
In situ hybridization of IP-10 (A and B) and MIG (C and D) probes with C26/IL-12 tumors from GKO (A and C) and BALB/c mice (B and D). (A) In GKO mice, IP-10 hybridization was weak and mostly restricted to tumor cells; whereas (B) C26/IL-12 tumors growing in BALB/c mice showed high level expression of IP-10 in tumor cells, some reactive cells, endothelium and epithelial cells of the subcutaneous sweat glands (SG). (D) Several lymphocytes of the inflammatory infiltrate were positive for MIG expression in BALB/c, whereas (C) MIG expression was undetectable in GKO. Positive cells were identified by the presence of a high accumulation of cytoplasmic black granules. Hematoxylin counterstaining. Original magnification: A–C, ×400; D, ×1,000.
Vascularization of the C26/IL-12 Tumor.
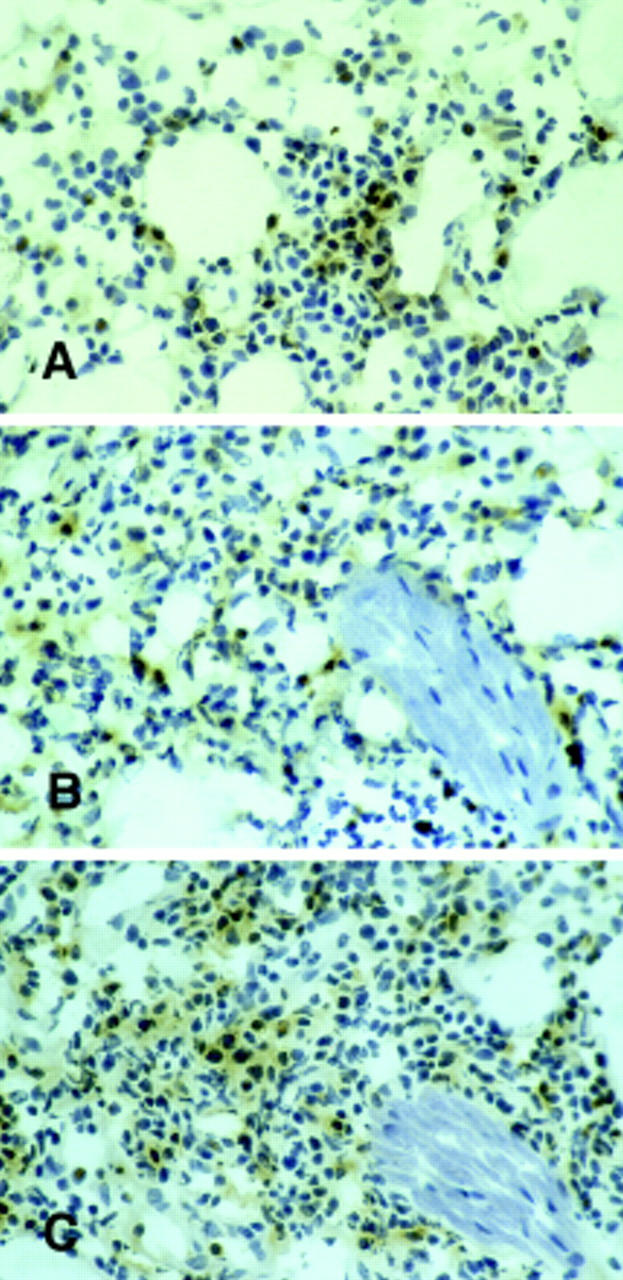
The parental C26 tumor grew in both BALB/c or GKO mice without differences; in comparison to them, C26/IL-12 tumors were characterized by vessels with an enlarged lumen filled with granulocytes and macrophages in BALB/c mice (Fig. 8), whereas in GKO mice, tumor vessels were more numerous (Table 4), although generally negative for expression of the angiogenesis-associated integrin αvβ3 (22), and were usually thinner in structure (Fig. 8).
Figure 8.
