Abstract
Alterations in the composition of intestinal commensal bacteria are associated with enhanced susceptibility to multiple inflammatory diseases, including those conditions associated with interleukin (IL)-17–producing CD4+ T helper (Th17) cells. However, the relationship between commensal bacteria and the expression of proinflammatory cytokines remains unclear. Using germ-free mice, we show that the frequency of Th17 cells in the large intestine is significantly elevated in the absence of commensal bacteria. Commensal-dependent expression of the IL-17 family member IL-25 (IL-17E) by intestinal epithelial cells limits the expansion of Th17 cells in the intestine by inhibiting expression of macrophage-derived IL-23. We propose that acquisition of, or alterations in, commensal bacteria influences intestinal immune homeostasis via direct regulation of the IL-25–IL-23–IL-17 axis.
The intestinal tract is a major site of colonization by commensal bacteria (1, 2). This bacterial population is acquired shortly after birth, is estimated to number 1014 organisms, and is remarkably diverse, being composed of at least 500–1,000 individual species (3). The host–commensal relationship is the product of millions of years of coevolution, and it can be influenced by the number and composition of bacterial species within the intestine. For example, studies using germ-free (GF) animals demonstrated that commensal bacteria are required for normal development of the immune system (2). Commensal bacteria have also been implicated in the pathogenesis of inflammatory bowel disease (IBD), as colitis-prone mice fail to develop intestinal inflammation if reared under GF conditions and patients suffering from IBD exhibit dysregulated immune responses against commensal bacteria (4, 5).
IL-17–producing CD4+ T (Th17) cells play a significant role in the pathogenesis of IBD (6–8). The differentiation of Th17 cells is promoted by IL-6– and TGF-β–dependent expression of the transcription factor retinoic acid–related orphan nuclear receptor-γt (RORγt [Rorc]), whereas IL-23 controls their expansion or survival (7, 9). Th17 cells are found constitutively in the small intestine of naive mice housed under conventional conditions (9), suggesting the presence of commensal bacteria may promote their development or maintenance in the intestine (9–11). An alternative model proposes that rather than promoting the presence of effector CD4+ T cell populations and cytokines in the intestine, commensal bacteria are recognized by Toll-like receptors, and MyD88-dependent NF-κB activation results in the maintenance of epithelial integrity that is associated with decreased proinflammatory cytokine and chemokine gene expression, leading to a state of immune hyporesponsiveness in the intestine (12–14). Consistent with this hypothesis, mice depleted of commensal bacteria by antibiotic treatment are highly susceptible to chemically induced colitis (12), and disruption of the NF-κB pathway in IECs results in exaggerated expression of proinflammatory cytokines and the development of spontaneous (15) and infection-induced intestinal inflammation before disruption of the epithelial barrier (16).
In this study, we show that intestinal commensal bacteria regulate expression of the IL-17 family of cytokines in the intestine. The frequency of Th17 cells was significantly increased in the large intestine of GF mice compared with conventionally reared (CNV) mice and was associated with heightened levels of IL-23. Expression of the IL-17 family member IL-25 (IL-17E) by intestinal epithelial cells (IECs) was dependent on the presence of commensal bacteria and administration of IL-25 to GF mice reduced expression of IL-23, and the frequency of Th17 cells in the large intestine. Consistent with a role for IL-25 in limiting IL-23 and Th17 cells in the large intestine, neutralization of IL-23 also resulted in decreased frequencies of Th17 cells in the large intestine of GF mice. Further, IL-25 directly inhibited LPS-induced IL-23 expression by macrophages in a STAT6-independent manner. Thus, these results demonstrate a functional link between commensal bacteria and the IL-25–IL-23–IL-17 axis and identify a novel commensal-dependent mechanism in the regulation of Th17 cells in the intestinal microenvironment.
