Abstract
The single-pass transmembrane protein Ryk (atypical receptor related tyrosine kinase) functions as a Wnt receptor. However, Ryk's correlation with Wnt/Frizzled (Fz) signaling is poorly understood. Here, we report that Ryk regulates Xenopus laevis convergent extension (CE) movements via the β-arrestin 2 (βarr2)-dependent endocytic process triggered by noncanonical Wnt signaling. During X. laevis gastrulation, βarr2-mediated endocytosis of Fz7 and dishevelled (Dvl/Dsh) actually occurs in the dorsal marginal zone tissues, which actively participate in noncanonical Wnt signaling. Noncanonical Wnt11/Fz7-mediated endocytosis of Dsh requires the cell-membrane protein Ryk. Ryk interacts with both Wnt11 and βarr2, cooperates with Fz7 to mediate Wnt11-stimulated endocytosis of Dsh, and signals the noncanonical Wnt pathway in CE movements. Conversely, depletion of Ryk and Wnt11 prevents Dsh endocytosis in dorsal marginal zone tissues. Our study suggests that Ryk functions as an essential regulator for noncanonical Wnt/Fz-mediated endocytosis in the regulation of X. laevis CE movements.
Introduction
Morphogenetic movements in gastrulation are essential for establishing basic germ layers and the body axis during early vertebrate development. The major driving forces for this process include convergent extension (CE) movements, by which cells polarize and elongate along the mediolateral axis and intercalate toward the midline (convergence), leading to extension of the anterior/posterior axis (Wallingford et al., 2002; Veeman et al., 2003). Although the precise molecular mechanisms of CE movements are not clearly understood, the noncanonical Wnt pathway is known to be important in the control of CE movements (Myers et al., 2002; Wallingford and Habas, 2005).
The noncanonical Wnt pathway, which is mediated by interaction between Frizzled (Fz) and members of the Wnt family of secreted glycoproteins, regulates cell shape, cell polarity, and cell adhesion without the induction of a secondary axis. This pathway is subdivided into the Wnt–Ca2+ pathway and the Wnt–planar cell polarity (PCP) pathway (Veeman et al., 2003). The former acts via a trimeric G protein to stimulate intracellular calcium release and activate PKCα and Cdc42 (Kohn and Moon, 2005). The latter PCP pathway is transmitted via dishevelled (Dsh), which has a dual role in the regulation of both the Wnt–β-catenin and PCP pathways (Wallingford and Habas, 2005), and requires β-arrestin 2 (βarr2), Daam1, RhoA, Rac1, Rho-associated kinase α, and Jun N-terminal kinase (JNK; Myers et al., 2002; Kim and Han, 2005, 2007; Wallingford and Habas, 2005).
Recent studies in the field of Wnt signaling have shown that receptor-mediated endocytosis is involved in the regulation of Wnt signaling (Kikuchi and Yamamoto, 2007). However, the molecular mechanism by which the endocytic process in noncanonical Wnt signaling is regulated or the physiological and functional importance of this process during embryonic development remains unclear. In the present study, we provide new insight that atypical receptor related tyrosine kinase (Ryk) acts as an Fz coreceptor and regulates the noncanonical Wnt-mediated endocytosis in Xenopus laevis CE movements.
Results and discussion
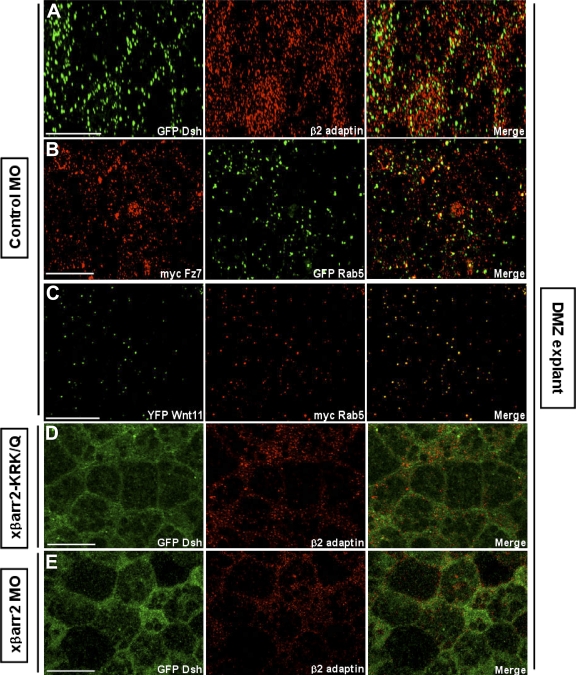
In Drosophila melanogaster and mammals, Fz and Dsh have been detected in the cytoplasmic puncta, which overlap with elements of the endocytic machinery within Wnt/Wg-responsive cells (Chen et al., 2003; Rives et al., 2006; Seto and Bellen, 2006). Association of Dsh with the μ-adaptin of AP2 has been needed for Fz4 internalization in culture cells and normal gastrulation in X. laevis (Yu et al., 2007). In a recent study, we demonstrated that βarr2, which acts as an essential adaptor in clathrin-mediated endocytosis (Claing et al., 2002), has a pivotal role in regulating X. laevis CE movements and requires endocytic activity for its function (Kim and Han, 2007). To further strengthen the claim that endocytic control in noncanonical Wnt signaling occurs in dorsal marginal zone (DMZ) tissues undergoing CE movements, we examined whether endocytosis of Dsh and the noncanonical Wnt/Fz, Wnt11, and Fz7, appears in X. laevis CE movements at the cellular level. Indeed, Dsh localized to punctate structures that colocalized with the cellular endocytic elements, Rab5 (an early endosomal marker; Fig. S1 A, available at http://www.jcb.org/cgi/content/full/jcb.200710188/DC1), and endogenous β2 adaptin (a subunit of the heterotetrameric AP2 adaptor complex that indicates the formation of endocytic clathrin-coated vesicles; Fig. 1 A), in DMZ tissues during gastrulation. Wnt11 and Fz7 also colocalized with Rab5 (Fig. 1, B and C). Interestingly, we found that knockdown of βarr2 (Kim and Han, 2007), which mediates Fz internalization in Wnt-stimulated cells (Chen et al., 2003), by the morpholino (xβarr2 morpholino oligonucleotide [MO]) and the endocytic mutant (xβarr2-KRK/Q) relocalizes Dsh to the cell membrane and inhibits colocalization with β2 adaptin in the cytoplasm of DMZ cells (Fig. 1, D and E). Likewise, βarr2 MO blocked the overlap between Fz7 and Rab5 (Fig. S1 B). A recent study showed that members of caveolin (Cav) family, Cav-1 and -2, which mediate clathrin-independent endocytosis, are expressed in entire regions of gastrula stages, including the involuting mesoderm during X. laevis early embryogenesis (Razani et al., 2002). We observed that Dsh does not colocalize with Cav-1 and -2 in DMZ cells (Fig. S1, C–E), which indicates that Dsh endocytosis is not involved in the caveolar pathway. Together, these results strongly support the idea that in X. laevis CE movements, internalization of Fz7 and Dsh is mediated by βarr2- and clathrin/AP2-dependent processes.