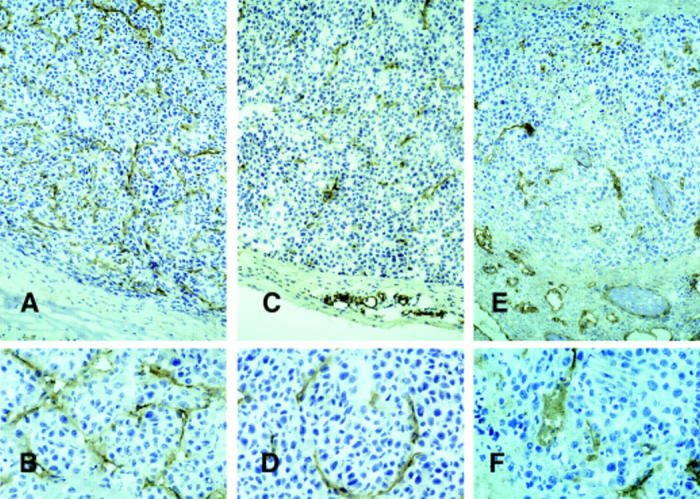
CD31 (PECAM-1) immunostaining of blood vessels associated with C26 (A and B) and C26/IL-12 (C–F) tumors in GKO (A–D) and BALB/c (E and F) mice. Vessels of the C26/IL-12 tumors in BALB/c mice were less numerous and showed an enlarged lumen as compared with those in GKO mice, which were more similar to those in C26 tumors, although less numerous. Large areas of tumor necrosis are more evident in C26/IL-12 tumors from BALB/c where the host response was stronger. PAP immunostaining, hematoxylin counterstain. Original magnification: A, C, and E, ×400; B, D, and F, ×1,000.
Table 4.
C26/IL-12 Tumor–associated Vessels Detected by Immunocytochemistry
| CD31 | CD34 | αvβ3 | ||||
|---|---|---|---|---|---|---|
| BALB/c | 5 ± 4* | 4 ± 4 | ND | |||
| + mAb to CD8 | 41 ± 11 | 33 ± 8 | 5 ± 2 (12%)‡ | |||
| + mAb to CD4 | 3 ± 2 | 5 ± 2 | ND | |||
| GKO | 20 ± 6 | 21 ± 15 | <1 | |||
| + mAb to CD8 | 42 ± 13 | 31 ± 3 | <1 | |||
| + mAb to CD4 | 69 ± 26 | 51 ± 18 | 9 ± 2 (13%) | |||
| C26 in BALB/c | 71 ± 10 | 53 ± 15 | 9 ± 3 (13%) | |||
| C26 in GKO | 55 ± 16 | 31 ± 2 | 8 ± 2 (14%) |
Frozen sections were stained with mAb by immunocytochemistry (PAP method) except for αvβ3, which required a fluorescein-conjugated Ab followed by antifluorescein for detection by peroxidase. Tumors were analyzed at day 10.
Mean number of cells/mm2 ± SD positive for immunostaining.
Percentage of CD31+ cells that were αvβ3 +.
Differential Requirement for CD4 T Cells and for GM-CSF in BALB/c and GKO Mice for C26/IL-12 Tumor Rejection.
Analysis of leukocyte infiltration (Table 3) indicated that CD4 cells were more numerous than CD8 lymphocytes in tumors from GKO mice, in contrast to tumors from BALB/c mice in which CD8 cells predominate. Unlike BALB/c mice, in which rejection of C26/IL-12 tumors was CD4 independent, GKO mice required CD4 lymphocytes to reject the C26/IL-12 tumor (Fig. 9). Moreover, CD4 T cell depletion was associated with disappearance or further reduction of the few CD8 T lymphocytes that infiltrated the C26/IL-12 tumor in GKO mice (Table 5). Both BALB/c and GKO mice required CD8 T lymphocytes for tumor rejection (Fig. 9). These results suggest that some factor(s) essential for CD8 T cell recruitment or survival at the tumor site in the absence of IFN-γ is produced by CD4 cells. This factor was identified as GM-CSF, whose expression was higher in GKO than BALB/c mice in response to C26/IL-12 cells, a result confirmed by in situ hybridization of tumor-draining lymph nodes (Fig. 10, A–D), and was not detectable in tumor sections from CD4-depleted GKO mice (Fig. 10 G). Moreover, treatment of BALB/c and GKO mice with mAbs against GM-CSF before injection with C26/IL-12 cells abrogated tumor inhibition in GKO but not in BALB/c mice (Fig. 9 C).