RESULTS AND DISCUSSION
The role of commensal bacteria in intestinal immune homeostasis remains unclear. One study identified the constitutive presence of Th17 cells primarily in the small intestinal lamina propria (LP) and hypothesized that commensal bacteria may be required for their presence in the intestine (9), whereas another reported that Th17 cells are found only in the large intestinal LP of CNV mice (17). We sought to test whether signals derived from commensal bacteria promote or inhibit the presence of Th17 cells in the intestine. We examined the frequency of Th17 cells in tissues isolated from CNV BALB/c or GF BALB/c mice by flow cytometry. In CNV mice, Th17 cells were present primarily in the LP of the small intestine (Fig. 1 A). Comparison of CNV and GF mice revealed equivalent frequencies of Th17 cells in the Peyer's patches and small intestinal LP of CNV and GF mice (Fig. 1 A). In contrast, the frequency of Th17 cells in the cecal patch (a lymphoid follicle associated with the cecum that is analogous to the human appendix) and large intestinal LP was three- to fourfold higher in GF mice compared with CNV animals (Fig. 1 A). Analysis of messenger RNA (mRNA) expression in whole sections of large intestinal tissue isolated from CNV or GF mice demonstrated elevated expression levels of mRNA for Il17 and Rorc (Fig. 1 B). Elevated levels of Il17 mRNA were independent of genetic background, as GF C57BL/6 mice also expressed significantly higher levels of Il17 than CNV C57BL/6 mice (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20080720/DC1). These results implicate signals derived from commensal bacteria in limiting the frequency of intestinal Th17 cells.
Figure 1.
Enteric commensal bacteria are required to limit the frequency of Th17 cells in the intestine. (A) Expression of IL-17 by CD4+ T cells in the Peyer's patches, cecal patch, and LP of the small or large intestine of CNV and GF mice was analyzed by flow cytometry. Flow cytometry plots depict log10 fluorescence. (B–D) Expression of mRNA for Il17 and Rorc (B), Il6 and Tgfb (C), or Il23a and Il12b (D) in the large intestine of CNV and GF mice was analyzed by quantitative real-time PCR. (E and F) Expression of mRNA for Il23a (E) and Il17 (F) in the large intestine of CNV and antibiotic-treated (Tx) CNV mice was analyzed by quantitative real-time PCR. Results are from 3 experiments (n = 6–9). Rel. units, relative units. *, P < 0.05. Error bars indicate the SEM.
The differentiation of Th17 cells is promoted by IL-6 and TGF-β, whereas IL-23 is required for the subsequent expansion or survival of committed Th17 cells (7). To test whether the absence of commensal bacteria influenced the development or survival/expansion of Th17 cells, we examined expression of IL-6, TGF-β, and IL-23 in the intestine of CNV and GF mice. Although the levels of Il6 and Tgfb mRNA were equivalent in the large intestine of mice housed under both conditions (Fig. 1 C), the levels of IL-23p19 (Il23a) and IL-12/23p40 (Il12b) mRNA were significantly higher in the large intestine of GF mice compared with CNV mice (Fig. 1 D). Secretion of IL-6 and IL-12/23p40 protein in large intestinal explant cultures confirmed mRNA expression patterns, with equivalent production of IL-6 (Fig. S2 A, available at http://www.jem.org/cgi/content/full/jem.20080720/DC1) by intestinal tissue of CNV and GF mice and increased production of IL-12/23p40 by GF intestinal tissue (Fig. S2 B). Expression of Il23a was also significantly increased in the large intestine of GF C57BL/6 mice (Fig. S3). These findings suggest that the commensal bacteria do not influence expression of cytokines that govern Th17 cell differentiation from naive precursors, but rather signals derived from commensal bacteria inhibit expression of IL-23 and the subsequent expansion and/or survival of Th17 cells in the large intestine.
An alternative approach was adopted to determine whether there was a link between commensal bacteria and expression of IL-23 and -17 in CNV mice. Oral administration of antibiotics has been shown to effectively reduce the numbers of bacteria in the intestine and leads to increased susceptibility to inflammation and food allergy (12, 18). To test whether heightened susceptibility to inflammation after antibiotic treatment was associated with altered expression of the IL-23–IL-17 axis, mice were treated orally with antibiotics for 6 wk, and segments of small and large intestine were analyzed for expression of Il23a and Il17. Antibiotic treatment of adult mice resulted in increased levels of Il23a and Il17 in the large intestine (Fig. 1, E and F). Critically, this effect was confined to the large intestine, as there were no significant changes in expression of Il23a, Il12b, or Il17 in the small intestine (Fig. S4, A–C, available at http://www.jem.org/cgi/content/full/jem.20080720/DC1). Thus, reduction in total numbers of commensal bacteria in adult mice results in heightened expression of IL-23 and -17 in the large intestine, suggesting that commensal bacteria are actively promoting inhibition of IL-23 and -17.
