Abstract
Golgi-localized, γ-Ear–containing, ADP-ribosylation factor-binding proteins (GGAs) and adaptor protein-1 (AP-1) mediate clathrin-dependent trafficking of transmembrane proteins between the trans-Golgi network (TGN) and endosomes. In yeast, the vacuolar sorting receptor Vps10p follows a direct pathway from the TGN to the late endosome/prevacuolar compartment (PVC), whereas, the processing protease Kex2p partitions between the direct pathway and an indirect pathway through the early endosome. To examine the roles of the Ggas and AP-1 in TGN–PVC transport, we used a cell-free assay that measures delivery to the PVC of either Kex2p or a chimeric protein (K-V), in which the Vps10p cytosolic tail replaces the Kex2p tail. Either antibody inhibition or dominant-negative Gga2p completely blocked K-V transport but only partially blocked Kex2p transport. Deletion of APL2, encoding the β subunit of AP-1, did not affect K-V transport but partially blocked Kex2p transport. Residual Kex2p transport seen with apl2Δ membranes was insensitive to dominant-negative Gga2p, suggesting that the apl2Δ mutation causes Kex2p to localize to a compartment that precludes Gga-dependent trafficking. These results suggest that yeast Ggas facilitate the specific and direct delivery of Vps10p and Kex2p from the TGN to the PVC and that AP-1 modulates Kex2p trafficking through a distinct pathway, presumably involving the early endosome.
INTRODUCTION
In the yeast Saccharomyces cerevisiae, the procarboxypeptidase Y (CPY) receptor Vps10p and the pro-α-factor processing enzyme Kex2p undergo rounds of transport between the TGN and the late endosome/prevacuolar compartment (PVC) (Cereghino et al., 1995; Cooper and Stevens, 1996; Brickner and Fuller, 1997). A central event in Vps10p and Kex2p transport is their incorporation into clathrin-coated vesicles bound for the PVC (Deloche et al., 2001). In current models, the process of cargo incorporation into incipient clathrin vesicles is mediated by adaptor proteins that associate with the cytoplasmic face of donor organelles. These adaptors couple cargo sorting with coat assembly through multivalent interactions with ADP-ribosylation factor (Arf)-guanosine triphosphate (GTP), C-tails of cargo proteins, clathrin, and accessory proteins involved in coat formation (Owen et al., 2004). Two different types of adaptors, adaptor protein-1 (AP-1) and Golgi-localized, γ-ear-containing, ADP-ribosylation factor-binding proteins (Ggas), have been implicated in clathrin-coated vesicle formation at the TGN and endosomes (Hinners and Tooze, 2003). AP-1, a member of the AP family of clathrin adaptors, is a heterotetrameric complex found at the trans-Golgi network (TGN) and endosomes. Ggas, originally identified by homology to the C-terminal domain of the γ-subunit of AP-1, are monomeric adaptors that are also found at these organelles (Dell'Angelica et al., 2000; Hirst et al., 2000).
Studies in Saccharomyces cerevisiae suggest that the Ggas and AP-1 facilitate distinct transport pathways between the TGN and endosomes. Deletion of the yeast GGA1 and GGA2 genes results in impaired proteolytic processing of CPY, proteinase A, and carboxypeptidase S, hydrolases that are sorted from the late-Golgi to the PVC en route to the vacuole (Dell'Angelica et al., 2000; Hirst et al., 2000; Costaguta et al., 2001). Gga function in vacuolar hydrolase sorting seems to involve transport steps upstream of the PVC (Costaguta et al., 2001). Studies of the PVC syntaxin, Pep12p, have shown that this protein is missorted to the early endosome in gga1Δ gga2Δ cells, supporting a role for Ggas in TGN-to-PVC transport (Black and Pelham, 2000). In contrast, AP-1 mutants do not display vacuolar sorting defects. AP-1 mutations, however, accentuate the pro-α-factor maturation defects in clathrin temperature-sensitive cells and exhibit synthetic growth defects with gga mutations (Rad et al., 1995; Costaguta et al., 2001), suggesting that AP-1 nevertheless functions in a clathrin-mediated transport at the TGN or endosomes. In support of such a role, there is evidence to suggest that AP-1 functions in transport between early endosomes and the TGN. This is based on the ability of AP-1 to regulate the retention of Chs3p (chitin synthase III) and Tlg1p (early endosomal t-SNARE) at the early endosome (Valdivia et al., 2002), and on the ability of AP-1 to slow delivery of a reporter protein, A(F→A)-alkaline phosphatase (ALP), to the PVC (Foote and Nothwehr, 2006). A 12-residue cytosolic motif in the C-tail of A(F→A)-ALP that functions to slow the transport of the protein into the PVC has been shown to bind directly to AP-1, suggesting that a specific sorting signal in this motif directs AP-1–dependent transport of A(F→A)-ALP from the early endosome to the TGN. Together, these observations suggest that the Ggas and AP-1 function in distinct TGN-PVC transport steps.
Cell-free reconstitution of vesicular transport events affords analysis of individual trafficking pathways between compartments of the cell's complex and dynamic endomembrane system (Bonifacino and Glick, 2004). Such reactions also provide direct, biochemical assays that can reliably establish requirements for molecular factors, bypassing difficulties inherent in resolving rapid and multiple trafficking steps by in vivo analysis alone. We have developed an assay that measures the cell-free transport of two TGN localized membrane proteins, Kex2p and Kex2-Vps10p (K-V), into the PVC (Blanchette et al., 2004; Abazeed et al., 2005). This reaction depends on multiple factors thought to be required for transport from the TGN to the PVC: clathrin, Vps1p, Vps21p, Vps45p, and Pep12p. Here, we used this assay to determine the roles of the Gga and AP-1 clathrin adaptors in TGN-to-PVC transport. We found that Gga function is required for direct transport of Kex2p and K-V from the TGN to the PVC. K-V transport was unaffected by mutation of AP-1, indicating that AP-1 is not involved in direct TGN–PVC transport. Moreover, AP-1 mutant membranes could not support direct TGN–PVC transport of Kex2p because loss of AP-1 function mislocalized Kex2p in vivo, likely due to a defect in transport from the early endosome to the TGN.
MATERIALS AND METHODS
Strains, Media, and Plasmids
The following strains used in this study were derived from CRY2 (Redding et al., 1991) and are listed with relevant genotype: KRY24-2D (MATα kex2::LEU2), JBY209 (MATα kex2::hisG dap2::kanr pep4::HIS3 ste13::LEU2) (Brickner et al., 2001), MAY4 (MATα kex2::hisG dap2::kanr pep4::HIS3 ste13::LEU2 gga1 gga2), MAY14 (MATα gga1::TRP1 gga2::HIS3), MAY17 (MATα kex2::hisG dap2::kanr pep4::HIS3 ste13::LEU2 gga1 GGA2::13myc-TRP1), and MAY8 (MATα kex2::hisG dap2::kanr pep4::HIS3 ste13::LEU2 apl2). DC14 (MATa his1) was used as a mating type tester. MAY4 was generated by consecutive transformations of JBY209 with DNA fragments containing the TRP1 gene from plasmid pFA6a-TRP1 (Longtine et al., 1998) and the URA3 gene from plasmid pUG72 (Gueldener et al., 2002) amplified using polymerase chain reaction (PCR) primers having homology upstream and downstream of GGA1 and GGA2, respectively. Ura− transformants were selected on 5-fluoroorotic acid (5-FOA), and a 5-FOAR isolate was selected (MAY4). MAY14 (MATα gga1::TRP1 gga2::HIS3) was generated by consecutive transformations of CRY2 (MATα) with DNA fragments containing the TRP1 gene from plasmid pFA6a-TRP1 (Longtine et al., 1998) and the HIS3 gene from plasmid pFA6-His3MX6 (Longtine et al., 1998) amplified using PCR primers having homology upstream and downstream of GGA1 and GGA2, respectively. MAY17, which has a gga1 deletion and expresses a tagged form of Gga2p (Gga2-13myc), was constructed as follows. JBY209 was transformed with a PCR-amplified URA3-marked DNA fragment from gga1 disruption plasmid, pMA-gga1. Ura− transformants were selected on 5-FOA, and a 5-FOAR isolate was selected (MAY16). MAY16 was subsequently transformed with a DNA fragment containing 13myc-TRP1 amplified using PCR from plasmid pMA-GGA2-13myc to generate MAY17. Strain MAY8 was generated by transforming JBY209 with a DNA fragment containing the URA3 gene from pUG72 (Gueldener et al., 2002) amplified using PCR primers having homology upstream and downstream of APL2. Colony PCR confirmed the deletion of APL2. Ura− transformants were selected on 5-FOA, and a 5-FOAR isolate was selected as MAY8.
Synthetic complete glucose medium lacking uracil (SDC-Ura) and synthetic complete galactose medium lacking uracil (SGC-Ura) were as described previously (Brickner and Fuller, 1997).