Figure 9.
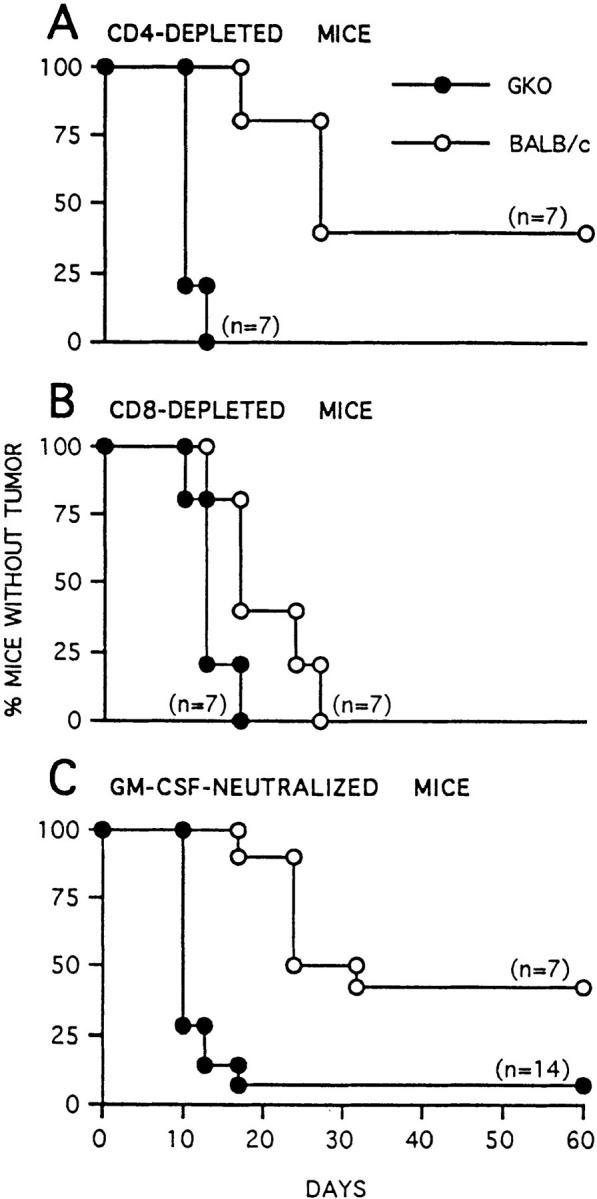
Tumorigenicity and latency of C26/IL-12 tumor cells injected at a dose of 5 × 104 cells into BALB/c or GKO mice depleted of CD4 (A) or CD8 (B) T cells or treated with mAbs against GM-CSF (C).
Table 5.
Immunocytochemical Characterization of Leukocytes Infiltrating C26/IL-12 Colon Carcinoma Injected into CD4-depleted BALB/c and GKO MICE
| Days | CD45 | CD4 | CD8 | MAC-3 | GR-1 | Eosinophils | AsialoGM1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BALB/c | 5 | 749 ± 102* | 0 | 252 ± 47 | 173 ± 32 | 191 ± 33 | 17 ± 3 | 197 ± 31 | ||||||||
| 10 | 838 ± 56 | 0 | 242 ± 73 | 150 ± 33 | 209 ± 27 | 36 ± 10 | 201 ± 31 | |||||||||
| GKO | 5 | 255 ± 53 | 0 | 0 | 160 ± 43 | 95 ± 33 | 4 ± 3 | 0 | ||||||||
| 10 | 892 ± 84 | 7 ± 5 | 13 ± 7 | 131 ± 7 | 121 ± 13 | 11 ± 4 | 4 ± 5 |
Frozen sections from two mice for each time point were analyzed by immunocytochemistry.
Mean number of cells/mm2 ± SD positive for immunostaining.
Figure 10.
