Abstract
Embryonic stem cells (ESCs) could potentially compensate for the lack of blood platelets available for use in transfusions. Here, we describe a new method for generating mouse ESC-derived platelets (ESPs) that can contribute to hemostasis in vivo. Flow cytometric sorting of cells from embryoid bodies on day 6 demonstrated that c-Kit+ integrin αIIb (αIIb)+ cells, but not CD31+ cells or vascular endothelial cadherin+ cells, are capable of megakaryopoiesis and the release of platelet-like structures by day 12. αIIbβ3-expressing ESPs exhibited ectodomain shedding of glycoprotein (GP)Ibα, GPV, and GPVI, but not αIIbβ3 or GPIbβ. ESPs showed impaired αIIbβ3 activation and integrin-mediated actin reorganization, critical events for normal platelet function. However, the administration of metalloproteinase inhibitors GM6001 or TAPI-1 during differentiation increased the expression of GPIbα, improving both thrombogenesis in vitro and posttransfusion recovery in vivo. Thus, the regulation of metalloproteinases in culture could be useful for obtaining high-quality, efficacious ESPs as an alternative platelet source for transfusions.
Platelet concentrates (PCs) from donated blood are required to treat severe thrombocytopenia in patients with various hematological diseases, such as those who have undergone cancer chemotherapy or are recovering from hematopoietic stem cell (HSC) transplantation (1, 2). Frequent transfusion of PCs is clinically necessary because the half-life of transfused human platelets is 4–5 d (3). Platelets cannot be stored frozen, thus the ability to generate platelets in vitro would provide significant advances for platelet replacement therapy in clinical settings. A novel culture method to generate human platelets from cord blood CD34+ cells was recently developed as an alternative source for PCs (4). However, technical difficulties in expanding ex vivo–cultured cord blood CD34+ cells on a large scale have limited this as a reasonable in vitro approach for generating platelets.
Human embryonic stem cells (ESCs) can be forced to differentiate along a megakaryocytic lineage and represent a promising in vitro source for platelets. Owing to their pluripotency, ESCs can potentially proliferate indefinitely in culture (5). Platelets, as anucleate fragments of cytoplasm, can be irradiated before transfusion to effectively eliminate any contaminating cell, such as an undifferentiated ESC. The possibility of irradiation is important, as ESCs can potentially form teratomas or, if present at high numbers, elicit an immune response (6, 7). Thus, although ESCs represent a potentially safe and unlimited source of platelets in vitro, there are technical obstacles that remain.
First, culture methods for efficient in vitro generation of platelets have not been established. Second, appropriate in vivo function of the in vitro–produced platelet must be achieved. In addition, contamination with nonhuman antigens resulting in immunological reactions must be prevented. We and other groups have developed a method to generate large numbers of megakaryocytes and platelets from mouse ESCs grown on OP9 stromal cells in vitro (8, 9). However, these methods have not consistently produced ESC-derived megakaryocytes or platelets in sufficient quantity or quality to be considered as an alternative platelet source. No pre-selection for megakaryocyte progenitors was included in these previous reports. Studies have shown that the in vitro generation of large numbers of mature megakaryocytes depends on increased numbers of ESC-obtained progenitors (7, 8). Therefore, the identification and selection of megakaryocyte progenitors might increase the efficiency of megakaryopoiesis.
The functional platelet paradigm in hemostasis and thrombosis is the initiation of platelet adhesion to the extracellular matrix (10). One key event in this process is the interaction between glycoprotein (GP)Ibα (the platelet receptor) and von Willebrand factor (VWF) present in the extracellular matrix (10). Simultaneously, platelets can interact with surface-bound collagen via platelet receptors GPVI and integrin α2β1 (11). The net result is an activation of integrin αIIbβ3 to become a competent fibrinogen receptor leading to the formation of platelet aggregates (10). A recent report has also suggested that GPIbα contributes to arterial thrombosis in vivo independently of binding to VWF (12). Indeed, other studies have demonstrated that GPIbα associates with thrombin, kininogen, coagulation factors XI and XII, and thrombospondin-1 (13). In addition, the GPIb–V-IX complex, consisting of GPIbα, GPIbβ, GPIX, and GPV (10, 14), can bind integrin αMβ2 on macrophages/monocytes or P-selectin on endothelial cells (13). Of note, aged human and mouse platelets shed GPV and an extracellular domain of GPIbα, which contains the binding sites for VWF and thrombin (13, 15). This process involves the action of a disintegrin and metalloproteinase (ADAM)17 (also referred to as tumor necrosis factor α converting enzyme) (16). This leads to decreased platelet function (16, 17).
In this study, we demonstrate that c-Kit+ integrin αIIb (αIIb)+ cells isolated from ESCs differentiate with high efficiency into megakaryocytes and ESC-derived platelets (ESPs) in the presence of thrombopoietin (TPO) and stromal cells. We also show that ESPs shed extracellular domains of GPIbα, GPV, and GPVI in culture, reducing αIIbβ3 activation and actin polymerization. Consequently, these ESPs are functionally impaired in thrombus growth (18, 19). However, the inhibition of metalloproteinases restores platelet function, making ESPs from induced pluripotent stem cells a source of platelets with therapeutic potential (20, 21).
RESULTS
Markers defining megakaryocyte differentiation from ESCs
The cell markers defining the megakaryocytic lineage in a culture system of ESCs or HSCs are not understood. It is known that megakaryocyte progenitors are highly enriched in the CD9+αIIb subunit+ (αIIb+)FcγRloc-Kit+Sca-1−Lin− fraction of bone marrow cells (22). The αIIb integrin subunit is an early primitive and definitive marker of hematopoiesis in the mouse embryo (17, 18) and a lineage-specific marker for postnatal megakaryocytes and platelets (23). We reasoned that CD31+, αIIb+, or c-Kit+ cells were candidates for megakaryocyte progenitors derived from ESCs because postnatal HSCs, mature megakaryocytes, and platelets all express CD31 and αIIbβ3, and postnatal megakaryocyte progenitors express both c-Kit and αIIbβ3 (22).
To determine which cells actually contribute to megakaryopoiesis, we chose to induce a liquid culture system of ESCs for embryoid body (EB) formation (24). Sorted cells were then applied onto OP9 stromal cells to differentiate along the megakaryocytic lineage (Fig. 1 A). This two-stage approach was used because EB formation has been reported to be more suitable than co-culture with OP9 cells in producing hematopoietic progenitors in vitro (25). Indeed, liquid culture for EB formation resulted in reproducibly higher expression of the αIIb integrin subunit than the use of the OP9 co-culture system (Fig. 1 B) (26). ESCs co-cultured with OP9 stromal cells preferentially expressed CD31 (Fig. 1 B, right) or vascular endothelial (VE)-cadherin (unpublished data) by days 4–5, as reported (27).
