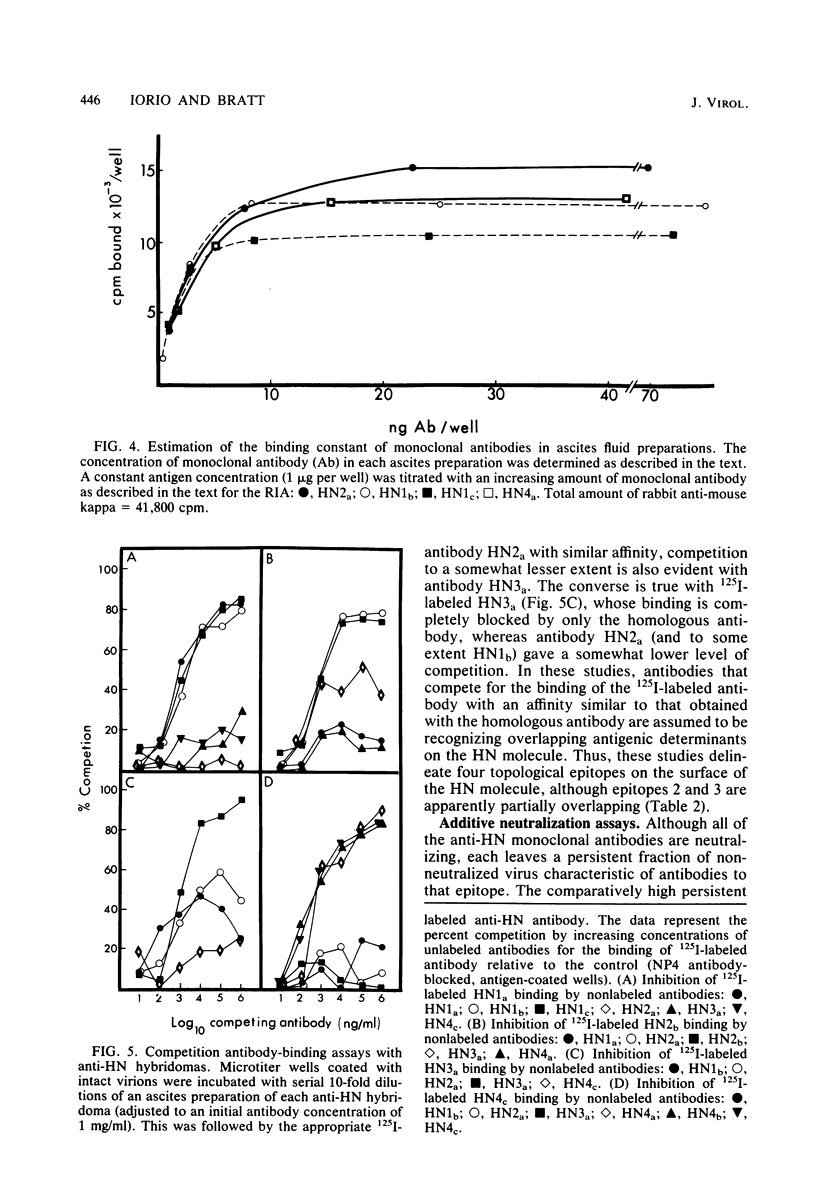
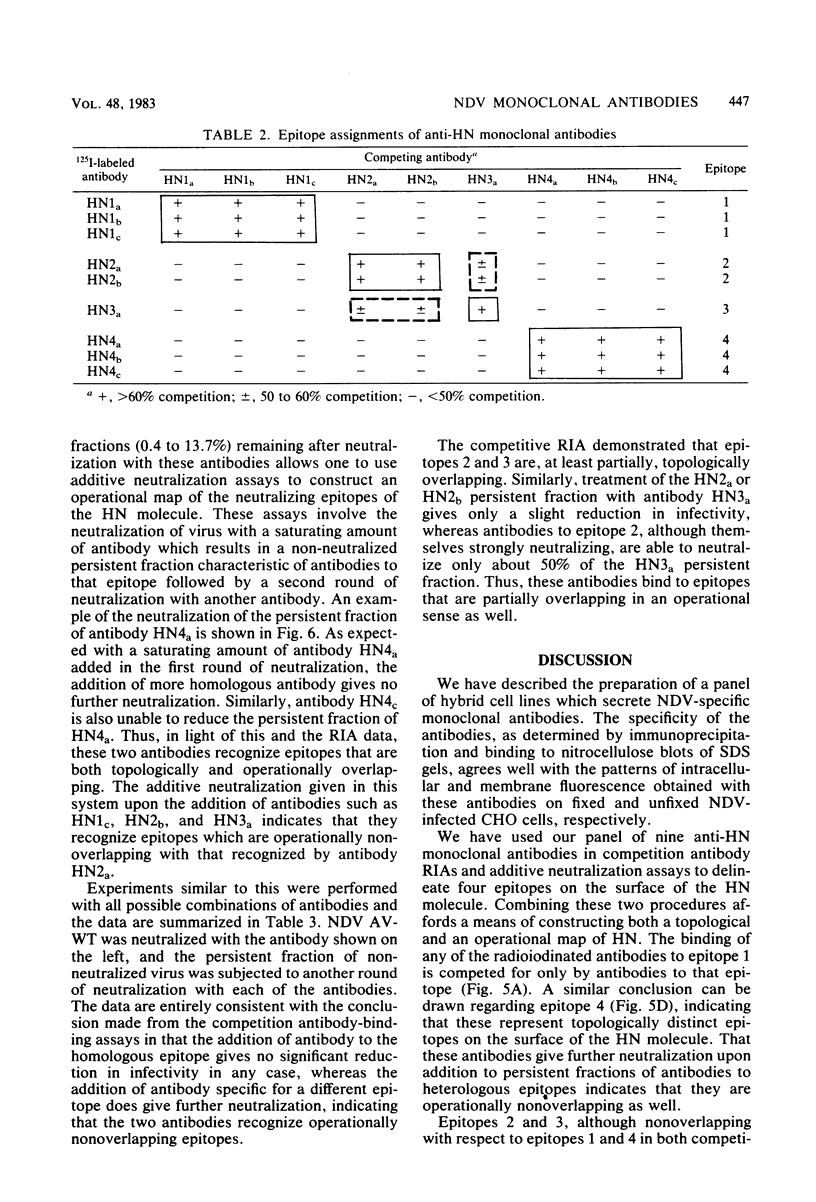
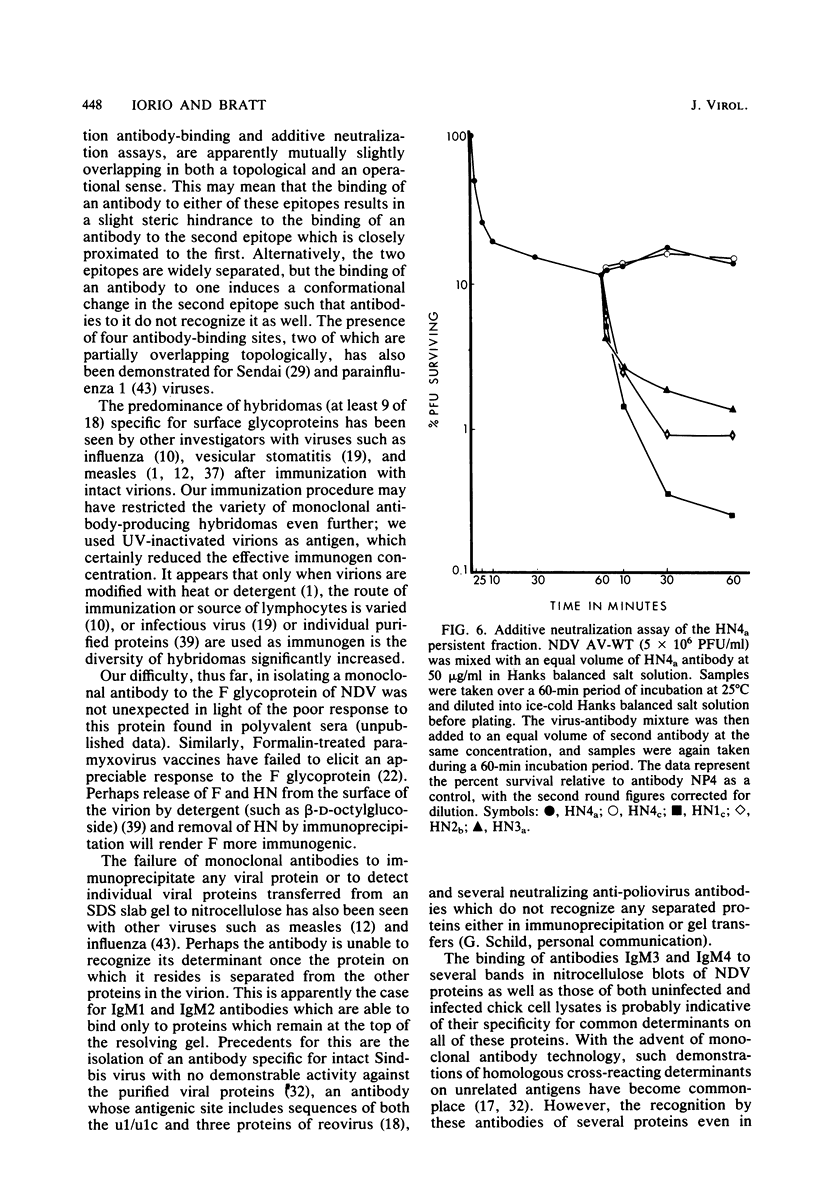
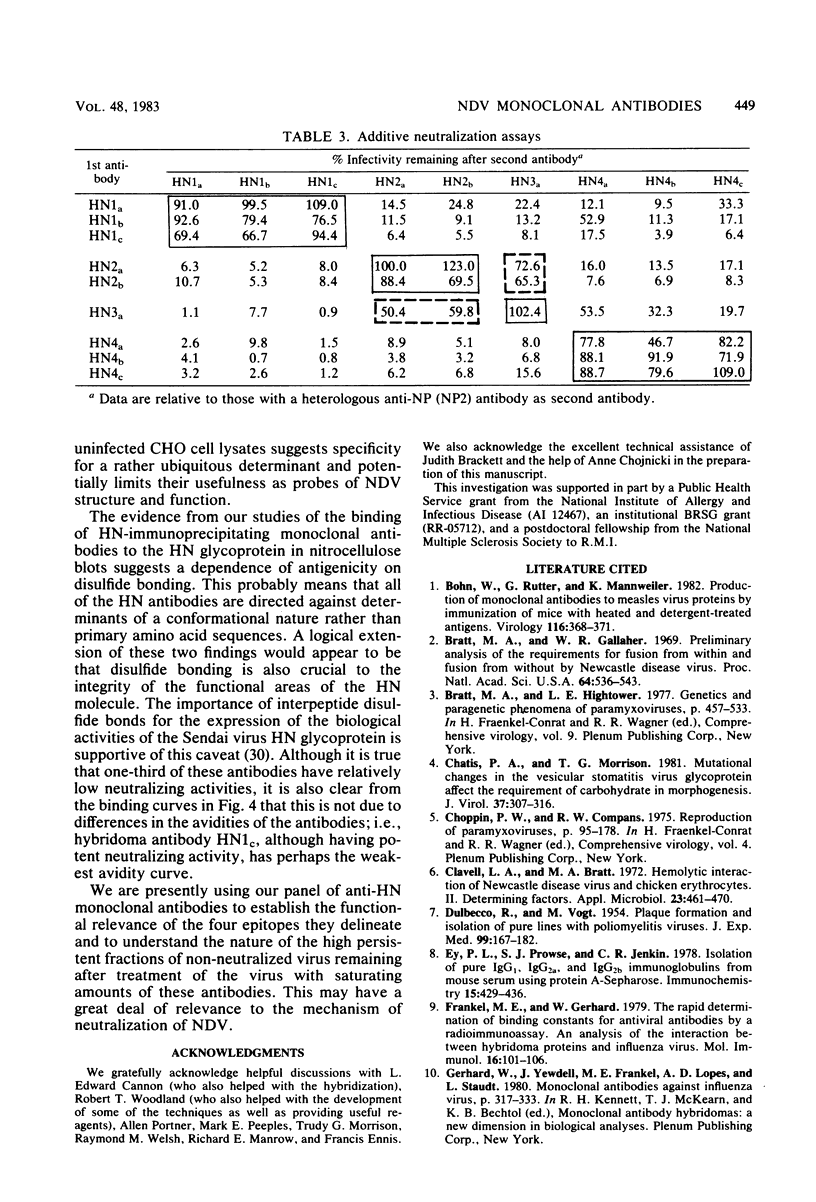
Abstract
Eighteen independent hybridomas producing monoclonal antibodies to Newcastle disease virus have been prepared by fusion of SP2 cells with spleen lymphocytes from a BALB/c mouse immunized with intact UV-inactivated Newcastle disease virus strain Australia-Victoria. They have been divided into three groups on the basis of radioimmunoprecipitation, infected cell surface and cytoplasmic fluorescence, and isotype. The anti-HN group is made up of nine antibodies which give surface fluorescence on infected cells and immunoprecipitate the HN glycoprotein. These antibodies bind to HN in nitrocellulose transfers of sodium dodecyl sulfate gels, but only if it has been neither reduced nor boiled. To varying degrees, all of these HN antibodies neutralize infectivity. These results suggest that they recognize exposed determinants of a conformational nature on the native HN molecule. They have been used in competition antibody-binding radioimmunoassays and additive neutralization assays, and on the basis of these studies the epitopes they recognize have been subdivided into four domains, two of which are overlapping, on the HN glycoprotein. The relatively weaker neutralizing activity observed with some of these antibodies cannot be explained by