Detection of GM-CSF expression by in situ hybridization. Tumor-draining lymph nodes (A–D) and C26/IL-12 tumors (E–G) were collected from BALB/c (A, B, and E), GKO (C, D, and F), and CD4-depleted GKO mice (G). Tumor-draining lymph nodes from BALB/c mice revealed GM-CSF expression in macrophages of the subcapsular sinus and in scattered lymphocytes of the T cell–dependent area, whereas the nodes from GKO mice were characterized by a much higher number of strongly positive lymphocytes in the paracortex. GM-CSF was expressed by scattered cells of the inflammatory infiltrate of BALB/c tumors (E), but by a large number of cells in tumors from GKO mice (F). Such positive cells disappeared after CD4 T cell depletion (G). F, primary follicles; PC, paracortical area; S, subcapsular sinus. [32P]dCTP-labeled GM-CSF autoradiography. Hematoxylin counterstain. Original magnification: A and C, ×250; G, ×400; B, D–F, ×1,000.
Discussion
Direct comparative data obtained using the same tumor transduced with several different cytokine genes, including IL-12, and using rIL-12 systemically, have shown that this cytokine is optimal in inducing antitumor activity in preclinical settings (23). The effects of IL-12 have been found to be IFN-γ dependent in several models (1). IFN-γ has been strongly associated with a favorable outcome of therapy given as rIL-12 (24) or as tumor cell vaccines based on IL-12 transduction (25). The intensity of IL-12–induced tumor inhibition is more directly associated with IFN-γ production than with CTL activity (26). Although IFN-γ has been associated with the antitumor activity of cytokines other than IL-12 (15), and even with the cure of metastasis by adoptive transfer of CD8 T cells (27), IFN-γ–secreting tumor cells were not inhibited to the same extent as were IL-12–secreting tumors. This observation might rest in the lower efficiency of IFN-γ released by engineered tumor cells as compared with IFN-γ physiologically produced as a secondary cytokine by infiltrating leukocytes and providing the correct immunological context (28). More likely, IL-12 induces secondary factors (in addition to IFN-γ) different from those induced by IFN-γ, depending on the type of leukocytes that infiltrate tumors transduced with either cytokine. In the TSA mammary tumor model, for example, TSA/IL-12 is rich in polymorphonuclear leukocytes that are rare or absent in TSA/IFN-γ (29). Depletion experiments have also underscored the role of granulocytes in cytokine-activated regression of established tumors (23).
Our study aimed to identify factors that might functionally substitute for IFN-γ rather than simply cooperate with it in mediating IL-12 antitumor effects. The use of GKO mice ensured the complete absence of IFN-γ throughout the experiments, a condition more difficult to obtain by using neutralizing antibodies. Since the first description of these mice (14), most of the studies to delineate the immunological defect have focused on the antimicrobial response, a setting in which IL-12 is an early mediator (30). The emerging picture is that GKO mice are susceptible to acute infection because of a defective early response, but are capable of a specific immune response and of a late response against chronic infection (30, 31). In these mice, no upregulation of Th2 cytokine mRNA was detected, but IL-12 was shown to enhance rather than suppress the Th2 type response (32), despite the recent description of IFN-γ–dependent regulation of IL-12Rβ2 (33). Our analysis of the impaired immune response of GKO mice to C26 carcinoma cells engineered to produce IL-12 revealed the expected defective early response and unimpaired CTL response as well as several unexpected findings. First, ∼50% of both BALB/c and GKO mice did not develop tumors after injection of 5 × 104 C26/IL-12 cells, but only GKO mice mounted a memory response. This result is compatible with the notion of an absent early response that allows late T cell activation, since the other 50% of GKO mice had tumors that formed more quickly than did those in BALB/c mice. CTL activation and infiltration of the challenge site by oligoclonal Vβ6 lymphocytes in GKO mice support such a notion, in accord with previous findings on tumors engineered to produce IL-2 at high (strong early NK response but no memory) and low (no early NK response but memory) levels (34). Thus, the rejection of incipient tumors is generally associated with immune memory (35), as also seen in BALB/c mice injected with 5 × 105 C26/IL-12 cells (Table 1 and reference 12).
