Abstract
N-acetyl aspartate (NAA) is an important marker of neuronal function and viability that can be measured using magnetic resonance spectroscopy (MRS). In this paper, we proposed a method to measure NAA synthesis using proton MRS with infusion of uniformly 13C-labeled glucose, and demonstrated its feasibility in an in vivo study of the rat brain. The rate of 13C-label incorporation into the acetyl group of NAA was measured using a localized, long echo-time proton MRS method. Signals from the 13C satellites of the main NAA methyl protons at 2.02 ppm were continuously monitored for 10 hours. Quantification of the data based on a linear kinetic model showed that NAA synthesis rate in isoflurane-anesthetized rats was 0.19 ± 0.02 µmol/g/h (mean ± standard deviation, n = 12).
Keywords: N-acetyl aspartate synthesis, proton magnetic resonance spectroscopy, in vivo
Introduction
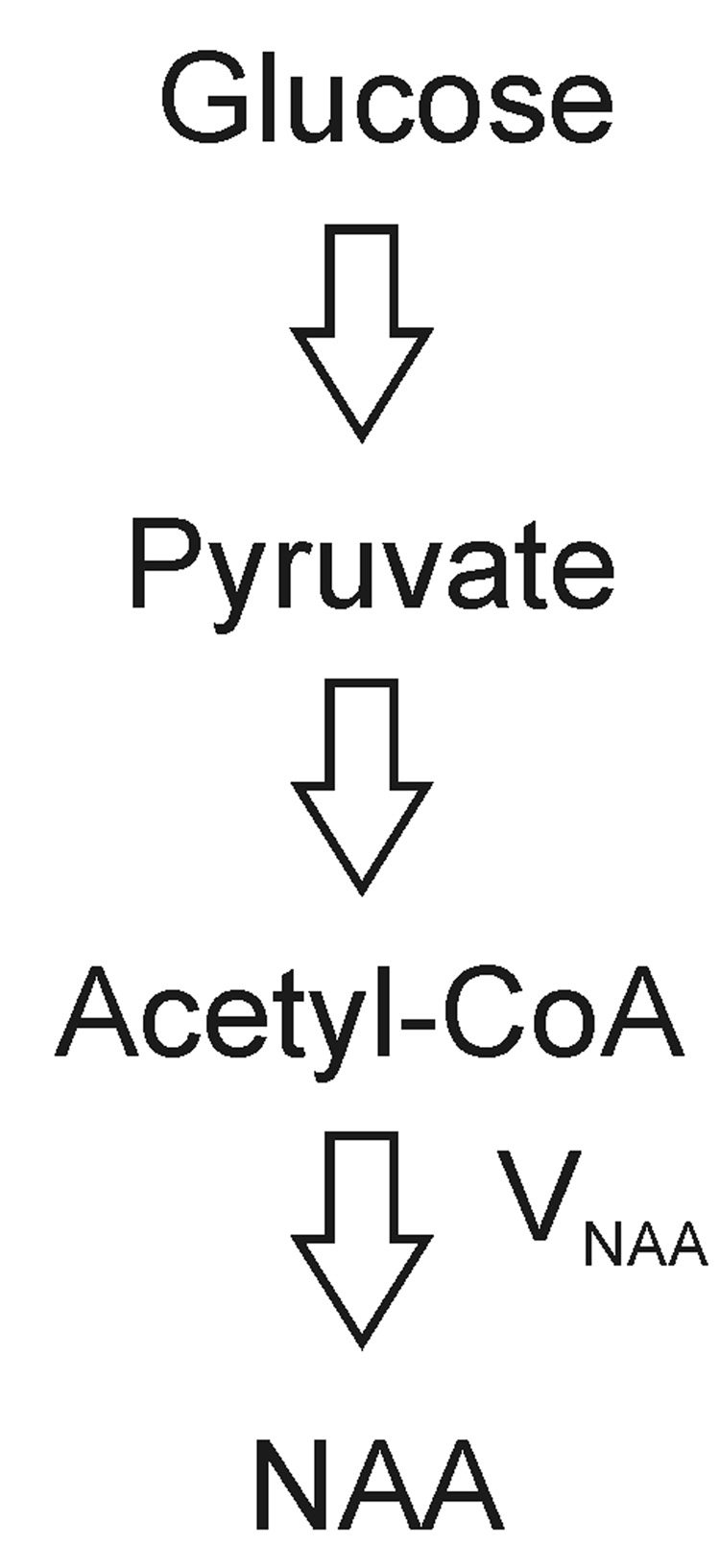
N-acetyl aspartate (NAA) is found in high concentration (~10 µmol/g) exclusively in the nervous system (Tallan, 1957; Miyake et al., 1981; Koller et al., 1984; Pan et al., 2005). It is primarily synthesized from acetyl coenzyme A (acetyl-CoA) and aspartate by NAA synthase (L-aspartate N-acetyltransferase; ANAT, EC 2.3.1.17) in neuronal mitochondria, or via cleaving N-acetylaspartylglutamate (NAAG) catalyzed by N-acetylated-α-linked-amino dipeptidase (NAALADase) along with glutamate, and subsequently exported to the cytoplasm (Patel et al., 1979, Bzdega et al. 1997, Luthi-Carter et al., 1998). The major NAA catabolic enzyme aspartoacylase (N-acetylaspartate amidohydrolase; EC 3.5.1.15) is located in oligodendrocytes (Kaul et al., 1991; Baslow et al., 1999). NAA has been widely used as a neuronal marker in the study of a variety of cerebral disorders using in vivo proton magnetic resonance spectroscopy (MRS) (see Moffett et al., 2007 for a recent review). Abnormalities in NAA synthesis, transport and/or breakdown may contribute to abnormal steady state NAA concentrations observable in proton MRS spectra of the brain (Clark, 1998; Moreno et al., 2001). Canavan disease, for example, is an NAA metabolic disorder due to N-aspartoacylase deficiency which results in elevation of the NAA signal in proton MRS spectra (Kvittingen et al., 1986; Matalon et al., 1988; Burns et al., 1992; Moreno et al., 2001). In contrast to the large body of literature on the total concentration of NAA in various brain disorders, the characterization of NAA synthesis remains scarce. Because NAA synthesis is dependent on mitochondrial metabolism