Abstract
A hallmark of eusociality in ants is the reproductive division of labor between queens and workers. Yet, nothing is known about the molecular mechanisms underlying reproduction in this group. We therefore compared the developmental genetic capacity of queens and workers to reproduce in several eusocially advanced species from the two largest subfamilies of ants, the Myrmicinae and Formicinae. In flies, the asymmetric localization of maternally encoded determinants (mRNAs and proteins) during oogenesis establishes oocyte polarity and subsequently ensures proper embryonic development. Vasa and nanos, two key maternal determinants, are properly localized in the posterior of queen oocytes, but their localization is impaired in those of the workers. This mislocalization leads to severe embryonic defects in worker progeny, and therefore, represents a constraint on worker reproduction that we call ‘reproductive constraint.’ We show that reproductive constraint is phylogenetically widespread, and is at high levels in most species tested. Reproductive constraint can simultaneously reduce or eliminate the workers' ability to produce viable eggs for reproduction, while preserving their ability to produce trophic eggs for nutrition, and thus, may have been the basis for the evolutionary retention of worker ovaries in the majority of ants. We propose that high levels of reproductive constraint has most likely evolved as a consequence of selection at the colony level to reduce or eliminate any potential conflict over worker reproduction, therefore maintaining harmony and colony efficiency in advanced ant societies.
Keywords: conflict, oogenesis, Vasa/nanos, worker reproduction
The reproductive division of labor in ants is a key feature of their eusocial organization. Workers in the vast majority of ant species, however, have retained ovaries and are thought to have significant reproductive potential (1–3). A major and widespread conflict will potentially arise in ant societies if workers selfishly engage in reproduction at the expense of colony tasks, such as foraging and brood rearing (4). An important challenge for evolutionary biology is to understand how highly social groups can reduce or prevent such costly conflicts from occurring. Although there exist a large body of theory to understand cooperation and conflict over reproduction in ants (5–11), little is known about the developmental and molecular mechanisms that control reproduction in queens and workers.
Reproduction in ants follows a haplo-diploid sex determination mechanism, whereby fertilized eggs develop into females, and unfertilized eggs develop into males (12). Workers in advanced ant societies cannot mate and have lost the spermatheca, and thus, they can only produce unfertilized eggs that develop either into males for reproduction (1–3) or trophic eggs for nutrition (12, 13). Hamilton's rule, which is based on both the genetic relatedness between individuals and the ecological benefits and costs these individuals incur, has been used to predict the potential for conflict over male production in ant societies (5). Because ecological costs and benefits are difficult to assess, relatedness alone has mainly been used to predict the potential for this conflict (3, 14). Relatedness predicts that in colonies headed by one queen that is singly mated, workers are more related to other workers' sons (nephews) than to the queen's sons (brothers), and therefore should rear their nephews over their brothers. Conversely, in colonies headed by multiple queens or a multiply mated queen, workers are more related to their brothers and therefore should refrain (through “self-restraint”) or prevent other workers (through “policing”) from reproducing. Empirical data, however, show that the actual levels of conflict over worker production of males in nature are much lower than expected (3, 14), as worker reproduction is usually reduced or eliminated in the majority of ant species. Because it is generally assumed that worker-produced eggs successfully develop into males (1, 2), the gap between actual and potential levels of this conflict has mainly been explained by behavioral control, such as self-restraint (15, 16) or policing (17–20).
Here, we tested the basic assumption that workers in advanced ant societies have a significant capacity to successfully produce males in three focal ant species: Aphaenogaster rudis, Myrmica americana, and Lasius niger. We examined the developmental genetic capacity of the workers in these species to perform oogenesis, as well as the capacity of their eggs to undergo proper embryonic development, both in the presence and in the absence of the queen. We uncovered a phylogenetically widespread developmental mechanism that can simultaneously reduce or eliminate the ability of the workers to produce viable eggs, while maintaining their ability to produce trophic eggs for nutrition.
Results
We first asked whether the process of oogenesis is generally conserved between ant queens and the fruit fly, Drosophila melanogaster. In Drosophila, oogenesis begins by the division of germ-line stem cells (GSCs), which give rise to cysts and then to egg chambers (21). The oocyte develops within the egg chamber, where it acquires its polarity through the precise localization of mRNAs and proteins known as maternal determinants (22, 23). Maternal determinants are produced by the nurse cells, and then transported to the oocyte where they are asymmetrically localized to the poles. Vasa and nanos, two highly conserved maternal determinants, are localized to the posterior pole of the oocyte. These molecules are necessary for patterning the posterior compartment of early embryos, and for specifying germ-line formation and differentiation (24, 25). Vasa mutant embryos lack the specialized posterior pole plasm and show impaired polarity (25), whereas nanos mutant embryos fail to form most of the abdomen altogether (24). We found that the cellular organization (Fig. 1 A and B) and the expression of Vasa protein (Figs. 1 and 2A) and nanos mRNA (Fig. 2B) are highly conserved in the ovarioles between ant queens and flies.
