Abstract
The Arabidopsis (Arabidopsis thaliana) cation calcium exchangers (CCXs) were recently identified as a subfamily of cation transporters; however, no plant CCXs have been functionally characterized. Here, we show that Arabidopsis AtCCX3 (At3g14070) and AtCCX4 (At1g54115) can suppress yeast mutants defective in Na+, K+, and Mn2+ transport. We also report high-capacity uptake of 86Rb+ in tonoplast-enriched vesicles from yeast expressing AtCCX3. Cation competition studies showed inhibition of 86Rb+ uptake in AtCCX3 cells by excess Na+, K+, and Mn2+. Functional epitope-tagged AtCCX3 fusion proteins were localized to endomembranes in plants and yeast. In Arabidopsis, AtCCX3 is primarily expressed in flowers, while AtCCX4 is expressed throughout the plant. Quantitative polymerase chain reaction showed that expression of AtCCX3 increased in plants treated with NaCl, KCl, and MnCl2. Insertional mutant lines of AtCCX3 and AtCCX4 displayed no apparent growth defects; however, overexpression of AtCCX3 caused increased Na+ accumulation and increased 86Rb+ transport. Uptake of 86Rb+ increased in tonoplast-enriched membranes isolated from Arabidopsis lines expressing CCX3 driven by the cauliflower mosaic virus 35S promoter. Overexpression of AtCCX3 in tobacco (Nicotiana tabacum) produced lesions in the leaves, stunted growth, and resulted in the accumulation of higher levels of numerous cations. In summary, these findings suggest that AtCCX3 is an endomembrane-localized H+-dependent K+ transporter with apparent Na+ and Mn2+ transport properties distinct from those of previously characterized plant transporters.
The plant vacuole and other endomembrane compartments play an important role in the sequestration of various compounds (Marschner, 1995; Marty, 1999). Concentration gradients of Na+, Ca2+, Cd2+, NO3−, and Mn2+ are established across these membranes by cation/H+ exchange activities (Schumaker and Sze, 1985; Salt and Wagner, 1993; Barkla and Pantoja, 1996; Gonzalez et al., 1999). Several genes encoding these transport activities have been identified (Shigaki and Hirschi, 2006). However, the biological functions of many of the individual transporters remain for the most part undefined.
CCXs (for calcium cation exchangers) were previously identified as CAX (for cation exchanger) homologs. Recently CAX7 to CAX11 were reclassified as CCX1 to CCX5 due to higher homology to mammalian K+-dependent Na+/Ca2+ antiporters (Shigaki et al., 2006). CAXs are cation/H+ antiporters that show high-capacity, low-affinity transport and have been characterized in a variety of plants (Blumwald and Poole, 1986; Kasai and Muto, 1990; Ettinger et al., 1999; Cheng et al., 2002; Luo et al., 2005). CAXs are energized by the pH gradient established by proton pumps such as H+-ATPase or H+-pyrophosphatase (Kamiya and Maeshima, 2004). Several plant CAXs have been characterized as vacuole-localized transporters, which function in H+-coupled antiport of Ca2+, Mg2+, and Mn2+, resulting in the accumulation of these cations in vacuoles (Hirschi, 1999; Pittman and Hirschi, 2001; Pittman et al., 2004a). CCXs have not been studied, and it would be interesting to compare and contrast their activities with those of CAXs and the less closely related Na+(K+)/H+ exchangers of the NHX family.
CAX proteins have N-terminal regulatory domains (Pittman and Hirschi, 2001), and AtCAX1 and AtCAX2 were originally cloned as functional N-terminal deletions (lacking the negative regulatory domain; Hirschi et al., 1996). We refer to these forms as sCAX1 and sCAX2 (Shigaki and Hirschi, 2006). Tobacco (Nicotiana tabacum) plants expressing AtsCAX1 exhibit Ca2+ deficiencies, leaf necrosis, tip burning, and hypersensitivity to ion imbalances, as well as increased tonoplast Ca2+/H+ transport activity (Hirschi, 1999). The N termini of CCXs lack homology with CAXs, and it is not known if the N terminus has a regulatory function.
Arabidopsis (Arabidopsis thaliana) CCXs are related to mammalian plasma membrane Na+/Ca2+ exchangers (NCXs). The NCXs mediate the exchange of Na+ for Ca2+ depending on the electrochemical gradients, and NCKXs (for Na2+/Ca2+-K+ exchangers) transport K+ and Ca2+ in exchange for Na+ (Lytton, 2007). Interestingly, both NCX and NCKX exchangers can operate in a forward (Ca2+ exit) or reverse (Ca2+ entry) mode, which is mediated by the change in Na+ gradients and the potential across the membrane (Cai and Lytton, 2004a). However, the extent to which CCX transporters from Arabidopsis transport Na+ or K+ has not been addressed.
To investigate the function of CCX transporters, we cloned AtCCX3 and the closely related AtCCX4. We expressed the transporters in various yeast strains in order to compare and contrast their functions to those of AtCAX1 and AtNHX1 transporters. We monitored the expression and localization of AtCCX3 in yeast cells and in planta. Finally, we overexpressed AtCCX3 in plants and examined ion uptake and plant growth. Collectively, these findings demonstrate that AtCCX3 is an endomembrane H+-dependent K+ transporter.
RESULTS
AtCCXs Show Homology to Mammalian NCKXs
A family of genes originally identified as members of plant CAXs (Maser et al., 2001) were recently found to have high similarity to mammalian NCKX6 (a member of the CCX family) and weak similarity to AtNHX1 (Supplemental Fig. S1A). Thus, these transporters were reclassified as AtCCXs (Shigaki and Hirschi, 2006). We hypothesized that these putative transporters might function in cation homeostasis. We prepared open reading frame (ORF) clones for AtCCX3 and AtCCX4 from Arabidopsis (Columbia ecotype) genomic DNA, since these genes were predicted to contain no introns. The cloned AtCCX3 and AtCCX4 open reading frames contained 1,935 and 1,938 nucleotides, which could encode 644 and 645 amino acids and produce putative proteins of 70.1 and 70.8 kD, respectively. AtCCX3 and AtCCX4 are 79.50% identical and 86.02% similar to one another. They share more identity (26.8% identical, 46.9% similar) with human (Homo sapiens) HsNCKX6 than with AtCAX1 (12.1% identical, 25.1% similar). In contrast to AtNHX1, a known plant Na+ transporter, the AtCCXs have very little sequence homology (Supplemental Fig. S1A). Both AtCCX3 and AtCCX4 contain short N-terminal hydrophilic domains, which are not related to the AtCAX1 N-terminal autoinhibitory domain (Supplemental Fig. S1B). Comparisons of the AtCCX3, AtCCX4, and HsNKX6 proteins show a short (25 amino acids) N-terminal region, followed by five transmembrane domains separated from another seven transmembrane domains by a long hydrophilic region (75 amino acids for HsNCKX6 and 115 amino acids for AtCCX4). By contrast, AtCAX1 has a long N-terminal domain containing 65 amino acids and has two sets of four transmembrane domains separated by a shorter hydrophilic domain (35 amino acids). Also, AtCCX3, AtCCX4, and HsNCKX6 have very short (15 amino acids) C-terminal domains compared with AtCAX1, which has a longer C-terminal region (25 amino acids; Supplemental Fig. S1C). Phylogenetic analysis of AtCCX1 to AtCCX5, AtCAXs, and the human K+-dependent Na+/Ca2+ antiporter HsNCKX6 clearly indicates that CCXs are more closely related to the K+-dependent Na+/Ca2+ antiporter than to any of the CAXs (Supplemental Fig. S1A; Shigaki et al., 2006). Specifically, AtCCX3 and AtCCX4 share the characteristic α-repeats GNG(A/S)PD in α1 and (G/S)(N/D)SxGD in α2 with HsNCKX6 (Supplemental Fig. S1B; Cai and Lytton, 2004a, 2004b); these are highly conserved Ca2+ and Na+ domains (Winkfein et al., 2003; Kang et al., 2005).
Expression of AtCCX3 and AtCCX4 in Yeast
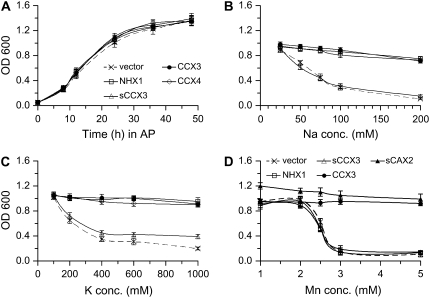
AtCCX3 and AtCCX4 were cloned into the piHGpd vector (Nathan et al., 1999) for expression in yeast under the control of the GPD promoter. To test the possibility of a CCX N-terminal regulatory domain, we made a truncation in the AtCCX3 ORF (AtsCCX3). These plasmids were then introduced into the yeast strains AXT3 (ena1∷HIS3∷ena4, nha1∷LEU2, nhx1∷TRP1, ura3-1; Yokoi et al., 2002) and wx1 (nhx1 mutant; Nass et al., 1997) with defects in vacuolar Na+/K+ transport. AtCCX3 and AtCCX4 expression did not alter growth of the yeast in Arg phosphate (AP) medium (Fig. 1A) compared with vector controls. Also, expression of AtsCCX3 or AtNHX1 did not affect the growth of the yeast strain (Fig. 1A). AtCCX3 and AtCCX4 suppressed the Na+ and K+ sensitivity of these yeast strains (AXT3 and wx1) deficient in vacuolar Na+/H+ transport (Fig. 1, B and C); however, AtsCCX3 did not suppress the Na+ and K+ sensitivity in these strains. We also tested both the hemagglutinin (HA)-AtCCX3 and AtCCX3-GFP tagged proteins, and they both showed similar phenotypes to the native AtCCX3 (data not shown). Yeast cells expressing all of the AtCCX3 and AtCCX4 variants were unable to suppress the Ca2+ sensitivity of yeast strains deficient in vacuolar Ca2+ transport (data not shown). Yeast cells expressing AtCAXs all show increased Ca2+ transport when truncated in their N-terminal domain (Hirschi, 1999; Shigaki et al., 2001, 2003; Pittman et al., 2004b). A lack of N-terminal regulation, the ability to suppress Na+-sensitive phenotypes, and the inability to suppress Ca2+-sensitive phenotypes in yeast suggests functions for AtCCX3 and AtCCX4 that differ from those of CAXs.
