Abstract
The small G protein K-Ras2A is rapidly induced by aldosterone in A6 epithelia. In these Xenopus sodium reabsorbing cells, aldosterone rapidly activates preexisting epithelial Na+ channels (XENaC) via a transcriptionally mediated mechanism. In the Xenopus oocytes expression system, we tested whether the K-Ras2A pathway impacts on XENaC activity by expressing XENaC alone or together with XK-Ras2A rendered constitutively active (XK-Ras2AG12V). As a second control, XENaC-expressing oocytes were treated with progesterone, a sex steroid that induces maturation of the oocytes similarly to activated Ras. Progesterone or XK-Ras2AG12V led to oocyte maturation characterized by a decrease in surface area and endogenous Na+ pump function. In both conditions, the surface expression of exogenous XENaC′s was also decreased; however, in comparison with progesterone-treated oocytes, XK-ras2AG12V-coinjected oocytes expressed a fivefold higher XENaC-mediated macroscopic Na+ current that was as high as that of control oocytes. Thus, the Na+ current per surface-expressed XENaC was increased by XK-Ras2AG12V. The chemical driving force for Na+ influx was not changed, suggesting that XK-Ras2AG12V increased the mean activity of XENaCs at the oocyte surface. These observations raise the possibility that XK-Ras2A, which is the first regulatory protein known to be transcriptionally induced by aldosterone, could play a role in the control of XENaC function in aldosterone target cells.
INTRODUCTION
The final urinary Na+ concentration is adjusted by the regulated reabsorption of Na+ across principal cells of the distal nephron. Aldosterone plays a central role in the control of this transport by regulating apical Na+ influx into the cells via the amiloride-sensitive epithelial Na+ channel (ENaC)1 as well as the basolateral extrusion by the Na+ pump (Na,K-ATPase) (Verrey et al., 1996; Garty and Palmer, 1997). ENaC is a tetramer formed by three homologous subunits (Canessa et al., 1994; Puoti et al., 1995; Garty and Palmer, 1997; Firsov et al., 1998). Mutations in its structure as well as defects of its regulation have been shown to lead to salt wasting or hypertension (White, 1994; Schild et al., 1995; Gründer et al., 1997). The structure and function of ENaC has been studied extensively in the Xenopus laevis oocyte system. Using an antibody binding assay combined with electrophysiological methods, Schild and coworkers (Firsov et al., 1996, 1998) estimated that, maximally, approximately one-tenth of ENaC molecules expressed at the surface of oocytes are active. Although not formally demonstrated, this could also be the case in native epithelia.
In Xenopus laevis A6 epithelia, the Na+ transport response to aldosterone is mediated by the mineralocorticoid and/or the glucocorticoid receptor (Geering et al., 1982; Schmidt et al., 1993; Verrey, 1995; Chen et al., 1998). This transcription- and translation-dependent response starts after a lag period of 20–60 min by a first two- to fivefold increase in Na+ transport. This early transcriptionally mediated effect on Na+ transport appears to result from the activation of preexisting Na+ channels and Na+ pumps (Kemendy et al., 1992; Beron et al., 1995; Garty and Palmer, 1997).
It is as yet unknown how the aldosterone-regulated changes in gene transcription lead to the early Na+ transport increase. In particular, no transcriptionally regulated mediator has been identified, despite many functional studies that point, among other possibilities, to the implication of a G protein–regulated methylation step (Sariban-Sohraby et al., 1984, 1995; Blazer-Yost et al., 1997).
Recently, we have cloned cDNAs corresponding to early aldosterone-regulated RNAs from A6 epithelia, using differential display PCR (Spindler et al., 1997). Adrenal steroid–upregulated RNA number 5 (ASUR5) encodes a Xenopus homologue of mammalian K-Ras2A, the splice variant of XK-Ras2 with a C-terminal region encoded by exon 4A, which contains a palmitoylation site, in contrast to the C-terminal region, encoded by exon 4B, which is characterized by a lysine-rich stretch. The induction of the mRNA of XK-Ras2A precedes the Na+ transport response and has a similar dose dependency. This induction does not require ongoing translation but does require ongoing transcription and also takes place in Xenopus kidney (Spindler et al., 1997; and our unpublished results).
Using the Xenopus oocyte expression system, we ask now whether the XK-Ras2A pathway interferes with the activity and/or surface expression of Xenopus epithelial Na+ channel (XENaC) and hence possibly could play a role in the regulation of ENaC function by aldosterone. To activate the XK-Ras2A pathway in oocytes, we expressed XK-Ras2A rendered constitutively active (XK-Ras2AG12V). The study was complicated by the fact that the activation of the Ras pathway induces the maturation of oocytes similar to protein kinase C, which has been shown to produce germinal vesicle breakdown (GVBD), reduction of the surface membrane area, and endocytosis of Na+ pumps (Schmalzing et al., 1991). To reveal maturation-independent effects of XK-Ras2AG12V on coexpressed XENaC, we compared the effect of its coexpression with that of a progesterone treatment, which also induces oocyte maturation but via a different pathway. We show here that XK-Ras2AG12V has a dual effect on coexpressed ENaC in oocytes: a decrease in the number of channels expressed at the cell surface, which is nearly as important as that of surface Na+ pumps, and in contrast to the decrease in Na+ pump current, an activation of ENaCs still expressed at the cell surface.
MATERIALS AND METHODS
Site-directed Mutagenesis
The XK-Ras2A cDNA (Spindler et al., 1997) was subcloned into the SalI site of pSDEasy (Puoti et al., 1997). Constitutively active Ras (XK-Ras2AG12V) was obtained by a point mutation in codon 12 (GGA to GTA) using the Megaprimer PCR protocol (White, 1993). The dominant negative Ras (XK-Ras2AS17N) was constructed by point mutation in codon 17 (AGC to AAC) with the QuikChange Kit (Stratagene, La Jolla, CA). The coding region of both constructs was sequenced in both directions with the T7Sequencing Kit (Pharmacia, Piscataway, NJ).
In Vitro Translation
cRNA was synthesized using SP6 RNA polymerase (Promega, Madison, WI). In vitro translation was performed with the rabbit reticulocyte lysate system plus or minus canine pancreatic microsomal membranes (Promega). The reaction was performed according to manufacturer’s protocol using 1 μg of cRNA in a final volume of 25 μl. Half of the microsomal reactions were washed with a twofold volume of 0.2 M sucrose, 10 mM Tris (pH 8.0), and centrifuged at 125,000 × g for 5 min at 4°C. The supernatant was saved, and the microsomes were washed a second time using the same amount of washing buffer. Analysis of in vitro–translated products was performed by SDS-PAGE according to standard procedures.
