Abstract
Analysis of the polar lipids of Toxoplasma gondii by electrospray ionization tandem mass spectrometry provides a detailed picture of the lipid molecular species of this parasitic protozoan. Most notably, T. gondii contains a relatively high level, estimated to about 2% of the total polar lipid, of ceramide phosphoethanolamine. The ceramide phosphoethanolamine has a fatty amide profile with only 16- and 18-carbon species. Compared with the host fibroblasts in which it was grown, T. gondii also has higher levels of phosphatidylcholine, but lower levels of sphingomyelin and phosphatidylserine. Analysis at the molecular species level indicated that T. gondii has greater amounts of shorter-chain fatty acid in its polar lipid molecular species than the host fibroblasts. Shorter-chain fatty acids with a combined total of 30 or fewer acyl carbons make up 21% of Toxoplasma’s, but only 3% of the host’s, diacyl phosphatidylcholine. Furthermore, diacyl phosphatidylcholine with two saturated acyl chains with 12, 14, or 16 carbons make up over 11% of parasite phosphatidylcholine, but less than 3% of the host phosphatidylcholine molecular species. The distinctive T. gondii tachyzoite lipid profile may be particularly suited to the function of parasitic membranes and the interaction of the parasite with the host cell and the host’s immune system. Combined with T. gondii genomic data, these lipidomic data will assist in elucidation of metabolic pathways for lipid biosynthesis in this important human pathogen.
Toxoplasma gondii infects 2–3 billion people throughout the world and is known to cause diseases that impair neurologic function and sight. A T. gondii infection can be life-threatening to those who are immunologically immature or immunologically impaired by AIDS, cancer, organ transplantation, or their therapy, and, in some instances, to persons without known immune compromise.
Potential differences in the lipid metabolism of T. gondii and mammalian hosts may provide promising targets for therapeutic drugs. Thus far, manipulation of fatty acid synthesis (1–5), phosphatidylcholine metabolism (6), and sphingolipid synthesis (7) have been shown to affect tachyzoite growth and survival. The origin of fatty acids for synthesis of acyl lipids has been intensely investigated by molecular and metabolic labeling approaches. The apicoplast harbors an active fatty acid synthase of type II (FASII) (1, 2, 8–11, 13). T. gondii also contains a cytosolic fatty acid synthase of type I (FAS I) and fatty acyl-elongases (FAEs) (1, 5, 12). Based on metabolic labeling experiments, Bisanz et al. (13) showed that in free T. gondii, de novo fatty acid synthesis was one of the sources for the acyl moiety of glycerolipids. Since acyl lipid labeling is abolished by treatment with haloxyfop, a reported inhibitor of the apicoplast acetyl CoA carboxylase, Bisanz et al. (13) concluded that an active FAS II was critical for the bulk of the acyl lipid synthesis. Combining conditional mutant analyses and metabolic labeling, Mazumdar et al. (5) demonstrated that FAS II was critical for the biogenesis of the apicoplast itself, and subsequently for parasite survival, but was unlikely the source of the acyl moiety of the bulk of acyl lipids. Rather, most glycerolipids from free T. gondii cells appears to be produced using acyl chains generated by FAS I or FAEs, which are resistant to the FAS II inhibitor thiolactomycin, but sensitive to the general FAS inhibitor cerulenin (5). Together, these analyses highlight the importance [1] of the parasite FAS for bulk acyl lipid syntheses in free stages and [2] of the FAS II activity for the apicoplast biogenesis. Bisanz et al. (13) surveyed lipid synthesizing gene candidates in T. gondii, and identified a glycerol-phosphate acyltransferase and a lysophosphatidate acyltransferase (phosphatidic acid synthase) that are predicted to be localized in the apicoplast, supporting the notion that fatty acids produced by FAS II are important for the organelle membrane expansion. Upon invasion, in spite of its autonomous capacity to synthesize its major membrane acyl lipids, T. gondii massively scavenges host cell lipid precursors for membrane biogenesis (6, 13, 14). Information about T. gondii lipids and their biosynthetic machinery and about the relationship between parasite and host cell lipids is still incomplete. Herein, analysis of the polar lipids of T. gondii tachyzoites (clonal type 1 RH) is described. Lipidomic analysis results in unprecedented compositional detail that provides new insight into the differences between host and parasite lipids. This information will facilitate metabolic reconstruction of lipid synthesis and metabolism in T. gondii following completion of genome sequence annotation.
MATERIALS AND METHODS
Toxoplasma cell pellet preparation
The RH strain of T. gondii was maintained by serial passage in human foreskin fibroblast (HFF) (15) that were grown to a confluent monolayer in glass Petri dishes (150 mm diameter). Cells were cultured in IMDM supplemented with 10% fetal calf serum (FCS) inactivated at 56°C for 1 hour, 1 IU of penicillin/ml, 100 μg/ml of streptomycin, 0.25 μg/ml of amphotericin B, and 10 mM L-glutamine at 37°C with 5% CO2. On day 4 of infection, tachyzoites were harvested from HFF cells by scraping the monolayer. The cell suspension was passed through a 25-gauge needle twice and a 27-gauge needle once. The cell suspension was then passed through a 3.0 micron filter (removing any HFF cells), before centrifuging at 1500 rpm (500 × g) for 15 min. The supernatant was removed and the parasite pellet was frozen and stored at −70°C.
Fibroblast preparation
Human foreskin fibroblasts (HFF) that were not infected were analyzed for comparison. They were grown to a confluent monolayer in glass Petri dishes (150 mm diameter) in IMDM supplemented with 10% FCS inactivated at 56°C for 1 hour, 1 IU of penicillin/ml, 100 μg/ml of streptomycin, 0.25 μg/ml of amphotericin B, and 10 mM L-glutamine at 37°C with 5% CO2. The cells were obtained from the Petri dish by scraping the monolayer and centrifuging at 1500 rpm (500 × g) for 15 min. The supernatant was removed and the cell pellet was frozen and stored at −70°C.
Lipid extraction
To 0.8 ml cells, 1 ml chloroform and 2 ml methanol were added. The sample was shaken, and 1 ml chloroform and 1 ml water were added. The samples were again shaken and centrifuged at approximately 1500 × g for 5 min. The lower phase was removed. One part chloroform was added, the mixture was shaken again and centrifuged and again the lower phase was removed. This was repeated, and the combined lower phases were washed once with 0.4 ml 1 M KCl and once with 0.4 ml water. The solvent was evaporated, and the sample was dissolved in 1 ml chloroform.
Lipidomics
An automated electrospray ionization-tandem mass spectrometry approach was used and data acquisition and analysis and acyl group identification were carried out as described previously (16, 17) with some modifications. An aliquot of extract (0.040 ml) was taken for mass spectrometry analysis. Internal standards were added, the solvent was evaporated, and the lipid was redissolved in chloroform/methanol/300 mM ammonium acetate in water (300/665/35) with a final volume of 1 mL. Internal standards, obtained and quantified as previously described (16), were 0.063 nmol di14:0 PC, 0.054 nmol di24:1 PC, 0.066 nmol 13:0 lyso PC, 0.066 nmol 19:0 lyso PC, 0.038 nmol di14:0 PE, 0.031 nmol di24:1 PE, 0.039 nmol 14:0 lyso PE, 0.034 nmol 18:0 lyso PE, 0.030 nmol di14:0 PA, 0.032 nmol di20:0(phytanoyl)-PA, 0.023 nmol di14:0 PS, 0.023 nmol di20:0(phytanoyl) PS, 0.015 nmol 16:0–18:0 PI, and 0.007 nmol di18:0 PI, 0.196 nmol d18:1/14:0 Cer, 0.155 nmol d18:1/12:0 Hex-cer and 0.124 nmol d18:1/12:0 Dihex-cer.