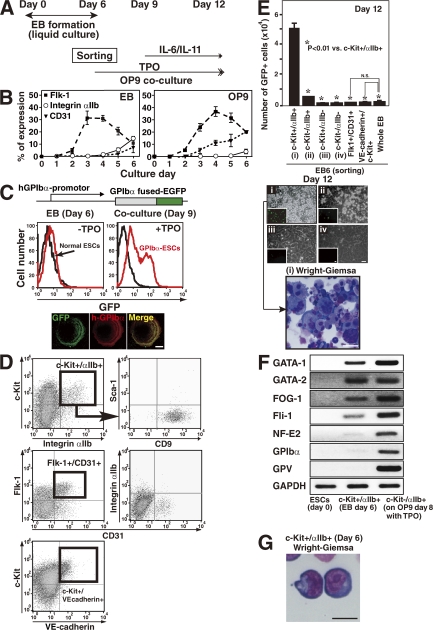
Figure 1.
c-Kit+αIIb+ cell selection from day 6 EB determines the megakaryocytic lineage. (A) Protocol used in this study. (B) EB formation or OP9 co-culture was used for ESC differentiation from day 0 through day 6 in vitro. On the indicated days of culture, expression of Flk-1, integrin αIIb subunit (CD41), or CD31 was examined in both groups of cultured cells. The graph shows mean ± SEM from three independent experiments. (C) GPIbα and GFP expression in GPIbα-ESCs is regulated by the human GPIbα promoter. GFP is detectable in cultured cells that exhibit megakaryopoiesis (day 9) but not in progenitors (day 6). In the bottom box, cells with surface-expressed GFP also express human GPIbα. The cells on day 9 were fixed and stained to mark human GPIbα to confirm the colocalization with GFP expression at the membrane of the cells. Bars, 10 μm. (D) Representative dot plots for the expression of individual antigens on 10,000 living cells from day 6 EB. c-Kit+αIIb+ cells corresponded with Flk-1+ cells (not depicted) but not with VE-cadherin+ cells. And >96% of c-Kit+αIIb+ cells are found in the CD9+Sca-1− fraction. (E) EB cells were sorted as shown in D, and 20,000 cells per well of a 6-well plate were seeded onto OP9 stroma cells in the presence of TPO. The left graph shows the number of generated GFP+ cells (mean ± SEM) per well of a 6-well plate on day 12 of culture. Representative images from day 12 culture dishes (phase or fluorescence microscopy [insets: bar, 50 μm] of four different fraction-derived cells at day 6; i, c-Kit+/αIIb+; ii, c-Kit−/αIIb+; iii, c-Kit+/αIIb−; iv, c-Kit−/αIIb− [phase: bar, 50 μm]) and of Wright-Giemsa–stained cytospin preparation on day 12 derived from c-Kit+/αIIb+ fraction of day 6 were also shown. Bar, 50 μm. Results indicate that c-Kit+/αIIb+ fraction of day 6 (i) selectively yields megakaryocytes on day 12. Similar results have been obtained from three independent experiments. (F) Expression of genes related to the megakaryocytic lineage as determined by RT-PCR of cells from day 0, 6, or 8 cultures as indicated. (G) Wright-Giemsa–stained cytospin preparation of sorted c-Kit+αIIb+ cells from EB at day 6 is shown. Bar, 10 μm.
To trace newly developed megakaryocytes and platelets in culture, we generated a novel ESC line in which the platelet-specific GPIbα promoter supports expression of a human GPIbα–EGFP fusion protein (Fig. 1 C, GPIb-ESCs) (28–30). Earlier reports showed selective expression in megakaryocytes and platelets using this system (29–31). The GFP-tagged cells were detectable by day 9 in the presence of TPO (Fig. 1 C). In addition, GFP expression was detectable only in αIIb+GPIbα+ megakaryocytes derived from cultures treated with TPO but not in Ter119+ erythroblasts isolated from erythropoietin-containing cultures (not depicted). We further separated EB cells on day 6 to identify which fraction could differentiate into GFP-expressing megakaryocytes (Fig. 1 D). Murine ESCs (2 × 105 per 100-mm dish) typically produced 106 c-Kit+αIIb+ cells by day 6 and 2.5 × 106 αIIb+ megakaryocytes by day 12. Cells derived from the c-Kit+αIIb+ fraction at day 6 expressed GFP on day 12 at 10-fold higher levels compared with cells in other fractions or in unfractionated whole EB-derived cells (Fig. 1 E). Fetal liver kinase (Flk)-1+CD31+ or VE-cadherin+c-Kit+ cells from day 6 EB did not contribute to megakaryopoiesis in this system (Fig. 1, D and E). ESC-derived VE-cadherin+c-Kit+CD45− cells in OP9 co-culture had been reported to contribute to definitive hematopoiesis (32).
Serial RT-PCR studies to detect GPIbα or GPV, both megakaryocytic-specific markers (33), along with the essential transcription factors for megakaryopoiesis, GATA-1, GATA-2, FOG-1, Fli-1, or NF-E2 (34, 35), indicated that day 6 EB c-Kit+αIIb+ cells were positive for these markers in the presence of TPO on OP9 stromal cells (Fig. 1 F) (34). Indeed, day 6 EB c-Kit+αIIb+ cells corresponded to CD9+Sca-1− cells in bone marrow–derived megakaryocyte progenitors (Fig. 1 D) (22), and their morphological features resembled immature hematopoietic progenitor cells derived from adult bone marrow (Fig. 1 G) (22). We concluded from these results that c-Kit+αIIb+ cells derived from ESCs can differentiate displaying several markers unique to the megakaryocytic lineage.
GPIbα expression on ESPs is reduced during in vitro culture
EB generation from ESC in liquid culture (6 d) followed by sorting for c-Kit+αIIb+ cells (Fig. 1 A) resulted in the constant generation of megakaryocytes and platelet-like particles, an improvement over our previously reported culture conditions (9). After 8–14 d in culture (2–8 d on co-culture with OP9 stromal cells), culture supernatants contained proplatelets (Fig. 2 A, i) and platelet-sized particles consistent with ESPs (Fig. 2 B). The granularity and size of these ESPs varied from those of circulating murine platelets as determined by transmission electron microscopy (TEM) (Fig. 2 A) and the forward scatter profiles produced by flow cytometry (Fig. 2 B). Thus, the culture conditions seem to yield various developmental stages of a platelet (36). ESPs displayed an extensive surface-connected open canalicular system and multiple α or dense granules (Fig. 2 A, ii and iii). As compared with a normal mouse platelet, ESPs had a reduced number of granules per ESP (unpublished data).
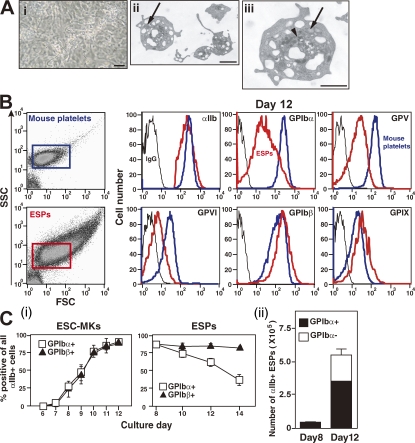
Figure 2.
GPIbα expression is reduced on cultured ESPs but not megakaryocytes. (A) On day 12 of the culture, ESC-derived megakaryocytes bearing proplatelets are represented in panel (i) (phase contrast image in culture dish). Bar, 100 μm. Panels (ii) and (iii) show TEM images of ESPs. The subcellular structure of ESPs showed an open canalicular system, dense granules (arrowhead), and α granules (arrow), which were similar to those of peripheral blood platelets. Bars, 1 μm. (B) Mouse platelets (12 wk old) or ESPs (day 12) were subjected to flow cytometry. Graphs show representative forward scatter (FSC) or side scatter (SSC) dot plots. ESPs vary in size compared with mouse platelets. The remaining six graphs show surface expression of αIIb integrin subunit, GPIbα, GPIbβ, GPV, GPVI, and GPIX on mouse platelets (red lines) or ESPs (blue lines) with control IgG (black lines). (C) (i) On the indicated days of culture (x axis), expression of GPIbα and GPIbβ in αIIb+ megakaryocytes derived from ESCs and ESPs is shown. Panel (ii) shows the number of αIIb+ ESPs on days 8 and 12 that are either GPIbα+ (black bar) or GPIbα− (white bar). All results are mean ± SEM from four independent experiments.
We examined the relative expression of receptors by flow cytometry comparing ESPs and freshly isolated murine platelets. Expression of the integrin αIIb, GPIbβ, and GPIX was similar in platelets from the two sources (Fig. 2 B). However, expression of GP Ibα, GPV, and GPVI was significantly less for ESPs as compared with mouse platelets (Fig. 2 B). Cells with an αIIb+GPIbα−GPIbβ+ phenotype composed 35–65% of the cellular population after 12–14 d in culture. Reduced GPIbα expression of ESPs was not detected at day 8 (Figs. 2 C, i and ii), consistent with a longer time in culture leading to a loss of GPIbα+ cells or shedding of GPIbα from the cell. Unlike ESPs, ESC-derived megakaryocytes did not exhibit reduced GPIbα expression, even when cultured for >8 d (Fig. 2 C, i).
