Abstract
C2H4 is associated with plant defense, but its role during the hypersensitive response (HR) remains largely uncharacterized. C2H4 production in tobacco (Nicotiana tabacum) following inoculation with HR-eliciting Pseudomonas syringae pathovars measured by laser photoacoustic detection was biphasic. A first transient rise (C2H4-I) occurred 1 to 4 h following inoculation with HR-eliciting, disease-forming, and nonpathogenic strains and also with flagellin (flg22). A second (avirulence-dependent) rise, at approximately 6 h (C2H4-II), was only seen with HR-eliciting strains. Tobacco leaves treated with the C2H4 biosynthesis inhibitor, aminoethoxyvinylglycine, suggested that C2H4 influenced the kinetics of a HR. Challenging salicylate hydroxylase-expressing tobacco lines and tissues exhibiting systemic acquired resistance suggested that C2H4 production was influenced by salicylic acid (SA). Disrupted expression of a C2H4 biosynthesis gene in salicylate hydroxylase tobacco plants implicated transcriptional control as a mechanism through which SA regulates C2H4 production. Treating leaves to increase oxidative stress or injecting with SA initiated monophasic C2H4 generation, but the nitric oxide (NO) donor sodium nitroprusside initiated biphasic rises. To test whether NO influenced biphasic C2H4 production during the HR, the NO synthase inhibitor NG-nitro-l-arginine methyl ester was coinoculated with the avirulent strain of P. syringae pv phaseolicola into tobacco leaves. The first transient C2H4 rise appeared to be unaffected by NG-nitro-l-arginine methyl ester, but the second rise was reduced. These data suggest that NO and SA are required to generate the biphasic pattern of C2H4 production during the HR and may influence the kinetics of HR formation.
Resistance to pathogens is often associated with localized cell death, the hypersensitive response (HR). The HR is initiated following host recognition of the pathogen-encoded avirulence (avr) gene product by a plant resistance (R) gene (Martin et al., 2003). In bacterial pathogens, AVR proteins are R gene product recognized members of a population of virulence effectors that are delivered into the plant via a hrp-pilus, a type III secretion system (TTSS). However, in the absence of an interacting R gene, this AVR protein will act as a virulence effector (Alfano and Collmer, 2004). Other non-AVR elicitors act on basal resistance mechanisms to influence plant defense, which can be suppressed by TTSS-delivered effectors to establish disease (Kim et al., 2005). Some non-TTSS-delivered elicitors appear analogous to pathogen-associated molecular patterns (PAMPs; Parker, 2003; also as microbial-associated molecular patterns; Ausubel, 2005) and include flagellin (flg22; Zipfel et al., 2004) and lipopolysaccharide (Zeidler et al., 2004).
The HR is initiated and regulated by calcium (Grant et al., 2000) and reactive oxygen species (ROS)—mainly the superoxide anion and H2O2 (Lamb and Dixon, 1997) and nitric oxide (NO; Delledonne et al., 1998). NO has been shown to activate proteases that appear to contribute to a HR-type cell death (Clarke et al., 2000; Belenghi et al., 2003), most likely by interacting with ROS-associated signals (Delledonne et al., 2001) as well as inducing defense gene expression (Grun et al., 2006).
The HR is influenced by the interaction of PAMP and AVR elicitors. This has been classically described within the context of the biphasic generation of H2O2 during the pathogen-elicited oxidative burst (Lamb and Dixon, 1997). Here, there is an initial transient rise in H2O2 (H2O2-I), induced by non-AVR elicitors, followed by a more persistent AVR-dependent rise in H2O2 (H2O2-II) some hours later. The kinetics and amplitude of H2O2-II influences the rate of HR cell death and, hence, the effectiveness of the associated defenses (Shirasu et al., 1997; Mur et al., 2000). Similar elicitation events and biphasic kinetics have also been noted for pathogen-elicited calcium fluxes (Grant et al., 2000). The synthesis of salicylic acid (SA) may be initiated by oxidative stress (Chamnongpol et al., 1998) and it may be that H2O2-I-initiated SA synthesis augments H2O2-II.
Ethylene has many roles in plant physiology and its biosynthesis and associated signaling have been extensively characterized (Bleecker and Kende, 2000). The biosynthetic pathway involves the conversion of S-adenosylmethione to 1-aminocyclopropane-1-carboxylic acid (ACC) by ACC synthase (ACS) and then to ethylene by ACC oxidase. Ethylene production in response to pathogens has been stated as being rapid (<4 h; Ecker and Davis, 1987), but only in treatments with isolated defense elicitors (Tong et al., 1986; Bailey et al., 1991; Kenyon and Turner, 1992). In response to pathogens, increased ethylene production was not observed until at least 24 h following challenge (Lund et al., 1998; Penninckx et al., 1998; Chen et al., 2003; O'Donnell et al., 2003).
Ethylene has diverse roles in plant defense, mostly associated with resistance to pathogens that adopt a necrotrophic lifestyle (Thomma et al., 1999; Norman-Setterblad et al., 2000; Berrocal-Lobo et al., 2002). Ethylene-mediated resistance can be exhibited through the induction of antimicrobial pathogenesis-related protein genes (van Loon et al., 2004), Hyp-rich protein genes (Ecker and Davis, 1987), and genes encoding key enzymes in the phenylpropanoid pathway leading to the production of antimicrobial phytoalexin compounds (Dixon et al., 2002). Ethylene has been shown to initiate cell death through the initiation of ROS generation and proteolytic enzyme activation (de Jong et al., 2002); however, analysis of a HR forming in the ethylene insensitive ein2 mutant in Arabidopsis (Arabidopsis thaliana) has suggested that ethylene plays, at best, a minor role in this type of cell death (Bent et al., 1992; Ciardi et al., 2000). Conversely, with many biotrophic (or partly biotrophic) pathogens, ethylene is a virulence factor. Host ethylene production is required for the full virulence of P. syringae pv (P. s. pv) glycinea, P. s. pv tomato, Xanthomonas campestris pv vesicatoria, Verticillium dahlia, and Cucumber mosaic virus on their hosts (Lund et al., 1998; van Loon et al., 2006). Some strains of P. syringae and X. campestris pathovars derive their own ethylene to serve a virulence function. Within these pathogens, ethylene is not synthesized from ACC, but from 2-oxoglutarate, by an ethylene-forming enzyme (EFE; Weingart and Volksch, 1997). The roles of ethylene in plant defense are therefore context specific, reflecting differing types of pathogen challenge and differing interactions with other defense signals.
We here extend our previous investigations on signal production during the nonhost HR elicited by P. s. pv phaseolicola (Psph) in tobacco (Nicotiana tabacum; Kenton et al., 1999; Mur et al., 2000, 2005a, 2005b) to describe the kinetics of ethylene production during interaction with various P. syringae pathovars, using laser photoacoustic detection (LPAD). These revealed biphasic patterns of ethylene production reflecting elicitation by PAMP and AVR elicitors in a manner reminiscent of biphasic H2O2 and calcium production (Lamb and Dixon, 1997; Grant et al., 2000). Further characterization suggested that the biphasic pattern of ethylene production could arise through the interaction of NO with SA and possibly H2O2.