Unfractionated lipid extracts were introduced by continuous infusion into an Applied Biosystems 4000 Q-TRAP (MDS Sciex, Ontario, Canada). Samples were introduced using an automated nanospray chip ion source Advion TriVersa NanoMate (Advion BioSciences, Ithaca, NY) at a flow rate of 0.11 μL/min. Ionization voltage was set to 1.8 kV or −1.8 and gas pressure to 0.1 psi and the source was controlled by ChipSoft 7.1.1 software. The collision gas pressure was set at 2 (arbitrary units) for phospholipids, 1 for glycosyldiacylglycerols, and 5 for Cer, Hex-cer, and Dihex-cer. The collision energies, with nitrogen in the collision cell, were 28 V for PE, 40 V for PC and SM, 47 V for Cer, Hex-cer, and Dihex-cer, −58 V for PI, −57 V for PA, and −34 V for PS. Declustering potentials were 100 V for PE, SM, PC, Cer, Hex-cer, and Dihex-cer and −100 V for PA, PI, and PS. Entrance potentials were 15 V for PE, 14 V for PC and SM, 10 V for Cer, Hex-cer, and Dihex-cer, and −10 V for PI and PA, and PS. Exit potentials were 11 V for PE, 14 V for PC, 15V for Cer, Hex-cer, and Dihex-cer, −15 V for PI, −14 V for PA, and −13 V for PS. The mass analyzers were adjusted to a resolution of 0.7 amu full width at half height. For each spectrum, 9 to 150 continuum scans were averaged in multiple channel analyzer (MCA) mode. The source temperature (heated nebulizer) was 40°C, the curtain gas was set at 10 (arbitrary units), and the two ion source gases were turned off.
Lipid species were detected, using the scans previously described, including neutral loss of 87 in the negative mode for PS, and additionally using precursor of 264.2 (sphingosine) in the positive mode for Cer, Hex-cer, and Dihex-cer (16, 18, 19). Sequential precursor and neutral loss scans of the extracts produce a series of spectra with each spectrum revealing a set of lipid species containing a common fragment. The specificity of precursor scan of 264.2 was confirmed using a neutral loss scan of 162.2 (mono-hexose) and 342.2 (di-hexose) for Hex-cer and Dihex-cer respectively; only peaks observed in both sphingosine and sugar scans were used for quantification. Two internal standards were used for quantification, except for Cer, Hex-cer, and Dihex-cer, for which one internal standard was used. Sphingomyelin was determined from the same mass spectrum as PC (precursors of m/z 184 in positive mode) (18–20) and by comparison with PC internal standards using a molar response factor for sphingomyelin (in comparison to PC) determined experimentally to be 0.36. PC 28:0 was determined in the absence of added internal standards by comparison with another naturally occurring PC species, which was determined in comparison to internal standards. Ceramide phosphoethanolamine was determined in a similar fashion to sphingomyelin, from the same mass spectrum as PE (neutral loss of m/z 141) and by comparison with PE internal standards. Because no authentic compound was available, no response factor for PE-cer was determined nor employed. The background of each spectrum was subtracted, the data were smoothed, and peak areas integrated using a custom script and Applied Biosystems Analyst software. Isotopic overlap corrections were applied, and the lipids in each class were quantified in comparison to the two internal standards of that class using corrected curves determined for the API 4000 mass spectrometer.
Acyl group identification and determination of ceramide phosphoethanolamine structure
The acyl groups of phospholipid species in the Toxoplasma lipid extract (without standards) were identified as acyl anions from the appropriate negative ion precursors. The collision energies were 20–55 V. The solvent was chloroform/methanol/300 mM ammonium acetate in water (300/665/35). PI and PE were analyzed as [M - H]− ions, and PC was analyzed as [M + OAc]−. The relative abundance of the fatty acyl ions was used to designate the position of the acyl chain, as the acyl group in the 2-position generally produces the more abundant ion, although this designation is somewhat equivocal (21). The data for determination of PE-cer structure were obtained on an API 4000 without addition of internal standard. The source temperature (heated nebulizer) was 100°C, the interface heater was on, +5.5 kV was applied to the electrospray capillary, the curtain gas was set at 20 (arbitrary units), the two ion source gases were set at 45 (arbitrary units), the collision gas pressure was 2, and the collision energy was 55 V.
RESULTS
Lipid classes
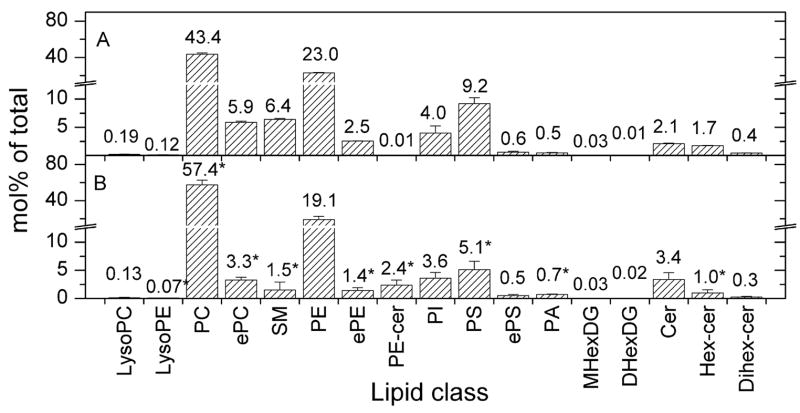
Quantitative analysis of polar lipids with masses less than 1000, including diacyl, ether-linked (ePC; i.e. alk(en)yl/acyl PC), and lyso PC, diacyl, ether-linked (ePE), and lyso PE, diacyl and ether-linked (ePS) PS, diacyl PI, diacyl PA, SM, PE-cer, MHexDG (monohexosyldiacylglycerol identified based on MGDG standards), DHexDG (dihexosyldiacylglycerol identified based on DGDG standards), Cer, Hex-cer, and Dihex-cer, of T. gondii and its host fibroblasts was performed, using electrospray ionization tandem mass spectrometry (ESI-MS/MS). As shown in Figure 1, when the lipid classes were considered as mol% of the total of these lipids from each source, Toxoplasma gondii was found to be enriched in PC (57.4% vs. 43.4%), PE-cer (2.4% vs. 0.01%), and PA (0.7% vs. 0.5%) relative to its host fibroblasts, while the host fibroblasts had (as mol%) relatively more ePC, ePE, lysoPE, diacyl PS, SM, and Hex-cer.
FIGURE 1.
Composition of lipid classes in host fibroblasts (A) and Toxoplasma gondii (B). Lipids are determined from the total of those lipid classes shown. Numbers above each column indicate the mol% value for that species. The detection of MHexDG and DHexDG was equivocal with trace amounts of several species just at the limit of detection and of uncertain significance. Error bars are standard deviation (n = 4). The asterisks indicate lipid classes that differed significantly (p < 0.05) in mol% in Toxoplasma gondii as compared to the host fibroblast.
Glycerolipid molecular species
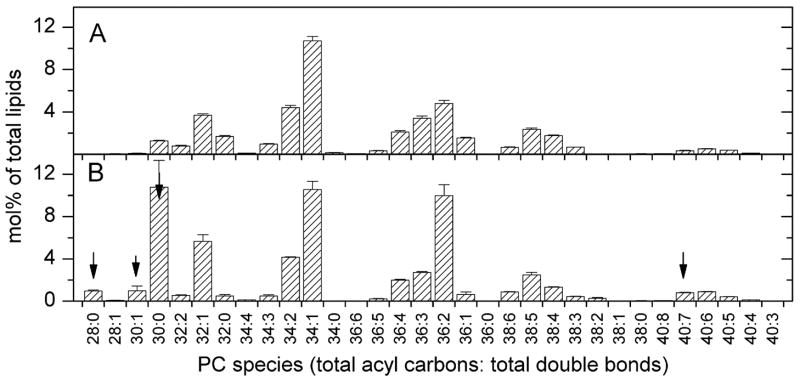
An advantage of ESI-MS/MS over traditional methodologies for lipid analysis is that individual lipid molecular species can be identified. In Figure 2, PC molecular species from T. gondii and host fibroblasts are depicted. From these data, it is obvious that there are significant differences between the molecular species profile of the parasite and its host. Most strikingly, there are high levels of shorter-chain (28-, 30- and 32-carbons in the combined two acyl chains) species in the parasite lipids. PCs with a combined count of 28 and 30 carbons make up about 21% of the T. gondii PC molecular species, while these species make up only 3% of the host molecular species. The most prominent of these 28 and 30 carbon species were identified by product ion analysis as 1–16:0,2–14:0 PC, 1–16:1,2–14:0 PC, di14:0 PC, and 1–16:0,2–12:0 PC (Table 1), indicating that saturated 14-carbon and even 12-carbon acyl species are used in phospholipids considerably more frequently by this parasite than by its host. While there are some qualitative differences, the amounts of 34-, 36- and 38-carbon (combined diacyl count) PC molecular species relative to the total polar lipids are roughly similar between the parasite and host. In the longer chain species, the parasite has more PC 40:7, which was shown to be 1–18:1,2–22:6 PC (Table 1). Overall, the PC of T. gondii appears to contain PC similar to the host with additional 28-, 30-, and 32-carbon species. Thus, the shorter-chain and mostly saturated species are fairly specific to the parasite.