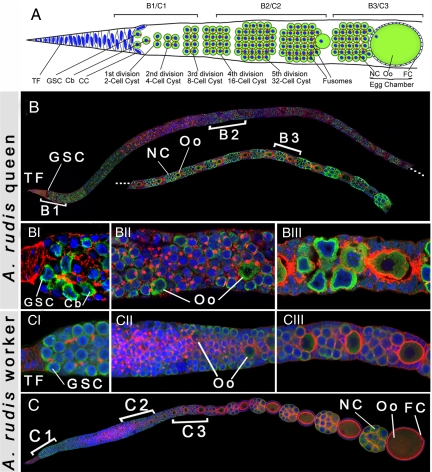
Fig. 1.
Cellular and molecular organization of queen and worker ovarioles in ants. Green color marks Vasa expression, red marks F-actin, and blue marks nuclei. (A) simplified schematic diagram showing the organization of the germ line in ant queen ovarioles. Germ-line stem cells (GSCs), located at the anterior tip of each adult ovariole, divide to give rise to cystoblasts (Cb), which in turn undergo five incomplete divisions to become 32-cell cysts. One cell acquires the oocyte fate whereas the remaining 31 cells become polyploid nurse cells. The cells forming each cyst are interconnected by fusomes; cytoskeletal bridges through which maternal determinants are transported from the nurse cells to the oocyte. Maternal determinants, including mRNAs and proteins, establish oocyte polarity and set up embryonic development. (B) A. rudis queen ovariole showing the anterior half from the terminal filament (TF) to early egg chambers. Dashed lines indicate the continuation of the ovariole. (C) A. rudis worker ovariole showing identical cellular and molecular organization to the queen. BI, BII, BIII, and CI, CII, CIII represent zooms of the regions indicated by B1, B2, B3, and C1, C2, C3, respectively. Both queens and workers show normal cystoblast production (B1 and C1), normal and continuous cyst production (B2 and C2), and proper establishment of oocyte identity and egg chamber formation (B3 and C3). Note that the ovariole of the queen (B) is longer than that of the worker (C), because of its higher activity. Cells from germ-line origin in both queens and workers, including GSC and their progeny, as well as the nurse cells express Vasa, whereas cells from somatic origin including TF, cap (CC), and follicle cells (FC) do not.
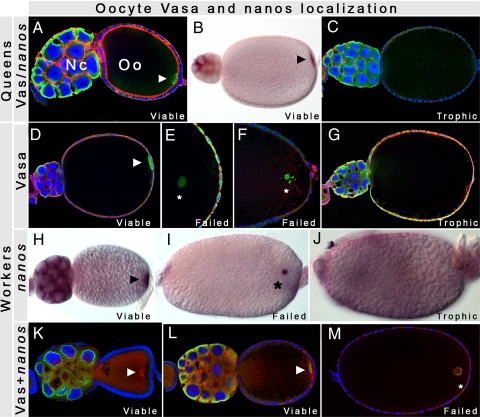
Fig. 2.
Patterns of Vasa and nanos expression and localization in queen and worker oocytes. Green color marks Vasa protein; red marks F-actin in A–G, and nanos mRNA in K–M; purple marks nanos mRNA in B and H–J; and blue marks nuclei. Arrow heads indicate correct localization, asterisks indicate impaired localization; NC nurse cells; Oo, oocyte. In queens, posterior localization of (A) Vasa and (B) nanos (red) indicate viable oocytes, whereas the lack of (C) Vasa localization indicate trophic oocytes. In workers, proper localization to the posterior of (D) Vasa and (H) nanos (purple) indicate viable oocytes; mis-localized (F) Vasa and (I) nanos (purple) indicate failed oocytes; and absence of (G) Vasa and (J) nanos (purple) indicate trophic oocytes. (K–M) double Vasa and nanos (red) in worker oocytes. (K) nanos is localized early in worker oocytes, followed later by the posterior (I) Vasa localization. (M) Both molecules remain colocalized while detaching from the posterior in late stages. Note that the yellow color in the follicle cells in E and G results from high background from the green channel, rather than Vasa expression in these cells.