Second, we identified GM-CSF as a possible substitute for IFN-γ in maintaining the immune response still present in GKO mice. This identification is based on repeated findings from different experiments in which GM-CSF was tested by ELISA in lymphocytes from lymph nodes draining the injection sites of either live or irradiated C26/IL-12 cells, as well as from spleens of mice injected with rIL-12. In addition, in situ hybridization showed that GM-CSF was highly expressed in C26/IL-12 tumor sections and draining nodes from GKO but not BALB/c mice. A functional role for GM-CSF was suggested by experiments in CD4-depleted GKO mice, where the complete abrogation of any residual response to C26/IL-12 cells was associated with a decrease in GM-CSF expression. Experiments using mAbs to neutralize GM-CSF confirmed this role. Elevated production of GM-CSF by lymphocytes from GKO mice was not restricted to C26/IL-12 response since a similar result was obtained by injecting the TSA/IL-12 mammary carcinoma (data not shown). Production of GM-CSF by tumor-infiltrating lymphocytes has been described as a predictor of tumor response in melanoma patients (36), and we have found that GM-CSF is associated with the therapeutic outcome of mice with C26 lung metastasis after treatment with a C26/IL-12 cell vaccine (our unpublished results). In CD4-depleted GKO mice, the decline in GM-CSF levels was accompanied by a reduction in the number of infiltrating granulocytes. These cells can mediate tumor cell killing through direct or bystander effects (37) and can participate in the cross-talk with CD8 T cells, which is instrumental in the rejection of established C26 colon carcinomas transduced to express G-CSF (15) and in the IL-12– mediated rejection of TSA mammary carcinoma (23). Such cross-talk was sustained by CD8 cell-produced IFN-γ (15), which is known to maintain granulocyte survival (38), a function that may well be supported by GM-CSF. The IFN-γ–independent late response to Leishmania donovani that has been attributed to TNF-α (39) may also require GM-CSF. Indeed, Taylor and Murray (39) noted that although TNF-α was probably the primary effector component, IL-12–induced GM-CSF in GKO mice likely acts as a compensatory factor for granuloma assembly in the absence of endogenous IFN-γ.
Third, we found that CD4 T cells, which were not necessary for IL-12–mediated tumor rejection in BALB/c mice or were even suppressive (11, 23), became essential for the IFN-γ–independent response to C26/IL-12 cells. Although CD8 T lymphocytes produced the higher amount of GM-CSF, CD4 T cells accounted for the larger difference in GM-CSF production between BALB/c and GKO lymphocytes. In addition, the CD4/CD8 ratio in C26/IL-12 tumors in GKO mice was reversed as compared with that in BALB/c tumors, with CD4 T cells as the predominant lymphocyte population. Depletion of CD4 T cells in GKO mice abolished the presence of CD8 T cells either directly or indirectly through the break in granulocyte-CD8 cross-talk (34, 40), possibly via the decrease in GM-CSF and consequent reduction of infiltrating granulocytes.
Finally, our studies revealed a correlation between expression of IFN-γ–inducible IP-10 and MIG and tumor vascularization, although this correlation does not entirely explain the IL-12–mediated antitumor effect still present in GKO mice. Although IL-12–mediated antitumor activity has also been observed in SCID and nu/nu mice, suggesting the activation of some nonimmunological events, nu/nu mice show a weaker response than that seen in euthymic mice, despite their 10-fold higher levels of serum IFN-γ (7). Such a setting should favor induction of IP-10, which reportedly elicits a potent thymus-dependent antitumor response in vivo (41) and is responsible for T cell recruitment when expressed at the tumor site (4). Moreover, IP-10 has been identified as a final inhibitor of neoangiogenesis induced by IL-12 (42). In GKO mice, C26/IL-12 tumors were characterized by a partial reduction in number of blood vessels as compared with tumors growing in BALB/c mice. This may be explained in part by the reduced expression of IP-10 and MIG in GKO mice, but other factors are undoubtedly required (perhaps GM-CSF), since CD4 T cell depletion in GKO but not in BALB/c mice reestablished vascularization conditions similar to those of IL-12– nonproducing C26 cells (Table 4). Tumor vessel status probably depends on a balance of factors that promote and inhibit neovascularization, as well as on granulocytes, which in the context of a local inflammatory response induce vessel wall injury and compromise the function of the vasculature and of the underlying tissues (43).