FIGURE 2.
Diacyl phosphatidylcholine molecular species of host fibroblasts and Toxoplasma gondii. Species shown in A and B are indicated by the total number of acyl carbons: the total number of double bonds. Species are indicated as mol% of total lipids in classes shown in Figure 1. Error bars are standard deviation (n = 4). (A) Diacyl phosphatidylcholine molecular species of host fibroblasts. (B) Diacyl phosphatidylcholine molecular species of Toxoplasma gondii. Species marked by the arrows, chosen because they are higher in Toxoplasma than in the host fibroblasts, were subjected to product ion analysis (Table 1).
Table 1.
Identification of lipid molecular species in Toxoplasma gondii tachyzoites. Selected lipid molecular species that represented a larger fraction of Toxoplasma gondii’s lipids than of fibroblast lipids were subjected to product ion analysis in the negative ion mode in order to identify the individual fatty acyl components.a
| Identification in head group scan | Acyl composition |
|---|---|
| PC 28:0 | 14:0-14:0 PC > 16:0-12:0 PC |
| PC 30:1 | 16:1-14:0 PC |
| PC 30:0 | 16:0-14:0 PC |
| PC 40:7 | 18:1-22:6 PC |
| PE 30:0 | 16:0-14:0 PE |
| PI 34:1 | 16:0/18:1 PIb |
The fatty acyl group presumed to be on the 1-position, due to lower signal abundance as compared to its paired acyl species, is listed first; this method is only suggestive with regard to acyl position (21).
Cannot determine acyl position. Intensities for 16:0 and 18:1 anions are similar.
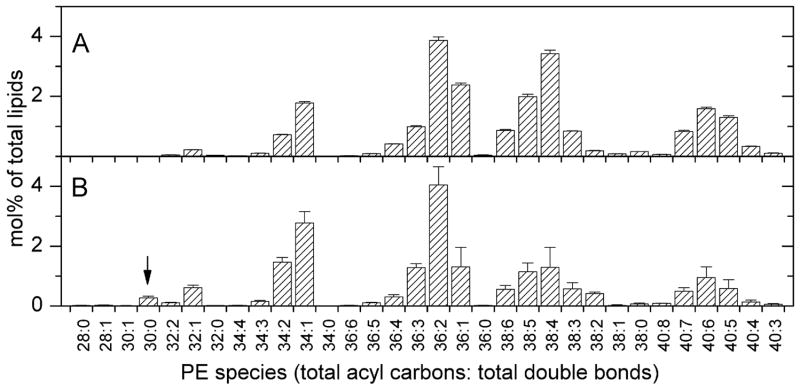
PE (Figure 3) of T. gondii is quite similar to its host in molecular species composition, but, as in PC, there are more 28- 30- and 32-carbon PE species in the parasite than in the host. Twenty-eight- and 30-carbon species make up about 1.6% of the parasite PE, but only 0.1% of the host PE. PE 30:0 from T. gondii was identified as 1–16:0,2–14:0 PE (Table 1).
FIGURE 3.
Diacyl phosphatidylethanolamine molecular species of host fibroblasts and Toxoplasma gondii. Species shown in A and B are indicated by the total number of acyl carbons: the total number of double bonds. Species are indicated as mol% of total lipids in classes shown in Figure 1. Error bars are standard deviation (n = 4). (A) Diacyl phosphatidylethanolamine molecular species of host fibroblasts. (B) Diacyl phosphatidylethanolamine molecular species of Toxoplasma gondii. The species marked by an arrow, chosen because it is higher in Toxoplasma than in the host fibroblasts, was subjected to product ion analysis (Table 1).
Whereas PI makes up a similar fraction of the polar lipids of T. gondii and host fibroblasts (Figure 1), the molecular species of PI (Figure 4A) of T. gondii are less dominated by the PI 38:4 molecular species, which makes up 48% of host PI and 10% of parasite PI. PI 34:1 makes up a greater percentage of the T. gondii PI (50%) than of the host PI (2%). This species was determined by product ion analysis (Table 1) to be PI containing 16:0 and 18:1 fatty acyl species.
FIGURE 4.
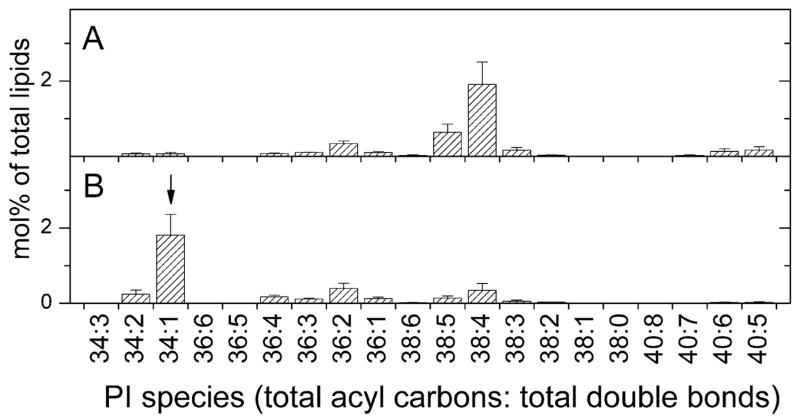
Diacyl phosphatidylinositol molecular species of host fibroblasts and Toxoplasma gondii. Species shown in A and B are indicated by the total number of acyl carbons: the total number of double bonds. Species are indicated as mol% of total lipids in classes shown in Figure 1. Error bars are standard deviation (n = 4). (A) Diacyl phosphatidylinositol molecular species of host fibroblasts. (B) Diacyl phosphatidylinositol molecular species of Toxoplasma gondii. The species marked by an arrow, chosen because it is higher in Toxoplasma than in the host fibroblasts, was subjected to product ion analysis (Table 1).
PS molecular species are shown in Figure 5. In general, T. gondii and host PS are similar, but host PS 36:1 species is common, making up about 24% of the total PS. T. gondii has only about half the relative amount of PS found in the host and has a more equitable distribution of PS among its molecular species, with the highest species (PS 36:2) making up 15% of the PS species.
FIGURE 5.
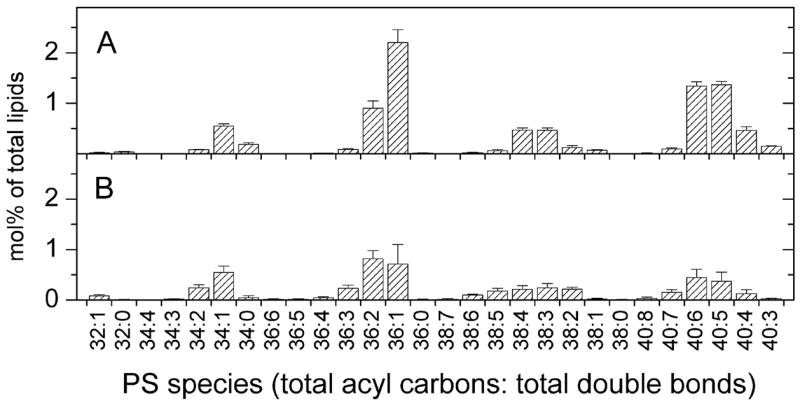
Diacyl phosphatidylserine molecular species of host fibroblasts and Toxoplasma gondii. Species are indicated by the total number of acyl carbons: the total number of double bonds. Species are indicated as mol% of total lipids in classes shown in Figure 1. Error bars are standard deviation (n = 4). (A) Diacyl phosphatidylserine molecular species of host fibroblasts. (B) Diacyl phosphatidylserine molecular species of Toxoplasma gondii.