RESULTS
Biphasic Ethylene Production in Tobacco in Response to Avirulent Bacterial Pathogens Contributes to the HR
Publicly held transcriptomic data suggest that genes encoding ethylene biosynthetic enzymes were up-regulated in Arabidopsis following challenge with avirulent bacteria (Supplemental Fig. S1). To investigate this further, we sought to exploit LPAD to measure online ethylene production in a tobacco-based pathosystem as the larger tobacco leaves offer a readily inoculable target tissue.
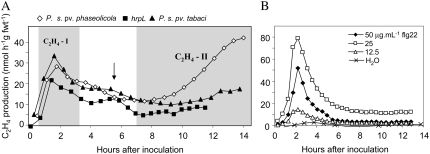
Examining ethylene production in tobacco leaves following inoculation with Psph strain 1448A revealed a biphasic pattern of ethylene production (Fig. 1A; Supplemental Table S1). The first rise in ethylene production (designated C2H4-I) appeared to be peak at about 2 h before declining. The second increase in ethylene production (designated C2H4-II) occurred 6 to 8 h after inoculation (hai) and persisted until at least 14 hai. P. s. pv tabaci (Pt) causes wild-fire disease symptoms in tobacco cv Samsun NN and elicited only a single peak in ethylene production, which corresponded closely in amplitude and timing to the C2H4-I seen when inoculating with Psph. A similar, single peak was measured when inoculating with a hrpL mutant (HrpL regulates expression of many genes involved in the Hrp/TTSS protein secretion machinery; Fouts et al., 2002) of Psph, which fails to elicit a HR. C2H4 production following inoculations with the flg22, which would be present in the Psph hrpL strain, was examined (Fig. 1B). Inoculating with various concentrations of flg22 initiated monophasic C2H4 production, which corresponded in timing to C2H4-I seen with the HR (Fig. 1A).
Figure 1.
Ethylene production in tobacco following challenge with P. syringae pathovars and flagellin. A, C2H4 production in tobacco leaves infiltrated with strains of P. syringae pathovars as detected using LPAD. The intercellular spaces in tobacco leaves were infiltrated with bacterial suspensions (2 × 106 colony-forming units mL−1) with the avirulent strain Psph, (⋄), the nonpathogenic and non-HR-eliciting derivative Psph hrpL (▪), and the virulent strain Pt (▴). The two rises associated with challenges with avirulent bacteria are indicated by shading and annotation as C2H4-I and C2H4-II. An increase in ethylene production, which may be elicited by the wounding associated with the injection procedure, is arrowed. B, C2H4 production elicited following infiltration with 50 (♦), 25 (□), and 12.5 (△) μg mL−1 Psph, flg22 with water (×) representing the negative control.
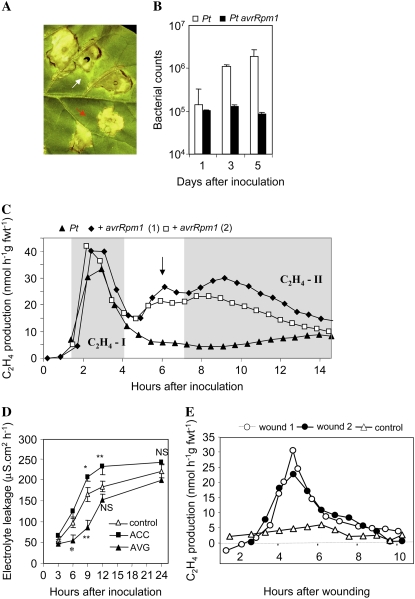
To investigate whether C2H4-II was specific to the HR, a Pt derivative into which the avirulence gene avrRpm1 had been introduced was generated. Several features of inoculation with Pt avrRpm1 suggested a HR was being elicited. Lesions formed when inoculating with Pt avrRpm1 lacked the chlorotic wild-fire symptoms seen with Pt (Fig. 2A) and in planta bacterial growth was only observed with populations of Pt, but not Pst avrRpm1 (Fig. 2B). Inoculation of tobacco with Pt avrRpm1 led to a major second period of production (Fig. 2C), suggesting that C2H4-II correlated with recognition of the avr gene that did not differ significantly (P = 0.272) from that elicited by Psph (Supplemental Table S1).
Figure 2.
The contribution of ethylene to the Psph-elicited HR in tobacco. A, Lesion phenotypes at 7 d following inoculation with Pt (white arrow; note, chlorotic halo) and a derived Pt avrRpm1 transconjugant (red arrow; note lack of chlorotic halo). B, Bacterial populations of Pt (□) and Pt avrRpm1 (▪) within 1-cm-diameter cores within infiltrated leaf areas of tobacco. Data are given as mean colony-forming units (n = 5 ± se). The black arrow indicates a subsidiary increase in ethylene production that may be due to wounding. See main text for details. C, Ethylene production in tobacco leaves infiltrated with Pt (▴) and Pt avrRpm1, for which the results of two replicates (+ avrRpm1, 1 [♦] and + avrRpm1, 2 [□]) are presented. The two rises associated with challenges with avirulent bacteria are indicated by shading and annotation as C2H4-I and C2H4-II. D, Electrolyte leakage from explants of tobacco leaves inoculated with either only the avirulent strain Psph (△) or with either 0.1 mm ACC (▪) or 0.1 mm AVG (▴). E, Ethylene production from tobacco leaves that had been multiply wounded by being struck with a wire brush. Data from two wounded leaves are given (wound 1, ○; wound 2, •) and from a detached leaf that had not been wounded with the wire brush (△).
Given the apparent AVR dependence of C2H4-II, a possible contribution of C2H4 to the HR was investigated. HR tobacco leaves were infiltrated with Psph with ACC or an ACS inhibitor, aminoethoxyvinylglycine (AVG). At 6 hai, AVG significantly (P < 0.01) decreased, whereas ACC increased (P < 0.05), electrolyte leakage elicited by Psph (Fig. 2D).
Because the inoculation procedure involved piercing with the syringe needle, the pattern of wound-associated ethylene was determined. Leaves were wounded by piercing with a wire brush and ethylene production increased after approximately 3 h and peaked at approximately 5 h, declining thereafter (Fig. 2E). This may correspond to a subsidiary peak in ethylene production seen at approximately 6 h following inoculation with Pt avrRpm1 (Fig. 2C, arrow).