lower avidities for their epitopes because there is not an inverse correlation between estimated binding constant and neutralizing activity. The four antibodies in the second group all give a predominantly cytoplasmic fluorescence pattern, immunoprecipitate the nucleocapsid protein, and bind to nucleocapsid protein in nitrocellulose transfers of reduced and nonreduced sodium dodecyl sulfate-polyacrylamide gels. All five of the antibodies in the third group are of the immunoglobulin M class, unlike the others which are all immunoglobulin G antibodies. Members of this group show variable fluorescence patterns, but none is able to immunoprecipitate or bind to a specific viral antigen transferred to nitrocellulose paper from sodium dodecyl sulfate-polyacrylamide gels.
Full text
PDF



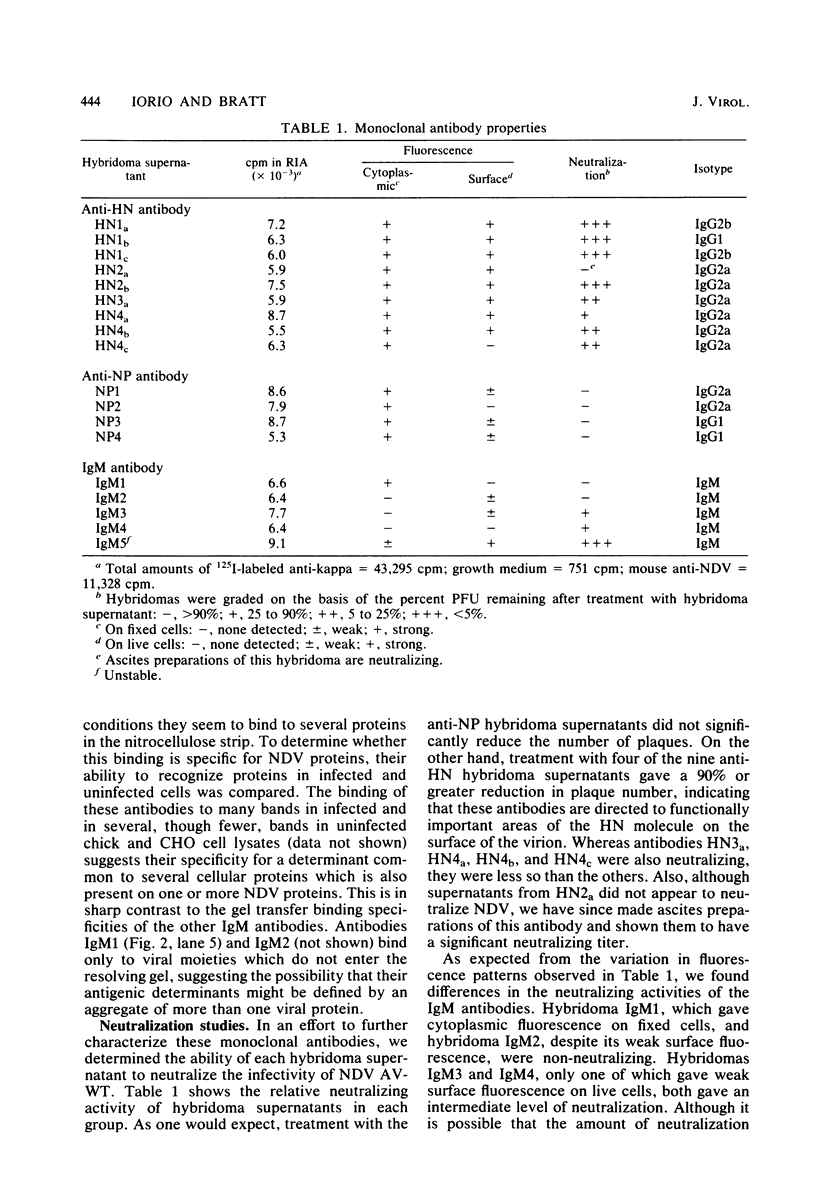
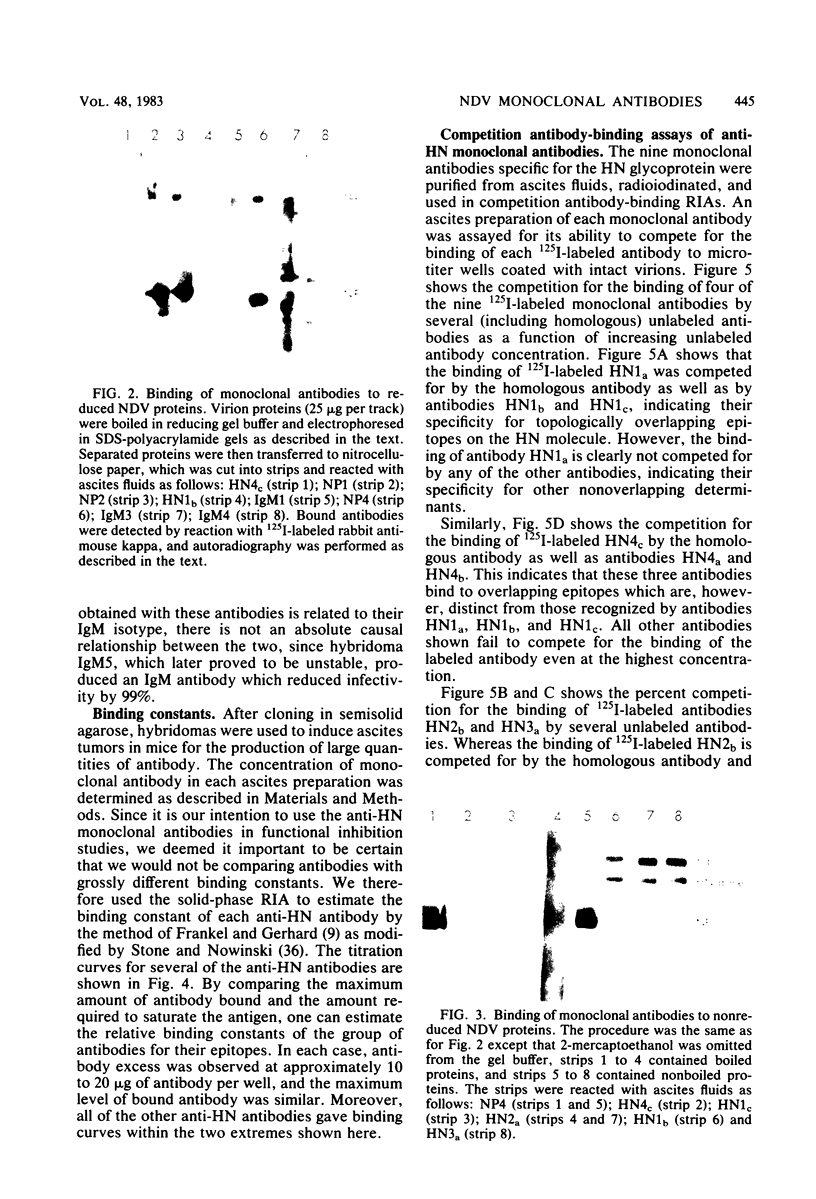

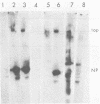
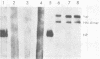
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohn W., Rutter G., Mannweiler K. Production of monoclonal antibodies to measles virus proteins by immunization of mice with heated and detergent-treated antigens. Virology. 1982 Jan 15;116(1):368–371. doi: 10.1016/0042-6822(82)90430-5. [DOI] [PubMed] [Google Scholar]