Figure 1.
Growth of AtCCX3-expressing yeast strains in AP and YPD media with different cation concentrations. A, Growth of yeast strain wx1 (nhx1) transformed with vector (p2UGpd; crosses), AtNHX1 (squares), AtsCCX3 (triangles), AtCCX3 (black circles), or AtCCX4 (diamonds) in AP over time. B, OD at 48 h for yeast strain AXT3 (ena1∷HIS3∷ena4, nha1∷LEU2, nhx1∷TRP1, ura3-1) grown in AP with different concentrations of NaCl + 1 mm KCl. C, OD at 48 h for yeast strain wx1 (nhx1) transformed with vector (p2UGpd), AtNHX1, AtCCX3, AtsCCX3, or AtCCX4 in AP with various concentrations of KCl. D, OD at 48 h for yeast strains (Δsmf1 + Δsmf2) transformed with vector (p2UGpd; crosses), AtNHX1 (squares), AtsCCX3 (triangles), AtCCX3 (black circles), or AtsCAX2(black triangles) in AP over time grown in YPD with different concentrations of MnCl2. Yeast strains expressing the different plasmids (n = 8) were serial diluted 125-fold, and 10 μL was placed into 190 μL of liquid selection medium containing NaCl, KCl, or MnCl2.
We also tested the possibility that AtCCX3 and AtCCX4 transport other metals, thus making the host yeast cells tolerant or hypersensitive to these metals. Expression of AtCCX3 in an Mg2+-requiring strain (CM66) did not suppress the ion sensitivity of these strains (MacDiarmid and Gardner, 1998). AtCCX3-expressing cells were also tested with a range of metals, such as Al3+, Cd2+, Cu2+, Ni2+, and Zn2+, in various mutant yeast strains (K661, K667; Salt and Wagner, 1993; Cunningham and Fink, 1996; Liu et al., 1997; Tuttle et al., 2003). In each case, AtCCX3 cells were indistinguishable from vector controls in our assay conditions. However, expression of AtCCX3 could suppress Mn2+ growth sensitivity in a yeast strain defective in both plasma and vacuolar membrane Mn2+ transport (smf1 + smf2; Supek et al., 1996; Cohen et al., 2000; Luk and Culotta, 2001; Fig. 1D). AtsCCX3 was unable to suppress the Mn2+ growth sensitivity, suggesting that truncating AtCCX3 does not activate a function that is different from that of the CAXs, where N-terminal truncations enhance function (Hirschi et al., 1996; Hirschi, 1999; Pittman and Hirschi, 2001; Pittman et al., 2002a, 2002b). This suppression of Mn2+ sensitivity phenocopied AtsCAX2 expression, a known vacuolar Mn2+ transporter (Fig. 1D; Pittman et al., 2004b). However, this is the only phenotype that overlaps with CAX transporters, and the other transport properties firmly establish distinct non-CAX functions for AtCCX3.
Transport Properties of AtCCX3 in Yeast
In order to determine whether AtCCX3 mediated K+ transport, we measured 86Rb+ uptake as a tracer for K+ into yeast cells and yeast microsomal membranes (Venema et al., 2002; Pittman et al., 2004a, 2004b). In wx1 yeast, only cells expressing AtCCX3 or AtNHX1 showed higher 86Rb+ uptake at high K+ concentrations (20 mm; data not shown) relative to vector controls. At low external K+ (0.02 mm) concentrations, 86Rb+ uptake by cells expressing AtCCX3 and AtNHX1 was similar to that of vector controls (data not shown). This suggests that AtCCX3 can facilitate K+ uptake with low affinity in yeast, although it is unclear whether K+ uptake is mediated directly or indirectly by CCX3.
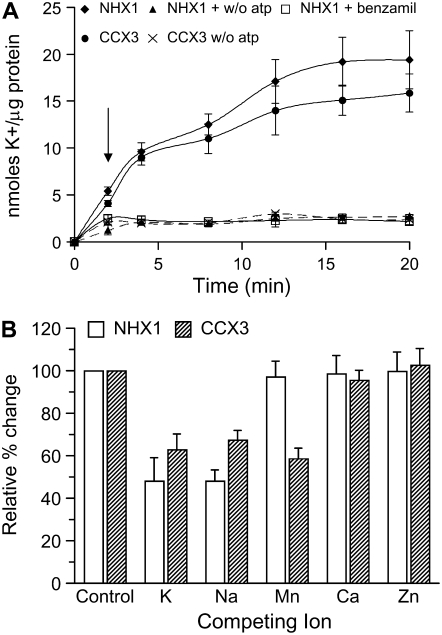
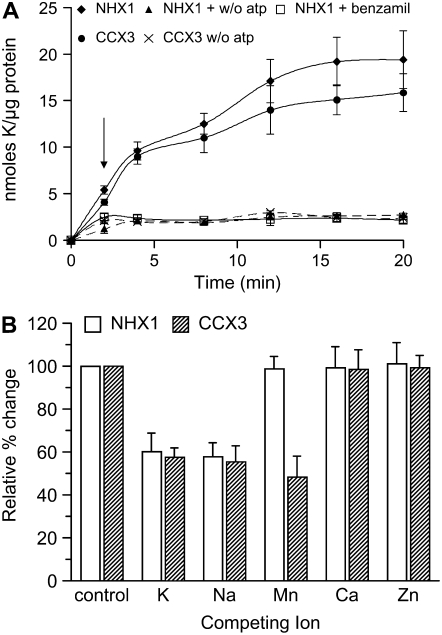
Transport assays were performed using vacuole membrane-enriched vesicles from yeast. Membranes isolated from wx1 cells expressing AtCCX3 or AtNHX1 showed increased 86Rb+ uptake at 50 mm K+ concentrations (Fig. 2A) than that of wx1 with vector only. At low external K+ (0.5 mm) concentrations, 86Rb+ uptake by cells expressing AtCCX3 and AtNHX1 was similar to that of vector controls (data not shown). As shown before, uptake of 86Rb+ by AtNHX1 was inhibited by benzamil (Venema et al., 2003). However, treatment of AtCCX3-expressing yeast cells with benzamil to measure 86Rb+ uptake inhibition was inconclusive (data not shown). Uptake of 86Rb+ from both AtNHX1 and AtCCX3 required MgCl2, as the removal of MgCl2 resulted in a reduction in 86Rb+ uptake (Fig. 2A). These results support the idea that Mg2+ is required to activate the vacuole-type H+-ATPase to generate a pH gradient across the vesicle. Thus, in yeast, AtCCX3 appears to behave as a low-affinity K+/proton exchanger in a manner similar to AtNHX1.
Figure 2.
AtCCX3 or AtNHX1 mediates cation uptake into yeast vacuolar vesicles. A, K+ (86Rb+) uptake into yeast vacuolar vesicles. Benzamil (10 μm; arrow) was added 2 min after addition of 86Rb+. K+ concentration was 50 mm; n = 6. Values shown are means ± se. B, Effect of various cation chlorides (300 μm) on K+ (86Rb+) uptake at 8 min. Values shown are means ± se from six replicate measurements.
These phenotypes suggest that AtCCX3 has a role in K+ transport, but the suppression of the Δsmf1, Δsmf2 Mn2+-sensitive yeast strain also suggests that AtCCX3 functions in Mn2+ transport (Cohen et al., 2000). To further investigate the yeast phenotypes, 86Rb+ uptake into vesicles was measured in the presence of excess nonradioactive KCl, NaCl, MnCl2, CaCl2, and ZnCl2. Excess (300 μm) KCl and NaCl reduced 86Rb+ uptake mediated by AtNHX1 and AtCCX3 by 50% and 40%, respectively (Fig. 2B), but no decrease in uptake was measured in the presence of excess CaCl2 or ZnCl2. Intriguingly, MnCl2 only reduced AtCCX3-mediated uptake of 86Rb+ but not that of AtNHX1 (Fig. 2B). Taken together, these results suggest that AtCCX3 mediates H+-dependent uptake of K+ and Na+, similar to NHX1, yet is distinct in its cation specificity to a divalent cation, such as Mn2+.
Metal Measurements in Yeast Expressing AtCCX3 and AtNHX1
We tested the alkali cation content in mutant alone or in yeast strains expressing AtCCX3 and AtNHX1 after cells were exposed to 100 mm NaCl or KCl. Yeast cells expressing AtCCX3 and AtNHX1 showed a 25% increase in Na+ and a 50% increase in K+ content, compared with yeast cells expressing only the vector (Fig. 3). Thus, CCX3 promotes Na+ or K+ uptake similar to NHX1. In a preliminary experiment of wild-type W303-1A yeast cells expressing AtCCX3, the Mn2+ content was 85% higher compared with that in cells expressing only vector. Thus, AtCCX3 expressed in yeast cells could play a role in Na+, K+, and Mn2+ homeostasis.
Figure 3.
K+ and Na+ contents in wx1 yeast expressing AtCCX3. Relative percentage change in K+ and Na+ between yeast wx1 vector control and wx1 transformed with AtNHX1 or AtCCX3 grown in medium containing 100 mm NaCl. ICP analysis was done on filtered whole yeast cells. Values shown are mean percentage differences ± se from four replicate measurements. Calculated se is for the relative percentage difference of the means. se was calculated as (mean CCX3/mean vector) × (square root of z) × 100 (http://www.census.gov/acs/www/Downloads/ACS/PercChg.pdf). Concentrations of K+ ions in wx1 yeast cells expressing various constructs were 25,107 μg g−1 vector, 35,643 μg g−1 AtNHX1, and 40,275 μg g−1 AtCCX3; concentrations of Na+ ions were 4,477 μg g−1 vector, 6,047 μg g−1 AtNHX1, and 5,873 μg g−1 AtCCX3.