and glucose is the major energy source of the brain under most conditions, 14C and 13C labeled glucoses have been used as primary substrates to determine the rate of NAA synthesis (VNAA) (Fig. 1). Using an in vitro enzymatic method, the activity of ANAT in adult rat brain homogenates was determined to be 0.19-0.58 µmol/g/h depending on specific brain anatomy. The cortex acitivity was found to be 0.29 µmol/g/h by Truckenmiller et al (1985). The activity of ANAT in the forebrain homogenate of thirty-five day old rats was determined to be 0.72 µmol/g/h (Burri et al., 1991). The in vivo cerebral VNAA has been directly measured using 13C MRS in both α-chloralose-anesthetized adult rats (VNAA = 0.7 ± 0.1 µmol/g/h) (Choi and Gruetter, 2004) and humans (VNAA = 0.55 ± 0.23 µmol/g/h) (Moreno et al., 2001).
Fig. 1.
Schematic representation of synthesis of NAA from glucose. VNAA, NAA synthesis rate.
The direct 13C methods are limited by the inherently low sensitivity of 13C MRS. The N-acetyl methyl group of NAA is the major resonance in water-suppressed proton MRS spectra. The three magnetically equivalent hydrogen atoms of the methyl group resonate in proton MRS spectra with a single, sharp peak at 2.02 ppm, and therefore NAA has been generally recognized as one of the most reliable markers in brain MRS studies (Fan et al., 1986; Luyten and den Hollander, 1986; Barany et al., 1987). Because of the prominence of the NAA proton signal in MRS and its significantly higher sensitivity compared to 13C-labeled metabolite signals, a potentially useful approach is to determine NAA synthesis by measuring the 13C satellite signals of NAA in the proton MRS spectra. The resonances of the 13C satellite peaks are symmetrically located with respect to the central resonance of the methyl protons attached to 12C. The intensity ratio of the satellite peaks to the total NAA signal reveals the 13C isotopic enrichment of the acetyl moiety of NAA. Unlike direct 13C methods which require a radio frequency (RF) coil tuned to 13C nuclei and a second RF channel as well as broadband amplifiers, proton MRS is in principle available on all clinical scanners, and by far the most widely used MRS technique. In this study, we demonstrate that VNAA can be measured in vivo using a localized long echo-time (TE) proton MRS method with infusion of uniformly 13C-labeled glucose ([U-13C]glucose) to label the N-acetyl methyl group of NAA without using a 13C channel. A similar approach for measuring the 13C labeling of glutamate was previously reported (Boumezbeur et al., 2004).