- Bratt M. A., Gallaher W. R. Preliminary analysis of the requirements for fusion from within and fusion from without by Newcastle disease virus. Proc Natl Acad Sci U S A. 1969 Oct;64(2):536–543. doi: 10.1073/pnas.64.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatis P. A., Morrison T. G. Mutational changes in the vesicular stomatitis virus glycoprotein affect the requirement of carbohydrate in morphogenesis. J Virol. 1981 Jan;37(1):307–316. doi: 10.1128/jvi.37.1.307-316.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavell L. A., Bratt M. A. Hemolytic interaction of Newcastle disease virus and chicken erythrocytes. II. Determining factors. Appl Microbiol. 1972 Mar;23(3):461–470. doi: 10.1128/am.23.3.461-470.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Frankel M. E., Gerhard W. The rapid determination of binding constants for antiviral antibodies by a radioimmunoassay. An analysis of the interaction between hybridoma proteins and influenza virus. Mol Immunol. 1979 Feb;16(2):101–106. doi: 10.1016/0161-5890(79)90051-8. [DOI] [PubMed] [Google Scholar]
- Gerhard W., Yewdell J., Frankel M. E., Webster R. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature. 1981 Apr 23;290(5808):713–717. doi: 10.1038/290713a0. [DOI] [PubMed] [Google Scholar]
- Giraudon P., Wild T. F. Monoclonal antibodies against measles virus. J Gen Virol. 1981 Jun;54(Pt 2):325–332. doi: 10.1099/0022-1317-54-2-325. [DOI] [PubMed] [Google Scholar]