Sphingolipid composition
Besides the PC species composition, the most notable difference between the polar lipid composition of T. gondii and that of its host fibroblasts is in the sphingolipids containing phosphate. Figure 6 shows a scan of the sphingosine-containing compounds (i.e., precursors of m/z 264 in positive ion mode) in host fibroblasts (Figure 6A) and T. gondii (Figure 6B). Both parasite and host contain Cer, Hex-cer, and, Dihex-cer, each with a predominance of 16- and 24-carbon fatty-amide species. Indeed, the sphingolipid spectra were qualitatively quite similar except for signals for SM (e.g., SM 16:0 at m/z 703) which was higher in the host cells than in T. gondii, and the presence of a large peak at m/z 661 and a smaller one at m/z 689, which were present in the parasite, but undetectable or at the limit of detection in the host cells. The SM 16:0 peak and other SM peaks were also detectable in a positive ion scan for precursors of phosphocholine (Pre 184) and peaks at m/z 661 and m/z 689 were also present in the positive ion scan for the neutral loss of phosphoethanolamine (NL 141) (data not shown). Peaks at m/z 661 and m/z 689 were identified by product ion analysis as PE-cer 16:0 and PE-cer 18:0; product ion spectra in the positive ion mode are shown in Figure 6C and 6D. The structures of the SM 16:0, the predominant phosphosphingolipid of host fibroblasts, and PE-cer 16:0, the predominant phosphosphingolipid of T. gondii, are shown in Figure 7. Sphingomyelin and PE-cer are related lipids with an ethanolamine moiety in PE-cer in contrast to a choline moiety in SM.
FIGURE 6.
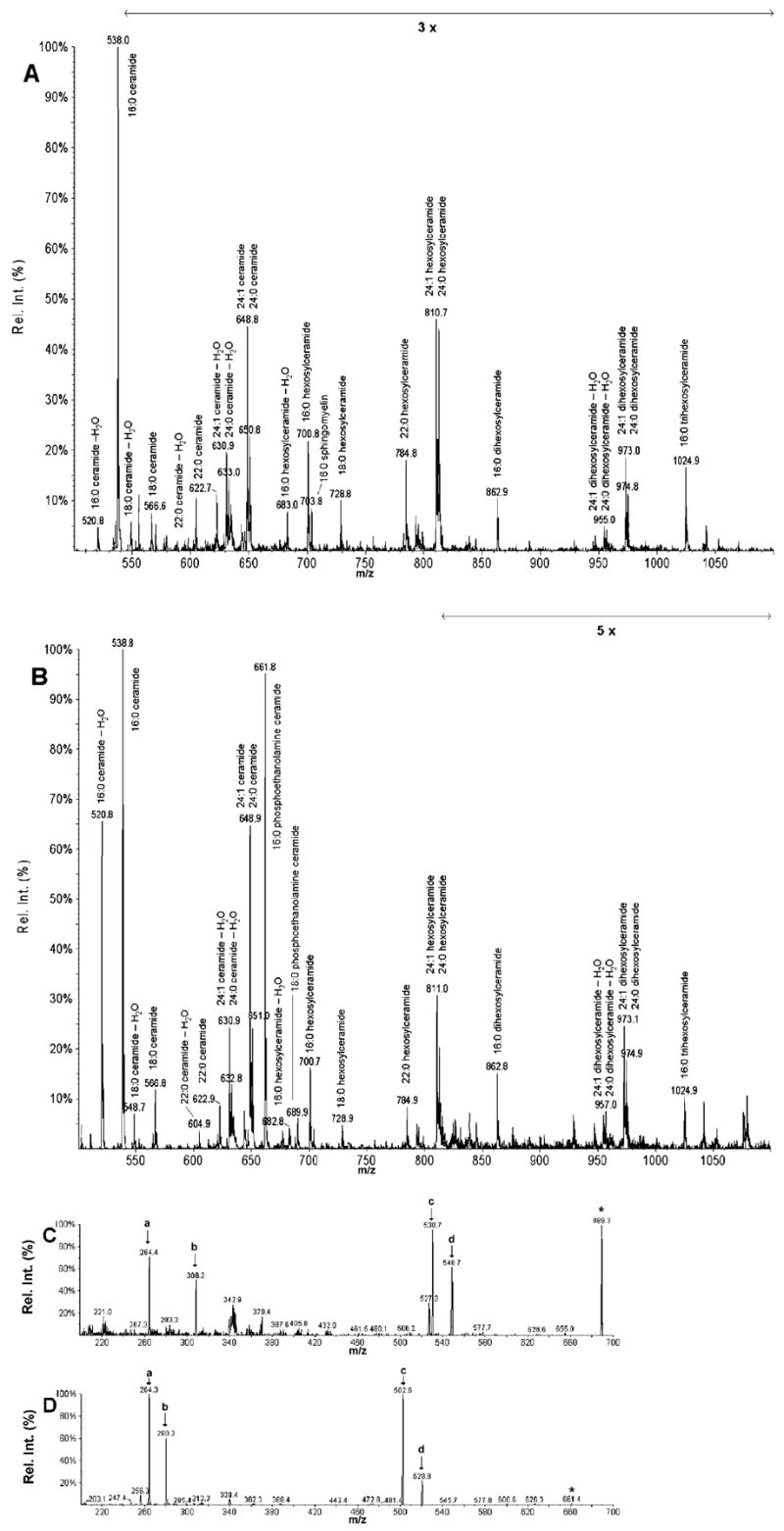
Sphingosine-containing molecular species of host fibroblasts and Toxoplasma gondii as shown by ESI MS/MS in the positive mode. (A and B) Precursors of m/z 264 (sphingosine) were identified as [M + H]+ ions. Ceramides, hexosyl ceramides, di-hexosyl ceramides, and tri-hexosyl ceramides are identified in both host fibroblasts and Toxoplasma gondii as indicated with their amide-linked fatty acyl species. SM 16:0 is identified in host fibroblasts at m/z 703, but produced only a very small signal in Toxoplasma. It should be noted that SM produces m/z 264 only as a minor fragment, in contrast to other sphingolipids, which produce m/z 264 as a major fragment; thus the signal for SM under-represents its amount in relation to the amounts of the other sphingolipids. (A) Sphingosine-containing molecular species of host fibroblasts. (B) Sphingosine-containing molecular species of Toxoplasma gondii. In the Toxoplasma gondii extract, PE-cer produced signals at m/z 689 for the 18:0 amide-linked species and at m/z 661 for the 16:0 amide-linked species. Product ion analysis of these species is shown in C and D. These species were absent from the precursors of m/z 264 scan of the host fibroblast extract (A). (C and D) Product ion analysis of the Toxoplasma phosphatidylethanolmine molecular species, “PE-cer 18:0” (m/z 689) (C) and “PE-cer 16:0” (m/z 661) (D). The species indicated by the arrow in B were subjected, as an [M + H]+ ions (*), to product ion analysis to confirm their identifications. Note that these product ion scans were performed on an unfractionated Toxoplasma extract, and so isobaric species, i.e. species with the same nominal mass as the molecular ion of interest may produce fragment ions in addition to those derived from the ion of interest. The m/z 264 ion, indicated as “a”, is the characteristic ion for sphingosine (a dihydroxy 18-carbon sphingoid base). The ions indicated as “b”, m/z 308 in A and m/z 280 in B, are characteristic of the fatty amide species, 18:0 and 16:0, respectively. Fragmentation of SM 16:0 produced the same m/z 280 ion, characteristic of the fatty amide (22). The ions labeled “c” and “d” are produced by a neutral loss of phosphoethanolamine (NL 141) (“d”) and an additional water loss (“c”).
FIGURE 7.