ADAM17 can cleave the extracellular domain of GPIbα on human and mouse platelets abrogating platelet function by its metalloproteinase activity (16, 17). Thus, we investigated whether a metalloproteinase supported the shedding of GPIbα in culture. Two potent metalloproteinase inhibitors, GM6001 (Ilomastat) or TAPI-1, were added to the co-culture system to evaluate the potential involvement of a matrix metalloproteinase (MMP). Because both inhibitors also inhibit MMP9, which has been reported to facilitate megakaryopoiesis (37), the drugs were administered only after day 10 (day 4 of co-culture) when megakaryocyte polyploidy is observed (9). Indeed, we found that the addition of GM6001 at the co-culture start impaired megakaryopoiesis (not depicted). GM6001 and TAPI-1 (Fig. 3 B, 1 and 10 μM) increased GPIbα expression on released ESPs at day 12 (Fig. 3, A and B). This increased expression did not affect the total number of αIIb+ ESPs (αIIb+GPIbα+ plus αIIb+GPIbα−) at days 12 (Fig. 3 C) or 14 (not depicted). But the reduction of GPIbα was not observed on megakaryocytes during the culture (Figs. 2 C, i, and 3 A). Similarly, GM6001 addition to the culture restored expression of GPV and GPVI in αIIb+ ESPs (Fig. 3 D) as reported for aged blood platelets (15, 38). These results suggest that metalloproteinase(s) impairs the retention of GPIbα, GPV, and GPVI on ESPs. Biochemical analysis confirmed that ESPs in culture shed the extracellular domain of GPIbα, referred to as “glycocalicin” (GC), in the absence of GM6001 but not in the presence of GM6001 (Fig. 3 E). As ESC-derived megakaryocytes do not show receptor shedding (Fig. 3, A and E, i), we removed them from the day 12 cell culture population. The remaining specimens were centrifuged, and pellets along with the corresponding supernatant were analyzed. Western blot analysis revealed GPIbα and GC antigen in the absence of GM6001, but a single band of GPIbα alone in the presence of GM6001 (Fig. 3 E, ii). A single band of GC was detectable only in the absence of GM6001 in ESP-derived supernatant (Fig. 3 E, iii).
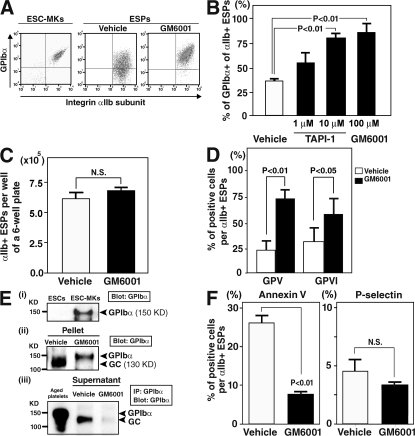
Figure 3.
Inhibition of metalloproteinases in culture restores GPIbα expression in ESPs. (A) On day 10 of ESC culture, 1% DMSO alone (vehicle) or 100 μM GM6001 dissolved with 1% DMSO was added to the culture medium. On day 12 of culture, representative flow cytometry dot plots of mature megakaryocytes derived from ESCs (ESC-MKs) in the absence of GM6001 (left) and ESPs in the absence or presence of GM6001 are shown. (B) The graph shows the percentage of GPIbα+ of all αIIb+ ESPs on day 12 in the absence or presence of 1–10 μM TAPI-1 or 100 μM GM6001. Results are shown as mean ± SEM from three independent experiments. (C) The graph summarizes total numbers of αIIb+ (αIIb+GPIbα+ plus αIIb+GPIbα−) ESPs per well of a 6-well plate on day 12. Results are the mean ± SEM from four independent experiments. (D) The graph shows the effects of the metalloproteinase inhibitor GM6001 on the surface expression of GPV or GPVI in αIIb+ ESPs on day12. Results are mean ± SEM from four independent experiments. (E) Expression of GPIbα or GC in lysates from ESC-MKs (i), in lysates from pellets containing ESPs depleted of MKs (ii), or in supernatant (iii). In panel (i) or (ii), lysates were analyzed by Western blot (7.5% SDS-PAGE) with an anti-GPIbα antibody. In panel (iii), supernatant derived from culture media of ESPs pretreated with or without GM6001 were subjected to immunoprecipitation followed by immunoblotting with anti-GPIbα. In vitro–aged platelets from an adult mouse were used as a positive control in panel (iii). Similar results were obtained from three independent experiments. (F) The graphs show the percentage of positive cells of annexin V or P-selectin from the total αIIb+ ESPs on day 12 in the absence or presence of GM6001. Results are the mean ± SEM from three independent experiments.
To study whether pre-activation by plasma membrane injury or the activation state of ESPs is associated with metalloproteinase activity during culture, we examined the annexin V binding and P-selectin expression in the absence or presence of GM6001. Annexin V is a marker of platelet activation that detects the translocation of phosphatidylserine to the outer membrane, while P-selectin expression is a hallmark marker of activation (13, 39). Annexin V binding, but not P-selection expression, was inhibited in the presence of GM6001 (Fig. 3 F). These data indicate that metalloproteinase-dependent cellular changes occurring during ESP generation leads to a reduced viability of these cells. Comparable results were obtained using a variety of different murine ESC lines (R1, EB3, TT2, or E14.1; unpublished data).
Inhibition of metalloproteinase activity restores integrin αIIbβ3 bidirectional signaling in ESPs
To explore whether metalloproteinases induce extracellular shedding of GPIbα and affect αIIbβ3 inside-out signaling, we used flow cytometry to assess specific fibrinogen binding upon agonist stimulation (9). GM6001 restored specific fibrinogen binding when washed ESPs were stimulated with thrombin or ADP (Fig. 4 A). To rule out the possibility that metalloproteinases directly impair integrin structure in ESPs, MnCl2 was also used (40). Binding of Alexa 488–conjugated fibrinogen to ESPs was comparable in the presence or absence of GM6001 (Fig. 4 A) and similar to that in blood platelets (not depicted). Moreover, αIIbβ3-based actin cytoskeletal changes (outside-in signaling) of ESPs in culture were unexpectedly enhanced in the presence of GM6001, apparent when lamellipodia formation was facilitated by the addition of thrombin (Fig. 4 B, arrow). To address whether our observation of defects in ESPs also applied to blood platelets, we generated in vitro–injured human platelets by incubation at 37°C for 24 h. Reduced GPIbα expression was determined by flow cytometry and Western blot analysis (unpublished data), and a defect in lamellipodia formation in these aged platelets was observed. Impaired lamellipodia formation, apparent even upon thrombin stimulation, in aged human platelets was partially restored by the administration of GM6001 in culture for 24 h (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20071482/DC1). These data demonstrate that deregulated αIIbβ3-mediated bidirectional signaling (both inside-out and outside-in) may be associated with an increase in metalloproteinase activity in both ESPs and human platelets.
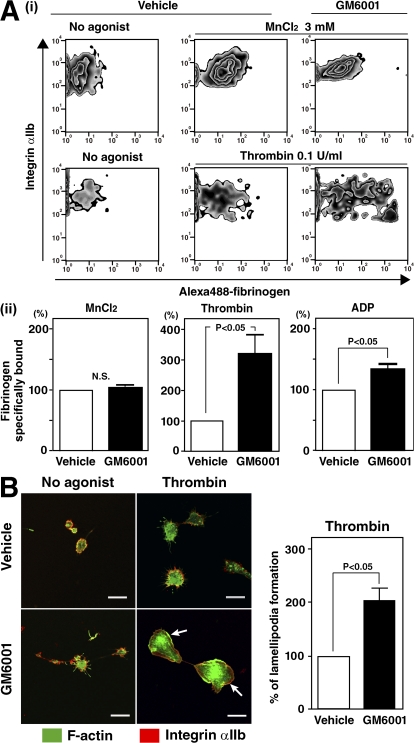
Figure 4.