Recent studies identified a reciprocal developmental pathway between Th17 cells and Foxp3-expressing regulatory T cells (19). Therefore, we examined whether the elevated frequency of Th17 cells in GF mice was associated with dysregulation of regulatory T cells. No significant differences in the frequency of CD4+ Foxp3+ regulatory T cells (Fig. 2 A) or IL-10–producing CD4+ T cells in the mesenteric LN (mLN), cecal patch, or large intestinal LP were observed between mice housed under CNV or GF conditions (Fig. S5, A–C, available at http://www.jem.org/cgi/content/full/jem.20080720/DC1). Additionally, there were no differences in the expression levels of Il10 mRNA in the large intestine between CNV and GF mice (Fig. S5 D). Consistent with normal frequencies of CD4+ Foxp3+ T cells in GF mice, there was no evidence of global dysregulation in expression of proinflammatory cytokines. Splenocytes from both CNV and GF mice produced equivalent levels of IFN-γ under neutral conditions (Fig. 2, B and C) or in the presence of exogenous IL-12 (Fig. 2, D and E). Further, there were no significant differences in the expression of IL-12p35 (Il12a) mRNA (Fig. S6 A) or the frequencies of CD4+ T cells producing IFN-γ in the mLN, cecal patch, and large intestinal LP of CNV and GF mice (Fig. S6 B). Collectively, these results suggest that the absence of commensal bacteria is associated with the selective overexpression of IL-23 and enhanced persistence, survival, or recruitment of Th17 cells in the intestinal microenvironment, rather than a general loss of regulatory T cell– and IL-10–dependent immune regulation.
Figure 2.
Equivalent regulatory T cell and Th1 cell responses in CNV and GF mice. (A) Cells isolated from the spleen, mLN, cecal patch, or large intestinal LP were stained for expression of CD4 and Foxp3 and analyzed by flow cytometry. (B–E) Splenocytes from CNV or GF mice were stimulated with anti-CD3 and -CD28 in the absence (B and C) or presence (D and E) of 1 ng/ml IL-12 for 72 h, followed by incubation with PMA, ionomycin, and brefeldin A for the final 5 h. Harvested cells were stained for expression of CD4 and IFN-γ by flow cytometry (B and D), and cell-free supernatants were analyzed by ELISA (C and E). Flow cytometry plots depict log10 fluorescence. Data are representative of 2 experiments (n = 8). Error bars indicate the SEM.
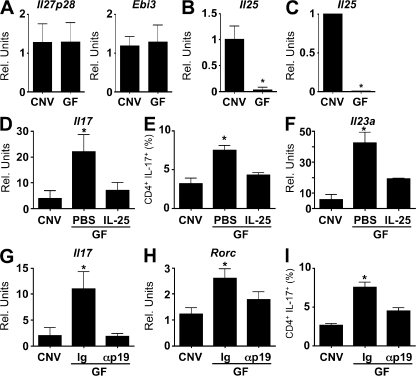
The absence of commensal bacteria resulted in elevated expression of IL-23 and an increased frequency of Th17 cells in the large intestine. Based on these findings, we sought to test the hypothesis that commensal bacteria promote expression of cytokines that could negatively regulate the frequency of Th17 cells in the intestine. IL-27 and -25 have both been shown to limit Th17 cell responses (20–23). IL-27 acts directly on CD4+ T cells to inhibit their differentiation into the Th17 cell lineage (24), whereas IL-25, which can be produced by a variety of cell types, including epithelial cells, mast cells, macrophages, and T cells, limits Th17 cell development by promoting expression of IL-13 that acts on DCs (22, 25–28). We observed no significant difference in mRNA expression levels of the IL-27 subunits p28 (Il27p28) and Epstein-Barr virus–induced (EBI)-3 (Ebi3) in the large intestine between CNV and GF mice (Fig. 3 A). In contrast, there was a significant reduction in the levels of Il25 mRNA in large intestinal tissue isolated from GF mice compared with CNV mice (Fig. 3 B). Consistent with a role for commensal bacteria in the expression of Il25, antibiotic treatment of CNV mice resulted in decreased expression of Il25 in the large intestine (Fig. S7, available at http://www.jem.org/cgi/content/full/jem.20080720/DC1). Expression of Il25 was detected in purified IECs from CNV but not GF mice (Fig. 3 C), suggesting that signals from commensal bacteria may be acting directly on IECs to induce expression of IL-25. Although we did not detect Il25 expression in the spleen, LNs, Peyer's patches, or LP in CNV or GF mice (unpublished data), we cannot rule out the possibility that other resident or infiltrating LP cell populations could also express Il25 in the intestine. Thus, absence of commensal bacteria was associated with decreased expression of IL-25 in IECs, heightened IL-23, and increased frequencies of Th17 cells in the large intestine.
Figure 3.