Expression in Oocytes and Two-Electrode Voltage-Clamp Measurements
Xenopus α, β, and γ ENaC (XENaC) cDNAs (Puoti et al., 1995) and the α and β Xenopus ENaC subunits tagged with FLAG epitope (XENaCF) were given by the group of B. C. Rossier in Lausanne (Firsov et al., 1996; and our unpublished results). Capped cRNAs of all cDNAs used were synthesized by SP6 RNA polymerase after linearization with BglII (for ras constructs and β ENaC) or AflIII (for α and γ ENaC subunits). cRNA (3.33 ng) of the different XK-Ras2A constructs and 1.33 ng of cRNA of each XENaC subunit were injected into the vegetal pole of stage V–VI Xenopus laevis oocytes. The dissection of Xenopus laevis ovaries and the collection and handling of the oocytes were performed as described by Busch et al. (1992). After injection, the oocytes were incubated in a low-Na+ Barth’s solution (ND10) containing (in mM) 10 NaCl, 86 N-methyl-d-glutamine-Cl (pH 7.4), 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES (pH 7.4), and 20 mg/l gentamicin sulfate for ∼20 h at 16°C in the presence or absence of 15 μM progesterone. Electrophysiological measurements were performed using a laboratory-built two-electrode voltage clamp, optimized for fast voltage clamping of the oocyte membrane using electronic compensation for the bath series resistance. Oocytes were continuously superfused in a small chamber (volume ∼200 μl) at 6 ml/min. Data were acquired using custom-built AD/DAC hardware and DATAC software (Bertrand and Bader, 1986). The capacitance was estimated from the integral of the capacitive transient, measured by stepping the membrane potential from −50 mV to −40 mV in ND10. The macroscopic amiloride-sensitive current (Iami) was defined as the difference between currents obtained in the presence (5 μM) and in the absence of amiloride at a resting potential of −100 mV in a high-Na+ Barth’s solution (ND96) (in mM): 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES (pH 7.4), and 20 mg/l gentamicin sulfate.
Macroscopic I–V curves were generated using a voltage ramp, duration 1 sec, from −80 mV to +60 mV.
Patch-Clamp Recordings
Oocytes were injected and incubated overnight as described above. After ∼20 h, oocytes were manually devitellinized in a solution containing (in mM): 1 MgCl2, 20 KCl, 10 HEPES (pH 7.4), and 200 Na+ glutamate (or 500 Na+ glutamate for XK-Ras2A–coinjected oocytes to compensate for their increased tonicity). Gigaseals were obtained with patch-clamp pipettes containing (in mM): 110 NaCl, 2.5 KCl, 1.8 CaCl2, and 10 HEPES (pH 7.4) (and 300 Na+ glutamate for XK-Ras2A–coinjected oocytes). The bath solution was identical to the pipette solution except that 110 mM KCl replaced NaCl (and 300 K+ glutamate replaced Na+ glutamate for XK-Ras2A coinjected oocytes). Data were sampled at 1000 samples per sec and low-pass–filtered at 400 Hz.
Na+ Pump Measurements and Ouabain Binding
After RNA injection, oocytes were incubated for a total of 2 h in a solution containing (in mM): 96 NaCl, 1 MgCl2, 5 HEPES (pH 7.4) (solution [sol] A), exchanging the solution every 15 min, and then incubated in sol A supplemented with 0.4 mM Ca2+ (sol B). After ∼20 h, the Na+ pump current was measured at a resting potential of −50 mV, filtered at 30 Hz by using the described laboratory-built two-electrode voltage clamp. The ouabain-inhibitable current was determined by measuring the current in a high-K+ solution (sol B + 5 mM KCl + 1 mM BaCl2) (sol C) and subtracting the current generated in a ouabain-containing sol (sol C + 10 μM ouabain) (sol D) (Horisberger et al., 1991).
For the 3H-ouabain binding, oocytes were incubated for 2 min in sol B at 25°C, and than binding was started by replacing the buffer with 100 μl of sol B containing 6.9 μCi 3H-ouabain and unlabelled ouabain to a final concentration of 10 μM or 1 mM (unspecific binding). After 10 min at 25°C, oocytes were washed five times in 3 ml of sol B containing 1 mM ouabain and distributed to separate vials. After lysis in 2% SDS, radioactivity was counted.
Iodination of M2Ab and Binding Assay
The procedures correspond essentially to those described by Firsov et al. (1996). Briefly, the Iodo-Bead (Pierce, Rockford, IL) was prewashed in 500 μl of 100 mM Pate (pH 6.5) for 1 min and dried on 3 MM paper (Whatman, Maidstone, England) for 1 min. After 10 min preincubation of the bead in 100 μl of 100 mM Na+ phosphate (pH 6.5) and 0.5 mCi 125I (NEN NEZ 033A, DuPont, Boston, MA), the iodination was started by adding 50 μg of M2AB antibody (Kodak, Rochester, NY) and incubating for 10 min at room temperature. Unincorporated 125I was separated by running the reaction mixture in ND10 over a preequilibrated (10 ml 2% BSA in PBS, 40 ml ND10) PD-10 column (Pharmacia). Five-hundred microliters of fraction were recovered and fractions six to eight, containing ∼80% of the iodinated antibody, were pooled and analyzed for specific activity that ranged between 0.5 and 2 × 1018 cpm/mol.
After electrophysiological measurements, oocytes were preincubated in 500 μl of ND10 + 10% FCS (ND10FCS) for 30 min on ice. Binding was in 100 μl of ND10FCS supplemented with 125I-labeled antibody (12 nM) on ice for 1 h. After washing the oocytes four times with ND10FCS and four times with ND10, the γ-radiation was determined in a gamma counter (LKB-Wallac, Gaithersburg, MD).
Immunofluorescence with Anti-FLAG Antibody
Twenty-four hours after injection, oocytes were fixed with 3% paraformaldehyde in PBS for 4 h. Fixed oocytes were placed on thin cork disks, embedded into cryo-embedding compound (Microm, Walldorf, Germany), frozen in liquid propane that was cooled by liquid nitrogen, and stored at −80°C until further use. Sections (6 μm) were cut in a cryostat and placed on chrom-alum gelatin-coated glass slides.
For immunocytochemistry we used the tyramide signal amplification (TSA-Direct) kit (NEN, Boston, MA) according to the manufacturer’s instructions. XENaCF was detected with an anti-FLAG IgG antibody (Kodak) that was diluted 1:100 in the TSA blocking buffer. Sections were rinsed with PBS containing 0.05% Tween (PBS–Tween) and were subsequently incubated with a 1:100 dilution of horseradish peroxidase–conjugated sheep anti-mouse Ig (Amersham, Arlington Heights, IL). After repeated washing with PBS–Tween, binding sites of the secondary antibody were revealed with FITC-tyramide conjugates diluted 1:50 in the TSA diluent. Sections were washed in PBS–Tween and mounted in DAKO-Glycergel (Dako, Glostrup, Denmark) containing 2.5% of 1,4-diazabicyclo (2.2.2)-octane as a fading retardant (Sigma, St. Louis, MO). All antibody incubations were performed for 1 h at room temperature. In some experiments a Cy3-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) diluted 1:1000 in PBS supplemented with 0.5% BSA was used as secondary antibody. Although the staining intensity with the Cy3-conjugated secondary antibody was less intense, the staining pattern was identical to the one obtained with the TSA method. No staining was observed in experiments in which the primary antibody was omitted.