Inhibition of metalloproteinases is required for platelet function mediated through integrin αIIbβ3 in ESPs. (A) (i) Representative flow cytometry dot plots showing three mM MnCl2-stimulated or 0.1 U/ml thrombin-stimulated fibrinogen binding to integrin αIIbβ3 in ESPs after pretreatment with 1% DMSO as a vehicle or 100 μM GM6001. (ii) The graphs show specific fibrinogen binding to αIIbβ3 stimulated with 3 mM MnCl2 (reference 40), 0.1 U/ml thrombin, or 500 μM adenosine diphosphate. The value of control (vehicle) is defined as 100%. Results are the mean ± SEM from three independent experiments. (B) On day 14 of culture, washed ESPs pretreated with 1% DMSO or 100 μM GM6001 were plated on fibrinogen-coated coverslips for 45 min. An aliquot of each preparation was assayed in the presence of 1 U/ml thrombin. Cells were fixed, permeabilized, and stained with Alexa 488–phalloidin to stain F-actin (green) and with an anti-αIIb antibody followed by Alexa 567 (red). Bar, 10 μm. The value of control (vehicle) is defined as 100%. The right graph summarizes three independent experiments (mean ± SEM).
Metalloproteinases may regulate thrombus formation under flow conditions and posttransfusion recovery in vivo by ESPs
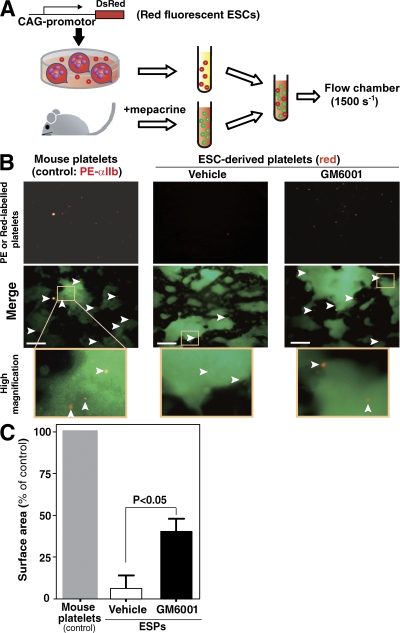
Thrombus formation is a dynamic process, and we sought to examine platelet function under physiologically relevant conditions found in the arterial circulation. An experimental system frequently used to study platelet thrombus formation is perfusion of whole blood over a monolayer of collagen at high shear rates, features mimicking those that occur in vivo (41). In this model, the initial event is tethering of platelets via binding of GPIbα to VWF, the latter being immobilized by collagen (10, 11, 13, 14). Direct interaction between integrin α2β1/GPVI on platelets and collagen follows (10). We prepared reconstituted whole blood consisting of mouse blood labeled with mepacrine, as a marker for platelets (green fluorescence), mixed (1,000:1) with Ds-red–labeled ESC-derived ESPs (red fluorescence). As a control, washed mouse platelets labeled with PE-conjugated anti-αIIb antibody were used (Fig. 5 A) (42). ESPs pretreated with 1% DMSO failed to adhere to collagen-VWF matrices, but pretreatment with GM6001 improved adhesion (Fig. 5, B and C). Most importantly, treating ESPs with GM6001-treated ESPs increased their ability to participate in thrombogenesis. However, their thrombus-forming potential was less than that of uninjured fresh platelets derived from adult mice (Fig. 5 C).
Figure 5.
ESPs treated with GM6001 but not without GM6001 contribute to thrombus formation under flow conditions. (A) Schema of the experimental design. (B) Representative two-dimensional fluorescence images at 6 min. Glass slides coated with type I collagen were perfused at a wall shear rate of 1,500 s−1 for 6 min. Samples included reconstituted whole blood composed of ESPs (red, arrowheads) in blood obtained from 10–12 mice (per experiment), or a mixture of PE-labeled mouse platelets (red, arrowheads) in whole blood pooled from 10–12 mice. All murine platelets or ESPs were stained with mepacrine (green). ESPs capture fluorescent mepacrine as indicated by their dual expression of green and red by flow cytometer. Bars, 100 μm. (C) After 6 min, platelet thrombi formed on the collagen surface were quantified using NIH Images software from a recorded video. The results summarize four independent experiments (mean ± SEM).
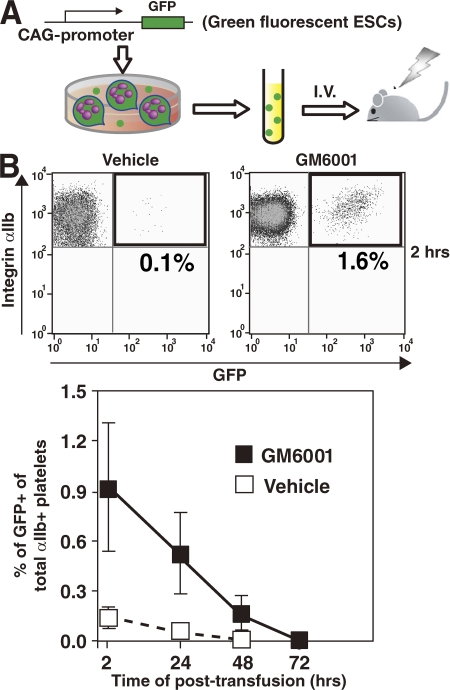
Aged or injured platelets are cleared out of the body after being trapped in the liver (unpublished data) and spleen (43). To confirm the effect of GM6001 on in vivo function of ESPs, we examined the time course kinetics of residual ESPs after transfusion. Because murine platelets do not express Ly5 antigen, we chose GFP-expressing ESCs for in vivo assays after transfusion (Fig. 6 A). More than 80% of ESPs on day 12 expressed GFP in culture. GM6001-treated ESPs were transfused into the tail veins of mice with significant thrombocytopenia (∼104/μl) as a result of irradiation 10 d earlier (Fig. 6 A). 2, 24, 48, or 72 h after transfusion, whole blood was obtained from recipient mice, and the percentage of GFP-expressing platelets among all αIIb+ platelets was determined. No difference in the size of endogenous platelets and ESPs transfused into recipient mice was observed (not depicted), suggesting that the in vivo circulation that may induce shear stress is an important determinant of platelet size (44). Preincubation with GM6001 consistently increased the percentage of GFP+ ESPs after transfusion compared with controls (Fig. 6 B).
Figure 6.
The effect of metalloproteinase inhibition on posttransfusion recovery by ESPs in mice. (A) Schema of the experimental design. (B) 2 h after transfusion of 3 × 106 ESPs, using a 129Ola strain mouse model in which platelet numbers are severely reduced, blood obtained from the retroorbital venous plexus was treated with 3.8% sodium citrate and subjected to flow cytometry to detect GFP+ platelets (ESPs) among all αIIb+ platelets. Additional serial evaluations were performed 24, 48, and 72 h after transfusion. Pretreatment with GM6001 increased the ratio of GFP+ ESPs per a recipient at all points examined. Results are the mean ± SEM from three independent experiments.