- Hightower L. E., Bratt M. A. Protein synthesis in Newcastle disease virus-infected chicken embryo cells. J Virol. 1974 Apr;13(4):788–800. doi: 10.1128/jvi.13.4.788-800.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. C., Murray J. M., White D. O., Gerhard W. U. Enumeration of antigenic sites of influenza virus hemagglutinin. Infect Immun. 1982 Sep;37(3):912–918. doi: 10.1128/iai.37.3.912-918.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane D., Koprowski H. Molecular recognition and the future of monoclonal antibodies. Nature. 1982 Mar 18;296(5854):200–202. doi: 10.1038/296200a0. [DOI] [PubMed] [Google Scholar]
- Lee P. W., Hayes E. C., Joklik W. K. Characterization of anti-reovirus immunoglobulins secreted by cloned hybridoma cell lines. Virology. 1981 Jan 15;108(1):134–146. doi: 10.1016/0042-6822(81)90533-x. [DOI] [PubMed] [Google Scholar]
- Lefrancois L., Lyles D. S. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. I. Analysis of neutralizing epitopes with monoclonal antibodies. Virology. 1982 Aug;121(1):157–167. [PubMed] [Google Scholar]
- Merz D. C., Scheid A., Choppin P. W. Immunological studies of the functions of paramyxovirus glycoproteins. Virology. 1981 Feb;109(1):94–105. doi: 10.1016/0042-6822(81)90474-8. [DOI] [PubMed] [Google Scholar]
- Merz D. C., Scheid A., Choppin P. W. Importance of antibodies to the fusion glycoprotein of paramyxoviruses in the prevention of spread of infection. J Exp Med. 1980 Feb 1;151(2):275–288. doi: 10.1084/jem.151.