We next examined whether the process of early oogenesis differs between queens (Fig. 1B) and workers (Fig. 1C) of the three species. Queen and worker castes of these species are determined environmentally sometime during larval development (12). As a result of caste determination, workers develop ovaries with a reduced number of ovarioles and no spermatheca (11). Using cellular and germ-line markers, however, we found that queens and workers show no difference in their division of GSCs (Fig. 1 B1 and C1), their production of cysts (Fig. 1 B2 and C2), as well as their establishment of oocyte identity (Fig. 1 B3 and C3).
We then asked whether there were any differences between queens and workers during later stages of oogenesis when oocytes acquire their polarity through the localization of maternal determinants. Queens and workers are known to produce both viable and trophic eggs (12, 13). Viable eggs undergo embryonic development, whereas trophic eggs are only used as a source of nutrition for the colony (12). Trophic egg production is particularly critical for young, newly mated, queens because it provides food to the growing colony. In queens and workers (Fig. 2) of all three species, we found two classes of oocytes based on their patterns of Vasa and nanos expression [see supporting information (SI) Figs. S1 and S2 and Table S1]. In the first class, Vasa and nanos were produced in the nurse cells, and localized to the posterior pole of the developing oocyte (Figs. 1B3 and 2 A, B, D, K, H, and L). This pattern, which is consistent with Vasa and nanos localization in Drosophila, indicates that these oocytes are destined to become embryos, and thus, we inferred this first class to be viable oocytes. In the second class, Vasa (Fig. 2 C and G and Fig. S1) and nanos (Fig. 2J and Fig. S2) were also produced by the nurse cells, transported to the oocyte, but their pattern appears diffuse throughout the ooplasm and are never localized to the posterior pole. This indicates that these oocytes lack the positional information necessary for further development, and are most likely destined to become food. We thus inferred this second class to be trophic oocytes.
We discovered, however, a unique class of oocytes in the workers that were never observed in the queens. In these oocytes, Vasa and nanos are produced in the nurse cells, transported to the oocyte posterior, but form aggregates (Fig. 2 H, K, and L) that detach from the cortex, disrupting proper localization (Figs. 2 E, F, I, and M and Figs. S1 and S2). We thus refer to this class as ‘failed’ oocytes. Unlike trophic oocytes, the posterior localization of Vasa and nanos in failed oocytes is initiated early, but not maintained in later stages (Fig. 2 K–M). This suggests that these oocytes are destined to reproduction, but fail in subsequent stages of development (Fig. 3). We interpret the presence of these oocytes as failed attempts by the workers to produce viable eggs.
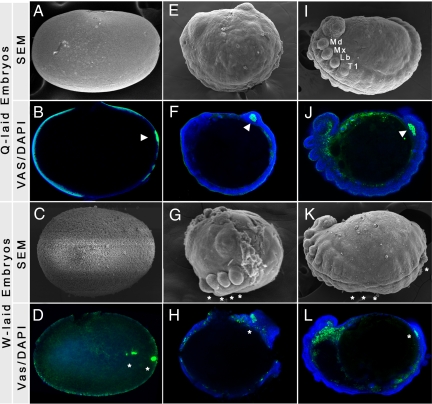
Fig. 3.
Molecular and morphological assessment of embryonic development in M. americana queen- and worker-laid eggs. Green marks Vasa protein; blue marks nuclei; arrow heads indicate normal structures, whereas asterisks indicate morphological or molecular defects. (A, E, and I) Embryos produced by the queen, shown at different developmental stages, undergo normal development. (B) Vasa protein, which is inherited from the mother during oogenesis, is properly localized in early embryos and is normally expressed in the germ line throughout development (F and J). Embryos produced by the workers show (C) disorganized cytoskeleton, as well as (G and K) severe axis defects during various stages of embryogenesis. (D) Worker embryos have inherited Vasa localization defects from their mothers, and fail to initiate or complete (H and L) subsequent embryogenesis stages.
To determine whether failed oocytes represent defects that are specific to either Vasa or nanos localization or a general defect in maintaining maternal determinants at the posterior cortex, we performed a double Vasa/nanos staining. nanos mRNA accumulation was detected first in early oocytes (Fig. 2K), followed later in development by posterior Vasa protein accumulation (Fig. 2L). When detached from the posterior pole of advanced oocyte stages (Fig. 2M), Vasa and nanos remained colocalized, suggesting that there is a general defect in maintaining maternal determinants at the posterior cortex. This impaired localization of maternal determinants in worker oocytes results in failed embryonic development (Fig. 3), and thus, represents a constraint on worker reproduction, which we call ‘reproductive constraint.’
