Abstract
Rab GTPases recruit myosin motors to endocytic compartments, which in turn are required for their motility. However, no Ypt/Rab GTPase has been shown to regulate the motility of exocytic compartments. In yeast, the Ypt31/32 functional pair is required for the formation of trans-Golgi vesicles. The myosin V motor Myo2 attaches to these vesicles through its globular-tail domain (GTD) and mediates their polarized delivery to sites of cell growth. Here, we identify Myo2 as an effector of Ypt31/32 and show that the Ypt31/32–Myo2 interaction is required for polarized secretion. Using the yeast-two hybrid system and coprecipitation of recombinant proteins, we show that Ypt31/32 in their guanosine triphosphate (GTP)-bound form interact directly with Myo2-GTD. The physiological relevance of this interaction is shown by colocalization of the proteins, genetic interactions between their genes, and rescue of the lethality caused by a mutation in the Ypt31/32-binding site of Myo2-GTD through fusion with Ypt32. Furthermore, microscopic analyses show a defective Myo2 intracellular localization in ypt31Δ/32ts and in Ypt31/32-interaction–deficient myo2 mutant cells, as well as accumulation of unpolarized secretory vesicles in the latter mutant cells. Together, these results indicate that Ypt31/32 play roles in both the formation of trans-Golgi vesicles and their subsequent Myo2-dependent motility.
INTRODUCTION
Orderly movement of proteins and membranes is essential for the proper function of all eukaryotic cells. In the exocytic pathway, traffic flows from the endoplasmic reticulum (ER), through the Golgi cisternae—cis, medial, and trans—to the plasma membrane (PM). In the endocytic pathway, transport occurs from the PM, via a set of endosomes, to the lysosome. In both pathways, membrane-bound vesicles transfer cargo proteins between compartments. These vesicles are formed at a donor compartment and move on the cytoskeleton to their destination, where they fuse with an acceptor compartment (Rothman, 1994). Ypt/Rabs GTPases are key regulators of these processes. In their GTP-bound form, they recruit divergent effectors that mediate selected steps of vesicle transport (Segev, 2001b). In addition to vesicle motility, which is required for transport between compartments, the compartments themselves also move to ensure their proper inheritance during cell division (Pruyne et al., 2004; Fagarasanu and Rachubinski, 2007).
Vesicle and compartment motility is mediated by molecular motors, which bind to membranes and move them on the cytoskeleton. Typically, long-range movement is mediated by the microtubule-based motors kinesins and dynein, whereas short-range movement is mediated by the actin motors myosins (Langford, 2002; Wu et al., 2006). Myosin V and myosin VI move cellular organelles to the plus and minus ends of actin filaments, respectively (Fehrenbacher et al., 2003; Buss and Kendrick-Jones, 2007). Whereas myosin V moves membrane-bound compartments toward the PM (Bretscher, 2003; Seabra and Coudrier, 2004; Desnos et al., 2007), myosin VI moves endocytic compartments and functions in Golgi anchoring (Sweeney and Houdusse, 2007).
The heavy chain of myosin V forms a homodimer in which the two monomers are held together at the coiled-coil domain. Myosin V contains three other domains: The actin-binding ATP-dependent motor domain; the lever arm domain, which contains six light-chain binding sites and whose length determines the speed of myosin movement on actin cables (Schott et al., 2002); and a globular tail. The globular tail domain (GTD) attaches myosin V to its cargoes (Catlett and Weisman, 1998; Trybus, 2008). Attachment of the GTD of myosin V and myosin VI to membranes requires a protein receptor. Recently, myosin V motors were shown to exist in two forms, an inactive-folded form and an open-active form that can move cargo along actin filaments (Thirumurugan et al., 2006; Taylor, 2007). Therefore, myosin V can be regulated at two levels: membrane recruitment and activity. Rab GTPases and their effectors were suggested to regulate myosin V function at both these levels (Hammer and Wu, 2002; Seabra and Coudrier, 2004; Li et al., 2005).
Myosin receptors on membranes of endocytic compartments—endosomes, lysosomes, or lysosome-related organelles such as melanosomes—were identified. In some cases, Rabs recruit myosin V to membranes, together with a binding partner (Desnos et al., 2007). For example, Rab27, through the bridging protein melanophilin, serves as a myosin V receptor on melanosomes (Hammer and Wu, 2002; Seabra and Coudrier, 2004); Rab11 and its effectors FIP2 were identified as myosin V receptors on recycling endosomes (Nedvetsky et al., 2007). In other cases, non-Rab receptors were implicated in this role. For example, the Vac8/Vac17 complex serves as the receptor for Myo2, a myosin V-type motor, on the yeast lysosome termed vacuole (Ishikawa et al., 2003; Tang et al., 2003). Rab8 and its binding partner optimanrium were identified as myosin VI receptors on the Golgi (Sahlender et al., 2005). Although receptors for myosin V-type motors have been identified for mitotic spindle orientation (Yin et al., 2000), the yeast vacuole (Weisman, 2006), and peroxisomes (Fagarasanu et al., 2006), they have not yet been identified for exocytic organelles. Moreover, there is currently a controversy in the field as to whether Rab GTPases interact with myosin V directly or whether Rab GTPases interact with bridging proteins that in turn interact with Rabs to recruit myosin V to membranes.
In yeast, the Ypt31/32 GTPase functional pair, which belongs to the Rab11 GTPase family, is required for the formation of trans-Golgi derived vesicles (Jedd et al., 1997) and the fusion of these vesicles with the PM is regulated by Sec4 GTPase (Goud et al., 1988). Myo2, one of the two myosin V-type motors in yeast, is required for the motility of these vesicles (Govindan et al., 1995; Schott et al., 1999; Karpova et al., 2000). Sec4 was implicated as a Myo2 receptor on these vesicles based on coprecipitation of the two proteins from yeast cell lysates (Wagner et al., 2002); however, neither direct interaction nor evidence for the role of such an interaction was shown for Sec4 and Myo2. Myo2 is also required for the polarized transport of late Golgi toward sites of cell growth (Rossanese et al., 2001). Here, we show that Ypt31/32 in their GTP-bound form interact directly with the GTD domain of the Myo2 motor and that this interaction is important for the polarized localization of Myo2 to sites of cell growth and for polarized secretion. These results suggest that Ypt31/32 regulate either the recruitment or the activation of Myo2, both of which are required for transport of late Golgi and/or Golgi-derived vesicles to sites of cell growth.
MATERIALS AND METHODS
Strains, Plasmids, and Reagents
Strains and plasmids used in this study are summarized in Supplemental Table S1. For the yeast two-hybrid assay, Ypt31, Ypt32, and Myo2-GTD constructs were cloned into pACT2 and pGBDU-C2 vectors by using polymerase chain reaction (PCR) followed by restriction and ligation at the SmaI and XhoI, or SmaI and SalI, sites, respectively. For expression in Escherichia coli, Ypt31 and Ypt32 were subcloned from pGEX-KG (Jedd et al., 1997) into pTrcHisA vector (Invitrogen, Carlsbad, CA), by using the BamHI and XhoI restriction sites. For genetic interaction experiments, Myo2 constructs (open reading frame including 600 bp upstream and 400 bp downstream) were cloned into pRS426 at the XhoI and SmaI restriction sites. The Myo2 wild type expressed from the 2μ vector is functional because it can rescue the temperature sensitivity of myo2-66 mutant cells. All yeast transformations were done using the lithium acetate method combined with heat shock. E. coli transformation was done using electroporation.
For construction of the pRS413 MYO2-YPT32 fusion plasmids, first an NheI site was generated at the C-terminal end of Myo2 by PCR. This was achieved by using primers 5′-CGT TCA AGA CGG CCA Cgc tag cTG ATG GCG CGA GAA AC-3′ and 5′-GTT TCT CGC GCC ATC Agc tag cGT GGC CGT CTT GAA CG-3′ to make pBlueScript myo2-tail-NheI from pBlueScript myo2-tail (pNLC15; pBlueScript EcoRI-EcoRI fragment of myo2-tail). Second, YPT32 and ypt32-SS fragments were amplified by PCR using primers 5′-AGA gct agc AGC AAC GAA GAT TAC GG-3′ and 5′-AGA tct aga TTA ACA ACA GTT GCT GG-3′ and 5′-AGA gct agc AGC AAC GAA GAT TAC GG-3′ and 5′-AGA tct aga TTA ACt ACt GTT GCT GGA TTT TTT CTT CTT G-3′, respectively. The YPT32 fragment was inserted into the pBS Myo2-tail-NheI at the NheI site to generate pBS Myo2-tail-YPT32. Finally, an EcoRI-EcoRI fragment was subcloned from pBS Myo2-tail-YPT32 into pRS413 MYO2 delta EcoRI-EcoRI (Catlett and Weisman, 1998).