Sections were studied by epifluorescence with a Polyvar microscope (Reichert Jung, Vienna, Austria). Digitized images were acquired with a VISICAM CCD camera (Visitron, Puchheim, Germany) and processed by Image-Pro Plus v3.0 software (Media Cybernetics, Silver Spring, MD).
RESULTS
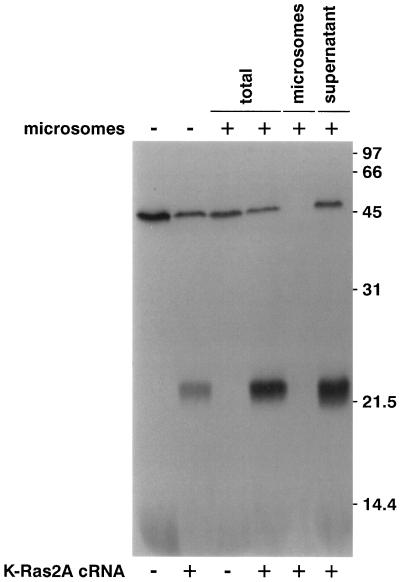
To determine whether the Ras pathway and specifically ASUR5 (XK-Ras2A) possibly impacts on the expression/function of the ENaC, we used the Xenopus laevis oocyte system to coexpress XK-Ras2A with ENaC, carrying a FLAG epitope in the extracellular domain of the α and β subunits in a similar position as described previously for rat ENaC (Firsov et al., 1996). Constitutively active (XK-Ras2AG12V) and dominant negative (XK-Ras2AS17N) forms were generated by site-directed mutagenesis. Figure 1 shows the result of an in vitro translation. As expected for p21ras, XK-Ras2A migrated on an SDS-PAGE gel as single band slightly above the 21-kDa marker protein and was not associated with microsomal membranes (Figure 1). The mutated forms XK-Ras2AS17N and XK-Ras2AG12V showed the same migration characteristics on SDS-PAGE (our unpublished results).
Figure 1.
In vitro translation of XK-ras2A cRNA. Products of in vitro translation performed in the presence or absence of canine pancreatic microsomal membranes were separated on a 15% SDS gel and submitted to fluorography. The molecular weight of marker proteins is indicated on the right in kilodaltons. XK-Ras2A protein migrated as a ∼22-kDa band and was not associated with microsomal membranes.
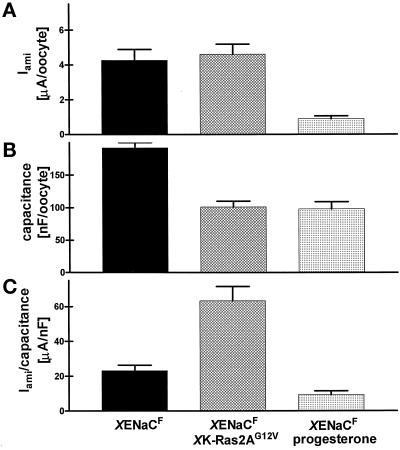
XENaC was expressed in oocytes as described by Puoti et al. (1995). Approximately 20 h after injection of 1.33 ng of cRNA of each channel subunit (αFLAG, βFLAG, and γ), Iami was measured by the two-electrode voltage-clamp technique. The mean Iami was 4.26 ± 0.63 μA/oocyte (n = 5) at a membrane potential of −100 mV and in buffer containing 96 mM Na+ (Figure 2A). The coinjection of 3.3 ng of cRNA coding for XK-Ras2AG12V did not significantly increase the Iami to 4.61 ± 0.58 μA/oocyte. In contrast, incubation of XENaCF-injected oocytes with progesterone (15 μM) reduced the Iami to 0.89 ± 0.17 μA/oocyte. Similar results were obtained in coinjection experiments with untagged XENaC (Iami [XENaC]: 4.57 ± 0.56 and Iami [XENaC+XK-Ras2AG12V]: 5.87 ± 0.7 μA/oocyte). Thus, progesterone decreased Iami in contrast to XK-Ras2AG12V coexpression.
Figure 2.
Effect of coexpressed XK-Ras2AG12V on XENaCF function and oocyte surface membrane area. (A) Amiloride-sensitive current (Iami) was measured by the two-electrode voltage-clamp technique at a membrane potential of −100 mV in oocytes expressing XENaCF, XENaCF + XK-Ras2AG12V, or XENaCF in the presence of 15 μM progesterone. Progesterone-treated cells showed an approximately fourfold lower Iami compared with control oocytes and XK-Ras2AG12V–coinjected oocytes. (B) The membrane area of the oocytes (measured as capacitance) was decreased in maturing oocytes such that XK-Ras2AG12V–coinjected oocytes had a higher Iami per surface area than those of the two other groups (C). Results are expressed as means of five independent experiments ± SEM with each of four to nine oocytes per group.
XK-Ras2AG12V Increases Iami per Surface Area
It is known that in Xenopus oocytes, activation of the Ras pathway (by insulin treatment or injection of oncogenic Ras protein or cRNA) and treatment with progesterone lead to maturation, but by different pathways. Oocyte maturation is characterized by GVBD (Birchmeier et al., 1985), which is visualized by the appearance of a white spot at the animal pole and also leads to a decrease in membrane area caused by an increase in endocytosis (Vasilets et al., 1990). Earlier studies have shown that progesterone leads to a decrease in activity of various transporters expressed at the oocyte surface (Richter et al., 1984). For example, the appearance of GVBD is paralleled by a decrease in Na+ pump activity and of the number of ouabain binding sites attributable to an endocytosis of Na+ pumps (Vasilets et al., 1990; Schmalzing et al., 1991).
We measured the capacitance of the oocyte membrane by integrating the capacitive current transient produced by a voltage step. Providing that the capacity per surface area remains constant, this represents a measure for the oocyte surface membrane area. As expected and in good agreement with the appearance of GVBD, the surface area was decreased by a factor of 2 in oocytes treated with progesterone as well as in those injected with XK-Ras2AG12V cRNA (Figure 2B).
Figure 2C shows the ratio of Iami per oocyte membrane area. In the case of the progesterone treatment, there was a decrease in Iami that was even larger than that of the surface area such that the current per membrane area was decreased compared with that of untreated oocytes injected with XENaCF cRNA alone. In contrast, oocytes coinjected with XK-Ras2AG12V cRNA showed a two- to threefold higher Iami per membrane area than control oocytes. The same was seen in coinjection experiments with untagged XENaC (3.2-fold higher Iami per membrane area in coinjected oocytes).
Neither the protooncogenic wild-type form of XK-Ras2A (ASUR5) nor the dominant negative form XK-Ras2AS17N impacted on the XENaCF-mediated Iami or induced maturation of the oocytes in the given time frame. It has been shown by others that injection of protooncogenic Ras protein can induce the maturation of oocytes, but less efficiently than the oncogenic form. For instance, injection of 200 ng of wild-type H-Ras protein has been reported to induce 50% GVBD after 40 h, in contrast to 10 ng of oncogenic H-RasG12V protein, which required only 10 h to produce the same effect (Birchmeier et al., 1985). We injected up to 37 ng of wild-type XK-Ras2A cRNA, but no significant induction of maturation was observed within 20 h.
