Abstract
Sex steroids modulate reproduction by altering the response of steroid-activated opioid circuits in the hypothalamus and limbic system, by inducing release of endogenous opioids and activation of their cognate receptors. Many studies have concentrated on steroid regulation of exogenous opioid peptides, but steroids also have important actions on opioid receptors inducing receptor trafficking. Opioid receptors are G protein-coupled receptors and their activation catalyzes the exchange of GTP for GDP initiating intracellular signaling cascades. Kinetics of G protein activation were studied using [35S]GTPγS binding. Catalytic amplification, the number of G proteins activated per occupied receptor, was used as a measure of receptor/transducer amplification. The present study examined whether estrogen and progesterone treatment altered the kinetics of nociceptin opioid receptor (ORL1) in plasma membranes from the medial preoptic area and mediobasal hypothalamus. These hypothalamic regions are important in the gonadal steroid hormone regulation of sexual receptivity. In the mediobasal hypothalamus, estrogen increased ORL1 (Bmax) receptor number 2-fold and maximal GTPγS binding (Emax) 3.9-fold. Subsequent progesterone treatment further increased ORL1 Emax 6.9-fold above baseline, despite a 2-fold decrease in the catalytic amplification factor. In the medial preoptic area, estrogen alone did not increase E max, but both estrogen and progesterone were able to increase ORL1 Bmax 2.2-fold and Emax 3-fold, despite having a 3-fold decrease in the catalytic amplification factor. These effects are interesting because they indicate actions of steroids that increase the number of ORL1 but decrease the catalytic amplification suggesting that the steroid effects on opioid receptors are complex and may involve modulation by other signals.
Keywords: GTPγS, G protein-coupled receptor, Opioid receptor 1, Mediobasal hypothalamus, Medial preoptic area
Introduction
Opioid receptor activation by endogenous and exogenous ligands results in a multitude of effects in the CNS, such as analgesia, euphoria, feeding, anxiety and reproduction. Our laboratory has been interested in the interactions of the sex steroids, estrogen and progesterone, with opioid receptors in hypothalamic and limbic circuits regulating sexual receptivity. Two important opioid receptors in the hypothalamus are the μ-opioid receptor and nociceptin receptor, MOP and ORL1, respectively [1]. Estrogen and progesterone have been shown to regulate MOP and ORL1 expression or function. For example, estrogen internalizes MOP, removing the receptor from rat brain plasma membranes [2, 3]. Whereas progesterone has been shown to reverse estrogen effects on MOP [4]. For ORL1 estrogen decreases the expression of ORL1 [5] and coupling to its G proteins [6]. Estrogen and progesterone together are required to increase OFQ/N mRNA levels in the medial preoptic area of the hypothalamus [7]. Previous experiments indicate that estrogen and progesterone regulate the behavioral response to opioid receptor agonists that can inhibit or facilitate lordosis. Estrogen activation of MOP in the medial preoptic area inhibits lordosis [4, 8]. However, subsequent progesterone blocks MOP inhibition and activates ORL1 in both the medial preoptic area and mediobasal hypothalamus [2]. ORL1 is structurally and functionally related to the opioid receptors [2, 9, 10] and has been demonstrated to play a role in nociception [11–13], learning and memory [14], cardiovascular functions [15], locomotion [16] and in the hypothalamus in neuroendocrine control [17] and feeding behavior [18].
Site-specific infusion of nociceptin, the endogenous ligand for ORL1 [9, 19, 20], into the medial preoptic area or the mediobasal hypothalamus facilitates lordosis behavior in estrogen-primed rats [21, 22]. Sex steroids increase in the expression of endogenous opioid peptide and nociceptin mRNAs in hypothalamic brain regions [7, 10], while others have shown decreased expression in the trigeminal region [5]. Additionally, estrogen and progesterone modulate opioid receptor internalization by inducing opioid peptide release. However, subsequent progesterone treatment blocks estrogen-induced MOP internalization [4]. These results suggest that sex steroids modulate opioid receptor function [2, 4, 23]. Thus, the manner by which steroids modulate ORL1 expression/function in the medial preoptic area and mediobasal hypothalamus is important to understand the mechanisms regulating lordosis behavior in the female rat.
Receptor function can be regulated by altering the number of binding sites, the affinity of the receptor, or by modulating the association of the activated receptor with G proteins. ORL1 is a member of the G protein-coupled receptor superfamily that interacts with heterotrimeric G proteins [2, 24]. Agonist activation of the receptor-G protein complex causes a conformational change in the G protein, which results in an exchange of GTP for GDP, leading to dissociation of the GαGTP protein and a Gβγ complex. The ensuing dissociation of trimeric G proteins initiates intracellular signaling cascades through the GαGTP and the Gβγ subunits. ORL1 acts via inhibitory G proteins to inhibit adenylyl cyclase [25–27] and regulate inward rectifying potassium channels [28, 29].
Using a physiological steroid replacement paradigm in female rats [4, 30, 31] to elicit sexual receptivity in ovariectomized (OVX) female rats, the purpose of the present study was to determine whether there is modulation of ORL1 binding or a catalytic amplification (receptor/transducer amplification) of the number of G proteins activated per occupied receptor [31–34]. Medial preoptic area and mediobasal hypothalamic membranes were prepared from OVX rats treated with oil, estrogen or estrogen + progesterone. Membranes were used to examine ORL1 activation of G proteins by measuring the binding of the hydrolysis-resistant GTP analog [35S]-guanylyl-5′-O-(thio)-triphosphate (GTPγS) [35, 36]. Enriched plasma membrane from the medial preoptic area and mediobasal hypothalamus were used for radioligand-binding assays with [3H]-OFQ/nociceptin as the ligand to determine the actions of estrogen and estrogen + progesterone on ORL1-binding kinetics. Comparison of the maximal numbers of activated G proteins with the numbers of ORL1 (GproteinBmax/ORL1 Bmax) allowed us to calculate a catalytic amplification factor. Some of the results herein reported are in preliminary form [37].