Kinetics of Ethylene Production Is Influenced by SA
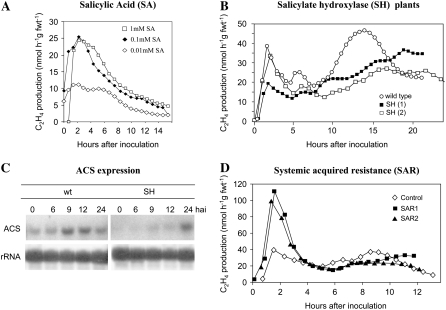
The biphasic pattern of the oxidative burst is influenced by SA (Shirasu et al., 1997; Mur et al., 2000); hence, the effects of SA on ethylene production were assessed. Different concentrations of SA were injected into tobacco leaves and stimulated a rapid rise in ethylene production, which declined after approximately 2 h (Fig. 3A). To examine the effect of SA on ethylene production within a HR, Psph was inoculated into leaves of cauliflower mosaic virus 35S salicylate hydroxylase (SH) transgenic tobacco plants, which degrade SA to catechol. Ethylene production was perturbed in SH tobacco leaves with C2H4-I being reduced and C2H4-II somewhat delayed and reduced in amplitude compared to wild-type plants (Fig. 3B).
Figure 3.
The influence of SA on Psph-elicited ethylene production and ACS expression in tobacco. A, Ethylene production in leaves of wild-type cv Samsun NN tobacco leaves injected with 0.01 (⋄), 0.1 (♦), and 1 (□) mm SA. B, Ethylene produced from wild type (○) and two replicates of SH (35S-SH, 1 [▪]; 35S-SH, 2 [□]) expressing tobacco plants challenged with Psph (C). Expression of ACS in tobacco at different hai of wild type and SH tobacco with Psph as detected using northern hybridization. A gene probe for the Arabidopsis ACS6 (At4g11280) was used as this exhibited high homology to stress-activated tobacco NtACS2. D, The lower-most leaves of tobacco plants were inoculated with Psph at several points over the leaf to form a patchwork of HR lesions. In control plants, the lower leaves were inoculated with water. After 7 d, the upper leaves of all plants were inoculated with Psph and ethylene production was measured. Results are given from two leaves from plants which had been first inoculated with Psph (SAR1, ▪; SAR2, ▴) and leaf from a plant which had been first injected with water (control, ⋄). Some of the data presented in Figure 3A have been published in conference proceedings (Mur et al., 2003).
SA could influence ethylene production, at least in part, by altering ACS transcription. Hence, ACS expression following Psph challenge in wild-type and SH tobacco was investigated by northern blotting. An Arabidopsis gene probe for ASC6 was used because this exhibits the highest homology to the stress-responsive NtACS2 gene (Lei et al., 2000). Psph induced ACS expression 6 hai, apparently peaking at approximately 12 h before reducing by 24 hai (Fig. 3C). In SH tobacco, the baseline expression of ACS appeared to be greatly reduced and only weak up-regulation was detected at 9 and 12 hai, although expression was much higher at 24 hai.
Some workers (e.g. Heck et al., 2003) have cautioned against relying solely on NahG transgenic lines to indicate SA effects. Therefore, ethylene production was examined in leaves exhibiting systemic acquired resistance (SAR), a SA-mediated phenomenon, following inoculation with Psph. The lower-most leaves of tobacco were inoculated with Psph or mock inoculated with water and then maintained under controlled environmental conditions for 5 d after inoculation (dai). Psph-inoculated tobacco exhibits SAR after 3 dai (Mur et al., 1996); hence, at 5 dai, single upper leaves of SAR-exhibiting tobacco plants and water-inoculated controls were injected with Psph. In Psph inoculation of SAR-exhibiting tissue, C2H4-I was significantly (P < 0.001) augmented compared to Psph inoculation of control plants. C2H4-II in SAR tissue was not significantly (P = 0.09) different from controls (Fig. 3D; Supplemental Table S2).
SA can act by influencing the generation of ROS to which C2H4 could act to augment (de Jong et al., 2002) or increase production in response to oxidative stress (Supplemental Fig. S1), likely in a positive feedback loop. Injections of either 1 mm methyl viologen or Glc:Glc oxidase (G:GO) elevated ethylene production (Supplemental Fig. S2). Hence, the observed patterns of ethylene production are likely to be linked to the well-established H2O2-SA interaction during defense (Lamb and Dixon, 1997).
NO Cooperates with SA to Initiate C2H4-II Ethylene Production during a Psph-Elicited HR in Tobacco
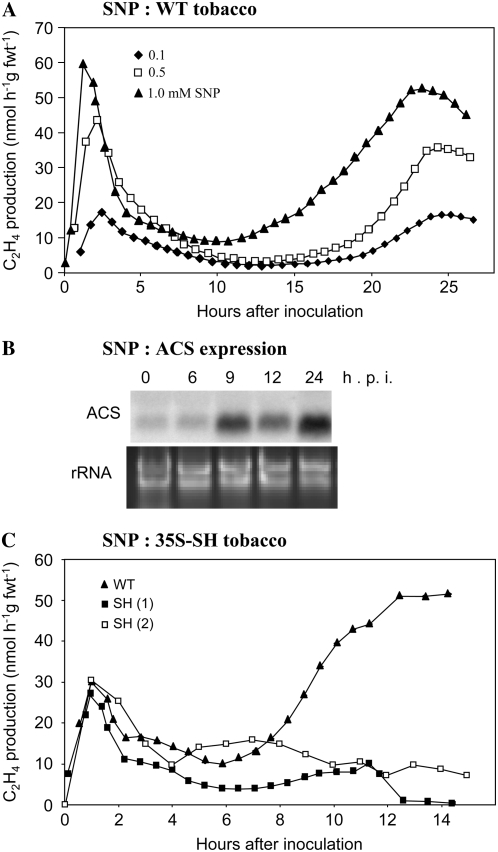
Along with SA and the oxidative burst, the generation of NO is also a feature of the HR. To investigate the effects of NO on ethylene production, various concentrations of the NO+ donor, sodium nitroprusside (SNP), were inoculated into tobacco leaves (Fig. 4A). Notably, injections of SNP, especially 1 and 0.5 mm SNP, induced a biphasic ethylene generation pattern. Injecting NO-exhausted solutions of SNP did not initiate ethylene production (data not shown). SNP was observed to up-regulate ACS expression, suggesting that, at least in part, increased ethylene production reflected up-regulation of biosynthetic genes (Fig. 4B).
Figure 4.
SNP-elicited ethylene production in tobacco leaves. A, Ethylene production was measured using LPAD from tobacco leaves following injection with 0.1 (♦); 0.5 (□), and 1 (▴) mm SNP. Inset, NtACS expression in tobacco following the application of SNP. B, Expression of ACS in tobacco at different hours postinjection of wild-type tobacco with 1 mm SNP. A gene probe for the Arabidopsis ACS6 (At4g11280) was used because this exhibited high homology to stress-activated tobacco NtACS2. C, Ethylene generation in wild-type Samsun (▴) and in two replicate leaves (SH, 1 [▪] and SH, 2 [□]) at various hai with 1 mm SNP. Some of the data presented in Figure 5A have been published in conference proceedings (Mur et al., 2003).