Localization of AtCCX3 in Yeast and Plants
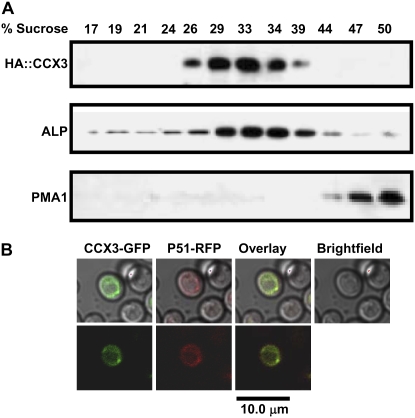
An N-terminal HA-tagged AtCCX3 construct conferred resistance to high Na+ and K+ stress in wx1 (data not shown). We utilized this construct to identify the cellular location of AtCCX3. As shown in Figure 4A, western-blot analysis of yeast membranes fractionated on Suc gradients showed that AtCCX3 colocalized with vacuolar membranes. The distribution of HA-AtCCX3 corresponded with the yeast vacuolar membrane marker alkaline phosphatase but not with the plasma membrane marker Pma1p. As further confirmation of yeast endomembrane localization, the AtCCX3-GFP expressed in yeast also appeared to reside on intracellular membranes, as the signal overlapped with vacuolar protein P51 fused to red fluorescent protein (Fig. 4B; Carter et al., 2004). These results suggest that AtCCX3 functions as an endomembrane cation transporter in yeast.
Figure 4.
Subcellular localization of AtCCX3 in yeast. A, Subcellular localization of epitope-tagged HA-AtCCX3 to the vacuole membrane. Yeast membranes were fractionated on 10% to 50% (w/w) Suc gradients, and equal amounts of protein (15 mg) were separated by SDS-PAGE, blotted, and subjected to western-blot analysis using antibodies against HA, the vacuolar membrane marker alkaline phosphatase (ALP), and the plasma membrane H+-ATPase (PMA1). B, Subcellular localization of CCX3-GFP. GFP was fused to the C terminus of full-length AtCCX3 and coexpressed with the yeast vacuolar membrane protein P51. The fusion protein and vacuolar marker were observed by confocal microscopy. The green channel (excitation at 488 nm, emission at 522 nm, and barrier filter at 522–535 nm) and red channel (excitation at 480 nm, emission at 530 nm with a fluorescent long-pass filter) images were captured with Fluoview software (Olympus America).
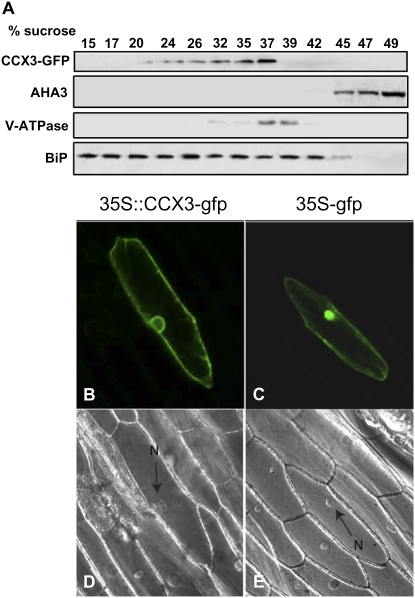
To investigate the subcellular localization of AtCAX3 in plants, microsomal membranes from transgenic lines harboring the AtCCX3-GFP fusion protein were fractionated. Centrifugation through a linear Suc gradient was first used to compare the distribution of the epitope-tagged transporter in transgenic Arabidopsis (Fig. 5A) with that of markers for the tonoplast, plasma membrane, and endoplasmic reticulum lumen. As shown in Figure 5A, the tagged proteins were located in fractions of 26% to 37% Suc. The AtCCX3-GFP protein was associated with fractions enriched in endomembranes, as indicated by the sedimentation profiles, which overlapped with a vacuole-type H+-ATPase (V-ATPase subunit B; Ward et al., 1992) but not with an endoplasmic reticulum (BiP; Cheng et al., 2002) or the plasma membrane (AHA3; Pardo and Serrano, 1989) protein. To provide further confirmation for the localization in plants, we examined the transient expression of AtCCX3-GFP in onion (Allium cepa) cells (Sivitz et al., 2007). Fluorescence associated with 35S∷GFP alone was localized to both the cytosol and the nucleus. In contrast, fluorescence was consistent with cytoplasmic vesicles and the vacuolar membrane, but some localization to the plasma membrane cannot be excluded in plant cells expressing 35S∷AtCCX3-GFP (Fig. 5B).
Figure 5.
AtCCX3 localization in plants. A, Localization of epitope-tagged CCX3-GFP to the tonoplast. Arabidopsis microsomal membranes were fractionated on 10% to 50% (w/w) Suc gradients. Aliquots were collected and equal amounts of protein (10 mg) were separated by SDS-PAGE, blotted, and subjected to western-blot analysis using antibodies against GFP and plant membrane markers: the vacuolar ATPase subunit B, the plasma membrane H+-ATPase (AHA3), and the endoplasmic reticulum protein (BiP; StressGen Biotechnologies). B and C, Projection confocal images of onion epidermis transiently expressing 35S∷CCX3-GFP (B) or 35S∷GFP (C). D and E, Differential interference contrast images of onion epidermis (D corresponds to B and E corresponds to C). The arrows indicate the position of the nucleus in D and E.
Expression of AtCCX3 and AtCCX4 in Arabidopsis
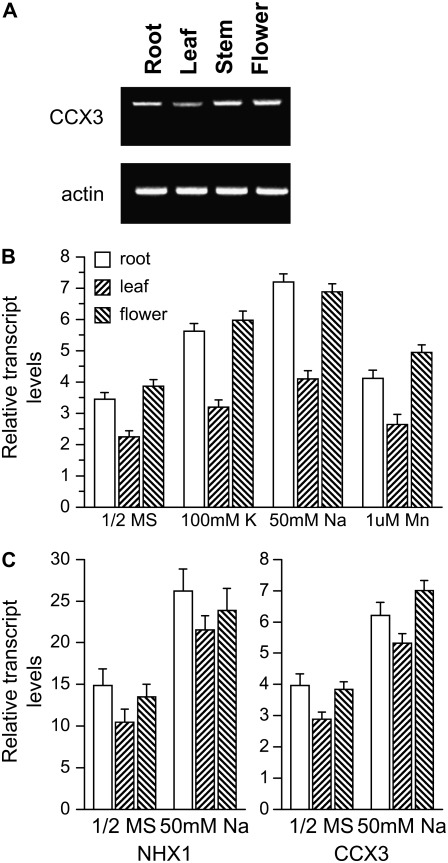
Analysis of publicly available Arabidopsis microarray data indicates that AtCCX3 is expressed at very low levels and predominantly in flowers and pollen grains (Bock et al., 2006; https://www.genevestigator.ethz.ch). This low level of expression made the use of northern blot and GUS promoter∷reporter analysis difficult (data not shown). In order to more precisely monitor expression, we conducted reverse transcription (RT)-PCR and detected the expression of AtCCX3 primarily in flowers, roots, and stems of wild-type Arabidopsis (Fig. 6A). AtCCX4 appears to be expressed in pollen and throughout the plant and at levels substantially higher than AtCCX3 (Bock et al., 2006; https://www.genevestigator.ethz.ch).
Figure 6.
Expression of AtCCX3 in Arabidopsis. A, RT-PCR shows AtCCX3 expression in Columbia wild-type tissues. B, The relative expression levels of AtCCX3 in roots, leaves, and flowers in response to various cation treatments were normalized to expression of the 18S subunit. The results were obtained from two independent RNA pools for each tissue and treatment combination analyzed. C, Comparison of AtCCX3 expression with AtNHX1 in roots, leaves, and flowers in response to 50 mm NaCl. Expression levels of AtCCX3 and AtNHX1 were normalized to expression of the 18S subunit. Values shown are means ± se from four replicate measurements.
The ability of AtCCX3 to suppress a yeast mutant sensitive to Na+ and K+ and the increased sensitivity of wild-type yeast to Mn2+ prompted us to examine whether these or other cations could induce AtCCX3 expression in Arabidopsis roots, leaves, and flowers. Expression of AtCCX3 increased 2- to 3-fold in response to exogenous Na+ and K+, and by 0.5-fold compared with Mn2+, in both roots and flowers (Fig. 6B). We then directly compared the changes in AtCCX3 expression with those of AtNHX1 under salt stress conditions. An increase in AtNHX1 in response to Na+ has been reported previously (Yokoi et al., 2002). Although AtCCX3 is expressed at much lower levels than AtNHX1, expression of both genes was induced by Na+ treatment in roots, leaves, and flowers (Fig. 6C). However, the basal level of AtNHX1 expression was much higher than that of AtCCX3.
Analysis of T-DNA Insertional Mutants of AtCCX3 and AtCCX4
To investigate the physiological function of AtCCX3 and AtCCX4 in Arabidopsis, we obtained two independent lines containing T-DNA insertions inside each open reading frame (Supplemental Fig. S2D). Homozygous lines were isolated by screening for the presence of the T-DNA insert and lack of native AtCCX. We isolated two different mutant alleles for AtCCX3, termed atccx3-1 and atccx3-2, and two alleles for AtCCX4, termed atccx4-1 and atccx4-2. RT-PCR analysis of the four different alleles showed no expression of AtCCX3 or AtCCX4 in the respective mutants (Supplemental Fig. S2, B and C). Pollen viability, pollen tube growth, and seed set were not altered in these mutants (data not shown), nor were there any other discernible growth abnormalities.
Ectopic Expression of AtCCX3
To further test the function of AtCCX3 in Arabidopsis, AtsCCX3 and AtCCX3 were expressed using the cauliflower mosaic virus 35S promoter. Despite increased expression of AtCCX3 RNA in the Arabidopsis lines, no visible changes in plant growth or development were detected (Supplemental Fig. S2A; J. Morris, unpublished data). However, lack of an AtCCX3-specific antibody prevented actual quantification of alterations of AtCCX3 protein amounts.