Figure 1.
Endocytosis of Fz7 and Dsh occurs in X. laevis CE movements. Embryos were microinjected into the two dorsal blastomeres at the four-cell stage with a combination of the indicated mRNAs (500 pg GFP Dsh, 500 pg myc Fz7, 500 pg YFP Wnt11, 500 pg myc Rab5, 500 pg GFP Rab5, 1 ng xβarr2-KRK/Q, 10 ng xβarr2 MO, and 10 ng Co MO). DMZ explants were dissected at stage 11–11.5 and then subjected to fluorescence analysis. (A) Dsh localized to punctate structures that colocalize with β2 adaptin in DMZ tissues. (B and C) Fz7 and Wnt11 also overlapped with Rab5. (D and E) Functional knockdown of βarr2 by xβarr2 MO and xβarr2-KRK/Q relocalized Dsh to the cell membrane and inhibited colocalization with β2 adaptin in cytoplasm. Bars, 20 μm.
Recent studies demonstrated that in cultured cells, Wnt5a triggers the internalization of Fz4 and Fz5 (Chen et al., 2003; Kurayoshi et al., 2007), and Wnt3a also induces Fz5 internalization (Yamamoto et al., 2006). Considering these data, the finding of the endocytosis of Fz7 and Dsh in CE movements prompted us to determine whether Fz7 and Dsh are internalized and present in endocytic vesicles as a result of noncanonical Wnt11 stimulation. The normal punctate localization of Dsh did not colocalize with these endocytic markers in X. laevis animal cap cells where both the CE and noncanonical Wnt signaling do not occur (Fig. S2, A and B, available at http://www.jcb.org/cgi/content/full/jcb.200710188/DC1), as described previously (Schwarz-Romond et al., 2005; Kim and Han, 2007). When GFP Dsh was expressed with Fz7 in animal cap cells, Dsh showed continuous staining at the cell membrane and did not colocalize with Rab5 (Fig. S2 C). Intriguingly, expression together with Wnt11, like zebrafish cells (Witzel et al., 2006), led to the coalescence of Dsh into puncta at the cell membrane that colocalized with Fz7 (Fig. S2 D) but not Rab5 and β2 adaptin (Fig. S2, E and F). Moreover, although βarr2 was present, the Wnt11-induced Fz7 aggregates (Fig. S2 G) were not internalized and were found only in punctate structures overlapping with βarr2 at the cell membrane (Fig. S2 H). We conclude that Dsh and βarr2 are recruited to the Wnt11-Fz7 calescence formed by the activation of noncanonical Wnt signaling and that the noncanonical Fz/Dsh endocytosis in X. laevis cells requires some additional component beyond Wnt stimulation.
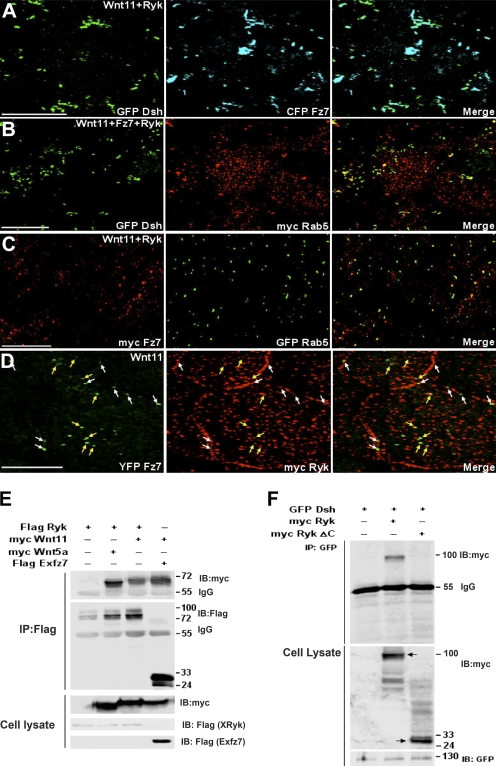
Recent advances in the signal transduction field have led to the claim that cell-membrane proteins serving as coreceptors have a role in promoting productive signal transduction via the interaction with receptor-mediated endocytosis. Low-density lipoprotein receptor-related protein 5/6 and Fz act as Wnt coreceptors and are essential for canonical Wnt signaling and Fz-mediated endocytosis in flies and mammals (Kikuchi and Yamamoto, 2007). Likewise, ADAM12, which acts through an association with TGF-β type II receptor (TβRII), mediates TGFβ signaling by controlling the endocytic trafficking of the TGFβ receptor (Atfi et al., 2007). Recently, it was found that Ryk, known as a homologue of Derailed in the fly and Lin-18 in the worm, is a plasma membrane protein that has important functions in Wnt signaling in several contexts (Keeble and Cooper, 2006). Ryk contains a PDZ-binding domain and a protein tyrosine kinase domain lacking kinase activity and has an extracellular Wnt inhibitory factor (WIF) domain that allows Ryk to interact with Wnt and act as its receptor. In fact, Ryk's WIF domain binds to Wnt1/3/3a/5a and forms a ternary complex with Fz8 and Wnt1 (Keeble and Cooper, 2006). However, the precise molecular mechanism by which Ryk correlates with Wnt/Fz signaling is poorly understood. To study the possibility that Ryk acts as a Fz coreceptor and cooperates with Fz7 to promote noncanonical Wnt11-mediated endocytosis, we first isolated a full-length cDNA of X. laevis Ryk (XRyk), which is highly similar to orthologues from other species (human, 