Together, our results point to GM-CSF as a cytokine that sustains an immune response to IL-12–producing tumors in the absence of IFN-γ. Moreover, the results suggest the existence of an alternative pattern of immune response that uses different regulatory leukocytes and chemokines to provide a similar outcome, i.e., tumor rejection.
Acknowledgments
We thank Dr. Giorgio Trinchieri for critically reviewing the manuscript. The technical expertise of Mr. Ivano Arioli is gratefully acknowledged. We also thank Mr. Mario Azzini for preparation of figures.
This work was supported by special programs on gene therapy from the Associazione Italiana per la Ricerca sul Cancro and the Italian Ministry of Health (ISS), and by Consiglio Nationale delle Ricerche.
Abbreviations used in this paper
- CMC
cell-mediated cytotoxicity
- GKO
IFN-γ knockout mice
- IP-10
IFN-inducible protein 10
- MIG
monokine induced by γ-IFN
- PAP
peroxidase antiperoxidase
References
- 1.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridges innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 2.Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, Gately MK. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993;178:1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou J-P, Yamamoto N, Fujii T, Takenaka H, Kobayashi M, Herrmann SH, Wolf SF, Fujiwara H, Hamaoka T. Systemic administration of rIL-12 induces complete tumor regression and protective immunity: response is correlated with a striking reversal of suppressed IFNγ production by anti-tumor T cells. Int Immunol. 1995;7:1135–1145. doi: 10.1093/intimm/7.7.1135. [DOI] [PubMed] [Google Scholar]
- 4.Tannenbaum CS, Wicker N, Armstrong D, Tubbs R, Finke J, Bukowski RM, Hamilton TA. Cytokine and chemokine expression in tumors of mice receiving systemic therapy with IL-12. J Immunol. 1996;156:693–699. [PubMed] [Google Scholar]
- 5.Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S, Kleinman HK, Reaman GH, Tosato G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182:155–162. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luster AD, Greenberg SM, Leder P. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J Exp Med. 1995;182:219–231. doi: 10.1084/jem.182.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunda MJ, Luistro L, Hendrzak JA, Fountoulakis M, Garotta G, Gately MK. Role of IFNγ in mediating the antitumor efficacy of interleukin-12. J Immunother. 1995;17:71–77. doi: 10.1097/00002371-199502000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Presky DH, Gubler U, Chizzonite RA, Gately MK. IL-12 receptors and receptor antagonists. Res Immunol. 1995;146:439–445. doi: 10.1016/0923-2494(96)83013-6. [DOI] [PubMed] [Google Scholar]
- 9.Trembleau S, Germann T, Gately MK, Adorini L. The role of IL-12 in the induction of organ-specific autoimmune diseases. Immunol Today. 1995;16:383–386. doi: 10.1016/0167-5699(95)80006-9. [DOI] [PubMed] [Google Scholar]
- 10.Hultgren B, Huang X, Dybdal N, Stewart TA. Genetic absence of gamma-interferon delays but does not prevent diabetes in NOD mice. Diabetes. 1996;45:812–817. doi: 10.2337/diab.45.6.812. [DOI] [PubMed] [Google Scholar]
- 11.Martinotti A, Stoppacciaro A, Vagliani M, Melani C, Spreafico F, Wysocka M, Parmiani G, Trinchieri G, Colombo MP. CD4 T cells inhibits in vivo the CD8-mediated immune response against murine colon carcinoma cells transduced with IL-12 genes. Eur J Immunol. 1995;25:137–146. doi: 10.1002/eji.1830250124. [DOI] [PubMed] [Google Scholar]
- 12.Colombo MP, Vagliani M, Spreafico F, Parenza M, Chiodoni C, Melani C, Stoppacciaro A. The amount of IL-12 available at the tumor site is critical for tumor regression. Cancer Res. 1996;56:2531–2534. [PubMed] [Google Scholar]
