Abstract
Bovine papillomavirus type 1 (BPV-1) induces fibropapillomas in its natural host and can transform fibroblasts in culture. The viral genome is maintained as an episome within fibroblasts, which has allowed extensive genetic analyses of the viral functions required for DNA replication, gene expression, and transformation. Much less is known about BPV-1 gene expression and replication in bovine epithelial cells because the study of the complete viral life cycle requires an experimental system capable of generating a fully differentiated stratified bovine epithelium. Using a combination of organotypic raft cultures and xenografts on nude mice, we have developed a system in which BPV-1 can replicate and produce infectious viral particles. Organotypic cultures were established with bovine keratinocytes plated on a collagen raft containing BPV-1-transformed fibroblasts. These keratinocytes were infected with virus particles isolated from a bovine wart or were transfected with cloned BPV-1 DNA. Several days after the rafts were lifted to the air interface, they were grafted on nude mice. After 6–8 weeks, large xenografts were produced that exhibited a hyperplastic and hyperkeratotic epithelium overlying a large dermal fibroma. These lesions were strikingly similar to a fibropapilloma caused by BPV-1 in the natural host. Amplified viral DNA and capsid antigens were detected in the suprabasal cells of the epithelium. Moreover, infectious virus particles could be isolated from these lesions and quantitated by a focus formation assay on mouse cells in culture. Interestingly, analysis of grafts produced with infected and uninfected fibroblasts indicated that the fibroma component was not required for productive infection or morphological changes characteristic of papillomavirus-infected epithelium. This system will be a powerful tool for the genetic analysis of the roles of the viral gene products in the complete viral life cycle.
Papillomaviruses induce proliferative epithelial lesions and can undergo vegetative replication only in terminally differentiated keratinocytes (reviewed in ref. 1). This fact has hampered the study of the viral life cycle because of the difficulties in generating a differentiated stratified epithelium in tissue culture. Considerable progress has been made in the production of human papillomaviruses (HPVs) in organotypic rafts in culture and in xenografts on immunodeficient mice (reviewed in refs. 2–4). Papillomaviruses are believed to infect basal epithelial cells via the α6β4 integrin receptor (5, 6). These cells proliferate, and the viral genome is maintained as an episome within the nucleus. As these cells differentiate and migrate upwards to the stratum spinosum, vegetative viral DNA replication begins. In the stratum granulosum, the next layer of stratified epithelium, the capsid antigens are expressed, and virion particles are assembled. The outermost layer of epithelium, the stratum corneum, contains virion particles that are sloughed from the epidermis.
Bovine papillomavirus type 1 (BPV-1) is a molecular prototype of the papillomaviruses, particularly in the study of viral DNA replication and transcriptional regulation (reviewed in ref. 1). However, little is known about the role of the viral gene products in the complete viral life cycle because of the restriction of vegetative DNA replication, late gene expression, and virion production to differentiated epithelial cells. The biology of BPV-1 has several features that may be advantageous in the development of a model system to study the lytic cycle. BPV-1 causes large productive lesions, and large quantities of BPV virions can be isolated from these lesions and used experimentally. BPV-1 causes fibropapillomas in its natural host. It is relatively easy to reproduce a fibroma in a mouse system (using BPV-1-transformed fibroblasts), and the fibroma component of the fibropapilloma may enhance the development of a productive epithelial infection. There are also a large number of BPV-1 mutations in specific gene functions that are available for genetic analysis of the complete viral life cycle. And finally, perhaps most useful, is the ability to quantitate infectious BPV-1 particles by using a focus formation assay in rodent cells (7). Using a combination of organotypic raft cultures and xenografts on nude mice, we have developed a system by which we can generate fully differentiated bovine epithelium in which BPV-1 can replicate and produce infectious viral particles. Furthermore, we can produce infectious BPV-1 from cloned viral DNA. Therefore this system will enable the genetic analysis of the complete viral life cycle.
Materials and Methods
Isolation of Bovine Skin, Keratinocytes, and Fibroblasts.
Thin sheets of fetal bovine skin used for grafting were removed by using a dermatome from the flanks of a second-trimester fetal calf (Pel-Freez Biologicals).
For culture, fetal keratinocytes were isolated from first-trimester fetal calves; sheets of epidermis from the flanks of the calf were floated dermis side down for 16 h at 4°C in a dish of 25 units/ml Dispase (Collaborative Research). The epithelial cells were gently scraped off, collected by centrifugation, and digested in 0.05% trypsin at 37°C for 10 min. Keratinocytes were cultured with irradiated Balb-3T3 feeder cells in F-medium (F12:DMEM, 3:1/5% FBS/0.4 μg/ml hydrocortisone/5 μg/ml insulin/8.4 ng/ml cholera toxin/10 ng/ml epidermal growth factor/24 μg/ml adenine). When the keratinocytes reached 70% confluence, the feeders were removed by differential trypsinization and the keratinocytes counted and frozen. Bovine dermal fibroblasts (BEF) were obtained by mincing pieces of bovine dermis and incubating them in 0.25% trypsin at 37°C for 15 min. Released fibroblasts were collected by centrifugation and cultured in DMEM/10% FBS.
