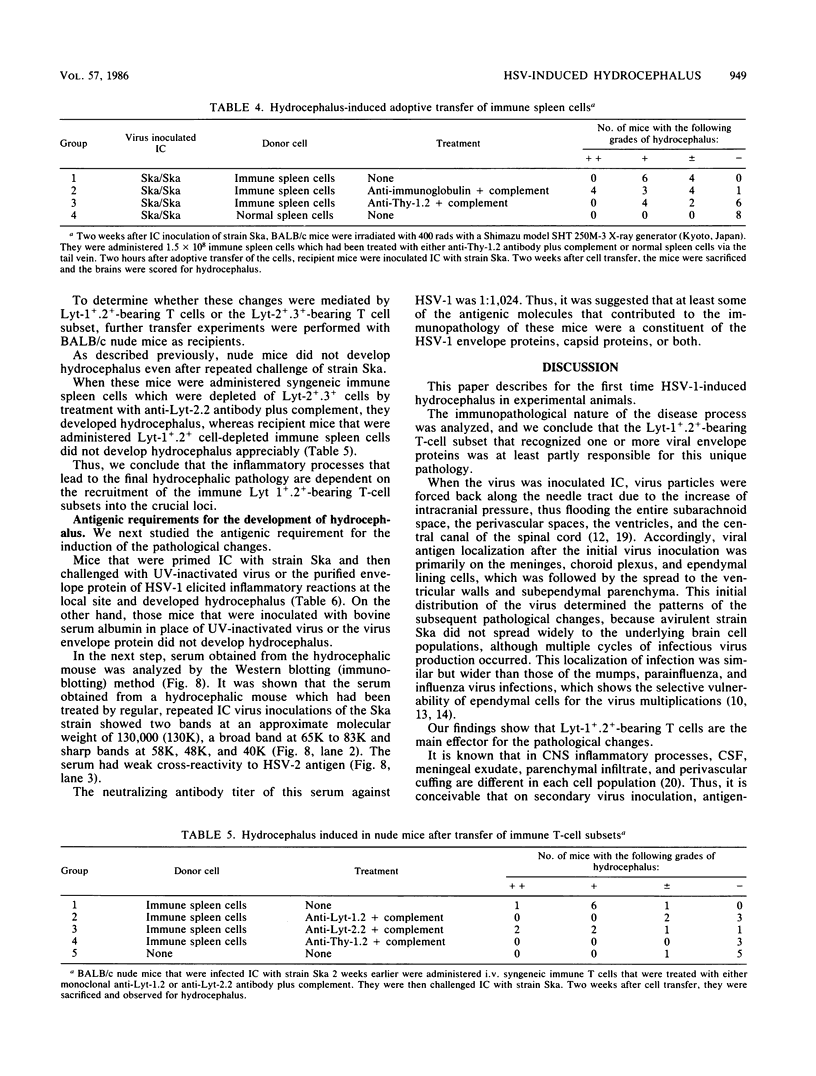
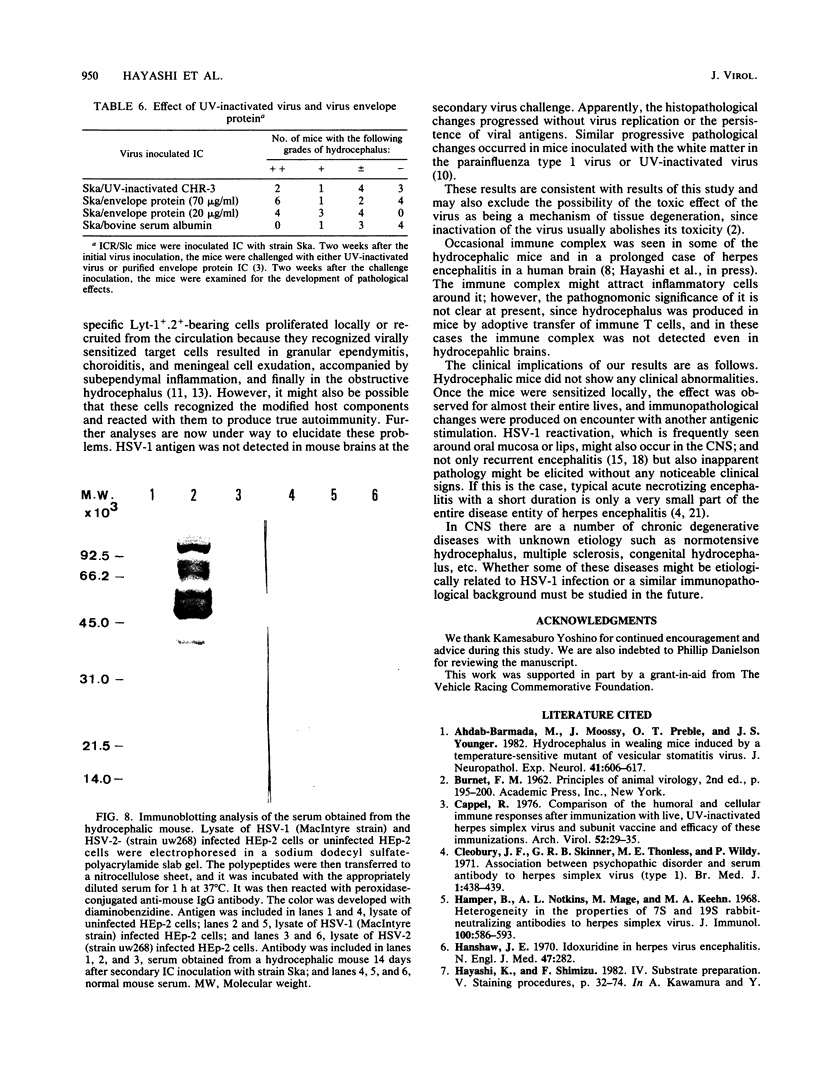
Abstract
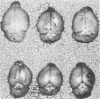
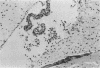
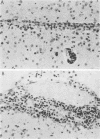
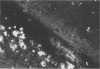
Adult ICR/Slc or BALB/c mice developed hydrocephalus when attenuated herpes simplex virus type 1 (HSV-1) (strain Ska) was injected intracerebrally 2 to 4 weeks earlier and then after mice were challenged with the same virus or virulent HSV-1. Initial inoculation of the Ska strain elicited acute meningitis and ependymitis with transient mild hydrocephalus. Viral antigen was seen in the meninges and subependymal areas, and the virus was titrated during the acute phase of infection. After the second virus inoculation, more prominent inflammation was evoked in the same area, and the animals developed hydrocephalus, although viral antigen and infectious virus were hardly detected. When the mice were immunosuppressed with cyclophosphamide, they ceased to develop hydrocephalus. BALB/c nude mice did not show the same pathology, even though they were treated in the same way. When irradiated mice, which had been infected with the Ska strain intracerebrally 2 weeks earlier, received syngeneic immune spleen cells, they developed hydrocephalus. The T-cell nature of the effector cells was confirmed by the elimination of the pathology after treatment of the donor cells with anti-Thy-1.2 plus complement. No hydrocephalic mice were observed after treatment of the donor cells with anti-Lyt-1.2 plus complement, which gave further evidence of the T-cell nature of the effector cells as the Lyt-1+.2+ antigen-bearing subsets. Intervals between priming and challenge virus inoculation could be more than 18 months. The presence of purified HSV-1 envelope protein was feasible for the development of the hydrocephalic animals.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahdab-Barmada M., Moossy J., Preble O. T., Youngner J. S. Hydrocephalus in weanling mice induced by a temperature-sensitive mutant of vesicular stomatitis virus. J Neuropathol Exp Neurol. 1982 Nov;41(6):606–617. doi: 10.1097/00005072-198211000-00004. [DOI] [PubMed] [Google Scholar]