35S∷AtCCX3 Arabidopsis plants treated with exogenous NaCl accumulated 35% more Na+ compared with controls (26,490 ± 370 μg g−1 dry weight for 35S∷AtCCX3, 17,305 ± 2,145 μg g−1 dry weight for controls). In contrast, when grown under normal conditions, there was only a modest Na+ accumulation in 35S∷AtCCX3 lines (6,044 ± 55 μg g−1 for 35S∷AtCCX3, 5,772 ± 73 μg g−1 for controls).
To determine the in planta K+ transport properties of AtCCX3, direct 86Rb uptake assays into whole plants and vacuole-enriched vesicles isolated from Arabidopsis roots were assayed (Pittman et al., 2004a). Given the difficulty of inferring function from whole plant uptake assays, we isolated tonoplast-enriched vesicles to better understand the function of AtCCX3 in planta. Vesicles from 35S∷AtCCX3 and AtNHX1 plants both showed higher 86Rb uptake at high K+ concentrations (50 mm; Fig. 7A) and no uptake at low external K+ concentration (0.05 mm; data not shown). Similar to the yeast membranes, uptake of 86Rb in membranes isolated from AtNHX1 plants was inhibited by benzamil (Fig. 7A; Venema et al., 2002). As in our yeast assay, benzamil showed less inhibition on 86Rb uptake into membranes from 35S∷AtCCX3 plants. Reduced uptake of 86Rb in both AtNHX1- and 35S∷AtCCX3-expressing plants was found when the H+ gradient was disrupted by removing MgCl2 from the reaction mixtures. Like our yeast assays, these plant membrane uptake values suggest that AtCCX3 is an H+-driven Na+, K+, and Mn2+ exchanger.
Figure 7.
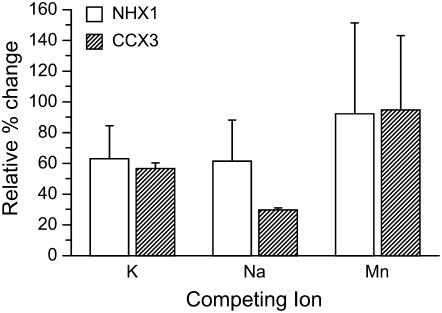
Cation uptake into Arabidopsis root vacuolar vesicles. A, K+ (86Rb+) uptake into vacuolar vesicles. Benzamil (10 μm; arrow) was added 2 min after addition of 86Rb. K+ concentration was 50 mm. B, Effect of various cation chlorides (300 μm) on K+ (86Rb+) uptake at 8 min. Values shown are means ± se from six replicate measurements.
We also tested the inhibition of 86Rb uptake by competition with excess (300 μm) nonlabeled cation chlorides. Similar to our results in yeast, excess KCl and NaCl reduced 86Rb uptake, while CaCl2 and ZnCl2 had no effect on 86Rb uptake into membranes isolated from cells expressing AtNHX1 and AtCCX3 (Fig. 7B). However, excess MnCl2 reduced the uptake of 86Rb by 57% in membranes from 35S∷AtCCX3-expressing membranes but showed no inhibition of 86Rb uptake in AtNHX1-expressing membranes (Fig. 7B). These transport measurements again suggest that AtCCX3 has a role in Na+, K+, and Mn2+ homeostasis in planta.
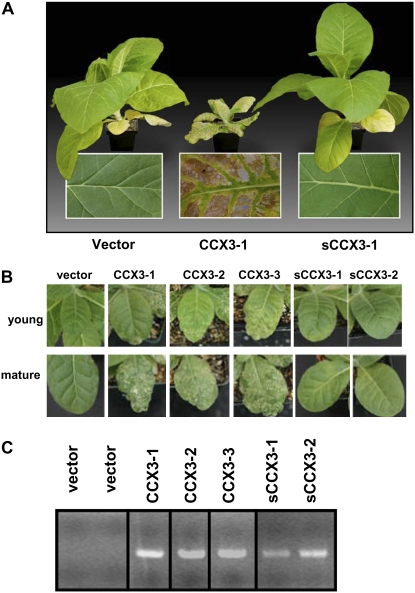
The Arabidopsis AtCCX3 and AtsCCX3 were also heterologously expressed in tobacco (KY14 variety). As shown in Figure 8C, AtCCX3 RNA accumulated in all 35S∷AtCCX3 transgenic lines. The inability to detect transcript in the vector lines indicates the specificity of the primers used during the amplification process. Visible alterations in plant growth were readily apparent in the 35S∷AtCCX3-expressing lines (Fig. 8C). After 3 weeks of growth in sterile conditions, 100% (17 of 17) of the primary transformants expressing 35S∷AtCCX3 formed leaves with small yellowing necrotic lesions (Fig. 8A). After being transferred to soil, the lines appeared to partially recover for a period of 3 to 5 d. After 1 week, these phenotypes reoccurred in all 17 of the primary transformants; however, the roots of these plants did not show altered growth. As shown in Figure 8, A and B, after 3 months, all of the 35S∷AtCCX3-expressing plants were severely stunted. The 35S∷AtCCX3 transgenic lines with the least dramatic growth changes always displayed low levels of 35S∷AtCCX3 expression (data not shown). In contrast to these dramatic phenotypes, the 12 35S∷AtsCCX3-expressing lines displayed phenotypes indistinguishable from the vector-expressing controls. Because the 35S∷AtCCX3 phenotypes were so dramatic, we repeated the transformation process and obtained identical results in a replicate experiment (data not shown).
Figure 8.
Ectopic expression of AtCCX3 in tobacco. A, Phenotypes of tobacco plants expressing vector, AtCCX3, or truncated AtCCX3 (AtsCCX3). Only AtCCX3-expressing lines displayed stunted growth, yellowing of the leaves, and necrosis of the leaf interveinal tissue. B, AtCCX3-expressing tobacco plants displayed yellow lesions at the tips in young leaves, and this became more apparent as the leaves aged (mature). These growth abnormalities were never seen in control lines regardless of age. C, AtCCX3 expression in the tobacco transgenic lines. Total RNA was isolated from 4-week-old leaf tissue, and 5 μg of RNA was used in the RT-PCR.
35S∷AtCCX3 lines were selected for further study on the basis of their T1 phenotype and their ability to make seeds. All lines analyzed that displayed the altered morphology were fertile (n = 13). The same growth abnormalities revisited all of the lines in the second generation. When grown from seed in tissue culture, the T2 plants appeared normal and unperturbed for the first 2 weeks, after which time the leaves began to display altered growth phenotypes (data not shown). T2 35S∷AtCCX3-transformed plants that were sown and grown in the greenhouse also displayed leaf symptoms after 3 weeks of growth (Fig. 8B). Once again, none of the 35S∷AtsCCX3- or vector control-expressing plants displayed these phenotypes.
Effects of AtCCX3 on Plant Growth
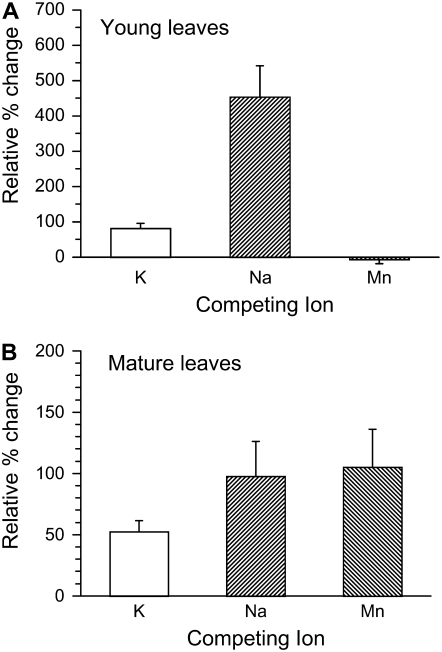
The symptoms of the 35S∷AtCCX3-expressing tobacco plants could not be phenocopied in vector control lines under any growth conditions tested (excess and depleted Na+, Mg2+, K+, Mn2+, and Ca2+; data not shown). Primary transformants and T2 35S∷AtCCX3-expressing plants displayed altered morphology regardless of the medium used for growth. Conceivably, constitutive AtCCX3 expression may alter various mineral levels simultaneously, making suppression of the phenotypes dependent on the addition or subtraction of multiple components. To ascertain whether 35S∷AtCCX3 expression altered ion content, we measured the total accumulation of ions in both mature and young leaves. In 35S∷AtCCX3-expressing plants, young leaves accumulated at least 40% more K+ and Na+ (Fig. 9A) compared with control lines and maintained these increased levels for K+ and Na+, even as the leaves matured (Fig. 9B). Mn2+ levels also increased as the plant leaves matured. One explanation for these growth defects is that the alterations in cellular Mn2+ and other cations may produce excess reactive oxygen species. To test this, we isolated total leaf protein from vector- and 35S∷AtCCX3-expressing plants and detected the carbonyl content of the proteins. As shown in Supplemental Figure S3A, the protein carbonyl content was higher in 35S∷AtCCX3-expressing plants compared with vector controls and AtsCCX3-expressing lines. In fact, these alterations were detectable before the onset of morphological changes in leaf architecture. Together, these findings suggest that AtCCX3 has a role in ion homeostasis in plants and that when AtCCX3 expression is heightened, increases in protein oxidation occur.
Figure 9.
Cation content in young and mature leaves of AtCCX3-expressing tobacco plants. ICP analysis from tobacco plants expressing AtCCX3. Relative percentage differences in the averages of seven independent lines are shown in the graphs. A, ICP analysis of young leaves from 28-d-old tobacco plants. B, ICP analysis of mature leaves from 40-d-old tobacco plants. se is for the relative percentage difference of the means. se was calculated as (mean CCX3/mean vector) × [square root [((se CCX3)2/(mean CCX3)2) + ((se vector)2/(mean vector)2)]] × 100 (http://www.census.gov/acs/www/Downloads/ACS/PercChg.pdf). Concentrations of K+, Na+, and Mn2+ ions in young leaves were 41,852, 2,150, and 170 μg g−1 for plants expressing vector compared with 75,800, 11,904, and 158 μg g−1 for plants expressing 35S∷AtCCX3. Concentrations of K+, Na+, and Mn2+ ions in mature leaves was 50,644, 3,127, and 98 μg g−1 for plants expressing vector compared with 77,051, 6,181, and 201 μg g−1 for plants expressing 35S∷AtCCX3.