Centromere plasmid pCWKX20 contains the KEX2 structural gene under control of the yeast GAL1 promoter (Wilcox et al., 1992). The KEX2-VPS10 fusion gene under the control of the GAL1 promoter were created by directional cloning of NarI-SalI–digested DNA fragments amplified by PCR using oligonucleotide primers containing NarI (5′ primer) and SalI (3′ primer) into pCWKX20. Centromere plasmid pCWKX10 contained KEX2 under its own promoter (Wilcox et al., 1992). Plasmids pKEX2-VPS10, encoding the K-V chimeric protein (Abazeed et al., 2005), and pPSHA (previously referred to as pPEP12-STE13ΔTMDα3xHA), encoding the PSHA chimeric protein (Blanchette et al., 2004), were as described. To generate a knockout construct for GGA1, the GGA1 open reading frame (ORF) was first amplified by PCR from yeast chromosomal DNA and inserted into pRS316 (Sikorski and Hieter, 1989). This plasmid was modified by EcoRI digestion and subsequent insertion of a fragment containing URA3 from plasmid pUG72 amplified with oligonucleotide primers containing EcoRI restriction sites, generating pMA-gga1. The GGA1 disruption DNA fragment was generated by PCR amplification from pMA-gga1 by using oligonucleotide primers containing the 5′ and 3′ ends of the ORF of GGA1. The gene replacement results in the loss of amino acids (aa) 103-557 in the Gga1p sequence. Plasmid pRS313 (Sikorski and Hieter, 1989) carrying an ∼1-kb GGA2 fragment was created by cloning a DNA fragment generated by overlap extension PCR containing sequences ∼500 base pairs upstream and ∼500 base pairs downstream of the GGA2 termination codon. Linker primers used in overlap extension contain a BamH I restriction site and lack the termination codon. To generate pMA-GGA2-13myc, pRS313-GGA2 was modified by BamHI digestion and insertion of a DNA fragment containing 13myc-TRP1 from plasmid pFA6a-13myc-TRP1 (Longtine et al., 1998) that was amplified with oligonucleotide primers containing BamHI sites. The gene replacement results in the fusion of 13 tandem c-myc epitope tags to the C terminus of Gga2p (aa 1-558) and creates the predicted amino acid sequence at the junction, Val558-Arg-Ile-ProGly-Leu-Asn. To generate Gga2-VHS-GAT expression vectors with a 6X His tag at the C-terminus, DNA encoding Gga2p VHS-GAT (aa 1-336) was amplified using oligonucleotide primers containing XhoI (5′ primer) and SalI (3′ primer) restriction sites and was cloned directionally into p426 based vectors under the promoters indicated in legends to Figure 3, B–D (Mumberg et al., 1995). To generate Gga2-VHS-GAT expression vectors with a 6X His-tag at the C terminus, DNA encoding Gga2p VHS-GAT (aa 1-336) was amplified using oligonucleotide primers containing six histidine codons (3′ primer), and BamHI I (5′ primer) and SalI (3′ primer) restriction sites. PCR products were cloned directionally into p426-based vectors under the promoters indicated in legends to Figure 3, B–D (Mumberg et al., 1995). To generate Gga2-VHS-GAT with a glutathione transferase (GST)-tag at the N terminus, DNA encoding Gga2p VHS-GAT (aa 1-336) was amplified using oligonucleotide primers containing BamHI (5′ primer) and NotI (3′ primer) restriction sites and was cloned directionally into pYEX 4T-1 (Ward et al., 1994). To generate pGEX-KG-VHS-GAT, DNA encoding Gga2p VHS-GAT (aa 1–336) was amplified using oligonucleotide primers containing BamH I (5′ primer) and Xho I (3′ primer) restriction sites and was cloned directionally into pGEX-KG (Guan and Dixon, 1991).
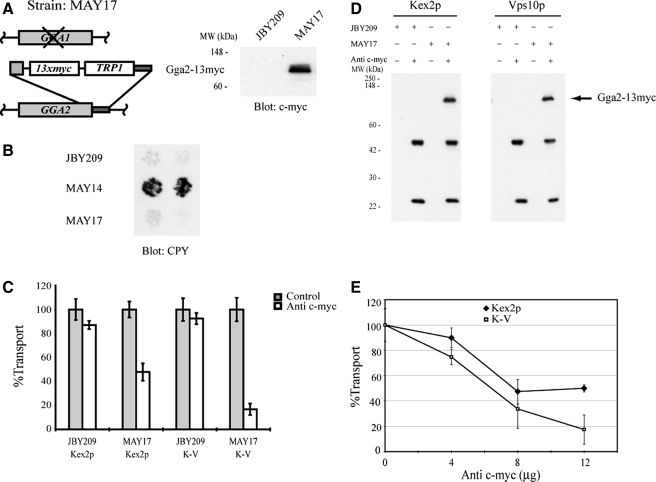
Figure 3.
Gga2p is required for cell-free TGN-to-PVC transport of Kex2p and K-V. (A) Schematic depiction of MAY17 construction (see Materials and Methods) and expression of Gga2p-myc. Whole-cell lysates were prepared from JBY209 (GGA1 GGA2) and MAY17 (gga1 GGA2::13xmyc). Proteins were resolved by SDS-PAGE (10% acrylamide body gel) and analyzed by immunoblotting using anti-c-myc. The position of Gga2p-myc and molecular mass size markers are indicated. (B) GGA2::13myc expresses Gga2-13myc and functionally replaces Gga2p. JBY209 (GGA1 GGA2), MAY14 (gga1 gga2), and MAY17 (gga1 GGA2::13myc) were analyzed for the Vps phenotype (CPY secretion; see Materials and Methods). (C) MSS was prepared from strains JBY209 (GGA1 GGA2) and MAY17 (gga1 GGA2::13myc) expressing Kex2p, K-V or PSHA. Donor and acceptor MSS samples were combined and preincubated with 12 μg of anti-c-myc for 1 h on ice. Cell-free transport reactions were then carried out (20 min at 30°C). Extents of transport observed for controls (no anti-c-myc) were 11.3%, JBY209 (Kex2p); 13.8%, MAY17 (Kex2p); 10.4%, JBY209 (K-V); and 11.9%, MAY17 (K-V), adjusted to 100% in the figure. Experimental values (+ anti-c-myc) are shown as percentage of the control values. (D) Anti-c-myc recognizes Gga2p-myc during the course of the reaction. An aliquot of each reaction shown in C was incubated with protein A-Sepharose for 1 h at 4°C. Protein A-Sepharose pellets were subsequently washed and solubilized in SDS-PAGE sample buffer. Eluted samples were resolved by SDS-PAGE and analyzed by immunoblotting using anti-c-myc. (E) Titration of anti-c-myc in transport reactions. Donor and acceptor MSS samples were combined and preincubated with the indicated amounts of anti-c-myc for 1 h on ice. Transport reactions were then carried out (20 min at 30°C). Data are expressed as percentage of control reactions as described in (C). Extents of transport in control (no anti-c-myc) reactions were 11.2% MAY17 (Kex2p) and 11.9% MAY17 (K-V).
Antibodies and Reagents
Anti-hemagglutinin (HA) monoclonal antibody (mAb) 12CA5 and anti-c-myc mAb (9E10) were from Roche Diagnostics (Indianapolis, IN). Anti-CPY mAb 10A5 and anti-Pep12p mAb (2C3) were from Invitrogen (Carlsbad, CA). Anti-6X His was from Novus Biologicals (Littleton, CO). Anti-GST was from Zymed Laboratories (South San Francisco, CA). Anti-Apl2p was a gift from G. Payne (UCLA). Glutathione Sepharose 4B and protein A-Sepharose were from GE Healthcare (Little Chalfont, Buckinghamshire, United Kingdom). Pansorbin was from Calbiochem (San Diego, CA). Immobilized papain was from Pierce Chemical (Rockford, IL). Bovine α-thrombin was from Hematologic Technologies (Essex Junction, VT). Restriction and DNA modification enzymes were from New England Biolabs (Ispwich, MA). Ala-ProMCA was from Bachem Biosciences (King of Prussia, PA). All other chemicals and reagents, unless otherwise indicated, were from Sigma-Aldrich (St. Louis, MO).
Phenotypic Assays
The plate-based mating (“onset of impotence”) assay was performed as described previously (Redding et al., 1996). Briefly, cells grown on SGC-Ura plates were replica plated to nitrocellulose filters (82 mm in diameter; 0.45-μm pore size) (BA85; Whatman Schleicher and Schuell, Keene, NH) on SGC-Ura plates. After overnight incubation at 30°C, filters were shifted to SDC-Ura plates for various times before replica-plating to SD plates spread with a late log phase culture of the MATa mating partner strain DC14. Growth of prototrophic diploids was scored after 2 d. The Vps phenotype was assessed by measuring CPY secretion by yeast strains grown in patches on nitrocellulose filters using the CPY colony immunoblotting assay (Rothman et al., 1986). Filters were probed by sequential incubations with monoclonal anti-CPY antibody, rabbit anti-mouse immunoglobulin G (IgG), and donkey anti-rabbit IgG conjugated to horseradish peroxidase and developed using enhanced chemiluminescence (GE Healthcare), detected on BioMax film (Eastman Kodak, Rochester, NY).