We then asked to what degree does reproductive constraint reduce or eliminate worker reproduction, and whether or not it is triggered by the presence of the queen in the three species. In the presence of the queen (queenright), workers are potentially subject to behavioral control, whereas in her absence (orphaned) workers are not, and thus, should be able to express their full reproductive potential. Therefore, we calculated the degree of reproductive constraint as the number of failed oocytes divided by the total number of failed and viable oocytes produced by both queenright and orphaned workers. We excluded trophic oocytes because they are destined for food and not reproduction.
In L. niger, queenright and orphaned workers differ significantly in their attempts to produce viable or failed oocytes (P = 0.00065; Table S1). Queenright workers produced mostly trophic oocytes (75.6%, n = 62) and failed oocytes (23.2%, n = 19), whereas viable oocytes were seen only occasionally (1.2%, n = 1). In contrast, orphaned workers decreased their trophic oocyte production to 47.8% (n = 33) and increased their reproductive attempts, most of which were failed (50.7%, n = 35). In orphaned workers, oocytes degenerated inside the ovaries (data not shown), and these workers never succeed in laying any eggs at all. The presence of failed oocytes in queenright workers indicates that these workers do not restrain themselves from reproducing in the presence of the queen, but rather fail because of reproductive constraint. Reproductive constraint in L. niger was high, and does not differ significantly between queenright (95%) and orphaned (97%) workers (P = 1), and thus, is intrinsic to the workers and not triggered by the presence of the queen. Reproductive constraint eliminates the capacity of the workers to produce males in this species.
In M. americana, we found no significant difference between queenright and orphaned workers in their attempts to produce viable or failed oocytes (P = 0.70044; Table S1). The majority of oocytes produced by both queenright (82%, n = 64) and orphaned (71.8%, n = 74) workers were failed, whereas a minority were viable (15.4%, n = 12 in queenright and 23.3%, n = 24 in orphaned).
We found no major difference in trophic oocyte production between queenright (2.6%, n = 2) and orphaned workers (4.8%, n = 5). To test whether failed oocytes will result in failed embryos, we assessed the condition of eggs laid by orphaned workers (Fig. 3 and Table S2). Indeed, 93.5% of orphaned worker-laid embryos failed to develop properly (Fig. 3). Many of these embryos were arrested early during the syncytium stage with abnormalities in external morphology indicating cytoskeleton defects (compare Fig. 3 A and C) and Vasa mislocalization (compare Fig. 3 B and D). Other embryos were arrested with a variety of defects, such as in axis or segment formation, during later developmental stages (Fig. 3 G, H, K, and L). This indicates that oogenesis and embryogenesis in the workers are highly correlated. Reproductive constraint in M. americana was relatively high, and does not differ significantly (P = 0.188) between queenright (84%) and orphaned workers (75.5%). Thus, reproductive constraint is the major mechanism through which worker reproduction is suppressed, and behavioral control may be low in this species.
In A. rudis (Table S1), queenright workers exclusively produced trophic oocytes (100%, n = 245), indicating that these workers do not attempt to reproduce in the presence of the queen. Orphaned workers, however, produced high numbers of viable (28%, n = 72) relative to failed (3.5%, n = 9) oocytes in addition to trophic oocytes (68.5%, n = 176). We again assessed the condition of embryos produced by orphaned workers (Table S2 and Fig. S3). The majority of orphaned worker-laid eggs (85.7%) developed normally, whereas a small fraction (4.9%) initiated embryogenesis, but were arrested with general morphological defects after gastrulation (Table S2 and Fig. S3). This confirms the high correlation between the defects observed during oogenesis and embryogenesis. Reproductive constraint is not the principal mechanism for suppressing worker reproduction in A. rudis because: (i) in the presence of the queen, workers did not produce any viable or failed oocytes; and (ii) in her absence they produced high numbers of viable, but low numbers of failed oocytes. Therefore, behavioral control, most likely in the form of policing, could be the principal mechanism for suppressing worker reproduction in this species (19, 20).
Discussion
We introduce reproductive constraint as a developmental mechanism that can reduce or eliminate a worker's capability to produce viable eggs for reproduction, while maintaining its ability to produce trophic eggs for nutrition. This mechanism acts through the mislocalization of maternal determinants in worker oocytes. The developmental genetic basis of reproductive constraint is likely to be found upstream of Vasa and nanos during earlier oocyte patterning steps, because both Vasa and nanos are expressed throughout worker oogenesis, but their disruption occurs only during the process of localization. Maternal determinants in insects are transported through microtubules to oocyte poles where they are anchored to the cytoskeleton (26). Therefore, in trophic oocytes, absence of localization is most likely because of defects in microtubules, whereas in failed oocytes, mislocalization may be associated to defects in the cytoskeleton. Indeed, disruption of microtubules and cytoskeleton in other insects (27–29) results in similar localization defects we found to occur naturally in the workers of ants.