Antibodies used in this study included rabbit anti-GAL4-AD, rabbit anti-GAL4-BD (Santa Cruz Biotechnology, Santa Cruz, CA); mouse monoclonal anti-hemagglutinin (HA) (Research Products, Princeton, NJ); rabbit anti-Emp47 (Schroder et al., 1995); rabbit anti-glutathione transferase (GST) (Invitrogen); mouse monoclonal anti-His6 (R&D Systems, Minneapolis, MN); affinity-purified rabbit anti-Ypt31/32 (Jedd et al., 1997); affinity-purified goat anti-Myo2 (Catlett et al., 2000); goat anti-rabbit-horseradish peroxidase (HRP) and goat anti-mouse-HRP (GE Healthcare, Chalfont St. Giles, United Kingdom); and Texas Red-conjugated anti-rabbit immunoglobulin G (IgG), fluorescein isothiocyanate (FITC)-conjugated rabbit anti-goat IgG, and FITC-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA).
All chemical reagents were purchased from Thermo Fisher Scientific (Fair Lawn, NJ), unless otherwise noted. Media components, other than amino acids, were purchased from US Biological (Swampscott, MA). ProtoGel for Western blots was purchased from National Diagnostics (Atlanta, GA). Amino acids, guanosine diphosphate (GDP), and guanosine 5′-O-(3-thio) triphosphate (GTPγS) and protease inhibitors were purchased from Sigma-Aldrich (St. Louis, MO). EDTA-free protease inhibitor cocktail (PIC) was purchased from Roche Diagnostics (Indianapolis, IN). Restriction enzymes and buffers were purchased from New England Biolabs, Ispwich, MA). Isopropil-β-d-thiogalactopyranoside (IPTG) was purchased from Acros Organics (Fair Lawn, NJ). Dithiothreitol (DTT) and Alexa Fluor 594 phalloidin were purchased from Invitrogen. Quantification of the bands on western blots was done using the Spot Denso option of the Alpha Imager (Alpha Innotech, San Leandro, CA).
Yeast Culture Conditions and Protein Expression Analysis
For yeast two-hybrid and genetic interaction assays yeast cultures were grown overnight at 26°C in minimal (SC) media, normalized to the same density by OD600, and spotted onto agar plates in serial dilutions of 1 to 5 or 1 to 10. To determine the expression level of yeast two-hybrid constructs, 4 OD600s of overnight culture cells were spun down, resuspended in 100 μl of Laemmli buffer, supplemented with PIC, boiled, vortexed with equal volume of glass beads (BioSpec Products, Bartlesville, OK), and subjected to Western blot analysis with anti-GAL4-AD, anti-HA, or anti-GAL4-BD. For endogenous Myo2 expression analysis, immunoelectron and immunofluorescence microscopy yeast cultures were grown at permissive temperature (24°C) in rich media (YPD) to log phase, and switched to a restrictive temperature (37°C) for 2 h, when needed. To test Myo2 expression in wild-type and ypt31Δ/32ts strains, cell pellets were resuspended in lysis buffer (0.8 M sorbitol in 10 mM triethanolamine and 1 mM EDTA, pH 7.2) (Walch-Solimena et al., 1997) containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin and chymostatin, 5 μg/ml pepstatin A and antipain, and 10 μg/ml aprotinin, 1 mM benzamidine) and lysed with glass beads. Protein levels in cell lysates were determined using immunoblot analysis and specific antibodies.
Expression and Coprecipitation of Recombinant Proteins
His6-Ypt1, His6-Ypt31, and His6-Ypt32 proteins were expressed in XL1 Blue and BL21 E. coli strains, respectively. GST and GST-Myo2-GTD were expressed in BL21 strain, and GST-Ypt32 was expressed in SURE strain. The expression was induced by 0.1 mM IPTG for 2 h at 37°C for GST-Ypt32, by 0.5 mM IPTG for 4 h at 30°C for His6-Ypts, and overnight at 24°C for GST/GST-Myo2 GTD. Cells were lysed by sonication as described previously (Jones et al., 1995) in PBS buffer containing 10 mM MgCl2 and 5 mM DTT.
For His6 pull-down, His6-tagged GTPases were bound to Ni2+-nitrilotriacetic acid agarose beads (QIAGEN, Hilden, Germany) in the presence of 2 mM imidazole (Avocado Research Chemicals, Heysham, United Kingdom), washed with preloading buffer (PBS containing 1 mg/ml bovine serum albumin (BSA), 1 mM EDTA, 1 mM MgCl2, and 1 mM DTT) and preloaded with GDP or GTPγS as described previously (Ortiz et al., 2002) with the following modifications. Briefly, 200–260 nM Ypt protein was incubated with 1 mM nucleotide in preloading buffer for 30 min at room temperature with rotation. MgCl2 concentration was adjusted to 5 mM, and incubation was continued for another 10 min. GTPases bound to the beads were than incubated with lysates containing GST or GST-Myo2-GTD diluted 100 times with buffer A (PBS containing 1 mg/ml BSA, 5 mM MgCl2, 1 mM DTT, 5 mM imidazole, and 0.2 mM GDP or GTPγS) to yield 100–130 nM His6-Ypt and 85 nM GST/GST-Myo2-GTD, at 4°C for 1.5 h. Pellets were washed sequentially with the following four wash buffers (WBs) by inverting each reaction tube 30 times: WB 1 (PBS containing 5 mM MgCl2 and 1 mM DTT), WB 2 (20 mM HEPES, pH 7.2, 100 mM NaCl, 10% glycerol, 0.1% Triton X-100, 1 mM DTT, 5 mM MgCl2, and 2 mM imidazole), WB 3 (WB 2, except 0.5% Triton X-100 and 20 mM imidazole), and WB 4 (WB 3, except 250 mM NaCl). The pellets were resuspended in Laemmli buffer, in 10% of the initial reaction volume, and were subjected to immunoblot analysis using anti-His and anti-GST antibodies.
For GST-pull-down, GST or GST-Myo2-GTD lysates (30 μg of total protein) were bound to glutathione-Sepharose beads (GE Healthcare) and incubated with 2.2 μg of Ypt32, in buffer B (50 mM Tris-HCl, pH 7.5, 50 mM NaCl, and 10 mM MgCl2) in a total volume of 300 μl for 1 h at 4°C. Beads were washed three times with wash buffer (buffer B containing 10% glycerol, 0.1% Triton X-100, and 1 mM DTT) and resuspended in Laemmli buffer, in 10% of the initial reaction volume, and then they were subjected to immunoblot analysis using anti-GST and anti-Ypt31/32 antibodies.
Microscopy
Immunofluorescence microscopy was performed as described previously (Jedd et al., 1997), with modifications as follows. Cells were fixed in 3.7% formaldehyde for 50 min. For spheroplasting, cells were incubated with rotation in the presence of 0.5% β-mercaptoethanol (Bio-Rad, Hercules, CA) and 0.3 mg/ml zymolase-20T (ICN Biomedicals, Aurora, OH) for 40 min at 30°C and pelleted at the lowest speed (100 × g). Pellets were resuspended in 1.5 M sorbitol containing 0.5% SDS and incubated with rotation for 3 min. All further steps were done in 0.05 M phosphate buffer, pH 7.5, containing 150 mM NaCl, 0.1% Tween (Sigma-Aldrich), and 10 mg/ml BSA. Spheroplasts were washed twice, 15 min each time, after which primary antibodies were added in a 1:500 dilution for anti-Myo2 and anti-HA, or 1:250 for anti-Ypt31. Spheroplasts were than washed twice, 15 min each time, and incubated with secondary antibodies, diluted 1:200 for anti-goat-FITC, 1:250 for anti-rabbit-Texas Red, and 1:500 for anti-mouse-FITC, 1 h at 30°C in the dark. After two consecutive 15-min washes, spheroplasts were immobilized on coverslips, coated with 0.1% poly-l-lysine (Sigma-Aldrich). Unattached spheroplasts were removed from the coverslips by a quick wash; the coverslips were covered with mounting solution and then were mounted on regular glass slides. Slides were visualized using deconvolution Axioscope microscope (Carl Zeiss, Thornwood, NY) as described previously (Liang et al., 2007). Briefly, a series of 7–12 Z-stacks, 275–450 nm each, were collected for each field by using a 63× objective, and they were deconvolved using Regularized Inverse Filter and Axiovision 4.3 software (Carl Zeiss).