Cell-free Transport
Details of the basic assay for cell-free TGN-to-PVC transport can be found in Blanchette et al. (2004). In summary, medium-speed supernatant (MSS) was prepared from semi-intact yeast cells by gentle freeze-thaw lysis of yeast spheroplasts followed by centrifugation. MSS prepared under these conditions contains cytosol and late Golgi and endosomal membranes but not endoplasmic reticulum or early Golgi vesicles (Brickner et al., 2001). Transport reactions were performed by combining equal volumes of 3× buffer (containing an ATP-regenerating system); donor MSS, prepared from JBY209 (or related strains) containing pCWKX10 (expressing Kex2p) or pKEX2-VPS10 (expressing K-V); and acceptor MSS prepared from JBY209 (or related strains) containing pPSHA (expressing PSHA). In some cases, MSS from JBY209 (kex2Δ) was added in place of donor MSS as a control. Mixtures were incubated at 30°C for various times to permit transport to occur. The PSHA chimera consists of the entire sequence of the PVC syntaxin Pep12p, which localizes the protein to the PVC and can replace endogenous Pep12p, followed by the catalytic domain of the Ste13p dipeptidyl aminopeptidase A (DPAP), a Kex2p cleavage site from pro-α-factor and a triple HA epitope tag. Delivery of Kex2 activity (either Kex2p or K-V) to PSHA-containing membranes results in cleavage of PSHA, releasing the 3xHA tag from the Pep12-Ste13p chimera. To measure the degree of PSHA cleavage, and thus of transport of Kex2p or K-V to the PVC, transport reactions were subjected to immunoprecipitation (IP) performed in the presence of 1% Triton X-100 by using anti-HA mAb, rabbit anti-mouse IgG, and Pansorbin. Parallel mock IP reactions lack antibodies. The extent of transport is determined as the ratio of DPAP activity in the supernatant fractions from the IP and the mock IP, expressed as a percentage. DPAP activity is measured using the fluorogenic peptide substrate Ala-ProMCA. Typically, incubation of PSHA and Kex2p MSS resulted in 10–12% of DPAP remaining in the supernatant after IP. Incubation of PSHA MSS with kex2Δ MSS as a control resulted in 1–3% of DPAP remaining in the supernatant after IP. Data points represent mean values of duplicates; all reactions were performed at least twice with comparable results. Error bars represent the SE of the mean of at least two reactions.
Preparation of Soluble Gga2p VHS-GAT
A GST-VHS-GAT fusion protein with a thrombin cleavage site positioned C-terminal to GST and N-terminal to the Gga2p VHS-GAT domain, was expressed in Escherichia coli BL21 grown at 37°C, from plasmid pGEX-KG-VHS-GAT. Four hours before harvest, GST-VHS-GAT expression was induced with 100 μM isopropyl β-d-thiogalactoside. An extract prepared by sonication (three 10-s bursts with intermittent cooling) followed by centrifugation of insoluble debris (12,000 rpm for 5 min) was incubated with glutathione-Sepharose 4B beads for 30 min at room temperature. Beads were washed three times (10 ml) with phosphate-buffered saline plus 0.05% Tween 20 and two times (1 ml) with thrombin cleavage buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 2.5 mM CaCl2, and 0.1% β-mercaptoethanol). GST fusion protein bound to beads was cleaved by bovine α-thrombin to release Gga2p VHS-GAT. Thrombin was inactivated with 1 mM phenymethylsulfonyl fluoride, and the soluble fraction and two 1-ml washes of thrombin-treated beads were pooled, concentrated, and dialyzed against phosphate-buffered saline. A control sample (mock sample in Figures 4A and 5E) was prepared identically from BL21 containing vector pGEX-KG.
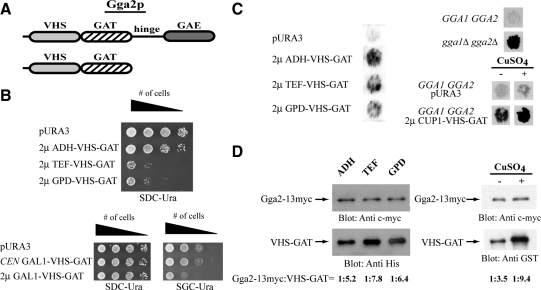
Figure 4.
Gga2p VHS-GAT functions as a dominant negative in vivo. (A) Schematic representation of Gga2p and Gga2p VHS-GAT. (B) High level expression of Gga2p VHS-GAT causes slow growth. Strain CRY2 was transformed with single-copy CEN vectors (p416) or high-copy 2μ vectors (p426) vectors expressing Gga2p VHS-GAT (aa 1-336) under the indicated promoters. Transformed strains were pronged at decreasing cell densities on SDC-Ura or SGC-Ura plates and tested for growth at 30°C for ∼2 d. (C) High level expression of Gga2p VHS-GAT causes secretion of CPY. Strain CRY2 was transformed with the indicated vectors and transformants were tested for the Vps phenotype (CPY secretion; see Materials and Methods). Also, strains CRY2 (GGA1 GGA2), MAY14 (gga1Δ gga2Δ), CRY2 (pURA3), and CRY2 (2 μ CUP1-VHS-GAT) were tested for the Vps phenotype (CPY secretion; see Materials and Methods). Filters were shifted to SDC-Ura or SDC-Ura supplemented containing 100 μM CuSO4 for 6 h before immunoblot analysis with anti-CPY antibody. (D) Whole-cell lysates were prepared from MAY17 (gga1 GGA2::13xmyc) expressing Gga2p VHS-GAT under ADH, TEF, GPD, or CUP1 promoters. Proteins were resolved by SDS-PAGE (10% acrylamide body gel) and analyzed by immunoblotting using anti-c-myc and anti-His or anti-GST. Quantification of ratios of Gga2-13myc:VHS-GAT was performed as described in Materials and Methods.
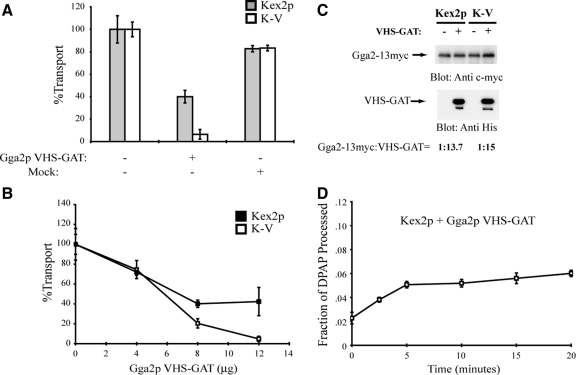
Figure 5.
Purified Gga2p VHS-GAT inhibits cell-free TGN-to-PVC transport reactions containing Kex2p and K-V donor membranes. (A) MSS was prepared from strain JBY209 expressing Kex2p, K-V, or PSHA. Donor and acceptor MSS samples were combined and preincubated with 12 μg of purified Gga2p VHS-GAT, with a mock-purified sample (equivalent volume) or with no addition (control), for 1 h on ice. Cell-free transport reactions were then carried out (20 min at 30°C). Extents of transport observed for the controls (no addition) were 10.8% JBY209 (Kex2p), 11.7% JBY209 (K-V), adjusted to 100% in the figure. Reactions containing Gga2p VHS-GAT or mock addition are shown as percentage of the control values. (B) Titration of Gga2p VHS-GAT in transport reactions. Donor and acceptor MSS samples were combined and preincubated with the indicated amounts of purified Gga2p VHS-GAT or with no addition (control) for 1 h on ice. Transport reactions were then carried out (20 min at 30°C). Data are expressed as percentage of control reactions as described in A. Extents of transport in control (no addition) reactions were 12.3% JBY209 (Kex2p), 9.9% JBY209 (K-V). (C) MSS was prepared from strain MAY17 (gga1 GGA2::13xmyc) expressing Kex2p, K-V, or PSHA. Donor and acceptor MSS samples were combined and incubated with 12 μg of purified Gga2p VHS-GAT with a C-terminal 6X His tag or with no addition. Proteins were resolved by SDS-PAGE (10% acrylamide body gel) and analyzed by immunoblotting using anti-c-myc and anti-His. Quantification of ratios of Gga2p:VHS-GAT was performed as described in Materials and Methods. (D) Donor and acceptor MSS from strain JBY209 expressing Kex2p or PSHA were combined and preincubated with Gga2p VHS-GAT as described in A. A time course of cell-free transport was then measured as Figure 1C.
Quantification of Expressed Gga2p and VHS-GAT
To analyze the expression of Gga2p and VHS-GAT, yeast protein extracts were prepared as described previously (Burke et al., 2000). Protein extracts were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE), followed by immunoblotting with antibodies against c-myc, 6X His, and GST. Nitrocellulose membranes were scanned on a Typhoon Trio+ Imager (GE Healthcare). Protein bands were quantified using ImageQuant software (GE Healthcare).