NO initiates SA synthesis (Durner et al., 1998) and SNP increased levels of SA accumulation at 24 h (data not shown). When injecting SNP into SH tobacco, C2H4-I was unaffected, but C2H4-II was clearly perturbed compared to SNP injected into wild-type tobacco controls (Fig. 4C). Given that NO generation from SNP will be unaffected by the SH transgene, we hypothesized that, during the HR, NO could influence the biphasic pattern of ethylene production through a SA-independent mechanism during C2H4-I and an SA-dependent mechanism during C2H4-II.
NO Production during the Psph-Elicited HR in Tobacco Influences C2H4-II
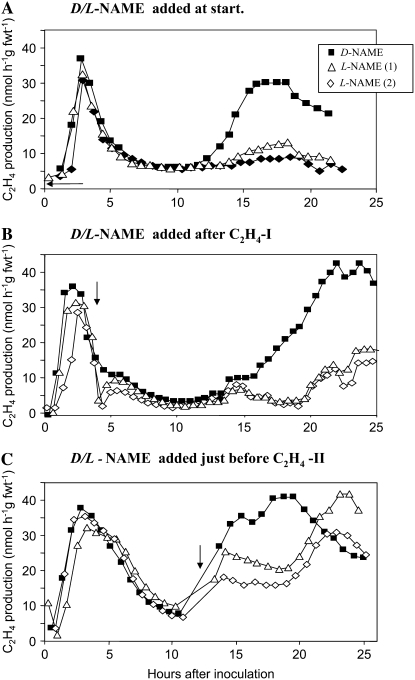
The effects of NO generation during the Psph-elicited HR on ethylene production were tested using the mammalian NO synthase (NOS) inhibitor NG-nitro-l-Arg methyl ester (l-NAME). This has proven to effectively suppress Psph-elicited NO generation, whereas the stereoisomer d-NAME had no detectable effect (Mur et al., 2005b). Addition of 1 mm l/d-NAME had no effect on the growth of Psph nutrient broth cultures and therefore was judged not to directly affect the bacteria (data not shown). When l-NAME was coinfiltrated with Psph, C2H4-I was not affected; however, C2H4-II was greatly suppressed compared to d-NAME-treated controls (Fig. 5A). To investigate whether only early NO generation was important for C2H4-II control, either l-NAME or d-NAME was injected into Psph-challenged tobacco leaves after C2H4-I (Fig. 5B) or just before the first signs of increased ethylene production linked to C2H4-II (Fig. 5C). When injecting with l-NAME at either time point, Psph-elicited C2H4-II was suppressed, indicating that contemporaneous NO generation was required for biphasic ethylene production. However, C2H4-II was more effectively suppressed when l-NAME was coinjected with Psph (Fig. 5A) or just after C2H4-I (Fig. 5B). Indeed, application of l-NAME appeared to only delay the C2H4-II rise (Fig. 5C). These series of experiments were repeated with the NO scavenger, CPTIO, which yielded similar data except that the extent of suppression was not as great as seen with l-NAME, most likely due to the photolability of CPTIO (Supplemental Table S2). Analysis of the l-NAME-perturbed patterns of ethylene production suggested that this reduced the effects of AVR recognition (Supplemental Fig. S3).
Figure 5.
The effects of l/d-NAME on ethylene production elicited in tobacco following challenge with Psph. The leaves of wild-type tobacco leaves were inoculated with the avirulent Psph strain and the ethylene production measured by LPAD. The effects of adding 1 mm d-NAME (▪) or in two replicates 1 mm l-NAME, 1 [△]; l-NAME, 2 [⋄]) at first injection with Psph (A), just after the first transient peak in ethylene generation (C2H4-I; B), or at the first sign of the second rise in production (C2H4-II; C). The times of injection with either l- or d-NAME are indicated with arrows.
DISCUSSION
The roles of ethylene in the HR remain somewhat obscure. Different groups have noted normal HR formation in Arabidopsis (Bent et al., 1992) and tomato (Solanum lycopersicum; Hoffman et al., 1999; Ciardi et al., 2000) lines where ethylene signaling was perturbed. However, HR-elicited ethylene production has also been frequently noted (Knoester et al., 1995; Lasserre et al., 1997) and tobacco mosaic virus-elicited HR lesion formation was delayed in ethylene-insensitive tobacco plants (Knoester et al., 2001). We noted that the kinetics of Psph-elicited HR, as revealed by electrolyte leakage, could be modulated by either adding the C2H4 precursor ACC or the ethylene biosynthesis inhibitor AVG (Fig. 2D).
To substantiate its link with Psph-elicited HR in tobacco, we determined C2H4 production following whole-leaf inoculation with bacterial suspensions. LPAD is a particularly appropriate method to do this because it allows online in planta measurements. LPAD indicated that C2H4 production from tobacco leaves challenged with avirulent Psph bacteria conformed to two main phases. This pattern was reminiscent of the biphasic oxidative burst and appeared to reflect similar elicitory steps (Lamb and Dixon, 1997). The first rise (C2H4-I) was common to leaves inoculated with avirulent (Psph, Pt avrRpm1), virulent (Pt), and hrp-compromised strains (Figs. 1A and 2C), implying a non-AVR elicitation event. Indeed, the PAMP-flg22 was an effective initiator of ethylene production, which was similar in its kinetics to C2H4-I (Figs. 1A and 2C; Supplemental Fig. S4). Another prediction of the biphasic model is that the second rise is AVR dependent. Data to support this were obtained from the C2H4-II observed with a Pt avrRpm1 transconjugant strain, but was not detected with the parental Pt strain (Fig. 2C).
It is possible that the bacteria contributed to the observed ethylene production. In P. syringae pathovars, ethylene can be produced by EFE acting on 2-oxoglutarate (Fukuda et al., 1993). With certain P. s. pv glycinea interactions with soybean (Glycine max), the majority of ethylene is derived from the pathogen and acts as a virulence factor, contributing to symptom development (Weingart et al., 2001). However, mutation of the Psph efe gene did not affect virulence and, more pertinently, screens of bean-adapted Psph strains failed to find evidence of ethylene production (Weingart and Volksch, 1997). Further, there is no efe-annotated open reading frame in the Psph 1448A genome sequence (http://pseudomonas-syringae.org); hence, it is probable that the contribution of Psph to the observed ethylene production was likely to be negligible. The paucity of Pt genomic sequence data means that ethylene production by this species cannot be ruled out, although Weingart and Volksch (1997) found no evidence of in vitro ethylene production in the four strains of Pt that they screened.
A feature of the biphasic oxidative burst is its modulation by SA (Shirasu et al., 1997; Mur et al., 2000); hence, an important priority was to establish how far SA also influenced biphasic ethylene production. SH-expressing transgenic plants have been used to show that ozone and X. campestris pv campestris-elicited ethylene production was influenced by SA (Rao et al., 2002; O'Donnell et al., 2003). Our experiments using SH transgenic plants demonstrated that Psph-elicited C2H4-II was influenced by SA (Fig. 3B). The link with SA was substantiated when Psph-elicited C2H4-I was potentiated in SAR-exhibiting tissue (Fig. 3D).