DISCUSSION
This study has shown that (a) GPIbα, GPV, and GPVI, but not integrin αIIbβ3 or GPIbβ, are shed from ESPs during culture; (b) the process is specific for ESPs and not relevant for megakaryocyte differentiation from ESCs; (c) the use of a metalloproteinase inhibitor retains the complete GPIb–V-IX complex on ESPs; and (d) both inside-out and outside-in signaling of αIIbβ3 might be associated with metalloproteinase activity. A link between metalloproteinase regulation and αIIbβ3-mediated signaling has not been reported previously. Preventing shedding of the α-subunit of the GPIb–V-IX complex retains key binding sites for VWF, thrombin, coagulation factors, P-selectin, and Mac-1, all potentially important for normal platelet function (13). The N-terminal motif of GPIbα is essential for arterial thrombosis independent of VWF (12), and the signaling cascade from the GPIb–V-IX complex to αIIbβ3 is well known to regulate integrin activation (45). However, the mechanisms whereby metalloproteinase activity regulates integrin signaling remain to be identified.
Metalloproteinases are functionally regulated by endogenous inhibitors, the tissue inhibitors of metalloproteinases (TIMPs) (46). When we considered the reason why megakaryocytes maintained GPIbα in culture, as opposed to ESPs, we hypothesized that TIMP-3, also known to inhibit ADAM17 (47), might be highly expressed in megakaryocytes but not in platelets (48). We therefore determined TIMP-3 expression in ESC-derived megakaryocytes and ESPs, but found no significant difference by RT-PCR (unpublished data). Alternatively, receptor shedding may be occurring with megakaryocytes but is not detectable owing to their gene and protein expression potential, which differs from the anucleate platelet (49).
Another interesting observation was that an administration of GM6001 or TAPI-1 from the beginning of culture impaired the production of c-Kit+αIIb+ cells by day 6 of the liquid culture (unpublished data). We observed that the use of inhibitors to metalloproteinases, which cover both MMPs and ADAMs, is advantageous for the production of efficacious platelets only after day 10 of co-culture. Nonspecific inhibition to metalloproteinases may affect the early phase of hematopoiesis from ESCs as demonstrated in postnatal hematopoiesis through MMP9-mediated mechanisms (50). In addition, it has been reported that MMP2 or MMP9 may have an effect on platelet function. MMP2 activates platelet aggregation through an increase in phospholipase C, protein kinase C, Ca2+ mobilization, and phosphatydylinositol 3 kinase (46, 51, 52). MMP9 blocks phospholipase C, protein kinase C, Ca2+ mobilization, and thromboxane A2 production leading to the inhibition of the effects of MMP2 (46, 52, 53). Integrins share some of these pathways and therefore might explain how both metalloproteinases and integrin activation might influence each other. In a search for specific MMPs regulating platelet function, we analyzed mice deficient in MMP9 and found comparable platelet numbers, integrin activation, and platelet spreading on fibrinogen comparing wild-type and MMP9-deficient mice (Fig. S2, A, C, and D, available at http://www.jem.org/cgi/content/full/jem.20071482/DC1). In addition, extracellular shedding of GPIbα was observed using in vitro–injured platelets from MMP9−/− mice as well as platelets from MMP9+/+ mice (Fig. S2 B). These results indicate that MMP9 per se is not implicated in thrombopoiesis and platelet function.
Our results suggest that the administration of metalloproteinase inhibitors prolongs the half-life of circulating ESPs in vivo (Fig. 6), presumably by maintaining the repertoire of membrane receptors, such as GPIb–V-IX and GPVI. In patients with thrombocytopenia, platelet destruction is proportional to plasma concentrations of GC, a shedding product that includes the N-terminal domain of GPIbα (54). The ectodomain of GPIbα, GPV, and GPVI is easily shed in stocked human PCs possibly due to increased activities of ADAM17 and ADAM10 (12, 13, 55). Consistent with our study, GM6001 has been recently shown to prevent inactivation of refrigerated platelets by inhibiting ADAM17 activity (56). Specific inhibition to ADAM17 (and ADAM10) with spatial-temporal regulation not affecting hematopoiesis in ESCs may increase generation and storage of efficacious ESPs.
In conclusion, the inhibition of metalloproteinases in murine cultures of ESC-derived c-Kit+αIIb+ primitive cells represents an improvement in the production of efficacious ESPs. Confirming these observations starting with human cells is a future direction potentially defining an experimental framework to produce ESPs in sufficient quantity for clinical application.
MATERIALS AND METHODS
Plasmid preparation, reagents, and mice.
All reagents were purchased from Sigma-Aldrich unless otherwise indicated. All animal and recombinant DNA experiments were approved by the Institutional Animal Care and Use Committee of the Institute of Medical Science, University of Tokyo. C57BL/6 mice were purchased from SLC. 129Ola mice (transfusion recipients) were purchased from The Jackson Laboratory. MMP9−/− mice were provided by Z. Werb (University of California, San Francisco, San Francisco, CA) (45). Rhodamine- and Alexa 488–conjugated phalloidin, Alexa 488–conjugated fibrinogen, Alexa 568–conjugated bovine IgG, and Alexa 647–conjugated mouse IgG antibodies were from Invitrogen. Purified human fibrinogen was from American Diagnostica Inc. FITC- and allophycocyanin (APC)-conjugated, PE-conjugated, and unconjugated anti–mouse integrin αIIb subunit and APC-conjugated anti–c-Kit, PE-conjugated anti-CD31, PE-conjugated anti–Sca-1, biotin-conjugated anti-CD9, FITC-conjugated anti–P-selectin, unconjugated human anti-GPIbα, and streptoavidin-APC–cyanine 7 (APC-Cy7) antibodies were from BD Biosciences. An annexin V–FITC apoptosis detection kit was purchased from BD Biosciences. PE-conjugated or unconjugated anti–mouse GPIbα, FITC-conjugated anti–mouse GPIbβ, GPV, GPVI, and GPIX antibodies were from Emfret. Biotin-conjugated anti–VE-cadherin antibody was provided by M. Ogawa (Kumamoto University, Kumamoto, Japan). Human TPO and erythropoietin were provided by H. Miyazaki (Kirin, Takasaki, Japan). Mouse leukemia inhibitory factor (RESGRO) was from Millipore. Human TPO, IL-6, and IL-11 were purchased from PeproTech. GM6001 and TAPI-1 (57) were from EMD.
Growth and differentiation of ES cells.
The murine ESC lines E14tg2A (58), E14 (5), R1 (8), EB3 (37), and TT2 (7) were maintained as described previously (9). For EB formation, 2 × 105 ESCs were placed in 100-mm bacterial Petri dishes containing Iscove's modified Dulbecco's medium supplemented with a cocktail of 300 μg/ml human transferrin, 0.45 mM monothioglycerol, 50 μg/ml ascorbic acid, and penicillin-streptomycin/l-glutamine solution (Invitrogen). On day 5 or 6 of culture, cells were dissociated with 0.25% trypsin/EDTA and subjected to sorting by FACS MoFlo (Dako). Sorted cells (2 × 104 per well) were seeded onto OP9 stroma cells in 6-well plates with 20 ng/ml TPO as described previously (9). After 3 d of culture, a cocktail of 10 ng/ml TPO, 5 ng/ml IL-6, and 10 ng/ml IL-11 was added. Cell surface antigen expression was examined by flow cytometry (FACS Aria; BD Biosciences).
Establishment of GPIb-ESC line.
An expression construct containing a human genomic DNA fragment containing the GPIbα promoter (28), followed by coding sequence for a human GPIbα––EGFP fusion, was inserted into pcDNA 3.1 (+)/zeo vector (Invitrogen). All sequences were subsequently confirmed by nucleotide sequencing. Completed pcDNA3.1 zeo vector was linearized with NheI site and transfected into E14tg2A ESCs by electroporation. After drug selection with 65 μg/ml zeocin for 7 d, resistant colonies were collected. Viable ESCs were confirmed by PCR to detect zeocin and human GPIbα–EGFP as a positive clone. To define useful ESC clones further, the intensity of GFP expression was assessed after differentiation into megakaryocytes (Fig. 1 C).
Quantification by RT-PCR.