Preparation of Fab Fragments
Fab fragments of monoclonal anti-Pep12p IgG were prepared by digestion with papain as described previously (Harlow and Lane, 1988) except as indicated below. Briefly, 100 μl of 0.5 mg/ml anti-Pep12p IgG was dialyzed against 100 mM sodium acetate, pH 5.5. Then, 1/20 volume of cysteine from a 1 M stock and 1/20 volume of EDTA from a 20 mM stock were added. Immobilized papain was added and mixed by inversion for 8–10 h at 37°C. Immobilized papain was separated from the digest by centrifugation. Iodoacetamide was added to a final concentration of 50 mM and was incubated with the digest for 30 min at room temperature. The digested anti-Pep12p mixture was dialyzed against PBS, pH 7.4. The Fab fragments were separated from undigested IgG and Fc fragments by incubation with protein A-Sepharose for 2 h at 4°C. Papain digestion and absence of IgG and Fc fragments was confirmed by SDS-PAGE.
RESULTS
Profiles of Kex2p and K-V Transport
We developed previously an assay that measures cell-free transport of Kex2p from TGN donor membranes to PVC acceptor membranes containing the chimeric Kex2p substrate PSHA (see Materials and Methods for a description; Blanchette et al., 2004). As an experimental refinement, we also reported that a Kex2p chimera (K-V) containing the cytosolic tail (C-tail) of Vps10p was also efficiently transported to the PVC (Abazeed et al., 2005; Figure 1A). Unlike Kex2p, Vps10p cycles directly between the TGN and the PVC in the absence of exchanges with the early endosome (Sipos et al., 2004). Thus, transport of the K-V chimera in the cell-free assay should only occur via the direct pathway from the TGN to the PVC. The ability to measure transport of two distinct C-tails to the PVC permits an extended analysis of protein trafficking in the TGN-endosome system.
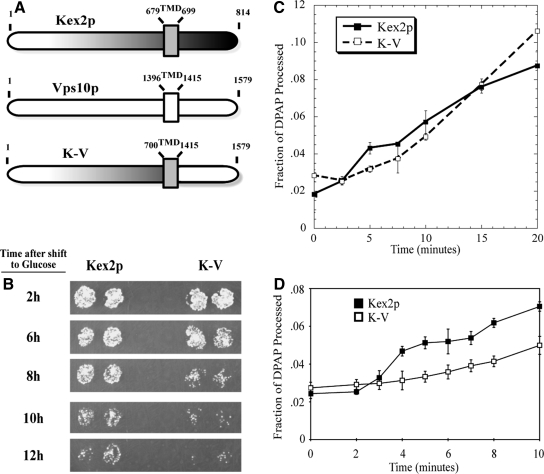
Figure 1.
The C-tails of Kex2p and Vps10p regulate the rate of transport between the TGN and the PVC. (A) Schematic depiction of the domain composition of Kex2p, Vps10p, and the chimeric protein K-V. The numbers above each construct designate the N- and C-terminal amino acids of lumenal, transmembrane, and cytosolic domains. TMD, transmembrane domain. (B) KRY24-2D (MATα kex2) cells expressing Kex2p or K-V under the control of the GAL1 promoter were shifted from galactose to glucose for the indicated times before testing mating competence as described in Materials and Methods. (C and D) Cell-free transport reactions were conducted as described in Materials and Methods by using MSS isolated from JBY209 transformed with pKEX2 (Kex2p; C and D) or transformed with pKEX2-VPS10 (K-V; C and D). Reactions were incubated at 30°C for the indicated times.
To compare trafficking of Kex2p and K-V in vivo, we measured the relative rates of exit of these proteins from the pro-α-factor processing compartment(s) (TGN and/or early endosome) using the onset of impotence assay, a well-characterized plate-based mating assay (Redding et al., 1996; Brickner and Fuller, 1997; Sipos et al., 2004). Patches of strain KRY24-2D (MATα kex2Δ), expressing wild-type Kex2p or K-V under GAL1 promoter control, were grown on galactose medium, shifted to glucose at various times to repress transcription, and then tested for their ability to mate with a MATa tester strain (Figure 1B). In this assay, as the concentration of Kex2p in the pro-α-factor processing compartment decreases below a level at which it becomes limiting for pro-α-factor cleavage, α-factor production, and thus mating efficiency, begins to decline as well (Wilcox et al., 1992). The mating efficiency of cells in which K-V expression was shut off began to decline after 8 h on glucose medium, with residual mating occurring at the 10- and 12-h time intervals. In contrast, cells in which Kex2p expression was shut off continued to mate at later times, with effective mating occurring into the 12-h time point. In comparable assays, cells containing Kex2p lacking the C-tail lose mating competence within 2–4 h (Redding et al., 1996). These results indicate that the Vps10 C-tail promotes localization of the Kex2p catalytic domain in the pro-α-factor processing compartment, but less efficiently than the Kex2p C-tail.
Next, we compared the function of the K-V chimera relative to wild-type Kex2p in cell-free transport. MSS containing Kex2 or K-V (Figure 1C) was incubated with MSS containing PSHA, and PSHA cleavage was measured as a function of time at 30°C. Cell-free processing of PSHA by K-V was time-dependent, with processing achieving a plateau at ∼20 min. Maximal cell-free processing by K-V was similar to that observed for Kex2p (∼10%). However, differences in the kinetics of transport were observed. The Kex2p reaction was biphasic, consisting of an initial rapid phase followed by a secondary phase that occurred after a short lag (this is best observed in the 0- to 10-min time course in Figure 1D). The K-V reaction exhibited a single phase, with PSHA processing occurring after an ∼10-min lag. Thus, the Kex2p and Vps10p C-tails confer distinct kinetics of cell-free transport, in line with their distinguishable effects on pro-α-factor processing in vivo.
Gga2p Is Required for Cell-free TGN-to-PVC Transport of Kex2p and K-V
The yeast S. cerevisiae contains two GGA genes, GGA1 and GGA2. Although deletion of either the GGA1 or GGA2 gene alone does not cause discernible sorting defects, cells lacking both GGA genes have been shown to exhibit several post-Golgi trafficking defects (Black and Pelham, 2000; Dell'Angelica et al., 2000; Hirst et al., 2000, 2001; Mullins and Bonifacino, 2001). For example, gga1Δ gga2Δ cells exhibit improper sorting of Vps10p, which binds both CPY and proteinase A as it cycles between the TGN and the PVC, and Kex2p, which also cycles between the TGN and endosomes and is required for the processing of pro-α-factor.
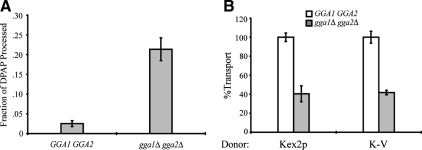
To study the role of the Gga proteins in Kex2p and K-V cell-free transport, a strain with a deletion of gga1Δ and gga2Δ was constructed. Previously, we found that deletion of several genes involved in vesicular transport resulted in elevated levels of Kex2p-independent cleavage of PSHA in vivo, preventing use of such strains in the cell-free reaction (our unpublished observation). To determine the level of in vivo PSHA cleavage in the gga1Δ gga2Δ background, MSS isolated from strains JBY209 (GGA1 GGA2) and MAY4 (gga1Δ gga2Δ) expressing the PSHA fusion protein was subjected to IP by using anti-HA antibody in the presence of 1% (vol/vol) Triton X-100. As shown in Figure 2A, deletion of gga1Δ gga2Δ resulted in in vivo PSHA cleavage well above the level in JBY209. Kex2p-independent cleavage of PSHA in gga null cells supports a role for the Gga proteins in Pep12p localization, consistent with previous reports that indicate a role for Gga proteins in the transport of Pep12p from the TGN to the PVC (Black and Pelham, 2000).
Figure 2.
Deletion of the GGAs results in increased PSHA processing and reduced cell-free transport of Kex2p and K-V. (A) PSHA is cleaved in vivo as a result of gga1 gga2 disruption. MSS samples prepared from JBY209 (kex2Δ GGA1+ GGA2+) and MAY8 (kex2Δ gga1Δ gga2Δ), both transformed with pPSHA, were incubated under transport reaction conditions and then subjected to IP with anti-HA antibody and mock IP in the presence of 1% Triton X-100. Supernatants were assayed for DPAP activity by using Ala-ProAMC (Brickner et al., 2001). The fraction of PSHA cleaved in vivo (i.e., lacking the HA epitopes) is the ratio of DPAP activity in the anti-HA IP to that in the mock IP. (B) Donor MSS were prepared from JBY209 (GGA1+ GGA2+) and MAY4 (gga1Δ gga2Δ) expressing Kex2p or K-V. Acceptor MSS were prepared from JBY209 (GGA1+ GGA2+) expressing PSHA. Cell-free transport reactions were then carried out (20 min at 30°C). Extents of transport observed for controls (GGA1+ GGA2+) were 9.2% JBY209 (Kex2p) and 10.3% JBY209 (K-V), adjusted to 100% in the figure. Experimental values (gga1Δ gga2Δ) are shown as percentage of the control values.