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G., Simpson D. Synthesis, stability, and cleavage of Newcastle disease virus glycoproteins in the absence of glycosylation. J Virol. 1980 Oct;36(1):171–180. doi: 10.1128/jvi.36.1.171-180.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G. Site of synthesis of membrane and nonmembrane proteins of vesicular stomatitis virus. J Biol Chem. 1975 Sep 10;250(17):6955–6962. [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Acute viral infection: tissue injury mediated by anti-viral antibody through a complement effector system. J Immunol. 1971 Nov;107(5):1274–1280. [PubMed] [Google Scholar]
- Orvell C., Grandien M. The effects of monoclonal antibodies on biologic activities of structural proteins of Sendai virus. J Immunol. 1982 Dec;129(6):2779–2787. [PubMed] [Google Scholar]
- Orvell C. Identification of paramyxovirus-specific haemolysis-inhibiting antibodies separate from haemagglutinating-inhibiting and neuraminidase-inhibiting antibodies. 1. Sendai virus haemolysis-inhibiting antibodies. Acta Pathol Microbiol Scand B. 1976 Dec;84B(6):441–450. [PubMed] [Google Scholar]
- Orvell C. Identification of paramyxovirus-specific haemolysis-inhibiting antibodies separate from haemagglutinating-inhibiting and neuraminidase-inhibiting antibodies. 2. NDV and mumps virus haemolysis-inhibiting antibodies. Acta Pathol Microbiol Scand B. 1976 Dec;84B(6):451–457. [PubMed] [Google Scholar]