DISCUSSION
The plant vacuole is a cation depot, and numerous transporters have been proposed to mediate influx and efflux from this endomembrane compartment (Marty, 1999; Maeshima, 2000; Ratajczak, 2000; Hirschi, 2001). Here, we use yeast suppression screens, in planta expression analysis, yeast and plant membrane transport studies, and AtCCX-generated phenotypes in transgenic tobacco to suggest that AtCCX3 expression is judiciously regulated and is part of an ensemble of transporters regulating K+, Na+, and possibly Mn2+ levels within plant cells.
Sequence Comparison of AtCCXs and HsNCKX6
Phylogenetic analysis recently identified five Arabidopsis CCX transporters as being closely related to mammalian K+-dependent Na+/Ca2+ exchangers (Supplemental Fig. S1A; Shigaki et al., 2006). The functions of AtCCX1 to AtCCX5 in plants are unknown, although they may functionally resemble NCKXs. NCKX exchangers are involved in mammalian signaling (Blaustein and Lederer, 1999; Lee et al., 2002) by catalyzing the electrogenic countertransport of four Na+ for one Ca2+ and one K+ (Cervetto et al., 1989; Dong et al., 2001). Like the HsNCKX6, AtCCX3 and AtCCX4 have similar conserved α-repeats (Supplemental Fig. S1B), which may play a critical role in maintaining a proper chemical microenvironment for ion binding (Cai and Lytton, 2004b). AtCCX3, AtCCX4, and HsNCKX6 also share similar membrane topologies, which differ from that of AtCAX1 (Supplemental Fig. S1C).
Function of AtCCX3 and AtCCX4 in Yeast
Our data show that AtCCX3 and AtCCX4 have functions distinct from AtNHX1. Results from transport assays in yeast are consistent with the phylogenetic analysis and indicate a possible Na+/K+ transport function for AtCCX3 and AtCCX4 (Fig. 1; Shigaki et al., 2006). Like AtNHX1, AtCCX3- and AtCCX4-expressing cells can suppress the Na+ and K+ sensitivities of mutant yeast strains defective in vacuolar Na+ and K+ transport (Fig. 1, B and C). Similarly, AtCCX3-mediated K+ (86Rb+) uptake in yeast cells was similar to that of AtNHX1-expressing cells (Fig. 2). However, benzamil had greater inhibition of 86Rb+ uptake in cells expressing AtNHX1 than in cells expressing AtCCX3. Furthermore, AtCCX3-expressing yeast cells facilitated the uptake of various cations at concentrations comparable to that of AtNHX1-expressing cells (Fig. 3). These results support the idea that AtCCX3 activity promotes K+ and Na+ accumulation into endomembrane compartments such as the vacuole.
AtCCX3- and AtCCX4-expressing yeast cells did not completely phenocopy AtNHX1-expressing yeast cells. AtCCX3- and AtCCX4-expressing cells could not suppress the hygromycin sensitivity of nhx1-deficient cells (data not shown; Nass and Rao, 1998; Darley et al., 2000). Both AtCCX3 and AtNHX1 are predominantly localized to the vacuole (see below), in contrast to the prevacuolar localization of yeast NHXp1 (Nass and Rao, 1998). A possible altered localization could explain the inability of AtCCX3-expressing cells to suppress this defect in the yeast secretory pathway. AtCCX3 expression suppressed the growth sensitivity in a yeast strain defective in both plasma and vacuolar membrane Mn2+ transport (Fig. 1D; Supek et al., 1996; Cohen et al., 2000; Luk and Culotta, 2001). The ability of AtCCX3 to suppress the growth defects of a Mn2+-sensitive yeast strain as well as excess Mn2+ decreasing 86Rb+ uptake in yeast vacuolar vesicles suggest that AtCCX3 is involved in Mn2+ homeostasis. The ability to suppress yeast growth defects in Na+ and Mn2+ homeostasis has not been observed with heterologous expression of AtNHX1 or AtCAX1 and suggests that AtCCX3 has distinct transport properties.
Our other yeast data further support AtCCX3 and AtCCX4 having biochemical functions distinct from CAX transporters. For example, N-terminal truncations of AtCAX1 and AtCAX2 suppress the Ca2+ sensitivity of yeast cells defective in vacuolar Ca2+ transport (Hirschi et al., 1996). In contrast, expression of both full-length and N-terminal truncations of AtCCX3 and AtCCX4 in yeast could not suppress these Ca2+ transport defects. Furthermore, the N-terminal domains of AtCCX3 and AtCCX4 were required for function. The requirement of the N-terminal domain in these yeast assays suggests that AtCCX3 and AtCCX4 do not contain a CAX-like N-terminal regulatory domain.
AtCCX3 Is an Endomembrane-Localized Transporter in Flowers
Functional epitope tags of AtCCX3 demonstrated that AtCCX3 localized to the endomembrane in both yeast and plants (Figs. 4 and 5). Furthermore, AtCCX3 appeared to function at the yeast vacuolar membrane as a cation transporter (Figs. 1 and 2). Whether AtCCX3 is localized exclusively on the plant vacuole or also on the trans-Golgi network and prevacuolar compartment is unclear at this time.
Although the precise role of AtCCX3 is still unclear, low AtCCX3 expression levels suggest that AtCCX3 might have a role in cation uptake in a specific subset of cells rather than be involved in bulk cation uptake (Fig. 6). Furthermore, the localization of AtCCX3 to the plant vacuole, and possibly other endomembrane compartments, combined with expression in floral tissue (Supplemental Fig. S2, A and B; publicly available microarray data) propose possible functions associated with the pollen vacuole during tube elongation and polarized tip growth (Cheung et al., 2003; Holdaway-Clarke et al., 2003).
AtCCX3 Phenotypes in Plants
AtCCX3 and AtCCX4 may function in concert with numerous other transporters to regulate pollen growth. The atccx3 and atccx4 lines displayed no altered pollen phenotypes, and general plant growth appeared robust in all our assays (Supplemental Fig. S2, B and C; data not shown). This lack of altered growth could be related to the expression of other AtCCX transporters during vegetative and pollen development (Sze et al., 2004; Bock et al., 2006). Many Arabidopsis T-DNA mutants lack any morphological phenotype, presumably due to functional redundancy (Krysan et al., 1999). Our working hypothesis is that AtCCX3 and AtCCX4 have similar functions because they arose from a gene duplication.
The strong phenotype resulting from overexpression of AtCCX3 in tobacco indicates that AtCCX3 must be carefully modulated. Tobacco plants ectopically expressing 35S∷AtCCX3 were stunted in growth and contained necrotic lesions in the leaf interveinal regions (Fig. 8A). These tobacco phenotypes differentiate AtCCX3 from both AtCAX1- and AtNHX1-overexpressing phenotypes. The AtCCX3 phenotypes were not observed in plants expressing AtsCCX3 (Fig. 8A). In contrast, ectopic expression of AtsCAX1 in tobacco produces dramatic phenotypes (Hirschi, 1999). This further suggests that AtCCX3 does not contain an N-terminal regulatory domain. The fundamental cause of AtCCX3-mediated tobacco phenotypes is less apparent than with the AtsCAX1-expressing lines. That is, AtsCAX1-expressing lines are Ca2+ deficient and application of exogenous Ca2+ can restore normal growth (Hirschi, 1999). In contrast, the AtCCX3-expressing lines could not be rescued by enhancing or reducing NaCl, KCl, or MnCl2 levels (data not shown). Possibly, the AtCCX3-expressing lines disrupt tonoplast V-type H+-translocating ATPase activity, causing a general disruption in pH homeostasis. In fact, altered expression of CAX transporters can produce alterations in vacuolar H+-ATPase activity (Shigaki and Hirschi, 2006); however, these CAX phenotypes are not as severe at those documented here. Like AtNHX1-expressing plants, AtCCX3 lines were able to accumulate Na+; however, unlike AtNHX1-expressing lines, these plants did not appear to be Na+ tolerant (data not shown; Apse et al., 1999, 2003).
Although no phenotypes related to changes in Mn2+ concentrations could be observed in Arabidopsis either lacking or expressing 35S∷AtCCX3, the Mn2+ inhibition of 86Rb uptake in vacuole-enriched vesicles suggests that AtCCX3 has a role in Mn2+ homeostasis (Fig. 7B). This lack of whole plant phenotypes could be related to functional redundancy that may prohibit fluctuations in Mn2+ content that could cause deleterious phenotypes in Arabidopsis (Pittman et al., 2004a).
Protein Oxidation Is Increased in Plants Expressing AtCCX3
AtCCX3 function may be related to plant reactive oxygen species signaling (Supplemental Fig. S3). Indeed, tobacco plants expressing 35S∷AtCCX3 showed much higher oxidation of proteins compared with controls (Supplemental Fig. S3). The drastic phenotypes observed in 35S∷AtCCX3 tobacco lines may be due to the perturbation of transient metal concentrations in AtCCX3-expressing tobacco lines (Figs. 8 and 9). Regulation of metal concentrations is essential for plant antioxidant systems (Halliwell and Gutteridge, 2006). When in excess, these metals can produce highly toxic hydroxyl radicals through a Fenton reaction, resulting in oxidative damage (Halliwell, 2006).
In summary, we have characterized what are to our knowledge the first CCX transporters from plants. We demonstrate that AtCCX3 resides predominantly on an endomembrane and may function as an H+-dependent K+ transporter that can also transport Na+ and Mn2+. The ability to transport both monovalent and divalent cations will need to be directly demonstrated, although it is analogous to yeast SMF1, which transports Fe2+ and is permeable by Na+, Li+, and K+ (Chen et al., 1999). This dual-capacity transport suggests a role in vacuolar cation homeostasis in planta, which might protect a subset of cells from metal ion overload (Chen et al., 1999).
MATERIALS AND METHODS
See Supplemental Table S1 for primer information.
Cloning of AtCCX3 and AtCCX4 cDNAs and Plasmid DNA Constructs
AtCCX3 and AtCCX4 were amplified from Arabidopsis (Arabidopsis thaliana) genomic DNA using PCR. Both AtCCX3 and AtCCX4 are predicted to have no introns. The primers are listed in Supplemental Table S1 (primers 1–5).