Electron microscopy was performed as described previously (Liang et al., 2007). Immunoelectron microscopy was performed as described previously (Mulholland et al., 1994) by using affinity-purified rabbit anti-Ypt31 and 10-nm gold-conjugated goat anti-rabbit IgG (BioCell, London, United Kingdom).
RESULTS
Ypt31/32 in Their GTP-bound Form Interact Directly with Myo2-GTD
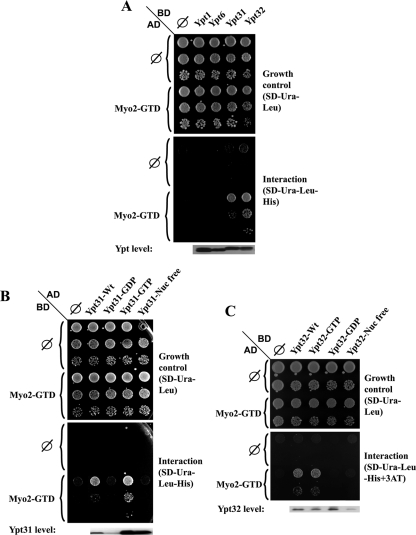
Because Rabs serve as myosin V receptors in the endocytic pathway, and Myo2 attaches to secretory vesicles via its GTD (Catlett and Weisman, 1998; Hammer and Wu, 2002; Seabra and Coudrier, 2004; Desnos et al., 2007), the interaction of Ypt31/32 with Myo2-GTD was tested in the yeast two-hybrid system. We found that Ypt31 and Ypt32 interact with Myo2-GTD. This interaction is specific to Ypt31/32 because two other Golgi Ypts, Ypt1 and Ypt6, do not interact with Myo2-GTD in this assay (Figure 1A). To determine whether the interaction with Myo2-GTD is dependent on the nucleotide-bound form of Ypt31/32, the two-hybrid assay was performed using various Ypt31 and Ypt32 mutants predicted to be restricted in nucleotide cycling (Olkkonen and Stenmark, 1997). Myo2-GTD interacts with the wild-type and the GTP-bound form of Ypt31 and Ypt32, but not with the GDP-bound or the nucleotide-free forms of these GTPases (Figure 1, B and C, respectively). Prenylation and attachment of the Ypt to the membrane is not required for this interaction because Ypt31 and Ypt32 that contain an SS modification at their C terminus, which cannot be prenylated (Khosravi-Far et al., 1991), also interact with Myo2-GTD (Supplemental Figure S1).
Figure 1.
Yeast two-hybrid interaction of Ypt31/32 GTPases and Myo2-GTD. (A) Ypt specificity: Ypt32 and Ypt31, but not Ypt1 and Ypt6, interact with the Myo2-GTD in the yeast two-hybrid mating assay. Yeast MATa (NSY468) cells expressing Myo2-GTD from the pACT2 (GAL4-AD, LEU2) vector were mated with MATα (NSY752) cells expressing the indicated Ypts from pAS1 (GAL4-BD, TRP1) plasmids: Ypt1, Ypt6, Ypt31, and Ypt32. Diploids were selected on SD-Trp-Leu medium. Growth control is shown on SD-Trp-Leu (top), and interaction is shown on SD-Trp-Leu-His (bottom). Cells were plated in 10-fold serial dilutions from top to bottom and incubated at 30°C. Empty vector (∅) for both plasmids are shown as negative controls. Immunoblot analysis shows similar Ypt expression levels by using anti-Gal4 binding domain antibody (bottom). (B) Nucleotide-bound form specificity of the Ypt31–Myo2-GTD interaction: Myo2-GTD interacts specifically with the wild-type and the GTP-bound form of Ypt31, but not with its GDP-bound and nucleotide-free form in the yeast two-hybrid assay. Yeast MATα cells expressing Myo2-GTD from the pGBDU-C2 (GAL4-BD, URA3) vector were mated with MATa cells expressing the various nucleotide-bound forms of Ypt31 from pACT2 (GAL4-AD, LEU2) plasmids: wild type, Q72L (GTP), S27D (GDP), and D129N (nucleotide free). Plating, negative controls, and immunoblot analysis were done as described in A, except for using anti-Gal4 activation domain. Growth control is shown on SD-Ura-Leu (top), and interaction is shown on SD-Ura-Leu-His (bottom). (C) Nucleotide-bound form specificity of the Ypt32–Myo2-GTD interaction: Myo2-GTD interacts specifically with the wild-type and the GTP-bound form of Ypt32, but not with its GDP-bound and nucleotide-free form in the yeast two-hybrid assay. Yeast cells were transformed with two plasmids: Myo2-GTD was expressed from the pACT (GAL4-AD, LEU2) vector and the indicated nucleotide-bound forms of Ypt32 were expressed from pGBDU-C2 (GAL4-BD, URA3) plasmids—wild type, Q72L (GTP), S27D (GDP), and D129N (nucleotide free). Growth control is shown on SD-Ura-Leu (top), and interaction is shown on SD-TUra-Leu-His + 0.1 mM 3AT (bottom). Plating, negative controls and immunoblot analysis were done as described in B, except cells were plated in fivefold dilutions. Results shown in this figure represent at least two independent experiments.
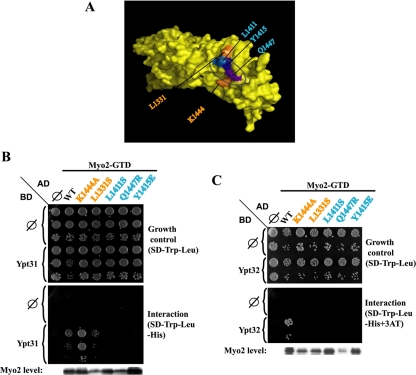
The vesicle-binding surface on the Myo2-GTD was mapped previously based on mutations in this domain that caused growth defects. Specifically, five conserved surface amino acids were implicated in vesicular transport (Pashkova et al., 2006). The effect of these mutations on the interaction of Myo2-GTD with Ypt31 and Ypt32 was examined using the yeast two-hybrid system. Three mutations—L1411S, Q1447R, and Y1415E—disrupt the interaction with both GTPases. The other two MYO2 mutations—L1331S and K1444A—exhibit defective interaction only with Ypt32 and not with Ypt31 (Figure 2). The three residues required for the two-hybrid interaction with both Ypt31 and Ypt32 form a patch on the vesicle-binding surface, indicating that they define the Ypt31/32 interaction site on Myo2-GTD. The two residues required for the Myo2-GTD interaction with Ypt32 (not Ypt31) are located on the two sides of that patch. Mutations in the three residues required for interaction with both Ypt31 and Ypt32 were used below for the disruption of the Ypt31/32–Myo2 interaction in the context of the cell.
Figure 2.
Mapping of the Ypt31/32 interaction domain on Myo2-GTD. (A) The cluster of residues that define the vesicle-binding site on the surface of Myo2-GTD is shown on a space-filling model of this domain (Pashkova et al., 2006). The three Myo2-GTD residues that interact with both Ypt31 and Ypt32 are found in a patch on the vesicle-binding surface of Myo2-GTD (blue and purple). The two residues required for the interaction with Ypt32, but not with Ypt31, are positioned at the two sides of this patch (orange). (B) Disruption of the Ypt31-Myo2-GTD yeast two-hybrid interaction by myo2-GTD mutations: yeast MATα (NSY752) cells expressing Ypt31 from pAS1 (GAL4-BD, TRP1) plasmid were mated with MATa (NSY468) cells expressing different Myo2-GTD alleles from the pACT2 (GAL4-AD, LEU2) vector. Diploids were selected on SD-Trp-Leu medium. Growth control is shown on SD-Trp-Leu (top), and interaction is shown on SD-Trp-Leu-His (bottom). Cells were plated in 10-fold serial dilutions from top to bottom and incubated at 30°C. Empty vector (∅) for both plasmids are shown as negative controls. Immunoblot analysis shows similar Myo2-GTD expression levels by using anti-Gal4 activation domain antibody (bottom). Wild-type (WT) Myo2-GTD and the two alleles—K1444A and L1331S—interact with Ypt31. In contrast, for three alleles—L1411S, Q1447R, and Y1415E—the interaction is defective. (C) Disruption of the Ypt32-Myo2-GTD yeast two-hybrid interaction by myo2-GTD mutations: This experiment was done as described in Figure 1C. The interaction of Ypt32 with Myo2-GTD is defective with all five alleles of Myo2. Results shown in this figure represent two independent experiments.