87% identity; mouse, 88% identity), from DMZ tissues of gastrula using a PCR-based approach (GenBank accession no. AAH74417). Temporally, Ryk is expressed both maternally and zygotically throughout the early development of X. laevis (Fig. S3 A, available at http://www.jcb.org/cgi/content/full/jcb.200710188/DC1). Spatially, Ryk is visible in the DMZ tissues of the embryo during the gastrula stages (Fig. S3 B). At later stages, Ryk expression was localized in the head region, including the eye, the otic vesicle, and the branchial arch (Fig. S3 C). We then tested whether, in the presence of Ryk, the Wnt11-induced accumulation of Fz7 and Dsh colocalizes with Rab5. To our surprise and contrary to the expression of Wnt11 alone, the punctate aggregates of Dsh and Fz7 (Fig. 2 A) overlapped with Rab5 in animal cap cells that coexpressed Wnt11 and Ryk (Fig. 2, B and C). Fz7 also colocalized with Ryk in cytoplasmic puncta (Fig. 2 D, yellow arrows) as well as in membrane (Fig. 2 D, white arrows). However, when Fz7 or Wnt11 was excluded, Ryk could not promote the cytoplasmic vesicles of Dsh (unpublished data). In addition, Ryk, like Fz7, interacted with the noncanonical Wnt11 and Wnt5a (Fig. 2 E). Furthermore, Ryk also bound to Dsh via its own cytoplasmic domain (Fig. 2 F), as described previously for mammalian Ryk (Lu et al., 2004). These results strongly suggest that Ryk is required for noncanonical Wnt11/Fz7-simulated internalization of Dsh and forms an endocytic complex with these components.
Figure 2.
Ryk promotes internalization of the Wnt11-induced Fz7 and Dsh aggregates in animal cap cells. (A–D) Four-cell stage embryos were injected in the animal pole with various mRNAs in combination as indicated (500 pg GFP Dsh, 500 pg myc Rab5, 1 ng Fz7, 1 ng myc Fz7, 1 ng CFP Fz7, 1 ng YFP Fz7, 500 pg GFP Rab5, 1 ng XRyk, 1 ng myc XRyk, and 1 ng Wnt11). Animal caps were dissected at stage 9–10 and then subjected to fluorescence analysis. In the presence of Ryk, the Wnt11-induced punctate aggregates of Dsh and Fz7 (A) overlapped with Rab 5 (B and C). (D) Fz7 colocalized with Ryk in both cytoplasmic (yellow arrows) and membrane (white arrows) puncta. Bars, 20 μm. (E and F) HEK293FT cells were transfected with various constructs, either alone or in combination as indicated. Cell lysates were immunoprecipitated with anti-Flag (E) or anti-GFP (F) antibodies and the immunocomplexes were blotted with specific antibodies. (E) Ryk bound to the noncanonical Wnt11 as well as Wnt5a. (F) Ryk and Ryk-ΔC, which lacks the cytoplasmic domain, interacted with Dsh. Arrows indicate a size of epitope-tagged Ryk or Ryk-ΔC. Numbers to the right of the gel blots indicate molecular mass standards in kD.
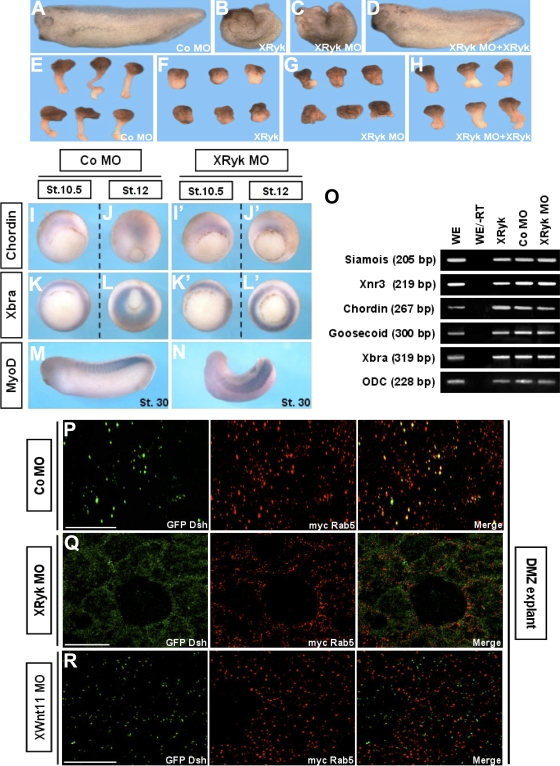
To assess the physiological importance of this novel finding, we used an antisense MO to knock down XRyk function in the embryo. The XRyk MO effectively blocked translation of mRNA containing the 5′ untranslated region sequences complementary to its MO, but translation of mRNA containing only the open reading frame (ORF) of the protein was not affected. Control (Co) MO had no effect on translation of the Ryk protein, and neither XRyk MO nor Co MO inhibited translation of the control, actin (Fig. S3 D). Interestingly, depletion of Ryk in X. laevis resulted in CE movement-defective phenotypes (Fig. 3 C), which included the delay of blastopore closure and the failure of neural tube closure and anterior/posterior axis formation in whole embryos, as well as the significant inhibition of the elongation of Keller (DMZ) sandwich explants (Fig. 3 G). Additionally, XRyk overexpression induced defects of CE movements that were indistinguishable from those of the XRyk knockdown (Fig. 3, B and F). To verify the specificity of the XRyk knockdown in CE movements, we performed a rescue assay with the ORF XRyk construct. Coinjection of XRyk MO with ORF XRyk mRNA rescued the CE defects caused by XRyk MO (Fig. 3, D and H; and Fig. S3, E and F).