Pathogen-challenge is perhaps too complicated a stimulus to allow the signal interactions influencing C2H4-I and C2H4-II to be readily deduced. Hence, the effect of adding various defense signals on ethylene production in tobacco leaves was assessed. H2O2 has been proposed to orchestrate plant defense (Levine et al., 1994) and could initiate the biosynthesis of ethylene. Equally, ethylene has been shown be required for the initiation of oxidative signaling in stomata (Desikan et al., 2006) and in tomato suspension cells (de Jong et al., 2002). Such data could suggest the existence of a positive feedback loop based on H2O2 and ethylene that could explain the observed biphasic generation pattern. Some evidence for this was provided by Moeder et al. (2002), who noted that ozone induced biphasic expression patterns in tomato ACS gene expression, with the second increase being dependent on previous ethylene production. In this study, G:GO was injected into the apoplast to generate H2O2 at levels that we had previously shown to be an effective initiator of plant defense (Mur et al., 2005a). G:GO only produced a single burst of ethylene production (Supplemental Fig. S2) in marked contrast to ethylene production following injection of SNP, an NO+ donor that is a potent nitrosylating agent, but subsequently releases gaseous NO following electrophilic attack (Membrillo-Hernandez et al., 1998), which we have previously measured (Mur et al., 2006). Crucially, addition of SNP resulted in biphasic patterns of ethylene biosynthesis (Fig. 4A) and, just as during the HR, the second rise in ethylene was perturbed in SH transgenic tobacco plants. As SNP induced SA biosynthesis, it could be that SA alone was required to initiate the biphasic pattern, but when this was injected into plants, only a single burst of C2H4 biosynthesis was initiated (Fig. 3A). Given that NO generation would be unaffected by the SH transgene, we interpret these data as suggesting that NO is required for the biphasic switch, but needed SA to set its kinetics. To substantiate this hypothesis, when l-NAME was added to developing HR lesions, only C2H4-II was affected (Fig. 5). Intriguingly, when plotting C2H4-I/ C2H4-II height and area ratios, infiltration with l-NAME shifted ethylene biosynthetic patterns toward interactions where there is no AVR recognition (Supplemental Fig. S3). This experiment assumed that l-NAME primarily affected plant-derived NO. Whereas no gene has been annotated as a NOS in the Psph strain 1448A genome, two are to be found within Psph B728a (Psyr 1024, Psyr 3724), which have orthologs in Psph (Pspph 1075; Pspph 3724; http://pseudomonas-syringae.org). Hence, although we have never noted NO production from Psph cultures, some in planta generation cannot be ruled out.
Psph-elicited C2H4-I appeared not to be affected by l-NAME (Fig. 5A), which was surprising given that SNP could initiate biphasic ethylene biosynthesis (Fig. 4A). It may be that C2H4-I is mostly regulated by H2O2 or linked signals (Supplemental Fig. S3) so that there is functional redundancy in an NO role in initiating C2H4-I. It is notable that timing (hai) if not amplitude of pathogen-elicited C2H4-I was similar and could be partially replicated by both SNP and G:GO (Supplemental Fig. S4). This could explain why C2H4-I is generated by the Psph hrpL mutant (but not C2H4-II) when this strain elicits negligible levels of NO (Mur et al., 2006) but a normal H2O2-I (Lamb and Dixon, 1997). Because AVG suppressed cell death at 6 h (Fig. 2D; i.e. prior to C2H4-II), this would implicate C2H4-I as a contributor to HR cell death. A contribution by H2O2 to C2H4-I to help drive C2H4-II could explain the latter's relative delay when elicited by SNP (where NO and SA are produced) compared to avirulent bacteria (where NO, SA, and ROS are produced). It seems likely that, as with the oxidative burst (Shirasu et al., 1997), an early transient rise influences the second peak in biphasic patterns of signal generation.
Our hypothesis is apparently at odds with the literature, which suggests an inverse relationship between NO and ethylene production during senescence (Leshem, 2000) and, further, in NO-deficient NO dioxygenase Arabidopsis plants. ACS6 expression was suppressed and senescence was delayed (Mishina et al., 2007). However, our data need not contradict such observations. Our measurements of NO generation from SNP show consistent production over at least a 24-h period (Mur et al., 2005). During this period, ethylene levels both increase and decrease, perhaps suggesting that NO has both initiatory and suppressive roles. It must also be acknowledged that the HR and senescence are very different phenomena. It seems likely that the outcomes of NO and ethylene interactions vary temporally and in different contexts.
A major task for future studies is to integrate PAMP/AVR elicitory events on ethylene production during a HR into a coherent regulatory pattern. Taking our data together, H2O2-I, SA, and possibly NO are likely to contribute to the generation of C2H4-I. It has been suggested that the second phase is initiated at AVR recognition (Draper, 1997); however, we alternatively suggest that this represents the triggering of a biphasic switch, as a result of a high level of NO generation (Mur et al., 2005b, 2006). Such a model could explain the biphasic waves observed in the absence of combinations of bacterial elicitors. For example, fumigation with ozone alone proved to be sufficient to trigger a biphasic oxidative burst (Schraudner et al., 1998) and, in line with our hypothesis, ozone has been shown to elicit a rapid rise in NO (Ederli et al., 2006). Further, it is possible to extrapolate from biphasic transcription of ethylene biosynthetic genes in tomato following treatment with ozone (Moeder et al., 2002) to suggest that a similar mechanism occurs during HR. Our data (Figs. 3C and 4C) also suggest that NO/SA influences the transcription of ACS; hence, the biphasic switch could arise from defined transcriptional events. More defined expression studies are required than carried out in this study to substantiate this hypothesis. The importance of the biphasic switch lies in its vital role in reiterating events occurring during the first phase, but in a more potent manner, and hence aids in conferring resistance. Applying this model to SAR tissue, the preexistence of SA would lead to the potentiated first phase seen in Figure 3D. It is currently unclear why a potentiated C2H4-II should not also feature during SAR, but it may be that this is already at maximal levels. Validation of such a model undoubtedly requires testing using various Arabidopsis mutants, but such interactions seem appropriate for further data mining mathematical modeling so that greater understanding of the actions of ethylene can be obtained.
MATERIALS AND METHODS
Plant Growth and Chemicals
Tobacco (Nicotiana tabacum ‘Samsun NN’) was germinated in Levingtons Universal Compost (Levingtons Horticulture) and transferred to John Innes Number 2 compost after 2 weeks. Tobacco plants were injected with bacterial suspensions or chemicals at 5 to 6 weeks following germination. All plants were grown at 22°C under a 16-h photoperiod. Solutions of SNP (Sigma-Aldrich Company Ltd.) where NO had been exhausted (Na2 [Fe (CN) 5 NO] + hv → [Fe (CN) 5]3− + ·NO) were generated by incubation under light for >48 h.
Phytopathogenic Bacteria Strains
Psph race 6 strain 1448 elicits a nonhost HR on tobacco, a trait that is abolished in the hrpL mutant derivative (Rahme et al., 1991). Pt strain 11528R forms disease symptoms on tobacco (Thilmony et al., 1995). The avrRpm1 avirulence gene, cloned on pVSP61B (Bisgrove et al., 1994), was introduced into Pt strain 11528R by triparental mating as described by Dangl et al. (1992).