In an independent approach, we found that bacterially expressed Myo2-GTD and Ypt31/32 coprecipitate. This finding shows that the interaction between Myo2 and Ypt31/32 is direct, because no other yeast proteins are present in this experiment. GST-tagged Myo2-GTD and His6-tagged Ypt31 or Ypt32 were expressed in bacterial cells. His6-tagged Ypt31, Ypt32, or Ypt1 as a negative control were precipitated from bacterial cell extracts by using Ni2+ beads. The Ypts on the Ni2+ beads were loaded with GDP or the nonhydrolyzable GTP analogue GTPγS incubated with GST-tagged Myo2-GTD or GST as a negative control, and the proteins attached to the beads were analyzed. Precipitation of the Ypt was verified by immunoblot analysis by using anti-His6 antibody. Coprecipitation of GST-Myo2-GTD was tested by immunoblot analysis with anti-GST antibody. GST-Myo2-GTD, but not GST, coprecipitates preferentially with Ypt31-GTP and Ypt32-GTP, but not with Ypt31-GDP or Ypt32-GDP. GST-Myo2-GTD also does not coprecipitate with Ypt1 bound to GTP or GDP (Figure 3). The direct interaction of bacterially expressed proteins was verified by the coprecipitation of Ypt32 with GST-Myo2. Specifically, purified Ypt32 coprecipitates with GST-Myo2-GTD, but not with GST, by using glutathione beads (Supplemental Figure S2). An interaction of Ypt32 with Myo2-GTD was recently reported in an independent study (Casavola et al., 2008). Together, these results indicate that Ypt31/32, in their GTP-bound form, interact directly with Myo2-GTD. Moreover, they suggest that the Myo2 motor acts as a downstream effector of the Ypt31/32 GTPases in targeting trans-Golgi vesicles to the PM.
Figure 3.
Specific interaction of bacterially expressed Ypt31/32-GTP with Myo2-GTD. His6-tagged Ypt31 (pNS619), Ypt32 (pNS620), or Ypt1 (pNS1082) as a negative control, were expressed in bacteria and purified using Ni2+-NTA beads. The Ypts on the beads were loaded with GTPγS (T) or GDP (D) and incubated with lysates from bacteria expressing GST-tagged Myo2-GTD (pNS734), or GST (pNS255) as a negative control (left). The Ypts were precipitated using Ni2+ beads (right), and their presence was detected using immunoblot analysis and anti-His6 antibodies (bottom). Coprecipitation of GST-Myo2-GTD or GST was detected using immunoblot analysis and anti-GST antibodies (top). GST-Myo2-GTD (∼5%), but not GST, coprecipitates with His6-Ypt31 (A) and His6-Ypt32 (B) preferentially in their GTP-bound form, but not with His6-Ypt1. Results shown in this figure represent three independent experiments.
Where in the Cell Do Ypt31/32 and Myo2 Interact?
Whereas Myo2 attaches to trans-Golgi-derived vesicles (Govindan et al., 1995; Schott et al., 1999; Karpova et al., 2000), we have previously shown that Ypt31/32 localize to the trans-Golgi based on their colocalization with a trans-Golgi marker by using live fluorescence microscopy (Chen et al., 2005; Sciorra et al., 2005). If Myo2 acts as a downstream effector of Ypt31/32, the two proteins should reside on the same intracellular compartment. Therefore, we first tested whether the two proteins colocalize in yeast cells. Colocalization of the Ypt31/32 GTPases and the HA-tagged Myo2 was shown using deconvolution immunofluorescence microscopy with anti-Ypt31/32 and anti-HA antibodies. Both Myo2-HA and Ypt31/32 localize to sites of cell growth—small buds and bud-mother necks—and there is a partial overlap between their localization in these regions, but not inside mother cells (Figure 4A). This colocalization supports the hypothesis that the Ypt31/32 interaction with Myo2 occurs in vivo in sites of cell growth.
Figure 4.
Ypt31/32 and Myo2 colocalize on secretory vesicles. (A) Colocalization of Ypt31/32 and Myo2 by immunofluorescence microscopy: yeast cells expressing endogenous HA-tagged Myo2 (NSY1268) were fixed and stained with anti-HA (left, green) and affinity-purified anti-Ypt31/32 antibodies (middle, red). The “merge” panels show colocalization in yellow. Differential interference contrast (DIC; right) shows the contour of the cells. Cells were visualized using a deconvolution microscope. Three sequential z-sections are shown to ensure colocalization of the two proteins. Arrows point to areas of colocalization; open arrows show areas with only Myo2. Results shown in this figure represent two independent experiments. Bar, 5 μm. B. Ypt31/32 localize to Golgi and trans-Golgi-derived vesicles by using immunoelectron microscopy: wild-type, sec15 (NY786), and ypt31Δ/32ts cells (NSY348) were grown at 26°, shifted to 37°C for 2 h and processed for immunoelectorn microscopy by using affinity-purified anti-Ypt31/32 antibody: i) wild type, Golgi, G (Preuss et al., 1992); ii) wild type, Golgi-to-PM vesicles, V; iii) sec15 mutant, Golgi-to-PM vesicles, V; and iv) ypt31Δ/32ts, negative control. Insets (top left corners) show an enlargement of the boxed areas. Bar, 1 μm.
To examine in which compartment the two proteins can interact, the intracellular localization of Ypt31/32 was determined in more detail by using immunoelectron microscopy. In wild-type cells, as well as in sec15 mutant cells that accumulate late-secretory vesicles, Ypt31/32 is found on Golgi cisternae and on ∼100-nm Golgi-derived vesicles (Figure 4B). Thus, both Ypt31/32 and Myo2 localize to trans-Golgi–derived vesicles, which are polarized to sites of cell growth, and they can interact there.
Physiological Relevance of the Ypt31/32–Myo2 Interaction
Showing a genetic interaction between genes supports the physiological relevance of a physical interaction between the proteins they encode. The effects of Myo2 overexpression on cells carrying the ypt31Δ/32ts mutations, and of Ypt31/32 overexpression on cells carrying a myo2ts mutation, were determined. Overexpression of Myo2 enhances the growth phenotype of ypt31Δ/32ts mutant cells, but not of ypt1ts mutant cells. In contrast, the growth defect of the myo2-Y1415E mutant cells can be suppressed by overexpression of Ypt31, but not of Ypt1 (Figure 5, A–C). Overexpression of Ypt32 showed a similar effect to that of Ypt31 (data not shown). Thus, overexpression of Ypt31/32 or Myo2 affects the growth phenotype of a mutation in the other gene and both interactions are specific to YPT31/32, because YPT1 does not exhibit these interactions with MYO2. The specific YPT31/32-MYO2 genetic interactions suggest that the physical interaction between the proteins they encode is physiologically relevant.
Figure 5.
Genetic interactions of YPT31/32 and MYO2. (A) Schematic presentation of the genetic interactions shown in B and C (flat arrow, phenotypic enhancement; arrow, phenotypic suppression). (B) Overexpression of Myo2 enhances the growth defect of ypt31Δ/32ts (NSY348) (left), but not ypt1ts (NSY161) (right), mutant cells. Overexpression of Myo2 from a 2μ plasmid has no effect on the growth of WT or ypt1ts mutant cells. However, when overexpressed in ypt31/Δ/32ts mutant cells, Myo2 causes an enhancement of their growth defect: at 36°C, mutant cells can grow (empty vector control, ∅) but not when Myo2 is overexpressed. The semirestrictive temperature for ypt31/Δ/32ts and ypt1ts mutant cells is 36 and 34°C, respectively. (C) Overexpression of Ypt31, but not Ypt1, suppresses the growth defect of a Myo2-GTD mutation in the vesicle-binding site. Overexpression of Ypt31 or Ypt1 from a 2μ plasmid has no effect on the growth of WT cells. However, when overexpressed in myo2-Y1415E mutant cells (NSY1169), Ypt31 suppresses their growth defect: at 36°C, mutant cells grow poorly (empty vector control, ∅) unless Ypt31 is overexpressed. In contrast, overexpression of Ypt1 from a 2μ plasmid does not suppress the phenotype of myo2 mutant cells. (D) Myo2-Ypt31/32 interaction-defective mutations disrupt the phenotypic enhancement of the ypt31Δ/32ts mutation by overexpression of Myo2. The experiment was done as explained in B, except that different MYO2 alleles were used and 34°C plate is shown. Myo2 alleles, which disrupt its interaction with both Ypt31 and Ypt32 (Figure 3), do not enhance the growth phenotype of the ypt31Δ/32ts mutant cells (compared with the empty vector control, ∅). Results shown in this figure represent at least two experiments.