- Ozawa M., Asano A., Okada Y. Importance of interpeptide disulfide bond in a viral glycoprotein with hemagglutination and neuraminidase activities. FEBS Lett. 1976 Nov;70(1):145–149. doi: 10.1016/0014-5793(76)80745-4. [DOI] [PubMed] [Google Scholar]
- Peeples M. E., Bratt M. A. UV irradiation analysis of complementation between, and replication of, RNA-negative temperature-sensitive mutants of Newcastle disease virus. J Virol. 1982 Mar;41(3):965–973. doi: 10.1128/jvi.41.3.965-973.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrig J. T., Corser J. A., Schlesinger M. J. Isolation and characterization of hybrid cell lines producing monoclonal antibodies directed against the structural proteins of Sindbis virus. Virology. 1980 Feb;101(1):41–49. doi: 10.1016/0042-6822(80)90481-x. [DOI] [PubMed] [Google Scholar]
- Seto J. T., Becht H., Rott R. Effect of specific antibodies on biological functions of the envelope components of Newcastle disease virus. Virology. 1974 Oct;61(2):354–360. doi: 10.1016/0042-6822(74)90273-6. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Smith G. W., Hightower L. E. Identification of the P proteins and other disulfide-linked and phosphorylated proteins of Newcastle disease virus. J Virol. 1981 Jan;37(1):256–267. doi: 10.1128/jvi.37.1.256-267.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone M. R., Nowinski R. C. Topological mapping of murine leukemia virus proteins by competition-binding assays with monoclonal antibodies. Virology. 1980 Jan 30;100(2):370–381. doi: 10.1016/0042-6822(80)90528-0. [DOI] [PubMed] [Google Scholar]
- Togashi T., Orvell C., Vartdal F., Norrby E. Production of antibodies against measles virions by use of the mouse hybridoma technique. Arch Virol. 1981;67(2):149–157. doi: 10.1007/BF01318598. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk W. A., Synder R. M., Benjamin D. C., Wagner R. R. Monoclonal antibodies to the glycoprotein of vesicular stomatitis virus: comparative neutralizing activity. J Virol. 1982 Apr;42(1):220–227. doi: 10.1128/jvi.42.1.220-227.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Bratt M. A. Polyadenylate sequences on Newcastle disease virus mRNA synthesized in vivo and in vitro. J Virol. 1974 Jun;13(6):1220–1230. doi: 10.1128/jvi.13.6.1220-1230.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Oldstone M. B. Inhibition of immunologic injury of cultured cells infected with lymphocytic choriomeningitis virus: role of defective interfering virus in regulating viral antigenic expression. J Exp Med. 1977 Jun 1;145(6):1449–1468. doi: 10.1084/jem.145.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Yewdell J., Gerhard W. Delineation of four antigenic sites on a paramyxovirus glycoprotein via which monoclonal antibodies mediate distinct antiviral activities. J Immunol. 1982 Jun;128(6):2670–2675. [PubMed] [Google Scholar]