In summary, the activated form of XK-Ras2A and progesterone produced a similar decrease in membrane area caused by oocyte maturation. In contrast to progesterone, XK-Ras2AG12V increased the Iami per membrane area carried by coexpressed XENaCF.
XK-Ras2AG12V Increases Iami per Surface-expressed XENaC
The effect of XK-Ras2AG12V cRNA injection reported above could be due to either a lack of change in XENaCF cell-surface expression and, hence, an increase in channel density or, alternatively, an increase in current per surface-expressed channel, if the number of channels was decreased as well during the reduction of surface area.
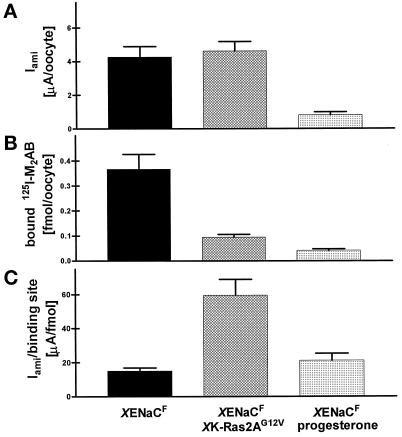
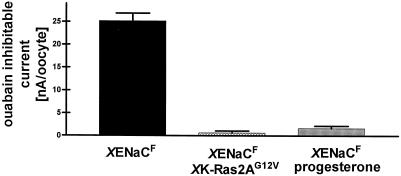
To dissociate these two possibilities we performed binding assays with anti-FLAG antibody and immunofluorescence experiments to visualize and quantitate XENaCF. Binding assays on single intact oocytes were performed after the electrophysiological measurement using 125I-labeled anti-FLAG antibody such that the number of binding sites, which is proportional to the number of surface-expressed channels, could be determined and correlated with the Iami (Firsov et al., 1996). The number of binding sites per oocyte (0.37 ± 0.06 fmol/oocyte) (Figure 3B) as well as the Iami per binding site (15.0 ± 1.9 μA/fmol) (Figure 3C) were in the range of values reported previously for rat ENaC (Firsov et al., 1996). In contrast, oocytes coinjected with XK-Ras2AG12V cRNA showed a three- to fourfold decrease in the number of binding sites (Figure 3B), indicating that the number of surface-expressed channels was decreased even to a larger extent than the surface area. The function of endogenous Na,K,-ATPases measured as pump current was completely inhibited (Figure 4), and specific ouabain binding was abolished (our unpublished results) (Richter et al., 1984; Vasilets et al., 1990; Schmalzing et al., 1991). Progesterone induced a decrease in anti-FLAG antibody binding sites that was approximately five times as large as the surface reduction. In this case and in contrast to the effect of XK-Ras2AG12V, the reduction of Iami was approximately parallel to that of surface channels measured by the binding assay (Figure 3). Similar to the effect of XK-Ras2AG12V coinjection, progesterone treatment induced a retrieval of nearly all endogenous surface Na+ pumps (our unpublished results) and fully inhibited the Na+ pump current (Figure 4). In summary, both pathways induce, in addition to the surface reduction, a retrieval of surface Na+ pumps, and to a lesser extent, of surface Na+ channels; however, only XK-Ras2AG12V induces a significant increase in Iami per surface-expressed channel.
Figure 3.
Quantitation of XENaCF surface expression with iodinated anti-FLAG antibody. (A) XENaCF and XENaCF + XK-Ras2AG12V–expressing oocytes showed a similarly high Iami, in contrast to the small current of the XENaCF–expressing ones treated with progesterone. (B) From the heterotetramer building the channel (2α, 1β, 1γ) (Firsov et al., 1998), three subunits out of four (2α and 1β) were tagged with a FLAG epitope. The antibody binding to surface XENaCF was quantitated as described by Firsov et al. (1996)for the rat ENaCF. Maturating oocytes showed a significantly lower number of binding sites such that Iami per surface-expressed XENaCF (C) was higher in XK-Ras2AG12V–coinjected oocytes than in those expressing XENaCF alone in the presence or absence of progesterone. Results are expressed as means of five independent experiments ± SEM with each of four to nine oocytes per group.
Figure 4.
Effect of XK-Ras2AG12V and progesterone on Na+ pump currents. The ouabain-inhibitable currents were measured by the two-electrode voltage-clamp technique at a membrane potential of −50 mV. Oocytes expressing XENaCF + XK-Ras2AG12V or XENaCF in the presence of 15 μM progesterone showed no Na+ pump activity in contrast to control oocytes expressing XENaCF.
Oocytes coinjected with the dominant negative form XK-Ras2AS17N expressed the same Iami and the same number of anti-FLAG antibody binding sites as control oocytes (our unpublished results). This suggested that a tonic stimulation by endogenous Ras via those pathways blocked by the S17N mutation was not required for the baseline Iami expression in oocytes injected with XENaC cRNA alone and that the action of activated XK-Ras2A was not due to an unspecific effect of RNA coinjection (Marais et al., 1998).
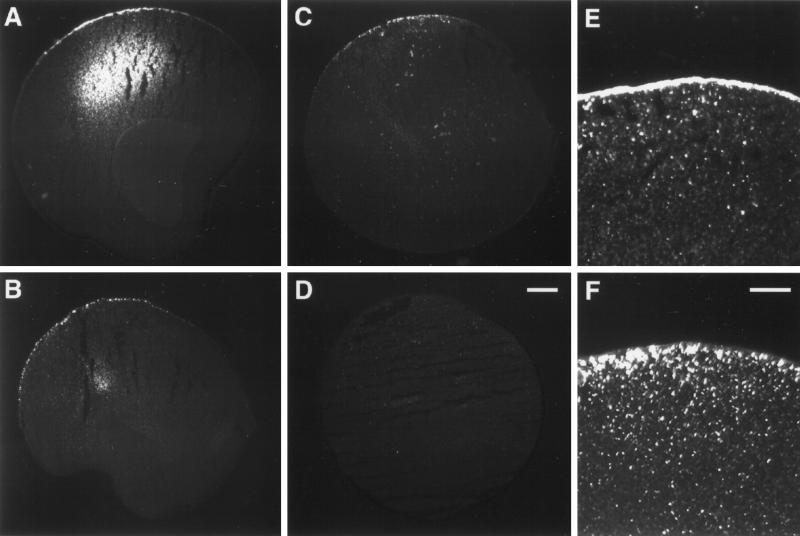
Immunofluorescence experiments using the same monoclonal anti-FLAG antibody revealed in low-power magnifications a polarized distribution of XENaCF in oocytes (Figure 5A) with a bright signal in the plasma membrane region at the vegetal pole. Immunofluorescence was also observed in an intracellular compartment. In oocytes coinjected with XK-Ras2AG12V (Figure 5B) or treated with progesterone (Figure 5C), XENaCF was also detected at the vegetal pole. At higher magnifications it became obvious that in untreated oocytes expressing only XENaCF (Figure 5E) the staining was seen over the plasma membrane and in a few submembranous vesicular structures. In XK-Ras2AG12V–coinjected oocytes (Figure 5F) as well as in oocytes treated with progesterone (our unpublished results), XENaCF-related immunofluorescence was almost absent in the plasma membrane and mainly visible in dotted structures just below the plasma membrane.