- 13.Corbett TH, Griswold DP, Jr, Roberts BJ, Peckham JC, Schabel FM., Jr Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assay, with a note on carcinogen structure. Cancer Res. 1975;35:2434–2437. [PubMed] [Google Scholar]
- 14.Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1745. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 15.Stoppacciaro A, Melani C, Parenza M, Mastracchio A, Bassi C, Baroni C, Parmiani G, Colombo MP. Regression of an established tumor genetically modified to release granulocyte colony–stimulating factor requires granulocyte–T cell cooperation and T cell–produced interferon γ. J Exp Med. 1993;178:151–161. doi: 10.1084/jem.178.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang AS, Gulden PH, Woods AS, Thomas MC, Tong CD, Wang W, Engelhard VH, Pasternack G, Cotter R, Hunt D, et al. The immunodominant major histocompatibility complex class I–restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc Natl Acad Sci USA. 1996;93:9730–9734. doi: 10.1073/pnas.93.18.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 18.Colombo MP, Forni G. Cytokine gene transfer in tumor inhibition and tentative tumor therapy: where are we now? . Immunol Today. 1994;15:48–51. doi: 10.1016/0167-5699(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 19.Rodolfo M, Castelli C, Bassi C, Accornero P, Sensi M, Parmiani G. Cytotoxic T lymphocytes recognize tumor antigens of a murine colonic carcinoma by using different T-cell receptors. Int J Cancer. 1994;57:440–447. doi: 10.1002/ijc.2910570324. [DOI] [PubMed] [Google Scholar]
- 20.Wigginton JM, Kuhns DB, Back TC, Brunda MJ, Wiltrout RH, Cox GW. Interleukin 12 primes macrophages for nitric oxide production in vivo and restores depressed nitric oxide production by macrophages from tumor-bearing mice: implications for the antitumor activity of interleukin 12 and/or interleukin 2. Cancer Res. 1996;56:1131–1136. [PubMed] [Google Scholar]
- 21.Voest EE, Kenyon BM, O'Reilly MS, Truitt G, D'Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995;87:581–586. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- 22.Brooks PC, Clark RAF, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 23.Cavallo F, Signorelli P, Giovarelli M, Musiani P, Modesti A, Brunda MJ, Colombo MP, Forni G. Antitumor efficacy of adenocarcinoma cells engineered to produce interleukin 12 (IL-12) or other cytokines compared with exogenous IL-12. J Natl Cancer Inst. 1997;89:1049–1058. doi: 10.1093/jnci/89.14.1049. [DOI] [PubMed] [Google Scholar]
- 24.Nastala CL, Edington HD, McKinney TG, Tahara H, Nalesnik MA, Brunda MJ, Gately MK, Wolf SF, Schreiber RD, Storkus WJ, Lotze MT. Recombinant IL-12 administration induces tumor regression in association with IFNγ production. J Immunol. 1994;153:1697–1706. [PubMed] [Google Scholar]
- 25.Rodolfo M, Zilocchi C, Melani C, Cappetti B, Arioli I, Parmiani G, Colombo MP. Immunotherapy of experimental metastases by vaccination with interleukin gene-transduced adenocarcinoma cells sharing tumor-associated antigens. Comparison between IL-12 and IL-2 gene-transduced tumor cell vaccines. J Immunol. 1996;157:5536–5542. [PubMed] [Google Scholar]
- 26.Vagliani M, Rodolfo M, Cavallo F, Parenza M, Melani C, Parmiani G, Forni G, Colombo MP. IL-12 potentiates the curative effect of a vaccine based on IL-2 transduced tumor cells. Cancer Res. 1996;56:467–470. [PubMed] [Google Scholar]