Figure 3.
Ryk is essential for CE movements. (A–H) Overexpression or depletion of XRyk caused the disruption of CE movements. Two blastomeres of four-cell stage embryos were injected at the dorsal equatorial region with 500 pg XRyk mRNA, 40 ng XRyk MO, or 40 ng Co MO. (A–C) Compared with the Co MO–injected embryos, embryos expressing XRyk mRNA or MO at stage 33 showed dorsal flexure and a failure to straighten the anteroposterior axis. (E–G) Keller sandwich explants from Co MO–injected embryos elongated, but the explants expressing XRyk mRNA or MO were significantly inhibited. (D and H) Rescue of XRyk knockdown by coinjection of ORF XRyk mRNA. (I–O) XRyk did not affect mesodermal cell fate specification. (I–N) Whole mount in situ hybridization with Chordin, Xbra, and MyoD as probes. (O) RT-PCR analysis using primers specific for Siamois, Xnr3, Chordin, Goosecoid, and Xbra. ODC, ornithine decarboxylase, (a loading control); −RT, minus reverse transcription control sample. (P–R) XRyk or Wnt11 depletion inhibited the Dsh puncta colocalization with Rab5 in DMZ cells. 60 ng Wnt11 MO, 40 ng XRyk MO, or 40 ng Co MO was coinjected with GFP Dsh and myc Rab5 into the two dorsal blastomeres at the four-cell stage. DMZ explants were dissected at stage 11–11.5 and then subjected to fluorescence analysis. Bars, 20 μm.
Dorsoventral patterning of the mesoderm affects CE movements indirectly by impacting cell fates in the DMZ (Keller and Danilchik, 1988). To examine whether the phenotypes resulting from XRyk MO were caused by a defect in mesodermal differentiation, we performed whole-mount in situ hybridization using several mesodermal genes. Before the onset of CE movements, XRyk MO-injected embryos (Fig. 3, I′ and K′) exhibited normal expression of the dorsal mesodermal marker Chordin and the panmesodermal marker Xbra (Fig. 3, I and K). However, convergent and extended localization of Chordin in the prechordal plate and notochord (Fig. 3 J) was blocked in midgastrula embryos expressing XRyk MO (Fig. 3 J′). The circumblastoporal localization of Xbra (Fig. 3 L) was wider in embryos injected with XRyk MO (Fig. 3 L′). However, Chordin and Xbra expression levels remained the same. Localization of MyoD in XRyk MO or Co MO-injected embryos at later stages also showed that muscle differentiation was not affected by XRyk depletion (Fig. 3, M and N). In addition, RT-PCR analysis demonstrated that XRyk does not affect the endogenous expression of dorsal mesoderm genes nor induce expression of these genes regulated by FGF, activin, and Wnt–β-catenin signaling (Fig. 3 O). Next, we determined the effect of XRyk MO on the normal internalization of Dsh in CE movements. Fluorescence analysis showed that the XRyk MO relocalized Dsh to the cell membrane and inhibited colocalization with Rab5 in the cytoplasm of DMZ cells (Fig. 3 Q). Moreover, depletion of Wnt11 (Pandur et al., 2002) blocked the colocalization between Dsh and Rab5 (Fig. 3 R), which indicates that Dsh endocytosis is dependent on endogenous Wnt11 signal in CE movements. Together, these results support the idea that Ryk is required for proper CE movements and normal endocytic regulation of noncanonical Wnt signaling without affecting cell fate during X. laevis gastrulation.