To test the role of the Gga proteins in cell-free transport, donor MSS from gga1Δ gga2Δ strains expressing either Kex2p or K-V and acceptor MSS from GGA1 GGA2 strains expressing PSHA were combined in transport reactions. Kex2p and K-V transport into the PVC was reduced by ∼60% in these reactions (Figure 2B). However, it was not clear whether the reduction in transport was due to the partial absence of Ggas in the cell-free reaction or to missorting of Kex2p and K-V in vivo.
To assess more directly whether Gga function was required during cell-free transport of Kex2p and K-V, we developed a strain with a disruption at the gga1 locus and an integrated 13 myc peptide tag at the C terminus of Gga2p (strain MAY17). This approach permits analysis of what seems to be the dominant Gga protein Gga2p (Costaguta et al., 2001). Gga2-13myc expression was confirmed by immunoblotting (Figure 3A). The function of Gga2-13myc in vivo was tested using the CPY filter immunoblot assay (Figure 3B). As expected, CPY was secreted by the gga1Δ gga2Δ strain (Vps− phenotype) (Figure 3B). In contrast, MAY17 was Vps+ and did not exhibit elevated CPY secretion, indicating that Gga2-13myc is functional in vivo.
MSS from strains expressing Gga2p or Gga2-13myc was used for cell-free transport of Kex2p and K-V into the PVC in the absence and presence of anti-c-myc antibody (Figure 3C). Anti-c-myc did not appreciably reduce transport in extracts prepared from Gga2p-expressing cells. However, in reactions containing MSS from Gga2-13myc expressing cells, Kex2p transport was reduced by ∼50% and K-V was reduced by >80%. IP analysis demonstrated that anti-c-myc was able to recognize Gga2p-13myc during the course of the reaction (Figure 3D). Because inhibition of Kex2p transport was incomplete, anti-c-myc was titrated into reactions containing Kex2p or K-V MSS, and dose–response curves were generated (Figure 3E). Inhibition of Kex2p transport reactions plateaued at ∼50%, suggesting that the residual transport represents a Gga2p-independent transport pathway for Kex2p. No plateau was observed in inhibition of K-V transport, suggesting a near complete dependence on Gga2p.
Dominant-Negative Gga2p VHS-GAT Inhibits Cell-free TGN-to-PVC Transport of Kex2p and K-V
Expression of a truncated form of human GGA1, consisting of the N-terminal VHS (VPS27, HRS, and STAM) and GAT (GGA and TOM1) domains, results in a dominant-negative phenotype, as measured by improper localization of the mannose-6-phosphate receptors (MPRs) (Puertollano et al., 2001). Based on this, we tested a similar VHS-GAT form of yeast Gga2p for dominant-negative activity both in vivo and in the cell-free assay. Gga2p VHS-GAT is predicted to be recruited to membranes by ARF-GTP and phosphatidylinositol 4-phosphate (PI4P) and bind cargo through its VHS domain, but to lack the elements required for the recruitment of clathrin and accessory proteins involved in vesicle formation, the hinge and C-terminal γ-adaptin ear domain (Bonifacino, 2004; Wang et al., 2007; Demmel et al., 2008).
To test the activity of Gga2p VHS-GAT in vivo, it was expressed under the control of constitutive and inducible promoters in wild-type cells. Expression from promoters with lower activity, the ADH promoter in a high copy number (2μ) plasmid and the GAL1 promoter in a low copy number (CEN ARS) plasmid, resulted in growth rates similar to wild-type cells. However, expression from 2μ vectors under the control of strong constitutive promoters (TEF and GPD) or the strong inducible GAL1 promoter resulted in a slow growth phenotype (Figure 4B). Given that deletion of two S. cerevisiae ARF genes, ARF1 and ARF2, is lethal (Stearns et al., 1990), it is likely that the slow growth phenotype observed upon high level expression of Gga2p VHS-GATp is a result of sequestration of Arf.
High-level expression of Gga2p VHS-GAT was then tested for its effect on CPY sorting. As shown in Figure 4C, expression of Gga2p VHS-GAT under ADH, TEF, or GPD promoter control led to secretion of CPY as measured by the colony immunoblot assay. Moreover, when Gga2p VHS-GAT was placed under the control of the inducible CUP1 promoter, growth on plates supplemented with 100 μM CuSO4 resulted in secretion of CPY (Figure 4D). Cellular extracts from strains expressing Gga2-13myc under the GGA2 promoter and Gga2p VHS-GAT-6X His under ADH, TEF, and GPD promoters or Gga2p GST-VHS-GAT under the CUP1 promoter were resolved by SDS-PAGE, analyzed by immunoblotting using anti-c-myc and anti-His or anti-GST, and ratios of antibody signals were measured as described in Materials and Methods. Gga2-13myc to Gga2p VHS-GAT antibody signal ratios for ADH, TEF, GPD, and CUP1 were 1:5.2, 1:7.8, 1:6.4, and 1:9.4, respectively (Figure 4D). Together, these results suggest that Gga2p VHS-GAT disrupts Vps10p transport between the TGN and the PVC in dominant-negative manner.
We then tested the ability of purified Gga2p VHS-GAT to inhibit cell-free transport of Kex2p and K-V into the PVC. Addition of bacterially expressed, purified Gga2p VHS-GAT (12 μg) to cell-free transport assays inhibited TGN-PVC transport reactions containing Kex2p donor membranes by ∼60%, but it inhibited reactions containing K-V donor membranes >90% (Figure 5A). Titration of Gga2p VHS-GAT protein showed that inhibition of Kex2p transport plateaued at ∼55% of control (Figure 5B). MSS containing Gga2-13myc expressed under the native GGA2 promoter and bacterially expressed, purified Gga2p VHS-GAT-6X His (12 μg) were resolved by SDS-PAGE, analyzed by immunoblotting using anti-c-myc and anti-His, and ratios of antibody signals were measured as described in Materials and Methods. Gga2-13myc to Gga2p VHS-GAT antibody signal ratios in cell-free transport assays were 1:13.7 (Kex2p) and 1:15 (K-V) (Figure 5C). These results indicate that the relative amount of Gga2p VHS-GAT required to inhibit TGN-PVC transport in vitro is comparable with the relative amount required for dominant-negative function within the cell as measured by CPY secretion.
Together, inhibition of transport by anti-c-myc in reactions containing Gga2-13myc and by the Gga2p dominant-negative VHS-GAT in reactions containing wild-type Gga2p provides direct biochemical evidence for a role of Ggas in transport of Kex2p and Vps10p to the PVC. The full requirement for Gga in K-V transport (>90% inhibition by Gga2p VHS-GAT) indicates that this class of adaptors is required for direct TGN to PVC transport. In the case of Kex2p; however, partial inhibition suggests that in the cell-free assay, Kex2p is delivered to the PVC by distinct Gga-dependent and Gga-independent trafficking pathways.
Given that reactions with Kex2p donor membranes exhibited an initial rapid phase not seen with K-V membranes, we sought to determine whether the initial rapid phase of Kex2p transport corresponded to the Gga2p-independent pathway. When a time course of TGN-PVC transport using MSS containing Kex2p was performed in the presence of purified Gga2p VHS-GAT (12 μg), only the first, rapid phase of the reaction was observed (Figure 5D). This result indicates that the early, rapid phase is Gga2p-independent. The second, slower phase resembles the time course with K-V donor membranes (Figure 1D) and is Gga2p dependent.
AP-1 Is Not Required for Direct TGN-to-PVC Transport
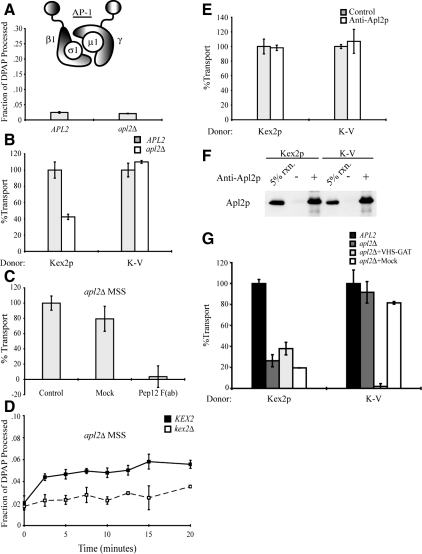
Yeast AP-1 is heterotetrameric complex consisting of large (γ and β1), medium (μ1), and small (σ1) subunits. In yeast, sequence comparison with mammalian adaptins, genetic interactions with chc1ts, and protein–protein interaction studies have established that Apl4p (γ), Apl2p (β1), Apm1p (μ1), and Aps1p (σ1) assemble to form AP-1 (Phan et al., 1994; Rad et al., 1995; Stepp et al., 1995; Yeung et al., 1999). It has also been shown that disruption of APL2 alone results in AP-1 mutant phenotypes with severity equivalent to that seen in cells that lack all four AP-1 subunits, suggesting a functional complex does not exist if APL2 is deleted (Yeung et al., 1999). The requirement for APL2 in complex assembly allows for the assessment of AP-1 function in cell-free transport by manipulation of a single locus.
