Abstract
When duplex DNA is altered in almost any way (replicated, recombined, or repaired), single strands of DNA are usually intermediates, and single-stranded DNA binding (SSB) proteins are present. These proteins have often been described as inert, protective DNA coatings. Continuing research is demonstrating a far more complex role of SSB that includes the organization and/or mobilization of all aspects of DNA metabolism. Escherichia coli SSB is now known to interact with at least 14 other proteins that include key components of the elaborate systems involved in every aspect of DNA metabolism. Most, if not all, of these interactions are mediated by the amphipathic C-terminus of SSB. In this review, we summarize the extent of the eubacterial SSB interaction network, describe the energetics of interactions with SSB, and highlight the roles of SSB in the process of recombination. Similar themes to those highlighted in this review are evident in all biological systems.
The genomes of all cellular organisms are organized as double-stranded (ds) DNA, with the information content, in the form of nucleotide bases, sequestered in the interior of the protective double helix 1; 2. To provide DNA replication, recombination, and repair machinery access to genomic information, dsDNA must be unwound to form single-stranded (ss) intermediates. Such processes are obligatory, but they are not without risks. ssDNA is prone to chemical and nucleolytic attacks that can produce breaks or lesions that are difficult to repair and can self-associate to create impediments to genome maintenance 3-9. To help preserve ssDNA intermediates, cells have evolved a specialized class of ssDNA-binding (SSB) proteins that associate with ssDNA with high affinity and in a sequence-independent manner 10-21. SSB binding protects ssDNA from degradation 10; 22-25, and, more globally, defines the nucleoprotein substrates upon which DNA replication, recombination, repair, and replication restart processes must act. With these central roles in genome maintenance, it is no surprise that SSB proteins are conserved throughout all kingdoms of life and are indispensable for cell survival 26-28.
Beyond their eponymous roles in DNA binding, SSB proteins have a second, less well-appreciated role in which they physically associate with a broad array of cellular genome maintenance proteins. SSB interaction with heterologous proteins targets enzymes to active genome maintenance sites and, in many cases, stimulates the biochemical activities of SSB's partner proteins. In this review, we focus on several aspects of eubacterial SSB interactions with heterologous proteins. First, we summarize the extent of SSB's interaction network by describing what is known about its many partner proteins. In this section, we focus primarily on E. coli SSB as a workhorse for understanding SSB structure and function. Second, we consider the thermodynamc mechanisms underlying the binding of heterologous proteins to SSB and the ways in which ssDNA binding by SSB influences its interaction with other proteins. These are important features for defining the specificity of binding to SSB. Finally, we summarize how heterologous proteins have adapted to carry out DNA recombination reactions in the cellular environment where mediator proteins regulate recombinase loading onto ssDNA/SSB nucleoprotein substrates. Although this review focuses on eubacterial SSBs, it is clear that eukaryotic SSB proteins have similarly evolved to interact with numerous genome maintenance enzymes as has been described in excellent reviews 26; 29. Thus, throughout the kingdoms of life ssDNA/SSB complexes are not merely inert particles but are instead dynamic centers that play a key role in choreographing the processes surrounding DNA replication, recombination, and repair.
SSB PROTEIN OVERVIEW
Eubacterial SSB proteins are linked by two common structural features. The first is the use of oligonucleotide/oligosaccharide-binding (OB) domains to bind ssDNA through a combination of electrostatic and base-stacking interactions with the phosphodiester backbone and nucleotide bases, respectively 30-37. The second is SSB oligomerization that brings together four DNA-binding OB folds in the protein's active form 34; 38-42. E. coli SSB, which encodes a single OB fold in each monomer and functions as a tetramer, has served as the prototypical SSB protein for decades 12; 19; 34; 35; 39; 43. Rare exceptions to the E. coli SSB-type arrangement exist, including the SSBs from the Deinococcus-thermus genera, which contain two OB folds per monomer and assemble as homodimers 44-47 (Figure 1). SSB proteins in non-eubacterial systems have distinct quaternary structures, including the heterotrimeric eukaryotic Replication Protein A (RPA) 29, which acts as a heterotrimer, and several bacteriophage and viral SSB proteins that function as monomers (T4 gp32) 41 and dimers (T7 gene 2.5) 41; 42.
Figure 1.
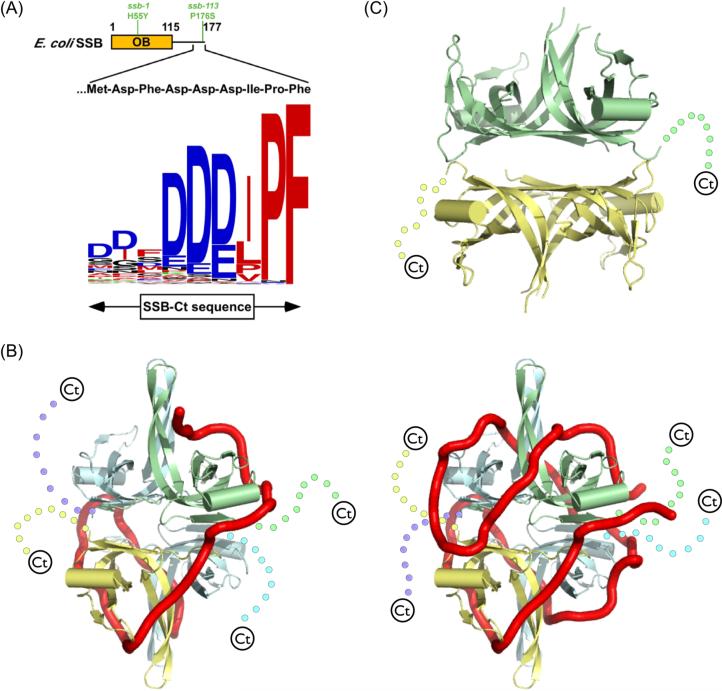
(A) Schematic representation of SSB. The E. coli SSB OB domain (residues 1−115) is shown as a box with its structurally dynamic C-terminal tail (residues 116−177) as a line. The sequence of the E. coli SSB-Ct element is displayed with its conservation across 280 eubacterial species represented as a logo424 in which the height of the residue relates to its frequency at the given position. Logo residues are colored to indicate the hydrophobic (red), electronegative (blue), polar (black), or electropositive (green) nature of their side chains. (B) Ribbon diagram of the proposed structures of the E. coli (SSB)35 (left) and (SSB)65 (right) ssDNA binding models 34. Each monomer in the tetramer is separately colored and its C-terminus is shown schematically as a dashed line. ssDNA is shown as a red tube. (C) Ribbon diagram of the crystal structure of D. radiodurans SSB 44. OB folds are colored as for E. coli SSB, but with two OB folds in each monomer of the dimer. C-terminal tails are displayed as dots.
Eubacterial SSB proteins can bind ssDNA in a highly cooperative manner, which leads to clustering of SSB protein tracts to form protein filaments on long ssDNA 12; 13; 15. However, E. coli SSB only binds ssDNA with high cooperativity in one of its binding modes (see below). The eukaryotic RPA SSB protein also does not display significant cooperativity in its binding to ssDNA 29; 48. Hence the general role of this cooperativity remains unclear. Due to the presence of four ssDNA binding sites, the E. coli SSB tetramer can bind to long stretches of ssDNA in multiple binding modes differing in the number of OB-folds that interact with the ssDNA 49-51. The primary ssDNA binding modes are denoted as the (SSB)65, (SSB)56 and (SSB)35 modes, where the subscript reflects the average number of nucleotide residues occluded by each tetramer in the complex. In the (SSB)65 mode, ∼65-nucleotides of ssDNA wrap around and interact with all four subunits of the tetramer, whereas in the (SSB)35 mode, ∼35-nucleotides interact with an average of only two subunits (Figure 1). The (SSB)65 binding mode is a limited cooperativity mode in which SSB shows little tendency to form protein clusters along ssDNA; the (SSB)35 binding mode, on the other hand, is a high, unlimited cooperativity mode in which SSB can form long protein clusters along ssDNA 14; 15; 50. The relative stabilities of the different SSB-DNA binding modes is influenced by monovalent salt concentration, Mg2+ concentration as well as the polyamines, spermine and spermidine 49; 51; 52, with the (SSB)65 mode being favored at monovalent salt concentrations above 200 mM. The (SSB)35 mode is also favored at high SSB to ssDNA ratios 15; 50. Whether these different SSB binding modes have specific functions in vivo is not clear, although it has been proposed that they may be used selectively in different processes in the cell. For example, under conditions where RecA protein stimulates DNA strand exchange in vitro, SSB binds primarily in the low cooperative, fully wrapped (SSB)65 binding mode 53. The (SSB)35 mode, which binds with high nearest neighbor cooperativity, has been proposed to function in DNA replication 37. The yeast RPA also undergoes a salt-dependent transition from a lower to a higher site size ssDNA binding mode 48.
Eubacterial SSB proteins have been shown to bind more than a dozen different proteins (Figure 2). For all cases tested thus far, complex formation requires the C-terminal region of SSB (SSBCt), suggesting a conserved mechanism by which proteins can recognize and bind to SSB. Indeed, the C-terminus of eubacterial SSB proteins, which ends in an Asp-Phe-Asp-Asp-Asp-Ile-Pro-Phe sequence in E. coli SSB, is very well conserved 54 (Figure 1). Owing to its high density of Asp residues, this region is often referred to as SSB's “acidic tail,” but the hydrophobic tripeptide that forms the extreme C-terminus is well conserved among SSBs and is critical for protein interactions (see below). Thus, the C-terminus of SSB should more accurately be considered as an amphipathic sequence element. In contrast to the well-folded OB domains, the C-termini of bacterial and bacteriophage SSB proteins appear to be structurally dynamic as they are readily removed by proteolysis 20; 55-57. Further, the C-terminus is not visible in the crystal structure of full-length E. coli SSB 35. Proteolysis of the SSB-Ct is stimulated by ssDNA binding, and deletion of the SSB-Ct influences the relative stabilities of the (SSB)35 and (SSB)65 binding modes 58. Recent studies have shown that the C-terminal tail of the phage T7 gene 2.5 SSB protein can compete with ssDNA for binding to the OB-fold 59.
Figure 2.
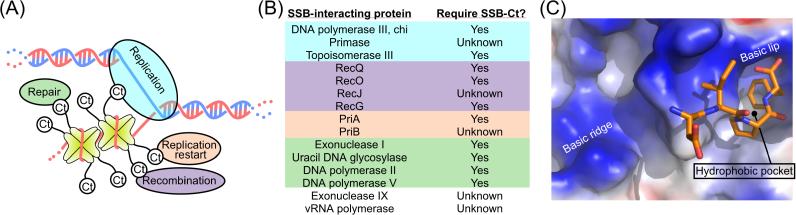
(A) Schematic representation of SSB interactions. SSB proteins (yellow) are depicted at tetramers with C-termini (Ct) interacting with ovals symbolizing proteins involved the major genome maintenance pathways of DNA replication (teal), recombination (purple), replication restart (orange), and repair (green). (B) List of proteins that are known to physically interact with SSB with their requirement for the SSB-Ct for interaction given. Citations for the interactions are given in the text. Highlighting colors indicate the major genome maintenance activities of the proteins (color coding as in (A)) and the sections in which each is described (except for Topoisomerase III, which is described in the recombination section with RecQ). (C) Binding site for the E. coli SSB C-terminus on Exonuclease I 54. Surface representation of Exonucelase I is stained in blue, red, and white to highlight positive, negative, and hydrophobic electrostatic features, respectively. The final four residues of the SSB C-terminus are shown in ball and stick form. Features shown to be critical for SSB binding by Exonuclease I are labeled.
Mutations within the SSB C-terminus have detrimental effects on E. coli cell survival. One well-studied mutation (ssb113) changes the penultimate Pro of the E. coli SSB C-terminus to a Ser 60; 61. This mutation confers temperature-sensitive lethality by producing an SSB variant that is competent to bind DNA but can no longer support DNA replication at non-permissive temperatures 60 and is hypersensitive to DNA damage even under permissive conditions 23; 62-65. A second set of mutations that alter the C-terminal-most E. coli SSB residue, Phe177, similarly impair viability 66-68. In both cases, mutations that alter the SSB-Ct sequence produce proteins that fail to interact properly with at least some of SSB's binding partners. Deletion of 10 amino acids from the C-terminus of SSB renders the cells expressing that protein inviable 69. These dramatic phenotypes have greatly illuminated the importance of SSB's interactions with heterologous proteins to cellular genome maintenance pathways.
I. INTERACTION OF SSB WITH DNA METABOLISM PROTEINS IN EUBACTERIA
In this section, we gauge the extent of SSB's interaction network by reviewing known eubacterial SSB-interacting proteins, with a particular emphasis on E. coli proteins. Members of the interaction network are grouped through their involvement in DNA replication, recombination, replication restart, repair, or as “other SSB-binding proteins”, but it should be recognized that in many cases these proteins function in several of the listed areas. Rather than provide an extensive background on each protein, we have limited discussion of each to focus primarily on what is known about the protein interactions with SSB and the biochemical and cellular consequences of such interactions.
DNA REPLICATION
DNA Polymerase III – χ subunit. (χ binds SSB via the SSB-Ct; disruption of this binding is lethal to cells)
The DNA polymerase III holoenzyme (Pol III HE) is the multi-subunit replicative DNA polymerase in E. coli 70-77. Within the Pol III HE, the γ clamp loader (comprised of γ, δ, δ', χ and ψ subunits) forms a subcomplex that loads the β processivity factor onto DNA and helps tie the holoenzyme together through a network of protein-protein interactions 74; 78; 79. Although χ and ψ are not required for clamp loading onto DNA 80, they do form a complex that facilitates the assembly of the clamp loader itself 81. Moreover, χψ binds SSB directly via the χ subunit 67; 68; 82; 83, which allows the Pol III HE to clear potentially inhibitory SSB proteins from lagging-strand DNA during DNA replication 62; 67; 82. SSB113 and an SSB truncation variant lacking the C-terminal 26 amino acids of the protein fail to bind χ, establishing the SSB C-terminus as the site of the protein-protein interaction 67; 68; 83. The χ/SSB interaction plays a crucial role in Pol III HE function by driving detachment of primase from RNA primers, which stimulates primer hand-off to the Pol III HE 68. This activity appears to be essential for cell viability in E. coli and accounts for the conditional-lethal ssb113 phenotype 67; 84.
Primase. (Primase binds SSB, possibly at the SSB-Ct)
E. coli Pol III HE cannot initiate DNA synthesis but instead extends preformed nucleic acid primers. RNA primers in bacteria are generated by a specialized RNA polymerase called primase (the product of the dnaG gene) 70; 77; 85-88. Primase constitutes the lone priming protein in E. coli 86; 89; 90, functioning in both leading- and lagging-strand synthesis in oriC-dependent replication and in replication restart processes 70; 87; 89; 91; 92.
E. coli primase interacts with the replicative helicase, DnaB, through its C-terminal protein interaction domain 92-101. DnaB/primase complex formation recruits primase to the replication fork, helps coordinate leading and lagging strand synthesis by Pol III by regulating Okazaki fragment synthesis, and initiates bi-directional replication at oriC 92; 96; 100; 102-105.
In addition to its association with DnaB, primase also interacts with SSB. Primase/SSB interaction strengthens the association between primase and the RNA primers it synthesizes 68 and is disrupted by the χ subunit, forming the basis of an involved handoff mechanism in which primase dissociates from the RNA-DNA duplex, allowing clamp-loader assembly to occur 68. The domains of primase and SSB that are required for complex assembly have not been identified, but apparent competition between primase and χ for SSB suggests that primase might bind to the C-terminus of SSB.
DNA RECOMBINATION
RecQ DNA helicase. (RecQ binds SSB via the SSB-Ct; interaction stimulates RecQ helicase activity)
E. coli RecQ functions as the DNA helicase in the RecF-recombination pathway 106-110, which helps repair gapped and UV-damaged DNA and can repair dsDNA breaks in recBC-deficient cells 111-119. Notably, many RecF-recombination pathway proteins interact with SSB, as will be described below and in section III. E. coli RecQ also plays roles in the SOS DNA damage response 120 and in the suppression of illegitimate recombination 121. RecQ promotes cell death in ruv recA(ts) uvrD E. coli cells, apparently by driving the accumulation of excessive recombination intermediates 122. RecQ-mediated recombination initiation 123, plasmid DNA catenation and supercoiling reactions 124; 125, and converging replication fork resolution 126 have been reconstituted in vitro. The latter two activities required the addition of Topoisomerase III, a type-Ia topoisomerase that appears to have coordinated activities with RecQ proteins in eukaryotes and bacteria 127-131. Interestingly each of RecQ's reconstituted reactions is stimulated by or requires SSB to proceed.
SSB has been shown to physically associate with RecQ and to stimulate RecQ DNA helicase activity 132-134. SSB interaction with RecQ is mediated by the 9 C-terminal-most residues of the SSB-Ct 132. SSB increases the efficiency of RecQ-mediated unwinding of a 71-basepair duplex formed between an oligonucleotide and M13 circular ssDNA; gp32 from bacteriophage T4 also stimulates this unwinding 133. SSB stimulates unwinding of a 30-basepair duplex DNA with a 70-base single-stranded 3’ overhang; in this case, gp32 and RPA inhibit unwinding, as does an E. coli SSB variant that lacks its SSB-Ct 132. These studies indicate that in some contexts interaction between RecQ and SSB is required for SSB stimulation. Moreover, E. coli Topoisomerase III has recently been shown to interact with SSB 126; 135, which could indicate that it acts with RecQ in a complex that is nucleated by SSB. Deletion analysis has shown that the RecQ winged-helix subdomain is the site of interaction with SSB 132. This subdomain appears to be utilized as a platform for protein interactions in eukaryotic RecQ helicases as well 136-138.
RecJ exonuclease. (RecJ DNA binding and exonuclease activities are stimulated by SSB in vitro)
E. coli RecJ is an exonuclease that degrades ssDNA in a 5’ to 3’ direction 139. It functions as a member of the RecF-recombination pathway 10; 11; 140-142 and, in conjunction with RecQ, RecJ acts at stalled replication forks to degrade nascent lagging strand DNA prior to resumption of replication 111; 112; 115; 143. Additional roles for RecJ in base excision repair 144; 145 and in the excision step of methyl-directed mismatch repair 146; 147 have been reported.
In vitro, RecJ binds to the 5’ end of ssDNA and requires a 5’ overhang for cleavage 148. Unlike most nucleases, RecJ DNA binding and degradation are stimulated by SSB 148. Because T4 gp32 does not provide similar enhancement and RecJ is able to supershift SSB-bound DNA, this enhancement is likely to be due to a specific physical interaction between E. coli SSB and RecJ 148. Strengthening this view, RecJ has been observed in complex with SSB in affinity purification studies 132; 135. The domains of RecJ and SSB that mediate their interaction have not been identified.
RecG DNA helicase. (RecG binds SSB (likely via the SSB-Ct); RecG DNA binding and ATPase activities are stimulated by SSB in vitro)
RecG is a monomeric DNA helicase 149-152 that binds forked DNA structures 153 and promotes regression of stalled replication forks 154-156. RecG has been implicated in a multitude of genome maintenance activities, including ssDNA gap repair and recombinational repair of dsDNA breaks 10; 157, chromosome segregation 158, stabilization of stalled replication forks 159, and resolution of Holliday junctions 11; 160-163. RecG binds and remodels numerous nucleic acid structures, including 3-way and 4-way DNA junctions160; 161, D-loops164, and R-loops149; 165. RecG can promote rapid, ATP-dependent regression of replication forks in vitro with low processivity 166 and can inhibit RecA-mediated strand exchange under conditions that are suboptimal for RecA 166-168.
SSB stabilizes E. coli RecG binding to negatively supercoiled DNA, the substrate upon which its ATPase activity is most highly stimulated 169. Maximal ATP hydrolysis also greatly increases when SSB is included in RecG reactions 169. In B. subtilis, RecG colocalizes with SSB at foci that are thought to be stalled replication forks 134. This colocalization is ablated in cells where SSB lacks its 35 C-terminal most amino acids, suggesting that RecG binds the SSB-Ct and requires SSB to associate with the replisome.
RecO. (RecO binds SSB (likely via SSB-Ct), which stimulates RecOR RecA loading)
RecO is a mediator protein in the RecF recombination pathway 106; 170; 171. Strains harboring mutations in recO exhibit numerous defects in DNA replication, recombination, and repair 107; 116; 172-180 and in the SOS response 181; 182, but recO mutations can also suppress illegitimate recombination caused by an excess of RecET 183; 184. Disruption of recO confers resistance to thymineless death 185 and sensitivity to UV irradiation 119; 171; 186-188. UV sensitivity in strains with mutations in recF 106 and recR 189 (also RecF-pathway genes) can be suppressed by over-expression of RecO and RecR 190 as well as by certain RecA mutants 191, suggesting that these proteins function in a common pathway. RecO binds ssDNA and dsDNA and possesses a DNA-annealing activity 192; 193. This annealing activity is stimulated by SSB and inhibited by RecR 194. Together with RecF and RecR, RecO functions as a modulator of RecA activity 10; 195-199. RecO and RecR facilitate RecA loading onto SSB-coated ssDNA 200-202. RecO and RecR produce an apparent stabilization of RecA filaments that is likely related to the continued association of RecR with the RecA filament after it forms 201; 202. The putative stabilization may reflect an actual suppression of RecA filament disassembly or an enhanced re-loading of any RecA protein that dissociates. Roles for RecO in DNA recombination are described in greater detail in section III of this review.
SSB directly binds RecO 200 and limits the formation of RecOR complexes on ssDNA 199. The ability of RecOR to load RecA is greatly reduced when RPA or an SSB variant lacking the C-terminal eight amino acids are substituted for wild-type protein 199, suggesting that direct physical interaction between SSB and RecO is necessary for maximal efficiency of the RecOR-stimulated reaction. A similar effect has been observed in Thermus thermophilus: RecO-assisted loading of RecA is achieved by means of the direct protein-protein interaction between RecO and SSB 203. In this case, RecO binds both SSB and ssDNA and in doing so displaces SSB from the DNA.
DNA REPLICATION RESTART
PriA DNA helicase. (PriA binds SSB via the SSB-Ct; assocaition with SSB stimulates PriA helicase activity)
The primosome was originally identified as a collection of E. coli proteins required for the conversion of the phage ϕX174 genome from its single-stranded form to its double-stranded (replicative) form 204. In total, the primosome consists of seven proteins: DnaB, DnaC, DnaG, PriA, PriB, PriC, and DnaT 76; 205. Of these, DnaB, DnaC, and DnaG (primase) are necessary for initiation of replication of the E. coli genome at oriC 70, whereas the remaining proteins drive origin-independent initiation (replication restart) at the sites of collapsed replication forks 206-208. PriA initiates assembly of the PriA/PriB/DnaT primosome by binding DNA structures that result from replication failure and attracting PriB and DnaT 164; 207; 209-218. PriA also appears to be an important anti-recombinase by binding stalled replication forks and preventing RecA binding and activity 219. In B. subtilis cells, PriA continuously colocalizes with the replication machinery 134. E. coli cells with deleterious priA mutations harbor numerous defects including UV sensitivity and defects in DNA repair, the SOS response, and chromosomal segregation 210; 217; 220-226.