BALB/c 3T3 A31 fibroblasts were obtained from American Tissue Culture Collection. BEF/BPV and BALB/BPV fibroblasts were obtained by infecting the respective fibroblasts with BPV-1 wart extract (see below).
Bovine Wart Extract and Tissue.
An extract of a BPV-1-infected wart was used as a source of BPV-1 virus. The epithelial portion of a frozen bovine wart was homogenized in 0.02 M Tris⋅HCl, pH 7.5/1.0 M NaCl/10 mM PMSF in a cooled Waring blender chamber. Cell debris was removed by centrifugation at 36,000 rpm in a Sorvall GSA rotor. The supernatant was stored at −70°C until use. The titer, as measured by using the C127 focus formation assay described below, was 4 × 106 infectious particles per milliliter. Bovine wart tissue was obtained from a cow inoculated with the sequenced isolate of BPV-1 by Carl Olsson (University of Wisconsin, Madison, WI).
Organotypic Raft Cultures.
The method used for organotypic raft cultures was derived from Dollard et al. (8). Collagen gels (1.5 ml; rat tail collagen type 1a, ×1 F12 media, 5% FBS) containing 3 × 105 fibroblasts were poured in a 12-well plate and covered with Raft medium (DMEM:F12, 3:1/10% FCS/0.4 μg/ml hydrocortisone/0.01 nM cholera toxin/5 μg/ml transferrin/0.5 ng/ml epidermal growth factor). After 5–16 h, 2 × 105 bovine keratinocytes were seeded on top of the raft. Keratinocytes were either transfected with viral DNA or infected with BPV-1 viral extract while still subconfluent. Keratinocytes were allowed to grow to confluence, at which time the collagen gel was raised to the air interface on either a metal grid or a Netwell (Corning Costar) in a 5% CO2 incubator at 37°C. Medium was changed every 24 h.
Grafting Bovine Skin or Rafts on Nude Mice.
Eight- to ten-week-old NIH Swiss nude mice (Tac:N:NIH(S)-nu/nu; Taconic Farms) were anesthetized, and a circular piece of skin the same size as the raft or graft was removed from the thoracic dorsum. Thin pieces of fetal bovine skin or organotypic rafts were placed directly on the exposed fascia. Rafts were grafted 4–5 days after being raised to the air interface. The graft was covered with Vaseline-impregnated gauze and dressed with Comfort Strip Band-Aids (3M Co.). The mice were checked frequently and the Band-Aids replaced as necessary. If grafts were exposed despite the dressing, a layer of Vaseline was applied daily. After 2 weeks, the Band-Aids were removed. Mice were typically euthanized by CO2 inhalation 6–10 weeks after grafting, and xenografts were excised and analyzed as described below.
Infection of Grafted Bovine Skin.
Two months after grafting, the surface of the xenograft was scarified, and 50 μl of wart extract was applied. The graft was bandaged for 1 week. Xenografts were analyzed 8 weeks after infection.
Infection and Transfection of Keratinocytes.
Cloned BPV-1 DNA (9) was cleaved from the prokaryotic vector and recircularized before transfection. Plasmid, p142–6, was digested with BamHI and religated at low concentration (5 μg/ml) to favor intramolecular recombination. DNA was introduced into keratinocytes by liposome-mediated transfection. Keratinocytes at 60% confluency were transfected on the collagen gel with 2 μg recircularized BPV-1 DNA and 6 μl Invitrogen lipid no. 6 in 1 ml of serum-free KGM (Life Technologies). The DNA/lipid mix was incubated on the raft for 3 to 4 h. The lipid mix was replaced with raft media. After 24 h, the rafts were lifted to the air interface. Keratinocytes at 60% confluency were infected on the collagen gel with 50 μl wart extract (2 × 105 focus-forming units).
Focus Formation Assay.
Small portions of xenografts were homogenized in DMEM by using a Tissumite homogenizer (IKA Works). Dishes (100 mm) of C127 cells were infected with the extract. Cells were cultured in DMEM/10% FBS and stained with methylene blue 2–3 weeks after transfection.
Histology, Immunohistochemistry, and in Situ Hybridization.
Xenografts were excised, fixed in formalin, and embedded in paraffin. Sections were stained with hematoxylin and eosin (H&E) for histological analysis. The L1 capsid antigen was detected by staining with an L1-specific antibody (MAB837, Chemicon) and the Vector ABC horseradish peroxidase detection kit (Vector Laboratories). Positive cells were identified by 3,3′-diaminobenzidine staining, and sections were counterstained with hematoxylin. For in situ hybridization, 4-μm-thick sections of formalin-fixed paraffin-embedded tissues were placed on positively charged slides. A digoxigenin (DIG)-labeled riboprobe transcribed from nucleotides 91–504 of the BPV genome was incubated on the slides in hybridization buffer (Qualtech Laboratories, Santa Barbara, CA) at a concentration of 0.7 ng/μl for 2 h at 54°C. Slides were washed in 2 × SSC (at 22°C), followed by 0.2 × SSC (at 54°C). Detection of the DIG riboprobes was with sheep anti-DIG conjugated to alkaline phosphatase, and 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium was used as the chromogen. The slides were counter stained with eosin.