Methods
Animals
Adult female Long-Evans rats (Charles River, Portage, Mich., USA), weighing 225–250 g, were bilaterally OVX by the supplier. Animals were housed in a partially reversed 12/12-hour light/dark cycle (lights on at 12 midnight) and provided food and water ad libitum. All procedures were approved by the UCLA Chancellor’s Animal Research Committee. Two weeks after ovariectomy, rats were injected with oil vehicle or 2 μg 17β-estradiol benzoate every 4 days for 3 ‘cycles’. Twenty-six hours after the third 17β-estradiol benzoate injection, animals were treated with either 500 μg progesterone or oil. The steroid treatments were derived from our gene expression and behavioral studies [4, 30, 31]. Four hours after progesterone or oil injection, rats were deeply anesthetized with sodium pentobarbital (100 mg/kg), decapitated, and brains rapidly removed and placed in ice-cold PBS. Medial preoptic areas (containing the medial preoptic nucleus) and mediobasal hypothalami (containing the ventromedial nucleus of the hypothalamus) were dissected. The medial preoptic area was defined as a region extending above the optic chiasm (rostrocaudally) and between the lateral borders of the stria medullaris (mediolaterally), and from the base of the brain to the dorsal extent of the stria medullaris (ventrodorsally). For the mediobasal hypothalamus, tissue was taken from a block defined by the rostrocaudal extent of the median eminence and the fornix mediolaterally and dorsally.
Membrane Preparation
Enriched plasma membrane from the medial preoptic area and mediobasal hypothalamus were pooled from 5 rats to generate a binding curve. Medial preoptic area and mediobasal hypothalamus tissues were individually homogenized in ice-cold lysis buffer (50 mM Tris-HCl, 10 mM sucrose, pH 7.4). The homogenates were centrifuged at 1,000 g for 10 min at 4 ° C and the supernatants removed and incubated in a shaking water bath for 30 min at 37 ° C to facilitate dissociation of endogenous opioid peptides and guanyl nucleotides. Subsequently, the supernatant was centrifuged at 46,000 g at 4 ° C for 15 min; the supernatant was resuspended in 2.5 ml ice-cold buffer (50 mM Tris-HCl, pH 7.4) and layered on top of a discontinuous sucrose density gradient with steps of 3.5 ml 55% (w/v), 3.5 ml 32% and 1.5 ml 5% sucrose in 50 mM Tris-HCl (pH 7.4). The sucrose density step-gradient was then centrifuged at 148,000 g for 60 min at 4 ° C. Membrane fractions were immediately resuspended, frozen and stored at −80 ° C until use. Protein content was measured using the Bradford assay (Bio-Rad Laboratories, Hercules, Calif., USA).
Radioligand-Binding Assays
For the nociceptin-binding assay and G-protein protein assays below, a dose-response curve for nociceptin was constructed to determine if the efficacy of specific opiate drug(s) was altered by steroid actions in estrogen- and progesterone-sensitive brain regions. 3 H-OFQ/N (Nociceptin; 33 Ci/mmol) was obtained from Amersham Pharmacia (Piscataway, N.J., USA). Membranes (10 μg in 100 μl Tris-HCl, pH 7.4, buffer), 50 μl radioligand (3H-Nociceptin for ORL1) and 100 μl protease inhibitors (10 μg/ml aprotinin, 0.1 mg/ml PMSF and 10 μg/ml pepstatin; Sigma-Aldrich, St. Louis, Mo., USA) were incubated at 25 ° C for 60 min in 50 mM Tris-HCl buffer (pH 7.4) in a final volume of 0.5 ml. Six concentration points for 3 H-nociceptin (0.01–0.8 nM) were used for Scatchard analysis. For nonspecific binding, 50 μl of unlabeled 1 μM nociceptin (Bachem, Torrance, Calif., USA) was used. A multichannel harvester (Brandell, Gaithersburg, Md., USA) was used to separate bound from free radiolabeled ligand by rapid filtration (3× with 1 ml ice-cold 50 mM Tris-HCl, pH 7.4, buffer) through glass fiber filters (Whatman GF/B, Brandell) presoaked in 0.1% BSA for 60 min at 4 ° C. Filters were air-dried, placed in scintillation vials with Ecolite scintillation fluid (National Diagnostic Inc., Atlanta, Ga., USA) and counted on a Wallac 1409 scintillation counter (Wallac Inc., Turku, Finland). Bound radioactivity was determined at 45% counting efficiency for 3H after overnight extraction of the filters in 5 ml of scintillation fluid. Ligand affinities (Kd) and receptor numbers (Bmax) for ORL1 were calculated using the Ligand program [38]. All binding experiments were carried out in duplicate, and the results are expressed as the mean ± SEM of 4–6 experiments. Statistical analysis was performed by one-way analysis of variance (ANOVA). The Student-Newman-Keuls test was used for post hoc comparisons with a significance level of p < 0.05.