Commensal-dependent expression of IL-25 inhibits IL-23 and the frequency of Th17 cells in the large intestine. (A) Expression of mRNA for Il27p28 and Ebi3 in the large intestine of CNV and GF mice was analyzed by quantitative real-time PCR. (B and C) Expression of mRNA for Il25 in the large intestine (B) and purified IECs (C) of CNV and GF mice was analyzed by quantitative real-time PCR. Data are from 3 experiments (n = 12). (D–F) Expression of mRNA for Il17 (D), the frequency of IL-17+ CD4+ cells (E), and expression of mRNA for Il23a (F) in the large intestine of CNV mice, GF mice, or GF mice treated with IL-25 (0.5 μg daily for 7 d) was analyzed by quantitative real-time PCR. Data are from 2 experiments (n = 6). (G–I) Expression of mRNA for Il17 (G) and Rorc (H) and the frequency of IL-17+ CD4+ cells (I) in the large intestine of CNV mice, GF mice, or GF mice treated with a neutralizing monoclonal antibody against IL-23p19 (αp19; 1 mg daily for 7 d) was analyzed by quantitative real-time PCR. Data are from 2 experiments (n = 4–6). Rel. units, relative units. *, P < 0.05. Error bars indicate the SEM.
Based on these findings, we next sought to examine whether there was a functional interaction between reduced expression of IL-25 and the exaggerated production of IL-23 and -17 in the intestine. To test this, exogenous IL-25 was administered to GF mice. IL-25 treatment resulted in decreased expression of Il17 (Fig. 3 D) and lower frequencies of Th17 cells in the LP of the large intestine (Fig. 3 E). IL-25 treatment also resulted in significantly decreased expression of Il23a mRNA (Fig. 3 F). The association between administration of IL-25 and reduced levels of IL-23 and -17 in GF mice suggested that the effects of IL-25 were mediated through the inhibition of IL-23. To directly address whether dysregulated expression of IL-23 in GF mice resulted in the increased frequency of Th17 cells observed, GF mice were treated with a neutralizing monoclonal antibody against IL-23 (αp19). Consistent with results obtained from administration of IL-25, neutralization of IL-23p19 in GF mice resulted in significantly decreased expression of Il17 and Rorc in the large intestine (Fig. 3, G and H), as well as a significant decrease in the frequency of Th17 cells in the large intestinal LP (Fig. 3 I). These results suggest that commensal-dependent expression of IL-25 is a component of the pathways that regulate expression of IL-23 and the frequency of Th17 cells in the intestinal microenvironment.
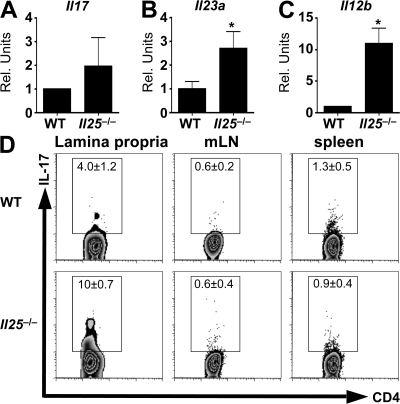
As GF mice and antibiotic-treated CNV mice expressed lower levels of IL-25, we hypothesized that the absence of IL-25 in CNV mice would recapitulate the heightened expression of IL-23 and increased frequency of Th17 cells observed in GF mice. To test this, expression of IL-17 and -23 in the large intestine of naive WT and IL-25–deficient (Il25−/−) mice housed under conventional conditions was examined. Expression of Il23a, Il12b, and Il17 was increased in naive Il25−/− mice compared with WT controls (Fig. 4, A–C), similar to results obtained in GF mice. Moreover, flow cytometric analysis of lymphocytes isolated from the large intestinal LP demonstrated an increased frequency of Th17 cells in Il25−/− mice under steady-state conditions (Fig. 4 D). The consequences of IL-25 deficiency on IL-17 expression were apparent primarily in the intestinal tissue, as the frequencies of Th17 cells in the mLN or spleen were similar in WT and Il25−/− mice (Fig. 4 D). Thus, the absence of endogenous IL-25 in CNV mice is associated with increased levels of IL-23 and heightened frequencies of Th17 cells in the large intestine of naive Il25−/− mice.
Figure 4.
Endogenous IL-25 is required to limit IL-23 and -17 expression in the large intestine. (A–C) Expression of mRNA for Il17 (A), Il23a (B), and Il12b (C) in the large intestine of CNV and GF mice was analyzed by quantitative real-time PCR. (D) Expression of IL-17 by CD4+ T cells in the large intestinal LP was analyzed by flow cytometry. Flow cytometry plots depict log10 fluorescence. Data are from 3 experiments (n = 6–9). Rel. units, relative units. *, P < 0.05. Error bars indicate the SEM.