To determine the level of in vivo PSHA cleavage in the apl2Δ background, MSS isolated from strains JBY209 (APL2) and MAY8 (apl2Δ) expressing the PSHA fusion protein was subjected to IP by using anti-HA antibody in the presence of 1% (vol/vol) Triton X-100. As shown in Figure 6A, deletion of APL2 did not result in increased cleavage of PSHA as had been observed in gga1Δ gga2Δ (Figure 2A).
Figure 6.
AP-1 is not required for cell-free TGN-to-PVC transport of Kex2p and K-V, but it is required for localization of Kex2p to the Gga-dependent donor compartment. (A) PSHA is not cleaved in vivo as a result of AP-1 disruption. MSS samples prepared from JBY209 (kex2Δ APL2+) and MAY8 (kex2Δ apl2Δ), both transformed with pPSHA, were incubated under transport reaction conditions then subjected to IP with anti-HA antibody and mock IP in the presence of 1% Triton X-100, and supernatants were assayed for DPAP activity by using Ala-ProAMC (Brickner et al., 2001). The fraction of PSHA cleaved in vivo (i.e., lacking the HA epitopes) is the ratio of DPAP activity in the anti-HA IP to that in the mock IP. (B) MSS were prepared from JBY209 (APL2+) and MAY8 (apl2Δ) expressing Kex2p, K-V, or PSHA. Cell-free transport reactions were then carried out (20 min at 30°C). Extents of transport observed for controls (APL2+) were 10.5% JBY209 (Kex2p), 9.7% JBY209 (K-V), adjusted to 100% in the figure. Experimental values (apl2Δ) are shown as percentage of the control values. (C) Residual Kex2p transport in apl2Δ MSS is dependent on Pep12p. Anti-Pep12p was subjected to papain cleavage to create F(ab) fragments against the cytosolic domain of Pep12p. A mock papain cleavage reaction (Mock) was performed to control for potential papain-dependent inhibition. Kex2p and PSHA MSS from strain MAY8 (apl2Δ) were combined and preincubated with 3.5 μg of anti-Pep12p F(ab), with a mock-purified sample (equivalent volume) or with no addition (control), for 1 h on ice. Cell-free transport reactions were then carried out (20 min at 30°C). Extent of transport observed for control [no Mock or anti-Pep12p F(ab)] was 6.2%, adjusted to 100% in the figure. Experimental values [Mock and anti-Pep12p F(ab)] are shown as percentage of the control values. (D) kex2Δ, KEX2+, or PSHA MSS from strain MAY8 (apl2Δ) were combined and a time course of cell-free transport was then measured as Figure 1C. (E) MSS was prepared from strain JBY209 (APL2) expressing Kex2p, K-V or PSHA. Donor and acceptor MSS samples were combined and preincubated with 14 μg of anti-Apl2p antibody or no antibody (control) for 1 h on ice. Cell-free transport reactions were then carried out (20 min at 30°C). Extents of transport observed for controls (no anti-Apl2p) were 12.4% JBY209 (Kex2p), 11.8% JBY209 (K-V), adjusted to 100% in the figure. Experimental values (+ anti-Apl2p) are shown as percentage of the control values. (F) Affinity-purified anti-Apl2p recognizes Apl2p during the course of the reaction. An aliquot of each reaction shown in E was incubated with protein A-Sepharose for 1 h at 4°C. Protein A-Sepharose pellets were subsequently washed and solubilized in SDS-PAGE sample buffer. Eluted samples were resolved by SDS-PAGE and analyzed by immunoblotting using anti-Apl2p. (G) Residual Kex2p transport in reactions containing donor and acceptor MSS from MAY8 (apl2Δ) is resistant to inhibition by purified Gga2 VHS-GAT. MSS were prepared from JBY209 (APL2+) and MAY8 (apl2Δ) expressing Kex2p, K-V, or PSHA. Donor and acceptor MSS samples from MAY8 (apl2Δ) were combined and preincubated with 12 μg of purified Gga2p VHS-GAT, with a mock-purified sample (equivalent volume) or with no addition, for 1 h on ice. Donor and acceptor MSS samples from JBY209 (APL2+) were combined and preincubated with no addition (control). Cell-free transport reactions were then carried out (20 min at 30°C). Extents of transport observed for the controls (APL2+, no addition) were 9.9% JBY209 (Kex2p), 10.1% JBY209 (K-V), adjusted to 100% in the figure. Reactions containing MSS from apl2Δ are shown as percentage of the control values.
To test the role of AP-1 in cell-free transport, MSS from apl2Δ strains expressing PSHA and either Kex2p or K-V were combined in transport reactions. In the apl2Δ reactions, Kex2p transport was diminished 60–80%, but K-V transport was unaffected compared with APL2+ controls (Figure 6, B and G). These results indicate that cell-free transport of K-V from the TGN to the PVC is independent of AP-1 and suggest, further, that localization of K-V in donor membranes was not altered in vivo in the apl2Δ strain.
To determine whether residual PSHA cleavage seen with Kex2p-containing MSS from apl2Δ cells represents transport into the PVC, we assayed for inhibition using F(ab) fragments of a monoclonal anti-Pep12p antibody. By phenotypic, structural, and sequence analysis, Pep12p is the likely heavy chain soluble N-ethylmaleimide-sensitive factor attachment protein receptor of the PVC and mediates the fusion of incoming vesicular traffic into this compartment (Becherer et al., 1996; Black and Pelham, 2000; Gerrard et al., 2000). Figure 6C illustrates that the addition of anti-Pep12p F(ab) reduced transport by >95%. We conclude that Pep12p function is required for the cell-free delivery of Kex2p into PSHA-containing compartments in apl2Δ MSS and that residual Kex2p transport in apl2Δ MSS represents Kex2p transport into the PVC.
Although Kex2p transport into the PVC was reduced in apl2Δ MSS, it was not clear whether reduction in transport was due to the absence of AP-1 function in the cell-free reaction or to missorting of Kex2p in vivo. The latter possibility would be consistent with studies demonstrating effects of AP-1 mutations both on Kex2-dependent processing in chc1ts cells and on trafficking of other marker proteins between the TGN and early endosome (Rad et al., 1995; Stepp et al., 1995; Valdivia et al., 2002; Foote and Nothwehr, 2006). Further characterization of the AP-1–sensitive pathway in apl2Δ MSS also permits an analysis of the role of AP-1 in anterograde traffic from the TGN/early endosome into the PVC; such a role for the AP-1 adaptor has remained elusive.
Given that reactions with Kex2p donor membranes exhibited an initial rapid phase not seen with K-V membranes (Figure 1, C and D), we sought to determine whether the initial rapid phase of Kex2p transport corresponded to an apl2Δ-independent pathway. When a time course of TGN-PVC transport using MSS from apl2Δ strains expressing Kex2p and PSHA was performed, only the first, rapid phase of the reaction was observed (Figure 6D). This result indicates that the early, rapid phase of Kex2p transport into the PVC is AP-1 independent.
As a direct test of a requirement for AP-1 function during the cell-free reaction, we determined the effect of adding an affinity-purified antibody against Apl2p. Even though this antibody recognized Apl2p when incubated with MSS under transport reaction conditions, addition of 14 μg of antibody had no effect on either Kex2p or K-V transport (Figure 6, E and F).
To extend this analysis, the residual PSHA cleavage seen with Kex2p-containing MSS from apl2Δ cells was assayed for inhibition by Gga2p VHS-GAT. Addition of Gga2p VHS-GAT had no significant effect on the residual Kex2p transport in reactions with apl2Δ MSS (Figure 5F). In contrast, addition of the Gga2p VHS-GAT inhibited transport reactions with K-V–containing MSS from apl2Δ cells to the same degree as seen with K-V–containing MSS from APL2+ cells (compare Figures 5A and 6F). These results indicate that the effect of apl2Δ on transport of Kex2p in the cell-free assay is an indirect consequence of Kex2p mislocalization in vivo and that AP-1 plays no direct role in cell-free transport of Kex2p from the TGN to the PVC. Conversely, apl2Δ has no effect on either the localization of K-V in vivo or on cell-free transport of K-V.
DISCUSSION
In the TGN–endosomal system, the identity of the multiple compartments (TGN, early and late endosomes) depends on their dynamic communication through cycles of fusion, fission, and vesicular trafficking. Interference with an individual transport step in vivo can have direct and indirect effects on both protein trafficking and organellar identity. Reconstitution of vesicular transport pathways in cell-free systems can resolve these difficulties by permitting direct biochemical analysis of individual transport steps. Sorting and trafficking of transmembrane proteins from the TGN to PVC is one step in a cycle of transport events required for both localization of proteins to the TGN and lysosomal/vacuolar biogenesis. Cell-free reconstitution of trafficking of Kex2p from the TGN to the PVC is authenticated by its dependence on a set of proteins implicated by genetic and cell-based studies; use of the K-V chimera in this system expands its utility by permitting analysis of Vps10p sorting as well (Blanchette et al., 2004; Abazeed et al., 2005).