The opposite effect of Ypt31/32 or Myo2 overexpression on the growth of cells mutated for the other might stem from a difference in the number of essential interactions in which they are involved. Thus, a possible reason for the enhancement of the ypt31Δ/32ts mutant phenotype by overexpression of Myo2 is that Ypt31/32 need to interact with multiple essential effectors, and an excess of Myo2 protein sequesters the mutant Ypt32ts protein from its other essential interactors. In contrast, suppression of the growth defect of the myo2 mutation by overexpression of Ypt31/32 can be attributed to the protein trafficking being the only known essential role of Myo2. If Ypt31/32 GTPases are important for this essential function of Myo2, their overexpression can help a cell growth defect caused by a myo2 mutation.
We expected that disruption of the Myo2–Ypt31/32 interaction by the MYO2-GTD mutations used in the yeast two-hybrid analysis would affect the genetic interactions as well. Indeed, whereas overexpression of wild-type Myo2 results in the enhancement of the ypt31Δ/32ts mutant phenotype, overexpression of MYO2 mutations that disrupt the Myo2–Ypt31/32 interaction did not affect the growth of this mutant (Figure 5D). This result implies that if a Myo2 mutant protein cannot interact effectively with the Ypt32ts mutant protein, it would not be able to sequester it from the other essential Ypt31/32 effectors and disrupt cell growth as a Myo2 wild-type protein does. Similarly, whereas overexpression of Ypt31 rescues the growth defect of MYO2 mutations that disrupt the Myo2–Ypt31 interaction, it does not rescue the growth defect of mutations that do not affect the Ypt31–Myo2 interaction (Supplemental Figure S3). This result implies that excess Ypt31 can only help the growth phenotype of myo2 mutants with compromised ability to interact with it, but not of myo2 mutants whose growth is defective due to a defect in Myo2 mutant protein interaction with Ypt32. The allele specificity of the genetic interactions between YPT31/32 and MYO2 further support the physiological relevance of the physical interaction between the proteins they encode.
The Role of the Ypt31/32–Myo2 Interaction
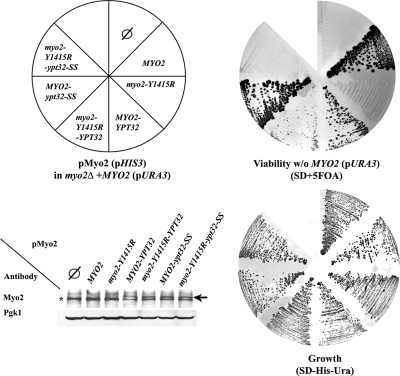
The essential role of Myo2 is to mobilize secretory vesicles to sites of cell growth (Schott et al., 2002). If the interaction with Ypt31/32 is required for this essential function, lethality caused by a MYO2 mutation that disrupts this interaction might be bypassed by fusion of the two proteins. Substitution of the Y1415 residue in the Ypt31/32 interaction site of Myo2-GTD with arginine is lethal (Pashkova et al., 2006). Importantly, fusing this Myo2-Y1415R mutant protein with Ypt32 at its C terminus rescues this lethality. Moreover, only Ypt32 that can attach to membranes rescues the myo2-Y1415R lethality, because fusing this mutant protein with Ypt32-SS, which cannot be prenylated and attached to membranes, does not rescue the lethality (Figure 6). This result indicates that interaction between Myo2 and Ypt31/32 is essential for cell viability.
Figure 6.
Interaction between Myo2 and Ypt32 is required for yeast cell viability. Fusion with Ypt32 suppresses the lethality caused by the myo2-Y1415R mutation. Cells carrying a deletion of MYO2 on the chromosome and a URA3 plasmid expressing wild type Myo2 (YCp50-MYO2; LWY2947) were transformed with HIS3 plasmids (pRS413) expressing Myo2 from the following vectors: empty vector (∅), MYO2, myo2-Y1415R, MYO2-YPT32, myo2-Y1415R-YPT32, MYO2-ypt32-SS, and myo2-Y1415E-ypt32-SS (schematic presentation of the plate, top, left). All strains can grow on SD-His-Ura plates (bottom, right). Cells expressing wild-type Myo2 proteins—Myo2, Myo2-Ypt32, or Myo2-Ypt32-SS—from the pRS413 plasmid can lose the YCp50-URA3-MYO2 plasmid and grow on the SD + 5-fluoroorotic acid (5-FOA) plate (top, right). Cells expressing the mutant proteins Myo2-Y1415R or the Myo2-Y1415R-Ypt32-SS cannot grow on SD + 5-FOA, whereas cells expressing the Myo2-Y1415R-Ypt32 fusion protein can lose the YCp50-URA3-MYO2 plasmid and grow on the SD + 5-FOA. Wild-type and mutant Myo2 proteins are expressed equally well (bottom, left). Strains were grown on SD-His-Ura, and lysates were analyzed for Myo2 expression by immunoblot analysis and antibodies against the Myo2-tail (top) or Pgk1p (loading control, bottom). Asterisk indicates Myo2p; arrow indicates the Myo2-Ypt32 fusion protein.
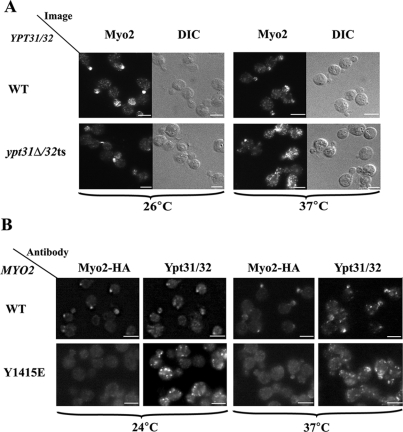
To determine the role of the interaction of Ypt31/32 with Myo2, the effect of YPT31 and MYO2 mutations on the cellular localization of Myo2 was examined using immunofluorescence microscopy. In wild-type cells, Myo2 is polarized to sites of cell growth: the tip of small buds during bud growth and the bud necks during cytokinesis. In ypt31Δ/32ts mutant cells at their restrictive temperature, Myo2 does not localize to sites of cell growth but is diffuse (Figure 7A), even though the Myo2 protein level is unchanged (Supplemental Figure S4). Disruption of the Ypt31/32–Myo2 interaction also results in diffused localization of Myo2. When the MYO2-HA mutated allele Y1415E, which disrupts the Ypt31/32–Myo2 interaction, was present as the sole copy in the cell, Myo2 localization was diffuse (Figure 7B). The level of the Myo2 mutant protein is similar to that of the wild-type protein (Pashkova et al., 2006). Therefore, the diffuse distribution of Myo2 in these mutant cells is caused by disruption of its attachment to secretory vesicles and/or of the targeting of these vesicles to sites of cell growth. Importantly, Ypt31/32 still localize to puncta even though the Ypt31/32 staining in the myo2 mutants is not polarized as in wild-type cells. The nonpolarized and brighter Ypt31/32 puncta seen in these mutant cells are probably a result of an accumulation of nonpolarized vesicles decorated with Ypt31/32. These results further support the hypothesis that Myo2 is a downstream effector of Ypt31/32 and show that the Ypt31/32–Myo2 interaction is required for the correct intracellular localization of Myo2.
Figure 7.