PriA is a helicase that unwinds DNA with a 3’-to-5’ polarity 227-229. PriA can unwind DNA duplexes of up to 40 bp on its own but requires SSB to process longer duplexes 228. PriA can bind SSB-coated DNA 230 and can displace SSB from DNA 231. Its helicase activity is stimulated by SSB on branched DNA substrates resembling replication fork lagging strands but is inhibited by SSB on partial duplex DNA 213; 232. SSB is able to weakly stimulate PriA-mediated unwinding of forked substrates that have no exposed ssDNA, suggesting that part of the enhancement effect is due to SSB sequestering DNA that PriA has already unwound 232.
SSB stimulation of PriA appears to be a consequence of the two proteins physically interacting via the SSB-Ct 232. Neither archaeal nor viral SSB are capable of stimulating PriA activity, and E. coli SSB variants with the ssb113 point mutation or the C-terminal 10 amino acids truncated fail to stimulate PriA activity 60; 232.
PriB. (PriB binds SSB in an undefined manner)
PriB is the second member of the PriA-primosome to assemble 230; 233. It acts to stabilize PriA binding to ssDNA and assists in primosome assembly by facilitating PriA binding to DnaT 233. Mutations in priB do not exhibit the UV sensitivity, recombination deficiency, or constitutive activation of the SOS response seen in priA mutants, but contribute to a very slow growth phenotype when combined with priC mutations 234 and can influence plasmid copy number 235. PriB is a 12 kDa protein that exists as a dimer in solution 236; 237 and shares extensive sequence and structural homology with SSB 236; 238; 239. Indeed, the similarities between SSB and PriB are of such a degree that it has been hypothesized that PriB arose due to a duplication of the ssb gene 240. Consistent with this hypothesis, PriB binds to ssDNA 236; 237, although in an unexpected fashion: while SSB binding of ssDNA is reliant upon base-stacking with the side chains of aromatic residues as well as electrostatic interactions 34; 241, PriB appears to utilize a mainly charge-based interaction made possible by multiple lysine residues 211, and is still able to strongly bind an oligonucleotide even when its lone surface-exposed tryptophan is mutated 242.
PriB is also able to bind SSB-coated DNA, suggesting a protein-protein interaction 237. Like SSB, PriB stimulates PriA helicase activity on forked DNA substrates and this stimulation is further increased when SSB is present 243. Consistent with previous observations, SSB inhibits PriA helicase activity on partial duplex DNA even when PriB is present 232; 243. PriB physically interacts with the helicase domain of PriA and bridges a ternary complex between PriA and DnaT 211. What role an interaction between PriB and SSB might play remains unclear.
DNA REPAIR
Exonuclease I. (Exonuclease I binds SSB via the SSB-Ct; interaction stimulates exonuclease activity; the structure of Exonuclease I in complex with SSB-Ct is known)
Exonuclease I (ExoI) is a DnaQ-family exonuclease that processively degrades ssDNA in a 3’-to-5’ direction 244-249. The gene that encodes ExoI in E. coli is known both as xonA and sbcB owing to the two distinct phenotypes that stem from different ExoI variants 249-251. sbcB (suppressors of recBC) mutations restore cellular recombination activity and reduce sensitivity to DNA damage from UV light in recBC- cells 249; 251; 252. The strong reduction of ExoI activity in these cells appears to allow 3’ ssDNA ends to remain intact and available as RecF pathway recombination substrates 106; 108. Ordinarily, ExoI would degrade the ssDNA ends, leaving DNA structures that cannot be efficiently recombined 106; 108; 245; 249. xonA mutants also acquire UV-resistance but are deficient in recombination activity compared to recBC- sbcB E. coli cells 249; 250; 253. The nucleolytic activity of ExoI is important for degradation of incorrectly base-paired DNA in mismatch repair 146; 147; 254. SSB also plays a central role in mismatch repair, which indicates that ExoI has adapted to act on SSB/ssDNA complexes 254. Like RecJ, ExoI activity is stimulated by the presence of SSB. This distinguishes both RecJ and ExoI from several other nucleases that are inhibited by SSB 25. ExoI also plays roles in the preservation of genome integrity by acting as a deoxyribophosphodiesterase at apurinic and apyrimidinic sites 255; 256 and by suppressing frameshift mutations 257; 258. The former activity is stimulated by SSB in vitro 259.
Several experiments have demonstrated a direct physical interaction between E. coli ExoI and SSB, and have shown that this interaction is mediated by the SSB-Ct 10; 16; 25; 54; 66; 259. This interaction is relatively strong (Ka∼ 7.1 × 106 M−1 54) and, consistent with an ExoI/SSB-Ct interaction, SSB113 and C-terminal deletion variants fail to interact with ExoI 54; 66. Recently, the structure of ExoI bound to a peptide composed of the nine C-terminal residues of SSB was determined 54 (Figure 2C). In this structure, the C-terminal-most phenylalanine of the SSB peptide packs into a hydrophobic pocket that is flanked by a basic surface that is thought to contact acidic SSB-Ct residues that lie N-terminal to the phenylalanine 54. Significantly, mutations that alter residues on the surface of ExoI that disrupt SSB binding also abolish SSB stimulation of ExoI activity. Similar results are seen when the SSB tail is altered or removed, suggesting that SSB acts to recruit ExoI to ssDNA 54.
Uracil DNA Glycosylase. (Uracil DNA glycosylase binds SSB via the SSB-Ct; interaction impacts uracil excision activity in a DNA-dependent manner)
Uracil DNA glycosylase (UDG) catalyzes the first step in a base excision repair pathway by creating an abasic site through removal of uracil from DNA 145; 260-265. The DNA harboring the abasic site is then degraded and resynthesized 144; 145; 262. UDG has also been implicated as a generator of dsDNA breaks when two uracils, located on opposite strands of a DNA duplex and separated by seven or fewer bases, are recognized and targeted for repair in rapid succession 266.
SSB affects uracil excision activity by E. coli UDG in different ways depending on substrate structure. On a ssDNA substrate that lacks secondary structure, SSB decreases excision up to three-fold, but in a ssDNA molecule containing a tetraloop, SSB enhances UDG activity 7- to 140-fold depending on the position of the uracil 267. SSB proteins from other bacterial species are also able to stimulate UDG uracil excision in a species-specific manner, but activity is decreased when a UDG from any of the tested species is mixed with a heterologous SSB 268; 269. Surface plasmon resonance experiments suggested that these changes in activity depend upon a physical interaction between UDG and SSB 268.
Handa and colleagues investigated UDG/SSB interactions in an exhaustive study 270. Interaction between E. coli and M. tuberculosis UDG and SSB proteins was demonstrated by yeast two-hybrid screens. In vitro, direct interaction between the proteins was demonstrated by far Western blot analysis and interaction on ssDNA was shown using electrophoretic mobility supershift assay 270. Surface plasmon resonance experiments yielded an association constant (Ka) for E. coli UDG and SSB of 5.9 × 106 M−1, which is similar to the Ka for ExoI/SSB complexes 54; 66; 270.
E. coli UDG can bind a chimeric SSB consisting of the C-terminal 47 amino acids from E. coli SSB appended to the N-terminal 130 amino acids of M. tuberculosis SSB. It binds this chimera, designated MtuEcoSSB, with lower affinity than wild type, but it does not bind the reciprocal chimera at all (EcoMtuSSB, in which the C-terminus of M. tuberculosis SSB is present). MtuEcoSSB is capable of stimulating E. coli UDG activity, but EcoMtuSSB has an inhibitory effect 270. Therefore, the C-terminus of SSB appears to be required for UDG binding and the attendant biochemical stimulation.
DNA Polymerase II. (DNA Polymerase II binds SSB; interaction enhances processivity and replication beyond abasic sites)
DNA polymerase II (Pol II) is a DNA repair polymerase 271-274. It is induced early in the SOS response up to 8-fold over basal levels 272-280. Pol II participates in repair of and synthesis across various lesions 281-286 including thymine dimers 287 and as such is especially important in replication and repair of UV-damaged DNA 179; 287; 288. Pol II plays a role in maintaining the fidelity of replication 281; 283; 289; 290, which is severely compromised when its proofreading exonuclease activity is removed 289; 291; 292.
Pol II was the first SSB interacting partner to be identified; indeed, in the manuscript announcing the isolation of SSB, Sigal and colleagues noted that the “DNA unwinding protein” that they purified to homogeneity had a strong stimulatory effect on DNA synthesis by Pol II 13; 18. Soon after, SSB was found to facilitate binding of Pol II to ssDNA, to stimulate the Pol II-associated nuclease activity, and to form a complex with Pol II in the absence of nucleic acid 19; 24.
When functioning alone, Pol II is poorly processive, synthesizing approximately five nucleotides before dissociating from the template strand; however, Pol III HE processivity factors (the β subunit and the clamp-loader complex) increase Pol II processivity to about 1600 nucleotides in an SSB-dependent manner 293; 294. The processivity factors and SSB are required for by-pass of abasic sites 293. β may play a role in determining when Pol II is activated in SOS-induced cells 295, as deactivation of Pol II in a strain with a mutant β restored viability to cells that would otherwise have been inviable 296.
DNA Polymerase V. (DNA Polymerase V binds SSB via the SSB-Ct; interaction with SSB is critical for translesion synthesis activity in vitro)
DNA polymerase V (Pol V) carries out translesion synthesis on damaged DNA 297; 298. Pol V is encoded by the genes umuD and umuC and forms when two molecules of UmuD undergo RecA-mediated cleavage to their active UmuD’ form 299-301 and assemble with one molecule of UmuC 302-305. In vitro, Pol V translesion synthesis activity requires RecA and SSB 303; 304; 306-310. Increasing concentrations of SSB increases initiation of Pol V bypass synthesis 308. This stimulation has recently been demonstrated to arise, in part, from a physical interaction between SSB and Pol V 311.
SSB increases Pol V access to the 3’ end of a DNA gap that is flanked by RecA filaments 311. The SSB113 protein and viral SSB proteins can substitute for E. coli SSB in this respect. However, when SSB113 is included in a translesion synthesis assay, little synthesis is observed, whereas the reaction is substantially more efficient in the presence of wild-type SSB 311. Interestingly, viral SSB proteins (gp32 from T4 phage and ICP8 from herpes simplex virus 1) allowed for attenuated activity in which DNA synthesis proceeds up to, but not beyond, the DNA lesion 311. When Pol III HE subunits β and γ (proteins that have been shown to assist with Pol V activity 308; 312) were included in reactions containing the viral SSBs, translesion synthesis occurred. Because Pol V coprecipitates with SSB but not SSB113, a physical interaction between the SSB C-terminus and Pol V is likely to play a crucial role in maximizing Pol V translesion synthesis activity 311. This conclusion is strengthened in light of the physical interaction between SSB and MucB, a plasmid-encoded UmuC homolog 313.
OTHER SSB-BINDING PROTEINS
Exonuclease IX. (Exonuclease IX binds SSB in an undefined manner)
E. coli Exonuclease IX (ExoIX) was initially identified as a putative exonuclease since it shares 60% identity with the DNA polymerase I 5’-to-3’ exonuclease domain 314. Indeed, partially purified preparations of ExoIX appeared to possess exonuclease activity 315; however it has since been shown that this activity is most likely due to an Exonuclease III contamination in ExoIX preparations, and that ExoIX itself is devoid of exonuclease activity 316. The function of ExoIX in the cell remains unclear, as it has no apparent enzymatic activity and strains harboring ExoIX deletions (xni-) are indistinguishable from wild type 317. However, ExoIX does interact directly with SSB as demonstrated by coprecipitation and crosslinking experiments in the absence of nucleic acid 316.
Bacteriophage N4 virion RNA polymerase. (N4 RNA polymerase binds SSB (most likely via the SSB-Ct) and requires SSB to stabilize a promoter hairpin)
RNA polymerase from bacteriophage N4 (vRNAP) specifically requires E. coli SSB for early transcription 318-320. N4 injects vRNAP into its host along with its genome in the initial stage of infection 321; 322. Even though N4 encodes its own SSB protein 323, its binding activity appears to be specialized to destabilize a hairpin structure that functions as a promoter, making the phage reliant on E. coli SSB as well 318. Interestingly, the N4 SSB functions as a transcriptional activator late in the phage's replication process, but does so through stimulation of E. coli RNA polymerase 324; 325. E. coli SSB not only allows the N4 promoter hairpin structure to remain intact, but also assists in displacing nascent RNA from the ssDNA template, a task vRNAP is unable to accomplish alone 320. SSB binds both the ssDNA template and the RNA product, preventing the formation of a DNA-RNA hybrid. The result of maintaining both species in their non-duplex form is increased access to ssDNA and template recycling 320.
The stimulation of transcription by SSB is dependent upon the presence of the SSB-Ct element. Wild type SSB increases vRNAP transcription by twenty-fold, but variants that lack the C-terminal ten residues of SSB fail to stimulate, but do not inhibit, transcription 320. Although a direct protein-protein interaction between vRNAP and SSB has not been explicitly demonstrated, experimental evidence strongly suggests that one exists.
PROTEOMIC INDENTIFICATION OF SSB-BINDING PROTEINS
Two large-scale studies that probe networks of interacting E. coli proteins have been published to date (Table 1). The first utilized dual affinity-tagged proteins to identify binding partners of essential proteins 135, whereas the second used hexahistidine affinity-tagged variants of the majority of the E. coli proteome to define interaction networks 326. Surprisingly, the His-tagging study detected just two of the known binding partners of SSB (RecG and UDG), which were only found when the partner proteins are the tagged bait (that is, tagged SSB failed to co-purify with either partner) 326. Two other His-tagged proteins (DNA photolyase and YbcN, a hypothetical protein) also were found to co-purify with SSB, but neither interaction has been confirmed outside of the co-purification study. Only two proteins co-purify with His-tagged SSB in the study: Peptidase D and RhlE, a putative helicase. It is possible that the purification conditions for His-tagged proteins are too stringent for most SSB-interacting proteins to remain stably bound to SSB throughout the purification method, which led to the large number of apparently false negative results for SSB-interacting proteins in the study.
Table 1.
SSB-interacting proteins found from proteomic studies.
| SSB-interacting protein | Found in dual-affinity experiment? | Found in His-tag experiment? |
|---|---|---|
| DNA polymerase III α | Yes, as bait | No |
| DNA polymerase III χ | Yes, as bait | No |
| PriA DNA helicase | Yes, as prey | No |
| RecG DNA helicase | Yes, as prey | Yes, as bait |
| RecQ DNA helicase | Yes, as bait/prey | No |
| RecJ exonuclease | Yes, as bait/prey | No |
| Exonuclease I | Yes, as prey | No |
| RNase H | Yes, as bait | No |
| DNA photolyase | No | Yes, as bait |
| Uracil DNA glycosylase | No | Yes, as bait |
| Topoisomerase I | Yes, as bait | No |
| Toposimerase III | Yes, as bait/prey | No |
| HU- α | Yes, as prey | No |
| SecA translocase | Yes, as prey | No |
| DnaK chaperone | Yes, as prey | No |
| Peptidase D | No | Yes, as prey |
| RhlE putatitve helicase | No | Yes, as prey |
| YbcN hypothetical protein | No | Yes, as bait |
In contrast to the His-tagged proteins screen, the dual affinity-tag study identified 52 interactions involving SSB: 37 when SSB was C-terminally tagged, and 15 others in which a partner protein was tagged 135. This study validated interactions in experiments in which co-purifying partner proteins were tagged and the same interaction was detected reciprocally in a second, separate purification. This reduced SSB interacting proteins to the 13 verified complexes listed in Table 1. It is worth noting that since this study used C-terminal affinity tags to identify protein complexes, many false negatives could arise since the SSB-Ct forms a critical binding site for its partner proteins.
While nearly all of the listed binding partners have a clear role in nucleic acid metabolism, there are some indications that neither study has sampled the complete SSB interaction network. First, between the dual-affinity and His-tag studies, only one common binding partner was detected (RecG). This indicates experimental conditions greatly altered the spectrum of identified interacting proteins. Given that interactions with SSB can be relatively weak and dependent upon solution conditions, this is not surprising. Second, at least one of the “validated” protein interaction partners, Topoisomerase I, is not believed to interact directly with SSB 327, consistent with these purification schemes detecting both direct and indirect binding partners. Finally, since validation requires that both protein partners be amenable to similar tagging and purification procedures, some interactions may be lost as false negatives in validation screens. In the dual affinity-tag screen, there were several candidates that one can imagine as interacting with SSB in the list of non-validated partner proteins, such as DNA gyrase and Topoisomerase IV. However, since the reciprocal interaction was not detected, they are considered non-validated. Selected non-validated proteins such as these may warrant further investigation.
II. THERMODYNAMICS OF SSB-PROTEIN INTERACTIONS
The proteins that have been shown to interact directly with E. coli SSB protein all appear to contact the unstructured C-terminal region of the SSB protein, in particular the last 9 amino acids. One question that arises is whether there is any specificity associated with these different interactions; i.e., is SSB binding to these different proteins determined solely by its unstructured C-terminus or are there other interactions that are specific to the protein partner? A second question is whether the stoichiometry of binding and/or specificity is influenced by SSB binding to DNA (either ssDNA or more complex junction structures) and furthermore whether the mode of SSB binding to ssDNA influences its interactions with these other proteins. Of course, answers to these questions require quantitative thermodynamics studies; however, to date, only a few of the proteins known to interact with SSB protein have been studied using direct quantitative methods (Table 2). Furthermore, even for those that have been studied quantitatively, the solution conditions used for those studies often differ and since solution conditions generally affect these interactions, questions of specificity are currently difficult to answer.
Table 2.
Thermodynamic binding data for SSB protein complexes*
|
(A) χ/SSB interaction | ||||
|---|---|---|---|---|
|
[Salt] |
Method |
SSB-Ct peptide binding data |
SSB binding data |
SSB/ssDNA binding data |
| High | ||||
| 300 mM | AU 83 | ND | N=4 | N=4 |
| K=(2+/−1) × 105 | K=(2+/−1) × 105 | |||
| 200 mM | ITC a | ND | ND | N=2.8+/−0.6 |
| K=(4.5+/−1.1) × 105 | ||||
| ΔH=−7.7+/−1.1 | ||||
| 150 mM | SPR 67;b | ND | N=2.9+/−0.1 | N=5.1+/−0.4 |
| K=(3.3+/−0.3) × 105 | K=(4.6+/−0.1) × 105 | |||
| 150 mM | SPR 83 | ND | N=2.5 | N=4.4 |
| K=(2.7+/−0.5) × 105 | K=(4.2+/−0.5) × 105 | |||
| 100 mM |
SPR 67; c |
ND |
K=3.7 × 105 |
K=(1 −3) × 108 d |
| Low | ||||
| 20 mM | ITC a | N=0.9+/−0.1 | ND | N=4.2+/−0.3 |
| K=(1.3+/−0.6) × 106 | K=(6.1+/−2.1) × 106 | |||
| ΔH=−8.6+/−1.8 | ΔH=−9.0+/−0.4 | |||
| N=4 | ||||
| 5 mM | AU 83 | ND | K=(4.0+/−1.0) × 105 | N=4 |
| K=(7.4+/−1.0) × 106 | ||||
| none | Gel filtration 67 | ND | ND | K=1.9 × 107 |
|
(B) PriA/SSB interaction | ||||
|---|---|---|---|---|
|
[Salt] |
Method |
SSB-Ct peptide binding data |
SSB binding data |
SSB/ssDNA binding data |
| High | ||||
| 200 mM | ITC a | ND | N=3.7+/−1.4 | N=4.6+/−0.5 |
| K=(1.0+/−0.8) × 106 | K=(1.8+/−0.5) × 106 | |||
| ΔH=−5.1+/−0.6 | ΔH=−6.8+/−0.3 | |||
| 150 mM |
SPR 232 |
K=(4.2+/−0.3) × 105 |
ND |
ND |
| Low | ||||
| 20 mM | ITC a | N=1.0+/−0.1 | N=1.7+/−0.2 | N=5.2+/−0.2 |
| K=(1.8+/−0.7) × 106 | K=(2.2+/−1.1) × 107 | K=(7.1+/−1.8) × 107 | ||
| ΔH=−6.9+/−0.6 | ΔH=−28.0+/−1.0 | ΔH=−28.7+/−1.2 | ||
|
(C) RecQ/SSB interaction | ||||
|---|---|---|---|---|
|
[Salt] |
Method |
SSB-Ct peptide binding data |
SSB binding data |
SSB/ssDNA binding data |
| High | ||||
| 150 mM | ITC 132 | N=0.90+/−0.02 | N=3.4+/−0.6 | ND |
| K=(1.5+/−0.3) × 105 | K=(1.5+/−0.4) × 105 | |||
| ΔH= −9.3 +/−1.1 | ΔH= −18.0 +/−2 | |||
|
(D) UDG/SSB interaction | ||||
|---|---|---|---|---|
|
[Salt] |
Method |
SSB-Ct peptide binding data |
SSB binding data |
SSB/ssDNA binding data |
| Intermediate | ||||
| 50 mM | SPR 268 | ND | ND | K= 5.9 × 106 |
- binding parameters: N - stoichiometry of protein binding, K (M−1) - association constant, ΔH (kcal/mol) - enthalpy change
Kozlov and Lohman, unpublished data
Parameters obtained fitting SPR data presented in Table I of Kelman et al (ref.67), to the N noninteracting sites model
Includes ψ protein
Determined in the presence of γδδ’ or τδδ’ subunits of pol III HE
Binding of SSB to the χ subunit of the Pol III HE in the presence and absence of ssDNA was investigated using Surface Plasmon Resonance (SPR) 67; 82; 83, analytical ultracentrifugation (AU) 83and gel filtration 67. The interactions of PriA helicase with the SSB C-terminal peptide232, RecO with SSB 200, and E. coli UDG with SSB 268 have also been examined using SPR methods, and, AU has been used to study the interaction of exoI with SSB and SSB with mutations in its C terminal mutants 66. Isothermal titration calorimetry (ITC) has been used to characterize the interactions of SSB and its C-terminal peptide with RecQ helicase 132, PriA helicase, and the χ subunit of the Pol III HE (Kozlov and Lohman, unpublished results).
As discussed above, E. coli SSB tetramers can bind to long ssDNA in a number of different binding modes that display distinct ssDNA binding properties, differing in the number of subunits that interact directly with the ssDNA, the inter-tetramer cooperativity, the affinity and the occluded site size 12; 34; 35; 66; 328. The transitions among these different binding modes can be modulated by the monovalent salt concentration 51, divalent and mutivalent cations 49; 52, as well as the SSB to ssDNA binding density 14; 50; 53; 58. It is therefore conceivable that the ability of SSB to recruit other proteins through interactions with its C terminus might be influenced by the particular mode of SSB binding to ssDNA. In addition, due to the acidic nature of the C-terminus of SSB, there is likely to be an electrostatic component to its interaction with other proteins. For these reasons, changes in solution conditions and especially salt concentration and type are likely to affect the binding properties of these proteins to SSB and its complexes with DNA.