Results
A Bovine Keratinocyte Organotypic Raft Culture System.
The aim of this study was to establish a system that would be amenable to the genetic analysis of the complete life cycle of BPV-1. The most straightforward way of doing this is to alter specific gene functions by mutation, transfect the mutated DNA into keratinocytes, and culture these keratinocytes in a system that supports differentiation and stratification of epithelial cells. Systems such as the organotypic raft culture system are proving useful for the genetic analysis of certain HPVs (reviewed in refs. 3 and 4). In these systems, a dermal equivalent consisting of a collagen matrix containing fibroblasts is established, and a keratinocyte monolayer is cultured on the surface. When the monolayer reaches confluency, the “raft” is raised on a grid to the air–liquid interface and cultured for several weeks. During this time, the keratinocytes differentiate and stratify. To establish a similar system for BPV-1, a method was developed to isolate bovine keratinocytes and fibroblasts (see Materials and Methods). Bovine keratinocytes could be cultured for several passages in FBS-containing medium in the presence of fibroblast feeder cells or in keratinocyte-specific serum-free medium. However, in the latter medium, they had to be cultured on collagen-coated flasks.
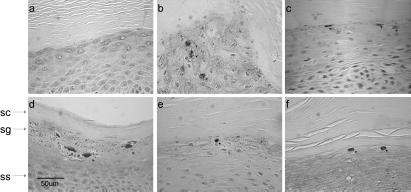
BPV-1 causes fibropapillomas in its natural host (10). The virus infects both epithelial cells and dermal fibroblasts; the infected fibroblasts proliferate and form a fibroma, but productive infection occurs only in the overlying epithelium. To mimic this situation in the organotypic raft system, we established fibroblast lines (from BALB/3T3 mouse cells and primary dermal bovine fibroblasts) that contained replicating episomal BPV-1 DNA. Organotypic rafts were established by using either BEF, BPV-1-containing bovine dermal fibroblasts (BEF/BPV), BALB/3T3 cells (BALB), or BALB/3T3 cells containing BPV-1 (BALB/BPV). Fig. 1 shows a time course of differentiation and stratification of bovine rafts on collagen containing mouse fibroblasts (no BPV). The bovine keratinocytes differentiated well, and all layers of epidermis could be identified. The lowermost basal layer (stratum basale) provides the germinal cells necessary for regeneration of the epidermis. In the next layer, desmosomes can be observed between cells, giving them a characteristic spiny appearance, hence this layer is designated the stratum spinosum. The cells of the stratum granulosum accumulate dense basophilic keratohyalin granules that contain proteins such as profilaggin, keratins, and loricrin. The outermost layer consists of dead cells filled with mature keratin. Differentiation of the bovine keratinocytes in organotypic rafts was comparable to that seen with human keratinocytes (11). The histological appearance of the raft seemed optimal approximately 8–10 days after it was lifted to the liquid–air interface. After 14 days, the cells equivalent to basal cells had deteriorated, and the raft decreased in thickness, perhaps because the differentiated keratinocytes were not replenished from the basal layer. There was little or no difference in appearance in rafts containing the four types of fibroblasts (data not shown).
Figure 1.
Time course of bovine organotypic raft development. Organotypic rafts were cultured by using mouse 3T3 fibroblasts and bovine keratinocytes. Samples were taken at the times shown after lifting the raft to the air–liquid interface. Sections of the rafts were stained with H&E. The stratum corneum (sc), stratum granulosum (sg), stratum spinosum (ss), and stratum basale (sb) are indicated by arrows.
It is often difficult to efficiently introduce DNA into early-passage keratinocytes by using established transfection procedures. A range of lipid reagents were tested for their ability to transfect a β-galactosidase reporter plasmid into primary bovine keratinocytes, and one reagent (Invitrogen Lipid no. 6) was found to have a transfection efficiency of 25–30%. On the basis of this efficiency, it was decided to transfect keratinocytes directly on the organotypic raft (see below) before stratification, rather than select for the transfected population of cells. BPV-1 did not appear to immortalize bovine keratinocytes, and preliminary studies indicated that BPV-1 genomes were not efficiently maintained under monolayer culture conditions (unpublished data). Therefore, our aim was to culture the infected/transfected cells under conditions that supported differentiation and stratification as soon as possible.