- 27.Barth RJ, Mulé JJ, Spiess PJ, Rosenberg SA. Interferon γ and tumor necrosis factor have a role in tumor regressions mediated by murine CD8+tumor-infiltrating lymphocytes. J Exp Med. 1991;173:647–653. doi: 10.1084/jem.173.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathan C, Sporn MJ. Cytokines in the context. J Cell Biol. 1991;113:981–985. doi: 10.1083/jcb.113.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musiani P, Allione A, Modica A, Lollini PL, Giovarelli M, Cavallo F, Belardelli F, Forni G, Modesti A. Role of neutrophils and lymphocytes in inhibition of a mouse mammary adenocarcinoma engineered to release IL-2, IL-4, IL-7, IL-10, IFN-alpha, IFN-gamma, and TNF-alpha. Lab Invest. 1996;74:146–157. [PubMed] [Google Scholar]
- 30.Locksley RM. Interleukin 12 in host defense against microbial pathogens. Proc Natl Acad Sci USA. 1993;90:5879–5880. doi: 10.1073/pnas.90.13.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenesin the absence of IFNγ. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z-E, Zheng S, Corry DB, Dalton DK, Seder RA, Reiner SL, Locksley RM. Interferon γ-independent effects of interleukin 12 administered during acute or established infection due to Leishmania major. . Proc Natl Acad Sci USA. 1994;91:12932–12936. doi: 10.1073/pnas.91.26.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12Rb2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cavallo F, Giovarelli M, Gulino A, Vacca A, Stoppacciaro A, Modesti A, Forni G. Role of neutrophils and CD4+ T lymphocytes in the primary and memory response to nonimmunogenic murine mammary adenocarcinoma made immunogenic by IL-2 gene transfection. J Immunol. 1992;149:3627–3635. [PubMed] [Google Scholar]
- 35.Musiani P, Modesti A, Giovarelli M, Cavallo F, Colombo MP, Lollini PL, Forni G. Cytokines, tumor-cell death and immunogenicity: a question of choice. Immunol Today. 1997;18:32–36. doi: 10.1016/s0167-5699(97)80012-6. [DOI] [PubMed] [Google Scholar]
- 36.Schwartzentruber DJ, Hom SS, Dadmarz M, White DE, Yannelli JR, Steinberg SM, Rosenberg SA, Topalian SL. In vitro predictors of therapeutic response in melanoma patients receiving tumor-infiltrating lymphocytes and interleukin-2. J Clin Oncol. 1994;12:1475–1482. doi: 10.1200/JCO.1994.12.7.1475. [DOI] [PubMed] [Google Scholar]
- 37.Ginsburg I, Gibbs DF, Varani J. Interaction of mammalian cells with polymorphonuclear leukocytes: relative sensitivity to monolayer disruption and killing. Inflammation. 1989;13:529–542. doi: 10.1007/BF00916759. [DOI] [PubMed] [Google Scholar]
- 38.Perussia B, Kobayashi M, Rossi ME, Anegon I, Trinchieri G. Immune interferon enhances functional properties of human granulocytes: role of Fc receptors and effect of lymphotoxin, tumor necrosis factor, and granulocyte-macrophage colony-stimulating factor. J Immunol. 1987;138:765–773. [PubMed] [Google Scholar]
- 39.Taylor AP, Murray HW. Intracellular antimicrobial activity in the absence of interferon-γ: effect of interleukin-12 in experimental visceral leishmaniasis in interferon-γ gene-disrupted mice. J Exp Med. 1997;185:1231–1239. doi: 10.1084/jem.185.7.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colombo MP, Modesti A, Parmiani G, Forni G. Local cytokine availability elicits tumor rejection and systemic immunity through granulocyte–T-lymphocyte cross-talk. Cancer Res. 1992;52:4853–4857. [PubMed] [Google Scholar]
- 41.Luster AD, Leder P. IP-10, a -C-X-C- chemokine, elicits a potent thymus-dependent antitumor response in vivo. J Exp Med. 1993;178:1057–1065. doi: 10.1084/jem.178.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sgadari C, Angiolillo AL, Tosatto G. Inhibition of angiogenesis by interleukin-12 is mediated by interferon-inducible protein 10. Blood. 1996;87:3877–3882. [PubMed] [Google Scholar]
- 43.Westling WF, Gimbrone MA. Neutrophil-mediated damage to human vascular endothelium. Role of cytokine activation. Am J Pathol. 1993;142:117–128. [PMC free article] [PubMed] [Google Scholar]