Sorted cells from day 6 EB or day 8 αIIb+c-Kit− megakaryocytes cultured on OP9 were lysed with Trizol-LS (Invitrogen) for total RNA extraction. cDNAs were obtained by using Thermo Script RT-PCR System and oligo-dT primer (Invitrogen). Final results were normalized with intrinsic GAPDH and PCR Taqman PCR probe in quantity (Applied Biosystems). RT-PCR was performed to determine expression levels of genes of interest. Amplification proceeded for 30–39 cycles. PCR products were separated on agarose gels and visualized by ethidium bromide staining. The primer sets used are shown in Table S1, which is available at http://www.jem.org/cgi/content/full/jem.20071482/DC1.
ESP preparation and TEM study.
Platelets in culture supernatant were gently collected. Acid citrate dextrose solution was added to yield final concentrations of 8.5 mM sodium citrate, 6.5 mM citric acid, and 10.4 mM glucose. The collected fluid was centrifuged at 150 g for 10 min to eliminate large cells (e.g., megakaryocytes). The supernatant was transferred into a new tube, 1 μM prostaglandin E1 and 1 U/ml apyrase were added to prevent platelet activation, and the mixture was centrifuged at 400 g for 10 min to sediment a platelet pellet. The pellet was resuspended in an appropriate volume of modified Tyrode-Hepes buffer, pH 7.4 (10 mM Hepes, 12 mM NaHCO3, 138 mM NaCl, 5.5 mM glucose, 2.9 mM KCl, and 1 mM MgCl2 without Ca2+), or in 2% fetal bovine serum in PBS. To determine the number of platelets in culture, cells were mixed with True Count Beads (BD Biosciences). To detect ESPs in flow cytometry dot plots, the side scatter and forward scatter gates for murine plasma platelets (from mice aged 8–12 wk) were used. TEM studies were performed by using a JEOL 1200EX transmission electron microscope operating at 80 kV (JOEL). Specimens were treated with a mixture of 0.5% glutaraldehyde and 2% paraformaldehyde, followed by 1% osmium tetroxide for observation.
Biochemical studies.
For immunoprecipitation, lysis buffer (2% Triton X-100 or 1% NP-40, 150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 0.5 mM EGTA, 0.5 mM EDTA, 1 mM Na3VO4, 0.5 mM NaF, 0.5 mM PMSF, and 50 μg/ml leupeptin) was used. Separated supernatant in culture at day 12 of culture was immunoprecipitated with anti-GPIbα antibody and immunoblotted with anti-GPIbα antibody (clone Xia G7).
Integrin activation and actin cytoskeletal changes.
To investigate integrin αIIbβ3 activation, 50-μl aliquots of ESPs were incubated with APC-conjugated anti-αIIb and 200 μg/ml Alexa 488–fibrinogen in the absence or presence of thrombin or ADP for 30 min at room temperature. Binding of Alexa 488–fibrinogen to ESPs was quantified using flow cytometry. Nonspecific binding was determined in the presence of 10 mM EDTA or 20 μg/ml 1B5, a specific inhibitor of mouse αIIbβ3 (provided by B. Coller, The Rockefeller University, New York, NY). Specific binding was defined as total minus nonspecific binding. To investigate outside-in signaling via αIIbβ3, all observations of cytoskeletal changes (morphology of spreading) in ESPs were performed using a confocal microscopic system (TCS SP2; Leica) as described previously (9).
Flow chamber study.
To study the effect of inhibition on metalloproteinases, reconstituted whole blood was prepared by combining ESPs and mouse blood. ESPs were generated from ESCs in which the CAG promoter consistently controls Ds-red expression (provided by H. Niwa, RIKEN, Kobe, Japan) (Fig. 5 A). ESPs in which red fluorescent protein was expressed were combined (1:1,000 ratio) with mouse whole blood that had been labeled with mepacrine to mark mouse platelets; the total volume per experiment was 7–8 ml. Washed platelets obtained from mice aged 10–12 wk were stained with 4 μg/ml of PE-labeled anti-αIIb antibody (to avoid blocking effect) (42). This whole blood was also obtained from 11–12 C57/BL6 mice aged 10–12 wk per single experiment; argatrovan, an anti-thrombin drug (Mitsubishi Pharma), was added at a final concentration of 100 μM. Samples of the treated blood, containing ESPs, were injected into the chamber with a syringe pump (Harvard Apparatus) at a constant flow rate to achieve high wall shear rates (i.e., 1,500 s−1) on collagen surfaces. Platelet thrombi forming on the collagen surfaces were visualized with an inverted-stage epifluorescence videomicroscope system (DM IRB; Leica) as described previously (59). The microscopic images were digitized online with a photosensitive color CCD camera (L-600; Leica). Image-J software (National Institutes of Health [NIH] Image) was used to quantify the percentages of surfaces covered by platelets.
Kinetics of residualESPs in vivo after transfusion.
ESPs were generated from ESCs in which the CAG promoter consistently controls GFP expression (provided by H. Niwa, RIKEN, Kobe, Japan) (Fig. 6 A). ESPs were generated in culture in the absence or presence of metalloproteinase inhibitor. On day 12 of culture, 3 × 106 ESPs were transfused into mice (129Ola strain) in which platelet numbers had been reduced by irradiation (650 cGy) 10 d beforehand. To detect GFP-expressing ESPs at the indicated time points, blood samples were collected with micro-capillaries from the retroorbital venous plexus and stained with APC-conjugated anti-αIIb antibody. The percentage of GFP+ ESPs was determined by flow cytometry for each specimen.
Statistical analysis.
Differences between experimental and control results (mean ± SE median) were analyzed by Student's t test. Probability values of P < 0.05 were considered significant.
Online supplemental material.
Table S1 depicts the primers for Fig. 1 F. Fig. S1 shows the effects of GM6001 on the spreading of aged human platelets on fibrinogen. Fig. S2 shows the platelet number and functions via an integrin bidirectional signaling in MMP9−/− mice and their control MMP9+/+ mice. The online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20071482/DC1.
Supplementary Material
Acknowledgments
The authors thank Drs. H. Miyazaki, M. Ogawa, H. Niwa, B. Coller, and Z. Werb for providing reagents and mice. We are also very grateful to H. Tsukui for her excellent help and Drs. A.S. Knisely and M. Mahaut-Smith for manuscript review.
H. Nishikii, K. Eto, N. Tamura, T. Kanaji, A. Sawaguchi, and S. Goto performed the study. K. Hattori, B. Heissig, and J. Ware provided critical reagents. H. Nishikii and K. Eto designed the study. K. Eto and H. Nakauchi wrote the manuscript.
This work was supported by grants from the Ministry of Education, Culture, Sport, Science and Technology of Japan (to K. Eto and H. Nakauchi) and by a grant from Vehicle Locomotion Foundation (Tokyo, Japan, to K. Eto and S. Goto).
The authors have no conflicting financial interests.
Abbreviations used: ADAM, a disintegrin and metalloproteinase; EB, embryoid body; ESC, embryonic stem cell; ESP, ESC-derived platelet; Flk, fetal liver kinase; GC, glycocalicin; GP, glycoprotein; HSC, hematopoietic stem cell; MMP, matrix metalloproteinase; PC, platelet concentrate; TEM, transmission electron microscopy; TIMP, tissue inhibitors of metalloproteinase; TPO, thrombopoietin; VE, vascular endothelial; VWF, von Willebrand factor.