Preliminary studies in which the bovine organotypic rafts were infected with a crude extract of BPV-1 virus or transfected with BPV-1 DNA showed no evidence of productive papillomavirus infection. However, the organotypic raft is a transient stratification system (Fig. 1). In contrast, in a natural infection it is thought that a papilloma arises several weeks after the initial infection and persists for several months. Xenograft systems more closely approximate this time frame, but in most cases tissue fragments must be infected with a viral inoculum, as it is difficult to introduce DNA efficiently into tissue. For genetic analysis of papillomavirus functions, it is necessary to start with genetically modified viral genomes. Therefore, we decided to combine the two approaches by grafting organotypic rafts generated in vitro onto the dorsa of immunocompromised mice. This method combines the ease of introducing DNA genomes into cultured epithelial cells with the longer-term tissue maintenance of the xenograft system.
BPV-1 Replicates in Cutaneous Bovine Xenografts on Nude Mice.
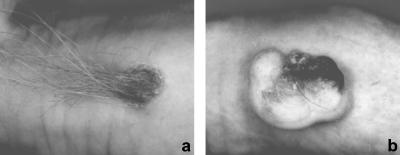
Before grafting bovine organotypic rafts onto immunocompromised mice, freshly isolated fetal bovine skin was grafted onto nude mice to ensure that BPV-1 could undergo a productive infection under these conditions. One-millimeter-thick sections of skin were removed from the flanks of a second-trimester fetal calf by using a dermatome. The dermatome was required because the calf dermis is very thick, and full-thickness skin did not graft well. A circular piece of skin was removed from the thoracic dorsum of the mouse, and the bovine graft was placed on the underlying fascia. After 2 months, the grafts had taken well (as evidenced by new hair growth characteristic of bovine skin; see Fig. 2a). The bovine skin was scarified and inoculated with bovine wart extract containing 2 × 105 infectious viral particles. Two months later, the infected skin had developed into large wart-like lesions (see Fig. 2b). A crude-viral extract was prepared from portions of the graft and used to infect C127 cells. After 2 weeks, many foci (>1,000 foci/plate) could be counted, suggesting that infectious BPV-1 viral particles had been produced in the graft.
Figure 2.
BPV-1 replicates in cutaneous bovine xenografts on nude mice. Fetal bovine skin was grafted onto nude mice and infected with BPV-1 wart extract. a shows an uninfected graft, and b shows a BPV-infected graft.
Infectious BPV-1 Can Be Produced in Organotypic Rafts Grafted on Nude Mice.
Because BPV-1 could successfully undergo productive infection in a xenograft of bovine skin, the next step was to see whether the viral life cycle could also be completed in a graft of artificial skin equivalent. Bovine rafts were prepared, and 4 days after being lifted to the air interface, they were grafted onto the dorsa of nude mice. The key to success with graft “take-rate” is to ensure that the raft remains moist for 2 weeks after grafting so that vascularization can take place and a barrier function can develop. This goal could be achieved by bandaging the mice well and monitoring the dressing on a daily basis. Success was also achieved by implanting the raft (covered with a silicone coverslip) under the skin of the dorsum for 2 weeks. At this time, the overlying skin was removed to expose the graft, which was covered by a bandage for several days. The latter method ensured that the graft remained moist but required two surgeries under anesthesia.
Another unique feature of the combination of organotypic rafts and xenografts is that different fibroblasts can be embedded in the dermal equivalent. It was found that grafts containing BALB or BALB/BPV fibroblasts had a much higher “take-rate” than those containing bovine dermal fibroblasts (BEF or BEF/BPV-1). Several strains of immunocompromised nude mice were used in the initial studies. A higher graft “take-rate” was obtained by using NIH Swiss nu/nu mice, as compared with NIH Balb/3T3 nu/nu mice or Balb athymic mice.
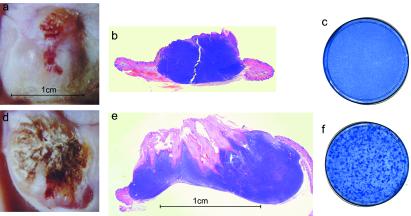
To test whether BPV-1 could undergo a productive infection in the organotypic raft/xenograft, keratinocytes were infected with 1 × 105 infectious BPV-1 particles while they were growing as a monolayer on the collagen dermal equivalent. The rafts were cultured and grafted as described above. To ensure that infectious virus isolated from the resulting grafts was not caused by input virus, an extract was prepared from rafts that were cultured for 4 days after being lifted to the air interface. Very few viral particles (0–0.005% of input) remained in these rafts. Fig. 3 shows an example of an uninfected (a and b) and an infected (d and e) graft from each group. In situ hybridization with a mouse- or bovine-specific probe determined that the xenografts were of bovine origin (data not shown). In most grafts, a large dermal fibroma developed because of the proliferation of BALB/BPV fibroblasts in the dermal equivalent. The overlying bovine epithelium was hyperplastic and hyperkeratotic in both uninfected and infected grafts. This pathology may be influenced by the presence of the underlying fibroma. However, the infected xenograft had much deeper invaginations of the epithelia into the fibroma characteristic of verrucous or papillomatous epidermal hyperplasia and produced abundant quantities of cornified cells, which gave the graft a “crusty” appearance. A small portion of each xenograft was homogenized and a portion used to infect C127 cells. After 2 weeks, numerous foci developed on the C127 monolayer infected with the extract from the BPV-1 infected xenograft (Fig. 3f) but not uninfected (c). This experiment indicated that the organotypic raft/xenograft could support the complete BPV-1 life cycle.