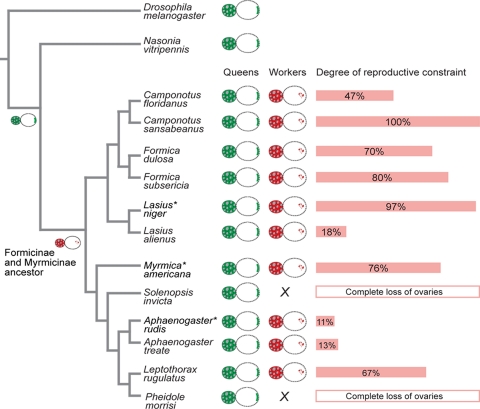
We surveyed 10 species from the two largest subfamilies of ants, the Myrmicinae and Formicinae. We found that reproductive constraint is present in all of the species tested (Fig. 4), indicating that it is a general mechanism in advanced ant societies. The level of reproductive constraint is high in the majority of species tested, although it can evolve dramatically between sister species, such as L. niger and L. alienus (Table S3 and Fig. 4), indicating that it is evolutionarily dynamic. Furthermore, our finding that reproductive constraint is intrinsic to the workers and not influenced by behavioral control, suggests that it may work in concert with behavioral control to reduce or eliminate worker reproduction. In species where reproductive constraint is high, such as L. niger or M. americana, behavioral control is likely to be weak or absent. Conversely, in species where reproductive constraint is low, such as A. rudis, behavioral control is likely to be strong. An illuminating case is that of Camponotus floridanus (Fig. 4), where worker policing is known to occur (16), and where we found intermediate levels of reproductive constraint (Fig. 4). The occurrence of both of these mechanisms in C. floridanus reinforces our conclusion that behavioral control and reproductive constraint may be complimentary forces for resolving conflicts over worker reproduction in ant societies.
Fig. 4.
The evolutionary dynamic of reproductive constraint in ants. Phylogenetic relationships were based on ref. 36. The asterisks indicate the three focal species. The unconstrained reproduction of solitary insects and ant queens is represented by a green oocyte diagram showing proper localization of maternal determinants. The constrained reproduction in the workers of several ant species within the formicines and myrmicines is represented by a red oocyte diagram showing impaired maternal determinant localization. The oocyte diagram at particular nodes represents the likely state of the ancestor of formicines and myrmicines, and of hymenopterans. The length of the red bars reflects the level and percentage of constraint, which was calculated as the number of failed oocytes divided by the total number of failed and viable oocytes produced by orphaned workers. Trophic oocytes were excluded.
Reproductive constraint can render workers that have retained ovaries nearly or fully sterile, thereby reducing or eliminating the risk of within group selfish behavior via worker production of males (30, 31), while retaining the benefit of cooperative sib-care through trophic egg production (13, 32). Therefore, reproductive constraint may have evolved in response to strong selection on worker ovaries for producing trophic eggs but against producing males. This has most likely facilitated the evolutionary retention and cooption of worker reproductive organs for nutritional but not reproductive purposes. This interpretation is consistent with the fact that only nine of the 283 currently known ant genera have completely lost ovaries (12, 33).
Finally, reproductive constraint may have important implications for understanding the evolutionary forces that govern cooperation and conflict in advanced ant societies. We propose that the appearance of high levels of reproductive constraint in the species we sampled may be a consequence of colony-level selection to reduce or eliminate the cost associated with worker reproduction. Indeed, colony efficiency is compromised when workers abandon their tasks and engage in reproductive activity (4). This interpretation is compatible with Hamilton's rule (5), and implies that costs and benefits are equal to, or more dominant than genetic relatedness in governing the conflict over male production. Relatedness alone is not sufficient to explain the evolution of reproductive constraint. If we assume that worker reproduction is cost-free, then workers should always be selected to produce males, because workers are always more closely related to their own sons than they are to their nephews or brothers. However, this prediction becomes invalid in the presence of high levels of reproductive constraint, because it reduces or eliminates worker reproduction in the first place. Furthermore, reproductive constraint occurs in all species we sampled regardless of their relatedness structure (Fig. 4). Alternatively, reproductive constraint may have evolved to help bias the sex ratio toward female production (34) in the case where: (i) workers are more closely related to their sisters than they are to their brothers or nephews; and (ii) the cost of eliminating males during early stages through reproductive constraint is lower than removing males during larval or pupal stages. However, this is not likely because reproductive constraint does not appear to be restricted only to species that may follow this particular case (Fig. 4). A further understanding of both sociogenetic and ecological conditions that favor the evolution of reproductive constraint will require testing for reproductive constraint in both ancestral and derived species where relatedness, behavioral control mechanisms, and colony-size have been well documented.