Figure 5.
Immunofluorescence detection of XENaCF with anti-FLAG antibody. (A) XENaCF-expressing control oocytes showed a bright signal over the plasma membrane of the vegetal pole and in an intracellular compartment. XK-Ras2AG12V–coinjected oocytes (B) as well as progesterone-treated control oocytes (C) showed a similarly polarized staining in the low-power magnification but in a dotted pattern. No signal was detected in uninjected oocytes (D). The high-power magnification revealed in XENaCF–injected oocytes a continuous staining over the plasma membrane and of a few submembranous dotted structures (E). In XK-Ras2AG12V–coinjected oocytes, a dotted staining pattern was localized almost entirely below the plasma membrane (F). Bars: D, 100 μm; F, 40 μm.
In conclusion, XK-Ras2AG12V and progesterone lead to a decrease in surface-expressed XENaCF caused by a shift to submembranous compartments. Because Iami is not altered in coinjected oocytes, XK-Ras2AG12V increases the Iami per surface-expressed Na+ channel.
XK-Ras2AG12V Increases the Activity of Surface-expressed ENaC
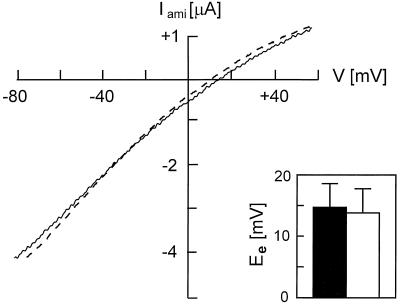
There were three possible explanations for the fact that the Iami per surface-expressed XENaCF was increased in XK-Ras2AG12V cRNA-injected oocytes. The first possibility was that the chemical component of the driving force for Na+ influx could have been increased. This appeared unlikely in view of the fact that the resting potential was very similar for XK-Ras2AG12V–coinjected and control XENaC-injected oocytes (our unpublished results). The determination of the equilibrium potential for sodium (Figure 6) showed that the Na+ activity in XK-Ras2AG12V–coinjected oocytes (13.8 ± 4.0 mV; n = 5) was comparable with XENaC-injected oocytes (14.7 ± 3.9 mV). This indicated that differences in intracellular Na+ concentrations and therefore the driving force for Na+ cannot be the explanation for the fourfold increase in the Iami per surface-expressed channel. The second possibility was that the conductance of single channels had been changed by the expression of XK-Ras2AG12V, and the third possibility was that the mean activity of the surface-expressed channels (Po (binding); see DISCUSSION) had been increased. To determine which of these two last possibilities was correct, we performed patch-clamp measurements of the expressed XENaCF to measure the single-channel conductance in the presence or absence of XK-Ras2AG12V. Preliminary recordings showed no difference in single-channel currents and slope conductance (our unpublished results), indicating that the difference in Iami per surface channel (measured by antibody binding) was probably not due to a difference in single-channel conductance. In conclusion, XK-RasG12V appears to increase the mean activity of the epithelial Na+ channels expressed at the surface of Xenopus laevis oocytes, most likely increasing the proportion of active channels within the surface-expressed pool.
Figure 6.
Macroscopic current–voltage relationship of XENaCF expressed alone or together with XK-Ras2AG12V in Xenopus oocytes. Macroscopic Iami was measured in ND96 solution in dependence of a voltage ramp at 100 mM extracellular Na+. Representative traces for XENaCF and XENaCF + XK-Ras2AG12V–injected oocytes are shown as straight and dotted lines, and mean equilibrium potential (Ee) is shown as black and white bars (n = 5), respectively.
DISCUSSION
Early Transcriptionally Mediated Regulatory Action of Aldosterone
The results of the present study show that XK-Ras2A, the small G protein that we have recently shown to be rapidly induced by aldosterone (Spindler et al. 1997), affects the surface expression and the function of XENaC when coexpressed in Xenopus laevis oocytes in its activated form.
Aldosterone is known to increase the reabsorption of Na+ in rat kidney after ∼0.5 h of treatment (Horisberger and Diezi, 1984). This early effect requires transcription and translation, and thus is likely mediated by induced proteins or, alternatively, the decrease in repressed proteins. The time frame of this early effect is similar in amphibian model epithelia (lag ∼30–60 min) in which it is assumed to be mediated by regulatory protein(s) acting on preexisting channels. This assumption is based, on the one hand, on indirect experimental evidence (Palmer and Edelman, 1981; Garty and Edelman, 1983) and, on the other hand, inferred from the observation that the early effect is proportional to the preexisting transport, such that it is entirely lacking in the absence of preexisting transport (Verrey, 1995). In contrast to this observation, de novo production of channels caused by transcriptional, translational, or posttranslational regulatory mechanisms would lead to an (absolute) increase in transport, independent of the preexisting one.
Multiple Effects of Aldosterone on ENaC Regulation
The mechanism by which channels are activated by aldosterone is not known. Regarding potential mechanisms of ENaC regulation, it is known that the function of the channel is modified in the presence of an extracellular protease (Vallet et al., 1997; Chraibi et al., 1998) and by the binding of protein(s), such as Nedd4 (Staub et al., 1996), at the level of the C-terminal region of the subunits that is mutated in the hyperactive ENaCs found in Liddle’s disease (Firsov et al., 1996). For the protease action, the physiological role is not yet known, and in the case of Nedd4, it appears that this mechanism is involved in mediating the feedback control by intracellular Na+ (Dinudom et al., 1998).
Concerning the aldosterone action, many indirect experiments have pointed to the possibility that G protein(s) could be involved in the mediation of the ENaC activation and that carboxymethylation of a prenylated protein would be required for this regulation to take place (Sariban-Sohraby et al., 1984, 1995; Blazer-Yost et al., 1997). These observations are compatible with the possibility that K-Ras2A is this GTP-binding protein, being itself also prenylated and carboxymethylated. In any case, functional experiments are required to examine the role of K-Ras2 in ENaC regulation.
Mechanistically it is important to elucidate whether regulation by aldosterone increases the open probability (Po) of already active channels or whether previously silent channels stored at the cell surface or in an intracellular pool are activated or translocated, respectively. Electrophysiological experiments have provided conflicting observations on this question.
The term “open probability” (Po) is generally used as above to characterize the activity of channels measurable electrophysiologically. This means that truly inactive channels are not considered. In contrast, using a function-independent approach to quantify the channels, for instance, the binding of an antibody at the oocyte surface (Firsov et al., 1996; this study), one can estimate an open probability relative to the “biochemical pool” of channels expressed at the cell surface. Here we call this open probability Po (binding).