Figure 3.
Infectious BPV-1 can be produced in organotypic rafts grafted on nude mice. Examples of an uninfected (a and b) and a BPV-1 infected (d and e) xenograft are shown. The dermal equivalent contained BALB/BPV fibroblasts that resulted in a fibroma in both cases. a and d show the gross pathology of the graft, and b and e show an H&E-stained section. The flanking mouse skin can be observed at the margins. The plates shown in c and f are 100-mm dishes of C127 cells stained with methylene blue. Foci derived from infectious BPV-1 obtained from an infected xenograft can be observed on the plate in f.
Infectious BPV-1 Can Be Produced from Cloned Viral DNA in Organotypic Rafts Grafted onto Nude Mice.
To determine whether infectious virus could be produced by transfecting bovine keratinocytes with cloned DNA, the BPV-1 insert was cleaved from the prokaryotic vector sequences and religated before transfection into a monolayer of keratinocytes on a collagen dermal equivalent. In parallel, groups of rafts were either uninfected or infected with wart extract. The rafts were cultured and grafted as described above. In this experiment, either BALB or BALB/BPV fibroblasts were cultured in the collagen base. Two months after grafting, mice were killed, and the xenografts were analyzed for virus production. Table 1 shows the number of xenografts obtained in each group and the proportion of these grafts that produced infectious BPV-1 as assayed by focus formation on C127 cells. A large percentage of both the DNA-transfected and virus-infected grafts synthesized infectious BPV. Occasionally, a graft became folded, and a keratinizing cyst formed underneath the mouse epidermis. These cysts also produced infectious viral particles. The amount of virus produced by the grafts could not be measured accurately because only a small portion of the graft was used to prepare a viral inoculum, and different regions of the grafts were not uniformly undergoing productive infection. However, several hundred foci could be obtained from a small section of the graft. Because only a small region of the graft was assayed for virus, the actual percentage of productive grafts is probably higher than measured.
Table 1.
Ability of xenografts to produce infectious BPV-1
| Group* | Fibroblasts | “Take-rate”† | Fibroma‡ | Virus production§ |
|---|---|---|---|---|
| None | BALB | 1 /3 | 0 /1 | 0/1 |
| BALB/BPV | 7 /14 | 4 /7 | 0/5 | |
| Total | 8 /17 | Total: 0/6 | ||
| DNA | BALB | 3 /5 | 0 /3 | 2/2 |
| BALB/BPV | 12 /13 | 11 /12 | 6/10 | |
| Total | 15 /18 | Total: 8/12 | ||
| Virus | BALB | 4 /4 | 1¶ /4 | 3/4 |
| BALB/BPV | 11 /14 | 10 /11 | 3/7 | |
| Total | 15 /18 | Total: 6/11 |
*Input BPV-1.
†Grafts per no. mice.
‡Fibromas per no. grafts.
§Grafts producing virus per no. grafts tested.
¶A small fibroma arose from infection of fibroblasts in the collagen.
Infectious BPV-1 Can Be Produced from Bovine Xenografts in the Absence of a Fibroma.
Several animal papillomaviruses cause fibropapillomas in their natural hosts; the viruses infect and cause proliferation of the underlying dermal fibroblasts. However, the fibroblasts are unable to support vegetative viral DNA amplification and late gene expression, and so the infection is nonproductive. To determine whether the presence of a fibroma is required for production of infectious virus by the overlying infected epithelium, the aforementioned grafting experiment was set up by using both BALB and BALB/BPV fibroblasts. As shown in Table 1, grafts with no fibroma were able to synthesize viral particles from both transfected DNA and wart extract. In one productive graft that had been infected with viral extract, a small fibroma developed. In this case, the BALB fibroblasts in the collagen matrix must have become infected with virus. However, in other grafts, clearly no fibroma was present, yet infectious viral particles were produced.
Histological Features of the Bovine Xenografts.