Agonist-Stimulated [35S]GTPγS-Binding Assays
The [35S]GTPγS-binding assay was used to identify receptor-activated G proteins [35, 39, 40]. This assay is based on the observation that in the inactive state, the α-subunit of the G protein has a relatively high affinity for GDP over GTP. Activation of a receptor by an agonist shifts the α-subunit to a higher affinity state for GTP compared with GDP. Excess GDP was used to shift G proteins to the receptor-uncoupled state and lower basal activity. Addition of [35S]GTPγ S and an agonist shifted the G protein affinity from GDP to GTP allowing the activated G protein to bind [35S]GTPγ S. The medial preoptic area and mediobasal hypothalamus were dissected from 2 animals for each hormonal treatment group, pooled and homogenized in 20 vol of buffer (50 mM Tris-HCl, 3 mM MgCl2, 1 mM EGTA, pH 7.4). This was repeated 4–6 times. The homogenates were centrifuged twice (15 min) at 48,000 g at 4 ° C and resuspended in assay buffer (50 mM Tris-HCl, 3 mM MgCl2, 0.2 mM EGTA, 100 mM NaCl, pH 7.4). The Bradford assay (Bio-Rad) was used to determine the protein concentration. For concentration-effect curves, membranes (10 μg protein) were incubated with 40 μM GDP, 0.05 nM [35S]GTPγS (1,200 Ci/mmol, New England Nuclear) and various concentrations of nociceptin (10 nM to 10 μM) [41], in a final volume of 1.0 ml at 30 ° C for 1 h. Nonspecific binding was measured in the presence of 10 μM unlabeled GTPγS and subtracted from total binding to get specific binding. Basal binding was assessed in the absence of agonist. Free radioligand was separated from bound radioligand by rapid filtration as described above. Bound radioactivity was determined by liquid scintillation spectrophotometry at 95% efficiency for [35S] after overnight extraction of the filters in 4 ml Ecolite scintillation fluid. The efficacy of G protein activation (Emax) was defined as the maximal percentage of stimulation by nociceptin determined from dose-response curves analyzed using nonlinear regression (GraphPad, Prism Software Inc., San Diego, Calif., USA) [42]. Statistical significance was determined by paired Student’s t test with a significance level of p < 0.05.
[35S]GTPγS Saturation Binding
For Scatchard analysis of effects on agonist-stimulated [35S]GTPγS binding, membranes (10 μg protein) were incubated 1 h with 40 μM GDP, 0.05 nM [35S]GTPγS and 0–20 nM unlabeled GTPγS with or without 10 μM nociceptin in 1 ml total volume. The reaction was terminated by vacuum filtration through Whatman GF/B glass filters followed by three washes with cold Tris buffer. Bound radioactivity was determined by liquid scintillation spectrophotometry at 95% efficiency after overnight extraction in Ecolite scintillation fluid. Data were analyzed with the Ligand program and are presented as mean ± SEM of at least 4 determinations which were performed in triplicate. Catalytic amplification, the number of G proteins activated per occupied receptor, was used as a measure of receptor/transducer amplification and was calculated by dividing the Bmax of agonist stimulated [35S]GTPγS binding by the Bmax of receptor binding. Statistical analysis of data was performed by one-way analysis of variance (ANOVA). The Student-Newman-Keuls test was used for post hoc comparisons with significance level at p < 0.05.
Results
Effect of Estrogen and Progesterone on [3H]-Nociceptin Binding in the Medial Preoptic Area and Mediobasal Hypothalamus
In the OVX oil-treated control animals, ORL1 Kd values were 41.3 and 45.5 pM in the mediobasal hypothalamus and medial preoptic area, respectively, which are consistent with previous studies measuring ORL1 Kd in male rat whole brain membrane [41, 43] and guinea pig whole brain membranes [44]. Specific binding of [3H]-Nociceptin at concentrations of 0.01–0.8 nM in medial preoptic area and mediobasal hypothalamic membranes revealed that ORL1 Bmax sites and Kd were differentially sensitive to hormonal treatment. In the medial preoptic area, estrogen treatment alone did not have an effect on ORL1 Bmax or Kd. Estrogen + progesterone treatment, however, resulted in a 2.2-fold increase in ORL1 Bmax and a 5.9-fold decrease in affinity of ORL1 (p < 0.05; table 1). In the mediobasal hypothalamus, estrogen and estrogen + progesterone treatment increased ORL1 Bmax 2-fold. Estrogen treatment also resulted in a 2-fold decrease in the affinity of nociceptin for ORL1 (p < 0.05; table 2), but subsequent progesterone treatment did not change either the Bmax or Kd (table 2).
Table 1.
Effect of in vivo sex steroid treatment on ORL1 and [35S]GTPγS binding in membranes from the medial preoptic area
| ORL1 |
G protein |
Catalytic amplification G Bmax/ORL1 Bmax | |||
|---|---|---|---|---|---|
| Kd, pM | Bmax, fmol/mg | G Kd, nM | G Bmax, fmol/mg | ||
| Oil | 41.3±5.6 | 173±38.9 | 3.68±0.8 | 4,871±630 | 32.3±5.9 |
| EB | 47.5±9.4 | 185±36.2 | 3.52±0.8 | 3,829±549 | 20.6±2.6 |
| EB + P | 242.3±28.4* | 377±93.6* | 2.93±0.74 | 3,598±1024 | 9.6±.7* |
EB = 17β-Estradiol benzoate; P = progesterone. Membranes were incubated with varying concentrations of [3H]N/OFQ (0.01–0.8 nM) to examine the Kd and Bmax values from saturation binding experiments. Nociceptin (N/OFQ) at 10 nM to 10 μM was used to examine the apparent G protein affinity (G Kd) and apparent G protein number (G Bmax) for net agonist-stimulated [35S]GTPγS binding. The ratio G Bmax/ORL1 Bmax gives the functional coupling (catalytic amplification factor). Data represent mean apparent Bmax and apparent Kd values ± SEM from 4–6 independent experiments.
p < 0.05, significantly different from control animals.
Table 2.