Materials and Methods
Ants.
A. rudis and L. niger colonies were collected at Montreal, Canada and M. americana colonies were collected in Medford, NY. The rest of the species in Fig. 4 were collected in Arizona, Florida, and New York. Colonies were maintained at 27°C, 70% humidity and a 12:12-h day:night cycle, and were fed on a combination of Crickets and Bachtar-Whitcomb diet. Three replicates of orphaned workers from each species were isolated for two to six months, and their egg production monitored. Orphaned A. rudis and M. americana workers were able to produce eggs, larvae, and adult males, but orphaned L. niger workers were not. Queenright and orphaned worker ovaries were then dissected and the number of viable, failed and trophic oocytes (as determined by Vasa localization) was counted. All percentages presented in the result section are the mean percentage for each three replicates.
Statistical Analysis.
We performed a Fisher's exact test (35) to determine: (i) if there is a significant difference in reproductive attempts between queenright and orphaned workers by comparing the frequency of trophic versus non trophic oocytes (viable plus failed) produced by these workers; and (ii) if there is a significant difference in reproductive constraint between queenright and orphaned workers by comparing the frequency of failed versus viable oocytes produced by these workers. We could not perform the test for A. rudis because queenright workers produced no reproductive oocytes (Table S1).
Nanos Cloning.
The nanos gene was cloned using degenerate and RACE PCR (Invitrogen) using the following primers: forward 5′-TGCGTWTTCTGYARAAATAA-3′ and reverse 5′-GGRCAATACTTKAYCGTRTGAG-3′ for degenerate PCR and Ant_nos Forward: 5′-TGATGGAAGAGTTTCGACACAATGG-3′ for RACE PCR. GenBank accession numbers for nanos sequences: A. rudis nanos, EU272791; L. niger nanos, EU272792; M. americana nanos, EU272793.
Ovary and Embryo Fixation.
Ovaries were dissected in PTW (1× PBS; 0.05% Tween-20) and kept on ice during the dissection process. The peritoneal sheet covering each ovariole was removed by using fine forceps. The ovarioles were fixed in 4% paraformaldehyde (200 μl) supplemented with 10% DMSO (20 μl), and heptane (600 μl) for 20 min at room temperature. Fixed ovarioles were then washed three times in PBT (1× PBS; 0.3% Triton X-100) and processed for subsequent staining.
Embryos are boiled for 45 seconds in PBT, and then quickly placed on ice. The chorion and vitelline membrane were removed manually by using fine forceps. Embryos were then fixed in 4% formaldehyde and heptane for 20 min and then washed three times in PBT.
Vasa Staining.
A cross-reacting anti-Vasa antibody, which was raised against Drosophila Vasa (25), was used to detect the Vasa protein in ants. Fixed ovarioles or embryos were permeabilized in PBT (1× PBS; 1% Triton X-100) for 1 h at room temperature. Permeabilization is followed by blocking step in PAT (1× PBS; 1% Triton X-100 and 1% bovine serum albumin) for 1 h at room temperature. Ovarioles/embryos were then incubated with a rabbit anti-Vasa antibody at a 1:100 dilution in PTW overnight at 4°C. Ovarioles/embryos were washed from excess antibody five times 10 min at room temperature in PBT (0.3% Triton X-100), then blocked again in PAT for one hour. A secondary, goat Cy2-conjugated anti-rabbit antibody (Jackson ImmunoResearch) was used to detect the rabbit anti-Vasa antibody at a 1:300 dilution in PTW. Ovarioles/embryos were incubated with the secondary antibody for two hours at room temperature in PTW. DAPI and Phalloidin were added at the same time as the secondary antibody. The ovarioles were finally washed five times 10 min in PTW then in increasing concentrations of glycerol in PBS and mounted in glycerol/1× PBS (80%/20%).
Nanos in situ hybridization.
Nanos staining was performed by using DIG-labeled nanos RNA probes (Roche). Fixed ovarioles were incubated one hour at 58°C in a hybridization solution (50% formamide, 5× SSC pH 6.5, 50 μg/ml salmon sperm DNA, 50 μg/ml heparin, 0.1% Tween-20, and 0.3% SDS). Ovarioles were then hybridized over-night at 58°C with the nanos DIG-labeled probe in the hybridization solution. Ovarioles were washed five times in PBT, blocked one hour in PAT then incubated two hours at room temperature with the anti-DIG antibody, conjugate with alkaline phosphatase. nanos expression was revealed by using NBT/BCIP (purple, Roche) or Fast-red (red, Sigma).