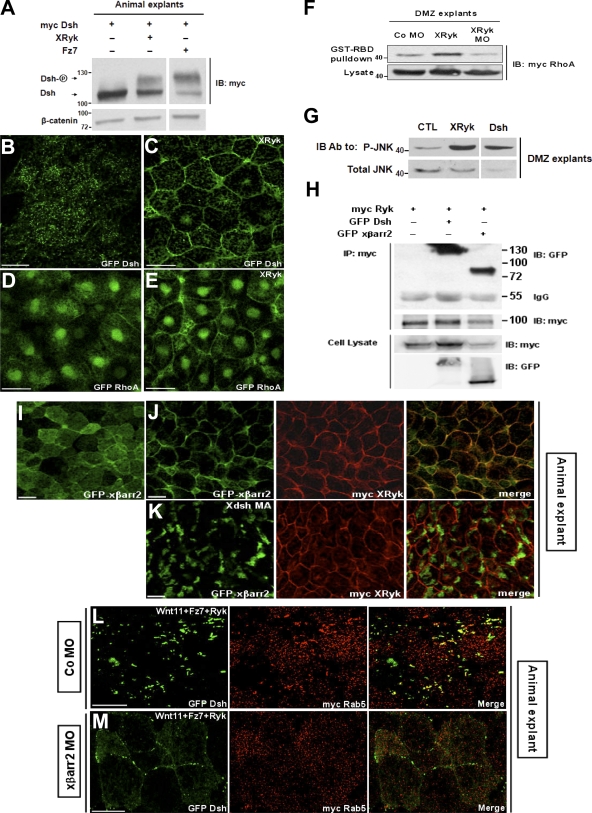
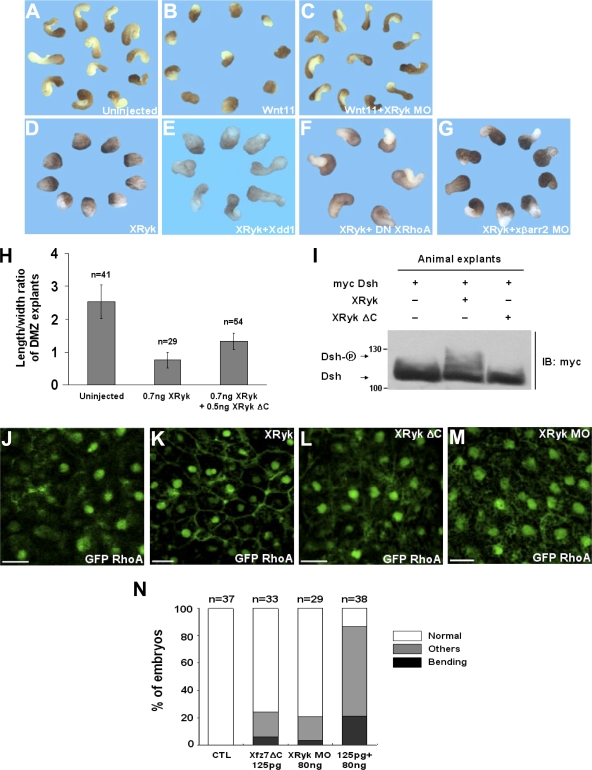
Next, we wanted to know whether Ryk signals the noncanonical Wnt pathway during CE movements. In the PCP pathway, phosphorylation of Dsh is important for its translocation to the cell membrane, and this translocation is a prerequisite for functional signaling activation (Park et al., 2005; Kim and Han, 2007). Therefore, it is possible that Ryk regulates the phosphorylation and subcellular localization of Dsh. In considering this possibility, we observed that Ryk induces the hyperphosphorylation (Fig. 4 A) and membrane accumulation of Dsh in animal cap cells (Fig. 4, B and C). In addition, we measured the activity of RhoA by using a GST-RBD fusion protein that recognizes the GTP-bound active RhoA and the phosphorylation level of JNK in CE movements. With theses assays, we found that XRyk is required for RhoA activation (Fig. 4 F) in concert with its translocation to the cell membrane of animal cap tissues (Fig. 4, D and E) and increases JNK phosphorylation in DMZ cells (Fig. 4 G). We then proceeded to determine the relationship between Ryk and βarr2. Coimmunoprecipitation and fluorescence analyses showed that Ryk is capable of binding to βarr2 (Fig. 4 H) and can redistribute βarr2 from the cytoplasm to the plasma membrane in animal cap cells (Fig. 4, I and J). Importantly, in the presence of Xdsh MA, a construct fused with the mitochondrial membrane–anchoring sequence (Park et al., 2005), the Ryk-dependent membrane accumulation of βarr2 is relocalized to intracellular clusters (Fig. 4 K), which implies that Ryk, like Fz, indirectly interacts with βarr2 via Dsh. Furthermore, we found that βarr2 knockdown inhibits the noncanonical Wnt11/Fz7-mediated Dsh endocytosis in the presence of Ryk (Fig. 4, L and M). Therefore, our observations strongly suggest that Ryk acts as a novel regulator that is essential for noncanonical Wnt/Fz-mediated endocytosis in the regulation of X. laevis CE movements. This idea was supported by investigating the biological epistasis between Ryk and noncanonical Wnt signaling components in more detail. For this purpose, rescue experiments using a DMZ elongation assay were performed. Wnt11 overexpression inhibited the elongation of DMZ explants, and, intriguingly, this inhibition was rescued by the coexpression of XRyk MO. Moreover, the CE movements that were significantly inhibited by Ryk overexpression were restored by the functional inhibition of cytoplasmic downstream molecules of noncanonical Wnt signaling using βarr2 MO and dominant-negative forms of Dsh (Xdd1) and RhoA (RhoA-N19) (Fig. 5, A–G; and Table S1, available at http://www.jcb.org/cgi/content/full/jcb.200710188/DC1). Consistently, the CE-defective phenotypes caused by Ryk depletion were also rescued by the overexpression of Dsh, βarr2, and the constitutively active form of RhoA (RhoA-V14; Fig. S3 F), which indicates that Ryk functions upstream of the cytoplasmic molecules activated by Wnt11 in CE movements. The biochemical experiments between Dsh and Ryk showed that the cytoplasmic domain of Ryk is important to interact with Dsh (Fig. 2 F). We further tested whether this domain is required for Ryk function in CE movements. Interestingly, the XRyk ΔC rescued the CE defects caused by XRyk (Fig. 5 H) and Wnt11 (Table S1). Moreover, XRyk ΔC did not induce the hyperphosphorylation of Dsh (Fig. 5 I) or the membrane accumulation of RhoA in animal cap cells (Fig. 5, J–M). Collectively, these observations suggest that Ryk mediates the noncanonical Wnt pathway by controlling Dsh in CE movements.
Figure 4.