The Ypt31/32-Myo2-GTD interaction is important for the polarized intracellular localization of Myo2. (A) Ypt31/32 GTPases are required for polarized intracellular distribution of Myo2. The effect of the ypt31Δ/32ts mutation on the cellular distribution of Myo2 was determined using immunofluorescence (IF) deconvolution microscopy and anti-Myo2 antibody. Cells were grown to mid-log at permissive temperature (24°C), shifted to 37°C for 2 h, and fixed and processed for IF (see Materials and Methods). In wild-type cells (NSY125), Myo2 localizes to sites of cell growth. In ypt31Δ/32ts mutant cells (NSY348) at the restrictive temperature (37°C), Myo2 staining is diffuse. (B) Ypt31/32 interaction mutation in Myo2-GTD results in an impaired Myo2 intracellular localization. The effect of the myo2-Y1415E mutation on the localization of Myo2 was determined by IF microscopy using anti-Myo2 and antiYpt31/32 antibodies. Cells were fixed and processed for IF (see Materials and Methods). In wild-type cells (NSY1268; top), Myo2 staining is sharp and localizes to sites of cell growth and the Ypt31/32 puncta are also mostly polarized to the buds. In cells carrying the myo2-Y1415E mutation (NSY1269, bottom), which disrupts the interaction with Ypt31 and Ypt32, at the permissive and restrictive temperatures Myo2 staining is diffuse even though the Ypt31/32 pattern is still punctate. Results shown in this figure represent at least two experiments. Bar, 5 μm.
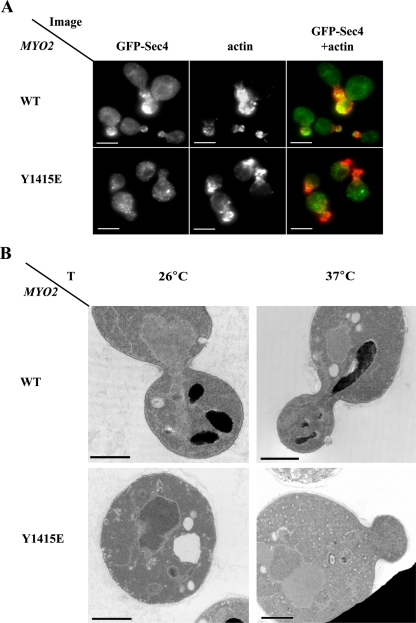
Because Myo2 is required for secretory vesicle motility, we predicted that the mislocalization of Myo2 in cells expressing a Ypt31/32–Myo2 interaction mutation would result in the accumulation of unpolarized secretory vesicles. We examined this possibility by using two independent approaches. First, green fluorescent protein (GFP)-tagged Sec4 was used as a marker of secretory vesicles. In wild-type cells, this protein localizes to small buds (Calero et al., 2003). In cells expressing the myo2-Y1415E mutation, which disrupts the Ypt31/32–Myo2 interactions, the GFP-Sec4 fluorescence is not polarized to small buds even though actin is still polarized (Figure 8A). Second, using electron microscopy, we showed the accumulation of unpolarized ∼100-nm secretory vesicles in myo2-Y1415E mutant cells (Figure 8B). Together, these results show that interaction between Ypt31/32 and Myo2-GTD is required for the essential role of the Myo2 motor in the polarization of secretory vesicles to sites of cell growth.
Figure 8.
Interaction between Ypt31/32 and Myo2-GTD is required for polarized secretion. (A) GFP-Sec4p is mislocalized in myo2-Y1415E mutant cells. Wild-type and myo2-Y1415E mutant cells containing a plasmid for GFP-Sec4p expression were grown at 24°C, shifted to 37°C for 2 h, fixed, stained with Alexa Fluor 594 phalloidin and visualized by fluorescence microscopy. GFP-Sec4 is shown on the left, actin in the middle, and merged images on the right. In wild-type cells (top), GFP-Sec4 is polarized to areas of cell growth: small buds and necks. In myo2-Y1415E mutant cells (bottom), the polarization of GFP-Sec4 to small buds is disrupted while actin is still polarized. Bar, 5 μm. (B) Unpolarized secretory vesicles accumulate in myo2-Y1415E mutant cells. Wild-type (NSY1168) and myo2-Y1415E mutant (NSY1169) cells were grown at 26°C (left), shifted to 37°C for 2 h (right), and processed for electron microscopy. Wild-type cells (top) grown at either temperature do not accumulate secretory vesicles. Mutant cells (bottom) accumulate some, ∼100 nm, secretory vesicles even at 26°C. At 37°C, >75% of mutant cells are filled with unpolarized secretory vesicles. Bar, 1 μm.
DISCUSSION
Here, we identify the myosin V-type motor Myo2 as an effector of the Ypt31/32 GTPases. Ypt31/32 in their GTP-bound form interact directly with the GTD of Myo2. The Ypt31/32 interaction site maps to a patch on the Myo2-GTD that was shown to be required for moving secretory vesicles, the essential cargo of Myo2 (Pashkova et al., 2006). The fact that Myo2 and Ypt31/32 colocalize and the existence of several allele-specific genetic interactions indicate that the physical interaction of Ypt31/32 with Myo2 is physiologically relevant. Indeed, fusion of Ypt32 to the C terminus of a Myo2 mutant protein defective in its interaction with Ypt31/32 rescues the lethality of this mutation. Moreover, the ypt31Δ/32ts double mutation as well as MYO2 mutations that affect the Myo2–Ypt31/32 interaction exhibit a defect in the polarized localization of Myo2. Finally, a MYO2 mutation that disrupts its interaction with Ypt31/32 results in the accumulation of unpolarized secretory vesicles. Together, these results indicate that Myo2 is a downstream effector of the Ypt31/32 GTPases and that the Ypt31/32 GTPases are required for the Myo2-dependent polarization of secretory vesicles.
The interaction of Myo2 with Ypt31/32 is likely to occur on the Golgi and Golgi-derived vesicles. Myo2 is required for the motility of both Golgi and trans-Golgi–derived vesicles toward the growing bud (Govindan et al., 1995; Schott et al., 1999; Karpova et al., 2000; Rossanese et al., 2001), and Ypt31/32 localize to both compartments (Figure 4B). Sec4 GTPase, which also resides on secretory vesicles, has been implicated in Myo2 recruitment to these organelles (Wagner et al., 2002). Even though to date there is no evidence of direct interaction between these two proteins (Beningo et al., 2000), or of a role for such interaction, the interaction of Myo2 with Ypt31/32 presented here does not preclude it.
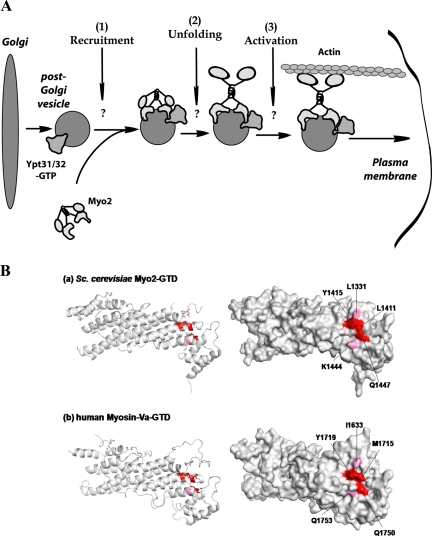
What is the role of Ypt31/32 interaction with Myo2? We have determined previously that Ypt31/32 GTPases are required for exit from the Golgi based on the accumulation of aberrant Golgi structures in ypt31Δ32ts mutant cells (Jedd et al., 1997). Interestingly, these mutant cells also contain more secretory vesicles than wild-type cells (Jedd et al., 1997; Figures 7–9). This last observation suggests that Ypt31/32 GTPases play an additional role in the life cycle of these vesicles. Here, we show that Ypt31/32 are required for the normal intracellular localization of the Myo2 motor and the Myo2-dependent motility of secretory vesicles essential for cell viability. We propose the recruitment or activation of the Myo2 motor, which is in turn required for the motility of secretory vesicles, as an additional role for Ypt31/32. Our data strongly suggest that the Ypt31/32 GTPases couple secretory vesicle formation and motility (Figure 9A). Proper inheritance of late-Golgi cisternae is dependent on Myo2 activity (Rossanese et al., 2001). Therefore, an additional role for the Ypt31/32-Myo2 interaction might be the motility of late Golgi, which is in turn required for the inheritance of this compartment.
Figure 9.