Protein Binding to SSB and its C-terminal peptides at high salt
Most studies of SSB binding to other proteins have been carried out under high salt conditions (100−300 mM NaCl)67; 83; 132. These are conditions that at equilibrium in vitro favor the fully wrapped high site size, (SSB)65 mode of binding to ssDNA. Under these conditions, the affinities of SSB (without DNA) for the χ subunit67; 83, and for RecQ helicase 132 are within the range of 2×105 to 4×105 M−1 (Table 2A and 2C). Similar values have been reported for the interaction of RecQ 132 and PriA 232 with peptides containing the last 9 or 15 amino acids of the SSB C-terminus also at similar high salt concentrations (Table 2B and 2C). Importantly, C-terminal deletion mutants of SSB that are missing the last 8 132 or 26 amino acids 83 as well as the SSB-113 mutant (Pro 176 to Ser) do not show any detectable affinity for χ 67; 83 or RecQ 132. The affinities of C–terminal peptides carrying the Pro to Ser substitution are also reduced dramatically for χ 67, RecQ 132 and PriA232. On the basis of these data it appears that at high salt concentrations (100−300 mM NaCl) SSB displays little specificity for χ vs. RecQ and interacts weakly ((2−4)×105 M−1) with these proteins using primarily its unstructured C-terminus. The reported stoichiometries of binding (proteins per SSB tetramer) are 4 for RecQ 132, but only 2−3 for χ67; 83. The lower values for χ might be explained by the fact that they were obtained using SPR83, where chemical coupling of SSB to the chip surface could result in some occlusion of potential binding sites.
Protein binding to ssDNA/SSB complexes at high salt
Under high salt conditions no differences in affinities were reported for the binding of χ or PriA to a pre-formed ssDNA/SSB complex compared to SSB alone (Tables 2A and 2B). The equilibrium constants reported for χ in the presence of poly(dT) using AU 83 and for SSB bound to (dT)65 using SPR67; 83, are within the same range as reported for SSB alone with stoichiometries of ∼ 4 χ proteins bound per SSB tetramer. This is also the case for PriA binding to SSB even in the presence of (dT)70. Therefore, it appears that the presence of ssDNA at high salt conditions has little effect on χ-SSB interactions, although PriA does display a higher affinity for SSB than does χ.
Interestingly, the presence of the ψ subunit, which is also a component of the Pol III HE and interacts with the χ subunit, does not affect the affinity of χ for SSB alone (3.7×105 M−1)82, whereas on a DNA template coated with SSB the affinity increases to 3×108 M−1 when the γδδ' subunits (clamp loader assembly) are also present. Hence, the presence of ssDNA and auxiliary proteins appears to increase the affinity between SSB and χ considerably (∼1000 fold).
Protein binding to ssDNA/SSB complexes and SSB at lower salt
In contrast to the results obtained at high NaCl concentrations, the binding of ssDNA to SSB has a more pronounced effect on SSB-χ binding affinity at lower salt concentrations. The equilibrium constant for χ binding to SSB determined by AU at 5 mM NaCl ((4.0±1.0)×105 M−1) increases ∼ 20 fold (7.4×106 M−1) when SSB is complexed with poly(dT) 83(Table 2A). PriA also shows an increase in affinity for SSB in the presence of ssDNA (Table 2B), with a binding affinity that is ten-fold higher than for χ, demonstrating some degree of SSB specificity for PriA.
With increasing salt concentration, the affinities of PriA and χ for ssDNA/SSB complexes decrease (Tables 2A and 2B). However, essentially no change in affinity is observed for the interaction of χ with SSB alone as the salt concentration increases from 5 mM to 300 mM NaCl83. This is somewhat surprising, especially since a ∼3−5 fold increase in affinity is observed for just the C-terminal peptide (9 or 15 amino acids long) binding to χ at low salt (20 mM NaCl) as determined by ITC (Table 2A). A significant decrease in affinity upon increasing salt concentration was also reported for SSB binding to Exonuclease I 66.
In summary, for χ and RecQ, the interaction with SSB at high salt concentrations (100−300 mM NaCl) is characterized by moderate affinities ((2−4)×105 M−1), which are unaffected by ssDNA (although this is shown only for χ) and are similar to the affinities determined for the interaction of these proteins with the C-terminal SSB peptide. Presently there is not enough data to estimate quantitatively the effect of low salt conditions on the equilibrium constants for SSB-protein binding, although strengthening of the interaction is expected 66. On the other hand it is evident that at low salt both χ 67; 83 and PriA (Kozlov and Lohman, unpublished results) interact with ssDNA/SSB complexes with much higher affinities than with SSB alone. The affinities of these proteins for SSB and ssDNA/SSB complexes also decrease with increasing salt concentrations. This latter effect may simply reflect an effect on the electrostatic component of the interaction or it could suggest that SSB bound in different binding modes (e.g., (SSB)35 at low salt or (SSB)65 at high salt) possesses different binding properties for these proteins.
III. ROLE OF SSB IN BACTERIAL RECOMBINATION PROCESSES
Recombination is a process focused on the repair of DNA strand breaks, primarily double strand breaks and single strand gaps. Both types of DNA damage are found most commonly at the sites of stalled or collapsed replication forks 7; 11; 329-334. The single strand gaps at stalled replication forks can be quite extensive 178; 208; 335; 336. Inevitably, SSB protein binds to the ssDNA in these gaps. Its role is not simply protective. As already described, SSB is a facilitator of ssDNA metabolism, and its interactions with the proteins of recombinational DNA repair are critical to the course of that repair. In this section, we consider the roles played by SSB in recombination both as a facilitator and an impediment to the overall process.
The late 1970s and early 1980s witnessed a renaissance in the understanding of bacterial recombination, centered on the functional characterization of the RecA protein, both in vivo and in vitro. The primary roles of RecA protein, in recombination 337-340, in the induction of the SOS response 341; 342, and in SOS mutagenesis 343-345, were established. In recombination, RecA protein promotes a series of DNA strand exchange reactions that lie at the heart of all recombinational processes 346-348. In the induction of the SOS response, RecA protein acts as a coprotease – facilitating the autocatalytic degradation of the LexA repressor of SOS genes 349; 350. In the mutagenesis that accompanies the SOS response, RecA protein acts as an essential activator and probable subunit of the error-prone translesion DNA polymerase V 309; 310.
Just out of the limelight, it became apparent at about the same time that SSB played a significant role in just about everything RecA protein did 346; 351-356. The RecA and SSB proteins were linked in their functions by three other proteins: RecF, RecO, and RecR 171; 195-197; 200; 357; 358. Notably, as was described above, RecO protein interacts with the SSB 194; 197; 200, and in particular the SSB C-terminus 199.
Two SSB mutations played key roles in elucidating the function of SSB in recombinational DNA repair. The first is ssb113, which was described above as producing a SSB-Ct variant mutation (Pro176Ser) that diminishes heterologous protein interactions with the SSB C-terminus 60. The ssb113 alteration results in a temperature-sensitive conditional lethality at 30 °C that is not suppressed by overexpression of the mutant protein 60. The second is the ssb1 mutation, which codes for an SSB with a mutation in the OB fold (His55Tyr) and confers a temperature-sensitive phenotype, with much SSB function abrogated at 42 °C 359. The ssb1 mutation is known to destabilize the SSB tetramer 359-361. Overproduction of the SSB1 protein suppresses the temperature-sensitive phenotype 359. Both mutant proteins confer a variety of defects in DNA metabolism in the strains expressing them, including sensitivity to UV irradiation, growth defects, and recombination defects 60; 61; 359; 362. Studies of SSB113 led to some of the first suggestions that SSB interacted directly with multiple other proteins 60. The ssb113 mutation produced severe defects in DNA synthesis and an increase in double strand breaks that led to chromosome degradation, while the ssb1 mutation produced more modest effects 61. Wang and Smith suggested that SSB played a key role in protecting exposed ssDNA during recombinational DNA repair 61. Lieberman and Witkin 354 noted that the DNA degradation and UV sensitivity seen at 42 °C in an ssb1 mutant cell was not rescued by the inactivation of recBCD (exonuclease V), or by overexpression of the wild type recA gene. This indicated that multiple nucleases were involved in the chromosomal degradation, and RecA did not function properly in recombinational DNA repair unless normal SSB was present. The ssb113 mutation blocks the induction of SOS at 30 °C (its restrictive temperature). The increase in UV sensitivity and decline of SOS-associated functions such as mutagenesis seen in the ssb113 strains was suppressed by the introduction of a recA allele (recA730 = recA E38K) that promotes constitutive SOS induction 354. The results implied that SSB played some direct role in the activation of RecA protein for SOS induction and SOS mutagenesis.
Although many proteins interact with the C-terminus of SSB, the details of the interaction are likely to vary from one protein to the next. A deletion of 10 C-terminal amino acids of SSB renders E. coli cells inviable 69. This implies that key interactions required for basic processes such as DNA replication occur at the SSB C-terminus. However, the alteration of the proline at position 176 in ssb113 allows cell growth while rendering the cell UV sensitive 60. This indicates that interactions required for DNA repair are disrupted by the Pro176Ser change in ssb113, but the interactions required in replication remain intact.
Effects of SSB on RecA protein function
SSB plays a complicated role in RecA reactions. RecA binding to ssDNA generally occurs in two phases, nucleation and filament extension. Nucleation must involve one or a few RecA monomers, and recent work suggests the number is about 4−5 363; 364. Under most conditions, RecA filament extension is relatively fast, allowing single filaments to coat long ssDNA molecules contiguously. Filament extension on ssDNA occurs in the 5' to 3' direction 201; 365, with little addition of subunits to the 5′-proximal end detectable 366. RecA disassembles from the 5'-proximal end 367, in a reaction that requires ATP hydrolysis 366. Disassembly from filaments bound to ssDNA occurs at a rate of about 70 subunits min-1 at 37 °C 366. This is substantially slower than filament extension, so that the growing ends of RecA filaments nucleated on a circular ssDNA soon encounter the disassembling end of the same or other filaments on the same DNA. Filament extension is blocked or impeded by secondary structure in the ssDNA substrate 352.
SSB has different effects on the two phases of RecA filament formation. RecA filament nucleation is inhibited, and under some conditions blocked entirely, if SSB is allowed to coat the DNA prior to RecA addition 200; 201; 346; 352; 367; 368. This inhibition is relieved in some RecA mutant proteins. These include RecA E38K (RecA 730 191; 369), RecA V37M (RecA 803 370), RecA T121I (RecA 2020 371; 372), RecA E38K, I298V (RecA 441 373-375), and a truncation of the RecA C-terminus of 17 amino acid residues (RecA ΔC17; E.A. Wood and M.M. Cox, unpublished results). The involvement of the RecA C-terminus (also highly negatively charged) in the suppression of RecA loading has some possible mechanistic implications. The RecA C-terminus (the last 25 amino acid residues) is a kind of autoregulatory flap, removal of which enhances a range of RecA functions 309; 310; 376-382. A plausible scheme is that the RecA C-terminus buries a surface on RecA protein that is required for a RecA interaction with SSB or the ssDNA bound to SSB, an interaction that is in turn necessary for SSB displacement and RecA nucleation. One or more of the other amino acid residues (E38, V37, T121) whose mutation also creates a protein more adept at bypassing the SSB block to nucleation may also be involved in interactions needed to cover the SSB displacement surface on RecA. For wild type RecA protein, mediator proteins alleviate the slow nucleation imposed by pre-bound SSB, as described below. These mediators may interact with the RecA C-terminus as part of their function.
When SSB is added to the ssDNA after, rather than before, RecA protein in an experiment that also includes ATP, the subsequent RecA reactions are enhanced rather than inhibited 383; 384. The early addition of RecA provides an opportunity to get past the slow nucleation step. In contrast to nucleation, the extension phase of RecA filament formation is facilitated by SSB. The wild type E. coli RecA protein is unable to disrupt secondary structure in ssDNA that is encountered during filament extension, leading to the formation of abbreviated filaments that do not uniformly coat the DNA. The SSB protein binds to and disrupts the ssDNA secondary structure. Extending RecA filaments readily displace SSB, allowing RecA to form a contiguous filament on the DNA. This general scheme, first proposed in the mid-1980s 352; 385; 386, is now seamlessly consistent with more than two decades of work on the reactions promoted by RecA and SSB 387; 388.
RecA protein promotes DNA strand exchange in vitro optimally under conditions that include relatively high concentrations of free Mg2+ ion (∼10 mM). The SSB binding mode under these conditions may play a major role in the course of the reactions. Under these conditions, SSB binds primarily in the low cooperative, fully wrapped (SSB)65 binding mode 15; 49; 50. The (SSB)35 mode, which binds with high nearest neighbor cooperativity, has been proposed to function in DNA replication 389.
There is some evidence for a persistent association of SSB with RecA protein filaments after contiguous RecA filaments are formed on ssDNA 384; 390. A similar interaction has been detected between the eukaryotic RecA-homologue Rad51 protein and the eukaryotic RPA protein 391. However, the positive effects of SSB on RecA filament formation (and of RPA on Rad51 filament formation in eukaryotes) are not limited to species-cognate SSBs 392; 393, and no indication of a persistent E. coli RecA-SSB complex has been evident in studies employing electron microscopy. If an association exists, it is relatively weak, does not occur between RecA and SSB when neither is bound to DNA 200, and does not play a role that is essential (or even stimulatory) to the RecA-mediated DNA strand exchange reactions commonly carried out in vitro. In those DNA strand exchange reactions, the RecA filaments form on ssDNA, and the bound DNA is then aligned with a homologous duplex DNA. A strand switch ensues, in which one strand of the duplex DNA is transferred to the original ssDNA to create a new duplex, and one strand of the original duplex is displaced. The SSB involved in facilitating the RecA filament formation prior to strand exchange is bound to the displaced ssDNA once strand exchange is complete 394. The RecA protein remains bound to the product duplex DNA, or dissociates, depending on solution conditions 395-397. In vivo, a persistent association of SSB with the RecA filaments could help choreograph the efficient transfer of the SSB to the displaced strand, and the transferred SSB could serve as a target for the binding of multiple other proteins involved in post-recombinational processes. In fact, direct transfer of SSB between two DNA strands has been documented and is facilitated when SSB is bound in the (SSB)35 binding mode such that two SSB OB-folds are unoccupied by ssDNA 398. A persistent association of SSB with a RecA nucleoprotein filament, one that leaves ssDNA binding surfaces on the SSB unoccupied, might serve a similar function. Such a role might not translate into a measurable advantage of a RecA filament-SSB interaction during in vitro reactions, but may merit further experimental investigation. Notably, the SSB protein of Mycobacterium smegmatis interacts directly and in a species-specific manner with M. smegmatis RecA nucleoprotein filaments 399. This interaction relies on the C-terminus of M. smegmatis SSB 399.
The E. coli mediators, RecF, RecO, and RecR proteins
The SSB barrier to RecA nucleation gives rise to a need for protein mediators – proteins that bypass the barrier and facilitate the nucleation process. The same problem exists in the loading of RecA-class recombinases in all organisms, and mediators and their critical loading functions are now recognized as common in bacteria, archaeans, and eukaryotes 400-403. There is potential for damaging genomic rearrangements inherent in recombination. Mediators provide a critical opportunity for every cell to regulate recombinase function at a point prior to the initiation of any recombinational process. The E. coli RecF, RecO, and RecR proteins are considered the prototypes of this class of proteins. However, considerable mechanistic variation may exist in different species and classes of organisms, as well as phages and viruses encoding recombination systems.
The genes coding for the RecF (40.5 kDa 106), RecO (27 kDa 186), and RecR (22 kDa 189; 404) proteins were discovered independently as functions that had modest effects on recombination and UV resistance in E. coli. The phenotypes of mutations in the three genes are very similar, and the effects of mutations in two or three of the genes are in many cases equivalent to the effects of any one of them, defining them as an epistatic group 170; 189. Several additional lines of evidence indicate that these three proteins function together early in recombinational processes, and tie them to a role in facilitating RecA filament assembly on SSB-coated ssDNA. Mutations in all three genes are suppressed by the recA E38K 191; 369, recA V37M 370, recA T121I 371; 372, recA E38K, I298V 373-375, and a truncation of the recA gene that removes 17 codons at the end encoding the C-terminus (E.A. Wood and M.M. Cox, unpublished results). As already noted, the mutant RecA proteins produced by these same genes generally exhibit an enhanced capacity to displace SSB and bind ssDNA. In addition, a gene in bacteriophage λ called ninB or orf can replace the functions of all three recFOR genes in lambda recombination 405; 406. Overexpression of SSB in E. coli produces a recFOR-like phenotype 358, providing another link between RecFOR and the SSB barrier to recombinase nucleation. Mutant bacteria missing any of the recFOR functions exhibit a delayed activation of the SOS response, most easily interpreted as a block to the formation of the RecA filaments required to facilitate the autocatalytic cleavage of the LexA repressor 182; 370.
Some results indicate that the recF, recO, and recR genes possess some functional distinctions. The three genes are not ubiquitous in bacteria, nor are they reliably coincident. A survey of recombination functions in 117 bacterial species demonstrates that bacteria tend to have all three genes, only the recO and recR genes, recR alone, or none 407. The recF gene is absent from 29 species in the survey, while recR is absent from only 10. There are only two cases where a recF gene is not accompanied by both recO and recR, and in both cases it is recO that appears to be missing 407. This may reflect a high substitution rate that seems to exist for recO, and an accompanying difficulty in identifying some recO homologues by classical search algorithms. A recO gene was subsequently identified in one of the two species in question (T. thermophilus), and its protein product has been studied at some length 203; 408. Taking the potential for discovery of a few more recO genes into account, the standard (or at least most common) complement of mediator functions in bacteria is either recFOR or recOR 407.
Even where all three genes are present, some results indicate that they do not always function together. In a strain lacking the function of PriA protein, the additional loss of recO produces different results than the loss of recF 175; 409. Mutation of recR or recF suppresses the strong effects of recO mutation, suggesting that RecF and RecR are deleterious to the cell in the absence of RecO 175. The RecF protein, but not RecO or RecR, is needed for the activation of DNA polymerase V and mutagenic translesion DNA synthesis (TLS) 179, providing one instance in which RecF may function without the other two proteins. SOS induction, UV resistance, and viability at 42°C are all reduced if RecF protein is overexpressed in vivo 410. Overexpression of the RecOR proteins suppresses many of the effects of either RecF overexpression 411 or a recF null mutation 190. These varied results indicate that RecOR may function on its own, or as part of a larger RecFOR system, and RecF may have a few independent functions.
This evidently complex situation is mirrored in vitro. Structural information about these proteins is becoming available, and this should promote mechanistic insight as it is coupled to ongoing biochemical analysis. All of the structures made available to date are from the RecFOR homologues of D. radiodurans. The D. radiodurans RecF protein exhibits an unexpected structural similarity with the head domain of the eukaryotic Rad50 protein 412. However, it lacks the long coiled-coil domain of Rad50 412. RecF is a member of the ATP-binding cassette (ABC) ATPase family of proteins, and possesses a weak ATPase activity 413-415. RecF binds to DNA, with increased affinity for dsDNA 413; 414; 416. ATP binding triggers RecF dimerization 412. ATP hydrolysis triggers dissociation from DNA 415. RecR protein forms a complex with RecF and improves the stability of RecF-DNA complexes 413; 414.
The D. radiodurans RecO protein contains an N-terminal domain that adopts an OB-fold, a novel α-helical domain, and a zinc-binding C-terminal domain 417; 418. RecO catalyzes complementary DNA strand annealing and invasion of duplex DNA by a complementary ssDNA 192-194. The RecO binds directly to ssDNA, a property established for the RecO proteins derived from E. coli, T. thermophilus, and D. radiodurans 192-194; 197; 203; 417; 418. Notably, it is the RecO protein that interacts with SSB 194; 197; 199; 200.
The RecR homologs in D. radiodurans and B. subtilis both bind DNA 419-421, although the E. coli RecR protein has no known intrinsic enzymatic or DNA binding activities. The D. radiodurans RecR structure is a tetrameric ring, with each monomer featuring a helix-hairpin-helix motif, a zinc finger motif, a Toprim domain, and a Walker B motif 420; 421.
In vitro, the E. coli and T. thermophilus RecR proteins bind to their cognate RecF and RecO proteins 197; 200; 202; 203; 408; 413; 414. Both T. thermophilus RecF and RecO proteins interact with the C-terminal TOPRIM domain of T. thermophilus RecR 408, providing a plausible explanation for an apparent competition between RecF and RecO for RecR binding that has been observed for the E. coli proteins 202. The structure of a D. radiodurans RecOR complex has also been elucidated 422. The proteins form a heterohexamer, with two RecO subunits on opposite faces of the RecR tetramer ring, and the OB domains of the RecO subunits proximal to the RecR ring 422. No structures of thse proteins with ssDNA or SSB are yet available.
The RecOR proteins clearly function together, and under many conditions these two proteins are necessary and sufficient to load RecA protein onto SSB-coated ssDNA 197; 199; 200; 202; 203. No conditions have yet been found in which one protein or the other alone can mediate the RecA loading process. As already noted, the RecO protein interacts directly with SSB 199; 200; 203. Significantly, removal of the 8 C-terminal residues of SSB eliminates most RecO function in the loading reaction 199, indicating that a RecO interaction with the SSB C-terminus is critical to the loading pathway. Early models indicated that RecOR does not displace SSB, but instead binds to it to form a RecO-RecR-SSB complex that facilitates RecA nucleation 200; 201. A recent examination of the loading process with the T. thermophilus proteins provided evidence for SSB displacement 203. The rate-limiting step in E. coli RecOR-mediated loading of RecA protein is the binding of RecO to ssDNA 199. This is inhibited by SSB, in spite of the direct interaction of RecO with the SSB C-terminus 199. The only set of conditions in which a small (8−10 min) lag in RecA loading was abolished was one in which the RecO was bound to ssDNA prior to the SSB 199. A model for the RecOR-mediated RecA loading process is presented in Figure 3.
Figure 3.
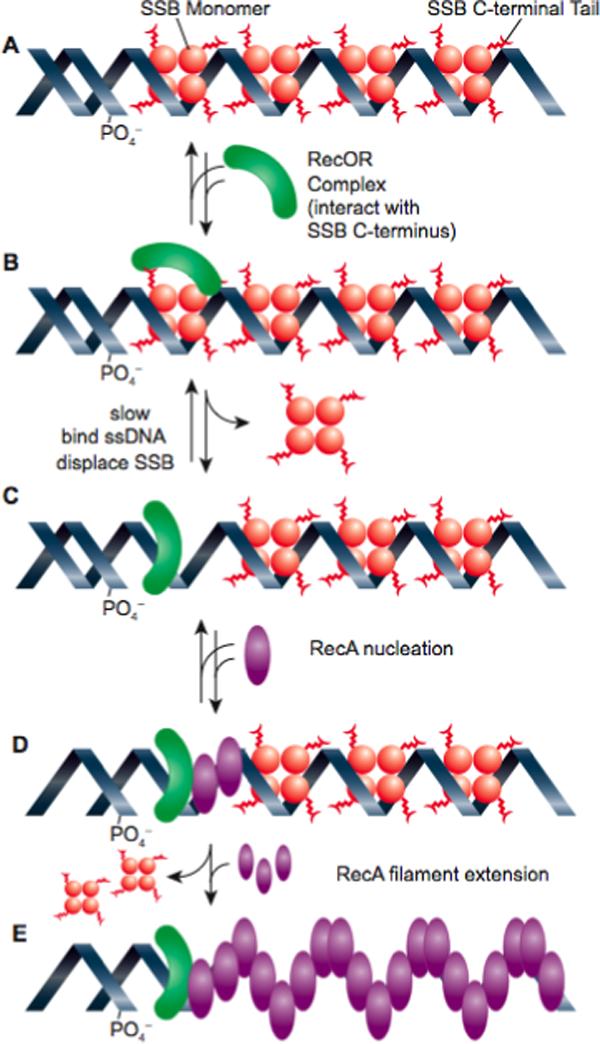
Loading of RecA protein onto SSB-coated ssDNA by the RecOR proteins. The RecO protein, in a complex with RecR, first binds to the C-terminus of SSB. The RecOR complex with SSB is then rearranged to permit direct binding of RecOR to the ssDNA and displacement of an SSB tetramer. Once RecOR is loaded, RecA interacts with RecOR (perhaps in a way that alters the conformation of the RecA C-terminus so as to expose an intrinsic loading surface), and RecA nucleation occurs. This is followed by rapid and unassisted RecA filament extension. This figure is based on recent studies of the loading process 199; 203.