Effect of in vivo sex steroid treatment on ORL1 and [35S]GTPγS binding in membranes from the mediobasal hypothalamus
| ORL1 |
G protein |
Catalytic amplification G Bmax/ORL1 Bmax | |||
|---|---|---|---|---|---|
| Kd, pM | Bmax, fmol/mg | G Kd, nM | G Bmax, fmol/mg | ||
| Oil | 45.5±8.1 | 276±29.1 | 3.0±0.64 | 2,144±496.3 | 7.7±1.8 |
| EB | 95±13.4* | 452±57.6* | 3.0±0.42 | 1,769±350.7 | 3.9±0.8* |
| EB + P | 122±29.9* | 432±40.4* | 3.6±0.9 | 2,059±466.4 | 3.4±0.7* |
EB = 17EB = 17β-Estradiol benzoate; P = progesterone. Membranes were incubated with varying concentrations of [3H]N/OFQ (0.01–0.8 nM) to examine the Kd and Bmax values from saturation binding experiments. Nociceptin (N/OFQ) at 10 nM to 10 μM was used to examine the apparent G protein affinity (G Kd) and apparent G protein number (G Bmax) for net agonist-stimulated [35S]GTPγS binding. The ratio G Bmax/ORL1 Bmax gives the functional coupling (catalytic amplification factor). Data represent mean apparent Bmax and apparent Kd values ± SEM from 4–6 independent experiments.
p < 0.05, significantly different from control animals.
Estrogen and Progesterone Effects on Nociceptin-Stimulated [35S]GTPγS Binding in Medial Preoptic Area and Mediobasal Hypothalamic Membranes
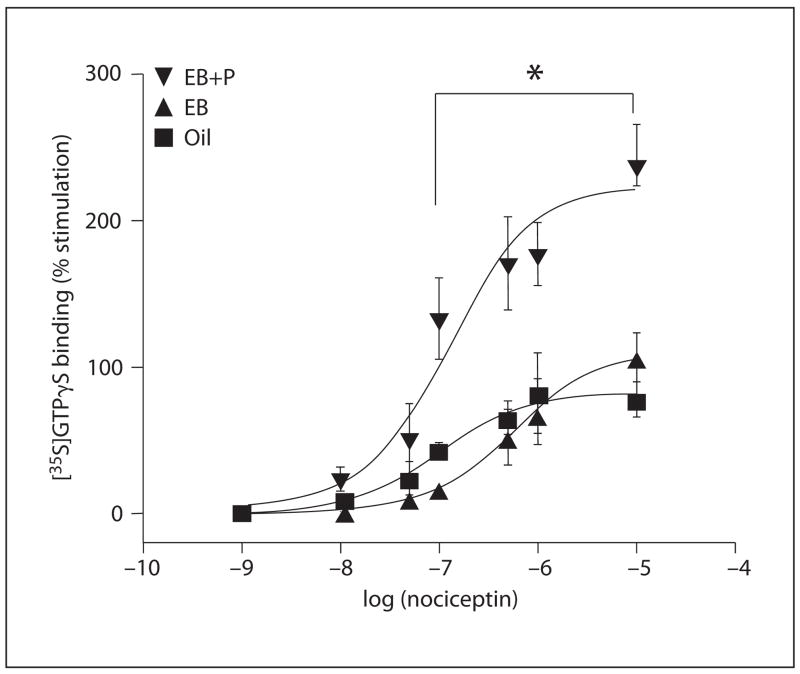
Nociceptin (0.01–10 μM) stimulated specific [35S]GTPγS binding in the medial preoptic area and mediobasal hypothalamic membranes in a concentration-dependent manner. In the medial preoptic area, estrogen treatment did not effect nociceptin-induced [35S]GTPγS binding compared with OVX oil-treated (control) animals (fig. 1). However, treatment with estrogen + progesterone significantly increased nociceptin-stimulated [35S]GTPγS binding with the Emax increasing 245% compared with baseline which was 82% (p < 0.05; fig. 1). Neither the total number of G protein binding nor G protein affinity were affected by hormone treatment (table 1). Since ORL1 Bmax was increased and GproteinBmaxremained unchanged, the catalytic amplification factor (ratio of apparent GproteinBmax/ORL1 Bmax) was significantly reduced in the estrogen + progesterone-treated group (p < 0.05, ANOVA; tables 1, 3).
Fig. 1.
Stimulation of ORL1 [35S]GTPγS binding in the medial preoptic area enriched plasma membrane. Concentration-response curves were determined for nociceptin as described in the Methods section. Nonspecific binding was determined using 10 μM cold GTPγ S and was subtracted from each data set. Each value represents the mean ± SEM of at least 4–6 independent experiments performed in triplicate. * Significant difference of 10 −7–10−5 nociceptin in the estradiol benzoate (EB) + progesterone (EB+P) group compared to the EB and oil values: p < 0.05.
Table 3.
Summary effects of steroid hormone on ORL1 and G protein kinetics in medial preoptic area and mediobasal hypothalamus membranes
| Medial preoptic area |
Mediobasal hypothalamus |
|||
|---|---|---|---|---|
| EB | EB + P | EB | EB + P | |
| Bmax | – | ↑ | ↑ | ↑ |
| Kd | – | ↑ | ↑ | ↑ |
| GproteinBmax | – | – | – | – |
| GproteinKd | – | – | – | – |
| Emax | – | ↑ | ↑ | ↑↑ |
| CA | – | ↓ | ↓ | ↓ |
EB = 17β-Estradiol benzoate; P = progesterone. Effects of steroid hormone on ORL1 kinetics and catalytic amplification (CA): no comparable differences compared to oil-treated animals (–); one arrow (↓ or ↑) significantly different compared to oil-treated animals (p < 0.05); two arrows (↑ ↑) significantly different compared to EB Emax mediobasal hypothalamus (p < 0.05).