A model for a conserved role for direct Ypt/Rab interaction with myosin V in coupling trans-Golgi vesicle formation and motility. (A) In yeast, the Ypt31/32 GTPases are required for the formation of trans-Golgi derived vesicles. Results presented here indicate that these GTPases play a subsequent role in ensuring the motility of these vesicles to the plasma membrane on the actin cytoskeleton. We show that Ypt31/32 interact directly with the cargo binding domain of the myosin V-type motor, Myo2, and we propose that this interaction is required for 1) recruiting Myo2 to these vesicles, and/or 2) the unfolding and 3) activation of Myo2. (B) Conservation of the Ypt/Rab interaction site on the myosin V-GTD. Top, the cluster of surface residues on subdomain II of the yeast Myo2-GTD, which define the secretory vesicle and the Ypt/Rab binding site (this study), are shown in red and pink on the 2.1-Å structure of this domain. Bottom, the predicted tertiary structure of human myosin Va (residues 1463-to-1853, C-terminal end) as calculated by Geno3D (http://geno3d-pbil.ibcp.fr/cgi-bin/geno3d_automat.pl?page=/GENO3D/geno3d_home.html). Geno3D selected the yeast Myo2-GTD as the closest solved structure. Shown are: ribbon (left) and space-filling (right) representations. Importantly, the cluster of the three amino acids (red) required for the Myo2–Ypt31/32 interaction is conserved from yeast (Y1415, L1411, and Q1447) to human (Y1719, M1715, and Q1750).
There are at least two possible mechanistic roles that Ypt31/32 play in the regulation of Myo2 function. Many effectors are regulated by Ypt/Rab GTPases via regulation of their recruitment to membranes (Pfeffer, 2001). Alternatively, Ypt/Rab GTPases may regulate effector activity (Segev, 2001a,b). In the absence of cargo, mammalian myosin V assumes a folded conformation, and the GTD binds to and inactivates the motor domain (Thirumurugan et al., 2006; Taylor, 2007). It is not yet clear whether Myo2 is regulated in a similar manner. Ypt31/32 may regulate either the recruitment of Myo2 to membranes of exocytic compartments or the unfolding and activation of Myo2 on these compartments (Figure 9A). It is also possible that Ypt31/32 regulate both the recruitment and the activation of Myo2. A possible scenario for the latter idea is as follows: Activation of myosin V is dependent on the attachment of myosin light chains, Mlc1 or calmodulin, to the six IQ motifs present in the arm domain of the myosin heavy chain (Krementsov et al., 2004). In yeast, the nucleotide exchanger for Sec4, Sec2, was shown to be required for the interaction of Mlc1 and Myo2 (Bielli et al., 2006). Because Ypt32 was suggested to recruit Sec2 to secretory vesicles (Ortiz et al., 2002), a possible model consistent with our data are that Ypt31/32 recruit both Myo2 and Sec2 to secretory vesicles, whereas Sec2 in turn recruits Mlc1 to activate Myo2.
In mammals, myosin Va is required for the motility of secretory granules carrying neuropeptides and hormones toward the PM and for moving melanosomes to dendritic tips of melanocytes. Thus, mutations in myosin Va in humans lead to Griscelli syndrome, which is manifested in hypopigmentation and neurological disorders (Eichler et al., 2006). Ypt/Rab GTPases and regions of myosin V motors are highly conserved. Notably, although the cargo-binding domain is the least conserved region of myosin V motors, the amino acid sequence surrounding the Ypt/Rab binding region is conserved (Pashkova et al., 2006). Importantly, a predicted three-dimensional structure of the human myosin Va-GTD by Geno3D reveals that the cluster of three residues required for the interaction of Myo2-GTD with Ypt31/32 is conserved from yeast to humans (Figure 9B). Based on the conservation of this interaction site, we propose direct contact of Rab GTPases with their myosin V effectors as a common mechanism for regulated transport of multiple cargoes.
Supplementary Material
ACKNOWLEDGMENTS
We thank R. Collins (Cornell University) for pAS1-Ypt and GFP-Sec4 plasmids, V. Lupashin (University of Arkansas) for His6-Ypt1 plasmid, H. Riezman (University of Geneva) for anti EMP47 antibody, C. Kaiser (Massachusetts Institute of Technology) for the sec15-1 strain, D. Suchkov for helpful discussions, and E. Segev for help in editing the manuscript. This research was supported by National Institutes of Health grants GM-45444 (to N. S.) and GM-62261 (to L. W.).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-02-0220) on July 23, 2008.
REFERENCES
- Beningo K. A., Lillie S. H., Brown S. S. The yeast kinesin-related protein Smy1p exerts its effects on the class V myosin Myo2p via a physical interaction. Mol. Biol. Cell. 2000;11:691–702. doi: 10.1091/mbc.11.2.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielli P., Casavola E. C., Biroccio A., Urbani A., Ragnini-Wilson A. GTP drives myosin light chain 1 interaction with the class V myosin Myo2 IQ motifs via a Sec2 RabGEF-mediated pathway. Mol. Microbiol. 2006;59:1576–1590. doi: 10.1111/j.1365-2958.2006.05041.x. [DOI] [PubMed] [Google Scholar]
- Bretscher A. Polarized growth and organelle segregation in yeast: the tracks, motors, and receptors. J. Cell Biol. 2003;160:811–816. doi: 10.1083/jcb.200301035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss F., Kendrick-Jones J. How are the cellular functions of myosin VI regulated within the cell? Biochem. Biophys. Res. Commun. 2007;369:165–175. doi: 10.1016/j.bbrc.2007.11.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calero M., Chen C. Z., Zhu W., Winand N., Havas K. A., Gilbert P. M., Burd C. G., Collins R. N. Dual prenylation is required for Rab protein localization and function. Mol. Biol. Cell. 2003;14:1852–1867. doi: 10.1091/mbc.E02-11-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casavola E. C., Catucci A., Bielli P., Di Pentima A., Porcu G., Pennestri M., Cicero D. O., Ragnini-Wilson A. Ypt32p and Mlc1p bind within the vesicle binding region of the class V myosin Myo2p globular tail domain. Mol. Microbiol. 2008;67:1051–1066. doi: 10.1111/j.1365-2958.2008.06106.x. [DOI] [PubMed] [Google Scholar]
- Catlett N. L., Duex J. E., Tang F., Weisman L. S. Two distinct regions in a yeast myosin-V tail domain are required for the movement of different cargoes. J. Cell Biol. 2000;150:513–526. doi: 10.1083/jcb.150.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlett N. L., Weisman L. S. The terminal tail region of a yeast myosin-V mediates its attachment to vacuole membranes and sites of polarized growth. Proc. Natl. Acad. Sci. USA. 1998;95:14799–14804. doi: 10.1073/pnas.95.25.14799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. H., Chen S., Tokarev A. A., Liu F., Jedd G., Segev N. Ypt31/32 GTPases and their novel F-box effector protein Rcy1 regulate protein recycling. Mol. Biol. Cell. 2005;16:178–192. doi: 10.1091/mbc.E04-03-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnos C., Huet S., Darchen F. ‘Should I stay or should I go?’: myosin V function in organelle trafficking. Biol. Cell. 2007;99:411–423. doi: 10.1042/BC20070021. [DOI] [PubMed] [Google Scholar]
- Eichler T. W., Kogel T., Bukoreshtliev N. V., Gerdes H. H. The role of myosin Va in secretory granule trafficking and exocytosis. Biochem. Soc. Trans. 2006;34:671–674. doi: 10.1042/BST0340671. [DOI] [PubMed] [Google Scholar]
- Fagarasanu A., Fagarasanu M., Eitzen G. A., Aitchison J. D., Rachubinski R. A. The peroxisomal membrane protein Inp2p is the peroxisome-specific receptor for the myosin V motor Myo2p of Saccharomyces cerevisiae. Dev. Cell. 2006;10:587–600. doi: 10.1016/j.devcel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Fagarasanu A., Rachubinski R. A. Orchestrating organelle inheritance in Saccharomyces cerevisiae. Curr. Opin. Microbiol. 2007;10:528–538. doi: 10.1016/j.mib.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher K. L., Boldogh I. R., Pon L. A. Taking the A-train: actin-based force generators and organelle targeting. Trends Cell Biol. 2003;13:472–477. doi: 10.1016/s0962-8924(03)00174-0. [DOI] [PubMed] [Google Scholar]