AtCCX3, AtsCCX3, and AtCCX4 cDNAs were subcloned into the yeast expression vector piHGpd (Nathan et al., 1999; Pittman and Hirschi, 2001; Pittman et al., 2002a).
The AtsCAX2 plasmid was cloned previously (Shigaki et al., 2003). The Arabidopsis Na+/H+ exchanger AtNHX1 was PCR cloned from plasmid DNA with the primers listed in Supplemental Table S1 (primers 6 and 7) and cloned into yeast expression vectors.
The AtCCX3 and AtsCCX3 ORFs were cloned into a modified pBIN19 vector (Dr. Toshiro Shigaki, Clontech, personal communication). The resulting constructs contained the cauliflower mosaic virus 35S promoter fragment driving expression of the transporters and the NOS terminator (Hull et al., 2000).
The triple HA epitope-tagged AtCCX3 (HA-AtCCX3) was constructed as described previously (Shigaki et al., 2001). The primers used are listed in Supplemental Table S1 (primers 8–11). The two fragments were ligated with the yeast expression vector piHGpd (Nathan et al., 1999).
The C-terminal GFP tag AtCCX3 (CCX3-GFP) was constructed as described previously (Cheng et al., 2004). The primers used are listed in Supplemental Table S1 (primers 12–15). After cloning into the appropriate yeast vectors, this expression cassette was also cloned into pBIN19.
Yeast Strains and Growth Conditions
The following Saccharomyces cerevisiae yeast strains were used in this study: W303-1A (MATa ade2-1 can1-100 his3-11,15 leu2-3, 112 trp1-1 ura3-1; Wallis et al., 1989) and R100 (Δnhx1∷URA3; Nass et al., 1997), which is isogenic to W303 and AXT3 (ena1∷HIS3∷ena4, nha1∷LEU2, nhx1∷TRP1, ura3-1; Yokoi et al., 2002). The Mn2+-sensitive strain Δsmf1 + Δsmf2 was used in yeast growth assays (Cohen et al., 2000). The Mg2+-sensitive yeast strain CM66 (MATa Δalr1∷HIS3, Δalr2∷TRP, his3-v200, ura3-52, leu2-v1, lys2-v202 trp1-v63; Liu et al., 2002) was used in yeast growth assays as well. The yeast strains K661 (MATa Δvcx1∷URA3, ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1; Cunningham and Fink, 1996) and K667 (cnb1∷LEU2 pmc1∷TRP1 vcx1Δ; Cunningham and Fink, 1996) were also used in yeast growth assays. Yeast metal-sensitive growth assays were performed as described previously (Hirschi et al., 1996; Nass et al., 1997; Nass and Rao, 1998; Darley et al., 2000; Shigaki et al., 2003; Padmanaban et al., 2007). Yeast samples for liquid culture assays were 5-fold serial diluted, and 10 μL of the 125-fold diluted inoculum was grown in 190 μL of medium supplemented with various concentrations of NaCl2, KCl, and MnCl2 at 30°C with continuous shaking. For the NaCl2 assays in the AXT3 strain, the medium was supplemented with 1 mm KCl (Yokoi et al., 2002). After 48 h, optical density at 600 nm (OD600) measurements were taken.
Yeast Sample Processing and Cation Analysis
Yeast culture conditions and sample processing were modified from previous studies (Lahner et al., 2003; Eide et al., 2005; Mei et al., 2007). Yeast strains expressing vector, AtNHX1, or AtCCX3 were grown overnight in AP medium. After measuring the OD600, equal numbers of cells were grown in 5 mL of yeast peptone dextrose (YPD) supplemented with 100 mm NaCl or 200 μm MnCl2 for 16 h.
Plant Materials and Growth
Arabidopsis ecotype Columbia was used as the wild type. Agrobacterium tumefaciens GV3101 was transformed with AtCCX3, AtsCCX3, or vector controls (Sambrook et al., 1989). Arabidopsis plants were transformed using the floral dip method (Clough and Bent, 1998). Tobacco (Nicotiana tabacum ‘KY14’; Koren'kov et al., 2007) transformation was done using the leaf disc method as described previously (Tarczynski et al., 1992).
Cation Analysis of Tobacco and Arabidopsis Plants
Arabidopsis seeds expressing 35S:AtCCX3 were planted on half-strength Murashige and Skoog (MS) + 1% Suc plates and grown for 10 d. Plants were transferred to soil and watered with 100 mL of 50 mm NaCl twice weekly. Plants were harvested at 35 d of age, dried at 68°C, and ground. Tobacco seeds were germinated on half-strength MS + 3% Suc and grown for 2 weeks, then they were transferred to soil. At 28 and 40 d of age, leaves were removed, dried at 68°C, and ground. Inductively coupled plasma (ICP) analysis was done as described previously (Franson, 1989).
86Rb+ Uptake Assay in Yeast and Arabidopsis Roots
A yeast strain (wx1) expressing AtCCX3, AtNHX1, and vector were grown for 16 h in AP selection medium, and membrane vesicles were isolated as described previously (Pittman and Hirschi, 2001; Cheng and Hirschi, 2003; Pittman et al., 2004b). Plants were grown hydroponically, and membrane vesicles were isolated as described previously (Pittman and Hirschi, 2001; Shigaki et al., 2001, 2003; Cheng et al., 2003).
Time-dependent 86Rb+ uptake measurements into membrane vesicles were performed as described previously (Venema et al., 2002; Cheng et al., 2003; Pittman et al., 2004a, 2004b). For the measurement of ΔpH-dependent KCl uptake, vacuole-enriched membrane (100 μg protein mL−1) vesicles were incubated in buffer containing 0.3 M sorbitol, 5 mm Tris-MES (pH 7.6), 25 mm KCl, 0.1 mm sodium azide, and 0.2 mm sodium orthovanadate. The vesicles were added to 1 mm MgSO4 and 1 mm ATP to reach a steady-state pH gradient for 5 min at 25°C before the addition of 86Rb+ (1 μCi mL−1; GE Bioscience). Benzamil was used at 10 μm to inhibit AtNHX1 uptake. For metal competition experiments, 25 μm 86Rb+ uptake was measured at the 8-min time point in the presence of 300 μm concentrations of the nonradioactive metals KCl, NaCl, CaCl2, MnCl2, and ZnCl2.
Protein Isolation and Western-Blot Analysis of Epitope-Tagged AtCCX3 from Yeast and Plants
Total protein was isolated from yeast expressing HA-AtCCX3 using the glass bead method (Ausubel et al., 1998). Suc fractionation and western blotting of membranes were done as described previously (Pittman et al., 2004a, 2004b). The vacuolar marker ALP (Molecular Probes) was used at a 1:2,000 dilution, and the plasma membrane H+-ATPase Pma1p was used at a 1:1,000 dilution. For the GFP-tagged protein, total plant protein was isolated as described previously (Fitzpatrick and Keegstra, 2001).
Microsomal membranes were prepared from AtCCX3-GFP-expressing Arabidopsis leaf tissues as described previously (Cheng et al., 2003). Immunoblotting was performed as described previously (Pittman and Hirschi, 2001). The GFP epitope and the membrane marker proteins were detected as described previously (Cheng et al., 2003).
Onion Epidermis Bombardment and Visualization of the GFP Subcellular Localization
Single epidermis layers were removed from white onion (Allium cepa) bulb and placed on the surface of a MS plate (Murashige and Skoog, 1962) containing 2% Suc and 50 μg mL−1 ampicillin. The constructs 35S∷CCX3-GFP and 35S∷GFP were introduced into onion epidermal cells by particle bombardment. The process of particle preparation, coating, and bombardment was as described previously (Sivitz et al., 2007). The GFP fluorescence acquisition was performed with a Plan Apo 20×/0.75 numerical aperture objective. Images were recorded with picture size of 512 × 512 pixels. Individual sections along whole cells were captured in IDS format and then transferred into TIFF files.
RNA Extraction and RT-PCR
RNA was isolated using the RNeasy Plant Kit (Qiagen) according to the instructions of the manufacturer. RT-PCR was performed to detect mRNA transcript in Arabidopsis of AtCCX3 and AtCCX4 knockout lines, Arabidopsis plants overexpressing AtCCX3 and AtsCCX3, and tobacco plants ectopically expressing AtCCX3 and AtsCCX3. One microliter of the first-strand cDNA was used to amplify an AtCCX3 gene-specific fragment and an Actin1 fragment (Geisler et al., 2000). Primers are listed in Supplemental Table S1 (primers 18–23). The relative intensities in different lanes within each individual experiment were independent of the number of PCR cycles performed.
Isolation of Homozygous T-DNA Insertional Lines
To isolate ccx3 and ccx4 null alleles, two T-DNA insertional lines were obtained from the SAIL T-DNA insertion collection (Sessions et al., 2002) for AtCCX3 (SAIL_F-09, ccx3-1; SAIL_C30-05, ccx3-2) and two from the SALK T-DNA insertion collection (Alonso et al., 2003) for AtCCX4 (SALK_113447, ccx4-1; SALK_040272, ccx4-2). Homozygous plants from each T3 generation were obtained by PCR screening using primers listed in Supplemental Table S1 (primers 24–27). For AtCCX4, primers 5 and 28 listed in Supplemental Table S1 were used.
Quantitative Real-Time PCR Analysis of AtCCX3
At 14 d of age, 25 plants were transferred to control medium or medium supplemented with 100 mm KCl, 50 mm NaCl, or 1 μm MnCl2. After 24 h, the plants were harvested and total plant RNA was extracted using the RNeasy Plant Kit (Qiagen). For floral RNA, 25 plants were transferred to soil and watered weekly with 50 mL of 100 mm KCl, 50 mm NaCl, or 1 μm MnCl2 solution. RNA was extracted using the RNeasy Plant Kit (Qiagen). Serial dilutions of RNA were made from 5 μg to 5 ng, and first-strand cDNA was synthesized as described previously (Geisler et al., 2000). The PCR amplification was performed with 5 μL of cDNA, 0.5 μm of each primer, and 1× SYBR Green PCR mix (Invitrogen). The primers used are listed in Supplemental Table S1 (primers 29–34). Quantitative PCR was done using the SYBR Green probe, 1 μg of cDNA, and the ABI 7900HT RT-PCR system (Applied Biosystems). Real-time PCR amplification was performed and calculations were made with the ABI Prism 7700 sequence detection system (Applied Biosystems). Relative transcript abundance was determined using the comparative ΔΔCT method with SDS software version 2.2.2 (Applied Biosystems). For a standard control, expression of the 18S ribosomal subunit was used.