Using A6 epithelia pretreated with aldosterone from which the hormone had been withdrawn, Kemendy et al. (1992) observed channels with low open probabilities (Po) by patch clamp. On readdition of the hormone, the Po returned to “normal”, that is, long open and closed periods with a mean Po between 0.24 and 0.38; however, it appears that low Po of the epithelial Na+ channel is not observed in other situations, and hence other authors only saw the appearance of “new channels” with a Po similar to the preexisting ones (if there were preexisting ones) on treatment of A6 epithelia with aldosterone or rats with low-sodium diet (Pácha et al., 1993; Helman et al., 1998). These latter results support the hypothesis that a population of surface channels is switched from a silent mode to an active one by aldosterone or that intracellular channels are translocated to the cell surface. The change in Po of ENaC observed in the first study in contrast could correspond to a regulation mode restricted to previously activated cell-surface channels. This complexity of the aldosterone action supports the hypothesis that more than a single, linear pathway is involved and that aldosterone rather produces several modifications in the channel-controlling network. The fact that K-Ras2A is rapidly transcriptionally regulated in A6 epithelia (Spindler et al., 1997) and in Xenopus kidney (our unpublished results) is in accordance with this view. We thus propose that this induction is part of the pleiotropic action of aldosterone on the intracellular signalling network. Such a pleiotropic action of aldosterone on a signalling cascade has been functionally described in the case of the aldosterone-induced potentiation of antidiuretic hormone-stimulated cAMP production. This effect is not very early but adds to the synergistic action of aldosterone and antidiuretic hormone at the level of ENaC (Verrey et al., 1993; Verrey, 1994). It will be interesting to establish which of the elements upstream of the adenylyl cyclase are (direct or indirect) targets of the transcriptional action of aldosterone.
Regulation of ENaC Surface Expression
In this study we took advantage of the oocyte system to ask whether the Ras pathway, or in particular the K-Ras2A pathway, might affect the function of ENaC. We have observed two effects that are apparently opposed: a reduction of channel surface expression that is related to the maturation of the oocytes and an increase in the activity of surface-expressed channels. As shown previously for other Ras molecules, XK-Ras2AG12V reproducibly induced oocyte maturation. The retrieval of surface membrane and transporters is known to be associated with maturation. In the case of the endogenous Na,K-ATPase, this leads to the disappearance of measurable Na+ pump function and molecules from the cell surface, as shown in this study for progesterone and XK-Ras2AG12V expression (Figure 4) and as shown previously for the action of protein kinase C (Vasilets et al., 1990; Schmalzing et al., 1991). Similarly, the exogenous XENaCs were found to be retrieved from the surface (or prevented from surface expression) to an unproportionally large extent compared with the membrane area reduction. This selective retrieval of membrane transport proteins is compatible with the fact that the enrichment or exclusion from sites of endocytosis, as well as similar processes in the exocytic pathway, control the traffic of proteins differentially from that of membranes. As expected from an increased endocytosis, exogenous ENaCs were found by immunofluorescence to be located mostly in a submembranous, intracellular compartment (Figure 5).
ENaC surface expression is also thought to be regulated in aldosterone target cells; however, translocation of ENaCs to the cell surface has not been shown to be involved in the effect of aldosterone itself, but rather in the regulation of ENaC by protein kinase A (Marunaka and Eaton, 1991; Verrey, 1994; Els and Helman, 1997). The considerable amount of literature on this question (translocation versus activation of ENaC) has to be viewed with caution, however, in particular in view of the fact that the number of active surface channels per cell (∼10–1000) is very low compared with the quite large number of channels produced in A6 cells and expressed in mammalian principal cells (May et al., 1997; our unpublished results).
Activity of Surface ENaC
In this study we show that XK-Ras2AG12V does not decrease the amiloride-sensitive current, despite a large decrease in XENaC surface expression discussed above. This suggests that the open probability of channels expressed at the cell surface (Po(binding)) is higher in XK-Ras2AG12V–expressing oocytes than in control oocytes. Similarly opposing effects on ENaC surface expression and activity are observed in the protein kinase A–mediated action of antidiuretic hormone. Protein kinase A appears to increase ENaC surface expression and concomitantly to inhibit its function via the cystic fibrosis transmembrane conductance regulator activation. These effects lead to a net increase in Na+ transport in the A6 system and frog skin (Verrey, 1995; Els and Helman, 1997), whereas in other systems the inhibitory action of cystic fibrosis transmembrane conductance regulator appears to dominate (Letz and Korbmacher, 1997).
How XK-Ras2AG12V mechanistically leads to an increase in the proportion of active channels at the cell surface of oocytes is not clear, but one could speculate that a similar mechanism operates in aldosterone target epithelia. Using antibody binding on oocytes, it has been clearly established that the number of surface-expressed channels is much larger than the number of electrically active ones (Firsov et al., 1996). Thus, an in situ activation of previously silent channels appears to be possible. Such a situation could also prevail in aldosterone target cells and would be compatible with the observation that the number of high-affinity amiloride-binding sites at the apical surface is much larger than the number of electrically active channels (Blazer-Yost and Helman, 1997). Estimates of the proportion of active channels lead to numbers in the order of 5–20% in control oocytes and approximately four times more in those coexpressing XK-Ras2AG12V.
Ras proteins are multifunctional switches, the downstream effects of which depend on the cell type and physiological state. Ras activation leads to the binding and hence membrane localization and activation of downstream effectors (Marshall, 1996). The physiological effect of Ras activation is mostly related to proliferation and/or differentiation depending on cell type and physiological state (Marshall, 1995). In this context, Ras activation has been shown to impact on the function of membrane channels or transporters (Houseknecht et al., 1996; Ma et al., 1996; Ritter et al., 1997), and in other cases Ras activation has been shown to lead to an increase in the number of transport proteins that are expressed in terminally differentiated cells (Pollock and Rane, 1996; Yoshikawa et al., 1996). It is conceivable that K-Ras2A plays a similar function in epithelial target cells of aldosterone, acting on the function and expression of Na+ channels.
Aldosterone increases K-Ras2 mRNA (Spindler et al., 1997) and p21ras protein biosynthesis in A6 epithelia (our unpublished results), and we show here that activated XK-Ras2A acts on ENaC surface expression and activity in oocytes. Thus, it will be interesting to evaluate the role of an increase in XK-Ras2 expression in the complex context of epithelial aldosterone target cells and to test whether observed effects are specific for this member of the Ras family. Indeed, the in vivo specificity of the effect of different Ras proteins has not yet been well characterized.
In conclusion, we show that the K-Ras2A pathway controls both surface localization and activity of ENaC expressed in oocytes, and we suggest that aldosterone, by increasing the quantity of newly synthesized K-Ras2 in target cells, could modify the regulatory impact of this signalling pathway on ENaC function.
ACKNOWLEDGMENTS
We thank Lea Kläusli in the laboratory of B. Kaissling (Institute of Anatomy, Zurich, Switzerland) and Ivan Gautschi in the laboratory of L. Schild (Institute of Pharmacology and Toxicology, Lausanne, Switzerland) for technical help. We also thank B. C. Rossier (Institute of Pharmacology and Toxicology, Lausanne), in whose laboratory Anne May worked, for the XENaC cDNAs, J. Biber and H. Murer (Institute of Physiology, Zurich) for the use of the Xenopus laevis oocyte expression system, and all three including L. Schild for helpful discussions, suggestions, and comments. We thank C. Gasser for the artwork. This study was supported by grant 31-49727.96 from the Swiss National Science Foundation to F.V.