Under most conditions, the RecF protein is either neutral or inhibitory for RecA loading on SSB-coated ssDNA when added to reactions containing RecOR 197; 199-202. The RecF protein has other demonstrable functions on the RecA filament formation process. RecFR complexes bind tightly to dsDNA, and can block the extension of RecA protein filaments initiated in ssDNA gaps into adjacent duplex DNA regions 414. RecF also interacts directly with the E. coli RecX protein, and antagonizes its function 423. The RecX protein blocks RecA filament extension, and the RecF function in this case may facilitate RecA protein extension in some instances. However, neither of these functions appears to fully explain the phenotypes of studied recF mutant strains.
When DNA substrates are used that incorporate short duplex regions on the ssDNA (generated by annealing short oligonucleotides to a bacteriophage ssDNA circle), the E. coli RecF protein has a positive effect on the RecA loading process in concert with RecR protein 117. This may reflect a special role for RecF protein in augmenting the loading process at the ends of DNA gaps. The positive effect of RecF is seen only when SSB is present at very high concentrations, corresponding to a 6−10 fold excess relative to available ssDNA binding sites (117; M. D. Hobbs and M. M. Cox, unpublished data), a requirement that is not yet explained.
With accumulating structural and biochemical data, this system seems poised for rapid advancement. Although RecF may have a special function in augmenting RecOR at the ends of gaps, there is no evidence that RecF binds specifically to those gap ends 414. Thus, there is potential for the discovery of additional targeting proteins in this system. A complete understanding of the RecFOR loading mechanism will facilitate studies of this critical function in all organisms. It should also facilitate an improved understanding of the dynamic nature of the SSB interaction with many other proteins.
IV. SUMMARY AND PERSPECTIVE
By binding both ssDNA and proteins central to every aspect of genome maintenance, eubacterial SSB proteins form a prominent interface at which genome maintenance pathways converge. As we have attempted to highlight in this review, the notion that SSB proteins are inert protective factors in bacterial cell biology vastly underestimates the contributions of this central scaffolding protein to genomic information storage and fidelity. From defining the substrates upon which DNA replication, recombination and repair must operate to playing an active role in nucleating complexes of enzymes, SSB proteins are central players in genome biology. Future work is needed to assess whether and how protein complex formation with SSB is regulated in vivo to determine which of the many competing interactions will predominate in a particular situation. In addition, the SSB-Ct-dependent nature of SSB/heterologous protein complexes could offer distinguishing features against which novel antibacterial therapies might be developed.
Acknowledgements
J.L.K acknowledges financial support from the NIH (GM068061). T.M.L. acknowledges financial support from the NIH (GM030498). M.M.C. acknowledges financial support from the NIH (GM032335 and GM0676085). R.D.S. is a Cremer Scholar. We apologize to any colleagues whose contributions to SSB studies might have been inadvertently overlooked in this review.
References
- 1.Watson JD, Crick FH. Genetical implications of the structure of deoxyribonucleic acid. Nature. 1953;171(4361):964. doi: 10.1038/171964b0. [DOI] [PubMed] [Google Scholar]
- 2.Watson JD, Crick FH. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953;171(4356):737. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- 3.Varghese AJ. Photochemistry of nucleic acids and their constituents. Photophysiology. 1972;(7):207. [PubMed] [Google Scholar]
- 4.Hanawalt PC. The U.V. sensitivity of bacteria: its relation to the DNA replication cycle. Photochem Photobiol. 1966;5(1):1. [PubMed] [Google Scholar]
- 5.Hanawalt PC. Normal replication of DNA after repair replication in bacteria. Nature. 1967;214(5085):269. doi: 10.1038/214269a0. [DOI] [PubMed] [Google Scholar]
- 6.Hanawalt PC, Haynes RH. The repair of DNA. Sci Am. 1967;216(2):36. doi: 10.1038/scientificamerican0267-36. [DOI] [PubMed] [Google Scholar]
- 7.Kuzminov A. DNA replication meets genetic exchange: chromosomal damage and its repair by homologous recombination. Proc Natl Acad Sci U S A. 2001;98(15):8461. doi: 10.1073/pnas.151260698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuzminov A. Single-strand interruptions in replicating chromosomes cause double-strand breaks. Proc Natl Acad Sci U S A. 2001;98(15):8241. doi: 10.1073/pnas.131009198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyrrell RM, Moss SH, Davies DJ. The variation in UV sensitivity of four K12 strains of Escherichia coli as a function of their stage of growth. Mutat Res. 1972;16(7):1. doi: 10.1016/0027-5107(72)90058-9. [DOI] [PubMed] [Google Scholar]
- 10.Kowalczykowski SC, Dixon DA, Eggleston AK, Lauder SD, Rehrauer WM. Biochemistry of homologous recombination in Escherichia coli. Microbiol Rev. 1994;58(3):401. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuzminov A. Recombinational repair of DNA damage in Escherichia coli and bacteriophage lambda. Microbiol Mol Biol Rev. 1999;63(4):751. doi: 10.1128/mmbr.63.4.751-813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohman TM, Ferrari ME. Escherichia coli single-stranded DNA-binding protein: multiple DNA-binding modes and cooperativities. Annu Rev Biochem. 1994;63:527. doi: 10.1146/annurev.bi.63.070194.002523. [DOI] [PubMed] [Google Scholar]
- 13.Meyer RR, Laine PS. The single-stranded DNA-binding protein of Escherichia coli. Microbiol Rev. 1990;54(4):342. doi: 10.1128/mr.54.4.342-380.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chrysogelos S, Griffith J. Escherichia coli single-strand binding protein organizes single-stranded DNA in nucleosome-like units. Proc Natl Acad Sci U S A. 1982;79(19):5803. doi: 10.1073/pnas.79.19.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lohman TM, Overman LB, Datta S. Salt-dependent changes in the DNA binding cooperativity of Escherichia coli single strand binding protein. J Mol Biol. 1986;187(4):603. doi: 10.1016/0022-2836(86)90338-4. [DOI] [PubMed] [Google Scholar]
- 16.Molineux IJ, Pauli A, Gefter ML. Physical studies of the interaction between the Escherichia coli DNA binding protein and nucleic acids. Nucleic Acids Res. 1975;2(10):1821. doi: 10.1093/nar/2.10.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider RJ, Wetmur JG. Kinetics of transfer of Escherichia coli single strand deoxyribonucleic acid binding protein between single-stranded deoxyribonucleic acid molecules. Biochemistry. 1982;21(4):608. doi: 10.1021/bi00533a002. [DOI] [PubMed] [Google Scholar]
- 18.Sigal N, Delius H, Kornberg T, Gefter ML, Alberts B. A DNA-unwinding protein isolated from Escherichia coli: its interaction with DNA and with DNA polymerases. Proc Natl Acad Sci U S A. 1972;69(12):3537. doi: 10.1073/pnas.69.12.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiner JH, Bertsch LL, Kornberg A. The deoxyribonucleic acid unwinding protein of Escherichia coli. Properties and functions in replication. J Biol Chem. 1975;250(6):1972. [PubMed] [Google Scholar]
- 20.Williams KR, Spicer EK, LoPresti MB, Guggenheimer RA, Chase JW. Limited proteolysis studies on the Escherichia coli single-stranded DNA binding protein. Evidence for a functionally homologous domain in both the Escherichia coli and T4 DNA binding proteins. J Biol Chem. 1983;258(5):3346. [PubMed] [Google Scholar]
- 21.Greipel J, Maass G, Mayer F. Complexes of the single-stranded DNA-binding protein from Escherichia coli (Eco SSB) with poly(dT). An investigation of their structure and internal dynamics by means of electron microscopy and NMR. Biophys Chem. 1987;26(2−3):149. doi: 10.1016/0301-4622(87)80018-2. [DOI] [PubMed] [Google Scholar]
- 22.Mackay V, Linn S. Selective inhibition of the dnase activity of the recBC enzyme by the DNA binding protein from Escherichia coli. J Biol Chem. 1976;251(12):3716. [PubMed] [Google Scholar]
- 23.Meyer RR, Glassberg J, Scott JV, Kornberg A. A temperature-sensitive single-stranded DNA-binding protein from Escherichia coli. J Biol Chem. 1980;255(7):2897. [PubMed] [Google Scholar]
- 24.Molineux IJ, Gefter ML. Properties of the Escherichia coli in DNA binding (unwinding) protein: interaction with DNA polymerase and DNA. Proc Natl Acad Sci U S A. 1974;71(10):3858. doi: 10.1073/pnas.71.10.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molineux IJ, Gefter ML. Properties of the Escherichia coli DNA-binding (unwinding) protein interaction with nucleolytic enzymes and DNA. J Mol Biol. 1975;98(4):811. doi: 10.1016/s0022-2836(75)80012-x. [DOI] [PubMed] [Google Scholar]
- 26.Fanning E, Klimovich V, Nager AR. A dynamic model for replication protein A (RPA) function in DNA processing pathways. Nucleic Acids Res. 2006;34(15):4126. doi: 10.1093/nar/gkl550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou Y, Liu Y, Wu X, Shell SM. Functions of human replication protein A (RPA): from DNA replication to DNA damage and stress responses. J Cell Physiol. 2006;208(2):267. doi: 10.1002/jcp.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Hippel PH, Delagoutte E. A general model for nucleic acid helicases and their “coupling” within macromolecular machines. Cell. 2001;104(2):177. doi: 10.1016/s0092-8674(01)00203-3. [DOI] [PubMed] [Google Scholar]
- 29.Wold MS. Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu Rev Biochem. 1997;66:61. doi: 10.1146/annurev.biochem.66.1.61. [DOI] [PubMed] [Google Scholar]
- 30.Casas-Finet JR, Khamis MI, Maki AH, Chase JW. Tryptophan 54 and phenylalanine 60 are involved synergistically in the binding of E. coli SSB protein to single-stranded polynucleotides. FEBS Lett. 1987;220(2):347. doi: 10.1016/0014-5793(87)80844-x. [DOI] [PubMed] [Google Scholar]
- 31.Curth U, Greipel J, Urbanke C, Maass G. Multiple binding modes of the single-stranded DNA binding protein from Escherichia coli as detected by tryptophan fluorescence and site-directed mutagenesis. Biochemistry. 1993;32(10):2585. doi: 10.1021/bi00061a016. [DOI] [PubMed] [Google Scholar]
- 32.Khamis MI, Casas-Finet JR, Maki AH, Murphy JB, Chase JW. Investigation of the role of individual tryptophan residues in the binding of Escherichia coli single-stranded DNA binding protein to single-stranded polynucleotides. A study by optical detection of magnetic resonance and site-selected mutagenesis. J Biol Chem. 1987;262(23):10938. [PubMed] [Google Scholar]
- 33.Merrill BM, Williams KR, Chase JW, Konigsberg WH. Photochemical cross-linking of the Escherichia coli single-stranded DNA-binding protein to oligodeoxynucleotides. Identification of phenylalanine 60 as the site of cross-linking. J Biol Chem. 1984;259(17):10850. [PubMed] [Google Scholar]
- 34.Raghunathan S, Kozlov AG, Lohman TM, Waksman G. Structure of the DNA binding domain of E. coli SSB bound to ssDNA. Nat Struct Biol. 2000;7(8):648. doi: 10.1038/77943. [DOI] [PubMed] [Google Scholar]
- 35.Savvides SN, Raghunathan S, Futterer K, Kozlov AG, Lohman TM, Waksman G. The C-terminal domain of full-length E. coli SSB is disordered even when bound to DNA. Protein Sci. 2004;13(7):1942. doi: 10.1110/ps.04661904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Overman LB, Bujalowski W, Lohman TM. Equilibrium binding of Escherichia coli single-strand binding protein to single-stranded nucleic acids in the (SSB)65 binding mode. Cation and anion effects and polynucleotide specificity. Biochemistry. 1988;27(1):456. doi: 10.1021/bi00401a067. [DOI] [PubMed] [Google Scholar]
- 37.Overman LB, Lohman TM. Linkage of pH, anion and cation effects in protein-nucleic acid equilibria. Escherichia coli SSB protein-single stranded nucleic acid interactions. J Mol Biol. 1994;236(1):165. doi: 10.1006/jmbi.1994.1126. [DOI] [PubMed] [Google Scholar]
- 38.Bochkarev A, Pfuetzner RA, Edwards AM, Frappier L. Structure of the single-stranded-DNA-binding domain of replication protein A bound to DNA. Nature. 1997;385(6612):176. doi: 10.1038/385176a0. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto T, Morimoto Y, Shibata N, Kinebuchi T, Shimamoto N, Tsukihara T, Yasuoka N. Roles of functional loops and the C-terminal segment of a single-stranded DNA binding protein elucidated by X-Ray structure analysis. J Biochem. 2000;127(2):329. doi: 10.1093/oxfordjournals.jbchem.a022611. [DOI] [PubMed] [Google Scholar]
- 40.Murzin AG. OB(oligonucleotide/oligosaccharide binding)-fold: common structural and functional solution for non-homologous sequences. EMBO J. 1993;12(3):861. doi: 10.1002/j.1460-2075.1993.tb05726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shamoo Y, Friedman AM, Parsons MR, Konigsberg WH, Steitz TA. Crystal structure of a replication fork single-stranded DNA binding protein (T4 gp32) complexed to DNA. Nature. 1995;376(6538):362. doi: 10.1038/376362a0. [DOI] [PubMed] [Google Scholar]
- 42.Suck D. Common fold, common function, common origin? Nat Struct Biol. 1997;4(3):161. doi: 10.1038/nsb0397-161. [DOI] [PubMed] [Google Scholar]
- 43.Molineux IJ, Friedman S, Gefter ML. Purification and properties of the Escherichia coli deoxyribonucleic acid-unwinding protein. Effects on deoxyribonucleic acid synthesis in vitro. J Biol Chem. 1974;249(19):6090. [PubMed] [Google Scholar]
- 44.Bernstein DA, Eggington JM, Killoran MP, Misic AM, Cox MM, Keck JL. Crystal structure of the Deinococcus radiodurans single-stranded DNA-binding protein suggests a mechanism for coping with DNA damage. Proc Natl Acad Sci U S A. 2004;101(23):8575. doi: 10.1073/pnas.0401331101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eggington JM, Haruta N, Wood EA, Cox MM. The single-stranded DNA-binding protein of Deinococcus radiodurans. BMC Microbiol. 2004;4:2. doi: 10.1186/1471-2180-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dabrowski S, Olszewski M, Piatek R, Brillowska-Dabrowska A, Konopa G, Kur J. Identification and characterization of single-stranded-DNA-binding proteins from Thermus thermophilus and Thermus aquaticus - new arrangement of binding domains. Microbiology. 2002;148(Pt 10):3307. doi: 10.1099/00221287-148-10-3307. [DOI] [PubMed] [Google Scholar]
- 47.Dabrowski S, Olszewski M, Piatek R, Kur J. Novel thermostable ssDNA-binding proteins from Thermus thermophilus and T. aquaticus-expression and purification. Protein Expr Purif. 2002;26(1):131. doi: 10.1016/s1046-5928(02)00504-1. [DOI] [PubMed] [Google Scholar]
- 48.Kumaran S, Kozlov AG, Lohman TM. Saccharomyces cerevisiae replication protein A binds to single-stranded DNA in multiple salt-dependent modes. Biochemistry. 2006;45(39):11958. doi: 10.1021/bi060994r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bujalowski W, Lohman TM. Escherichia coli single-strand binding protein forms multiple, distinct complexes with single-stranded DNA. Biochemistry. 1986;25(24):7799. doi: 10.1021/bi00372a003. [DOI] [PubMed] [Google Scholar]
- 50.Griffith JD, Harris LD, Register J., 3rd Visualization of SSB-ssDNA complexes active in the assembly of stable RecA-DNA filaments. Cold Spring Harb Symp Quant Biol. 1984;49:553. doi: 10.1101/sqb.1984.049.01.062. [DOI] [PubMed] [Google Scholar]
- 51.Lohman TM, Overman LB. Two binding modes in Escherichia coli single strand binding protein-single stranded DNA complexes. Modulation by NaCl concentration. J Biol Chem. 1985;260(6):3594. [PubMed] [Google Scholar]
- 52.Wei TF, Bujalowski W, Lohman TM. Cooperative binding of polyamines induces the Escherichia coli single-strand binding protein-DNA binding mode transitions. Biochemistry. 1992;31(26):6166. doi: 10.1021/bi00141a029. [DOI] [PubMed] [Google Scholar]
- 53.Bujalowski W, Overman LB, Lohman TM. Binding mode transitions of Escherichia coli single strand binding protein-single-stranded DNA complexes. Cation, anion, pH, and binding density effects. J Biol Chem. 1988;263(10):4629. [PubMed] [Google Scholar]
- 54.Lu D, Keck JL. Structural basis of E. coli single-stranded DNA-binding protein stimulation of Exonuclease I. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0800741105. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lonberg N, Kowalczykowski SC, Paul LS, von Hippel PH. Interactions of bacteriophage T4-coded gene 32 protein with nucleic acids. III. Binding properties of two specific proteolytic digestion products of the protein (G32P*I and G32P*III) J Mol Biol. 1981;145(1):123. doi: 10.1016/0022-2836(81)90337-5. [DOI] [PubMed] [Google Scholar]
- 56.Burke RL, Alberts BM, Hosoda J. Proteolytic removal of the COOH terminus of the T4 gene 32 helix-destabilizing protein alters the T4 in vitro replication complex. J Biol Chem. 1980;255(23):11484. [PubMed] [Google Scholar]
- 57.Hosoda J, Moise H. Purification and physicochemical properties of limited proteolysis products of T4 helix destabilizing protein (gene 32 protein) J Biol Chem. 1978;253(20):7547. [PubMed] [Google Scholar]
- 58.Roy R, Kozlov AG, Lohman TM, Ha T. Dynamic Structural Rearrangements Between DNA Binding Modes of E. coli SSB Protein. J Mol Biol. 2007;369(5):1244. doi: 10.1016/j.jmb.2007.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marintcheva B, Marintchev A, Wagner G, Richardson CC. Acidic C-terminal tail of the ssDNA-binding protein of bacteriophage T7 and ssDNA compete for the same binding surface. Proc Natl Acad Sci U S A. 2008;105(6):1855. doi: 10.1073/pnas.0711919105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chase JW, L'Italien JJ, Murphy JB, Spicer EK, Williams KR. Characterization of the Escherichia coli SSB-113 mutant single-stranded DNA-binding protein. Cloning of the gene, DNA and protein sequence analysis, high pressure liquid chromatography peptide mapping, and DNA-binding studies. J Biol Chem. 1984;259(2):805. [PubMed] [Google Scholar]
- 61.Wang TC, Smith KC. Effects of the ssb-1 and ssb-113 mutations on survival and DNA repair in UV-irradiated delta uvrB strains of Escherichia coli K-12. J Bacteriol. 1982;151(1):186. doi: 10.1128/jb.151.1.186-192.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greenberg J, Berends LJ, Donch J, Green MH. exrB: a malB-linked gene in Escherichia coli B involved in sensitivity to radiation and filament formation. Genet Res. 1974;23(2):175. doi: 10.1017/s0016672300014798. [DOI] [PubMed] [Google Scholar]
- 63.Greenberg J, Donch J. Sensitivity to elevated temperatures in exrB strains of Escherichia coli. Mutat Res. 1974;25(3):403. doi: 10.1016/0027-5107(74)90070-0. [DOI] [PubMed] [Google Scholar]
- 64.Johnson BF. Two-dimensional electrophoretic analysis of the regulation of SOS proteins in three ssb mutants. Arch Microbiol. 1984;138(2):106. doi: 10.1007/BF00413009. [DOI] [PubMed] [Google Scholar]
- 65.Meyer RR, Rein DC, Glassberg J. The product of the lexC gene of Escherichia coli is single-stranded DNA-binding protein. J Bacteriol. 1982;150(1):433. doi: 10.1128/jb.150.1.433-435.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Genschel J, Curth U, Urbanke C. Interaction of E. coli single-stranded DNA binding protein (SSB) with exonuclease I. The carboxy-terminus of SSB is the recognition site for the nuclease. Biol Chem. 2000;381(3):183. doi: 10.1515/BC.2000.025. [DOI] [PubMed] [Google Scholar]
- 67.Kelman Z, Yuzhakov A, Andjelkovic J, O'Donnell M. Devoted to the lagging strand-the χ subunit of DNA polymerase III holoenzyme contacts SSB to promote processive elongation and sliding clamp assembly. EMBO J. 1998;17:2436. doi: 10.1093/emboj/17.8.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yuzhakov A, Kelman Z, O'Donnell M. Trading places on DNA--a three-point switch underlies primer handoff from primase to the replicative DNA polymerase. Cell. 1999;96(1):153. doi: 10.1016/s0092-8674(00)80968-x. [DOI] [PubMed] [Google Scholar]
- 69.Curth U, Genschel J, Urbanke C, Greipel J. In vitro and in vivo function of the C-terminus of Escherichia coli single-stranded DNA binding protein. Nucleic Acids Res. 1996;24(14):2706. doi: 10.1093/nar/24.14.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaguni JM, Kornberg A. Replication initiated at the origin (oriC) of the E. coli chromosome reconstituted with purified enzymes. Cell. 1984;38(1):183. doi: 10.1016/0092-8674(84)90539-7. [DOI] [PubMed] [Google Scholar]
- 71.Kornberg A. Enzyme studies of replication of the Escherichia coli chromosome. Adv Exp Med Biol. 1984;179:3. doi: 10.1007/978-1-4684-8730-5_1. [DOI] [PubMed] [Google Scholar]
- 72.Baker TA, Bell SP. Polymerases and the replisome: machines within machines. Cell. 1998;92(3):295. doi: 10.1016/s0092-8674(00)80923-x. [DOI] [PubMed] [Google Scholar]
- 73.Davey MJ, O'Donnell M. Mechanisms of DNA replication. Curr Opin Chem Biol. 2000;4(5):581. doi: 10.1016/s1367-5931(00)00134-4. [DOI] [PubMed] [Google Scholar]
- 74.Johnson A, O'Donnell M. Cellular DNA replicases: components and dynamics at the replication fork. Annu Rev Biochem. 2005;74:283. doi: 10.1146/annurev.biochem.73.011303.073859. [DOI] [PubMed] [Google Scholar]
- 75.O'Donnell M. Replisome architecture and dynamics in Escherichia coli. J Biol Chem. 2006;281(16):10653. doi: 10.1074/jbc.R500028200. [DOI] [PubMed] [Google Scholar]