Transport of Vps10p from the TGN to the PVC seems to follow a direct pathway that does not include transit of early endosomal compartments (Ha et al., 2003; Sipos et al., 2004). Kex2p, in contrast, seems to follow a more complex itinerary, partitioning between the direct pathway followed by Vps10p and an indirect pathway that does traverse the early endosome (Sipos et al., 2004). Consistent with these considerations, we have shown here that although membranes containing Kex2p and the K-V chimera are both active in the cell-free transport reaction, reactions containing Kex2p and K-V donor membranes exhibit clear differences in kinetics and adaptor protein requirements.
Cell-free transport reactions using K-V donor membranes were completely dependent on Gga2p function, as judged by antibody inhibition and by sensitivity to addition of a Gga2p dominant-negative protein, Gga2p VHS-GAT. Conversely, K-V reactions were unaffected by deletion of the APL2 gene and resistant to anti-Apl2p antibody. These results are further corroborated by the observation that the residual reaction seen with Gga− (ggaΔ) donor membranes containing K-V and Gga+ acceptor membranes containing PSHA was largely inhibited by addition of either anti-Pep12p F(ab) antibodies or purified Gga2p VHS-GAT but insensitive to anti-Apl2p antibodies (Supplemental Figure S1). Thus, with K-V serving as a proxy for Vps10p, these results provide a direct, biochemical demonstration that the pathway of transport of Vps10p from the TGN to the PVC is Gga dependent and AP-1 independent.
Transport reactions using Kex2p donor membranes were more complex. Kex2p transport exhibited a Gga-dependent component that corresponds kinetically to K-V transport and thus reflects the direct TGN–PVC pathway. Reactions using Kex2p-containing MSS from an apl2Δ strain were significantly reduced in extent; required Pep12p for delivery of Kex2p into PSHA-containing compartments; displayed kinetics corresponding to the early, rapid phase of Kex2p transport; and were resistant to the Gga2p dominant-negative protein. These results indicate that Kex2p localizes to an additional compartment, one that that does not require AP-1 or Gga for entry into the PVC. This compartment is likely to be the early endosome. Evidence that AP-1 is required for transport of both Chs3p and Ste13p to the TGN from the early endosome also supports the conclusion that this compartment is the early endosome (Valdivia et al., 2002; Foote and Nothwehr, 2006). Thus, the Gga-independent component of Kex2p transport in reactions using APL2+ MSS presumably reflects a step in delivery of Kex2p from the early endosome to the PVC.
The Gga-independent component of Kex2p transport corresponded to a rapid kinetic phase, suggesting a relatively simple fusion reaction. In contrast, Gga-dependent transport of K-V and the Gga-dependent component of Kex2p transport exhibited a kinetic lag that may reflect more complex molecular events involved in sorting and the formation of a transport intermediate. The nature of the sorting events and the putative transport intermediate remain to be identified.
Previous studies have suggested that GGA and AP-1 function in parallel to package cargo molecules into clathrin-containing carriers at the TGN. Indeed, binding studies reveal an interaction between GGA and AP-1 in both mammalian and yeast cells (Costaguta et al., 2001; Doray et al., 2002). The colocalization of GGA and AP-1 in clathrin-coated buds of the TGN and the lack of MPR defective in GGA binding in AP-1–positive clathrin-coated vesicles suggested a model in which GGA and AP-1 cooperate to mediate cargo transport at the TGN in mammals (Doray et al., 2002). Here, we show that AP-1 is not required for direct TGN-to-PVC transport of either Kex2p or Vps10p (i.e., K-V), indicating that the interaction of AP-1 and Gga cannot be required for transport of these two cargo molecules from the TGN to the PVC.
On the basis of the experiments presented and by analogy to mammalian GGA function, the yeast Ggas are expected to interact with the C-tails of Kex2p and Vps10p. Human GGAs have been shown to bind directly to sequence elements (acidic dileucine motif-DXXLL) found in the C-tail of the cation-dependent and -independent MPRs (Puertollano et al., 2001; Takatsu et al., 2001; Zhu et al., 2001) as well as to a distinct sequence element in sortilin (sorLA) (Jacobsen et al., 2002). No interactions have yet been reported between Ggas and C-tails of TGN-localized membrane proteins in yeast. Vps10p and Kex2p do not have canonical DXXLL signals, nor are the VHS residues involved in interaction with the MPR conserved in Gga1/2p (Misra et al., 2002; Shiba et al., 2003). This suggests that the yeast Ggas are likely to function through recognition of a different type of sorting signal(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank G. S. Payne (University of California, Los Angeles, CA) and S. D. Emr (Cornell University, Ithaca, NY) for antibodies, plasmids, and strains; J. H. Brickner for construction of the K-V chimera; and members of the Fuller laboratory for helpful comments on the manuscript. This work was supported in part by National Institutes of Health grants GM-50915 and GM-39697 (to R.S.F.), University of Michigan Medical Scientist Training Program grant GM-0786 (to M.E.A.), Genetics Training Program grant GM-07544 (to M.E.A.), a University of Michigan Rackham Graduate School predoctoral fellowship (to M.E.A.), and P30 CA46592 to the University of Michigan Comprehensive Cancer Center.
Abbreviations used:
- aa
amino acid
- ALP
alkaline phosphatase
- AP-1
adaptor protein-1
- C-tail
cytosolic tail
- CPY
carboxypeptidase Y
- DPAP
dipeptidyl aminopeptidase
- GAT
GGA and TOM1
- GGA
Golgi-localized, γ-ear-containing, ADP-ribosylation factor binding protein
- GST
glutathione transferase
- HA
hemagglutinin
- IP
immunoprecipitation
- K-V
Kex2-Vps10p chimera
- MPR
mannose-6-phosphate receptor
- MSS
medium-speed supernatant
- PI4P
phosphatidylinositol 4-phosphate
- PSHA
Pep12Ste13αHA fusion substrate
- PVC
prevacuolar compartment
- TGN
trans-Golgi network
- VHS
Vps27, Hrs, and Stam.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-05-0442) on September 10, 2008.
REFERENCES
- Abazeed M. E., Blanchette J. M., Fuller R. S. Cell-free transport from the trans-Golgi network to late endosome requires factors involved in formation and consumption of clathrin-coated vesicles. J. Biol. Chem. 2005;280:4442–4450. doi: 10.1074/jbc.M412553200. [DOI] [PubMed] [Google Scholar]
- Becherer K. A., Rieder S. E., Emr S. D., Jones E. W. Novel syntaxin homologue, Pep12p, required for the sorting of lumenal hydrolases to the lysosome-like vacuole in yeast. Mol. Biol. Cell. 1996;7:579–594. doi: 10.1091/mbc.7.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M. W., Pelham H. R. A selective transport route from Golgi to late endosomes that requires the yeast GGA proteins. J. Cell Biol. 2000;151:587–600. doi: 10.1083/jcb.151.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette J. M., Abazeed M. E., Fuller R. S. Cell-free reconstitution of transport from the trans-Golgi network to the late endosome/prevacuolar compartment. J. Biol. Chem. 2004;279:48767–48773. doi: 10.1074/jbc.M406368200. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S. The GGA proteins: adaptors on the move. Nat. Rev. Mol. Cell Biol. 2004;5:23–32. doi: 10.1038/nrm1279. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Glick B. S. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- Brickner J. H., Blanchette J. M., Sipos G., Fuller R. S. The Tlg SNARE complex is required for TGN homotypic fusion. J. Cell Biol. 2001;155:969–978. doi: 10.1083/jcb.200104093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickner J. H., Fuller R. S. SOI1 encodes a novel, conserved protein that promotes TGN-endosomal cycling of Kex2p and other membrane proteins by modulating the function of two TGN localization signals. J. Cell Biol. 1997;139:23–36. doi: 10.1083/jcb.139.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Dawson D., Stearns T., editors. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Cereghino J. L., Marcusson E. G., Emr S. D. The cytoplasmic tail domain of the vacuolar protein sorting receptor Vps10p and a subset of VPS gene products regulate receptor stability, function, and localization. Mol. Biol. Cell. 1995;6:1089–1102. doi: 10.1091/mbc.6.9.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. A., Stevens T. H. Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J. Cell Biol. 1996;133:529–541. doi: 10.1083/jcb.133.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costaguta G., Stefan C. J., Bensen E. S., Emr S. D., Payne G. S. Yeast Gga coat proteins function with clathrin in Golgi to endosome transport. Mol. Biol. Cell. 2001;12:1885–1896. doi: 10.1091/mbc.12.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Angelica E. C., Puertollano R., Mullins C., Aguilar R. C., Vargas J. D., Hartnell L. M., Bonifacino J. S. GGAs: a family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex. J. Cell Biol. 2000;149:81–94. doi: 10.1083/jcb.149.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloche O., Yeung B. G., Payne G. S., Schekman R. Vps10p transport from the trans-Golgi network to the endosome is mediated by clathrin-coated vesicles. Mol. Biol. Cell. 2001;12:475–485. doi: 10.1091/mbc.12.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmel L., et al. The clathrin adaptor Gga2p is a phosphatidylinositol 4-phosphate effector at the Golgi exit. Mol. Biol. Cell. 2008;19:1991–2002. doi: 10.1091/mbc.E06-10-0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doray B., Ghosh P., Griffith J., Geuze H. J., Kornfeld S. Cooperation of GGAs and AP-1 in packaging MPRs at the trans-Golgi network. Science. 2002;297:1700–1703. doi: 10.1126/science.1075327. [DOI] [PubMed] [Google Scholar]