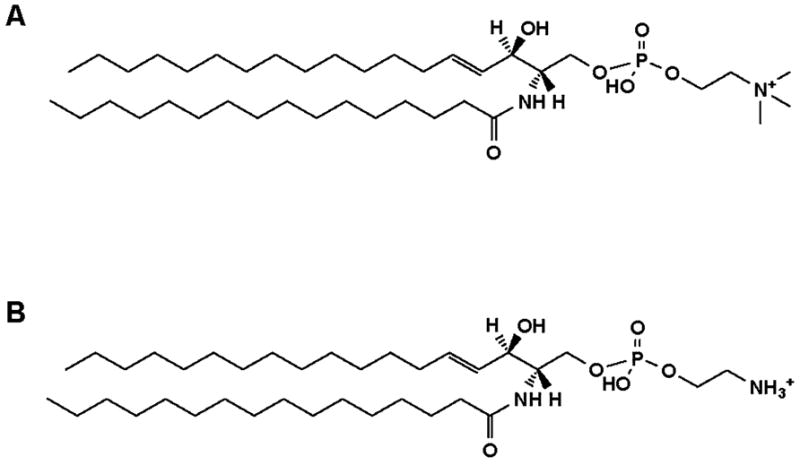
Structures of SM 16:0 and PE-cer 16:0 as [M + H]+ ions. (A) SM 16:0, the predominant phosphosphingolipid of host fibroblasts. (B) PE-cer 16:0, the predominant phosphosphingolipid of Toxoplasma gondii tachyzoites.
PE-cer and SM molecular species were quantified (Figure 8A and B). Because an authentic compound for PE-cer is not available, PE was quantified in relation to diacyl PE internal standards. Our lack of knowledge of a “response factor” for PE-cer only allows us to estimate the PE-cer content at about 2% of the polar lipids. Because the same method was used to analyze PE-cer in T. gondii and in the host cells, we were able to ascertain that T. gondii has a much higher, on the order of 100-fold higher, concentration of PE-cer than its host.
FIGURE 8.
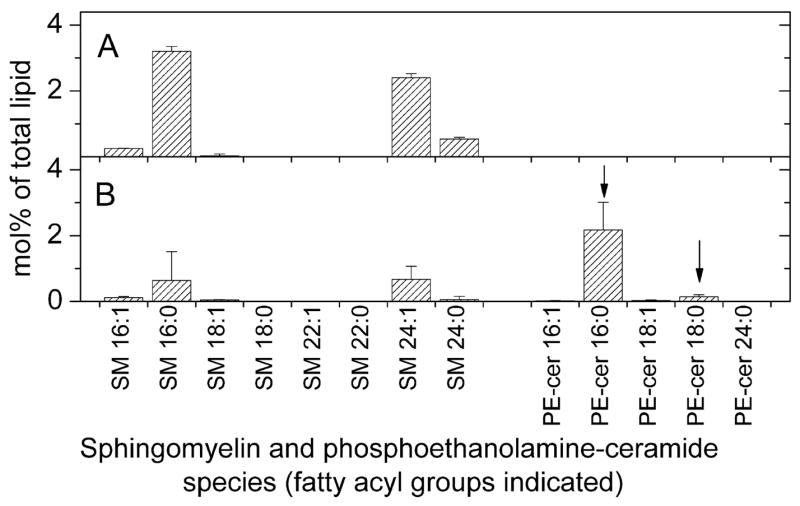
Quantification of sphingomyelin and phosphoethanolamine molecular species of host fibroblasts and Toxoplasma gondii. Species are indicated as mol% of total lipids in classes shown in Figure 1 and are indicated by the number of acyl carbons: the number of double bonds, assuming that the ceramide contains a dihydroxy18:1 base (sphingosine); this is demonstrated in Figure 6C and 6D for the species indicated. Error bars are standard deviation (n = 5). (A) Sphingomyelin and phosphoethanolamine molecular species of host fibroblasts. (B) Sphingomyelin and phosphoethanolamine molecular species of Toxoplasma gondii. The species marked by the arrows, chosen because they are higher in Toxoplasma than in the host fibroblasts, were subjected to product ion analysis.
Correction for host cell membrane contamination
In separate studies (data not shown), uninfected host cells were passed through a needle and a 3-micron filter in precisely the same manner as the infected cells. The lipidomic data obtained were subtracted from those obtained with the infected cells (i.e., those from the isolated parasites). Because the amount of filtered fibroblast lipids was less than 4% of that of the filtered T. gondii lipid species, employing this correction did not significantly change the lipid composition determined for the isolated parasites.
DISCUSSION
Defining the composition and abundance of T. gondii lipids provides not only information about the nature of plasma and organelle membranes, but also insight into nutrient requirements for parasite metabolism and host parasite interactions (5, 23). Collectively, this information might inform design of new antimicrobials. Herein, a broad-based analysis of composition and relative abundance of T. gondii RH strain intracellular tachyzoite polar lipids is reported. Overall the relative abundance of the lipid classes is in reasonable agreement with a previous study (6), but, in addition to information about the relative abundance of the various lipid classes, the current study provides information about lipid molecular species and, in particular, about diacyl, ether-linked (i.e., alk(en)yl/acyl) and ceramide-based polar lipids. In addition, this study identifies a class of sphingolipids, PE-cer, which is common in the parasite but, is found at very low levels in host cells.
Phosphatidylcholines
Higher levels of PC were found in the parasite as compared with the host cells. Gupta et al. (6) noted the high PC levels in T. gondii and found that replacing PC with phosphatidyldimethylethanolamine drastically inhibited parasite growth. The difference in PC quantity between host and parasites is largely due to the presence of parasite PC species with relatively short acyl chains, i.e., containing only a combined 28 or 30 carbons in the two acyl species. The 28:0, 30:1, and 30:0 lipids are comprised of 12-, 14-, and 16-carbon fatty acids. Shorter-chained PC species potentially could be derived by scavenging from the host or by either type I or type II fatty acid synthesis (FAS), since T. gondii is capable of acquiring lipids by all three routes (24–26). Shorter-chained fatty acids or shorter-chain PC species are more water-soluble than longer-chained species and so could be transferred from the host more easily than longer-chain molecular species. Recent studies on Typanosoma brucei indicate that this organism synthesizes 14-carbon fatty acid by an unexpected pathway, involving 2 of 3 modular elongases (27). The three elongases yield 10-, 14- and 18-carbon fatty acids sequentially. Trypanosomes regulate this pathway to produce varied length fatty acids under differing environmental conditions. It remains speculation that Toxoplasma might also regulate the length of its fatty acyl chains under differing environmental conditions or in different stages of its life cycle.
In addition to the T. gondii PC species being shorter-chained than is typical for those of mammalian membranes, the PC species are also more saturated. PC 30:0, which was determined by product ion analysis to be 16:0/14:0 PC, makes up about 11% of T. gondii PC species, but only about 1% of host PC species. While the relative intensity of the product ion spectral peaks suggests that 16:0 is in the predominant species in the 1-position of 16:0/14:0 PC, determination of position by peak intensity can provide ambiguous or incorrect positional assignments (27), and it is also possible that PC 30:0 may be a mixture of positional isomers. 1–16:0,2–14:0 PC has a gel-liquid crystalline phase transition temperature of 27.3°C, and 1–14:0,2–16:0 has a transition temperature of 35.1°C (28). It is speculated that this/these PC 30:0 species might contribute to the growth arrest caused by replacement of PC with phosphatidyldimethylethanolamine (6), since phosphatidyldimethylethanolamines have gel-to-liquid crystalline transition temperatures approximately 8°C higher than PC species with the same acyl chains (29). Thus, if head group alteration occurred without significant changes in fatty acyl species in this group of lipids, phosphatidyldimethylethanolamine replacement for PC could potentially result in the formation of gel-phase lipid in the membranes of T. gondii. This might be related to the observed intracellular accumulation of lipids and the negative effect of phosphatidyldimethylethanolamine on T. gondii multiplication (6). On the other hand, many organisms are able to alter fatty acid composition to compensate for altered phospholipid head group composition (e.g., (30)), but the partial dependence of T. gondii on host cell lipid biosynthesis may not permit the parasite to make compensatory changes in its lipid composition quickly.