- 76.Marians KJ. Enzymology of DNA in replication in prokaryotes. CRC Crit Rev Biochem. 1984;17(2):153. doi: 10.3109/10409238409113604. [DOI] [PubMed] [Google Scholar]
- 77.Kornberg A, Baker TA. DNA Replication. W. H. Freeman; New York: 1992. [Google Scholar]
- 78.Jeruzalmi D, O'Donnell M, Kuriyan J. Crystal structure of the processivity clamp loader gamma (gamma) complex of E. coli DNA polymerase III. Cell. 2001;106(4):429. doi: 10.1016/s0092-8674(01)00463-9. [DOI] [PubMed] [Google Scholar]
- 79.Pritchard AE, Dallmann HG, Glover BP, McHenry CS. A novel assembly mechanism for the DNA polymerase III holoenzyme DnaX complex: association of deltadelta’ with DnaX(4) forms DnaX(3)deltadelta'. EMBO J. 2000;19(23):6536. doi: 10.1093/emboj/19.23.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Onrust R, Finkelstein J, Naktinis V, Turner J, Fang L, O'Donnell M. Assembly of a chromosomal replication machine: two DNA polymerases, a clamp loader, and sliding clamps in one holoenzyme particle. I. Organization of the clamp loader. J Biol Chem. 1995;270(22):13348. doi: 10.1074/jbc.270.22.13348. [DOI] [PubMed] [Google Scholar]
- 81.Olson MW, Dallmann HG, McHenry CS. DnaX complex of Escherichia coli DNA polymerase III holoenzyme. The chi psi complex functions by increasing the affinity of tau and gamma for delta.delta’ to a physiologically relevant range. J Biol Chem. 1995;270(49):29570. [PubMed] [Google Scholar]
- 82.Glover BP, McHenry CS. The chi psi subunits of DNA polymerase III holoenzyme bind to single-stranded DNA-binding protein (SSB) and facilitate replication of an SSB-coated template. J Biol Chem. 1998;273(36):23476. doi: 10.1074/jbc.273.36.23476. [DOI] [PubMed] [Google Scholar]
- 83.Witte G, Urbanke C, Curth U. DNA polymerase III chi subunit ties single-stranded DNA binding protein to the bacterial replication machinery. Nucleic Acids Res. 2003;31(15):4434. doi: 10.1093/nar/gkg498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Quinones A, Neumann S. The ssb-113 allele suppresses the dnaQ49 mutator and alters DNA supercoiling in Escherichia coli. Mol Microbiol. 1997;25(2):237. doi: 10.1046/j.1365-2958.1997.4531718.x. [DOI] [PubMed] [Google Scholar]
- 85.Brutlag D, Schekman R, Kornberg A. A possible role for RNA polymerase in the initiation of M13 DNA synthesis. Proc Natl Acad Sci U S A. 1971;68(11):2826. doi: 10.1073/pnas.68.11.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rowen L, Kornberg A. Primase, the dnaG protein of Escherichia coli. An enzyme which starts DNA chains. J Biol Chem. 1978;253(3):758. [PubMed] [Google Scholar]
- 87.Griep MA. Primase structure and function. Indian J Biochem Biophys. 1995;32(4):171. [PubMed] [Google Scholar]
- 88.Benkovic SJ, Valentine AM, Salinas F. Replisome-mediated DNA replication. Annu Rev Biochem. 2001;70:181. doi: 10.1146/annurev.biochem.70.1.181. [DOI] [PubMed] [Google Scholar]
- 89.van der Ende A, Baker TA, Ogawa T, Kornberg A. Initiation of enzymatic replication at the origin of the Escherichia coli chromosome: primase as the sole priming enzyme. Proc Natl Acad Sci U S A. 1985;82(12):3954. doi: 10.1073/pnas.82.12.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kitani T, Yoda K, Ogawa T, Okazaki T. Evidence that discontinuous DNA replication in Escherichia coli is primed by approximately 10 to 12 residues of RNA starting with a purine. J Mol Biol. 1985;184(1):45. doi: 10.1016/0022-2836(85)90042-7. [DOI] [PubMed] [Google Scholar]
- 91.Ogawa T, Baker TA, van der Ende A, Kornberg A. Initiation of enzymatic replication at the origin of the Escherichia coli chromosome: contributions of RNA polymerase and primase. Proc Natl Acad Sci U S A. 1985;82(11):3562. doi: 10.1073/pnas.82.11.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zechner EL, Wu CA, Marians KJ. Coordinated leading- and lagging-strand synthesis at the Escherichia coli DNA replication fork. III. A polymerase-primase interaction governs primer size. J Biol Chem. 1992;267(6):4054. [PubMed] [Google Scholar]
- 93.Keck JL, Roche DD, Lynch AS, Berger JM. Structure of the RNA polymerase domain of E. coli primase. Science. 2000;287(5462):2482. doi: 10.1126/science.287.5462.2482. [DOI] [PubMed] [Google Scholar]
- 94.Podobnik M, McInerney P, O'Donnell M, Kuriyan J. A TOPRIM domain in the crystal structure of the catalytic core of Escherichia coli primase confirms a structural link to DNA topoisomerases. J Mol Biol. 2000;300(2):353. doi: 10.1006/jmbi.2000.3844. [DOI] [PubMed] [Google Scholar]
- 95.Sun W, Tormo J, Steitz TA, Godson GN. Domains of Escherichia coli primase: functional activity of a 47-kDa N-terminal proteolytic fragment. Proc Natl Acad Sci U S A. 1994;91(24):11462. doi: 10.1073/pnas.91.24.11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tougu K, Peng H, Marians KJ. Identification of a domain of Escherichia coli primase required for functional interaction with the DnaB helicase at the replication fork. J Biol Chem. 1994;269(6):4675. [PubMed] [Google Scholar]
- 97.Tougu K, Marians KJ. The extreme C terminus of primase is required for interaction with DnaB at the replication fork. J Biol Chem. 1996;271(35):21391. doi: 10.1074/jbc.271.35.21391. [DOI] [PubMed] [Google Scholar]
- 98.Oakley AJ, Loscha KV, Schaeffer PM, Liepinsh E, Pintacuda G, Wilce MC, Otting G, Dixon NE. Crystal and solution structures of the helicase-binding domain of Escherichia coli primase. J Biol Chem. 2005;280(12):11495. doi: 10.1074/jbc.M412645200. [DOI] [PubMed] [Google Scholar]
- 99.Bailey S, Eliason WK, Steitz TA. Structure of hexameric DnaB helicase and its complex with a domain of DnaG primase. Science. 2007;318(5849):459. doi: 10.1126/science.1147353. [DOI] [PubMed] [Google Scholar]
- 100.Lu YB, Ratnakar PV, Mohanty BK, Bastia D. Direct physical interaction between DnaG primase and DnaB helicase of Escherichia coli is necessary for optimal synthesis of primer RNA. Proc Natl Acad Sci U S A. 1996;93(23):12902. doi: 10.1073/pnas.93.23.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tougu K, Marians KJ. The interaction between helicase and primase sets the replication fork clock. J Biol Chem. 1996;271(35):21398. doi: 10.1074/jbc.271.35.21398. [DOI] [PubMed] [Google Scholar]
- 102.Wu CA, Zechner EL, Reems JA, McHenry CS, Marians KJ. Coordinated leading- and lagging-strand synthesis at the Escherichia coli DNA replication fork. V. Primase action regulates the cycle of Okazaki fragment synthesis. J Biol Chem. 1992;267(6):4074. [PubMed] [Google Scholar]
- 103.Hiasa H, Marians KJ. Initiation of bidirectional replication at the chromosomal origin is directed by the interaction between helicase and primase. J Biol Chem. 1999;274(38):27244. doi: 10.1074/jbc.274.38.27244. [DOI] [PubMed] [Google Scholar]
- 104.Bhattacharyya S, Griep MA. DnaB helicase affects the initiation specificity of Escherichia coli primase on single-stranded DNA templates. Biochemistry. 2000;39(4):745. doi: 10.1021/bi991555d. [DOI] [PubMed] [Google Scholar]
- 105.Johnson SK, Bhattacharyya S, Griep MA. DnaB helicase stimulates primer synthesis activity on short oligonucleotide templates. Biochemistry. 2000;39(4):736. doi: 10.1021/bi991554l. [DOI] [PubMed] [Google Scholar]
- 106.Horii Z, Clark AJ. Genetic analysis of the recF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J Mol Biol. 1973;80(2):327. doi: 10.1016/0022-2836(73)90176-9. [DOI] [PubMed] [Google Scholar]
- 107.Tseng YC, Hung JL, Wang TC. Involvement of RecF pathway recombination genes in postreplication repair in UV-irradiated Escherichia coli cells. Mutat Res. 1994;315(1):1. doi: 10.1016/0921-8777(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 108.Wang TC, Smith KC. Mechanism of sbcB-suppression of the recBC-deficiency in postreplication repair in UV-irradiated Escherichia coli K-12. Mol Gen Genet. 1985;201(2):186. doi: 10.1007/BF00425658. [DOI] [PubMed] [Google Scholar]
- 109.Nakayama H, Nakayama K, Nakayama R, Irino N, Nakayama Y, Hanawalt PC. Isolation and genetic characterization of a thymineless death-resistant mutant of Escherichia coli K12: identification of a new mutation (recQ1) that blocks the RecF recombination pathway. Mol Gen Genet. 1984;195(3):474. doi: 10.1007/BF00341449. [DOI] [PubMed] [Google Scholar]
- 110.Nakayama K, Irino N, Nakayama H. The recQ gene of Escherichia coli K12: molecular cloning and isolation of insertion mutants. Mol Gen Genet. 1985;200(2):266. doi: 10.1007/BF00425434. [DOI] [PubMed] [Google Scholar]
- 111.Courcelle J, Carswell-Crumpton C, Hanawalt PC. recF and recR are required for the resumption of replication at DNA replication forks in Escherichia coli. Proc Natl Acad Sci U S A. 1997;94(8):3714. doi: 10.1073/pnas.94.8.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Courcelle J, Crowley DJ, Hanawalt PC. Recovery of DNA replication in UV-irradiated Escherichia coli requires both excision repair and recF protein function. J Bacteriol. 1999;181(3):916. doi: 10.1128/jb.181.3.916-922.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Courcelle J, Donaldson JR, Chow KH, Courcelle CT. DNA damage-induced replication fork regression and processing in Escherichia coli. Science. 2003;299(5609):1064. doi: 10.1126/science.1081328. [DOI] [PubMed] [Google Scholar]
- 114.Hegde SP, Qin MH, Li XH, Atkinson MA, Clark AJ, Rajagopalan M, Madiraju MV. Interactions of RecF protein with RecO, RecR, and single-stranded DNA binding proteins reveal roles for the RecF-RecO-RecR complex in DNA repair and recombination. Proc Natl Acad Sci U S A. 1996;93(25):14468. doi: 10.1073/pnas.93.25.14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Courcelle CT, Chow KH, Casey A, Courcelle J. Nascent DNA processing by RecJ favors lesion repair over translesion synthesis at arrested replication forks in Escherichia coli. Proc Natl Acad Sci U S A. 2006;103(24):9154. doi: 10.1073/pnas.0600785103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ivancic-Bace I, Salaj-Smic E, Brcic-Kostic K. Effects of recJ, recQ, and recFOR mutations on recombination in nuclease-deficient recB recD double mutants of Escherichia coli. J Bacteriol. 2005;187(4):1350. doi: 10.1128/JB.187.4.1350-1356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morimatsu K, Kowalczykowski SC. RecFOR proteins load RecA protein onto gapped DNA to accelerate DNA strand exchange: a universal step of recombinational repair. Mol Cell. 2003;11(5):1337. doi: 10.1016/s1097-2765(03)00188-6. [DOI] [PubMed] [Google Scholar]
- 118.Chow KH, Courcelle J. RecBCD and RecJ/RecQ initiate DNA degradation on distinct substrates in UV-irradiated Escherichia coli. Radiat Res. 2007;168(4):499. doi: 10.1667/RR1033.1. [DOI] [PubMed] [Google Scholar]
- 119.Centore RC, Sandler SJ. UvrD limits the number and intensities of RecA-green fluorescent protein structures in Escherichia coli K-12. J Bacteriol. 2007;189(7):2915. doi: 10.1128/JB.01777-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hishida T, Han YW, Shibata T, Kubota Y, Ishino Y, Iwasaki H, Shinagawa H. Role of the Escherichia coli RecQ DNA helicase in SOS signaling and genome stabilization at stalled replication forks. Genes Dev. 2004;18(15):1886. doi: 10.1101/gad.1223804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hanada K, Ukita T, Kohno Y, Saito K, Kato J, Ikeda H. RecQ DNA helicase is a suppressor of illegitimate recombination in Escherichia coli. Proc Natl Acad Sci U S A. 1997;94(8):3860. doi: 10.1073/pnas.94.8.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Magner DB, Blankschien MD, Lee JA, Pennington JM, Lupski JR, Rosenberg SM. RecQ promotes toxic recombination in cells lacking recombination intermediate-removal proteins. Mol Cell. 2007;26(2):273. doi: 10.1016/j.molcel.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harmon FG, Kowalczykowski SC. RecQ helicase, in concert with RecA and SSB proteins, initiates and disrupts DNA recombination. Genes Dev. 1998;12(8):1134. doi: 10.1101/gad.12.8.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Harmon FG, Brockman JP, Kowalczykowski SC. RecQ helicase stimulates both DNA catenation and changes in DNA topology by topoisomerase III. J Biol Chem. 2003;278(43):42668. doi: 10.1074/jbc.M302994200. [DOI] [PubMed] [Google Scholar]
- 125.Harmon FG, DiGate RJ, Kowalczykowski SC. RecQ helicase and topoisomerase III comprise a novel DNA strand passage function: a conserved mechanism for control of DNA recombination. Mol Cell. 1999;3(5):611. doi: 10.1016/s1097-2765(00)80354-8. [DOI] [PubMed] [Google Scholar]
- 126.Suski C, Marians KJ. Resolution of converging replication forks by RecQ and Topoisomerase III. 2008. in press. [DOI] [PMC free article] [PubMed]
- 127.Bachrati CZ, Hickson ID. RecQ helicases: suppressors of tumorigenesis and premature aging. Biochem J. 2003;374(Pt 3):577. doi: 10.1042/BJ20030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bennett RJ, Keck JL. Structure and function of RecQ DNA helicases. Crit Rev Biochem Mol Biol. 2004;39(2):79. doi: 10.1080/10409230490460756. [DOI] [PubMed] [Google Scholar]
- 129.Brosh RM, Jr., Bohr VA. Human premature aging, DNA repair and RecQ helicases. Nucleic Acids Res. 2007;35(22):7527. doi: 10.1093/nar/gkm1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cobb JA, Bjergbaek L. RecQ helicases: lessons from model organisms. Nucleic Acids Res. 2006;34(15):4106. doi: 10.1093/nar/gkl557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hanada K, Hickson ID. Molecular genetics of RecQ helicase disorders. Cell Mol Life Sci. 2007;64(17):2306. doi: 10.1007/s00018-007-7121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shereda RD, Bernstein DA, Keck JL. A Central Role for SSB in Escherichia coli RecQ DNA Helicase Function. J Biol Chem. 2007;282(26):19247. doi: 10.1074/jbc.M608011200. [DOI] [PubMed] [Google Scholar]
- 133.Umezu K, Nakayama H. RecQ DNA helicase of Escherichia coli. Characterization of the helix-unwinding activity with emphasis on the effect of single-stranded DNA-binding protein. J Mol Biol. 1993;230(4):1145. doi: 10.1006/jmbi.1993.1231. [DOI] [PubMed] [Google Scholar]
- 134.Lecointe F, Serena C, Velten M, Costes A, McGovern S, Meile JC, Errington J, Ehrlich SD, Noirot P, Polard P. Anticipating chromosomal replication fork arrest: SSB targets repair DNA helicases to active forks. EMBO J. 2007;26(19):4239. doi: 10.1038/sj.emboj.7601848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Butland G, Peregrin-Alvarez JM, Li J, Yang W, Yang X, Canadien V, Starostine A, Richards D, Beattie B, Krogan N, Davey M, Parkinson J, Greenblatt J, Emili A. Interaction network containing conserved and essential protein complexes in Escherichia coli. Nature. 2005;433(7025):531. doi: 10.1038/nature03239. [DOI] [PubMed] [Google Scholar]
- 136.Doherty KM, Sommers JA, Gray MD, Lee JW, von Kobbe C, Thoma NH, Kureekattil RP, Kenny MK, Brosh RM., Jr. Physical and functional mapping of the replication protein a interaction domain of the werner and bloom syndrome helicases. J Biol Chem. 2005;280(33):29494. doi: 10.1074/jbc.M500653200. [DOI] [PubMed] [Google Scholar]
- 137.Hu JS, Feng H, Zeng W, Lin GX, Xi XG. Solution structure of a multifunctional DNA-and protein-binding motif of human Werner syndrome protein. Proc Natl Acad Sci U S A. 2005;102(51):18379. doi: 10.1073/pnas.0509380102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sun JZ, Feng HQ, Lin GX, Zeng W, Hu JS. NMR assignments of the winged-helix domain of human werner syndrome protein. J Biomol NMR. 2005;32(3):261. doi: 10.1007/s10858-005-7960-6. [DOI] [PubMed] [Google Scholar]
- 139.Lovett ST, Kolodner RD. Identification and purification of a single-stranded-DNA-specific exonuclease encoded by the recJ gene of Escherichia coli. Proc Natl Acad Sci U S A. 1989;86(8):2627. doi: 10.1073/pnas.86.8.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lovett ST, Clark AJ. Genetic analysis of the recJ gene of Escherichia coli K-12. J Bacteriol. 1984;157(1):190. doi: 10.1128/jb.157.1.190-196.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lloyd RG, Porton MC, Buckman C. Effect of recF, recJ, recN, recO and ruv mutations on ultraviolet survival and genetic recombination in a recD strain of Escherichia coli K12. Mol Gen Genet. 1988;212(2):317. doi: 10.1007/BF00334702. [DOI] [PubMed] [Google Scholar]
- 142.Asai T, Kogoma T. The RecF pathway of homologous recombination can mediate the initiation of DNA damage-inducible replication of the Escherichia coli chromosome. J Bacteriol. 1994;176(22):7113. doi: 10.1128/jb.176.22.7113-7114.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Courcelle J, Hanawalt PC. RecQ and RecJ process blocked replication forks prior to the resumption of replication in UV-irradiated Escherichia coli. Mol Gen Genet. 1999;262(3):543. doi: 10.1007/s004380051116. [DOI] [PubMed] [Google Scholar]
- 144.Dianov G, Sedgwick B, Daly G, Olsson M, Lovett S, Lindahl T. Release of 5'-terminal deoxyribose-phosphate residues from incised abasic sites in DNA by the Escherichia coli RecJ protein. Nucleic Acids Res. 1994;22(6):993. doi: 10.1093/nar/22.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dianov G, Lindahl T. Reconstitution of the DNA base excision-repair pathway. Curr Biol. 1994;4(12):1069. doi: 10.1016/s0960-9822(00)00245-1. [DOI] [PubMed] [Google Scholar]
- 146.Viswanathan M, Burdett V, Baitinger C, Modrich P, Lovett ST. Redundant exonuclease involvement in Escherichia coli methyl-directed mismatch repair. J Biol Chem. 2001;276(33):31053. doi: 10.1074/jbc.M105481200. [DOI] [PubMed] [Google Scholar]
- 147.Burdett V, Baitinger C, Viswanathan M, Lovett ST, Modrich P. In vivo requirement for RecJ, ExoVII, ExoI, and ExoX in methyl-directed mismatch repair. Proc Natl Acad Sci U S A. 2001;98(12):6765. doi: 10.1073/pnas.121183298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Han ES, Cooper DL, Persky NS, Sutera VA, Jr., Whitaker RD, Montello ML, Lovett ST. RecJ exonuclease: substrates, products and interaction with SSB. Nucleic Acids Res. 2006;34(4):1084. doi: 10.1093/nar/gkj503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fukuoh A, Iwasaki H, Ishioka K, Shinagawa H. ATP-dependent resolution of R-loops at the ColE1 replication origin by Escherichia coli RecG protein, a Holliday junction-specific helicase. EMBO J. 1997;16(1):203. doi: 10.1093/emboj/16.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Singleton MR, Scaife S, Wigley DB. Structural analysis of DNA replication fork reversal by RecG. Cell. 2001;107(1):79. doi: 10.1016/s0092-8674(01)00501-3. [DOI] [PubMed] [Google Scholar]
- 151.Mahdi AA, Briggs GS, Sharples GJ, Wen Q, Lloyd RG. A model for dsDNA translocation revealed by a structural motif common to RecG and Mfd proteins. EMBO J. 2003;22(3):724. doi: 10.1093/emboj/cdg043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McGlynn P, Mahdi AA, Lloyd RG. Characterisation of the catalytically active form of RecG helicase. Nucleic Acids Res. 2000;28(12):2324. doi: 10.1093/nar/28.12.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Briggs GS, Mahdi AA, Wen Q, Lloyd RG. DNA binding by the substrate specificity (wedge) domain of RecG helicase suggests a role in processivity. J Biol Chem. 2005;280(14):13921. doi: 10.1074/jbc.M412054200. [DOI] [PubMed] [Google Scholar]
- 154.McGlynn P, Lloyd RG. Genome stability and the processing of damaged replication forks by RecG. Trends Genet. 2002;18(8):413. doi: 10.1016/s0168-9525(02)02720-8. [DOI] [PubMed] [Google Scholar]
- 155.Gregg AV, McGlynn P, Jaktaji RP, Lloyd RG. Direct rescue of stalled DNA replication forks via the combined action of PriA and RecG helicase activities. Mol Cell. 2002;9(2):241. doi: 10.1016/s1097-2765(02)00455-0. [DOI] [PubMed] [Google Scholar]
- 156.Briggs GS, Mahdi AA, Weller GR, Wen Q, Lloyd RG. Interplay between DNA replication, recombination and repair based on the structure of RecG helicase. Philos Trans R Soc Lond B Biol Sci. 2004;359(1441):49. doi: 10.1098/rstb.2003.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Meddows TR, Savory AP, Lloyd RG. RecG helicase promotes DNA double-strand break repair. Mol Microbiol. 2004;52(1):119. doi: 10.1111/j.1365-2958.2003.03970.x. [DOI] [PubMed] [Google Scholar]
- 158.Ishioka K, Iwasaki H, Shinagawa H. Roles of the recG gene product of Escherichia coli in recombination repair: effects of the delta recG mutation on cell division and chromosome partition. Genes Genet Syst. 1997;72(2):91. doi: 10.1266/ggs.72.91. [DOI] [PubMed] [Google Scholar]
- 159.Tanaka T, Masai H. Stabilization of a stalled replication fork by concerted actions of two helicases. J Biol Chem. 2006;281(6):3484. doi: 10.1074/jbc.M510979200. [DOI] [PubMed] [Google Scholar]
- 160.Whitby MC, Lloyd RG. Targeting Holliday junctions by the RecG branch migration protein of Escherichia coli. J Biol Chem. 1998;273(31):19729. doi: 10.1074/jbc.273.31.19729. [DOI] [PubMed] [Google Scholar]
- 161.McGlynn P, Lloyd RG. RecG helicase activity at three- and four-strand DNA structures. Nucleic Acids Res. 1999;27(15):3049. doi: 10.1093/nar/27.15.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lloyd RG. Conjugational recombination in resolvase-deficient ruvC mutants of Escherichia coli K-12 depends on recG. J Bacteriol. 1991;173(17):5414. doi: 10.1128/jb.173.17.5414-5418.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sharples GJ, Ingleston SM, Lloyd RG. Holliday junction processing in bacteria: insights from the evolutionary conservation of RuvABC, RecG, and RusA. J Bacteriol. 1999;181(18):5543. doi: 10.1128/jb.181.18.5543-5550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McGlynn P, Al-Deib AA, Liu J, Marians KJ, Lloyd RG. The DNA replication protein PriA and the recombination protein RecG bind D-loops. J Mol Biol. 1997;270(2):212. doi: 10.1006/jmbi.1997.1120. [DOI] [PubMed] [Google Scholar]