Each pathogen was grown at 28°C in nutrient agar (Oxoid Limited). The culture was washed twice with sterile distilled water and finally diluted to 106 colony-forming units mL−1 based on spectrophotometric readings (Mur et al., 2000). The resulting bacterial suspensions were injected into the intercellular spaces of the entire leaf using a 5-mL syringe (Asahi Techno Glass) with a 2.5-gauge 5/8 needle (Microlance; Becton-Dickinson & Co. Ltd.).
Northern Hybridization
RNA extraction, northern blotting, and hybridization were undertaken as described in Draper et al. (1988). A 1.2-kb probe for the Arabidopsis (Arabidopsis thaliana) ACS6 (At4g11280) using the specific primers 5′-AAATCAACTTGATAGTCG-3′ and 5′-TCTGTTTAGCTAATCCCGGC-3′ had been previously generated (David Chrimes, Aberystwyth, UK) and exhibited the highest homology (E-value 2 × 10−8) to stress-activated NtACS2 (Lei et al., 2000), which was used to suggest tobacco ACS transcript accumulation. Each northern hybridization experiment was undertaken at least twice, yielding similar results.
Estimations of Cell Death by Electrolyte Leakage
Changes in the conductivity of the solution bathing 1-cm-diameter leaf explants were determined as stated in Mur et al. (2000). Significance testing employed ANOVA, using MiniTab version 13.
LPAD
Ethylene production was monitored in real time by LPAD, basically as described by Cristescu et al. (2002). Briefly, a line-tunable CO2 laser emits 9- to 11-μm infrared light into a photoacoustic cell. A line with carrier gas (scrubbed air) passed through the cuvette with the infected tobacco leaves into the photoacoustic cell. The evolved gases in the air flow were detected via their absorption of rapidly chopped infrared light, which generated pressure variations, resulting in acoustic energy detected by a miniature microphone (Bijnen et al., 1996). The amplitude of the acoustic waves is directly proportional to the concentration of ethylene in the photoacoustic cell. Ethylene gas mixtures are sensitively measured by the laser-based ethylene detector due to the distinct fingerprint-like spectrum of ethylene in the CO2 laser wavelength range (Brewer et al., 1982). A system of valves allows three cuvettes with biological samples to be measured in sequence. Each cuvette was measured for 20 min. When not being measured, the gas flow through the cuvette was maintained, but was vented into the atmosphere rather than passed into the photoacoustic chamber. All data are corrected for weight. Repeated inoculations with water or 10 mm MgCl2 elicited trivial levels of C2H4 (approximately 5 nmol h−1 g−1 fresh weight), which was monophasic (data not shown).
Replication of LPAD Measurements
The photoacoustic system was organized to sample from one of three cuvettes for 20 min before moving on to the next. Hence, each figure gives the results from a single experiment (i.e. three traces), one from each cuvette. Each experiment was repeated at least three times, on separate days and plants, giving similar trends. Although plant/leaf age and the stage of bacterial culture used as an inoculum period was standardized between experiments, the interval between the first and second peaks of ethylene when challenging with identical strains varied between experiments (e.g. compare the results with Psph in Figs. 1A, 3B, and 5) when undertaken on separate days. As a result, the data from different experiments could not be pooled if the pattern of ethylene production, which was the major theme of this work, was to be clearly discerned. Other parameters describing features in the patterns of ethylene production where determined using Origin Pro 7 (Origin Lab Corporation) and are given as supplemental data.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Expression of ethylene biosynthetic genes in Arabidopsis following pathogenic challenge or treatment with defense-associated chemicals.
Supplemental Figure S2. Ethylene production initiated by oxidative stress.
Supplemental Figure S3. The effect of suppressing NO levels on the patterns of ethylene production during a HR elicited by Psph in tobacco.
Supplemental Figure S4 The timing and maximal rates of ethylene production of C2H4-I.
Supplemental Table S1. Peak characteristics of ethylene production in tobacco in response to P. syringae pathovars.
Supplemental Table S2. The influence of SA on ethylene production in tobacco in response to P. syringae.
Supplemental Table S3. The effect of NO suppression on characteristics of ethylene production in tobacco in response to Psph.
Supplementary Material
Acknowledgments
We thank Gerard van der Weerden and Walter Hendrickx in Nijmegen, The Netherlands, and Tom Thomas (University of Wales, Aberystwyth, UK) for growing and maintaining the tobacco plants. The P. syringae strains were the kind gift of Prof. John Mansfield (Wye College, Imperial College, UK). We appreciate the help provided by Dr. Amanda Lloyd and Dr. Paul Kenton (Aberystwyth, UK) and Dr. Galya Novikova (Timiriazev Institute of Plant Physiology, Moscow) with ideas and manuscript preparation.. We are grateful to Prof. Lozanka Popova, Editor in Chief of General and Applied Plant Physiology, for permission to include data from Mur et al. (2003) from the Proceedings of the European Workshop on Environmental Stress and Sustainable Agriculture, September 7-12, 2002, Varna, Bulgaria.
This work was supported by UK license PHF 123A/3624 and by the European Union (EU), Access to Research Infrastructure Action of the Improving Human Potential Program. The Nijmegen facility has been funded by the EU to act as a service unit for the measurement of trace gases. Scientists may apply to http://www.tracegasfac.science.ru.nl/index.html for use.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Luis A.J. Mur (lum@aber.ac.uk).
The online version of this article contains Web-only data.