Abbreviations used:
- ENaC
epithelial sodium channel
- GVBD
germinal vesicle breakdown
- Iami
amiloride-sensitive current
- Po
open probability
- TSA
tyramide signal amplification
REFERENCES
- Beron J, Mastroberardino L, Spillmann A, Verrey F. Aldosterone modulates sodium kinetics of Na, K-ATPase containing an alpha1 subunit in A6 kidney cell epithelia. Mol Biol Cell. 1995;6:261–271. doi: 10.1091/mbc.6.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Bader C. DATAC: a multipurpose biological data analysis program based on a mathematical interpreter. Int J Biomed Comput. 1986;18:193–202. doi: 10.1016/0020-7101(86)90016-4. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Broek D, Wigler M. RAS protein can induce meiosis in Xenopus oocytes. Cell. 1985;43:615–621. doi: 10.1016/0092-8674(85)90233-8. [DOI] [PubMed] [Google Scholar]
- Blazer-Yost BL, Helman SI. The amiloride-sensitive epithelial Na+ channel-binding sites and channel densities. Am J Physiol. 1997;41:C761–C769. doi: 10.1152/ajpcell.1997.272.3.C761. [DOI] [PubMed] [Google Scholar]
- Blazer-Yost BL, Hughes CL, Nolan PL. Protein prenylation is required for aldosterone-stimulated Na+ transport. Am J Physiol. 1997;41:C1928–C1935. doi: 10.1152/ajpcell.1997.272.6.C1928. [DOI] [PubMed] [Google Scholar]
- Busch AE, Kavanaugh MP, Varnum MD, Adelman JP, North RA. Regulation by second messenger of the slowly-activating, voltage-dependent potassium current in Xenopus oocytes. Am J Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Chen SY, Wang J, Liu WH, Pearce D. Aldosterone responsiveness of A6 cells is restored by cloned rat mineralocorticoid receptor. Am J Physiol. 1998;43:C39–C46. doi: 10.1152/ajpcell.1998.274.1.C39. [DOI] [PubMed] [Google Scholar]
- Chraibi A, Vallet V, Firsov D, Hess SK, Horisberger JD. Protease modulation of the activity of the epithelial sodium channel expressed in Xenopus oocytes. J Gen Physiol. 1998;111:127–138. doi: 10.1085/jgp.111.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinudom A, Harvey KF, Komwatana P, Young JA, Kumar S, Cook DI. Nedd4 mediates control of an epithelial Na+ channel in salivary duct cells by cytosolic Na+ Proc Natl Acad Sci USA. 1998;95:7169–7173. doi: 10.1073/pnas.95.12.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Els WJ, Helman SI. Dual role of prostaglandins (PGE2) in regulation of channel density and open probability of epithelial Na+ channels in frog skin (R-pipiens) J Membr Biol. 1997;155:75–87. doi: 10.1007/s002329900159. [DOI] [PubMed] [Google Scholar]
- Firsov D, Gautschi I, Merillat AM, Rossier BC, Schild L. The heterotetrameric architecture of the epithelial sodium channel (ENaC) EMBO J. 1998;17:344–352. doi: 10.1093/emboj/17.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firsov D, Schild L, Gautschi I, Merillat AM, Schneeberger E, Rossier BC. Cell surface expression of the epithelial Na channel and a mutant causing Liddle syndrome: a quantitative approach. Proc Natl Acad Sci USA. 1996;93:15370–15375. doi: 10.1073/pnas.93.26.15370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty H, Edelman IS. Amiloride-sensitive trypsinisation of apical sodium channels. J Gen Physiol. 1983;81:785–803. doi: 10.1085/jgp.81.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- Geering K, Girardet M, Bron C, Kraehenbühl JP, Rossier BC. Hormonal regulation of (Na+, K+)-ATPase biosynthesis in the toad bladder. J Biol Chem. 1982;257:10338–10343. [PubMed] [Google Scholar]
- Gründer S, Firsov D, Chang SS, Jaeger NF, Gautschi I, Schild L, Lifton RP, Rossier BC. A mutation causing pseudohypoaldosteronism type 1 identifies a conserved glycine that is involved in the gating of the epithelial sodium channel. EMBO J. 1997;16:899–907. doi: 10.1093/emboj/16.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman SI, Liu X, Baldwin K, Blazer-Yost B, Els WJ. Time-dependent stimulation by aldosterone of blocker-sensitive ENaCs in A6 epithelia. Am J Physiol. 1998;43:C947–C957. doi: 10.1152/ajpcell.1998.274.4.C947. [DOI] [PubMed] [Google Scholar]
- Horisberger JD, Diezi J. Inhibition of aldosterone-induced antinatriuresis and kaliuresis by actinomycin D. Am J Physiol. 1984;246:F201–F204. doi: 10.1152/ajprenal.1984.246.2.F201. [DOI] [PubMed] [Google Scholar]
- Horisberger JD, Jaunin P, Good PJ, Rossier BC, Geering K. Coexpression of alpha-1 with putative beta-3 subunits results in functional Na+/K+ pumps in Xenopus oocytes. Proc Natl Acad Sci USA. 1991;88:8397–8400. doi: 10.1073/pnas.88.19.8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseknecht KL, Zhu AX, Gnudi L, Hamann A, Zierath JR, Tozzo E, Flier JS, Kahn BB. Overexpression of Ha-ras selectively in adipose tissue of transgenic mice: evidence for enhanced sensitivity to insulin. J Biol Chem. 1996;271:11347–11355. doi: 10.1074/jbc.271.19.11347. [DOI] [PubMed] [Google Scholar]
- Kemendy AE, Kleyman TR, Eaton DC. Aldosterone alters the open probability of amiloride-blockable sodium channels in A6 epithelia. Am J Physiol. 1992;263:C825–C837. doi: 10.1152/ajpcell.1992.263.4.C825. [DOI] [PubMed] [Google Scholar]
- Letz B, Korbmacher C. cAMP stimulates CFTR-like Cl− channels and inhibits amiloride-sensitive Na+ channels in mouse CCD cells. Am J Physiol. 1997;41:C657–C666. doi: 10.1152/ajpcell.1997.272.2.C657. [DOI] [PubMed] [Google Scholar]
- Ma HP, Matsunaga H, Li B, Schieffer B, Marrero MB, Ling BN. Ca2+ channel activation by platelet-derived growth factor-induced tyrosine phosphorylation and Ras guanine triphosphate-binding proteins in rat glomerular mesangial cells. J Clin Invest. 1996;97:2332–2341. doi: 10.1172/JCI118676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais R, Light Y, Mason C, Paterson H, Olson MF, Marshall CJ. Requirement of Ras-GTP-Raf complexes for activation of Raf-1 by protein kinase C. Science. 1998;280:109–111. doi: 10.1126/science.280.5360.109. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signalling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. Ras effectors. Curr Opin Cell Biol. 1996;8:197–204. doi: 10.1016/s0955-0674(96)80066-4. [DOI] [PubMed] [Google Scholar]