- Foote C., Nothwehr S. F. The clathrin adaptor complex 1 directly binds to a sorting signal in Ste13p to reduce the rate of its trafficking to the late endosome of yeast. J. Cell Biol. 2006;173:615–626. doi: 10.1083/jcb.200510161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard S. R., Levi B. P., Stevens T. H. Pep12p is a multifunctional yeast syntaxin that controls entry of biosynthetic, endocytic and retrograde traffic into the prevacuolar compartment. Traffic. 2000;1:259–269. doi: 10.1034/j.1600-0854.2000.010308.x. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Gueldener U., Heinisch J., Koehler G. J., Voss D., Hegemann J. H. A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic Acids Res. 2002;30:e23. doi: 10.1093/nar/30.6.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha S. A., Torabinejad J., DeWald D. B., Wenk M. R., Lucast L., De Camilli P., Newitt R. A., Aebersold R., Nothwehr S. F. The synaptojanin-like protein Inp53/Sjl3 functions with clathrin in a yeast TGN-to-endosome pathway distinct from the GGA protein-dependent pathway. Mol. Biol. Cell. 2003;14:1319–1333. doi: 10.1091/mbc.E02-10-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- Hinners I., Tooze S. A. Changing directions: clathrin-mediated transport between the Golgi and endosomes. J. Cell Sci. 2003;116:763–771. doi: 10.1242/jcs.00270. [DOI] [PubMed] [Google Scholar]
- Hirst J., Lindsay M. R., Robinson M. S. GGAs: roles of the different domains and comparison with AP-1 and clathrin. Mol. Biol. Cell. 2001;12:3573–3588. doi: 10.1091/mbc.12.11.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J., Lui W. W., Bright N. A., Totty N., Seaman M. N., Robinson M. S. A family of proteins with gamma-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome. J. Cell Biol. 2000;149:67–80. doi: 10.1083/jcb.149.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen L., Madsen P., Nielsen M. S., Geraerts W. P., Gliemann J., Smit A. B., Petersen C. M. The sorLA cytoplasmic domain interacts with GGA1 and -2 and defines minimum requirements for GGA binding. FEBS Lett. 2002;511:155–158. doi: 10.1016/s0014-5793(01)03299-9. [DOI] [PubMed] [Google Scholar]
- Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Misra S., Puertollano R., Kato Y., Bonifacino J. S., Hurley J. H. Structural basis for acidic-cluster-dileucine sorting-signal recognition by VHS domains. Nature. 2002;415:933–937. doi: 10.1038/415933a. [DOI] [PubMed] [Google Scholar]
- Mullins C., Bonifacino J. S. The molecular machinery for lysosome biogenesis. Bioessays. 2001;23:333–343. doi: 10.1002/bies.1048. [DOI] [PubMed] [Google Scholar]
- Mumberg D., Muller R., Funk M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene. 1995;156:119–122. doi: 10.1016/0378-1119(95)00037-7. [DOI] [PubMed] [Google Scholar]
- Owen D. J., Collins B. M., Evans P. R. Adaptors for clathrin coats: structure and function. Annu. Rev. Cell Dev. Biol. 2004;20:153–191. doi: 10.1146/annurev.cellbio.20.010403.104543. [DOI] [PubMed] [Google Scholar]
- Phan H. L., Finlay J. A., Chu D. S., Tan P. K., Kirchhausen T., Payne G. S. The Saccharomyces cerevisiae APS1 gene encodes a homolog of the small subunit of the mammalian clathrin AP-1 complex: evidence for functional interaction with clathrin at the Golgi complex. EMBO J. 1994;13:1706–1717. doi: 10.1002/j.1460-2075.1994.tb06435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puertollano R., Aguilar R. C., Gorshkova I., Crouch R. J., Bonifacino J. S. Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science. 2001;292:1712–1716. doi: 10.1126/science.1060750. [DOI] [PubMed] [Google Scholar]
- Rad M. R., Phan H. L., Kirchrath L., Tan P. K., Kirchhausen T., Hollenberg C. P., Payne G. S. Saccharomyces cerevisiae Apl2p, a homologue of the mammalian clathrin AP beta subunit, plays a role in clathrin-dependent Golgi functions. J. Cell Sci. 1995;108:1605–1615. doi: 10.1242/jcs.108.4.1605. [DOI] [PubMed] [Google Scholar]
- Redding K., Brickner J. H., Marschall L. G., Nichols J. W., Fuller R. S. Allele-specific suppression of a defective trans-Golgi network (TGN) localization signal in Kex2p identifies three genes involved in localization of TGN transmembrane proteins. Mol. Cell. Biol. 1996;16:6208–6217. doi: 10.1128/mcb.16.11.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding K., Holcomb C., Fuller R. S. Immunolocalization of Kex2 protease identifies a putative late Golgi compartment in the yeast Saccharomyces cerevisiae. J. Cell Biol. 1991;113:527–538. doi: 10.1083/jcb.113.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. H., Hunter C. P., Valls L. A., Stevens T. H. Overproduction-induced mislocalization of a yeast vacuolar protein allows isolation of its structural gene. Proc. Natl. Acad. Sci. USA. 1986;83:3248–3252. doi: 10.1073/pnas.83.10.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba T., Kawasaki M., Takatsu H., Nogi T., Matsugaki N., Igarashi N., Suzuki M., Kato R., Nakayama K., Wakatsuki S. Molecular mechanism of membrane recruitment of GGA by ARF in lysosomal protein transport. Nat. Struct. Biol. 2003;10:386–393. doi: 10.1038/nsb920. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipos G., Brickner J. H., Brace E. J., Chen L., Rambourg A., Kepes F., Fuller R. S. Soi3p/Rav1p functions at the early endosome to regulate endocytic trafficking to the vacuole and localization of trans-Golgi network transmembrane proteins. Mol. Biol. Cell. 2004;15:3196–3209. doi: 10.1091/mbc.E03-10-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T., Kahn R. A., Botstein D., Hoyt M. A. ADP ribosylation factor is an essential protein in Saccharomyces cerevisiae and is encoded by two genes. Mol. Cell. Biol. 1990;10:6690–6699. doi: 10.1128/mcb.10.12.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp J. D., Pellicena-Palle A., Hamilton S., Kirchhausen T., Lemmon S. K. A late Golgi sorting function for Saccharomyces cerevisiae Apm1p, but not for Apm2p, a second yeast clathrin AP medium chain-related protein. Mol. Biol. Cell. 1995;6:41–58. doi: 10.1091/mbc.6.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu H., Katoh Y., Shiba Y., Nakayama K. Golgi-localizing, gamma-adaptin ear homology domain, ADP-ribosylation factor-binding (GGA) proteins interact with acidic dileucine sequences within the cytoplasmic domains of sorting receptors through their Vps27p/Hrs/STAM (VHS) domains. J. Biol. Chem. 2001;276:28541–28545. doi: 10.1074/jbc.C100218200. [DOI] [PubMed] [Google Scholar]
- Valdivia R. H., Baggott D., Chuang J. S., Schekman R. W. The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev. Cell. 2002;2:283–294. doi: 10.1016/s1534-5807(02)00127-2. [DOI] [PubMed] [Google Scholar]
- Wang J., Sun H. Q., Macia E., Kirchhausen T., Watson H., Bonifacino J. S., Yin H. L. PI4P promotes the recruitment of the GGA adaptor proteins to the trans-Golgi network and regulates their recognition of the ubiquitin sorting signal. Mol. Biol. Cell. 2007;18:2646–2655. doi: 10.1091/mbc.E06-10-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A. C., Castelli L. A., Macreadie I. G., Azad A. A. Vectors for Cu(2+)-inducible production of glutathione S-transferase-fusion proteins for single-step purification from yeast. Yeast. 1994;10:441–449. doi: 10.1002/yea.320100403. [DOI] [PubMed] [Google Scholar]
- Wilcox C. A., Redding K., Wright R., Fuller R. S. Mutation of a tyrosine localization signal in the cytosolic tail of yeast Kex2 protease disrupts Golgi retention and results in default transport to the vacuole. Mol. Biol. Cell. 1992;3:1353–1371. doi: 10.1091/mbc.3.12.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung B. G., Phan H. L., Payne G. S. Adaptor complex-independent clathrin function in yeast. Mol. Biol. Cell. 1999;10:3643–3659. doi: 10.1091/mbc.10.11.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Doray B., Poussu A., Lehto V. P., Kornfeld S. Binding of GGA2 to the lysosomal enzyme sorting motif of the mannose 6-phosphate receptor. Science. 2001;292:1716–1718. doi: 10.1126/science.1060896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.