- 165.Vincent SD, Mahdi AA, Lloyd RG. The RecG branch migration protein of Escherichia coli dissociates R-loops. J Mol Biol. 1996;264(4):713. doi: 10.1006/jmbi.1996.0671. [DOI] [PubMed] [Google Scholar]
- 166.Robu ME, Inman RB, Cox MM. Situational repair of replication forks: roles of RecG and RecA proteins. J Biol Chem. 2004;279(12):10973. doi: 10.1074/jbc.M312184200. [DOI] [PubMed] [Google Scholar]
- 167.Whitby MC, Ryder L, Lloyd RG. Reverse branch migration of Holliday junctions by RecG protein: a new mechanism for resolution of intermediates in recombination and DNA repair. Cell. 1993;75(2):341. doi: 10.1016/0092-8674(93)80075-p. [DOI] [PubMed] [Google Scholar]
- 168.Whitby MC, Lloyd RG. Branch migration of three-strand recombination intermediates by RecG, a possible pathway for securing exchanges initiated by 3'-tailed duplex DNA. EMBO J. 1995;14(14):3302. doi: 10.1002/j.1460-2075.1995.tb07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Slocum SL, Buss JA, Kimura Y, Bianco PR. Characterization of the ATPase activity of the Escherichia coli RecG protein reveals that the preferred cofactor is negatively supercoiled DNA. J Mol Biol. 2007;367(3):647. doi: 10.1016/j.jmb.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Clark AJ, Sandler SJ. Homologous genetic recombination: the pieces begin to fall into place. Crit Rev Microbiol. 1994;20(2):125. doi: 10.3109/10408419409113552. [DOI] [PubMed] [Google Scholar]
- 171.Kolodner R, Fishel RA, Howard M. Genetic recombination of bacterial plasmid DNA: effect of RecF pathway mutations on plasmid recombination in Escherichia coli. J Bacteriol. 1985;163(3):1060. doi: 10.1128/jb.163.3.1060-1066.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Belle JJ, Casey A, Courcelle CT, Courcelle J. Inactivation of the DnaB helicase leads to the collapse and degradation of the replication fork: a comparison to UV-induced arrest. J Bacteriol. 2007;189(15):5452. doi: 10.1128/JB.00408-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chow KH, Courcelle J. RecO acts with RecF and RecR to protect and maintain replication forks blocked by UV-induced DNA damage in Escherichia coli. J Biol Chem. 2004;279(5):3492. doi: 10.1074/jbc.M311012200. [DOI] [PubMed] [Google Scholar]
- 174.Fujii S, Isogawa A, Fuchs RP. RecFOR proteins are essential for Pol V-mediated translesion synthesis and mutagenesis. EMBO J. 2006;25(24):5754. doi: 10.1038/sj.emboj.7601474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Grompone G, Sanchez N, Dusko Ehrlich S, Michel B. Requirement for RecFOR-mediated recombination in priA mutant. Mol Microbiol. 2004;52(2):551. doi: 10.1111/j.1365-2958.2004.03997.x. [DOI] [PubMed] [Google Scholar]
- 176.Ivancic-Bace I, Peharec P, Moslavac S, Skrobot N, Salaj-Smic E, Brcic-Kostic K. RecFOR function is required for DNA repair and recombination in a RecA loading-deficient recB mutant of Escherichia coli. Genetics. 2003;163(2):485. doi: 10.1093/genetics/163.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Luisi-DeLuca C, Lovett ST, Kolodner RD. Genetic and physical analysis of plasmid recombination in recB recC sbcB and recB recC sbcA Escherichia coli K-12 mutants. Genetics. 1989;122(2):269. doi: 10.1093/genetics/122.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.McInerney P, O'Donnell M. Replisome fate upon encountering a leading strand block and clearance from DNA by recombination proteins. J Biol Chem. 2007;282(35):25903. doi: 10.1074/jbc.M703777200. [DOI] [PubMed] [Google Scholar]
- 179.Rangarajan S, Woodgate R, Goodman MF. Replication restart in UV-irradiated Escherichia coli involving pols II, III, V, PriA, RecA and RecFOR proteins. Mol Microbiol. 2002;43(3):617. doi: 10.1046/j.1365-2958.2002.02747.x. [DOI] [PubMed] [Google Scholar]
- 180.Ryder L, Whitby MC, Lloyd RG. Mutation of recF, recJ, recO, recQ, or recR improves Hfr recombination in resolvase-deficient ruv recG strains of Escherichia coli. J Bacteriol. 1994;176(6):1570. doi: 10.1128/jb.176.6.1570-1577.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hegde S, Sandler SJ, Clark AJ, Madiraju MV. recO and recR mutations delay induction of the SOS response in Escherichia coli. Mol Gen Genet. 1995;246(2):254. doi: 10.1007/BF00294689. [DOI] [PubMed] [Google Scholar]
- 182.Whitby MC, Lloyd RG. Altered SOS induction associated with mutations in recF, recO and recR. Mol Gen Genet. 1995;246(2):174. doi: 10.1007/BF00294680. [DOI] [PubMed] [Google Scholar]
- 183.Ikeda H, Shiraishi K, Ogata Y. Illegitimate recombination mediated by double-strand break and end-joining in Escherichia coli. Adv Biophys. 2004;38:3. [PubMed] [Google Scholar]
- 184.Shiraishi K, Hanada K, Iwakura Y, Ikeda H. Roles of RecJ, RecO, and RecR in RecET-mediated illegitimate recombination in Escherichia coli. J Bacteriol. 2002;184(17):4715. doi: 10.1128/JB.184.17.4715-4721.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nakayama K, Shiota S, Nakayama H. Thymineless death in Escherichia coli mutants deficient in the RecF recombination pathway. Can J Microbiol. 1988;34(7):905. doi: 10.1139/m88-157. [DOI] [PubMed] [Google Scholar]
- 186.Morrison PT, Lovett ST, Gilson LE, Kolodner R. Molecular analysis of the Escherichia coli recO gene. J Bacteriol. 1989;171(7):3641. doi: 10.1128/jb.171.7.3641-3649.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mendonca VM, Matson SW. Genetic analysis of delta helD and delta uvrD mutations in combination with other genes in the RecF recombination pathway in Escherichia coli: suppression of a ruvB mutation by a uvrD deletion. Genetics. 1995;141(2):443. doi: 10.1093/genetics/141.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Veaute X, Delmas S, Selva M, Jeusset J, Le Cam E, Matic I, Fabre F, Petit MA. UvrD helicase, unlike Rep helicase, dismantles RecA nucleoprotein filaments in Escherichia coli. EMBO J. 2005;24(1):180. doi: 10.1038/sj.emboj.7600485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mahdi AA, Lloyd RG. Identification of the recR locus of Escherichia coli K-12 and analysis of its role in recombination and DNA repair. Mol Gen Genet. 1989;216(2−3):503. doi: 10.1007/BF00334397. [DOI] [PubMed] [Google Scholar]
- 190.Sandler SJ, Clark AJ. RecOR suppression of recF mutant phenotypes in Escherichia coli K-12. J Bacteriol. 1994;176(12):3661. doi: 10.1128/jb.176.12.3661-3672.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang TC, Chang HY, Hung JL. Cosuppression of recF, recR and recO mutations by mutant recA alleles in Escherichia coli cells. Mutat Res. 1993;294(2):157. doi: 10.1016/0921-8777(93)90024-b. [DOI] [PubMed] [Google Scholar]
- 192.Luisi-DeLuca C, Kolodner R. Purification and characterization of the Escherichia coli RecO protein. Renaturation of complementary single-stranded DNA molecules catalyzed by the RecO protein. J Mol Biol. 1994;236(1):124. doi: 10.1006/jmbi.1994.1123. [DOI] [PubMed] [Google Scholar]
- 193.Luisi-DeLuca C. Homologous pairing of single-stranded DNA and superhelical double-stranded DNA catalyzed by RecO protein from Escherichia coli. J Bacteriol. 1995;177(3):566. doi: 10.1128/jb.177.3.566-572.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kantake N, Madiraju MV, Sugiyama T, Kowalczykowski SC. Escherichia coli RecO protein anneals ssDNA complexed with its cognate ssDNA-binding protein: A common step in genetic recombination. Proc Natl Acad Sci U S A. 2002;99(24):15327. doi: 10.1073/pnas.252633399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Madiraju MV, Clark AJ. Use of recA803, a partial suppressor of recF, to analyze the effects of the mutant Ssb (single-stranded DNA-binding) proteins in vivo and in vitro. Mol Gen Genet. 1990;224(1):129. doi: 10.1007/BF00259459. [DOI] [PubMed] [Google Scholar]
- 196.Madiraju MV, Lavery PE, Kowalczykowski SC, Clark AJ. Enzymatic properties of the RecA803 protein, a partial suppressor of recF mutations. Biochemistry. 1992;31(43):10529. doi: 10.1021/bi00158a016. [DOI] [PubMed] [Google Scholar]
- 197.Umezu K, Chi NW, Kolodner RD. Biochemical interaction of the Escherichia coli RecF, RecO, and RecR proteins with RecA protein and single-stranded DNA binding protein. Proc Natl Acad Sci U S A. 1993;90(9):3875. doi: 10.1073/pnas.90.9.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cox MM. Regulation of bacterial RecA protein function. Crit Rev Biochem Mol Biol. 2007;42(1):41. doi: 10.1080/10409230701260258. [DOI] [PubMed] [Google Scholar]
- 199.Hobbs MD, Sakai A, Cox MM. SSB protein limits RecOR binding onto single-stranded DNA. J Biol Chem. 2007;282(15):11058. doi: 10.1074/jbc.M611007200. [DOI] [PubMed] [Google Scholar]
- 200.Umezu K, Kolodner RD. Protein interactions in genetic recombination in Escherichia coli. Interactions involving RecO and RecR overcome the inhibition of RecA by single-stranded DNA-binding protein. J Biol Chem. 1994;269(47):30005. [PubMed] [Google Scholar]
- 201.Shan Q, Bork JM, Webb BL, Inman RB, Cox MM. RecA protein filaments: end-dependent dissociation from ssDNA and stabilization by RecO and RecR proteins. J Mol Biol. 1997;265(5):519. doi: 10.1006/jmbi.1996.0748. [DOI] [PubMed] [Google Scholar]
- 202.Bork JM, Cox MM, Inman RB. The RecOR proteins modulate RecA protein function at 5' ends of single-stranded DNA. EMBO J. 2001;20(24):7313. doi: 10.1093/emboj/20.24.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Inoue J, Honda M, Ikawa S, Shibata T, Mikawa T. The process of displacing the single-stranded DNA-binding protein from single-stranded DNA by RecO and RecR proteins. Nucleic Acids Res. 2008;36(1):94. doi: 10.1093/nar/gkm1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wickner S, Hurwitz J. Association of phiX174 DNA-dependent ATPase activity with an Escherichia coli protein, replication factor Y, required for in vitro synthesis of phiX174 DNA. Proc Natl Acad Sci U S A. 1975;72(9):3342. doi: 10.1073/pnas.72.9.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Marians KJ. Prokaryotic DNA replication. Annu Rev Biochem. 1992;61:673. doi: 10.1146/annurev.bi.61.070192.003325. [DOI] [PubMed] [Google Scholar]
- 206.Heller RC, Marians KJ. Unwinding of the nascent lagging strand by Rep and PriA enables the direct restart of stalled replication forks. J Biol Chem. 2005;280(40):34143. doi: 10.1074/jbc.M507224200. [DOI] [PubMed] [Google Scholar]
- 207.Ng JY, Marians KJ. The ordered assembly of the phiX174-type primosome. I. Isolation and identification of intermediate protein-DNA complexes. J Biol Chem. 1996;271(26):15642. doi: 10.1074/jbc.271.26.15642. [DOI] [PubMed] [Google Scholar]
- 208.Heller RC, Marians KJ. The disposition of nascent strands at stalled replication forks dictates the pathway of replisome loading during restart. Mol Cell. 2005;17(5):733. doi: 10.1016/j.molcel.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 209.Shlomai J, Kornberg A. An Escherichia coli replication protein that recognizes a unique sequence within a hairpin region in phi X174 DNA. Proc Natl Acad Sci U S A. 1980;77(2):799. doi: 10.1073/pnas.77.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lee EH, Kornberg A. Replication deficiencies in priA mutants of Escherichia coli lacking the primosomal replication n’ protein. Proc Natl Acad Sci U S A. 1991;88(8):3029. doi: 10.1073/pnas.88.8.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lopper M, Boonsombat R, Sandler SJ, Keck JL. A hand-off mechanism for primosome assembly in replication restart. Mol Cell. 2007;26(6):781. doi: 10.1016/j.molcel.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zavitz KH, Marians KJ. ATPase-deficient mutants of the Escherichia coli DNA replication protein PriA are capable of catalyzing the assembly of active primosomes. J Biol Chem. 1992;267(10):6933. [PubMed] [Google Scholar]
- 213.Jones JM, Nakai H. Duplex opening by primosome protein PriA for replisome assembly on a recombination intermediate. J Mol Biol. 1999;289(3):503. doi: 10.1006/jmbi.1999.2783. [DOI] [PubMed] [Google Scholar]
- 214.Marians KJ. PriA: at the crossroads of DNA replication and recombination. Prog Nucleic Acid Res Mol Biol. 1999;63:39. doi: 10.1016/s0079-6603(08)60719-9. [DOI] [PubMed] [Google Scholar]
- 215.Marians KJ. PriA-directed replication fork restart in Escherichia coli. Trends Biochem Sci. 2000;25(4):185. doi: 10.1016/s0968-0004(00)01565-6. [DOI] [PubMed] [Google Scholar]
- 216.Nurse P, Liu J, Marians KJ. Two modes of PriA binding to DNA. J Biol Chem. 1999;274(35):25026. doi: 10.1074/jbc.274.35.25026. [DOI] [PubMed] [Google Scholar]
- 217.Sandler SJ, Marians KJ. Role of PriA in replication fork reactivation in Escherichia coli. J Bacteriol. 2000;182(1):9. doi: 10.1128/jb.182.1.9-13.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Xu L, Marians KJ. PriA mediates DNA replication pathway choice at recombination intermediates. Mol Cell. 2003;11(3):817. doi: 10.1016/s1097-2765(03)00061-3. [DOI] [PubMed] [Google Scholar]
- 219.Mahdi AA, Buckman C, Harris L, Lloyd RG. Rep and PriA helicase activities prevent RecA from provoking unnecessary recombination during replication fork repair. Genes Dev. 2006;20(15):2135. doi: 10.1101/gad.382306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kogoma T, Cadwell GW, Barnard KG, Asai T. The DNA replication priming protein, PriA, is required for homologous recombination and double-strand break repair. J Bacteriol. 1996;178(5):1258. doi: 10.1128/jb.178.5.1258-1264.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Masai H, Asai T, Kubota Y, Arai K, Kogoma T. Escherichia coli PriA protein is essential for inducible and constitutive stable DNA replication. EMBO J. 1994;13(22):5338. doi: 10.1002/j.1460-2075.1994.tb06868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sandler SJ, Samra HS, Clark AJ. Differential suppression of priA2::kan phenotypes in Escherichia coli K-12 by mutations in priA, lexA, and dnaC. Genetics. 1996;143(1):5. doi: 10.1093/genetics/143.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zavitz KH, Marians KJ. Dissecting the functional role of PriA protein-catalysed primosome assembly in Escherichia coli DNA replication. Mol Microbiol. 1991;5(12):2869. doi: 10.1111/j.1365-2958.1991.tb01846.x. [DOI] [PubMed] [Google Scholar]
- 224.Cox MM. Recombinational DNA repair in bacteria and the RecA protein. Prog Nucleic Acid Res Mol Biol. 1999;63:311. doi: 10.1016/s0079-6603(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 225.Grompone G, Bidnenko V, Ehrlich SD, Michel B. PriA is essential for viability of the Escherichia coli topoisomerase IV parE10(Ts) mutant. J Bacteriol. 2004;186(4):1197. doi: 10.1128/JB.186.4.1197-1199.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Grompone G, Ehrlich D, Michel B. Cells defective for replication restart undergo replication fork reversal. EMBO Rep. 2004;5(6):607. doi: 10.1038/sj.embor.7400167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lee MS, Marians KJ. Escherichia coli replication factor Y, a component of the primosome, can act as a DNA helicase. Proc Natl Acad Sci U S A. 1987;84(23):8345. doi: 10.1073/pnas.84.23.8345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lasken RS, Kornberg A. The primosomal protein n’ of Escherichia coli is a DNA helicase. J Biol Chem. 1988;263(12):5512. [PubMed] [Google Scholar]
- 229.Heller RC, Marians KJ. Non-replicative helicases at the replication fork. DNA Repair (Amst) 2007;6(7):945. doi: 10.1016/j.dnarep.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 230.Allen GC, Jr., Kornberg A. Assembly of the primosome of DNA replication in Escherichia coli. J Biol Chem. 1993;268(26):19204. [PubMed] [Google Scholar]
- 231.Arai K, Low RL, Kornberg A. Movement and site selection for priming by the primosome in phage phi X174 DNA replication. Proc Natl Acad Sci U S A. 1981;78(2):707. doi: 10.1073/pnas.78.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cadman CJ, McGlynn P. PriA helicase and SSB interact physically and functionally. Nucleic Acids Res. 2004;32(21):6378. doi: 10.1093/nar/gkh980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Liu J, Nurse P, Marians KJ. The ordered assembly of the phiX174-type primosome. III. PriB facilitates complex formation between PriA and DnaT. J Biol Chem. 1996;271(26):15656. doi: 10.1074/jbc.271.26.15656. [DOI] [PubMed] [Google Scholar]
- 234.Sandler SJ, Marians KJ, Zavitz KH, Coutu J, Parent MA, Clark AJ. dnaC mutations suppress defects in DNA replication- and recombination-associated functions in priB and priC double mutants in Escherichia coli K-12. Mol Microbiol. 1999;34(1):91. doi: 10.1046/j.1365-2958.1999.01576.x. [DOI] [PubMed] [Google Scholar]
- 235.Berges H, Oreglia J, Joseph-Liauzun E, Fayet O. Isolation and characterization of a priB mutant of Escherichia coli influencing plasmid copy number of delta rop ColE1-type plasmids. J Bacteriol. 1997;179(3):956. doi: 10.1128/jb.179.3.956-958.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lopper M, Holton JM, Keck JL. Crystal structure of PriB, a component of the Escherichia coli replication restart primosome. Structure. 2004;12(11):1967. doi: 10.1016/j.str.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 237.Low RL, Shlomai J, Kornberg A. Protein n, a primosomal DNA replication protein of Escherichia coli. Purification and characterization. J Biol Chem. 1982;257(11):6242. [PubMed] [Google Scholar]
- 238.Shioi S, Ose T, Maenaka K, Shiroishi M, Abe Y, Kohda D, Katayama T, Ueda T. Crystal structure of a biologically functional form of PriB from Escherichia coli reveals a potential single-stranded DNA-binding site. Biochem Biophys Res Commun. 2005;326(4):766. doi: 10.1016/j.bbrc.2004.11.104. [DOI] [PubMed] [Google Scholar]
- 239.Liu JH, Chang TW, Huang CY, Chen SU, Wu HN, Chang MC, Hsiao CD. Crystal structure of PriB, a primosomal DNA replication protein of Escherichia coli. J Biol Chem. 2004;279(48):50465. doi: 10.1074/jbc.M406773200. [DOI] [PubMed] [Google Scholar]
- 240.Ponomarev VA, Makarova KS, Aravind L, Koonin EV. Gene duplication with displacement and rearrangement: origin of the bacterial replication protein PriB from the single-stranded DNA-binding protein Ssb. J Mol Microbiol Biotechnol. 2003;5(4):225. doi: 10.1159/000071074. [DOI] [PubMed] [Google Scholar]
- 241.Bujalowski W, Lohman TM. Negative co-operativity in Escherichia coli single strand binding protein-oligonucleotide interactions. II. Salt, temperature and oligonucleotide length effects. J Mol Biol. 1989;207(1):269. doi: 10.1016/0022-2836(89)90455-5. [DOI] [PubMed] [Google Scholar]
- 242.Huang CY, Hsu CH, Sun YJ, Wu HN, Hsiao CD. Complexed crystal structure of replication restart primosome protein PriB reveals a novel single-stranded DNA-binding mode. Nucleic Acids Res. 2006;34(14):3878. doi: 10.1093/nar/gkl536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Cadman CJ, Lopper M, Moon PB, Keck JL, McGlynn P. PriB stimulates PriA helicase via an interaction with single-stranded DNA. J Biol Chem. 2005;280(48):39693. doi: 10.1074/jbc.M508521200. [DOI] [PubMed] [Google Scholar]
- 244.Lehman IR. The deoxyribonucleases of Escherichia coli. I. Purification and properties of a phosphodiesterase. J Biol Chem. 1960;235:1479. [PubMed] [Google Scholar]
- 245.Lehman IR, Nussbaum AL. The Deoxyribonucleases of Escherichia Coli. V. On the Specificity of Exonuclease I (Phosphodiesterase) J Biol Chem. 1964;239:2628. [PubMed] [Google Scholar]
- 246.Thomas KR, Olivera BM. Processivity of DNA exonucleases. J Biol Chem. 1978;253(2):424. [PubMed] [Google Scholar]
- 247.Brody RS, Doherty KG, Zimmerman PD. Processivity and kinetics of the reaction of exonuclease I from Escherichia coli with polydeoxyribonucleotides. J Biol Chem. 1986;261(16):7136. [PubMed] [Google Scholar]
- 248.Brody RS. Nucleotide positions responsible for the processivity of the reaction of exonuclease I with oligodeoxyribonucleotides. Biochemistry. 1991;30(29):7072. doi: 10.1021/bi00243a006. [DOI] [PubMed] [Google Scholar]
- 249.Phillips GJ, Prasher DC, Kushner SR. Physical and biochemical characterization of cloned sbcB and xonA mutations from Escherichia coli K-12. J Bacteriol. 1988;170(5):2089. doi: 10.1128/jb.170.5.2089-2094.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kushner SR, Nagaishi H, Clark AJ. Indirect suppression of recB and recC mutations by exonuclease I deficiency. Proc Natl Acad Sci U S A. 1972;69(6):1366. doi: 10.1073/pnas.69.6.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kushner SR, Nagaishi H, Clark AJ. Isolation of exonuclease VIII: the enzyme associated with sbcA indirect suppressor. Proc Natl Acad Sci U S A. 1974;71(9):3593. doi: 10.1073/pnas.71.9.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sunshine MG, Kelly B. Extent of host deletions associated with bacteriophage P2-mediated eduction. J Bacteriol. 1971;108(2):695. doi: 10.1128/jb.108.2.695-704.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Allgood ND, Silhavy TJ. Escherichia coli xonA (sbcB) mutants enhance illegitimate recombination. Genetics. 1991;127(4):671. doi: 10.1093/genetics/127.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lahue RS, Au KG, Modrich P. DNA mismatch correction in a defined system. Science. 1989;245(4914):160. doi: 10.1126/science.2665076. [DOI] [PubMed] [Google Scholar]