Phosphosphingolipids
Gupta et al. (6) found that radiolabeled serine was incorporated into two unknown alkaline-stable materials and suggested that these unidentified materials might be sphingolipids. In the current work, a major phosphosphingolipid class was determined to be PE-cer. While the mol% of sphingomyelin in T. gondii is less than half that of the host fibroblasts, there is a large amount of PE-cer in T. gondii in comparison to host cells. PE-cer is generally found only in trace quantities in mammalian cells, but it has been previously detected in the rumen ciliate commensal Entodinium caudatum and oomycete plant pathogens (31, 32). This might suggest that the production of PE-cer and the route to its biosynthesis is conserved, or is an ancestral characteristic of the Chromoalveolates. PE-cer has also been reported in marine invertebrates (33), in insects (34, 35) and in low amounts in mammalian cells (36, 37). In the current studies the level of PE-cer in uninfected host fibroblasts was determined to be very low, about 0.01% of total polar lipids. Interestingly, the fatty amides associated with PE-cer included only 16- and 18-carbon species. 24-Carbon species, which are common (15–40%) in the other sphingolipid classes (Cer, Hex-cer, Dihex-cer, and SM), were not detectable in the PE-cer class.
PE-cer synthesis may occur through a transfer of a phosphoethanolamine group from PE to ceramide; this pathway was suggested to occur in rumen ciliate commensal Entodinium caudatum (31). This reaction might be catalyzed by a protozoan-encoded protein or could conceivably be due to an upregulation of the synthesis of PE-cer by the host as a result of the commensal interaction or parasite infection, as this lipid is known to occur at low levels in mammalian cells (36, 37). Indeed, Apicomplexa genomes contain genes for putative sphingomyelin synthase (one candidate gene in T. gondii, i.e. 50.m03113 and two putative homologues in P. falciparum, i.e. the PFF1210w and PFF1215w contiguous genes), and we do not know if these enzymes might be capable of synthesizing ceramide phophoethanolamine as well or if a substantial amount of SM and ceramide phosphoethanolamine might be diverted from host cells.
Fouts and Boothroyd (38) and Kim et al. (39) recently analyzed the global transcriptomic responses of HFF cells after infection by T. gondii. A list of 46 "lipid biosynthesis" genes with expression induced or repressed by T. gondii infection was reported (38); of these, 10 are reported in the other study (39). Both analyses confirm a strong remodeling of host cell lipid metabolism upon infection.
In the study by Fouts and Boothroyd (38), a human gene encoding a phosphoserine aminotransferase 1 (AI015679) is shown to be repressed in tachyzoite infected HFF cells. Suppression of phosphoserine aminotransferase (if confirmed) would support the diversion of the phosphoserine metabolism toward serine production. Amongst different metabolic usages, serine can enter both glycerolipid and sphingolipid metabolism, since it is the substrate for both phosphatidylserine synthase (generating PS) and for serine palmitoyltransferase (entry point for the synthesis of sphinganine). In the study by Kim et al. (39), the phosphatidylserine synthase 1 (H28984) is consistently enhanced after infection by T. gondii.
In addition, Kim et al. (39) provide evidence for remodelling of host cell sphingolipid metabolism: in particular, expression of a sphingosine kinase (AI341901) is stimulated, supporting the synthesis of sphingosine 1-phosphate, which is the direct substrate for the sphinganine-1-phosphate aldolase that generates phosphoethanolamine. Three human genes encoding sphingomyelin synthases (transmembrane protein 23: AI261602, AA459293 and AA693488) are markedly repressed after infection. These proteins are bidirectional and capable of converting PC and ceramide to SM and diacylglycerol (DAG) and vice versa; direction is dependent on the relative concentrations of DAG and ceramide as phosphocholine acceptors. It is therefore difficult to deduce from the reduced expression of sphingomyelin synthase genes whether they are correlated to a decrease or an increase of HFF SM. It is also possible that some of these enzymes could be involved in ceramide phosphoethanolamine synthesis. If the enzyme involved in PE-cer synthesis belongs to the protozoan, its uncommon specificity for ceramides with 16C and 18C amides might suggest that the enzyme has an unusual active site that might provide a parasite-specific target.
PE-cer and other ceramide derivatives have structural resemblance to lipopolysaccharide and represent alternative ligands for host toll receptors (40). If PE-cer does bind to toll receptors, PE-cer may be made by the parasite to modulate host cell function.
Hexosylceramides
Figure 6, a discovery scan for sphingolipids, indicates that, besides the phosphosphingolipids, the major ceramide-containing species in the mass range between 500 and 1100 are ceramides, (mono)Hex-cers, and Dihex-cers. A peak at m/z 1024, consistent with trihexosyl ceramide 16:0, was also detected. The current data are consistent with the detection of mongalactosylceramide synthesis after metabolic labeling of T. gondii cells with radiolabled UDP-galactose (41). These data further confirm the synthesis of mono-,di- and trihexosyl ceramides detected by Bisanz et al. (13) after acetate metabolic labeling.
Ceramide
The amount of free ceramide in T. gondii relative to the composition of the uninfected host cell is also of interest in that both ceramide and lipopolysaccharide bind to CD14 and CD36 which appear to co-associate with toll receptors 2 and 4 (40). These findings again are suggestive of a way in which the parasite lipids could modulate host cell functions.
Glycosyldiacylglycerols
The amounts of MHexDG and DHexDG in T. gondii tachyzoites are at or below the limit of detection. Maréchal et al. (41) could detect the synthesis of both MGDG and DGDG in T. gondii cells incubated with radiolabelled UDP-galactose and could detect a glycolipid reacting with an anti-DGDG antibody in T. gondii lipid extracts. Bisanz et al. (13) further detected a glycolipid comigrating with DGDG. Here, MHexDG and DHexDG with masses corresponding to diacyl galactolipids, similar to those found in plant chloroplasts, are, at most, very minor lipid species.
Significance of PE-cer and distinctive PC species
The work herein precisely characterizes lipid species in T. gondii, focusing initially on one clonal type and the tachyzoite life cycle stage. Bradyzoites have more lipid and more abundant lipid bodies than tachyzoites (42), and whether lipid species in tachyzoites and bradyzoites differ remains to be defined. It will also be of interest to compare lipid content in different isolates (clonal and atypical parasite types) and in T. gondii organelles (43). Mass spectrometry-based lipidomics is a robust methodology for comparing parasite and host cell lipid composition in detail. It identifies a novel sphingolipid, ceramide phosphoethanolamine, which is likely to play a role in the interaction between the parasite and the host. Ceramide phosphoethanolamine might also be an important component of T. gondii membrane rafts.
Occurrence of ceramide phosphoethanolamine suggests the existence of multiple, and possibly non-redundant, pathways for the synthesis of sphingolipids. SM can be generated either by conventional PC-ceramide phosphocholine transferases (one putative homologous gene in T. gondii, i.e. 50.m03113 and two putative homologous genes in P. falciparum, i.e. the PFF1210w and PFF1215w contiguous genes) or by a stepwise process involving PE-ceramide phosphoethanolamine transferases, generating ceramide phosphoethanolamine, and methyltransferases, generating SM. PE-ceramide phosphoethanolamine transferase activity might be harboured by PC-ceramide phosphocholine transferases with loose substrate specificity or by as yet unidentified enzymes. Subsequent methylation of the phosphoethanolamine polar head into phosphocholine might be catalyzed by a phosphoethanolamine methyltransferase (one gene characterized in P. falciparum, i.e. PfPMT or MAL13P1.214; no clear homologue in T. gondii genome). In P. falciparum, the PfPMT enzyme was shown to catalyze the in vitro conversion of phosphethanolamine into phosphocholine and is suspected to also catalyze the transmethylation of PE into PC in vivo (reviewed in 44). Such an enzyme might therefore also catalyze the transmethylation of ceramide phosphoethanolamine into SM. PfPMT has attracted attention because it is inhibited by phosphocholine and synthetic analogs (miltefosine (45) and 1,12-bis-(N,N'-acetamidinyl)dodecane derivatives (46)), which represent some of the most promising future generations of anti-malarial drugs. In T. gondii, the accumulation of ceramide phosphoethanolamine might be related to the apparent lack of a PfPMT homologue or a methyl transferase of different substrate affinity. In future studies, it would be of interest for comparisons of T. gondii and P. falciparum lipidomic profiles to focus on the ceramide phosphoethanolamine and SM contents, and on the enzymes that are involved in the transmethylations of phosphoethanolamine, ceramide phosphoethanolamine and PE, in both T. gondii and P. falciparum.