Image capture was performed by using either 510 confocal or Axiovert Zeiss microscopes. Large samples, which do not fit in a 40× or 20× objective field were assembled from multiple files and processed by using Adobe Photoshop.
Supplementary Material
Acknowledgments.
We thank P. Lasko (McGill University) for providing the anti-Vasa antibody; A. Francoeur (Université du Québec à Chicoutimi, Saguenay, QC, Canada), R. Johnson (Arizona State University, Tempe, AZ), R. Sanwald (Medford, NY), and W. Tschinkel (Florida State University, Tallahassee, FL) for ant identification or collection; J.J. Boomsma, C. Desplan, M.B. Dijkstra, J. Heinze, B. Hölldobler, L. Nilson, D. Schoen, A. Vincent, E.O. Wilson, the members of the Abouheif, Nilson, Shoek, and Lasko labs, the Behavioral Ecology and Drosophila groups at McGill, and two anonymous reviewers for discussion or critical reading of the manuscript; and McGill University for permitting us to work on the Gault Nature Reserve. This work was supported by Natural Sciences and Engineering Research Council and Alfred P. Sloan funding (to E.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. EU272791, EU272792, and EU272793).
This article contains supporting information online at www.pnas.org/cgi/content/full/0807351105/DCSupplemental.
References
- 1.Bourke AFG. Worker reproduction in the higher eusocial hymenoptera. Q Rev Biol. 1988:291–311. [Google Scholar]
- 2.Ratnieks FLW, Foster KR, Wenseleers T. Conflict resolution in insect societies. Annu Rev Entomol. 2006;51:581–608. doi: 10.1146/annurev.ento.51.110104.151003. [DOI] [PubMed] [Google Scholar]
- 3.Wenseleers T, Ratnieks FL. Comparative analysis of worker reproduction and policing in eusocial hymenoptera supports relatedness theory. Am Nat. 2006;168:E163–179. doi: 10.1086/508619. [DOI] [PubMed] [Google Scholar]
- 4.Wenseleers T, Helantera H, Hart A, Ratnieks FL. Worker reproduction and policing in insect societies: An ESS analysis. J Evol Biol. 2004;17:1035–1047. doi: 10.1111/j.1420-9101.2004.00751.x. [DOI] [PubMed] [Google Scholar]
- 5.Bourke AFG, Franks NR. Social Evolution in Ants. Princeton: Princeton Univ Press; 1995. [Google Scholar]
- 6.Hamilton WD. The genetical evolution of social behaviour. J Theor Biol. 1964;7:1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 7.Korb J, Heinze J. Multilevel selection and social evolution of insect societies. Naturwissenschaften. 2004;91:291–304. doi: 10.1007/s00114-004-0529-5. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann L, Keller L, West S, Roze D. Group selection and kin selection: Two concepts but one process. Proc Natl Acad Sci USA. 2007;104:6736–6739. doi: 10.1073/pnas.0700662104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reeve HK, Holldobler B. The emergence of a superorganism through intergroup competition. Proc Natl Acad Sci USA. 2007;104:9736–9740. doi: 10.1073/pnas.0703466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson EO. One giant leap: How insects achieved altruism and colonial life. BioScience. 2008;58:17–25. [Google Scholar]
- 11.Wilson EO, Holldobler B. Eusociality: Origin and consequences. Proc Natl Acad Sci USA. 2005;102:13367–13371. doi: 10.1073/pnas.0505858102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hölldobler B, Wilson EO. The Ants. Cambridge, Mass.: Belknap Press of Harvard Univ Press; 1990. [Google Scholar]
- 13.Gobin B, Ito F. Queens and major workers of Acanthomyrmex ferox redistribute nutrients with trophic eggs. Naturwissenschaften. 2000;87:323–326. doi: 10.1007/s001140050731. [DOI] [PubMed] [Google Scholar]
- 14.Hammond RL, Keller L. Conflict over male parentage in social insects. PLoS Biol. 2004;2:E248. doi: 10.1371/journal.pbio.0020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dijkstra MB, Nash DR, Boomsma JJ. Self-restraint and sterility in workers of Acromyrmex and Atta leafcutter ants. Insect Soc. 2005;52:67–76. [Google Scholar]