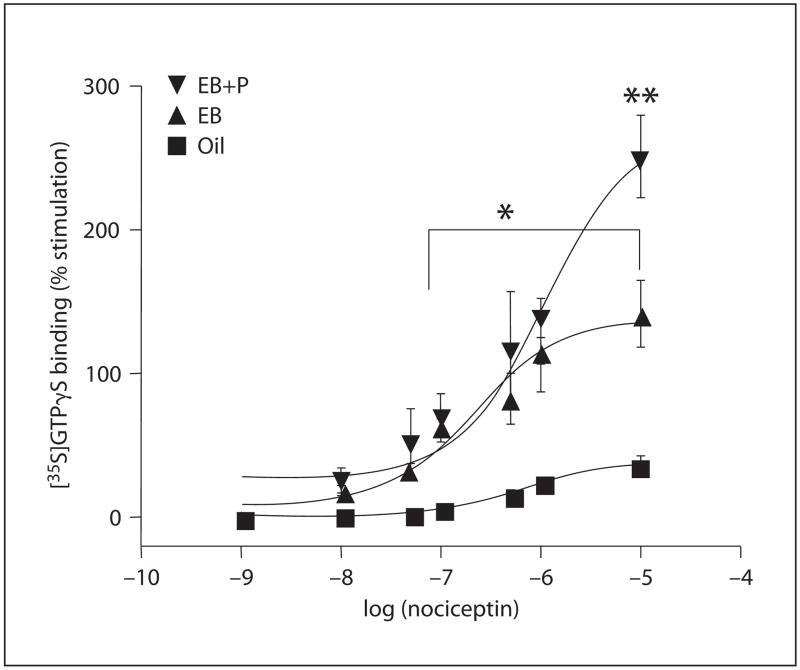
In the mediobasal hypothalamus, estrogen treatment resulted in a significant increase in nociceptin-stimulated [35S]GTPγS binding. Estrogen increased the Emax to 142% compared with 36% Emax in oil-treated rats (p < 0.05; fig. 2). Subsequent progesterone treatment significantly increased nociceptin-stimulated [35S]GTPγS binding compared with estrogen-treated animals, further increasing Emax from 142 to 251% (p < 0.05; fig. 2). No differences in apparent GproteinBmax or apparent GproteinKd were detected in any steroid treatment group tissue derived from the mediobasal hypothalami (table 2). Thus, as in the medial preoptic area, the catalytic amplification factor (GproteinBmax/ORL1 Bmax) was significantly reduced in the estrogen- and estrogen + progesterone-treated groups compared with controls (p < 0.05, ANOVA; tables 2, 3).
Fig. 2.
Stimulation of ORL1 [35S]GTPγS binding in the mediobasal hypothalamus enriched plasma membrane. Concentration-response curves were determined for nociceptin as described in the Methods section. Nonspecific binding was determined using 10 μM cold GTPγ S and was subtracted from each data set. Each value represents the mean ± SEM of at least 4–6 independent experiments performed in triplicate. * Significant difference of 10−7–10−5 nociceptin in estradiol benzoate (EB), EB + progesterone (EB+P) groups compared to oil results: p < 0.05. ** Significant difference of 10 μM nociceptin from EB+P group compared to EB values: p < 0.05.
Discussion
We examined steroid actions on ORL1, in both the medial preoptic and mediobasal hypothalamus. These experiments grew out of earlier studies demonstrating that site-specific infusions of nociceptin in either area facilitates receptivity in estrogen-primed rats [21, 22]. In this study, the effects of physiological concentrations of gonadal steroids on ORL1 binding and apparent G protein activation were examined in the hypothalamus. The major finding of the present experiments is that steroid hormones influence ORL1 number, affinity and the catalytic amplification of ORL1s in the medial preoptic area and mediobasal hypothalamus.
In the medial preoptic area, estrogen alone did not alter ORL1 number or affinity. However, treatment with estrogen + progesterone significantly increased ORL1 binding while decreasing ORL1 affinity for nociceptin. [35S]GTPγ S-binding assays revealed that treatment with estrogen + progesterone caused an increased in maximal stimulation of [35S]GTPγS, without affecting the number of G proteins being activated or a change in G protein affinity for [35S]GTPγS. Because estrogen + progesterone increased the number of ORL1-binding sites without affecting the number of activated G proteins, there was a decrease in the catalytic amplification factor. These data indicate that each activated receptor is less efficacious in catalyzing the exchange of GDP for GTP, which may be due to the decrease in ORL1 affinity for nociceptin. Despite the decrease in ORL1 affinity, the increase in the maximal stimulation of [35S]GTPγ S is likely due to the estrogen + progesterone-induced increase in ORL1-binding sites and not catalytic activity.
In distinction to the mediobasal hypothalamus, in the medial preoptic area estrogen treatment alone increased the number of ORL1-binding sites and decreased ORL1 affinity for nociceptin. Subsequent progesterone treatment did not further increase ORL1-binding sites, suggesting that estrogen and not progesterone affect ORL1 expression in this region. Despite the loss of receptor affinity, estrogen treatment increased the maximal stimulation of [35S]GTPγ S and subsequent progesterone treatment further enhanced the maximal stimulation of [35S]GTPγ S. Neither the apparent G protein number nor the apparent G protein affinity was affected in the mediobasal hypothalamus, resulting in a decreased catalytic amplification factor. This result was unexpected because ORL1 affinity and catalytic amplification were reduced. Progesterone did not alter these parameters, but did increase maximal stimulation of [35S]GTPγS, suggesting that the maximal binding value may be influenced by receptors other than ORL1. Progesterone receptors have been considered to be steroid nuclear receptors. Recently, however, progesterone receptors were localized in the plasma membrane [45]. Thus, it is probable that progesterone treatment may activate these receptors producing a significantly enhanced maximal stimulation of [35S]GTPγS. Further work currently being done in the laboratory is attempting to determine whether membrane progesterone receptors account for this result. Our results indicate that the number of ORL1 sites, and not their affinity or catalytic amplification factor, determine the increase in total GTPγ S binding in the medial preoptic area and mediobasal hypothalamus.