Ryk acts as an essential regulator of noncanonical Wnt signaling on CE movements. (A) Ryk, like Fz7, phosphorylated Dsh. Animal caps expressing myc Dsh (1 ng) alone or with the combinations of XRyk (1 ng) and Fz7 (1 ng) as indicated were subjected to Western blotting using anti-myc and anti–β-catenin antibodies. (B–E) The normal Dsh (B) and RhoA (D) distribution was translocated by Ryk to the cell membrane in animal cap cells (C and E). XRyk (1 ng) were injected with GFP Dsh (500 pg) or GFP RhoA (500 pg) into the animal regions of embryos at the four-cell stage, either alone or in combination as indicated. (F and G) Two blastomeres of four-cell stage embryos were injected at the dorsal marginal region with the indicated mRNAs or MO (1 ng myc RhoA, 1 ng Dsh, 1 ng XRyk, and 40 ng XRyk MO). The DMZ explants were dissected at stage 10.25 and cultured until stage 12. (F) Ryk activated RhoA in DMZ tissues during gastrulation, but Ryk MO inactivated them. GTP-bound RhoA in DMZ lysates was precipitated using GST-RBD and visualized by immunoblotting with an anti-myc antibody. (G) Ryk, like Dsh, induced JNK phosphorylation in CE movements. The explant lysates were blotted with anti-phospho JNK and anti-JNK antibodies. (H) Ryk physically interacted with βarr2 in vivo. HEK293FT cells were transfected with myc XRyk, GFP xβarr2, or GFP Dsh, either alone or in combination as indicated. Cell lysates were immunoprecipitated with anti-myc antibody, and the immunocomplexes were blotted with specific antibodies. Numbers to the sides of the gel blots indicate molecular mass standards in kD. (I–M) Four-cell stage embryos were injected in the animal pole with various mRNA in combination as indicated (500 pg GFP xβarr2, 1 ng Xdsh MA, 1 ng, myc XRyk, 1 ng Wnt11, 1 ng Fz7, 500 pg GFP Dsh, 10 ng xβarr2 MO, 10 ng Co MO, and 500 pg myc Rab5). Animal caps were dissected at stage 9–10 and then subjected to fluorescence analysis. (I) Normal distribution of βarr2 in animal cap cells. (J and K) Ryk-induced membrane distribution of βarr2 was relocalized by Xdsh-MA to the intracellular cluster. (L and M) βarr2 knockdown inhibited the noncanonical Wnt11/Fz7-mediated Dsh endocytosis in the presence of Ryk. Bars, 20 μm.
Figure 5.
Ryk is required for Dsh to regulate the activation of noncanonical Wnt signaling. (A–G) Rescue assays between Ryk and noncanonical Wnt components. Two blastomeres of four-cell stage embryos were injected at the dorsal marginal region with the indicated reagents. Quantitative results of DMZ elongation assay are explained in Table S1 (available at http://www.jcb.org/cgi/content/full/jcb.200710188/DC1). (H) Quantitative assays of rescue between Ryk and Ryk ΔC in DMZ elongation assay. n, total number of explants. Error bars indicate the mean ± SD. (I) Ryk ΔC, unlike Ryk, did not phosphorylate Dsh. Animal caps expressing myc Dsh (1 ng) alone or with the combinations of XRyk (1 ng) and XRyk ΔC (1 ng), as indicated, were subjected to Western blotting using anti-myc. Numbers to the left of the gel blot indicate molecular mass standards in kD. (J–M) The normal RhoA distribution was not translocated by Ryk ΔC and Ryk MO to the cell membrane in animal cap cells. XRyk (1 ng), XRyk MO (80 ng), or XRyk ΔC (1 ng) were injected with GFP RhoA (500 pg) into the animal regions of embryos at the four-cell stage, either alone or in combination as indicated. Bar, 20 μm. (N) Ryk and Fz7 have a cooperative effect in CE movements. Others indicate a truncated and mild kinked axis. n, total number of embryos.
The roles for Ryk-regulating CE movements in noncanonical Wnt signaling resemble those for Fz7 (Djiane et al., 2000; Kim and Han, 2007). To further investigate the relationship between Ryk and Fz7, we examined the interaction between XRyk MO and Ex-Xfz7, the dominant-negative form of Xfz7 (Djiane et al., 2000). Suboptimal amounts of XRyk MO or Ex-Xfz7 caused no CE defects in whole embryos when injected separately. Importantly, when both XRyk MO and Ex-Xfz7 were expressed, a cooperative effect was found (Fig. 5 N), which is consistent with collaboration in the noncanonical Wnt11-mediated endocytosis.
In conclusion, our data demonstrate a novel function for Ryk in the positive control of noncanonical Wnt signaling by modulating βarr2-dependent endocytic trafficking. At present, there are no satisfactory descriptions explaining the molecular mechanism by which the endocytic process in noncanonical Wnt signaling is regulated or the relationship between noncanonical Wnt signaling-mediated endocytosis and development or disease. Thus, the demonstration that Ryk and Fz7 are Wnt coreceptors in regulation of X. laevis CE movements provides new insight into noncanonical Wnt-mediated endocytosis, which is necessary to trigger productive signal transduction in noncanonical Wnt signaling.
Materials and methods
X. laevis embryos and microinjection
X. laevis was obtained from Xenopus I, Inc., and Nasco. Eggs were obtained from X. laevis primed with 800 units of human chorionic gonadotropin (Sigma-Aldrich). In vitro fertilization was performed, and developmental stages of the embryos were determined according to Nieuwkoop and Faber (1967). Microinjection was performed in 0.33× Modified Ringer's (MR) containing 4% Ficoll-400 (GE Healthcare) using a Nanoliter Injector (Watson Pharmaceuticals, Inc.). Injected embryos were cultured in 0.33× MR until stage 8 and then transferred to 0.1× MR until they had reached the appropriate stage.