- Cappel R. Comparison of the humoral and cellular immune response after immunization with live, UV inactivated herpes simplex virus and a subunit vaccine and efficacy of these immunizations. Arch Virol. 1976;52(1-2):29–35. doi: 10.1007/BF01317862. [DOI] [PubMed] [Google Scholar]
- Cleobury J. F., Skinner G. R., Thouless M. E., Wildy P. Association between psychopathic disorder and serum antibody to herpes simplex virus (type 1). Br Med J. 1971 Feb 20;1(5746):438–439. doi: 10.1136/bmj.1.5746.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampar B., Notkins A. L., Mage M., Keehn M. A. Heterogeneity in the properties of 7 S and 19S rabbit-neutralizing antibodies to herpes simplex virus. J Immunol. 1968 Mar;100(3):586–593. [PubMed] [Google Scholar]
- Hayashi K., Takagi S., Sekine N., Kurihara I. Clinical and experimental study of the immune complex (herpes simplex virus type 1-IgM) in herpes encephalitis brain. Ann N Y Acad Sci. 1983;420:147–158. doi: 10.1111/j.1749-6632.1983.tb22199.x. [DOI] [PubMed] [Google Scholar]
- Holtz A., Borman G., Li C. P. Hydrocephalus in mice infected with polyoma virus. Proc Soc Exp Biol Med. 1966 Apr;121(4):1196–1200. doi: 10.3181/00379727-121-31003. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y., McMichael J., Koprowki H. Experimental parainfluenza type 1 virus-induced encephalomyelopathy in adult mouse: a histopathological study. Acta Neuropathol. 1975;31(4):315–324. doi: 10.1007/BF00687926. [DOI] [PubMed] [Google Scholar]
- Johnson K. P., Johnson R. T. Granular ependymitis. Occurrence in myxovirus infected rodents and prevalence in man. Am J Pathol. 1972 Jun;67(3):511–526. [PMC free article] [PubMed] [Google Scholar]
- Johnson R. T., Johnson K. P. Hydrocephalus as a sequela of experimental myxovirus infections. Exp Mol Pathol. 1969 Feb;10(1):68–80. doi: 10.1016/0014-4800(69)90049-5. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Johnson K. P. Hydrocephalus following viral infection: the pathology of aqueductal stenosis developing after experimental mumps virus infection. J Neuropathol Exp Neurol. 1968 Oct;27(4):591–606. [PubMed] [Google Scholar]