- Goud B., Salminen A., Walworth N. C., Novick P. J. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988;53:753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Govindan B., Bowser R., Novick P. The role of Myo2, a yeast class V myosin, in vesicular transport. J. Cell Biol. 1995;128:1055–1068. doi: 10.1083/jcb.128.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer J. A., 3rd, Wu X. S. Rabs grab motors: defining the connections between Rab GTPases and motor proteins. Curr. Opin. Cell Biol. 2002;14:69–75. doi: 10.1016/s0955-0674(01)00296-4. [DOI] [PubMed] [Google Scholar]
- Ishikawa K., Catlett N. L., Novak J. L., Tang F., Nau J. J., Weisman L. S. Identification of an organelle-specific myosin V receptor. J. Cell Biol. 2003;160:887–897. doi: 10.1083/jcb.200210139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd G., Mulholland J., Segev N. Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J. Cell Biol. 1997;137:563–580. doi: 10.1083/jcb.137.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S., Litt R. J., Richardson C. J., Segev N. Requirement of nucleotide exchange factor for Ypt1 GTPase mediated protein transport. J. Cell Biol. 1995;130:1051–1061. doi: 10.1083/jcb.130.5.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova T. S., Reck-Peterson S. L., Elkind N. B., Mooseker M. S., Novick P. J., Cooper J. A. Role of actin and Myo2p in polarized secretion and growth of Saccharomyces cerevisiae. Mol. Biol. Cell. 2000;11:1727–1737. doi: 10.1091/mbc.11.5.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi-Far R., Lutz R. J., Cox A. D., Conroy L., Bourne J. R., Sinensky M., Balch W. E., Buss J. E., Der C. J. Isoprenoid modification of rab proteins terminating in CC or CXC motifs. Proc. Natl. Acad. Sci. USA. 1991;88:6264–6268. doi: 10.1073/pnas.88.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krementsov D. N., Krementsova E. B., Trybus K. M. Myosin V: regulation by calcium, calmodulin, and the tail domain. J. Cell Biol. 2004;164:877–886. doi: 10.1083/jcb.200310065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford G. M. Myosin-V, a versatile motor for short-range vesicle transport. Traffic. 2002;3:859–865. doi: 10.1034/j.1600-0854.2002.31202.x. [DOI] [PubMed] [Google Scholar]
- Li X. D., Ikebe R., Ikebe M. Activation of myosin Va function by melanophilin, a specific docking partner of myosin Va. J. Biol. Chem. 2005;280:17815–17822. doi: 10.1074/jbc.M413295200. [DOI] [PubMed] [Google Scholar]
- Liang Y., Morozova N., Tokarev A. A., Mulholland J. W., Segev N. The role of Trs65 in the Ypt/Rab guanine nucleotide exchange factor function of the TRAPP II complex. Mol. Biol. Cell. 2007;18:2533–2541. doi: 10.1091/mbc.E07-03-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland J., Preuss D., Moon A., Wong A., Drubin D., Botstein D. Ultrastructure of the yeast actin cytoskeleton and its association with the plasma membrane. J. Cell Biol. 1994;125:381–391. doi: 10.1083/jcb.125.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedvetsky P. I., et al. A role of myosin Vb and Rab11-FIP2 in the aquaporin-2 shuttle. Traffic. 2007;8:110–123. doi: 10.1111/j.1600-0854.2006.00508.x. [DOI] [PubMed] [Google Scholar]
- Olkkonen V. M., Stenmark H. Role of Rab GTPases in membrane traffic. Int. Rev. Cytol. 1997;176:1–85. doi: 10.1016/s0074-7696(08)61608-3. [DOI] [PubMed] [Google Scholar]
- Ortiz D., Medkova M., Walch-Solimena C., Novick P. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J. Cell Biol. 2002;157:1005–1015. doi: 10.1083/jcb.200201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashkova N., Jin Y., Ramaswamy S., Weisman L. S. Structural basis for myosin V discrimination between distinct cargoes. EMBO J. 2006;25:693–700. doi: 10.1038/sj.emboj.7600965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 2001;11:487–491. doi: 10.1016/s0962-8924(01)02147-x. [DOI] [PubMed] [Google Scholar]
- Preuss D., Mulholland J., Franzusoff A., Segev N., Botstein D. Characterization of the Saccharomyces Golgi complex through the cell cycle by immunoelectron microscopy. Mol. Biol. Cell. 1992;3:789–803. doi: 10.1091/mbc.3.7.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D., Legesse-Miller A., Gao L., Dong Y., Bretscher A. Mechanisms of polarized growth and organelle segregation in yeast. Annu. Rev. Cell Dev. Biol. 2004;20:559–591. doi: 10.1146/annurev.cellbio.20.010403.103108. [DOI] [PubMed] [Google Scholar]
- Rossanese O. W., Reinke C. A., Bevis B. J., Hammond A. T., Sears I. B., O'Connor J., Glick B. S. A role for actin, Cdc1p, and Myo2p in the inheritance of late Golgi elements in Saccharomyces cerevisiae. J. Cell Biol. 2001;153:47–62. doi: 10.1083/jcb.153.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Sahlender D. A., Roberts R. C., Arden S. D., Spudich G., Taylor M. J., Luzio J. P., Kendrick-Jones J., Buss F. Optineurin links myosin VI to the Golgi complex and is involved in Golgi organization and exocytosis. J. Cell Biol. 2005;169:285–295. doi: 10.1083/jcb.200501162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott D., Ho J., Pruyne D., Bretscher A. The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J. Cell Biol. 1999;147:791–808. doi: 10.1083/jcb.147.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott D. H., Collins R. N., Bretscher A. Secretory vesicle transport velocity in living cells depends on the myosin-V lever arm length. J Cell Biol. 2002;156:35–39. doi: 10.1083/jcb.200110086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder S., Schimmoller F., Singer-Kruger B., Riezman H. The Golgi-localization of yeast Emp47p depends on its di-lysine motif but is not affected by the ret1–1 mutation in alpha-COP. J. Cell Biol. 1995;131:895–912. doi: 10.1083/jcb.131.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciorra V. A., Audhya A., Parsons A. B., Segev N., Boone C., Emr S. D. Synthetic genetic array analysis of the PtdIns 4-kinase Pik1p identifies components in a Golgi-specific Ypt31/rab-GTPase signaling pathway. Mol. Biol. Cell. 2005;16:776–793. doi: 10.1091/mbc.E04-08-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabra M. C., Coudrier E. Rab GTPases and myosin motors in organelle motility. Traffic. 2004;5:393–399. doi: 10.1111/j.1398-9219.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- Segev N. Cell biology. A TIP about Rabs. Science. 2001a;292:1313–1314. doi: 10.1126/science.1061114. [DOI] [PubMed] [Google Scholar]
- Segev N. Ypt/rab GTPases: regulators of protein trafficking. Sci STKE. 2001b;2001:RE11. doi: 10.1126/stke.2001.100.re11. [DOI] [PubMed] [Google Scholar]
- Sweeney H. L., Houdusse A. What can myosin VI do in cells? Curr. Opin. Cell Biol. 2007;19:57–66. doi: 10.1016/j.ceb.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Tang F., Kauffman E. J., Novak J. L., Nau J. J., Catlett N. L., Weisman L. S. Regulated degradation of a class V myosin receptor directs movement of the yeast vacuole. Nature. 2003;422:87–92. doi: 10.1038/nature01453. [DOI] [PubMed] [Google Scholar]
- Taylor K. A. Regulation and recycling of myosin V. Curr. Opin. Cell Biol. 2007;19:67–74. doi: 10.1016/j.ceb.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Thirumurugan K., Sakamoto T., Hammer J. A., 3rd, Sellers J. R., Knight P. J. The cargo-binding domain regulates structure and activity of myosin 5. Nature. 2006;442:212–215. doi: 10.1038/nature04865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trybus K. M. Myosin V from head to tail. Cell Mol Life Sci. 2008;65:1378–1389. doi: 10.1007/s00018-008-7507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W., Bielli P., Wacha S., Ragnini-Wilson A. Mlc1p promotes septum closure during cytokinesis via the IQ motifs of the vesicle motor Myo2p. EMBO J. 2002;21:6397–6408. doi: 10.1093/emboj/cdf650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C., Collins R. N., Novick P. J. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J. Cell Biol. 1997;137:1495–1509. doi: 10.1083/jcb.137.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman L. S. Organelles on the move: insights from yeast vacuole inheritance. Nat. Rev. 2006;7:243–252. doi: 10.1038/nrm1892. [DOI] [PubMed] [Google Scholar]
- Wu X., Xiang X., Hammer J. A., 3rd Motor proteins at the microtubule plus-end. Trends Cell Biol. 2006;16:135–143. doi: 10.1016/j.tcb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Yin H., Pruyne D., Huffaker T. C., Bretscher A. Myosin V orientates the mitotic spindle in yeast. Nature. 2000;406:1013–1015. doi: 10.1038/35023024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.