- 255.Sandigursky M, Franklin WA. DNA deoxyribophosphodiesterase of Escherichia coli is associated with exonuclease I. Nucleic Acids Res. 1992;20(18):4699. doi: 10.1093/nar/20.18.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sandigursky M, Lalezari I, Franklin WA. Excision of sugar-phosphate products at apurinic/apyrimidinic sites by DNA deoxyribophosphodiesterase of Escherichia coli. Radiat Res. 1992;131(3):332. [PubMed] [Google Scholar]
- 257.Viswanathan M, Lovett ST. Single-strand DNA-specific exonucleases in Escherichia coli. Roles in repair and mutation avoidance. Genetics. 1998;149(1):7. doi: 10.1093/genetics/149.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Bzymek M, Saveson CJ, Feschenko VV, Lovett ST. Slipped misalignment mechanisms of deletion formation: in vivo susceptibility to nucleases. J Bacteriol. 1999;181(2):477. doi: 10.1128/jb.181.2.477-482.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sandigursky M, Mendez F, Bases RE, Matsumoto T, Franklin WA. Protein-protein interactions between the Escherichia coli single-stranded DNA-binding protein and exonuclease I. Radiat Res. 1996;145(5):619. [PubMed] [Google Scholar]
- 260.Lindahl T. DNA repair enzymes. Annu Rev Biochem. 1982;51:61. doi: 10.1146/annurev.bi.51.070182.000425. [DOI] [PubMed] [Google Scholar]
- 261.Sakumi K, Sekiguchi M. Structures and functions of DNA glycosylases. Mutat Res. 1990;236(2−3):161. doi: 10.1016/0921-8777(90)90003-n. [DOI] [PubMed] [Google Scholar]
- 262.Mosbaugh DW, Bennett SE. Uracil-excision DNA repair. Prog Nucleic Acid Res Mol Biol. 1994;48:315. doi: 10.1016/s0079-6603(08)60859-4. [DOI] [PubMed] [Google Scholar]
- 263.Lindahl T, Ljungquist S, Siegert W, Nyberg B, Sperens B. DNA N-glycosidases: properties of uracil-DNA glycosidase from Escherichia coli. J Biol Chem. 1977;252(10):3286. [PubMed] [Google Scholar]
- 264.Lindahl T. An N-glycosidase from Escherichia coli that releases free uracil from DNA containing deaminated cytosine residues. Proc Natl Acad Sci U S A. 1974;71(9):3649. doi: 10.1073/pnas.71.9.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Savva R, McAuley-Hecht K, Brown T, Pearl L. The structural basis of specific base-excision repair by uracil-DNA glycosylase. Nature. 1995;373(6514):487. doi: 10.1038/373487a0. [DOI] [PubMed] [Google Scholar]
- 266.D'Souza D,I, Harrison L. Repair of clustered uracil DNA damages in Escherichia coli. Nucleic Acids Res. 2003;31(15):4573. doi: 10.1093/nar/gkg493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kumar NV, Varshney U. Contrasting effects of single stranded DNA binding protein on the activity of uracil DNA glycosylase from Escherichia coli towards different DNA substrates. Nucleic Acids Res. 1997;25(12):2336. doi: 10.1093/nar/25.12.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Purnapatre K, Handa P, Venkatesh J, Varshney U. Differential effects of single-stranded DNA binding proteins (SSBs) on uracil DNA glycosylases (UDGs) from Escherichia coli and mycobacteria. Nucleic Acids Res. 1999;27(17):3487. doi: 10.1093/nar/27.17.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Acharya N, Varshney U. Biochemical properties of single-stranded DNA-binding protein from Mycobacterium smegmatis, a fast-growing mycobacterium and its physical and functional interaction with uracil DNA glycosylases. J Mol Biol. 2002;318(5):1251. doi: 10.1016/s0022-2836(02)00053-0. [DOI] [PubMed] [Google Scholar]
- 270.Handa P, Acharya N, Varshney U. Chimeras between single-stranded DNA-binding proteins from Escherichia coli and Mycobacterium tuberculosis reveal that their C-terminal domains interact with uracil DNA glycosylases. J Biol Chem. 2001;276(20):16992. doi: 10.1074/jbc.M100393200. [DOI] [PubMed] [Google Scholar]
- 271.Chen H, Bryan SK, Moses RE. Cloning the polB gene of Escherichia coli and identification of its product. J Biol Chem. 1989;264(34):20591. [PubMed] [Google Scholar]
- 272.Iwasaki H, Nakata A, Walker GC, Shinagawa H. The Escherichia coli polB gene, which encodes DNA polymerase II, is regulated by the SOS system. J Bacteriol. 1990;172(11):6268. doi: 10.1128/jb.172.11.6268-6273.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lewis LK, Jenkins ME, Mount DW. Isolation of DNA damage-inducible promoters in Escherichia coli: regulation of polB (dinA), dinG, and dinH by LexA repressor. J Bacteriol. 1992;174(10):3377. doi: 10.1128/jb.174.10.3377-3385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Qiu Z, Goodman MF. The Escherichia coli polB locus is identical to dinA, the structural gene for DNA polymerase II. Characterization of Pol II purified from a polB mutant. J Biol Chem. 1997;272(13):8611. doi: 10.1074/jbc.272.13.8611. [DOI] [PubMed] [Google Scholar]
- 275.Bonner CA, Hays S, McEntee K, Goodman MF. DNA polymerase II is encoded by the DNA damage-inducible dinA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1990;87(19):7663. doi: 10.1073/pnas.87.19.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Schnarr M, Oertel-Buchheit P, Kazmaier M, Granger-Schnarr M. DNA binding properties of the LexA repressor. Biochimie. 1991;73(4):423. doi: 10.1016/0300-9084(91)90109-e. [DOI] [PubMed] [Google Scholar]
- 277.Lewis LK, Harlow GR, Gregg-Jolly LA, Mount DW. Identification of high affinity binding sites for LexA which define new DNA damage-inducible genes in Escherichia coli. J Mol Biol. 1994;241(4):507. doi: 10.1006/jmbi.1994.1528. [DOI] [PubMed] [Google Scholar]
- 278.Napolitano R, Janel-Bintz R, Wagner J, Fuchs RP. All three SOS-inducible DNA polymerases (Pol II, Pol IV and Pol V) are involved in induced mutagenesis. EMBO J. 2000;19(22):6259. doi: 10.1093/emboj/19.22.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Pham P, Rangarajan S, Woodgate R, Goodman MF. Roles of DNA polymerases V and II in SOS-induced error-prone and error-free repair in Escherichia coli. Proc Natl Acad Sci U S A. 2001;98(15):8350. doi: 10.1073/pnas.111007198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Bonner CA, Randall SK, Rayssiguier C, Radman M, Eritja R, Kaplan BE, McEntee K, Goodman MF. Purification and characterization of an inducible Escherichia coli DNA polymerase capable of insertion and bypass at abasic lesions in DNA. J Biol Chem. 1988;263(35):18946. [PubMed] [Google Scholar]
- 281.Escarceller M, Hicks J, Gudmundsson G, Trump G, Touati D, Lovett S, Foster PL, McEntee K, Goodman MF. Involvement of Escherichia coli DNA polymerase II in response to oxidative damage and adaptive mutation. J Bacteriol. 1994;176(20):6221. doi: 10.1128/jb.176.20.6221-6228.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Berardini M, Foster PL, Loechler EL. DNA polymerase II (polB) is involved in a new DNA repair pathway for DNA interstrand cross-links in Escherichia coli. J Bacteriol. 1999;181(9):2878. doi: 10.1093/gao/9781884446054.article.t031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Becherel OJ, Fuchs RP. Mechanism of DNA polymerase II-mediated frameshift mutagenesis. Proc Natl Acad Sci U S A. 2001;98(15):8566. doi: 10.1073/pnas.141113398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Fuchs RP, Koffel-Schwartz N, Pelet S, Janel-Bintz R, Napolitano R, Becherel OJ, Broschard TH, Burnouf DY, Wagner J. DNA polymerases II and V mediate respectively mutagenic (−2 frameshift) and error-free bypass of a single N-2-acetylaminofluorene adduct. Biochem Soc Trans. 2001;29(Pt 2):191. doi: 10.1042/0300-5127:0290191. [DOI] [PubMed] [Google Scholar]
- 285.Al Mamun AA, Humayun MZ. Escherichia coli DNA polymerase II can efficiently bypass 3,N(4)-ethenocytosine lesions in vitro and in vivo. Mutat Res. 2006;593(1−2):164. doi: 10.1016/j.mrfmmm.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 286.Pages V, Janel-Bintz R, Fuchs RP. Pol III proofreading activity prevents lesion bypass as evidenced by its molecular signature within E.coli cells. J Mol Biol. 2005;352(3):501. doi: 10.1016/j.jmb.2005.07.063. [DOI] [PubMed] [Google Scholar]
- 287.Sedliakova M, Slezarikova V, Masek F, Vizvaryova M, Pirsel M. Role of DNA polymerase II in the tolerance of thymine dimers remaining unexcised in UV-irradiated Escherichia coli exposed to pre-UV nutritional stress. J Photochem Photobiol B. 2001;65(2−3):145. doi: 10.1016/s1011-1344(01)00256-1. [DOI] [PubMed] [Google Scholar]
- 288.Rangarajan S, Woodgate R, Goodman MF. A phenotype for enigmatic DNA polymerase II: a pivotal role for pol II in replication restart in UV-irradiated Escherichia coli. Proc Natl Acad Sci U S A. 1999;96(16):9224. doi: 10.1073/pnas.96.16.9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Foster PL, Gudmundsson G, Trimarchi JM, Cai H, Goodman MF. Proofreading-defective DNA polymerase II increases adaptive mutation in Escherichia coli. Proc Natl Acad Sci U S A. 1995;92(17):7951. doi: 10.1073/pnas.92.17.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Nowosielska A, Janion C, Grzesiuk E. Effect of deletion of SOS-induced polymerases, pol II, IV, and V, on spontaneous mutagenesis in Escherichia coli mutD5. Environ Mol Mutagen. 2004;43(4):226. doi: 10.1002/em.20019. [DOI] [PubMed] [Google Scholar]
- 291.Banach-Orlowska M, Fijalkowska IJ, Schaaper RM, Jonczyk P. DNA polymerase II as a fidelity factor in chromosomal DNA synthesis in Escherichia coli. Mol Microbiol. 2005;58(1):61. doi: 10.1111/j.1365-2958.2005.04805.x. [DOI] [PubMed] [Google Scholar]
- 292.Rangarajan S, Gudmundsson G, Qiu Z, Foster PL, Goodman MF. Escherichia coli DNA polymerase II catalyzes chromosomal and episomal DNA synthesis in vivo. Proc Natl Acad Sci U S A. 1997;94(3):946. doi: 10.1073/pnas.94.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Bonner CA, Stukenberg PT, Rajagopalan M, Eritja R, O'Donnell M, McEntee K, Echols H, Goodman MF. Processive DNA synthesis by DNA polymerase II mediated by DNA polymerase III accessory proteins. J Biol Chem. 1992;267(16):11431. [PubMed] [Google Scholar]
- 294.Dalrymple BP, Kongsuwan K, Wijffels G, Dixon NE, Jennings PA. A universal protein-protein interaction motif in the eubacterial DNA replication and repair systems. Proc Natl Acad Sci U S A. 2001;98(20):11627. doi: 10.1073/pnas.191384398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Becherel OJ, Fuchs RP, Wagner J. Pivotal role of the beta-clamp in translesion DNA synthesis and mutagenesis in E. coli cells. DNA Repair (Amst) 2002;1(9):703. doi: 10.1016/s1568-7864(02)00106-4. [DOI] [PubMed] [Google Scholar]
- 296.Sutton MD, Duzen JM, Maul RW. Mutant forms of the Escherichia colibeta sliding clamp that distinguish between its roles in replication and DNA polymerase V-dependent translesion DNA synthesis. Mol Microbiol. 2005;55(6):1751. doi: 10.1111/j.1365-2958.2005.04500.x. [DOI] [PubMed] [Google Scholar]
- 297.Goodman MF. Coping with replication ‘train wrecks’ in Escherichia coli using Pol V, Pol II and RecA proteins. Trends Biochem Sci. 2000;25(4):189. doi: 10.1016/s0968-0004(00)01564-4. [DOI] [PubMed] [Google Scholar]
- 298.Livneh Z. DNA damage control by novel DNA polymerases: translesion replication and mutagenesis. J Biol Chem. 2001;276(28):25639. doi: 10.1074/jbc.R100019200. [DOI] [PubMed] [Google Scholar]
- 299.Nohmi T, Battista JR, Dodson LA, Walker GC. RecA-mediated cleavage activates UmuD for mutagenesis: mechanistic relationship between transcriptional derepression and posttranslational activation. Proc Natl Acad Sci U S A. 1988;85(6):1816. doi: 10.1073/pnas.85.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Peat TS, Frank EG, McDonald JP, Levine AS, Woodgate R, Hendrickson WA. Structure of the UmuD’ protein and its regulation in response to DNA damage. Nature. 1996;380(6576):727. doi: 10.1038/380727a0. [DOI] [PubMed] [Google Scholar]
- 301.McDonald JP, Maury EE, Levine AS, Woodgate R. Regulation of UmuD cleavage: role of the amino-terminal tail. J Mol Biol. 1998;282(4):721. doi: 10.1006/jmbi.1998.2044. [DOI] [PubMed] [Google Scholar]
- 302.Bruck I, Woodgate R, McEntee K, Goodman MF. Purification of a soluble UmuD'C complex from Escherichia coli. Cooperative binding of UmuD'C to single-stranded DNA. J Biol Chem. 1996;271(18):10767. doi: 10.1074/jbc.271.18.10767. [DOI] [PubMed] [Google Scholar]
- 303.Reuven NB, Arad G, Maor-Shoshani A, Livneh Z. The mutagenesis protein UmuC is a DNA polymerase activated by UmuD', RecA, and SSB and is specialized for translesion replication. J Biol Chem. 1999;274(45):31763. doi: 10.1074/jbc.274.45.31763. [DOI] [PubMed] [Google Scholar]
- 304.Tang M, Shen X, Frank EG, O'Donnell M, Woodgate R, Goodman MF. UmuD'(2)C is an error-prone DNA polymerase, Escherichia coli pol V. Proc Natl Acad Sci U S A. 1999;96(16):8919. doi: 10.1073/pnas.96.16.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Schlacher K, Jiang Q, Woodgate R, Goodman MF. Purification and characterization of Escherichia coli DNA polymerase V. Methods Enzymol. 2006;408:378. doi: 10.1016/S0076-6879(06)08023-2. [DOI] [PubMed] [Google Scholar]
- 306.Rehrauer WM, Bruck I, Woodgate R, Goodman MF, Kowalczykowski SC. Modulation of RecA nucleoprotein function by the mutagenic UmuD'C protein complex. J Biol Chem. 1998;273(49):32384. doi: 10.1074/jbc.273.49.32384. [DOI] [PubMed] [Google Scholar]
- 307.Tang M, Bruck I, Eritja R, Turner J, Frank EG, Woodgate R, O'Donnell M, Goodman MF. Biochemical basis of SOS-induced mutagenesis in Escherichia coli: reconstitution of in vitro lesion bypass dependent on the UmuD'2C mutagenic complex and RecA protein. Proc Natl Acad Sci U S A. 1998;95(17):9755. doi: 10.1073/pnas.95.17.9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Maor-Shoshani A, Livneh Z. Analysis of the stimulation of DNA polymerase V of Escherichia coli by processivity proteins. Biochemistry. 2002;41(48):14438. doi: 10.1021/bi0262909. [DOI] [PubMed] [Google Scholar]
- 309.Schlacher K, Cox MM, Woodgate R, Goodman MF. RecA acts in trans to allow replication of damaged DNA by DNA polymerase V. Nature. 2006;442(7105):883. doi: 10.1038/nature05042. [DOI] [PubMed] [Google Scholar]
- 310.Schlacher K, Leslie K, Wyman C, Woodgate R, Cox MM, Goodman MF. DNA polymerase V and RecA protein, a minimal mutasome. Mol Cell. 2005;17(4):561. doi: 10.1016/j.molcel.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 311.Arad G, Hendel A, Urbanke C, Curth U, Livneh Z. Single-stranded DNA-binding Protein Recruits DNA Polymerase V to Primer Termini on RecA-coated DNA. J Biol Chem. 2008;283(13):8274. doi: 10.1074/jbc.M710290200. [DOI] [PubMed] [Google Scholar]
- 312.Tang M, Pham P, Shen X, Taylor JS, O'Donnell M, Woodgate R, Goodman MF. Roles of E. coli DNA polymerases IV and V in lesion-targeted and untargeted SOS mutagenesis. Nature. 2000;404(6781):1014. doi: 10.1038/35010020. [DOI] [PubMed] [Google Scholar]
- 313.Sarov-Blat L, Livneh Z. The mutagenesis protein MucB interacts with single strand DNA binding protein and induces a major conformational change in its complex with single-stranded DNA. J Biol Chem. 1998;273(10):5520. doi: 10.1074/jbc.273.10.5520. [DOI] [PubMed] [Google Scholar]
- 314.Sayers JR. Computer aided identification of a potential 5'−3' exonuclease gene encoded by Escherichia coli. J Theor Biol. 1994;170(4):415. doi: 10.1006/jtbi.1994.1202. [DOI] [PubMed] [Google Scholar]
- 315.Shafritz KM, Sandigursky M, Franklin WA. Exonuclease IX of Escherichia coli. Nucleic Acids Res. 1998;26(11):2593. doi: 10.1093/nar/26.11.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Hodskinson MR, Allen LM, Thomson DP, Sayers JR. Molecular interactions of Escherichia coli ExoIX and identification of its associated 3'−5' exonuclease activity. Nucleic Acids Res. 2007;35(12):4094. doi: 10.1093/nar/gkm396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Lombardo MJ, Aponyi I, Ray MP, Sandigursky M, Franklin WA, Rosenberg SM. xni-deficient Escherichia coli are proficient for recombination and multiple pathways of repair. DNA Repair (Amst) 2003;2(11):1175. doi: 10.1016/s1568-7864(03)00135-6. [DOI] [PubMed] [Google Scholar]
- 318.Glucksmann-Kuis MA, Dai X, Markiewicz P, Rothman-Denes LB. E. coli SSB activates N4 virion RNA polymerase promoters by stabilizing a DNA hairpin required for promoter recognition. Cell. 1996;84(1):147. doi: 10.1016/s0092-8674(00)81001-6. [DOI] [PubMed] [Google Scholar]
- 319.Markiewicz P, Malone C, Chase JW, Rothman-Denes LB. Escherichia coli single-stranded DNA-binding protein is a supercoiled template-dependent transcriptional activator of N4 virion RNA polymerase. Genes Dev. 1992;6(10):2010. doi: 10.1101/gad.6.10.2010. [DOI] [PubMed] [Google Scholar]
- 320.Davydova EK, Rothman-Denes LB. Escherichia coli single-stranded DNA-binding protein mediates template recycling during transcription by bacteriophage N4 virion RNA polymerase. Proc Natl Acad Sci U S A. 2003;100(16):9250. doi: 10.1073/pnas.1133325100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Falco SC, Laan KV, Rothman-Denes LB. Virion-associated RNA polymerase required for bacteriophage N4 development. Proc Natl Acad Sci U S A. 1977;74(2):520. doi: 10.1073/pnas.74.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Davydova EK, Santangelo TJ, Rothman-Denes LB. Bacteriophage N4 virion RNA polymerase interaction with its promoter DNA hairpin. Proc Natl Acad Sci U S A. 2007;104(17):7033. doi: 10.1073/pnas.0610627104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Lindberg G, Kowalczykowski SC, Rist JK, Sugino A, Rothman-Denes LB. Purification and characterization of the coliphage N4-coded single-stranded DNA binding protein. J Biol Chem. 1989;264(21):12700. [PubMed] [Google Scholar]
- 324.Miller A, Dai X, Choi M, Glucksmann-Kuis MA, Rothman-Denes LB. Single-stranded DNA-binding proteins as transcriptional activators. Methods Enzymol. 1996;274:9. doi: 10.1016/s0076-6879(96)74004-1. [DOI] [PubMed] [Google Scholar]
- 325.Cho NY, Choi M, Rothman-Denes LB. The bacteriophage N4-coded single-stranded DNA-binding protein (N4SSB) is the transcriptional activator of Escherichia coli RNA polymerase at N4 late promoters. J Mol Biol. 1995;246(4):461. doi: 10.1006/jmbi.1994.0098. [DOI] [PubMed] [Google Scholar]
- 326.Arifuzzaman M, Maeda M, Itoh A, Nishikata K, Takita C, Saito R, Ara T, Nakahigashi K, Huang HC, Hirai A, Tsuzuki K, Nakamura S, Altaf-Ul-Amin M, Oshima T, Baba T, Yamamoto N, Kawamura T, Ioka-Nakamichi T, Kitagawa M, Tomita M, Kanaya S, Wada C, Mori H. Large-scale identification of protein-protein interaction of Escherichia coli K-12. Genome Res. 2006;16(5):686. doi: 10.1101/gr.4527806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Sikder D, Unniraman S, Bhaduri T, Nagaraja V. Functional cooperation between topoisomerase I and single strand DNA-binding protein. J Mol Biol. 2001;306(4):669. doi: 10.1006/jmbi.2000.4384. [DOI] [PubMed] [Google Scholar]
- 328.Raghunathan S, Ricard CS, Lohman TM, Waksman G. Crystal structure of the homotetrameric DNA binding domain of Escherichia coli single-stranded DNA-binding protein determined by multiwavelength x-ray diffraction on the selenomethionyl protein at 2.9-A resolution. Proc Natl Acad Sci U S A. 1997;94(13):6652. doi: 10.1073/pnas.94.13.6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Cox MM. Historical overview: Searching for replication help in all of the rec places. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(15):8173. doi: 10.1073/pnas.131004998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Cox MM, Goodman MF, Kreuzer KN, Sherratt DJ, Sandler SJ, Marians KJ. The importance of repairing stalled replication forks. Nature. 2000;404(6773):37. doi: 10.1038/35003501. [DOI] [PubMed] [Google Scholar]
- 331.Kuzminov A. Unraveling the late stages of recombinational repair: metabolism of DNA junctions in Escherichia coli. [78 refs] Bioessays. 1996;18(9):757. doi: 10.1002/bies.950180911. [DOI] [PubMed] [Google Scholar]
- 332.Michel B. Replication fork arrest and DNA recombination. Trends in Biochemical Sciences. 2000;25:173. doi: 10.1016/s0968-0004(00)01560-7. [DOI] [PubMed] [Google Scholar]
- 333.Michel B, Flores MJ, Viguera E, Grompone G, Seigneur M, Bidnenko V. Rescue of arrested replication forks by homologous recombination. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(15):8181. doi: 10.1073/pnas.111008798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Michel B, Grompone G, Flores MJ, Bidnenko V. Multiple pathways process stalled replication forks. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(35):12783. doi: 10.1073/pnas.0401586101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Cordeiro-Stone M, Makhov AM, Zaritskaya LS, Griffith JD. Analysis of DNA replication forks encountering a pyrimidine dimer in the template to the leading strand. Journal of Molecular Biology. 1999;289(5):1207. doi: 10.1006/jmbi.1999.2847. [DOI] [PubMed] [Google Scholar]
- 336.Higuchi K, Katayama T, Iwai S, Hidaka M, Horiuchi T, Maki H. Fate of DNA replication fork encountering a single DNA lesion during oriC plasmid DNA replication in vitro. Genes to Cells. 2003;8(5):437. doi: 10.1046/j.1365-2443.2003.00646.x. [DOI] [PubMed] [Google Scholar]