The increased expression of IL-23 in GF mice and the ability of IL-25 to inhibit IL-23 and -17 expression in vivo led to the hypothesis that IL-25 could act on accessory cells to limit expression of IL-23 and subsequent expansion or survival of Th17 cells. To test this, CD11b+ cells were isolated from the large intestinal LP of CNV and GF mice and analyzed for ex vivo expression of Il23a and Il12b mRNA. CD11b+ cells isolated from GF mice expressed higher levels of Il23a and Il12b than those isolated from CNV mice (Fig. 5 A). To examine whether IL-25 could directly act on macrophages to limit IL-23 expression, bone marrow–derived macrophages were activated with LPS, a TLR ligand that is known to induce IL-23, in the absence or presence of IL-25. Examination of mRNA levels revealed that IL-25 inhibited LPS-induced Il23a gene expression (Fig. 5 B). This effect was specific for IL-23, as no down-regulation of Il12a, Tgfb, Il6, or Il10 gene expression was observed after exposure to IL-25 (Fig. 5, C–F). A previous study demonstrated that IL-25–induced IL-13 production by DCs that could inhibit IL-23 production (22). However, treatment of macrophages with IL-25 failed to induce Il4 or Il13 mRNA (unpublished data), and IL-25 inhibited LPS-induced expression of Il23a in macrophages derived from mice deficient in STAT6 (Stat6−/− mice), which is the primary signaling molecule downstream of IL-4 and -13 (Fig. 5 G). Consistent with this, despite higher levels of Il23a mRNA in GF mice, we did not detect any difference in Il13 mRNA in the large intestine of mice housed under CNV or GF conditions (Fig. S8, available at http://www.jem.org/cgi/content/full/jem.20080720/DC1). Therefore, IL-25 can act directly on macrophages to inhibit TLR-induced IL-23 expression independently of the IL-4–IL-13–STAT6 pathway.
Figure 5.
IL-25 limits accessory cell-derived IL-23 independently of STAT6. (A) Increased expression of Il23a and Il12b by CD11b+ cells isolated from the large intestinal LP of GF mice. (B–F) Expression of mRNA for Il23a (B), Il12a (C), Tgfb (D), Il6 (E), and Il10 (F) in macrophages 4 h after stimulation with LPS in the absence or presence of IL-25 was analyzed by quantitative real-time PCR. Results are from four experiments. (G) mRNA expression of IL-23p19 (Il23a) in WT or Stat6−/− macrophages was analyzed by real-time PCR after stimulation with media alone, LPS, or LPS and IL-25. Results are representative of two experiments. *, P < 0.05. Error bars indicate the SEM.
Collectively, these results identify a previously unrecognized commensal-dependent immunoregulatory pathway associated with the maintenance of intestinal immune homeostasis. Specifically, recognition of commensal bacteria promotes expression of IL-25 by IECs that is a component of the cytokine network necessary to limit expression of IL-23 and the size of the Th17 cell pool in the intestine under steady-state conditions (Fig. S9, available at http://www.jem.org/cgi/content/full/jem.20080720/DC1). Several studies suggest that commensal bacteria can promote immune cell hyporesponsiveness to maintain intestinal immune homeostasis. For example, commensal bacteria can actively inhibit the activation of NF-κB and expression of proinflammatory cytokines by several mechanisms, including sequestration of signaling components such as PPAR-γ and RelA and inhibition of IκB degradation (13, 14, 29). The absence of these signals in GF mice could contribute to a mechanism that results in increased IL-23 and -17 expression in the absence of commensal bacteria. Supporting a model in which innate recognition of commensal bacteria shapes the local immune environment, a recent study in Drosophila highlighted the influence of signals derived from commensal bacteria in the maintenance of intestinal immune homeostasis (30). When NF-κB–dependent expression of antimicrobial peptides was disrupted in intestinal cells of the fly, there was an outgrowth of a population of pathogenic commensal bacteria that led to fly mortality. In mammals, disruption of innate recognition of commensal bacteria via IEC-specific deletion of the NF-κB pathway by targeting IκB kinase-β (IKK-β) or IKK-γ/NEMO, resulted in spontaneous and infection-induced intestinal inflammation (15, 16). Collectively, these studies highlight the growing recognition of the influence of commensal bacteria-derived signals that promote immunoregulatory pathways in the intestine. Thus, in addition to established immunoregulatory cytokines such as IL-10 and TGF-β, the demonstration of a role for commensal bacteria in influencing IL-25 expression identifies a previously unrecognized pathway through which commensal bacteria can regulate intestinal immune responses.