References
- Alfano JR, Collmer A (2004) Type III secretion system effector proteins: double agents in bacterial disease and plant defense. Annu Rev Phytopathol 42 385–414 [DOI] [PubMed] [Google Scholar]
- Ausubel FM (2005) Are innate immune signaling pathways in plants and animals conserved? Nat Immunol 6 973–979 [DOI] [PubMed] [Google Scholar]
- Bailey BA, Taylor R, Dean JF, Anderson JD (1991) Ethylene biosynthesis-inducing endoxylanase is translocated through the xylem of Nicotiana tabacum cv Xanthi Plants. Plant Physiol 97 1181–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenghi B, Acconcia F, Trovato M, Perazzolli M, Bocedi A, Polticelli F, Ascenzi P, Delledonne M (2003) AtCYS1, a cystatin from Arabidopsis thaliana, suppresses hypersensitive cell death. Eur J Biochem 270 2593–2604 [DOI] [PubMed] [Google Scholar]
- Bent A, Innes R, Ecker J, Staskawitcz B (1992) Disease development in ethylene-insensitive Arabidopsis thaliana infected with virulent and avirulent Pseudomonas and Xanthomonas pathogens. Mol Plant Microbe Interact 5 372–378 [DOI] [PubMed] [Google Scholar]
- Berrocal-Lobo M, Molina A, Solano R (2002) Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J 29 23–32 [DOI] [PubMed] [Google Scholar]
- Bijnen FGC, Reuss J, Harren FJM (1996) Geometrical optimization of a longitudinal resonant photoacoustic cell for sensitive and fast trace gas detection. Rev Sci Instrum 67 2914–2923 [Google Scholar]
- Bisgrove SR, Simonich MT, Smith NM, Sattler A, Innes RW (1994) A disease resistance gene in Arabidopsis with specificity for two different pathogen avirulence genes. Plant Cell 6 927–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16 1–18 [DOI] [PubMed] [Google Scholar]
- Brewer RJ, Bruce CW, Mater JL (1982) Optoacoustic spectroscopy of C2H4. Appl Opt 21 4092–4100 [DOI] [PubMed] [Google Scholar]
- Chen N, Goodwin PH, Hsiang T (2003) The role of ethylene during the infection of Nicotiana tabacum by Colletotrichum destructivum. J Exp Bot 54 2449–2456 [DOI] [PubMed] [Google Scholar]
- Ciardi JA, Tieman DM, Lund ST, Jones JB, Stall RE, Klee HJ (2000) Response to Xanthomonas campestris pv. vesicatoria in tomato involves regulation of ethylene receptor gene expression. Plant Physiol 123 81–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A, Desikan R, Hurst RD, Hancock J, Neill SJ (2000) NO way back: nitric oxide and programmed cell death in Arabidopsis thaliana suspension cultures. Plant J 24 667–677 [DOI] [PubMed] [Google Scholar]
- Chamnongpol S, Willekens H, Moeder W, Langebartels C, Sandermann H Jr, Van Montagu M, Inze D, Van Camp W (1998) Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proc Natl Acad Sci USA 95 5818–5823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristescu SM, De Martinis D, Te Lintel Hekkert S, Parker DH, Harren FJ (2002) Ethylene production by Botrytis cinerea in vitro and in tomatoes. Appl Environ Microbiol 68 5342–5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl JL, Ritter C, Gibbon MJ, Mur LAJ, Wood JR, Goss S, Mansfield JW, Taylor JD, Vivian A (1992) Functional homologs of the Arabidopsis RPM1 disease resistance gene in bean and pea. Plant Cell 4 1359–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong AJ, Yakimova ET, Kapchina VM, Woltering EJ (2002) A critical role for ethylene in hydrogen peroxide release during programmed cell death in tomato suspension cells. Planta 214 537–545 [DOI] [PubMed] [Google Scholar]
- Delledonne M, Xia Y, Dixon RA, Lamb C (1998) Nitric oxide functions as a signal in plant disease resistance. Nature 394 585–588 [DOI] [PubMed] [Google Scholar]
- Delledonne M, Zeier J, Marocco A, Lamb C (2001) Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA 98 13454–13459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Last K, Harrett-Williams R, Tagliavia C, Harter K, Hooley R, Hancock JT, Neill SJ (2006) Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J 47 907–916 [DOI] [PubMed] [Google Scholar]
- Dixon RA, Achnine L, Kota P, Liu CJ, Reddy MSS, Wang L (2002) The phenylpropanoid pathway and plant defence—a genomics perspective. Mol Plant Pathol 3 371–390 [DOI] [PubMed] [Google Scholar]
- Draper J (1997) Salicylate, superoxide synthesis and suicide in plant defence. Trends Plant Sci 2 162–165 [Google Scholar]
- Durner J, Wendehenne D, Klessig DF (1998) Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Natl Acad Sci USA 95 10328–10333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker JR, Davis RW (1987) Plant defense genes are regulated by ethylene. Proc Natl Acad Sci USA 84 5202–5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ederli L, Morettini R, Borgogni A, Wasternack C, Miersch O, Reale L, Ferranti F, Tosti N, Pasqualini S (2006) Interaction between nitric oxide and ethylene in the induction of alternative oxidase in ozone-treated tobacco plants. Plant Physiol 142 595–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouts DE, Abramovitch RB, Alfano JR, Baldo AM, Buell CR, Cartinhour S, Chatterjee AK, D'Ascenzo M, Gwinn ML, Lazarowitz SG, et al (2002) Genome wide identification of Pseudomonas syringae pv. tomato DC3000 promoters controlled by the HrpL alternative sigma factor. Proc Natl Acad Sci USA 19 2275–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H, Ogawa T, Tanase S (1993) Ethylene production by micro-organisms. Adv Microb Physiol 35 275–306 [DOI] [PubMed] [Google Scholar]
- Grant M, Brown I, Adams S, Knight M, Ainslie A, Mansfield JW (2000) The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant J 23 441–450 [DOI] [PubMed] [Google Scholar]
- Grun S, Lindermayr C, Sell S, Durner J (2006) Nitric oxide and gene regulation in plants. J Exp Bot 57 507–516 [DOI] [PubMed] [Google Scholar]
- Heck S, Grau T, Buchala A, Metraux JP, Nawrath C (2003) Genetic evidence that expression of NahG modifies defence pathways independent of salicylic acid biosynthesis in the Arabidopsis-Pseudomonas syringae pv. tomato interaction. Plant J 36 342–352 [DOI] [PubMed] [Google Scholar]
- Hoffman T, Schmidt JS, Zheng X, Bent AF (1999) Isolation of ethylene-insensitive soybean mutants that are altered in pathogen susceptibility and gene-for-gene disease resistance. Plant Physiol 119 935–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenton P, Mur LAJ, Wasternack C, Atzorn R, Draper J (1999) (-)-Jasmonic acid rises during the hypersensitive response in tobacco. Mol Plant Microbe Interact 12 74–78 [Google Scholar]
- Kenyon JS, Turner JG (1992) The stimulation of ethylene synthesis in Nicotiana tabacum leaves by the phytotoxin coronatine. Plant Physiol 100 219–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MG, da Cunha L, McFall AJ, Belkhadir Y, DebRoy S, Dangl JL, Mackey D (2005) Two Pseudomonas syringae type III effectors inhibit RIN4-regulated basal defense in Arabidopsis. Cell 121 749–759 [DOI] [PubMed] [Google Scholar]
- Knoester M, Bol JF, van Loon LC, Linthorst HJ (1995) Virus-induced gene expression for enzymes of ethylene biosynthesis in hypersensitively reacting tobacco. Mol Plant Microbe Interact 8 177–180 [DOI] [PubMed] [Google Scholar]
- Knoester M, Linthorst HJM, Bol JF, Van Loon LC (2001) Involvement of ethylene in lesion development and systemic acquired resistance in tobacco during the hypersensitive reaction to tobacco mosaic virus. Physiol Mol Plant Pathol 59 45–57 [Google Scholar]
- Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48 251–275 [DOI] [PubMed] [Google Scholar]
- Lasserre E, Godard F, Bouquin T, Hernandez JA, Pech JC, Roby D, Balague C (1997) Differential activation of two ACC oxidase gene promoters from melon during plant development and in response to pathogen attack. Mol Gen Genet 256 211–222 [DOI] [PubMed] [Google Scholar]
- Lei G, Liu JZ, Wong WS, Hsiao WLW, Chong K, Xu CZK, Yang SF, Kung SD, Li N (2000) Identification of a novel multiple environmental factor responsive 1-aminocyclopropano-1-carboxylatosythase gene, NT-ACS2, from tobacco. Plant Cell Environ 23 1169–1182 [Google Scholar]
- Leshem YY (2000) Nitric Oxide in Plants. Function, Occurrence and Use. Kluwer Academic Publishers, Dordrecht, The Netherlands
- Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79 583–593 [DOI] [PubMed] [Google Scholar]
- Lund ST, Stall RE, Klee HJ (1998) Ethylene regulates the susceptible response to pathogen infection in tomato. Plant Cell 10 371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GB, Bogdanove AJ, Sessa G (2003) Understanding the functions of plant disease resistance proteins. Annu Rev Plant Biol 54 23–61 [DOI] [PubMed] [Google Scholar]
- Membrillo-Hernandez J, Coopamah MD, Channa A, Hughes MN, Poole RK (1998) A novel mechanism for upregulation of the Escherichia coli K-12 hmp (flavohaemoglobin) gene by the ‘NO releaser’, S-nitrosoglutathione: nitrosation of homocysteine and modulation of MetR binding to the glyA-hmp intergenic region. Mol Microbiol 29 1101–1112 [DOI] [PubMed] [Google Scholar]
- Mishina TE, Lamb C, Zeier J (2007) Expression of a nitric oxide degrading enzyme induces a senescence programme in Arabidopsis. Plant Cell Environ 30 39–52 [DOI] [PubMed] [Google Scholar]
- Moeder W, Barry CS, Tauriainen AA, Betz C, Tuomainen J, Utriainen M, Grierson D, Sandermann, H, Langebartels C, Kangasjarvi J (2002) Ethylene synthesis regulated by biphasic induction of 1-aminocyclopropane-1-carboxylic acid synthase and 1-aminocyclopropane-1-carboxylic acid oxidase genes is required for hydrogen peroxide accumulation and cell death in ozone-exposed tomato. Plant Physiol 130 1918–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur LA, Carver TL, Prats E (2006) NO way to live; the various roles of nitric oxide in plant-pathogen interactions. J Exp Bot 57 489–505 [DOI] [PubMed] [Google Scholar]
- Mur LAJ, Brown IR, Darby RM, Bestwick CS, Bi YM, Mansfield JW, Draper J (2000) A loss of resistance to avirulent bacterial pathogens in tobacco is associated with the attenuation of a salicylic acid-potentiated oxidative burst. Plant J 23 609–621 [DOI] [PubMed] [Google Scholar]
- Mur LAJ, Kenton P, Draper J (2005. a) In planta measurements of oxidative bursts elicited by avirulent and virulent bacterial pathogens suggests that H2O2 is insufficient to elicit cell death in tobacco. Plant Cell Environ 28 548–561 [Google Scholar]
- Mur LAJ, Naylor G, Warner SAJ, Sugars JM, White RF, Draper J (1996) Salicylic acid potentiates defence gene expression in tissue exhibiting acquired resistance to pathogen attack. Plant J 9 559–571 [Google Scholar]
- Mur LAJ, Santosa EJ, Laarhoven LJ, Harren FJ, Smith AR (2003) A new partner in the danse macabre: The role of nitric oxide in the hypersensitive response. Bull J Plant Physiol (Special Issue) 110–123
- Mur LAJ, Santosa IE, Laarhoven LJ, Holton NJ, Harren FJ, Smith AR (2005. b) Laser photoacoustic detection allows in planta detection of nitric oxide in tobacco following challenge with avirulent and virulent Pseudomonas syringae pathovars. Plant Physiol 138 1247–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman-Setterblad C, Vidal S, Palva ET (2000) Interacting signal pathways control defense gene expression in Arabidopsis in response to cell wall-degrading enzymes from Erwinia carotovora. Mol Plant Microbe Interact 13 430–438 [DOI] [PubMed] [Google Scholar]
- O'Donnell PJ, Schmelz EA, Moussatche P, Lund ST, Jones JB, Klee HJ (2003) Susceptible to intolerance-a range of hormonal actions in a susceptible Arabidopsis pathogen response. Plant J 33 245–257 [DOI] [PubMed] [Google Scholar]
- Parker JE (2003) Plant recognition of microbial patterns. Trends Plant Sci 8 245–247 [DOI] [PubMed] [Google Scholar]
- Penninckx IA, Thomma BP, Buchala A, Metraux JP, Broekaert WF (1998) Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 10 2103–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahme LG, Mindrinos MN, Panopoulos NJ (1991) Genetic and transcriptional organization of the hrp cluster of Pseudomonas syringae pv. phaseolicola. J Bacteriol 173 575–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Lee HI, Davis KR (2002) Ozone-induced ethylene production is dependent on salicylic acid, and both salicylic acid and ethylene act in concert to regulate ozone-induced cell death. Plant J 32 447–456 [DOI] [PubMed] [Google Scholar]
- Schraudner M, Moeder W, Wiese Van Camp W, Inzém D, Langebartelsm C, Sandermann H (1998) Ozone-induced oxidative burst in the ozone biomonitor plant tobacco Bel W3. Plant J 16 235–245 [DOI] [PubMed] [Google Scholar]
- Shirasu K, Nakajima H, Rajasekhar VK, Dixon RA, Lamb C (1997) Salicylic acid potentiates an agonist-dependent gain control that amplifies pathogen signals in the activation of defense mechanisms. Plant Cell 9 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilmony RL, Chen Z, Bressan RA, Martin GB (1995) Expression of the tomato Pto gene in tobacco enhances resistance to Pseudomonas syringae pv tabaci expressing avrPto. Plant Cell 7 1529–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BP, Eggermont K, Tierens KF, Broekaert WF (1999) Requirement of functional ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea. Plant Physiol 121 1093–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong CB, Labavitch JM, Yang SF (1986) The induction of ethylene production from pear cell culture by cell wall fragments. Plant Physiol 81 929–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon LC, Geraats BP, Linthorst HJ (2006) Ethylene as a modulator of disease resistance in plants. Trends Plant Sci 11 184–191 [DOI] [PubMed] [Google Scholar]
- van Loon LC, Rep M, Pieterse CM (2004) Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol 44 135–162 [DOI] [PubMed] [Google Scholar]
- Weingart H, Ullrich H, Geider K, Völksch B (2001) The role of ethylene production in virulence of Pseudomonas syringae pvs. glycinea and phaseolicola. Phytopathology 91 511–518 [DOI] [PubMed] [Google Scholar]
- Weingart H, Volksch B (1997) Ethylene production by Pseudomonas syringae pathovars in vitro and in planta. Appl Environ Microbiol 63 156–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler D, Zahringer U, Gerber I, Dubery I, Hartung T, Bors W, Hutzler P, Durner J (2004) Innate immunity in Arabidopsis thaliana: lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proc Natl Acad Sci USA 101 15811–15816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JD, Felix G, Boller T (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428 764–767 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.