Xenograft tissues were sectioned and stained by H&E. The xenografts ranged in size from 2 mm to 2 cm in diameter with a large underlying fibroma. Fig. 4 shows sections of adult bovine skin (a), a bovine fibropapilloma (c, e, and g), a control xenograft in which neither the BALB fibroblasts nor keratinocytes contained BPV-1 (b), and a productive BPV-1-infected xenograft that contained a BPV-1 induced fibroma (d, f, and h). Both uninfected and infected grafts were hyperplastic and hyperkeratotic compared with normal mouse skin or bovine skin, but these features were much more prominent in the productive grafts. The productive xenografts showed many features characteristic of papillomavirus infection. The grafts displayed papillomatosis (undulating epithelium), acanthosis (hyperplasia in the spinous layer), and prominent clumped keratohyalin granules in the stratum granulosum. In the stratum granulosum, there is marked perinuclear vacuolization (koilocytosis) with marginal sickle-shaped nuclei, a hallmark of papillomavirus infection. As shown in Fig. 4, there is pronounced hyperkeratosis and parakeratosis (nuclei retained in the stratum corneum). All of these features are observed in naturally occurring warts (Fig. 4 c, e, and g).
Figure 4.
Histological features of the bovine xenografts. a shows an H&E-stained section of adult bovine skin. c, e, and f show an H&E-stained section from a bovine fibropapilloma. The sections shown in b, d, f, and h are from bovine organotypic raft/xenografts. The control graft shown in b was generated with uninfected bovine keratinocytes and BALB fibroblasts. The graft shown in d, f, and h was generated with BPV-1-infected keratinocytes, and the dermal equivalent of this graft contained BALB/BPV fibroblasts that resulted in a fibroma. Characteristic features of papillomavirus infection are indicated with arrows: acanthosis (a); koilocytosis (ky); prominent keratohyalin granules (g); parakeratosis (pk); and hyperkeratosis (hk). Flanking mouse skin (ms) is shown in b. Magnifications are indicated.
Sections of the xenografts were analyzed for vegetative viral DNA replication by in situ hybridization with a DIG-labeled RNA probe from the sense strand of the E6 region of the BPV-1 genome. Fig. 5 shows that amplified BPV-1 DNA could be detected in the stratum spinosum and granulosum of the xenografts. Examples are shown of grafts with (Fig. 5 d and e) and without (b and c) fibroma and resulting from transfection of viral DNA (b and d) or infection with wart extract (c and e). The staining was not uniform throughout the epithelium, indicating that only portions of the xenograft were undergoing productive infection. The staining pattern observed is very similar (although positive staining cells are less abundant and less intense) to that detected in a bovine wart (Fig. 5a).
Figure 5.
In situ hybridization for amplified BPV-1 DNA. Examples of grafts with (e and f) and without (a–c) fibroma (because of BALB/BPV fibroblasts) and either uninfected (a), transfected with viral DNA (b and e), or infected with a wart extract (c and f). d shows a natural bovine fibropapilloma. Positive cells containing viral DNA are stained with 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium and counterstained with eosin.
BPV-1 capsid antigen could also be detected in the stratum granulosum of the grafts by using a monoclonal antibody against the L1 protein. Fig. 6 shows examples of grafts with (Fig. 6 d and e) and without (b and c) fibroma and resulting from transfection of viral DNA (b and d) or infection with wart extract (c and e). This staining was observed in the nuclei of cells in the stratum granulosum and was similar (but less abundant and less intense than that observed in the control bovine wart (Fig. 6a). No BPV-1 DNA- or L1-specific staining could be observed in uninfected xenografts or in the surrounding mouse epithelium (data not shown).
Figure 6.
Immunohistochemistry of BPV-1 L1 antigen in xenografts. Examples of grafts with (e and f) and without (a–c) fibroma (because of BALB/BPV fibroblasts) and either uninfected (a), transfected with viral DNA (b and e), or infected with a wart extract (c and f). d shows a bovine fibropapilloma. Positive cells were detected with 3,3′-diaminobenzidine and counterstained with hematoxylin. The stratum corneum (sc), stratum granulosum (sg), and stratum spinosum (ss) are indicated by arrows.
Discussion
We have developed a system to study the complete viral life cycle of BPV-1. In this system, organotypic rafts that have been transfected with viral DNA or infected with viral particles are grafted onto the dorsa of immunocompromised mice. Infectious BPV particles can be isolated from these grafts and assayed by using a C127 focus formation assay that capitalizes on the ability of BPV-1 to transform rodent fibroblasts. Within these grafts, the pattern of vegetative viral DNA amplification and capsid protein synthesis is tightly regulated and is similar to that of a BPV-1 bovine fibropapilloma.
This system will allow the genetic analysis of the complete infectious cycle of BPV-1. BPV-1 has been studied extensively because of its ability to transform fibroblasts in culture and to maintain its genome as an episome indefinitely within these cells (12), and many well-characterized mutations that disrupt specific viral functions have been described. The system described here will be very useful in determining the role of individual viral gene products and/or specific gene functions in the vegetative viral life cycle.