MATERIALS AND METHODS
Mice.
BALB/c, C57BL/6, and BALB/c Stat6−/− mice were obtained from The Jackson Laboratory. Il25−/− mice have been described previously (22, 23). GF BALB/c and C57BL/6 mice were maintained in plastic isolator units and fed autoclaved feed and water. Conventional animals were maintained in a specific pathogen–free facility and routinely tested negative for pathogens. In individual experiments, mice were age matched and used at 6–8 wk of age or 12–14 wk of age. All experiments were performed under the guidelines of the Institutional Animal Care and Use Committee at the University of Pennsylvania.
Cytokine and antibody treatments.
IL-25 (R&D Systems) was administered daily (0.5 μg i.p.) to GF mice for 7 d. Control mice received PBS. Monoclonal anti–IL-23p19 was produced at Schering-Plough Biopharma, and 1 mg was administered i.p. daily. Control mice received 1 mg of control antibody i.p. daily.
Isolation of cells.
At necropsy, mLNs, spleen, cecal patches, and Peyer's patches were harvested, and single-cell suspensions were prepared. IECs and LP lymphocytes were isolated as previously described (9). Purity of IECs was determined by flow cytometric analysis using anti–Ep-Cam antibody (G8.8, obtained from Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, Iowa) and was routinely >90%.
Macrophage isolation, culture, and stimulation.
CD11b+ cells were isolated from LP of CNV and GF mice using magnetic beads (Miltenyi Biotech). Bone marrow macrophages were cultured for 7 d, plated overnight at 106 cells/ml, and pulsed with 10 ng/ml LPS (Salmonella typhimurium; Sigma-Aldrich) in the absence or presence of 50 ng/ml IL-25 the next day.
Cell stimulations and cytokine assays.
Spleen cells were plated in medium alone or in the presence of anti-CD3 and -CD28 (1 μg/ml each) in the presence or absence of 1 ng/ml IL-12 (eBioscience). Large intestinal tissue explant cultures were performed as previously described (12). Cell-free supernatants were harvested after 72 h, and analyzed for cytokine secretion by sandwich ELISA (eBioscience). Cells were stimulated by incubation for 4 h with 50 ng/ml PMA (Sigma-Aldrich) and 750 ng/ml ionomycin (Sigma-Aldrich) in the presence of 10 μg/ml Brefeldin A (Sigma-Aldrich), and then surface stained with fluorochrome-conjugated antibodies against CD4 and CD3 fixed in 2% paraformaldehyde. Fixed cells were permeabilized with 0.5% saponin (Sigma-Aldrich) and stained intracellularly for IL-17, IFN-γ, TNF-α, IL-10 or Foxp3 (eBioscience), acquired on a FACSCalibur using CellQuest Pro software (BD Biosciences) and analyzed with FlowJo software (Version 8.5; Tree Star, Inc.).
RNA isolation and real-time PCR.
RNA was isolated from tissues using RNeasy Spin columns (QIAGEN) after disruption in a homogenizer (TissueLyzer; QIAGEN). RNA was reverse transcribed into cDNA, and quantitative real-time PCR was performed on cDNA using primer sets for Il10, Il12a, Il12b, Il23a, Tgfb, Il6, Il27p28, and Ebi3 (QIAGEN) using SYBR Green chemistry. Primers for RORγt (Rorc) have been previously described (9). Il17 and Il25 were analyzed using TaqMan primer/probe pairs (Applied Biosystems). All reactions were run on an ABI 7500 Fast Real-Time PCR System (Applied Biosystems). Samples were normalized to naive controls unless specifically stated.
Statistics.
Results represent the mean ± SEM. Statistical significance was determined by Student's t test (between two groups or conditions) or analysis of variance with a post-hoc test (three or more groups or conditions) using Prism 4.0 (GraphPad Software).
Online supplemental material.