- Marunaka Y, Eaton DC. Effects of vasopressin and cAMP on single amiloride-blockable Na channels. Am J Physiol. 1991;260:C1071–C1084. doi: 10.1152/ajpcell.1991.260.5.C1071. [DOI] [PubMed] [Google Scholar]
- May A, Puoti A, Gaeggeler HP, Horisberger JD, Rossier BC. Early effect of aldosterone on the rate of synthesis of the epithelial sodium channel alpha subunit in A6 renal cells. J Am Soc Nephrol. 1997;8:1813–1822. doi: 10.1681/ASN.V8121813. [DOI] [PubMed] [Google Scholar]
- Pácha J, Frindt G, Antonian L, Silver RB, Palmer LG. Regulation of Na channels of the rat cortical collecting tubule by aldosterone. J Gen Physiol. 1993;102:25–42. doi: 10.1085/jgp.102.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LG, Edelman IS. Control of apical sodium permeability in the toad urinary bladder by aldosterone. Ann NY Acad Sci. 1981;372:1–14. doi: 10.1111/j.1749-6632.1981.tb15453.x. [DOI] [PubMed] [Google Scholar]
- Pollock JD, Rane SG. p21(ras) signaling is necessary but not sufficient to mediate neurotrophin induction of calcium channels in PC12 cells. J Biol Chem. 1996;271:8008–8014. doi: 10.1074/jbc.271.14.8008. [DOI] [PubMed] [Google Scholar]
- Puoti A, May A, Canessa CM, Horisberger JD, Schild L, Rossier BC. The highly selective low-conductance epithelial Na channel of Xenopus laevis A6 kidney cells. Am J Physiol. 1995;38:C188–C197. doi: 10.1152/ajpcell.1995.269.1.C188. [DOI] [PubMed] [Google Scholar]
- Puoti A, May A, Rossier BC, Horisberger JD. Novel isoforms of the beta and gamma subunits of the Xenopus epithelial Na channel provide information about the amiloride binding site and extracellular sodium sensing. Proc Natl Acad Sci USA. 1997;94:5949–5954. doi: 10.1073/pnas.94.11.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter HP, Jung D, Passow H. Regulatory changes of membrane transport and ouabain binding during progesterone-induced maturation of Xenopus oocytes. J Membr Biol. 1984;79:203–210. doi: 10.1007/BF01871059. [DOI] [PubMed] [Google Scholar]
- Ritter M, Woll E, Haller T, Dartsch PC, Zwierzina H, Lang F. Activation of Na+/H+-exchanger by transforming Ha-ras requires stimulated cellular calcium influx and is associated with rearrangement of the actin cytoskeleton. Eur J Cell Biol. 1997;72:222–228. [PubMed] [Google Scholar]
- Sariban-Sohraby S, Burg M, Wiesmann WP, Chiang PK, Johnson JP. Methylation increases sodium transport into A6 apical membrane vesicles: possible mode of action of aldosterone action. Science. 1984;225:745–746. doi: 10.1126/science.6463652. [DOI] [PubMed] [Google Scholar]
- Sariban-Sohraby S, Mies F, Abramow M, Fisher RS. Aldosterone stimulation of GTP hydrolysis in membranes from renal epithelia. Am J Physiol. 1995;37:C557–C562. doi: 10.1152/ajpcell.1995.268.3.C557. [DOI] [PubMed] [Google Scholar]
- Schild L, Canessa CM, Shimkets RA, Gautschi I, Lifton RP, Rossier BC. A mutation in the epithelial sodium channel causing Liddle disease increases channel activity in the Xenopus laevis oocyte expression system. Proc Natl Acad Sci USA. 1995;92:5699–5703. doi: 10.1073/pnas.92.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalzing G, Mädefessel K, Haase W, Geering K. Immunocytochemical evidence for Protein kinase C-induced internalization of sodium pumps in Xenopus laevis oocytes. In: Kaplan JH, editor. The Sodium Pump, Recent Developments. P. De Weer, New York: The Rockefeller University Press; 1991. pp. 465–469. [Google Scholar]
- Schmidt TJ, Husted RF, Stokes JB. Steroid hormone stimulation of Na+ transport in A6 cells is mediated via glucocorticoid receptors. Am J Physiol. 1993;264:C875–C884. doi: 10.1152/ajpcell.1993.264.4.C875. [DOI] [PubMed] [Google Scholar]
- Spindler B, Mastroberardino L, Custer M, Verrey F. Characterization of early aldosterone-induced RNAs identified in A6 kidney epithelia. Pflügers Arch. 1997;434:323–331. doi: 10.1007/s004240050403. [DOI] [PubMed] [Google Scholar]
- Staub O, Dho S, Henry PC, Correa J, Ishikawa T, McGlade J, Rotin D. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle’s syndrome. EMBO J. 1996;15:2371–2380. [PMC free article] [PubMed] [Google Scholar]
- Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature. 1997;389:607–610. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]
- Vasilets LA, Schmalzing G, Mädefessel K, Haase W, Schwarz W. Activation of protein kinase C by phorbol ester induces downregulation of the Na+/K+-ATPase in oocytes of Xenopus laevis. J Membr Biol. 1990;118:131–142. doi: 10.1007/BF01868470. [DOI] [PubMed] [Google Scholar]
- Verrey F. Antidiuretic hormone action in A6 cells: effect on apical Cl and Na conductances and synergism with aldosterone for NaCl reabsorption. J Membr Biol. 1994;138:65–76. doi: 10.1007/BF00211070. [DOI] [PubMed] [Google Scholar]
- Verrey F. Transcriptional control of sodium transport in tight epithelia by adrenal steroids. J Membr Biol. 1995;144:93–110. doi: 10.1007/BF00232796. [DOI] [PubMed] [Google Scholar]
- Verrey F, Beron J, Spindler B. Corticosteroid regulation of renal Na, K-ATPase. Miner Electrolyte Metab. 1996;22:279–292. [PubMed] [Google Scholar]
- Verrey F, Digicaylioglu M, Bolliger U. Polarized membrane movements in A6 kidney cells are regulated by aldosterone and vasopressin/vasotocin. J Membr Biol. 1993;133:213–226. doi: 10.1007/BF00232021. [DOI] [PubMed] [Google Scholar]
- White BA. PCR protocols, current methods and applications. Methods in Molecular Biology. New Jersey: Human Press; 1993. [Google Scholar]
- White PC. Mechanisms of disease: disorders of aldosterone biosynthesis and action. New Engl J Med. 1994;331:250–258. doi: 10.1056/NEJM199407283310408. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H, Yasukawa Y, Okino S, Takamori M, Iida K. Calcitonin gene-related peptide increases K-ras expression in primary culture cell of rat skeletal muscle. Biomed Res Tokyo. 1996;17:165–169. [Google Scholar]