The present results are consistent with behavioral data on lordosis after nociceptin injection into the ventromedial nucleus and into the medial preoptic nucleus. In both regions, nociceptin facilitated female sexual receptivity [21, 46]. However, lordosis data indicate that the medial preoptic area requires three times the amount of nociceptin to facilitate E2 -dependent sexual receptivity compared with a behaviorally relevant dose into the mediobasal hypothalamus [22]. In this previous study, mice received E2 (2 μg) at a subthreshold level that does not induce lordosis [22]. This higher exogenous dosage of nociceptin into the medial preoptic area indirectly suggests that progesterone in a normal receptive female may produce the release of endogenous nociceptin. Thus, these studies with our own demonstrate that, in the medial preoptic area, estrogen activation by itself was not sufficient to fully stimulate ORL1 signaling, but estrogen + progesterone was needed to drive the maximal stimulation of [35S]GTPγS. These data suggest that in the medial preoptic area, both estrogen and progesterone are necessary to increase the efficacy of nociceptin activation of sexual receptivity. However, in the ventromedial nucleus, which is located within the mediobasal hypothalamus, estrogen increased levels of ORL1 mRNA, immunoreactivity, binding and G protein activation, events that are positively correlated with a facilitation of female sexual receptivity [2, 21]. One possible model for ORL1 action in the mediobasal hypothalamus is to facilitate sexual receptivity by turning off the estrogen-induced arcuate nucleus-medial preoptic nucleus β-endorphin pathway [8]. In this model, nociceptin may inhibit β-endorphin neurons in the arcuate nucleus [6]. Thus, released from this β-endorphin-MOP inhibitory tone, the animal will display a facilitation of lordosis.
Moreover, the present experiments show that the number of ORL1-binding sites in the cell surface membrane was not decreased by steroid treatments. A reduction of receptors in the plasma membrane fraction is an indicator of receptor internalization. This lack of internalization suggests that ORL1 desensitization may be regulated differently than MOP, which is internalized following agonist stimulation [2, 4, 8, 23]. ORL1 desensitization appears to depend on a decreased affinity rather than internalization. The present results show that under steroid conditions that activate ORL1 (probably through the release of endogenous nociceptin), receptor affinity is decreased (tables 1, 2).
The present experiments demonstrate the disparity of receptor affinity and receptor activation of G proteins, the initial step of intracellular signaling. The affinity of ORL1 was in the nanomolar range and is well within the previously reported Kd for these receptors [41, 43, 44]. However, significantly higher doses of opioids were needed to stimulate [35S]-GTPγ S binding. At this point the reasons are not clear, but this phenomenon has been seen in other experiments which compare receptor occupancy and stimulation of [35S]-GTPγS binding [42, 48]. In addition to previous results, the present results demonstrate that receptor affinity may not be a good measure of agonist concentrations needed to activate G protein-dependent signaling cascades, which is a measure of receptor activity and not simply binding.
To our knowledge, we are the first to compare the modulation of catalytic amplification of ORL1 by physiological levels of steroid hormones in the medial preoptic area and mediobasal hypothalamus, regions in which nociceptin regulates sexual receptivity. Estrogen in the mediobasal hypothalamus and estrogen + progesterone in the medial preoptic area increased the maximal G protein binding which is consistent with behavioral data showing that nociceptin facilitates lordosis in both areas [21, 22]. Overall, these studies demonstrate that an increase in receptor number and Emax are better predictors of ORL1 activity rather than catalytic amplification (table 3). Furthermore, these results are consistent with our hypothesis that the initial action of estrogen activates the inhibitory MOP system and that subsequent progesterone treatment activates the facilitatory ORL1 system [2]. Thus steroid hormones, by modulating MOP and ORL1 signaling, are able to switch the response of lordosis regulating circuitry from inhibition modulated by MOP to stimulation modulated by ORL1. Furthermore, this study also has important pharmacological implications since the efficacy of specific opiate drug(s) may be altered by steroid actions in estrogen- and progesterone-sensitive brain regions.
Acknowledgments
We thank Drs. Kevin Sinchak and Paul Popper for the helpful discussions. This work was supported by DA13185.