Increased expression of Il17 in C57BL/6 GF mice is shown in Fig. S1. Production of IL-6 and -12p40 in large intestinal tissue explant cultures is shown in Fig. S2. Increased expression of Il23a in C57BL/6 GF mice is shown in Fig. S3. Fig. S4 shows that there are minimal antibiotic-induced changes in gene expression in the small intestine of CNV mice. Equivalent Il10 mRNA expression and frequencies of IL-10–producing CD4+ T cells in CNV and GF mice are shown in Fig. S5. Fig. S6 depicts expression of IL-12 and IFN-γ in CNV and GF mice. Fig. S7 shows that expression of Il25 mRNA is decreased in the large intestine of antibiotic-treated CNV mice. Similar levels of Il13 expression in CNV and GF mice are shown in Fig. S8. Fig. S9 depicts a model of how commensal-dependent IL-25 regulates the IL-23–IL-17 axis in the intestine. The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20080720/DC1.
Supplementary Material
Acknowledgments
The authors thank E.J. Pearce, F.A. Marshall, and D.M. Schifferli for the critical reading of the manuscript.
This work was supported by the National Institutes of Health (AI61570 and AI74878 to D. Artis, AI37108 to Y. Yu, F31-GM82187 to S.A. Saenz, T32-AI007532-08 to J.G. Perrigoue, F32-AI72943 to A.E. Troy, and T32-CA09140-30 to B.C. Taylor), the Burroughs Wellcome Fund (Investigator in Pathogenesis of Infectious Disease Award to D. Artis), the Crohn's and Colitis Foundation of America (William and Shelby Modell Family Foundation Research Award to D. Artis), and pilot grants from the University of Pennsylvania (Center for Infectious Diseases and University Research Fund to D. Artis). Schering-Plough Biopharma (formerly DNAX Research Inc.) is funded by the Schering-Plough Corporation. C. Zaph is funded by the Irvington Institute Fellowship Program of the Cancer Research Institute.
The authors have no conflicting financial interests.
C. Zaph's present address is The Biomedical Research Centre, University of British Columbia, Vancouver, BC, V6T 1Z3, Canada.
References
- 1.Hooper, L.V., and J.I. Gordon. 2001. Commensal host-bacterial relationships in the gut. Science. 292:1115–1118. [DOI] [PubMed] [Google Scholar]
- 2.Macpherson, A.J., and N.L. Harris. 2004. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 4:478–485. [DOI] [PubMed] [Google Scholar]
- 3.Ley, R.E., D.A. Peterson, and J.I. Gordon. 2006. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 124:837–848. [DOI] [PubMed] [Google Scholar]
- 4.Strober, W., I.J. Fuss, and R.S. Blumberg. 2002. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 20:495–549. [DOI] [PubMed] [Google Scholar]
- 5.Sellon, R.K., S. Tonkonogy, M. Schultz, L.A. Dieleman, W. Grenther, E. Balish, D.M. Rennick, and R.B. Sartor. 1998. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 66:5224–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yen, D., J. Cheung, H. Scheerens, F. Poulet, T. McClanahan, B. McKenzie, M.A. Kleinschek, A. Owyang, J. Mattson, W. Blumenschein, et al. 2006. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 116:1310–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weaver, C.T., R.D. Hatton, P.R. Mangan, and L.E. Harrington. 2007. L-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25:821–852. [DOI] [PubMed] [Google Scholar]
- 8.Kolls, J.K., and A. Linden. 2004. Interleukin-17 family members and inflammation. Immunity. 21:467–476. [DOI] [PubMed] [Google Scholar]
- 9.Ivanov, I.I., B.S. McKenzie, L. Zhou, C.E. Tadokoro, A. Lepelley, J.J. Lafaille, D.J. Cua, and D.R. Littman. 2006. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 126:1121–1133. [DOI] [PubMed] [Google Scholar]
- 10.Becker, C., S. Wirtz, M. Blessing, J. Pirhonen, D. Strand, O. Bechthold, J. Frick, P.R. Galle, I. Autenrieth, and M.F. Neurath. 2003. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J. Clin. Invest. 112:693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazmanian, S.K., C.H. Liu, A.O. Tzianabos, and D.L. Kasper. 2005. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 122:107–118. [DOI] [PubMed] [Google Scholar]
- 12.Rakoff-Nahoum, S., J. Paglino, F. Eslami-Varzaneh, S. Edberg, and R. Medzhitov. 2004. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 118:229–241. [DOI] [PubMed] [Google Scholar]