References
- 1.Webb, I.J., and K.C. Anderson. 1999. Risks, costs, and alternatives to platelet transfusions. Leuk. Lymphoma. 34:71–84. [DOI] [PubMed] [Google Scholar]
- 2.McCullough, J. 2000. Current issues with platelet transfusion in patients with cancer. Semin. Hematol. 37:3–10. [DOI] [PubMed] [Google Scholar]
- 3.Berger, G., D.W. Hartwell, and D.D. Wagner. 1998. P-Selectin and platelet clearance. Blood. 92:4446–4452. [PubMed] [Google Scholar]
- 4.Matsunaga, T., I. Tanaka, M. Kobune, Y. Kawano, M. Tanaka, K. Kuribayashi, S. Iyama, T. Sato, Y. Sato, R. Takimoto, et al. 2006. Ex vivo large-scale generation of human platelets from cord blood CD34+ cells. Stem Cells. 24:2877–2887. [DOI] [PubMed] [Google Scholar]
- 5.Keller, G. 2005. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 19:1129–1155. [DOI] [PubMed] [Google Scholar]
- 6.Asano, T., K. Sasaki, Y. Kitano, K. Terao, and Y. Hanazono. 2006. In vivo tumor formation from primate embryonic stem cells. Methods Mol. Biol. 329:459–467. [DOI] [PubMed] [Google Scholar]
- 7.van der Meer, P.F., and R.N. Pietersz. 2005. Gamma irradiation does not affect 7-day storage of platelet concentrates. Vox Sang. 89:97–99. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto, T.T., S. Kohata, H. Suzuki, H. Miyazaki, and K. Fujimura. 2003. Production of functional platelets by differentiated embryonic stem (ES) cells in vitro. Blood. 102:4044–4051. [DOI] [PubMed] [Google Scholar]
- 9.Eto, K., R. Murphy, S.W. Kerrigan, A. Bertoni, H. Stuhlmann, T. Nakano, A.D. Leavitt, and S.J. Shattil. 2002. Megakaryocytes derived from embryonic stem cells implicate CalDAG-GEFI in integrin signaling. Proc. Natl. Acad. Sci. USA. 99:12819–12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruggeri, Z.M. 2002. Platelets in atherothrombosis. Nat. Med. 8:1227–1234. [DOI] [PubMed] [Google Scholar]
- 11.Nieswandt, B., and S.P. Watson. 2003. Platelet-collagen interaction: is GPVI the central receptor? Blood. 102:449–461. [DOI] [PubMed] [Google Scholar]
- 12.Bergmeier, W., C.L. Piffath, T. Goerge, S.M. Cifuni, Z.M. Ruggeri, J. Ware, and D.D. Wagner. 2006. The role of platelet adhesion receptor GPIbalpha far exceeds that of its main ligand, von Willebrand factor, in arterial thrombosis. Proc. Natl. Acad. Sci. USA. 103:16900–16905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canobbio, I., C. Balduini, and M. Torti. 2004. Signalling through the platelet glycoprotein Ib-V-IX complex. Cell. Signal. 16:1329–1344. [DOI] [PubMed] [Google Scholar]
- 14.Berndt, M.C., Y. Shen, S.M. Dopheide, E.E. Gardiner, and R.K. Andrews. 2001. The vascular biology of the glycoprotein Ib-IX-V complex. Thromb. Haemost. 86:178–188. [PubMed] [Google Scholar]
- 15.Rabie, T., A. Strehl, A. Ludwig, and B. Nieswandt. 2005. Evidence for a role of ADAM17 (TACE) in the regulation of platelet glycoprotein V. J. Biol. Chem. 280:14462–14468. [DOI] [PubMed] [Google Scholar]
- 16.Bergmeier, W., C.L. Piffath, G. Cheng, V.S. Dole, Y. Zhang, U.H. von Andrian, and D.D. Wagner. 2004. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates GPIbalpha shedding from platelets in vitro and in vivo. Circ. Res. 95:677–683. [DOI] [PubMed] [Google Scholar]
- 17.Bergmeier, W., P.C. Burger, C.L. Piffath, K.M. Hoffmeister, J.H. Hartwig, B. Nieswandt, and D.D. Wagner. 2003. Metalloproteinase inhibitors improve the recovery and hemostatic function of in vitro-aged or -injured mouse platelets. Blood. 102:4229–4235. [DOI] [PubMed] [Google Scholar]
- 18.Goto, S., N. Tamura, H. Ishida, and Z.M. Ruggeri. 2006. Dependence of platelet thrombus stability on sustained glycoprotein IIb/IIIa activation through adenosine 5′-diphosphate receptor stimulation and cyclic calcium signaling. J. Am. Coll. Cardiol. 47:155–162. [DOI] [PubMed] [Google Scholar]
- 19.Shattil, S.J., and P.J. Newman. 2004. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 104:1606–1615. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi, K., and S. Yamanaka. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126:663–676. [DOI] [PubMed] [Google Scholar]
- 21.Hanna, J., M. Wernig, S. Markoulaki, C.W. Sun, A. Meissner, J.P. Cassady, C. Beard, T. Brambrink, L.C. Wu, T.M. Townes, and R. Jaenisch. 2007. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 318:1920–1923. [DOI] [PubMed] [Google Scholar]
- 22.Nakorn, T.N., T. Miyamoto, and I.L. Weissman. 2003. Characterization of mouse clonogenic megakaryocyte progenitors. Proc. Natl. Acad. Sci. USA. 100:205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang, J., F. Varas, M. Stadtfeld, S. Heck, N. Faust, and T. Graf. 2007. CD41-YFP mice allow in vivo labeling of megakaryocytic cells and reveal a subset of platelets hyperreactive to thrombin stimulation. Exp. Hematol. 35:490–499. [DOI] [PubMed] [Google Scholar]
- 24.Kyba, M., R.C. Perlingeiro, and G.Q. Daley. 2002. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 109:29–37. [DOI] [PubMed] [Google Scholar]
- 25.Zhang, W.J., C. Park, E. Arentson, and K. Choi. 2005. Modulation of hematopoietic and endothelial cell differentiation from mouse embryonic stem cells by different culture conditions. Blood. 105:111–114. [DOI] [PubMed] [Google Scholar]
- 26.Nakano, T., H. Kodama, and T. Honjo. 1994. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 265:1098–1101. [DOI] [PubMed] [Google Scholar]
- 27.Nishikawa, S.I., S. Nishikawa, M. Hirashima, N. Matsuyoshi, and H. Kodama. 1998. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 125:1747–1757. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto, Y., and J. Ware. 1995. Identification of essential GATA and Ets binding motifs within the promoter of the platelet glycoprotein Ib alpha gene. J. Biol. Chem. 270:24532–24539. [DOI] [PubMed] [Google Scholar]
- 29.Ohmori, T., J. Mimuro, K. Takano, S. Madoiwa, Y. Kashiwakura, A. Ishiwata, M. Niimura, K. Mitomo, T. Tabata, M. Hasegawa, et al. 2006. Efficient expression of a transgene in platelets using simian immunodeficiency virus-based vector harboring glycoprotein Ibalpha promoter: in vivo model for platelet-targeting gene therapy. FASEB J. 20:1522–1524. [DOI] [PubMed] [Google Scholar]
- 30.Lavenu-Bombled, C., B. Izac, F. Legrand, M. Cambot, A. Vigier, J.M. Masse, and A. Dubart-Kupperschmitt. 2007. Glycoprotein Ibalpha promoter drives megakaryocytic lineage-restricted expression after hematopoietic stem cell transduction using a SIN lentiviral vector. Stem Cells. 25:1571–1577. [DOI] [PubMed] [Google Scholar]
- 31.Fujita, H., Y. Hashimoto, S. Russell, B. Zieger, and J. Ware. 1998. In vivo expression of murine platelet glycoprotein Ibalpha. Blood. 92:488–495. [PubMed] [Google Scholar]
- 32.Fraser, S.T., M. Ogawa, R.T. Yu, S. Nishikawa, M.C. Yoder, and S. Nishikawa. 2002. Definitive hematopoietic commitment within the embryonic vascular endothelial-cadherin(+) population. Exp. Hematol. 30:1070–1078. [DOI] [PubMed] [Google Scholar]