References
- 1.Guide to Receptors and Channels. Br J Pharmacol. 2007;147:S61–S62. doi: 10.1038/sj.bjp.0706651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinchak K, Micevych P. Visualizing activation of opioid circuits by internalization of G protein-coupled receptors. Mol Neurobiol. 2003;27:197–222. doi: 10.1385/MN:27:2:197. [DOI] [PubMed] [Google Scholar]
- 3.Quesada A, Micevych P. Estrogen and CCK1 receptor modification of mu-opioid receptor binding in the cortex of female rats. Brain Res. 2006;1073–1074:316–320. doi: 10.1016/j.brainres.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 4.Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of mu-opioid receptors regulates reproductive behavior. J Neurosci. 2001;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flores CA, Shughrue P, Petersen SL, Mokha SS. Sex-related differences in the distribution of opioid receptor-like 1 receptor mRNA and colocalization with estrogen receptor mRNA in neurons of the spinal trigeminal nucleus caudalis in the rat. Neuroscience. 2003;118:769–778. doi: 10.1016/s0306-4522(02)01000-x. [DOI] [PubMed] [Google Scholar]
- 6.Wagner EJ, Ronnekleiv OK, Grandy DK, Kelly MJ. The peptide orphanin FQ inhibits beta-endorphin neurons and neurosecretory cells in the hypothalamic arcuate nucleus by activating an inwardly-rectifying K+ conductance. Neuroendocrinology. 1998;67:73–82. doi: 10.1159/000054301. [DOI] [PubMed] [Google Scholar]
- 7.Sinchak K, Romeo HE, Micevych PE. Site-specific estrogen and progestin regulation of orphanin FQ/nociceptin and nociceptin opioid receptor mRNA expression in the female rat limbic hypothalamic system. J Comp Neurol. 2006;496:252–268. doi: 10.1002/cne.20949. [DOI] [PubMed] [Google Scholar]
- 8.Mills RH, Sohn RK, Micevych PE. Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci. 2004;24:947–955. doi: 10.1523/JNEUROSCI.1366-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darland T, Heinricher MM, Grandy DK. Orphanin FQ/nociceptin: a role in pain and analgesia, but so much more. Trends Neurosci. 1998;21:215–221. doi: 10.1016/s0166-2236(97)01204-6. [DOI] [PubMed] [Google Scholar]
- 10.Sinchak K, Hendricks DG, Baroudi R, Micevych PE. Orphanin FQ/nociceptin in the ventromedial nucleus facilitates lordosis in female rats. Neuroreport. 1997;8:3857–3860. doi: 10.1097/00001756-199712220-00004. [DOI] [PubMed] [Google Scholar]
- 11.Flores CA, Wang XM, Zhang KM, Mokha SS. Orphanin FQ produces gender-specific modulation of trigeminal nociception: behavioral and electrophysiological observations. Neuroscience. 2001;105:489–498. doi: 10.1016/s0306-4522(01)00179-8. [DOI] [PubMed] [Google Scholar]
- 12.Claiborne J, Nag S, Mokha SS. Activation of opioid receptor like-1 receptor in the spinal cord produces sex-specific antinociception in the rat: estrogen attenuates antinociception in the female, whereas testosterone is required for the expression of antinociception in the male. J Neurosci. 2006;26:13048–13053. doi: 10.1523/JNEUROSCI.4783-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meunier JC. Nociceptin/orphanin FQ and the opioid receptor-like ORL1 receptor. Eur J Pharmacol. 1997;340:1–15. doi: 10.1016/s0014-2999(97)01411-8. [DOI] [PubMed] [Google Scholar]
- 14.Manabe T, Noda Y, Mamiya T, Katagiri H, Houtani T, Nishi M, Noda T, Takahashi T, Sugimoto T, Nabeshima T, Takeshima H. Facilitation of long-term potentiation and memory in mice lacking nociceptin receptors. Nature. 1998;394:577–581. doi: 10.1038/29073. [DOI] [PubMed] [Google Scholar]
- 15.Champion HC, Pierce RL, Kadowitz PJ. Nociceptin, a novel endogenous ligand for the ORL1 receptor, dilates isolated resistance arteries from the rat. Regul Pept. 1998;78:69–74. doi: 10.1016/s0167-0115(98)00117-7. [DOI] [PubMed] [Google Scholar]
- 16.Devine DP, Taylor L, Reinscheid RK, Monsma FJ, Jr, Civelli O, Akil H. Rats rapidly develop tolerance to the locomotor-inhibiting effects of the novel neuropeptide orphanin FQ. Neurochem Res. 1996;21:1387–1396. doi: 10.1007/BF02532380. [DOI] [PubMed] [Google Scholar]
- 17.Bryant W, Janik J, Baumann M, Callahan P. Orphanin FQ stimulates prolactin and growth hormone release in male and female rats. Brain Res. 1998;807:228–233. doi: 10.1016/s0006-8993(98)00802-6. [DOI] [PubMed] [Google Scholar]
- 18.Olszewski PK, Levine AS. Minireview: characterization of influence of central nociceptin/orphanin FQ on consummatory behavior. Endocrinology. 2004;145:2627–2632. doi: 10.1210/en.2004-0016. [DOI] [PubMed] [Google Scholar]
- 19.Nothacker HP, Reinscheid RK, Mansour A, Henningsen RA, Ardati A, Monsma FJ, Jr, Watson SJ, Civelli O. Primary structure and tissue distribution of the orphanin FQ precursor. Proc Natl Acad Sci USA. 1996;93:8677–8682. doi: 10.1073/pnas.93.16.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anton B, Fein J, To T, Li X, Silberstein L, Evans CJ. Immunohistochemical localization of ORL-1 in the central nervous system of the rat. J Comp Neurol. 1996;368:229–251. doi: 10.1002/(SICI)1096-9861(19960429)368:2<229::AID-CNE5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Sinchak K, Hendricks DG, Baroudi R, Micevych PE. Orphanin FQ/nociceptin in the ventromedial nucleus facilitates lordosis in female rats. Neuroreport. 1997;8:3857–3860. doi: 10.1097/00001756-199712220-00004. [DOI] [PubMed] [Google Scholar]
- 22.Sinchak K, Dewing P, Cook M, Micevych P. Release of orphanin FQ/nociceptin in the medial preoptic nucleus and ventromedial nucleus of the hypothalamus facilitates lordosis. Horm Behav. 2007;51:406–412. doi: 10.1016/j.yhbeh.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhawan BN, Cesselin F, Raghubir R, Reisine T, Bradley PB, Portoghese PS, Hamon M. International Union of Pharmacology. XII Classification of opioid receptors. Pharmacol Rev. 1996;48:567–592. [PubMed] [Google Scholar]