- 337.McEntee K. Protein X is the product of the recA gene of Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America. 1977;74(12):5275. doi: 10.1073/pnas.74.12.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.McEntee K, Weinstock GM, Lehman IR. Initiation of general recombination catalyzed in vitro by the RecA protein of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 1979;76(6):2615. doi: 10.1073/pnas.76.6.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Shibata T, Cunningham RP, Das Gupta C, Radding CM. Homologous pairing in genetic recombination: complexes of RecA protein and DNA. Proc. Natl. Acad. Sci. U.S.A. 1979;76(10):5100. doi: 10.1073/pnas.76.10.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Shibata T, Das Gupta C, Cunningham RP, Radding CM. Purified Escherichia coli RecA protein catalyzes homologous pairing of superhelical DNA and single-stranded fragments. Proc. Natl. Acad. Sci. U.S.A. 1979;76(4):1638. doi: 10.1073/pnas.76.4.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Roberts JW, Roberts CW, Craig NL. Escherichia coli recA gene product inactivates phage lambda repressor. Proceedings of the National Academy of Sciences of the United States of America. 1978;75(10):4714. doi: 10.1073/pnas.75.10.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Roberts JW, Roberts CW, Craig NL, Phizicky EM. Activity of the Escherichia coli recA-gene product. Cold Spring Harbor Symp. Quant. Biol. 1979;43:917. doi: 10.1101/sqb.1979.043.01.100. [DOI] [PubMed] [Google Scholar]
- 343.Witkin EM. RecA protein in the SOS response: milestones and mysteries. Biochimie. 1991;73(2−3):133. doi: 10.1016/0300-9084(91)90196-8. [DOI] [PubMed] [Google Scholar]
- 344.Witkin EM, Kogoma T. Involvement of the activated form of RecA protein in SOS mutagenesis and stable DNA replication in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America. 1984;81(23):7539. doi: 10.1073/pnas.81.23.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Witkin EM, McCall JO, Volkert MR, Wermundsen IE. Constitutive expression of SOS functions and modulation of mutagenesis resulting from resolution of genetic instability at or near the recA locus of Escherichia coli. Mol. Gen. Genet. 1982;185(1):43. doi: 10.1007/BF00333788. [DOI] [PubMed] [Google Scholar]
- 346.Cox MM, Lehman IR. recA protein of Escherichia coli promotes branch migration, a kinetically distinct phase of DNA strand exchange. Proc Natl Acad Sci U S A. 1981;78(6):3433. doi: 10.1073/pnas.78.6.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Cunningham RP, Wu AM, Shibata T, Das Gupta C, Radding CM. Homologous pairing and topological linkage of DNA molecules by combined action of E. coli RecA protein and topoisomerase I. Cell. 1981;24(1):213. doi: 10.1016/0092-8674(81)90517-1. [DOI] [PubMed] [Google Scholar]
- 348.West SC, Cassuto E, Howard-Flanders P. RecA protein promotes homologous-pairing and strand-exchange reactions between duplex DNA molecules. Proc. Natl. Acad. Sci. U.S.A. 1981;78(4):2100. doi: 10.1073/pnas.78.4.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Little JW. Autodigestion of LexA and phage lambda repressors. Proc. Natl. Acad. Sci. U.S.A. 1984;81(5):1375. doi: 10.1073/pnas.81.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Little JW. Mechanism of specific LexA cleavage - autodigestion and the role of RecA coprotease. Biochimie. 1991;73(4):411. doi: 10.1016/0300-9084(91)90108-d. [DOI] [PubMed] [Google Scholar]
- 351.Cox MM, Soltis DA, Livneh Z, Lehman IR. On the role of single-stranded DNA binding protein in recA protein-promoted DNA strand exchange. J Biol Chem. 1983;258(4):2577. [PubMed] [Google Scholar]
- 352.Kowalczykowski SC, Clow J, Somani R, Varghese A. Effects of the Escherichia coli SSB protein on the binding of Escherichia coli RecA protein to single-stranded DNA. Demonstration of competitive binding and the lack of a specific protein-protein interaction. J Mol Biol. 1987;193(1):81. doi: 10.1016/0022-2836(87)90629-2. [DOI] [PubMed] [Google Scholar]
- 353.Lieberman HB, Witkin EM. Variable Expression of the Ssb-1 Allele in Different Strains of Escherichia-Coli-K12 and B - Differential Suppression of Its Effects on DNA-Replication, DNA-Repair and Ultraviolet Mutagenesis. Molecular & General Genetics. 1981;183(2):348. doi: 10.1007/BF00270639. [DOI] [PubMed] [Google Scholar]
- 354.Lieberman HB, Witkin EM. DNA degradation, UV sensitivity and SOS-mediated mutagenesis in strains of Escherichia coli deficient in single-strand DNA binding protein: effects of mutations and treatments that alter levels of exonuclease V or RecA protein. Mol. Gen. Genet. 1983;190(1):92. doi: 10.1007/BF00330329. [DOI] [PubMed] [Google Scholar]
- 355.McEntee K, Weinstock GM, Lehman IR. recA protein-catalyzed strand assimilation: stimulation by Escherichia coli single-stranded DNA-binding protein. Proc Natl Acad Sci U S A. 1980;77(2):857. doi: 10.1073/pnas.77.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.West SC, Cassuto E, Howard-Flanders P. Role of SSB protein in RecA promoted branch migration reactions. Mol. Gen. Genet. 1982;186(3):333. doi: 10.1007/BF00729451. [DOI] [PubMed] [Google Scholar]
- 357.Dri AM, Moreau PL. Properties of RecA441 protein reveal a possible role for RecF and SSB proteins in Escherichia coli. Mol Gen Genet. 1991;227(3):488. doi: 10.1007/BF00273942. [DOI] [PubMed] [Google Scholar]
- 358.Moreau PL. Overproduction of single-stranded-DNA-binding protein specifically inhibits recombination of UV-irradiated bacteriophage DNA in Escherichia coli. J. Bacteriol. 1988;170(6):2493. doi: 10.1128/jb.170.6.2493-2500.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Williams KR, Murphy JB, Chase JW. Characterization of the structural and functional defect in the Escherichia coli single-stranded DNA binding protein encoded by the ssb-1 mutant gene. Expression of the ssb-1 gene under lambda pL regulation. J Biol Chem. 1984;259(19):11804. [PubMed] [Google Scholar]
- 360.Bujalowski W, Lohman TM. Monomer-tetramer equilibrium of the Escherichia coli ssb-1 mutant single strand binding protein. J Biol Chem. 1991;266(3):1616. [PubMed] [Google Scholar]
- 361.Bujalowski W, Lohman TM. Monomers of the Escherichia coli SSB-1 mutant protein bind single-stranded DNA. J Mol Biol. 1991;217(1):63. doi: 10.1016/0022-2836(91)90611-9. [DOI] [PubMed] [Google Scholar]
- 362.Tessman ES, Peterson PK. Suppression of the ssb-1 and ssb-113 mutations of Escherichia coli by a wild-type rep gene, NaCl, and glucose. J Bacteriol. 1982;152(2):572. doi: 10.1128/jb.152.2.572-583.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Galletto R, Amitani I, Baskin RJ, Kowalczykowski SC. Direct observation of individual RecA filaments assembling on single DNA molecules. Nature. 2006;443(7113):875. doi: 10.1038/nature05197. [DOI] [PubMed] [Google Scholar]
- 364.Joo C, McKinney SA, Nakamura M, Rasnik I, Myong S, Ha T. Real-time observation of RecA filament dynamics with single monomer resolution. Cell. 2006;126(3):515. doi: 10.1016/j.cell.2006.06.042. [DOI] [PubMed] [Google Scholar]
- 365.Register JC, III, Griffith J. The direction of RecA protein assembly onto single strand DNA is the same as the direction of strand assimilation during strand exchange. J. Biol. Chem. 1985;260(22):12308. [PubMed] [Google Scholar]
- 366.Arenson TA, Tsodikov OV, Cox MM. Quantitative analysis of the kinetics of end-dependent disassembly of RecA filaments from ssDNA. Journal of Molecular Biology. 1999;288(3):391. doi: 10.1006/jmbi.1999.2705. [DOI] [PubMed] [Google Scholar]
- 367.Bork JM, Cox MM, Inman RB. RecA protein filaments disassemble in the 5 ' to 3 ' direction on single-stranded DNA. Journal of Biological Chemistry. 2001;276(49):45740. doi: 10.1074/jbc.M109247200. [DOI] [PubMed] [Google Scholar]
- 368.Lavery PE, Kowalczykowski SC. Properties of recA441 protein-catalyzed DNA strand exchange can be attributed to an enhanced ability to compete with SSB protein. J Biol Chem. 1990;265(7):4004. [PubMed] [Google Scholar]
- 369.Ennis DG, Levine AS, Koch WH, Woodgate R. Analysis of recA mutants with altered SOS functions. Mutation Research. 1995;336(1):39. doi: 10.1016/0921-8777(94)00045-8. [DOI] [PubMed] [Google Scholar]
- 370.Madiraju MV, Templin A, Clark AJ. Properties of a mutant recA-encoded protein reveal a possible role for Escherichia coli recF-encoded protein in genetic recombination. Proc. Natl. Acad. Sci. U.S.A. 1988;85(18):6592. doi: 10.1073/pnas.85.18.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Wang TC, Smith KC. recA (Srf) suppression of recF deficiency in the postreplication repair of UV-irradiated Escherichia coli K-12. J Bacteriol. 1986;168(2):940. doi: 10.1128/jb.168.2.940-946.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Wang TCV, Madiraju MVVS, Templin A, Clark AJ. Cloning and preliminary characterization of srf-2020 and srf-801, the recF partial suppressor mutations which map in recA of Escherichia coli K-12. Biochimie. 1991;73(2−3):335. doi: 10.1016/0300-9084(91)90221-l. [DOI] [PubMed] [Google Scholar]
- 373.Knight KL, Aoki KH, Ujita EL, McEntee K. Identification of the amino acid substitutions in two mutant forms of the RecA protein from Escherichia coli: RecA441 and RecA629. J. Biol. Chem. 1984;259(18):11279. [PubMed] [Google Scholar]
- 374.Thomas A, Lloyd RG. Control of recA dependent activities in Escherichia coli: a possible role for the recF product. J Gen Microbiol. 1983;129(Pt 3):681. doi: 10.1099/00221287-129-3-681. [DOI] [PubMed] [Google Scholar]
- 375.Volkert MR, Margossian LJ, Clark AJ. Two-component suppression of recF143 by recA441 in Escherichia coli K-12. Journal of Bacteriology. 1984;160(2):702. doi: 10.1128/jb.160.2.702-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Drees JC, Lusetti SL, Chitteni-Pattu S, Inman RB, Cox MM. A RecA filament capping mechanism for RecX protein. Mol Cell. 2004;15(5):789. doi: 10.1016/j.molcel.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 377.Drees JC, Lusetti SL, Cox MM. Inhibition of RecA protein by the Escherichia coli RecX protein: modulation by the RecA C terminus and filament functional state. J Biol Chem. 2004;279(51):52991. doi: 10.1074/jbc.M409050200. [DOI] [PubMed] [Google Scholar]
- 378.Eggler AL, Lusetti SL, Cox MM. The C terminus of the Escherichia coli RecA protein modulates the DNA binding competition with single-stranded DNA-binding protein. J Biol Chem. 2003;278(18):16389. doi: 10.1074/jbc.M212920200. [DOI] [PubMed] [Google Scholar]
- 379.Lusetti SL, Shaw JJ, Cox MM. Magnesium ion-dependent activation of the RecA protein involves the C terminus. J Biol Chem. 2003;278(18):16381. doi: 10.1074/jbc.M212916200. [DOI] [PubMed] [Google Scholar]
- 380.Lusetti SL, Wood EA, Fleming CD, Modica MJ, Korth J, Abbott L, Dwyer DW, Roca AI, Inman RB, Cox MM. C-terminal deletions of the Escherichia coli RecA protein. Characterization of in vivo and in vitro effects. J Biol Chem. 2003;278(18):16372. doi: 10.1074/jbc.M212917200. [DOI] [PubMed] [Google Scholar]
- 381.Kurumizaka H, Aihara H, Ikawa S, Kashima T, Bazemore LR, Kawasaki K, Sarai A, Radding CM, Shibata T. A possible role of the C-terminal domain of the RecA protein. A gateway model for double-stranded DNA binding. J Biol Chem. 1996;271(52):33515. doi: 10.1074/jbc.271.52.33515. [DOI] [PubMed] [Google Scholar]
- 382.Yu X, Egelman EH. Removal of the RecA C-terminus results in a conformational change in the RecA-DNA filament. J Struct Biol. 1991;106(3):243. doi: 10.1016/1047-8477(91)90074-7. [DOI] [PubMed] [Google Scholar]
- 383.Cox MM, Lehman IR. Enzymes of general recombination. Annu Rev Biochem. 1987;56:229. doi: 10.1146/annurev.bi.56.070187.001305. [DOI] [PubMed] [Google Scholar]
- 384.Morrical SW, Lee J, Cox MM. Continuous association of Escherichia coli single-stranded DNA binding protein with stable complexes of recA protein and single-stranded DNA. Biochemistry. 1986;25(7):1482. doi: 10.1021/bi00355a003. [DOI] [PubMed] [Google Scholar]
- 385.Flory SS, Tsang J, Muniyappa K, Bianchi M, Gonda D, Kahn R, Azhderian E, Egner C, Shaner S, Radding CM. Intermediates in homologous pairing promoted by RecA protein and correlations of recombination in vitro and in vivo. Cold Spring Harb Symp Quant Biol. 1984;49:513. doi: 10.1101/sqb.1984.049.01.058. [DOI] [PubMed] [Google Scholar]
- 386.Muniyappa K, Shaner SL, Tsang SS, Radding CM. Mechanism of the concerted action of recA protein and helix-destabilizing proteins in homologous recombination. Proc Natl Acad Sci U S A. 1984;81(9):2757. doi: 10.1073/pnas.81.9.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Cox JM, Abbott SN, Chitteni-Pattu S, Inman RB, Cox MM. Complementation of one RecA protein point mutation by another. Evidence for trans catalysis of ATP hydrolysis. J Biol Chem. 2006;281(18):12968. doi: 10.1074/jbc.M513736200. [DOI] [PubMed] [Google Scholar]
- 388.Lusetti SL, Cox MM. The bacterial RecA protein and the recombinational DNA repair of stalled replication forks. Annu Rev Biochem. 2002;71:71. doi: 10.1146/annurev.biochem.71.083101.133940. [DOI] [PubMed] [Google Scholar]
- 389.Lohman TM, Bujalowski W, Overman LB. E. coli single strand binding protein: a new look at helix-destabilizing proteins. Trends Biochem Sci. 1988;13(7):250. [PubMed] [Google Scholar]
- 390.Muniyappa K, Williams K, Chase JW, Radding CM. Active nucleoprotein filaments of single-stranded binding protein and recA protein on single-stranded DNA have a regular repeating structure. Nucleic Acids Res. 1990;18(13):3967. doi: 10.1093/nar/18.13.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Golub EI, Gupta RC, Haaf T, Wold MS, Radding CM. Interaction of human rad51 recombination protein with single-stranded DNA binding protein, RPA. Nucleic Acids Res. 1998;26(23):5388. doi: 10.1093/nar/26.23.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Egner C, Azhderian E, Tsang SS, Radding CM, Chase JW. Effects of various single-stranded-DNA-binding proteins on reactions promoted by RecA protein. J. Bacteriol. 1987;169(8):3422. doi: 10.1128/jb.169.8.3422-3428.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Namsaraev EA, Berg P. Rad51 uses one mechanism to drive DNA strand exchange in both directions. Journal of Biological Chemistry. 2000;275(6):3970. doi: 10.1074/jbc.275.6.3970. [DOI] [PubMed] [Google Scholar]
- 394.Lavery PE, Kowalczykowski SC. A postsynaptic role for single-stranded DNA-binding protein in recA protein-promoted DNA strand exchange. J Biol Chem. 1992;267(13):9315. [PubMed] [Google Scholar]
- 395.Cox JM, Tsodikov OV, Cox MM. Organized unidirectional waves of ATP hydrolysis within a RecA filament. PLoS Biology. 2005;3(2):231. doi: 10.1371/journal.pbio.0030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Lee JW, Cox MM. Inhibition of RecA protein-promoted ATP hydrolysis. II. Longitudinal assembly and disassembly of RecA protein filaments mediated by ATP and ADP. Biochemistry. 1990;29(33):7677. doi: 10.1021/bi00485a017. [DOI] [PubMed] [Google Scholar]
- 397.Pugh BF, Cox MM. RecA protein binding to the heteroduplex product of DNA strand exchange. J. Biol. Chem. 1987;262(3):1337. [PubMed] [Google Scholar]
- 398.Kozlov AG, Lohman TM. Kinetic mechanism of direct transfer of Escherichia coli SSB tetramers between single-stranded DNA molecules. Biochemistry. 2002;41(39):11611. doi: 10.1021/bi020361m. [DOI] [PubMed] [Google Scholar]
- 399.Reddy MS, Guhan N, Muniyappa K. Characterization of single-stranded DNA-binding proteins from Mycobacteria. The carboxyl-terminal of domain of SSB is essential for stable association with its cognate RecA protein. J Biol Chem. 2001;276(49):45959. doi: 10.1074/jbc.M103523200. [DOI] [PubMed] [Google Scholar]
- 400.Beernink HT, Morrical SW. RMPs: recombination/replication mediator proteins. Trends Biochem Sci. 1999;24(10):385. doi: 10.1016/s0968-0004(99)01451-6. [DOI] [PubMed] [Google Scholar]
- 401.Gasior SL, Olivares H, Ear U, Hari DM, Weichselbaum R, Bishop DK. Assembly of RecA-like recombinases: Distinct roles for mediator proteins in mitosis and meiosis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(15):8411. doi: 10.1073/pnas.121046198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Song B, Sung P. Functional interactions among yeast Rad51, recombinase, Rad52 mediator and replication protein A in DNA strand exchange. Journal of Biological Chemistry. 2000;275(21):15895. doi: 10.1074/jbc.M910244199. [DOI] [PubMed] [Google Scholar]
- 403.Sung P. Function of yeast Rad52 protein as a mediator between replication protein A and the Rad51 recombinase. Journal of Biological Chemistry. 1997;272(45):28194. doi: 10.1074/jbc.272.45.28194. [DOI] [PubMed] [Google Scholar]
- 404.Mahdi AA, Lloyd RG. The recR locus of Escherichia coli K-12: molecular cloning, DNA sequencing and identification of the gene product. Nucleic Acids Research. 1989;17(17):6781. doi: 10.1093/nar/17.17.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Sawitzke JA, Stahl FW. Phage lambda has an analog of Escherichia coli recO, recR and recF genes. Genetics. 1992;130(1):7. doi: 10.1093/genetics/130.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Sawitzke JA, Stahl FW. The phage lambda orf gene encodes a trans-acting factor that suppresses Escherichia coli recO, recR, and recF mutations for recombination of lambda but not of E. coli. Journal of Bacteriology. 1994;176(21):6730. doi: 10.1128/jb.176.21.6730-6737.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Rocha EPC, Cornet E, Michel B. Comparative and evolutionary analysis of the bacterial homologous recombination systems. PLoS Genetics. 2005;1(2):e15. doi: 10.1371/journal.pgen.0010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Honda M, Inoue J, Yoshimasu M, Ito Y, Shibata T, Mikawa T. Identification of the RecR Toprim domain as the binding site for both RecF and RecO. A role of RecR in RecFOR assembly at double-stranded DNA-single-stranded DNA junctions. J Biol Chem. 2006;281(27):18549. doi: 10.1074/jbc.M512658200. [DOI] [PubMed] [Google Scholar]
- 409.Sandler SJ. Overlapping functions for recF and priA in cell viability and UV-inducible SOS expression are distinguished by dnaC809 in Escherichia coli K-12. Molec. Microbiol. 1996;19(4):871. doi: 10.1046/j.1365-2958.1996.429959.x. [DOI] [PubMed] [Google Scholar]
- 410.Sandler SJ, Clark AJ. Use of high and low level overexpression plasmids to test mutant alleles of the recF gene of Escherichia coli K-12 for partial activity. Genetics. 1993;135(3):643. doi: 10.1093/genetics/135.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Sandler SJ, Clark AJ. Mutational analysis of sequences in the recF gene of Escherichia coli K-12 that affect expression. J Bacteriol. 1994;176(13):4011. doi: 10.1128/jb.176.13.4011-4016.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Koroleva O, Makharashvili N, Courcelle CT, Courcelle J, Korolev S. Structural conservation of RecF and Rad50: implications for DNA recognition and RecF function. Embo Journal. 2007;26(3):867. doi: 10.1038/sj.emboj.7601537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Webb BL, Cox MM, Inman RB. An interaction between the Escherichia coli RecF and RecR proteins dependent on ATP and double-stranded DNA. Journal of Biological Chemistry. 1995;270(52):31397. doi: 10.1074/jbc.270.52.31397. [DOI] [PubMed] [Google Scholar]
- 414.Webb BL, Cox MM, Inman RB. Recombinational DNA repair: the RecF and RecR proteins limit the extension of RecA filaments beyond single-strand DNA gaps. Cell. 1997;91(3):347. doi: 10.1016/s0092-8674(00)80418-3. [DOI] [PubMed] [Google Scholar]
- 415.Webb BL, Cox MM, Inman RB. ATP hydrolysis and DNA binding by the Escherichia coli RecF protein. J Biol Chem. 1999;274(22):15367. doi: 10.1074/jbc.274.22.15367. [DOI] [PubMed] [Google Scholar]
- 416.Griffin T.J.t., Kolodner RD. Purification and preliminary characterization of the Escherichia coli K-12 recF protein. Journal of Bacteriology. 1990;172(11):6291. doi: 10.1128/jb.172.11.6291-6299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Leiros I, Timmins J, Hall DR, McSweeney S. Crystal structure and DNA-binding analysis of RecO from Deinococcus radiodurans. Embo Journal. 2005;24(5):906. doi: 10.1038/sj.emboj.7600582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Makharashvili N, Koroleva O, Bera S, Grandgenett DP, Korolev S. A novel structure of DNA repair protein RecO from Deinococcus radiodurans. Structure. 2004;12(10):1881. doi: 10.1016/j.str.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 419.Alonso JC, Stiege AC, Dobrinski B, Lurz R. Purification and properties of the RecR protein from Bacillus subtilis 168. J Biol Chem. 1993;268(2):1424. [PubMed] [Google Scholar]
- 420.Lee BI, Kim KH, Park SJ, Eom SH, Song HK, Suh SW. Ring-shaped architecture of RecR: implications for its role in homologous recombinational DNA repair. EMBO J. 2004;23(10):2029. doi: 10.1038/sj.emboj.7600222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Lee BI, Kim KH, Shim SM, Ha KS, Yang JK, Yoon HJ, Ha JY, Suh SW. Crystallization and preliminary X-ray crystallographic analysis of the RecR protein from Deinococcus radiodurans, a member of the RecFOR DNA-repair pathway. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 2):379. doi: 10.1107/S0907444903028191. [DOI] [PubMed] [Google Scholar]
- 422.Timmins J, Leiros I, McSweeney S. Crystal structure and mutational study of RecOR provide insight into its mode of DNA binding. Embo Journal. 2007;26(13):3260. doi: 10.1038/sj.emboj.7601760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Lusetti SL, Hobbs MD, Stohl EA, Chitteni-Pattu S, Inman RB, Seifert HS, Cox MM. The RecF protein antagonizes RecX function via direct interaction. Mol Cell. 2006;21(1):41. doi: 10.1016/j.molcel.2005.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14(6):1188. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]