Plasmids, RNA synthesis, and MO
For expression in X. laevis embryos, the entire coding region of XRyk was cloned into the EcoRI site of the pCS2+ vector and linearized with NotI. XRyk-ΔC was cloned into the ClaI site of the myc-pCS2+ vector. GFP- and myc-Dsh, Xdd1, and Wnt11 were obtained from R. Moon (University of Washington, Seattle, WA). Xdsh-MA and Myc-Fz7 were received from J. Wallingford (University of Texas, Austin, TX) and H. Steinbeisser (University Heidelberg, Heidelberg, Germany), respectively. hCav-1 and mCav-2 were obtained from S.H. Cha (Inha University, Incheon, South Korea). YFP-Wnt11 and YFP/CFP-Fz7 were provided by C.P. Heisenberg (Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany). Antisense MO were obtained from Gene Tools, LLC. The MO sequences were: 5′-CCGGGCAGCATGTCTCTCACAGGC-3′ for XRyk MO and 5′-CCTCTTACCTCAGTTACAATTTATA-3′ for Co MO. For the specificity assay of XRyk MO, XRyk was cloned into the ClaI site of the myc-pCS2+ vector. myc XRyk was linearized with NotI. Capped mRNAs were synthesized from linearized plasmids using the mMessage mMachine kit (Ambion).
In situ hybridization and RT-PCR
Whole-mount in situ hybridization and RT-PCR analyses were performed as described previously (Kim and Han, 2005). The forward and reverse primers for XRyk were 5′-ATGCTGCCCGGGCGGCCGGA-3′ and 5′-GGCAACATTGACCGGATCGA-3′, respectively. Primers for Chordin, Goosecoid, ODC, Siamois, Xbra, and Xnr3 were used as described online by D. Robertis (www.hhmi.ucla.edu/derobertis/index.html).
RhoA activity assay
Isolation of activated RhoA from DMZ tissues was performed by affinity for the protein-binding domain of rhotekin (GST-C21). The GST-RBD binding assay was performed as described previously (Park et al., 2006). The quantity of protein in each sample was determined using a bicinchoninic acid protein assay reagent (Thermo Fisher Scientific).
Immunoprecipitation and immunoblotting
HEK293FT cells (Invitrogen) were transfected with the indicated constructs for 48 h using Lipofectamine Plus reagent (Invitrogen) and then resuspended in immunoprecipitation buffer (50 mM Hepes/NaOH, pH 7.5), 3 mM EDTA, 3 mM CaCl2, 80 mM NaCl, 1% Triton X-100, and 5 mM DTT). Immunoprecipitation and immunoblot analysis were performed as described previously (Kim et al., 2006). Mouse anti-myc monoclonal, rabbit anti-myc polyclonal, mouse anti-GFP monoclonal (Santa Cruz Biotechnology, Inc.), and mouse anti-flag monoclonal antibodies (Sigma-Aldrich) were used.
Immunofluorescence microscopy and immunostaining
Dissected animal caps and DMZ cells were fixed in 4% paraformaldehyde in PBS for 2 h, rinsed in PBS, and directly mounted for GFP-, YFP-, and CFP-tagged proteins. Alternatively, they were incubated in PBSTB (PBS, 0.1% Triton X-100, and 2% BSA) to block nonspecific binding, followed by a standard immunostaining procedure. Image analysis was performed using a confocal laser-scanning microscope (FluoView 1000; Olympus), a 60×/1.35 NA oil immersion objective lens (Olympus), and an immersion oil objective lens (ND = 1.516, 23°C; Olympus). Images were processed with Photoshop (Adobe). The antibodies for immunofluorescence were mouse anti-myc (Santa Cruz Biotechnology, Inc.), rabbit anti–Cav-1 (BD Biosciences), and mouse anti-β2 adaptin (BD Biosciences) primary antibodies; and TRITC anti–rabbit (Sigma-Aldrich) and Alexa Fluor 594 anti–mouse (Invitrogen) secondary antibodies.
DMZ elongation assay
Embryos were injected with mRNA into either DMZ at the four-cell stage embryos. DMZ explants were excised at stage 10.5 and were cultured in 1× MR containing 10 μg/ml of bovine serum albumin, 50 μg/ml of gentamycin, and 5 μg/ml of streptomycin, until stage 18.
Online supplemental material
Fig. S1 shows that Dsh is not mediated by Cav-dependent processes. Fig. S2 shows the fluorescence analyses in X. laevis animal cap cells. Fig. S3 shows the developmental expression of XRyk and the specificity of XRyk MO. Table S1 shows the rescue assays between Ryk and noncanonical Wnt components. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200710188/DC1.
Supplementary Material
Acknowledgments
We are grateful to Dr. R. Moon, J. Wallingford, S.H. Cha, C.P. Heisenberg, and H. Steinbeisser for the generous gifts of plasmids. We also acknowledge other members of our laboratory for reading this manuscript and providing constructive criticism.
This work was supported by the Advanced Basic Research Laboratory Program (grant R14-2002-012-01001-0), the Pure Basic Research Group (grant 070-2005-C00115) of the Korean Research Foundation, and the Brain Korea 21 project.
Abbreviations used in this paper: βarr2, β-arrestin 2; Cav, caveolin; CE, convergent extension; Co, control; DMZ, dorsal marginal zone; Dsh, dishevelled; Fz, Frizzled; JNK, Jun N-terminal kinase; MO, morpholino oligonucleotide; ORF, open reading frame; PCP, planar cell polarity; Ryk, atypical receptor related tyrosine kinase; XRyk, Xenopus laevis Ryk.