- 16.Endler A, et al. Surface hydrocarbons of queen eggs regulate worker reproduction in a social insect. Proc Natl Acad Sci USA. 2004;101:2945–2950. doi: 10.1073/pnas.0308447101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Ettorre P, Heinze J, Ratnieks FL. Worker policing by egg eating in the ponerine ant Pachycondyla inversa. Proc Biol Sci. 2004;271:1427–1434. doi: 10.1098/rspb.2004.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster KR, Ratnieks FL. Facultative worker policing in a wasp. Nature. 2000;407:692–693. doi: 10.1038/35037665. [DOI] [PubMed] [Google Scholar]
- 19.Hölldobler B, Carlin NF. Colony founding, queen control, and worker reproduction in the ant Aphaenogaster (=Novomessor) cockerelli (Hymenoptera: Formicidae) Psyche. 1989;96:131–151. [Google Scholar]
- 20.Iwanishi S, Hasegawa E, Ohkawara K. Worker oviposition and policing behaviour in the myrmicine ant Aphaenogaster smythiesi japonica Forel. Anim Behav. 2003;66:513–519. [Google Scholar]
- 21.van Eeden F, St Johnston D. The polarisation of the anterior–posterior and dorsal-ventral axes during Drosophila oogenesis. Curr Opin Genet Dev. 1999;9:396–404. doi: 10.1016/S0959-437X(99)80060-4. [DOI] [PubMed] [Google Scholar]
- 22.Nüsslein-Volhard C, Frohnhöfer HG, Lehmann R. Determination of anteroposterior polarity in Drosophila. Science. 1987;238:1675–1681. doi: 10.1126/science.3686007. [DOI] [PubMed] [Google Scholar]
- 23.Riechmann V, Ephrussi A. Axis formation during Drosophila oogenesis. Curr Opin Genet Dev. 2001;11:374–383. doi: 10.1016/s0959-437x(00)00207-0. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann R, Nüsslein-Volhard C. The maternal gene nanos has a central role in posterior pattern formation of the Drosophila embryo. Development. 1991;112:679–691. doi: 10.1242/dev.112.3.679. [DOI] [PubMed] [Google Scholar]
- 25.Styhler S, Nakamura A, Swan A, Suter B, Lasko P. vasa is required for GURKEN accumulation in the oocyte, and is involved in oocyte differentiation and germline cyst development. Development. 1998;125:1569–1578. doi: 10.1242/dev.125.9.1569. [DOI] [PubMed] [Google Scholar]
- 26.Ephrussi A, Lehmann R. Induction of germ cell formation by oskar. Nature. 1992;358:387–392. doi: 10.1038/358387a0. [DOI] [PubMed] [Google Scholar]
- 27.Huynh JR, St Johnston D. The origin of asymmetry: Early polarisation of the Drosophila germline cyst and oocyte. Curr Biol. 2004;14:R438–449. doi: 10.1016/j.cub.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 28.Olesnicky EC, Desplan C. Distinct mechanisms for mRNA localization during embryonic axis specification in the wasp Nasonia. Dev Biol. 2007;306:134–142. doi: 10.1016/j.ydbio.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinhauer J, Kalderon D. Microtubule polarity and axis formation in the Drosophila oocyte. Dev Dyn. 2006;235:1455–1468. doi: 10.1002/dvdy.20770. [DOI] [PubMed] [Google Scholar]
- 30.Clutton-Brock TH, Vincent AC. Sexual selection and the potential reproductive rates of males and females. Nature. 1991;351:58–60. doi: 10.1038/351058a0. [DOI] [PubMed] [Google Scholar]
- 31.Dijkstra MB, Boomsma JJ. The economy of worker reproduction in Acromyrmex leafcutter ants. Animal Behaviour. 2007;47:519–529. [Google Scholar]
- 32.Wradlaw JC, Elmes GW. Trophic eggs laid by fertile Myrmica queens (Hymenoptera: Formicidae) Insect Soc. 1995;42:303–308. [Google Scholar]
- 33.Oster GF, Wilson EO. Caste and ecology in the social insects. Monogr Popul Biol. 1978;12:1–352. [PubMed] [Google Scholar]
- 34.Foster KR, Ratnieks FL. Convergent evolution of worker policing by egg eating in the honeybee and common wasp. Proc Biol Sci. 2001;268:169–174. doi: 10.1098/rspb.2000.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sokal RR, Rohlf FJ. Biometry. New York: W. H. Freeman and Company; 1995. [Google Scholar]
- 36.Brady SG, Schultz TR, Fisher BL, Ward PS. Evaluating alternative hypotheses for the early evolution and diversification of ants. Proc Natl Acad Sci USA. 2006;103:18172–18177. doi: 10.1073/pnas.0605858103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.