Protein Oxidation Analysis
Carbonyl assays for the analysis of oxidized proteins in plant cells were performed as described previously (Levine et al., 1994; Davletova et al., 2005; Cheng et al., 2006). Total protein was isolated from young and old leaves of 6-week-old tobacco plants ectopically expressing AtCCX3, AtsCCX3, and vector only, as described previously (Cheng et al., 2006). The oxidized proteins were detected by protein gel blotting using anti-dinitrophenylhydrazone antibody (Davletova et al., 2005).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AtCCX3: NM_112262 and AtCCX4: NM_104289.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. AtCCX3 and AtCCX4 amino acid comparison with the CAX family of transporters and HsNCKX6.
Supplemental Figure S2. Altered expression of AtCCX3 in Arabidopsis mutants.
Supplemental Figure S3. Protein oxidation from tobacco plants expressing AtCCX3.
Supplemental Table S1. Oligonucleotide primers used in the experiments.
Supplementary Material
Acknowledgments
We thank Dr. Ramon Gonzalez for providing AtNHX1 in pDR196. We thank Bronwyn Barkla and Heven Sze for critical reading of the manuscript. We also thank Adam Gillum for his help with the preparation of the figures.
This work was supported by the U.S. Department of Agriculture/Agricultural Research Service (Cooperative Agreement no. 58–62650–6001), the National Science Foundation (grant nos. NSF 0344350 and NSF 020977), and the U.S. Department of Agriculture Cooperative State Research, Education, and Extension Service (grant no. 2005–34402–17121 to K.D.H.). The National Science Foundation (grant no. 0209792) funded work in the laboratory of J.M.W.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Kendal D. Hirschi (kendalh@bcm.tmc.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657 [DOI] [PubMed] [Google Scholar]
- Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285 1256–1258 [DOI] [PubMed] [Google Scholar]
- Apse MP, Sottosanto JB, Blumwald E (2003) Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. Plant J 36 229–239 [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidmena JG, Smith JA, Struhl K (1998) Current Protocols in Molecular Biology. Green Publishing Associates/Wiley International, New York
- Barkla BJ, Pantoja O (1996) Physiology of ion transport across the tonoplast of higher plants. Annu Rev Plant Physiol Plant Mol Biol 47 159–184 [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ (1999) Sodium/calcium exchange: its physiological implications. Physiol Rev 79 763–854 [DOI] [PubMed] [Google Scholar]
- Blumwald E, Poole RJ (1986) Kinetics of Ca2+/H+ antiport in isolated tonoplast vesicles from storage tissue of Beta vulgaris L. Plant Physiol 80 727–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock KW, Honys D, Ward JM, Padmanaban S, Nawrocki EP, Hirschi KD, Twell D, Sze H (2006) Integrating membrane transport with male gametophyte development and function through transcriptomics. Plant Physiol 140 1151–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Lytton J (2004. a) The cation/Ca2+ exchanger superfamily: phylogenetic analysis and structural implications. Mol Biol Evol 21 1692–1703 [DOI] [PubMed] [Google Scholar]
- Cai X, Lytton J (2004. b) Molecular cloning of a sixth member of the K+-dependent Na+/Ca2+ exchanger gene family, NCKX6. J Biol Chem 279 5867–5876 [DOI] [PubMed] [Google Scholar]
- Carter C, Pan S, Zouhar J, Avila EL, Girke T, Raikhel NV (2004) The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell 16 3285–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L, Lagnado L, Perry RJ, Robinson DW, McNaughton PA (1989) Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature 337 740–743 [DOI] [PubMed] [Google Scholar]
- Chen XZ, Peng JB, Cohen A, Nelson H, Nelson N, Hediger MA (1999) Yeast SMF1 mediates H+-coupled iron uptake with concomitant uncoupled cation currents. J Biol Chem 274 35089–35094 [DOI] [PubMed] [Google Scholar]
- Cheng N, Pittman JK, Shigaki T, Hirschi KD (2002) Characterization of CAX4, an Arabidopsis H+/cation antiporter. Plant Physiol 128 1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH, Hirschi KD (2003) Cloning and characterization of CXIP1, a novel PICOT domain-containing Arabidopsis protein that associates with CAX1. J Biol Chem 278 6503–6509 [DOI] [PubMed] [Google Scholar]
- Cheng NH, Liu JZ, Brock A, Nelson RS, Hirschi KD (2006) AtGRXcp, an Arabidopsis chloroplastic glutaredoxin, is critical for protection against protein oxidative damage. J Biol Chem 281 26280–26288 [DOI] [PubMed] [Google Scholar]
- Cheng NH, Liu JZ, Nelson RS, Hirschi KD (2004) Characterization of CXIP4, a novel Arabidopsis protein that activates the H+/Ca2+ antiporter, CAX1. FEBS Lett 559 99–106 [DOI] [PubMed] [Google Scholar]
- Cheng NH, Pittman JK, Barkla BJ, Shigaki T, Hirschi KD (2003) The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. Plant Cell 15 347–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Chen CY, Tao LZ, Andreyeva T, Twell D, Wu HM (2003) Regulation of pollen tube growth by Rac-like GTPases. J Exp Bot 54 73–81 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Cohen A, Nelson H, Nelson N (2000) The family of SMF metal ion transporters in yeast cells. J Biol Chem 275 33388–33394 [DOI] [PubMed] [Google Scholar]
- Cunningham KW, Fink GR (1996) Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol Cell Biol 16 2226–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley CP, van Wuytswinkel OC, van der Woude K, Mager WH, de Boer AH (2000) Arabidopsis thaliana and Saccharomyces cerevisiae NHX1 genes encode amiloride sensitive electroneutral Na+/H+ exchangers. Biochem J 351 241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R (2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17 268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Light PE, French RJ, Lytton J (2001) Electrophysiological characterization and ionic stoichiometry of the rat brain K+-dependent Na+/Ca2+ exchanger, NCKX2. J Biol Chem 276 25919–25928 [DOI] [PubMed] [Google Scholar]
- Eide D, Clark S, Nair TM, Gehl M, Gribskov M, Guerinot M, Harper J (2005) Characterization of the yeast ionome: a genome-wide analysis of nutrient mineral and trace element homeostasis in Saccharomyces cerevisiae. Genome Biol 6 R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger WF, Clear AM, Fanning KJ, Peck ML (1999) Identification of a Ca2+/H+ antiport in the plant chloroplast thylakoid membrane. Plant Physiol 119 1379–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick LM, Keegstra K (2001) A method for isolating a high yield of Arabidopsis chloroplasts capable of efficient import of precursor proteins. Plant J 27 59–65 [DOI] [PubMed] [Google Scholar]
- Franson MAH (1989) 3120 metals by plasma emission spectroscopy. In Standard Methods for the Examination of Water and Wastewater. American Public Health Association, Washington, DC, pp 3.53–3.62
- Geisler M, Frangne N, Gomes E, Martinoia E, Palmgren MG (2000) The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol 124 1814–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Koren'Kov V, Wagner GJ (1999) A comparison of Zn, Mn, Cd, and Ca transport mechanisms in oat root tonoplast vesicles. Physiol Plant 106 203–209 [Google Scholar]
- Halliwell B (2006) Reactive species and antioxidants: redox biology is a fundamental theme of aerobic life. Plant Physiol 141 312–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC (2006) Free Radicals in Biology and Medicine, Ed 4. Clarendon Press, Oxford
- Hirschi K (2001) Vacuolar H+/Ca2+ transport: who's directing the traffic? Trends Plant Sci 6 100–104 [DOI] [PubMed] [Google Scholar]
- Hirschi KD (1999) Expression of Arabidopsis CAX1 in tobacco: altered calcium homeostasis and increased stress sensitivity. Plant Cell 11 2113–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD, Zhen RG, Cunningham KW, Rea PA, Fink GR (1996) CAX1, an H+/Ca2+ antiporter from Arabidopsis. Proc Natl Acad Sci USA 93 8782–8786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clarke TL, Weddle NM, Kim S, Robi A, Parris C, Kunkel JG, Hepler PK (2003) Effect of extracellular calcium, pH and borate on growth oscillations in Lilium formosanum pollen tubes. J Exp Bot 54 65–72 [DOI] [PubMed] [Google Scholar]
- Hull AK, Vij R, Celenza JL (2000) Arabidopsis cytochrome P450s that catalyze the first step of tryptophan-dependent indole-3-acetic acid biosynthesis. Proc Natl Acad Sci USA 97 2379–2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya T, Maeshima M (2004) Residues in internal repeats of the rice cation/H+ exchanger are involved in the transport and selection of cations. J Biol Chem 279 812–819 [DOI] [PubMed] [Google Scholar]
- Kang KJ, Kinjo TG, Szerencsei RT, Schnetkamp PPM (2005) Residues contributing to the Ca2+ and K+ binding pocket of the NCKX2 Na+/Ca2+-K+ exchanger. J Biol Chem 280 6823–6833 [DOI] [PubMed] [Google Scholar]
- Kasai M, Muto S (1990) Ca2+ pump and Ca2+/H+ antiporter in plasma membrane vesicles isolated by aqueous two-phase partitioning from corn leaves. J Membr Biol 114 133–142 [DOI] [PubMed] [Google Scholar]