- KLUVER H., BARRERA E. A method for the combined staining of cells and fibers in the nervous system. J Neuropathol Exp Neurol. 1953 Oct;12(4):400–403. doi: 10.1097/00005072-195312040-00008. [DOI] [PubMed] [Google Scholar]
- Koenig H., Rabinowitz S. G., Day E., Miller V. Post-infectious encephalomyelitis after successful treatment of herpes simplex encephalitis with adenine arabinoside: ultrastructural observations. N Engl J Med. 1979 May 10;300(19):1089–1093. doi: 10.1056/NEJM197905103001906. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MIMS C. A. Intracerebral injections and the growth of viruses in the mouse brain. Br J Exp Pathol. 1960 Feb;41:52–59. [PMC free article] [PubMed] [Google Scholar]
- Margolis G., Kilham L. Hydrocephalus in hamsters, ferrets, rats, and mice following inoculations with reovirus type I. II. Pathologic studies. Lab Invest. 1969 Sep;21(3):189–198. [PubMed] [Google Scholar]
- Milstein J. M., Biggs H. E., Jr Recurrent encephalitis with elevated titers for herpes simplex. Arch Neurol. 1977 Jul;34(7):434–436. doi: 10.1001/archneur.1977.00500190068010. [DOI] [PubMed] [Google Scholar]
- Moench T. R., Griffin D. E. Immunocytochemical identification and quantitation of the mononuclear cells in the cerebrospinal fluid, meninges, and brain during acute viral meningoencephalitis. J Exp Med. 1984 Jan 1;159(1):77–88. doi: 10.1084/jem.159.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEARER M. L., FINCH S. M. PERIODIC ORGANIC PSYCHOSIS ASSOCIATED WITH RECURRENT HERPES SIMPLEX. N Engl J Med. 1964 Sep 3;271:494–497. doi: 10.1056/NEJM196409032711004. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino K., Andoh T., Hashimoto M., Aoyama Y. Absence of correlation between an attenuated strain of type 1 herpes simplex virus and tumors produced in neonatally infected mice. Jpn J Exp Med. 1976 Apr;46(2):139–144. [PubMed] [Google Scholar]
- Yoshino K., Yanagi K., Abe K. Efficacy of intradermal administration of herpes simplex virus subunit vaccine. Microbiol Immunol. 1982;26(8):753–757. doi: 10.1111/j.1348-0421.1982.tb00220.x. [DOI] [PubMed] [Google Scholar]