The functional significance of the high-level of 12-, 14- and 16-carbon saturated T. gondii PCs and the effect of these lipids on the properties of the parasite’s membranes remain to be determined. However, if their role is demonstrated to be essential, the enzymes involved in the synthesis of distinctive lipids may also provide unique molecular targets for chemotherapeutics (7, 47, 48).
Acknowledgments
We thank Levi Kinderknecht for technical assistance.
Footnotes
This work was supported by NIH NIAID R01s AI27530, AI43228, AI071319, the Research to Prevent Blindness Foundation, and gifts from the Kieweit, Blackmon, Brennan, Koshland, Langel, Morel, Rosenstein, Cussen, Kapnick, and Rooney-Alden families. Work and instrument acquisition at the Kansas Lipidomics Research Center Analytical Laboratory were supported by grants from NSF (MCB 0455318 and DBI 0521587) and NSF's EPSCoR program (EPS-0236913), with matching support from the State of Kansas through Kansas Technology Enterprise Corporation and Kansas State University, as well from NIH grant P20 RR016475 from the INBRE program of the National Center for Research Resources. This is contribution 07-308-J from the Kansas Agricultural Experiment Station.
Abbreviations: ceramide, Cer; ceramide phosphoethanolamine, PE-cer; dihexosylceramide, Dihex-cer; electrospray ionization, ESI; mass spectrometry, MS; phosphatidic acid, PA; phosphatidylcholine, PC; (alkyl or alkenyl)/acyl glycerophosphocholine, ePC; phosphatidylethanolamine, PE; (alkyl or alkenyl)/acyl glycerophosphoethanolamine, ePE; phosphatidylinositol, PI; (alkyl or alkenyl)/acyl glycerophosphoserine, ePS; Hexosyl ceramide, Hex-cer; phosphatidylserine, PS; sphingomyelin, SM; tandem mass spectrometry, MS/MS.
References
- 1.Zuther E, Johnson JJ, Haselkorn R, McLeod R, Gornicki P. Growth of Toxoplasma gondii is inhibited by aryloxyphenoxypropionate herbicides targeting acetyl-CoA carboxylase. Proc Natl Acad Sci USA. 1999;96:13387–13392. doi: 10.1073/pnas.96.23.13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLeod R, Muench SP, Rafferty JB, Kyle DE, Mui EJ, Kirisits MJ, Mack DG, Roberts CW, Samuel BU, Lyons RE, Dorris M, Milhous WK, Rice DW. Triclosan inhibits the growth of Plasmodium falciparum and Toxoplasma gondii by inhibition of Apicomplexan Fab. I Int J Parasitol. 2001;31:109–113. doi: 10.1016/s0020-7519(01)00111-4. [DOI] [PubMed] [Google Scholar]
- 3.Roberts CW, McLeod R, Rice DW, Ginger M, Chance ML, Goad LJ. Fatty acid and sterol metabolism: potential antimicrobial targets in apicomplexan and trypanosomatic parasitic protozoa. Mol & Biochem Parasitol. 2003;126:129–142. doi: 10.1016/s0166-6851(02)00280-3. [DOI] [PubMed] [Google Scholar]
- 4.Samuel BJ, Hearn B, Mack DG, Wender P, Rothbard J, Kirisits M, Mui E, Roberts C, Prigge S, Rice D, Muench SP, Law A, McLeod R. Delivery of antimicrobials into parasites. Proc Natl Acad Sci USA. 2003;100:14281–14286. doi: 10.1073/pnas.2436169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazumdar JH, Wilson E, Masek KA, Hunter C, Striepen B. Apicoplast fatty acid synthesis is essential for organelle biogenesis and parasite survival in Toxoplasma gondii. Proc Natl Acad Sci USA. 2006;103:13192–13197. doi: 10.1073/pnas.0603391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta N, Zahn MM, Coppens I, Joiner KA, Voelker DR. Selective disruption of PC metabolism of the intracellular parasite, Toxoplasma gondii arrests its growth. J Biol Chem. 2005;280:16345–16353. doi: 10.1074/jbc.M501523200. [DOI] [PubMed] [Google Scholar]
- 7.Sonda S, Ting LM, Novak S, Kim K, Maher JJ, Farese RV, Jr, Ernst JD. Cholesterol esterification by host and parasite is essential for optimal proliferation of Toxoplasma gondii. J Biol Chem. 2001;275:34434–34440. doi: 10.1074/jbc.M105025200. [DOI] [PubMed] [Google Scholar]
- 8.Waller RF, Keeling PJ, Donald RGK, Striepen B, Handman E, Lang-Unnasch N, Cowman AF, Besra GS, Roos DS, McFadden GI. Nuclear-encoded proteins target to the plastid in Toxoplasma gondii and Plasmodium falciparum. Proc Natl Acad Sci USA. 1998;95:12352–12357. doi: 10.1073/pnas.95.21.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waller RF, McFadden GI. The apicoplast: a review of the derived plastid of apicomplexan parasites. Curr Issues Mol Biol. 2005;7:57–79. [PubMed] [Google Scholar]
- 10.Gleeson MT. The plastid in Apicomplexa: what use is it? Int J Parasitol. 2000;30:1053–1070. doi: 10.1016/s0020-7519(00)00100-4. [DOI] [PubMed] [Google Scholar]
- 11.Maréchal E, Cesbron-Delauw MF. The apicoplast: a new member of the plastid family. Trends Plant Sci. 2001;6:200–205. doi: 10.1016/s1360-1385(01)01921-5. [DOI] [PubMed] [Google Scholar]
- 12.Zhu G. Current progress in the fatty acid metabolism in Cryptosporidium parvum. J Eukaryot Microbiol. 2004;51:381–388. doi: 10.1111/j.1550-7408.2004.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 13.Bisanz C, Bastien O, Grando D, Jouhet J, Maréchal E, Cesbron-Delauw MF. Toxoplasma gondii acyl-lipid metabolism: de novo synthesis from apicoplast-generated fatty acids versus scavenging of host cell precursors. Biochem J. 2006;394:197–205. doi: 10.1042/BJ20050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charron AJ, Sibley LD. Host cells: mobilizable lipid resources for the intracellular parasite Toxoplasma gondii. J Cell Science. 2002;115:3049–3059. doi: 10.1242/jcs.115.15.3049. [DOI] [PubMed] [Google Scholar]
- 15.Mui EJ, Jacobus D, Milhous WK, Schiehser G, Hsu H, Roberts CW, Kirisits MJ, McLeod R. Triazines inhibit Toxoplasma gondii tachyzoites in vitro and in vivo. Antimicrob Ag Chemother. 2005;49:3463–3467. doi: 10.1128/AAC.49.8.3463-3467.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou H–E, Rajashekar CB, Williams TD, Wang X. Profiling of membrane lipids in plant stress responses. J Biol Chem. 2002;277:31994–32002. doi: 10.1074/jbc.M205375200. [DOI] [PubMed] [Google Scholar]
- 17.Wanjie SW, Welti R, Moreau RA, Chapman KD. Identification and quantification of glycerolipids in cotton fibers: Reconciliation with metabolic pathway predictions from DNA databases. Lipids. 2005;40:773–785. doi: 10.1007/s11745-005-1439-4. [DOI] [PubMed] [Google Scholar]
- 18.Brügger B, Erben G, Sandhoff R, Wieland FT, Lehmann WD. Quantitative analysis of biological membrane lipids at the low picomole level by nano-electrospray ionization tandem mass spectrometry. Proc Natl Acad Sci U S A. 1997;94:2339–2344. doi: 10.1073/pnas.94.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welti R, Shah J, LeVine S, Esch SW, Williams TD, Wang X. High throughput lipid profiling to identify and characterize genes involved in lipid metabolism, signaling, and stress response. In: Feng L, Prestwich GD, editors. Functional Lipidomics. Marcel Dekker; New York: 2005. pp. 307–322. [Google Scholar]
- 20.Liebisch G, Lieser B, Rathenberg J, Drobnik W, Schmitz G. High-throughput quantification of phosphatidylcholine and sphingomyelin by electrospray ionization tandem mass spectrometry coupled with isotope correction algorithm. Biochim Biophys Acta. 2004;1686:108–117. doi: 10.1016/j.bbalip.2004.09.003. Erratum: (2005) Biochim Biophys Acta 1734:86–89. [DOI] [PubMed] [Google Scholar]