- 33.Lepage, A., M. Leboeuf, J.P. Cazenave, C. de la Salle, F. Lanza, and G. Uzan. 2000. The alpha(IIb)beta(3) integrin and GPIb-V-IX complex identify distinct stages in the maturation of CD34(+) cord blood cells to megakaryocytes. Blood. 96:4169–4177. [PubMed] [Google Scholar]
- 34.Shivdasani, R.A. 2001. Molecular and transcriptional regulation of megakaryocyte differentiation. Stem Cells. 19:397–407. [DOI] [PubMed] [Google Scholar]
- 35.Schulze, H., and R.A. Shivdasani. 2005. Mechanisms of thrombopoiesis. J. Thromb. Haemost. 3:1717–1724. [DOI] [PubMed] [Google Scholar]
- 36.Tober, J., A. Koniski, K.E. McGrath, R. Vemishetti, R. Emerson, K.K. de Mesy-Bentley, R. Waugh, and J. Palis. 2007. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood. 109:1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lane, W.J., S. Dias, K. Hattori, B. Heissig, M. Choy, S.Y. Rabbany, J. Wood, M.A. Moore, and S. Rafii. 2000. Stromal-derived factor 1-induced megakaryocyte migration and platelet production is dependent on matrix metalloproteinases. Blood. 96:4152–4159. [PubMed] [Google Scholar]
- 38.Stephens, G., Y. Yan, M. Jandrot-Perrus, J.L. Villeval, K.J. Clemetson, and D.R. Phillips. 2005. Platelet activation induces metalloproteinase-dependent GP VI cleavage to down-regulate platelet reactivity to collagen. Blood. 105:186–191. [DOI] [PubMed] [Google Scholar]
- 39.Clarke, M.C., J. Savill, D.B. Jones, B.S. Noble, and S.B. Brown. 2003. Compartmentalized megakaryocyte death generates functional platelets committed to caspase-independent death. J. Cell Biol. 160:577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nieswandt, B., M. Moser, I. Pleines, D. Varga-Szabo, S. Monkley, D. Critchley, and R. Fassler. 2007. Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo. J. Exp. Med. 204:3113–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yap, C.L., K.E. Anderson, S.C. Hughan, S.M. Dopheide, H.H. Salem, and S.P. Jackson. 2002. Essential role for phosphoinositide 3-kinase in shear-dependent signaling between platelet glycoprotein Ib/V/IX and integrin alpha(IIb)beta(3). Blood. 99:151–158. [DOI] [PubMed] [Google Scholar]
- 42.Falati, S., P. Gross, G. Merrill-Skoloff, B.C. Furie, and B. Furie. 2002. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat. Med. 8:1175–1181. [DOI] [PubMed] [Google Scholar]
- 43.Hoffmeister, K.M., T.W. Felbinger, H. Falet, C.V. Denis, W. Bergmeier, T.N. Mayadas, U.H. von Andrian, D.D. Wagner, T.P. Stossel, and J.H. Hartwig. 2003. The clearance mechanism of chilled blood platelets. Cell. 112:87–97. [DOI] [PubMed] [Google Scholar]
- 44.Junt, T., H. Schulze, Z. Chen, S. Massberg, T. Goerge, A. Krueger, D.D. Wagner, T. Graf, J.E. Italiano Jr., R.A. Shivdasani, and U.H. von Andrian. 2007. Dynamic visualization of thrombopoiesis within bone marrow. Science. 317:1767–1770. [DOI] [PubMed] [Google Scholar]
- 45.Kasirer-Friede, A., M.R. Cozzi, M. Mazzucato, L. De Marco, Z.M. Ruggeri, and S.J. Shattil. 2004. Signaling through GP Ib-IX-V activates alpha IIb beta 3 independently of other receptors. Blood. 103:3403–3411. [DOI] [PubMed] [Google Scholar]
- 46.Santos-Martinez, M.J., C. Medina, P. Jurasz, and M.W. Radomski. 2007. Role of metalloproteinases in platelet function. Thromb. Res. 121:535–542. [DOI] [PubMed] [Google Scholar]
- 47.Amour, A., P.M. Slocombe, A. Webster, M. Butler, C.G. Knight, B.J. Smith, P.E. Stephens, C. Shelley, M. Hutton, V. Knauper, et al. 1998. TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett. 435:39–44. [DOI] [PubMed] [Google Scholar]
- 48.Radomski, A., P. Jurasz, E.J. Sanders, C.M. Overall, H.F. Bigg, D.R. Edwards, and M.W. Radomski. 2002. Identification, regulation and role of tissue inhibitor of metalloproteinases-4 (TIMP-4) in human platelets. Br. J. Pharmacol. 137:1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimmerman, G.A., and A.S. Weyrich. 2008. Signal-dependent protein synthesis by activated platelets: new pathways to altered phenotype and function. Arterioscler. Thromb. Vasc. Biol. 28:s17–s24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heissig, B., K. Hattori, S. Dias, M. Friedrich, B. Ferris, N.R. Hackett, R.G. Crystal, P. Besmer, D. Lyden, M.A. Moore, et al. 2002. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 109:625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Falcinelli, E., G. Guglielmini, M. Torti, and P. Gresele. 2005. Intraplatelet signaling mechanisms of the priming effect of matrix metalloproteinase-2 on platelet aggregation. J. Thromb. Haemost. 3:2526–2535. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez-Patron, C., M.A. Martinez-Cuesta, E. Salas, G. Sawicki, M. Wozniak, M.W. Radomski, and S.T. Davidge. 1999. Differential regulation of platelet aggregation by matrix metalloproteinases-9 and -2. Thromb. Haemost. 82:1730–1735. [PubMed] [Google Scholar]
- 53.Sheu, J.R., T.H. Fong, C.M. Liu, M.Y. Shen, T.L. Chen, Y. Chang, M.S. Lu, and G. Hsiao. 2004. Expression of matrix metalloproteinase-9 in human platelets: regulation of platelet activation in in vitro and in vivo studies. Br. J. Pharmacol. 143:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Houwerzijl, E.J., N.R. Blom, J.J. van der Want, M.T. Esselink, J.J. Koornstra, J.W. Smit, H. Louwes, E. Vellenga, and J.T. de Wolf. 2004. Ultrastructural study shows morphologic features of apoptosis and para-apoptosis in megakaryocytes from patients with idiopathic thrombocytopenic purpura. Blood. 103:500–506. [DOI] [PubMed] [Google Scholar]
- 55.Gardiner, E.E., D. Karunakaran, Y. Shen, J.F. Arthur, R.K. Andrews, and M.C. Berndt. 2007. Controlled shedding of platelet glycoprotein (GP)VI and GPIb-IX-V by ADAM family metalloproteinases. J. Thromb. Haemost. 5:1530–1537. [DOI] [PubMed] [Google Scholar]
- 56.Josefsson, E., V. Rumjantseva, C. Dahlgren, W. Bergmeier, D. Wagner, J. Hartwig, and K. Hoffmeister. 2007. Metalloproteinase Inhibitors Increase the Survival of Long-Term Refrigerated Platelets in Mice. Blood. (ASH Annual Meeting Abstracts) 110:419. [Google Scholar]
- 57.Mohler, K.M., P.R. Sleath, J.N. Fitzner, D.P. Cerretti, M. Alderson, S.S. Kerwar, D.S. Torrance, C. Otten-Evans, T. Greenstreet, K. Weerawarna, et al. 1994. Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature. 370:218–220. [DOI] [PubMed] [Google Scholar]
- 58.Doetschman, T., R.G. Gregg, N. Maeda, M.L. Hooper, D.W. Melton, S. Thompson, and O. Smithies. 1987. Targetted correction of a mutant HPRT gene in mouse embryonic stem cells. Nature. 330:576–578. [DOI] [PubMed] [Google Scholar]
- 59.Goto, S., N. Tamura, S. Handa, M. Arai, K. Kodama, and H. Takayama. 2002. Involvement of glycoprotein VI in platelet thrombus formation on both collagen and von Willebrand factor surfaces under flow conditions. Circulation. 106:266–272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.