- 25.Childers SR. Opioid receptor-coupled second messenger systems. Life Sci. 1991;48:1991–2003. doi: 10.1016/0024-3205(91)90154-4. [DOI] [PubMed] [Google Scholar]
- 26.Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- 27.Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O, Orphanin FQ. A neuropeptide that activates an opioid-like G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- 28.Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dollae P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 29.Vaughan CW, Christie MJ. Increase by the ORL1 receptor (opioid receptor-like1) ligand, nociceptin, of inwardly rectifying K conductance in dorsal raphe nucleus neurones. Br J Pharmacol. 1996;117:1609–1611. doi: 10.1111/j.1476-5381.1996.tb15329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Micevych PE, Eckersell CB, Holland KL, Smith A. Induction of CCK mRNA levels in the limbic-hypothalamic circuit: time course and site-specific effects of estrogen. J Neurobiol. 1996;30:465–479. doi: 10.1002/(SICI)1097-4695(199608)30:4<465::AID-NEU3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 31.Micevych PE, Eckersell CB, Sarafzadeh K. Acute Estrogen Stimulates Cholecystokinin (CCK) mRNA Expression in the Limbic-Hypothalamic Circuit of the Female Rat. Washington: Society for Neuroscience; 1996. p. 1416. [Google Scholar]
- 32.Micevych PE, Eckersell CB, Brecha N, Holland K. Estrogenic modulation of opiate and cholecystokinin systems in the limbic-hypothalamic circuit. Brain Res Bull. 1997;44:335–343. doi: 10.1016/s0361-9230(97)00212-8. [DOI] [PubMed] [Google Scholar]
- 33.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity. Horm Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- 34.Childers SR. Opioid receptors: pinning down the opiate targets. Curr Biol. 1997;7:R695–R697. doi: 10.1016/s0960-9822(06)00358-7. [DOI] [PubMed] [Google Scholar]
- 35.Hilf G, Gierschik P, Jakobs KH. Muscarinic acetylcholine receptor-stimulated binding of guanosine 5′-O-3-thiotriphosphate to guanine-nucleotide-binding proteins in cardiac membranes. Eur J Biochem. 1989;186:725–731. doi: 10.1111/j.1432-1033.1989.tb15266.x. [DOI] [PubMed] [Google Scholar]
- 36.Traynor JR, Nahorski SR. Modulation by mu-opioid agonists of guanosine-5′-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol Pharmacol. 1995;47:848–854. [PubMed] [Google Scholar]
- 37.Micevych PE, Rissman EF, Gustafsson JA, Sinchak K. Estrogen receptor-alpha is required for estrogen-induced mu-opioid receptor internalization. J Neurosci Res. 2003;71:802–810. doi: 10.1002/jnr.10526. [DOI] [PubMed] [Google Scholar]
- 38.Munson PJ, Rodbard D. Ligand. a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 39.Sim LJ, Childers SR. Anatomical distribution of mu, delta, and kappa opioid- and nociceptin/orphanin FQ stimulated [35S]Guanylyl-5′-O-(–thio)-triphosphate binding in guinea pig brain. J Comp Neurol. 1997;386:562–572. doi: 10.1002/(sici)1096-9861(19971006)386:4<562::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 40.Sim LJ, Xiao R, Childers SR. Identification of opioid receptor-like (ORL1) peptide-stimulated [35S]GTP gamma S binding in rat brain. Neuroreport. 1996;7:729–733. doi: 10.1097/00001756-199602290-00012. [DOI] [PubMed] [Google Scholar]
- 41.Yamada S, Kusaka T, Urayama A, Kimura R, Watanabe Y. In vitro and ex vivo effects of a selective nociceptin/orphanin FQ (N/OFQ) peptide receptor antagonist, CompB, on specific binding of [3H]N/OFQ and [35S]GTPgammaS in rat brain and spinal cord. Br J Pharmacol. 2003;139:1462–1468. doi: 10.1038/sj.bjp.0705371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Breivogel CS, Childers SR. Cannabinoid agonist signal transduction in rat brain: comparison of cannabinoid agonists in receptor binding, G-protein activation, and adenylyl cyclase inhibition. J Pharmacol Exp Ther. 2000;295:328–336. [PubMed] [Google Scholar]
- 43.Unterwald EM, Rubenfeld JM, Imai Y, Wang JB, Uhl GR, Kreek MJ. Chronic opioid antagonist administration upregulates mu opioid receptor binding without altering mu opioid receptor mRNA levels. Brain Res Mol Brain Res. 1995;33:351–355. doi: 10.1016/0169-328x(95)00143-g. [DOI] [PubMed] [Google Scholar]
- 44.Adapa ID, Toll L. Relationship between binding affinity and functional activity of nociceptin/orphanin FQ. Neuropeptides. 1997;31:403–408. doi: 10.1016/s0143-4179(97)90032-9. [DOI] [PubMed] [Google Scholar]
- 45.Younglai EV, Wu Y, Foster WG, Lobb DK, Price TM. Binding of progesterone to cell surfaces of human granulosalutein cells. J Steroid Biochem Mol Biol. 2006;101:61–67. doi: 10.1016/j.jsbmb.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 46.Sinchak K, Hipschman S, Cook M, Micevych P. Nociceptin/Orphanin FQ Afferents of the Medial Preoptic Nucleus. San Diego: Society for Neuroscience; 2004. [Google Scholar]
- 47.Sim LJ, Selley DE, Dworkin SI, Childers SR. Effects of chronic morphine administration on 4-opioid receptor-stimulated [35S]GTPSS autoradiography in rat brain. J Neurosci. 1996;16:2684–2692. doi: 10.1523/JNEUROSCI.16-08-02684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaqura MA, Zollner C, Mousa SA, Stein C, Schafer M. Characterization of mu opioid receptor binding and G protein coupling in rat hypothalamus, spinal cord, and primary afferent neurons during inflammatory pain. J Pharmacol Exp Ther. 2004;308:712–718. doi: 10.1124/jpet.103.057257. [DOI] [PubMed] [Google Scholar]