- Koren'kov V, Park S, Cheng NH, Sreevidya C, Lachmansingh J, Morris J, Hirschi K, Wagner G (2007) Enhanced Cd2+-selective root-tonoplast-transport in tobaccos expressing Arabidopsis cation exchangers. Planta 225 403–411 [DOI] [PubMed] [Google Scholar]
- Krysan PJ, Young JC, Sussman MR (1999) T-DNA as an insertional mutagen in Arabidopsis. Plant Cell 11 2283–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahner B, Gong J, Mahmoudian M, Smith EL, Abid KB, Rogers EE, Guerinot ML, Harper JF, Ward JM, McIntyre L, et al (2003) Genomic scale profiling of nutrient and trace elements in Arabidopsis thaliana. Nat Biotechnol 21 1215–1221 [DOI] [PubMed] [Google Scholar]
- Lee SH, Kim MH, Park KH, Earm YE, Ho WK (2002) K+-dependent Na+/Ca2+ exchange is a major Ca2+ clearance mechanism in axon terminals of rat neurohypophysis. J Neurosci 22 6891–6899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL, Williams JA, Stadtman EP, Shacter E, Lester P (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233 346–357 [DOI] [PubMed] [Google Scholar]
- Liu GJ, Martin DK, Gardner RC, Ryan PR (2002) Large Mg2+-dependent currents are associated with the increased expression of ALR1 in Saccharomyces cerevisiae. FEMS Microbiol Lett 213 231–237 [DOI] [PubMed] [Google Scholar]
- Liu XF, Supek F, Nelson N, Culotta VC (1997) Negative control of heavy metal uptake by the Saccharomyces cerevisiae BSD2 gene. J Biol Chem 272 11763–11769 [DOI] [PubMed] [Google Scholar]
- Luk EEC, Culotta VC (2001) Manganese superoxide dismutase in Saccharomyces cerevisiae acquires its metal co-factor through a pathway involving the Nramp metal transporter, Smf2p. J Biol Chem 276 47556–47562 [DOI] [PubMed] [Google Scholar]
- Luo GZ, Wang HW, Huang J, Tian AG, Wang YJ, Zhang JS, Chen SY (2005) A putative plasma membrane cation/proton antiporter from soybean confers salt tolerance in Arabidopsis. Plant Mol Biol 59 809–820 [DOI] [PubMed] [Google Scholar]
- Lytton J (2007) Na+/Ca2+ exchangers: three mammalian gene families control Ca2+ transport. Biochem J 406 365–382 [DOI] [PubMed] [Google Scholar]
- MacDiarmid CW, Gardner RC (1998) Overexpression of the Saccharomyces cerevisiae magnesium transport system confers resistance to aluminum ion. J Biol Chem 273 1727–1732 [DOI] [PubMed] [Google Scholar]
- Maeshima M (2000) Vacuolar H+-pyrophosphatase. Biochim Biophys Acta 1465 37–51 [DOI] [PubMed] [Google Scholar]
- Marschner H (1995) Mineral Nutrition of Higher Plants, Ed 2. Academic Press, San Diego
- Marty F (1999) Plant vacuoles. Plant Cell 11 587–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJM, Sanders D, et al (2001) Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol 126 1646–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei H, Zhao J, Pittman JK, Lachmansingh J, Park S, Hirschi KD (2007) In planta regulation of the Arabidopsis Ca2+/H+ antiporter CAX1. J Exp Bot 58 3419–3427 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15 473–497 [Google Scholar]
- Nass R, Cunningham KW, Rao R (1997) Intracellular sequestration of sodium by a novel Na+/H+ exchanger in yeast is enhanced by mutations in the plasma membrane H+-ATPase: insights into mechanisms of sodium tolerance. J Biol Chem 272 26145–26152 [DOI] [PubMed] [Google Scholar]
- Nass R, Rao R (1998) Novel localization of a Na+/H+ exchanger in a late endosomal compartment of yeast: implications for vacuole biogenesis. J Biol Chem 273 21054–21060 [DOI] [PubMed] [Google Scholar]
- Nathan DF, Vos MH, Lindquist S (1999) Identification of SSF1, CNS1, and HCH1 as multicopy suppressors of a Saccharomyces cerevisiae Hsp90 loss-of-function mutation. Proc Natl Acad Sci USA 96 1409–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanaban S, Chanroj S, Kwak JM, Li X, Ward JM, Sze H (2007) Participation of endomembrane cation/H+ exchanger AtCHX20 in osmoregulation of guard cells. Plant Physiol 144 82–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JM, Serrano R (1989) Structure of a plasma membrane H+-ATPase gene from the plant Arabidopsis thaliana. J Biol Chem 264 8557–8562 [PubMed] [Google Scholar]
- Pittman JK, Cheng NH, Shigaki T, Kunta M, Hirschi KD (2004. a) Functional dependence on calcineurin by variants of the Saccharomyces cerevisiae vacuolar Ca2+/H+ exchanger Vcx1p. Mol Microbiol 54 1104–1116 [DOI] [PubMed] [Google Scholar]
- Pittman JK, Hirschi KD (2001) Regulation of CAX1, an Arabidopsis Ca2+/H+ antiporter: identification of an N-terminal autoinhibitory domain. Plant Physiol 127 1020–1029 [PMC free article] [PubMed] [Google Scholar]
- Pittman JK, Shigaki T, Cheng NH, Hirschi KD (2002. a) Mechanism of N-terminal autoinhibition in the Arabidopsis Ca2+/H+ antiporter CAX1. J Biol Chem 277 26452–26459 [DOI] [PubMed] [Google Scholar]
- Pittman JK, Shigaki T, Marshall JL, Morris JL, Cheng NH, Hirschi KD (2004. b) Functional and regulatory analysis of the Arabidopsis thaliana CAX2 cation transporter. Plant Mol Biol 56 959–971 [DOI] [PubMed] [Google Scholar]
- Pittman JK, Sreevidya CS, Shigaki T, Ueoka-Nakanishi H, Hirschi KD (2002. b) Distinct N-terminal regulatory domains of Ca2+/H+ antiporters. Plant Physiol 130 1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak R (2000) Structure, function and regulation of the plant vacuolar H+-translocating ATPase. Biochim Biophys Acta 1465 17–36 [DOI] [PubMed]
- Salt DE, Wagner GJ (1993) Cadmium transport across tonoplast of vesicles from oat roots: evidence for a Cd2+/H+ antiport activity. J Biol Chem 268 12297–12302 [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Schumaker KS, Sze H (1985) A Ca2+/H+ antiport system driven by the proton electrochemical gradient of a tonoplast H+-ATPase from oat roots. Plant Physiol 79 1111–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions A, Burke E, Presting G, Aux G, McElver J, Patton D, Dietrich B, Ho P, Bacwaden J, Ko C, et al (2002) A high-throughput Arabidopsis reverse genetics system. Plant Cell 14 2985–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigaki T, Cheng N-h, Pittman JK, Hirschi K (2001) Structural determinants of Ca2+ transport in the Arabidopsis H+/Ca2+ antiporter CAX1. J Biol Chem 276 43152–43159 [DOI] [PubMed] [Google Scholar]
- Shigaki T, Hirschi KD (2006) Diverse functions and molecular properties emerging for CAX cation/H+ exchangers in plants. Plant Biol 8 419–429 [DOI] [PubMed] [Google Scholar]
- Shigaki T, Pittman JK, Hirschi KD (2003) Manganese specificity determinants in the Arabidopsis metal/H+ antiporter CAX2. J Biol Chem 278 6610–6617 [DOI] [PubMed] [Google Scholar]
- Shigaki T, Rees I, Nakhleh L, Hirschi KD (2006) Identification of three distinct phylogenetic groups of CAX cation/proton antiporters. J Mol Evol 63 815–825 [DOI] [PubMed] [Google Scholar]
- Sivitz AB, Reinders A, Johnson ME, Krentz AD, Grof CPL, Perroux JM, Ward JM (2007) Arabidopsis sucrose transporter AtSUC9: high-affinity transport activity, intragenic control of expression, and early flowering mutant phenotype. Plant Physiol 143 188–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supek F, Supekova L, Nelson H, Nelson N (1996) A yeast manganese transporter related to the macrophage protein involved in conferring resistance to mycobacteria. Proc Natl Acad Sci USA 93 5105–5110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H, Padmanaban S, Cellier F, Honys D, Cheng N-H, Bock KW, Conejero G, Li X, Twell D, Ward JM, et al (2004) Expression patterns of a novel AtCHX gene family highlight potential roles in osmotic adjustment and K+ homeostasis in pollen development. Plant Physiol 136 2532–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarczynski MC, Jensen RG, Bohnert HJ (1992) Expression of a bacterial mtlD gene in transgenic tobacco leads to production and accumulation of mannitol. Proc Natl Acad Sci USA 89 2600–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle MS, Radisky D, Li L, Kaplan J (2003) A dominant allele of PDR1 alters transition metal resistance in yeast. J Biol Chem 278 1273–1280 [DOI] [PubMed] [Google Scholar]
- Venema K, Belver A, Marin-Manzano MC, Rodriguez-Rosales MP, Donaire JP (2003) A novel intracellular K+/H+ antiporter related to Na+/H+ antiporters is important for K+ ion homeostasis in plants. J Biol Chem 278 22453–22459 [DOI] [PubMed] [Google Scholar]
- Venema K, Quintero FJ, Pardo JM, Donaire JP (2002) The Arabidopsis Na+/H+ exchanger AtNHX1 catalyzes low affinity Na+ and K+ transport in reconstituted liposomes. J Biol Chem 277 2413–2418 [DOI] [PubMed] [Google Scholar]
- Wallis JW, Chrebet G, Brodsky G, Rolfe M, Rothstein R (1989) A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell 58 409–419 [DOI] [PubMed] [Google Scholar]
- Ward JM, Reinders A, Hsu HT, Sze H (1992) Dissociation and reassembly of the vacuolar H+-ATPase complex from oat roots. Plant Physiol 99 161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkfein RJ, Szerencsei RT, Kinjo TG, Kang KJ, Perizzolo M, Eisner L, Schnetkamp PPM (2003) Scanning mutagenesis of the alpha repeats and of the transmembrane acidic residues of the human retinal cone Na/Ca-K exchanger. Biochemistry 42 543–552 [DOI] [PubMed] [Google Scholar]
- Yokoi S, Quintero FJ, Cubero B, Ruiz MT, Bressan RA, Hasegawa PM, Pardo JM (2002) Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J 30 529–539 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.