References
- Atfi, A., E. Dumont, F. Colland, D. Bonnier, A. L'Helgoualc'h, C. Prunier, N. Ferrand, B. Clement, U.M. Wewer, and N. Theret. 2007. The disintegrin and metalloproteinase ADAM12 contributes to TGF-β signaling through interaction with the type II receptor. J. Cell Biol. 178:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W., D. ten Berge, J. Brown, S. Ahn, L.A. Hu, W.E. Miller, M.G. Caron, L.S. Barak, R. Nusse, and R.J. Lefkowitz. 2003. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 301:1391–1394. [DOI] [PubMed] [Google Scholar]
- Claing, A., S.A. Laporte, M.G. Caron, and R.J. Lefkowitz. 2002. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog. Neurobiol. 66:61–79. [DOI] [PubMed] [Google Scholar]
- Djiane, A., J. Riou, M. Umbhauer, J. Boucaut, and D. Shi. 2000. Role of frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 127:3091–3100. [DOI] [PubMed] [Google Scholar]
- Keeble, T.R., and H.M. Cooper. 2006. Ryk: a novel Wnt receptor regulating axon pathfinding. Int. J. Biochem. Cell Biol. 38:2011–2017. [DOI] [PubMed] [Google Scholar]
- Keller, R., and M. Danilchik. 1988. Regional expression, pattern and timing of convergence and extension during gastrulation of Xenopus laevis. Development. 103:193–209. [DOI] [PubMed] [Google Scholar]
- Kikuchi, A., and H. Yamamoto. 2007. Regulation of Wnt signalling by receptor-mediated endocytosis. J. Biochem. 141:443–451. [DOI] [PubMed] [Google Scholar]
- Kim, G.H., and J.K. Han. 2005. JNK and ROKalpha function in the noncanonical Wnt/RhoA signaling pathway to regulate Xenopus convergent extension movements. Dev. Dyn. 232:958–968. [DOI] [PubMed] [Google Scholar]
- Kim, G.H., and J.K. Han. 2007. Essential role for beta-arrestin 2 in the regulation of Xenopus convergent extension movements. EMBO J. 26:2513–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, G.H., E. Park, Y.Y. Kong, and J.K. Han. 2006. Novel function of POSH, a JNK scaffold, as an E3 ubiquitin ligase for the Hrs stability on early endosomes. Cell. Signal. 18:553–563. [DOI] [PubMed] [Google Scholar]
- Kohn, A.D., and R.T. Moon. 2005. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 38:439–446. [DOI] [PubMed] [Google Scholar]
- Kurayoshi, M., H. Yamamoto, S. Izumi, and A. Kikuchi. 2007. Post-translational palmitoylation and glycosylation of Wnt-5a are necessary for its signalling. Biochem. J. 402:515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, W., V. Yamamoto, B. Ortega, and D. Baltimore. 2004. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 119:97–108. [DOI] [PubMed] [Google Scholar]
- Myers, D.C., D.S. Sepich, and L. Solnica-Krezel. 2002. Convergence and extension in vertebrate gastrulae: cell movements according to or in search of identity? Trends Genet. 18:447–455. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop, P.D., and J. Faber, editors. 1967. Normal Table of Xenopus laevis (Daudin). Second edition. North Holland Publishing Co.: Amsterdam. 252 pp.
- Pandur, P., M. Lasche, L.M. Eisenberg, and M. Kuhl. 2002. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature. 418:636–641. [DOI] [PubMed] [Google Scholar]
- Park, T.J., R.S. Gray, A. Sato, R. Habas, and J.B. Wallingford. 2005. Subcellular localization and signaling properties of dishevelled in developing vertebrate embryos. Curr. Biol. 15:1039–1044. [DOI] [PubMed] [Google Scholar]
- Park, E., G.H. Kim, S.C. Choi, and J.K. Han. 2006. Role of PKA as a negative regulator of PCP signaling pathway during Xenopus gastrulation movements. Dev. Biol. 292:344–357. [DOI] [PubMed] [Google Scholar]
- Razani, B., D.S. Park, Y. Miyanaga, A. Ghatpande, J. Cohen, X.B. Wang, P.E. Scherer, T. Evans, and M.P. Lisanti. 2002. Molecular cloning and developmental expression of the caveolin gene family in the amphibian Xenopus laevis. Biochemistry. 41:7914–7924. [DOI] [PubMed] [Google Scholar]
- Rives, A.F., K.M. Rochlin, M. Wehrli, S.L. Schwartz, and S. DiNardo. 2006. Endocytic trafficking of Wingless and its receptors, Arrow and DFrizzled-2, in the Drosophila wing. Dev. Biol. 293:268–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Romond, T., C. Merrifield, B.J. Nichols, and M. Bienz. 2005. The Wnt signalling effector Dishevelled forms dynamic protein assemblies rather than stable associations with cytoplasmic vesicles. J. Cell Sci. 118:5269–5277. [DOI] [PubMed] [Google Scholar]
- Seto, E.S., and H.J. Bellen. 2006. Internalization is required for proper Wingless signaling in Drosophila melanogaster. J. Cell Biol. 173:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman, M.T., J.D. Axelrod, and R.T. Moon. 2003. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev. Cell. 5:367–377. [DOI] [PubMed] [Google Scholar]
- Wallingford, J.B., and R. Habas. 2005. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 132:4421–4436. [DOI] [PubMed] [Google Scholar]
- Wallingford, J.B., S.E. Fraser, and R.M. Harland. 2002. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev. Cell. 2:695–706. [DOI] [PubMed] [Google Scholar]
- Witzel, S., V. Zimyanin, F. Carreira-Barbosa, M. Tada, and C.P. Heisenberg. 2006. Wnt11 controls cell contact persistence by local accumulation of Frizzled 7 at the plasma membrane. J. Cell Biol. 175:791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, H., H. Komekado, and A. Kikuchi. 2006. Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of beta-catenin. Dev. Cell. 11:213–223. [DOI] [PubMed] [Google Scholar]
- Yu, A., J.F. Rual, K. Tamai, Y. Harada, M. Vidal, X. He, and T. Kirchhausen. 2007. Association of Dishevelled with the clathrin AP-2 adaptor is required for Frizzled endocytosis and planar cell polarity signaling. Dev. Cell. 12:129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.