- 21.Murphy RC. Mass Spectrometry of Lipids. In: Snyder F, editor. Handbook of Lipid Research. Vol. 7. Plenum Press; New York: 1993. pp. 223–226. [Google Scholar]
- 22.Hsu F–F, Turk J. Structural determination of sphingomyelin by tandem mass spectrometry with electrospray ionization. J Am Soc Mass Spectrom. 2000;11:437–449. doi: 10.1016/S1044-0305(99)00150-6. [DOI] [PubMed] [Google Scholar]
- 23.Botté C, Saïdani N, Mondragon R, Gonzales S, Isaac G, Mui E, McLeod R, Dubremetz J-F, Vial H, Welti R, Cesbron-Delauw M–F, Mercier C, Maréchal E. Subcellular localization and dynamics of a digalactolipid-like lipid along the life cycle of the apicomplexan parasite Toxoplasma gondii. 2007 doi: 10.1194/jlr.M700476-JLR200. Submitted. [DOI] [PubMed] [Google Scholar]
- 24.Coppens I, Sinai AP, Joiner KA. Toxoplasma gondii exploits host low-density lipoprotein receptor-mediated endocytosis for cholesterol acquisition. J Cell Biol. 2000;149:167–180. doi: 10.1083/jcb.149.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coppens I, Vielemeyer O. Insights into un ique physiological features of neutral lipids in Apicomplexa: from storage to potential mediation in parasite metabolic activities. Int J Parasitol. 2005;35:597–615. doi: 10.1016/j.ijpara.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Coppens I. Contribution of host lipids to Toxoplasma pathogenesis. Cell Microbiol. 2006;8:1–9. doi: 10.1111/j.1462-5822.2005.00647.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee SH, Stephens JL, Paul KS, Englund PT. Fatty acid synthesis by elongases in trypanosomes. Cell. 2006;126:691–699. doi: 10.1016/j.cell.2006.06.045. [DOI] [PubMed] [Google Scholar]
- 28.Chen SC, Sturtevant JM. Thermotropic behavior of bilayers formed from mixed-chain PCs. Biochemistry. 1981;20:713–718. doi: 10.1021/bi00507a007. [DOI] [PubMed] [Google Scholar]
- 29.Mulukutia S, Shipley GG. Structure and thermotropic properties of phosphatidylethanolamine and its N-methyl derivatives. Biochemistry. 1984;23:2514–2519. doi: 10.1021/bi00306a030. [DOI] [PubMed] [Google Scholar]
- 30.Schroeder F, Holland JF, Vagelos PR. Physical properties of membranes isolated from tissue culture cells with altered phospholipids composition. J Biol Chem. 1976;251:6747–6756. [PubMed] [Google Scholar]
- 31.Broad TE, Dawson RMC. Formation of ceramide phosphorylethanolamine from phosphatidylethanolamine in the rumen protozoan Entodinium caudatum. Biochem J. 1973;134:659–662. doi: 10.1042/bj1340659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreau RA, Young DH, Danis PO, Powell MJ, Quinn CJ, Beshah K, Slawecki RA, Dilliplane RL. Identification of ceramide-phosphorylethanolamine in oomycete plant pathogens: Pythium ultimum, Phytophthora infestans, and Phytophthora capsici. Lipids. 1998;33:307–317. doi: 10.1007/s11745-998-0210-1. [DOI] [PubMed] [Google Scholar]
- 33.Hori T, Sugita M, Arakawa I. Structural elucidation of sphingoethanolamine and its distribution in aquatic animals. Biochim Biophys Acta. 1968;152:211–213. [PubMed] [Google Scholar]
- 34.Hildenbradt GR, Abraham T, Bieber LL. Metabolism of ceramide phosphorylethanolamine, phosphatidylinositol, phosphatidylserine and phosphatidylglycerol by housefly larvae. Lipids. 1971;6:508–516. doi: 10.1007/BF02531237. [DOI] [PubMed] [Google Scholar]
- 35.Dawson RM, Kemp P. Isolation of ceramide phosphorylethanolamine from the blowfly Calliphora erythrocephala. Biochem J. 1968;106:319–320. doi: 10.1042/bj1060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muehlenberg BA, Sribney M, Duffe MK. Occurrence and biosynthesis of ceramide phosphorylethanolamine in chicken and rat liver. Can J Biochem. 1972;50:166–173. doi: 10.1139/o72-022. [DOI] [PubMed] [Google Scholar]
- 37.Maurice A, Malgat M. Evidence for the biosynthesis of ceramide-phosphoethanolamine in brain synaptic plasma membrane vesicles and in sciatic nerve microsomes from normal and Trembler mice. Neurosci Lett. 1990;118:177–180. doi: 10.1016/0304-3940(90)90620-o. [DOI] [PubMed] [Google Scholar]
- 38.Fouts AE, Boothroyd JC. Infection with Toxoplasma gondii bradyzoites has a diminished impact on host transcript levels relative to tachyzoite infection. Infect Immun. 2007;75:634–642. doi: 10.1128/IAI.01228-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim SK, Fouts AE, Boothroyd JC. Toxoplasma gondii dysregulates IFN-gamma-inducible gene expression in human fibroblasts: insights from a genome-wide transcriptional profiling. J Immunol. 2007;178:5154–5165. doi: 10.4049/jimmunol.178.8.5154. [DOI] [PubMed] [Google Scholar]
- 40.Fischer H, Ellstrom P, Ekstrom K, Gustafsson L, Gustafsson M, Svanborg C. Ceramide as a TLR4 agonist; a putative signalling intermediate between sphingolipid receptors for microbial ligands and TLR4. Cell Microbiol. 2007;9:1239–1251. doi: 10.1111/j.1462-5822.2006.00867.x. [DOI] [PubMed] [Google Scholar]
- 41.Maréchal E, Azzouz N, de Macedo CS, Block MA, Feagin JE, Schwarz RT, Joyard J. Synthesis of chloroplast galactolipids in apicomplexan parasites. Eukaryot Cell. 2002;1:653–656. doi: 10.1128/EC.1.4.653-656.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev. 1998;11:267–299. doi: 10.1128/cmr.11.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foussard F, Leriche MA, Dubremetz JF. Characterization of the lipid content of Toxoplasma gondii rhoptries. Parasitology. 1991;102:367–370. doi: 10.1017/s0031182000064313. [DOI] [PubMed] [Google Scholar]
- 44.Vial H, Ben Mamoun C. Plasmodium lipids: metabolism and function. In: Sherman IW, editor. Molecular Approaches to Malaria. ASM Press; Washington, D.C.: 2005. pp. 327–352. [Google Scholar]
- 45.Pessi G, Kociubinski G, Ben Mamoun C. A pathway for phosphatidylcholine biosynthesis in Plasmodium falciparum involving phosphoethanolamine methylation. Proc Natl Acad Sci USA. 2004;101:6206–6211. doi: 10.1073/pnas.0307742101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ouattara M, Wein S, Calas M, Hoang YV, Vial H, Escale R. Synthesis and antimalarial activity of new 1,12-bis(N, N′-acetamidinyl)dodecane derivatives. Bioorg Med Chem Lett. 2007;17:593–596. doi: 10.1016/j.bmcl.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 47.Ferguson DJP, Henriquez FL, Kirisits MJ, Muench SP, Prigge ST, Rice DW, Roberts CW, McLeod RL. Maternal inheritance and stage specific variation of the apicoplast in Toxoplasma gondii during development in the intermediate and definitive host. Euk Cell. 2005;4:814–826. doi: 10.1128/EC.4.4.814-826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muench S, Prigge S, Kirisits MJ, McLeod R, Rafferty JB, Kirisitis MJ, Roberts CW, Mui EJ, Rice D. Studies of Toxoplasma gondii and Plasmodium falciparum enoyl acyl carrier protein reductase and implications for the development of antiparasitic agents. Acta Crystallog D Biol Crystallogr. 2007;63:328–338. doi: 10.1107/S0907444906053625. [DOI] [PMC free article] [PubMed] [Google Scholar]