- 13.Kelly, D., J.I. Campbell, T.P. King, G. Grant, E.A. Jansson, A.G. Coutts, S. Pettersson, and S. Conway. 2004. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-γ and RelA. Nat. Immunol. 5:104–112. [DOI] [PubMed] [Google Scholar]
- 14.Neish, A.S., A.T. Gewirtz, H. Zeng, A.N. Young, M.E. Hobert, V. Karmali, A.S. Rao, and J.L. Madara. 2000. Prokaryotic regulation of epithelial responses by inhibition of IκB-α ubiquitination. Science. 289:1560–1563. [DOI] [PubMed] [Google Scholar]
- 15.Nenci, A., C. Becker, A. Wullaert, R. Gareus, G. van Loo, S. Danese, M. Huth, A. Nikolaev, C. Neufert, B. Madison, et al. 2007. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 446:557–561. [DOI] [PubMed] [Google Scholar]
- 16.Zaph, C., A.E. Troy, B.C. Taylor, L.D. Berman-Booty, K.J. Guild, Y. Du, E.A. Yost, A.D. Gruber, M.J. May, F.R. Greten, et al. 2007. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature. 446:552–556. [DOI] [PubMed] [Google Scholar]
- 17.Niess, J.H., F. Leithauser, G. Adler, and J. Reimann. 2008. Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J. Immunol. 180:559–568. [DOI] [PubMed] [Google Scholar]
- 18.Bashir, M.E., S. Louie, H.N. Shi, and C. Nagler-Anderson. 2004. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J. Immunol. 172:6978–6987. [DOI] [PubMed] [Google Scholar]
- 19.Bettelli, E., M. Oukka, and V.K. Kuchroo. 2007. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 8:345–350. [DOI] [PubMed] [Google Scholar]
- 20.Batten, M., J. Li, S. Yi, N.M. Kljavin, D.M. Danilenko, S. Lucas, J. Lee, F.J. de Sauvage, and N. Ghilardi. 2006. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat. Immunol. 7:929–936. [DOI] [PubMed] [Google Scholar]
- 21.Stumhofer, J.S., A. Laurence, E.H. Wilson, E. Huang, C.M. Tato, L.M. Johnson, A.V. Villarino, Q. Huang, A. Yoshimura, D. Sehy, et al. 2006. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 7:937–945. [DOI] [PubMed] [Google Scholar]
- 22.Kleinschek, M.A., A.M. Owyang, B. Joyce-Shaikh, C.L. Langrish, Y. Chen, D.M. Gorman, W.M. Blumenschein, T. McClanahan, F. Brombacher, S.D. Hurst, et al. 2007. IL-25 regulates Th17 function in autoimmune inflammation. J. Exp. Med. 204:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owyang, A.M., C. Zaph, E.H. Wilson, K.J. Guild, T. McClanahan, H.R. Miller, D.J. Cua, M. Goldschmidt, C.A. Hunter, R.A. Kastelein, and D. Artis. 2006. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J. Exp. Med. 203:843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kastelein, R.A., C.A. Hunter, and D.J. Cua. 2007. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 25:221–242. [DOI] [PubMed] [Google Scholar]
- 25.Angkasekwinai, P., H. Park, Y.H. Wang, Y.H. Wang, S.H. Chang, D.B. Corry, Y.J. Liu, Z. Zhu, and C. Dong. 2007. Interleukin 25 promotes the initiation of proallergic type 2 responses. J. Exp. Med. 204:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda, K., H. Nakajima, K. Suzuki, S. Kagami, K. Hirose, A. Suto, Y. Saito, and I. Iwamoto. 2003. Mast cells produce interleukin-25 upon FcεRI-mediated activation. Blood. 101:3594–3596. [DOI] [PubMed] [Google Scholar]
- 27.Kang, C.M., A.S. Jang, M.H. Ahn, J.A. Shin, J.H. Kim, Y.S. Choi, T.Y. Rhim, and C.S. Park. 2005. Interleukin-25 and interleukin-13 production by alveolar macrophages in response to particles. Am. J. Respir. Cell Mol. Biol. 33:290–296. [DOI] [PubMed] [Google Scholar]
- 28.Fort, M.M., J. Cheung, D. Yen, J. Li, S.M. Zurawski, S. Lo, S. Menon, T. Clifford, B. Hunte, R. Lesley, et al. 2001. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 15:985–995. [DOI] [PubMed] [Google Scholar]
- 29.Collier-Hyams, L.S., V. Sloane, B.C. Batten, and A.S. Neish. 2005. Cutting edge: bacterial modulation of epithelial signaling via changes in neddylation of cullin-1. J. Immunol. 175:4194–4198. [DOI] [PubMed] [Google Scholar]
- 30.Ryu, J.H., S.H. Kim, H.Y. Lee, J.Y. Bai, Y.D. Nam, J.W. Bae, D.G. Lee, S.C. Shin, E.M. Ha, and W.J. Lee. 2008. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science. 319:777–782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.