The capacity of BPV-1 to replicate in and transform certain nonepithelial cells when compared with other epithliotrophic papillomaviruses likely corresponds to its ability to cause fibropapillomas. This property is thought to be caused by the E5 ORF that encodes a 44-aa hydrophobic polypeptide. This ORF is very homologous between BPV-1 and other fibropapillomaviruses, such as reindeer, deer, and European elk papillomavirus (13). In this study, we find that a BPV-1-induced fibroma is not required for productive infection of the overlying epithelium in the xenograft model. Nonproductive infection of dermal fibroblasts is of no advantage to the virus unless it increases the amount or spread of virus produced from the overlying papilloma. The fibroma may increase the proliferation of the epithelium in a natural infection or may help in evasion from the immune system. We do notice a marked proliferation of the epithelium overlying the fibroma, even in the absence of BPV-1 infection. Alternatively, by raising the surface of BPV-1-infected epithelium above the densely-haired skin of the natural host, the fibroma may facilitate transmission of viral particles. In BPV fibropapillomas, a fibroblastic reaction is observed within a week of infection, but proliferation of the epithelium does not become visible for 4–6 weeks (10). It has been shown that no dermis at all is required for the development of cottontail rabbit papillomavirus (CRPV)-induced papillomas in autografts of rabbit skin (14).
HPVs can maintain their genomes episomally in human keratinocytes in monolayer culture, but this requires specialized cell culture conditions, and the genomes are very prone to integration (15). We find that BPV-1 genomes are also not efficiently maintained in bovine keratinocytes in monolayer culture (A.M., unpublished observations). However, when these infected keratinocytes are immediately cultured in a stratified epithelium, the virus can undergo the complete productive life cycle.
Papillomaviruses, including BPV-1, have been successfully propagated in chips of epithelial tissue implanted in the renal capsule of immunocompromised mice (16–19). However, the surgery is technically difficult and requires a viral extract to infect tissue, as it is difficult to efficiently transfect DNA into tissue chips. Brandsma et al. have successfully introduced HPV16 DNA into human skin grafts and CRPV DNA into domestic rabbit skin by using a particle-mediated jet injector (20, 21). Using the latter system, Brandsma et al. have been able to assess the role of different viral gene products on the formation of a papilloma, but because CRPV is nonproductive in domestic rabbits, they could not assess virus production (22–24). Injection of CRPV DNA into cottontail rabbit skin does result in infectious viral particles (25). The advantage of the cutaneous systems described by Brandsma and us is that the graft can be assessed easily at different time periods during the development of the papilloma. It should be possible to take punch biopsies of the grafts so that they can be analyzed without sacrificing the mouse, allowing the graft to continue to develop. Laimins and colleagues have used organotypic rafts extensively to study the requirements for HPV31 productive infection (26–29). However, this system is transient, and the artificial epithelium deteriorates after 10–14 days.
Grafting the organotypic rafts onto the panniculus carnosus instead of the dorsal fascia could probably increase the efficiency of grafting further. The panniculus carnosus is a thin sheet of muscle that is attached to the dermis, and grafting here may improve vascular ingrowth of the graft. Cotransfection of keratinocytes with BPV-1 DNA and a drug-selectable marker and subsequent selection of the transfected cell population could also increase the percentage of infected cells in the graft. However, as shown in this study, these steps are not necessary to obtain a bovine xenograft that produces infectious BPV-1 particles. This system has the advantage that genetically modified viral genomes can be tested, and both keratinocytes and fibroblasts can be manipulated in the laboratory before grafting. The long-term tissue maintenance and persistent infection is much more analogous to natural infection than the short-term in vitro organotypic raft system, and the use of immunocompromised mice eliminates the problems of regression of virally infected tissue.
This system could be adapted to study other differentiation-dependent epitheliotropic viruses, such as molluscum contagiosum. It will also be useful for nonviral applications. For example, keratinocyte gene therapy requires efficient methods of gene transfer and persistent gene expression in keratinocytes. This system is amenable to manipulation, and Kolodka et al. have developed a similar system to demonstrate long-term engraftment and expression of transgenes (30). The ability of the artificial xenograft to support the papillomavirus life cycle demonstrates that it constitutes a functionally differentiated stratified epithelium.
Acknowledgments
We thank Louise Chow (University of Alabama, Birmingham, AL) for the organotypic raft protocol and Jonathon Garlick (State University of New York, Stony Brook, NY) for advice on grafting.
Abbreviations
- HPV
human papillomavirus
- BPV
bovine papillomavirus
- BEF
bovine dermal fibroblasts
- DIG
digoxigenin
- H&E
hematoxylin and eosin
- CRPV
cottontail rabbit papillomavirus
References
- 1.Howley P M. In: Virology. Fields B N, Knipe D M, Howley P M, editors. Philadelphia: Lippincott; 1995. pp. 2045–2076. [Google Scholar]
- 2.Howett M K, Christensen N D, Kreider J W. Clin Dermatol. 1997;15:229–236. doi: 10.1016/s0738-081x(96)00166-6. [DOI] [PubMed] [Google Scholar]
- 3.Meyers C, Laimins L A. Curr Top Microbiol Immunol. 1994;186:199–215. doi: 10.1007/978-3-642-78487-3_11. [DOI] [PubMed] [Google Scholar]
- 4.Chow L T, Broker T R. Clin Dermatol. 1997;15:217–227. doi: 10.1016/s0738-081x(97)00069-2. [DOI] [PubMed] [Google Scholar]
- 5.McMillan N A, Payne E, Frazer I H, Evander M. Virology. 1999;261:271–279. doi: 10.1006/viro.1999.9825. [DOI] [PubMed] [Google Scholar]
- 6.Evander M, Frazer I H, Payne E, Qi Y M, Hengst K, McMillan N A. J Virol. 1997;71:2449–2456. doi: 10.1128/jvi.71.3.2449-2456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarver N, Rabson M S, Yang Y C, Byrne J C, Howley P M. J Virol. 1984;52:377–388. doi: 10.1128/jvi.52.2.377-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dollard S C, Wilson J L, Demeter L M, Bonnez W, Reichman R C, Broker T R, Chow L T. Genes Dev. 1992;6:1131–1142. doi: 10.1101/gad.6.7.1131. [DOI] [PubMed] [Google Scholar]
- 9.Sarver N, Byrne J C, Howley P M. Proc Natl Acad Sci USA. 1982;79:7147–7151. doi: 10.1073/pnas.79.23.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheville N F, Olson C. Pathol Vet. 1964;1:248–257. [Google Scholar]
- 11.McCance D J, Kopan R, Fuchs E, Laimins L A. Proc Natl Acad Sci USA. 1988;85:7169–7173. doi: 10.1073/pnas.85.19.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Law M F, Lowy D R, Dvoretzky I, Howley P M. Proc Natl Acad Sci USA. 1981;78:2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno-Lopez J, Ahola H, Eriksson A, Bergman P, Pettersson U. J Virol. 1987;61:3394–3400. doi: 10.1128/jvi.61.11.3394-3400.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breedis C, Kreider J W. Cancer Res. 1970;30:974–979. [PubMed] [Google Scholar]
- 15.Jeon S, Allen-Hoffmann B L, Lambert P F. J Virol. 1995;69:2989–2997. doi: 10.1128/jvi.69.5.2989-2997.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreider J W, Howett M K, Leure-Dupree A E, Zaino R J, Weber J A. J Virol. 1987;61:590–593. doi: 10.1128/jvi.61.2.590-593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoler M H, Whitbeck A, Wolinsky S M, Broker T R, Chow L T, Howett M K, Kreider J W. J Virol. 1990;64:3310–3318. doi: 10.1128/jvi.64.7.3310-3318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreider J W, Patrick S D, Cladel N M, Welsh P A. Virology. 1990;177:415–417. doi: 10.1016/0042-6822(90)90503-j. [DOI] [PubMed] [Google Scholar]
- 19.Ghim S, Christensen N D, Kreider J W, Jenson A B. Int J Cancer. 1991;49:285–289. doi: 10.1002/ijc.2910490224. [DOI] [PubMed] [Google Scholar]
- 20.Brandsma J L, Yang Z H, Barthold S W, Johnson E A. Proc Natl Acad Sci USA. 1991;88:4816–4820. doi: 10.1073/pnas.88.11.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandsma J L, Brownstein D G, Xiao W, Longley B J. J Virol. 1995;69:2716–2721. doi: 10.1128/jvi.69.4.2716-2721.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Defeo-Jones D, Vuocolo G A, Haskell K M, Hanobik M G, Kiefer D M, McAvoy E M, Ivey-Hoyle M, Brandsma J L, Oliff A, Jones R E. J Virol. 1993;67:716–725. doi: 10.1128/jvi.67.2.716-725.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandsma J L, Yang Z H, DiMaio D, Barthold S W, Johnson E, Xiao W. J Virol. 1992;66:6204–6207. doi: 10.1128/jvi.66.10.6204-6207.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu X, Xiao W, Brandsma J L. J Virol. 1994;68:6097–6102. doi: 10.1128/jvi.68.9.6097-6102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas J T, Hubert W G, Ruesch M N, Laimins L A. Proc Natl Acad Sci USA. 1999;96:8449–8454. doi: 10.1073/pnas.96.15.8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stubenrauch F, Colbert A M, Laimins L A. J Virol. 1998;72:8115–8123. doi: 10.1128/jvi.72.10.8115-8123.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stubenrauch F, Lim H B, Laimins L A. J Virol. 1998;72:1071–1077. doi: 10.1128/jvi.72.2.1071-1077.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klumpp D J, Stubenrauch F, Laimins L A. J Virol. 1997;71:8186–8194. doi: 10.1128/jvi.71.11.8186-8194.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frattini M G, Lim H B, Laimins L A. Proc Natl Acad Sci USA. 1996;93:3062–3067. doi: 10.1073/pnas.93.7.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolodka T M, Garlick J A, Taichman L B. Proc Natl Acad Sci USA. 1998;95:4356–4361. doi: 10.1073/pnas.95.8.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]