Materials and Methods
Male young adult Sprague-Dawley rats (169–217 g, n = 12) were fasted overnight (> 12 hours) with free access to drinking water. For preparation, the rats were anaesthetized with isoflurane (1.5%) in a mixture of 70% N2O/30% O2. The left femoral artery was cannulated for periodically sampling of arterial blood to monitor blood gases (pO2, pCO2), pH, and glucose concentration using a blood analyzer (Bayer Rapidlab 860, East Walpole, MA), and for monitoring arterial blood pressure levels. The left femoral vein was also cannulated for intravenous infusion of [U-13C]glucose (99% enrichment, Cambridge Isotope Labs, Andover, MA, 20 % wt/vol). The glucose infusion protocol consisted of an initial bolus of 162 mg/kg/min of 1.1 M [U-13C]glucose in the first ten minutes followed by an approximately constant infusion rate of 62.8 mg/kg/min. Plasma glucose level was maintained at 19.6 ± 1.6 mM during the experiment. Arterial blood pO2, pCO2, mean blood pressure, and pH were maintained at approximately 129 ± 16 mm Hg, 36 ± 4 mmHg, 100–145 mm Hg, and 7.34 ± 0.04, respectively. All procedures were approved by the National Institute of Mental Health Animal Care and Use Committee.
The in vivo MRS experiments were performed on a Bruker 11.7 Tesla spectrometer interfaced to an 89 mm inner-diameter vertical bore magnet. A 15-mm inner diameter proton surface coil was used for excitation and detection, and was positioned ~0–1 mm posterior to bregma. Adjustment of all first- and second-order shims was accomplished using a fully automatic procedure described previously (Chen et al., 2004) which was based on a fast automatic shimming technique by mapping along projections method (Gruetter, 1993). The single-shot adiabatic 3D localization pulse sequence (Slotboom et al., 1991) was similar to that used in our previously study (Xu et al., 2005) but with a lengthened echo-time (TE = 100 ms). Specifically, the proton MRS pulse sequence used slice-selective adiabatic refocusing with two hyperbolic secant pulses per dimension (Conolly et al., 1991) (2 ms sech pulse, µ = 5, 1% truncation). Chemical shift selective (CHESS) water suppression (Gueron et al., 1991) was used together with outer volume suppression using nominal 90° hyperbolic secant pulses along the x (10-mm slab), −x (10-mm slab), y (3-mm slab), −y (5-mm slab), z (10-mm slab), and −z (10-mm slab) directions. The CHESS and the outer volume suppression pulses were repeated three times. Orthogonal gradient pairs or triplets carefully adjusted to minimize the resultant B0 shifts were used as crushers for CHESS, outer volume suppression, and for the slice-selective refocusing adiabatic pulses. Proton MRS spectra were acquired from a 6 × 3 × 6 mm3 voxel centered on the midline of the brain with 4096 data points, a spectral width of 4000 Hz, and a repetition time (TR) of 3.2 s. For each data block, 440 acquisitions were accumulated over 24 minutes. After acquisition of each data block, a 6-minute interval was used for re-shimming to maintain B0 homogeneity over a total experimental duration of 10 hours. Satellite NAA methyl peaks were analyzed using the MATLAB curve-fitting toolbox (The MathWorks, Inc., Natick, MA). Since NAAG cannot be reliably quantified, the total NAA signal (= NAA + NAAG) was used instead. To minimize interference from the glutamate and glutamine H3 methylene group at 2.09 – 2.14 ppm regions, the upfield NAA satellite peak at 1.89 ppm was used in the quantification of 13C-labeled NAA with its intensity multiplied by a factor of 2.
Results
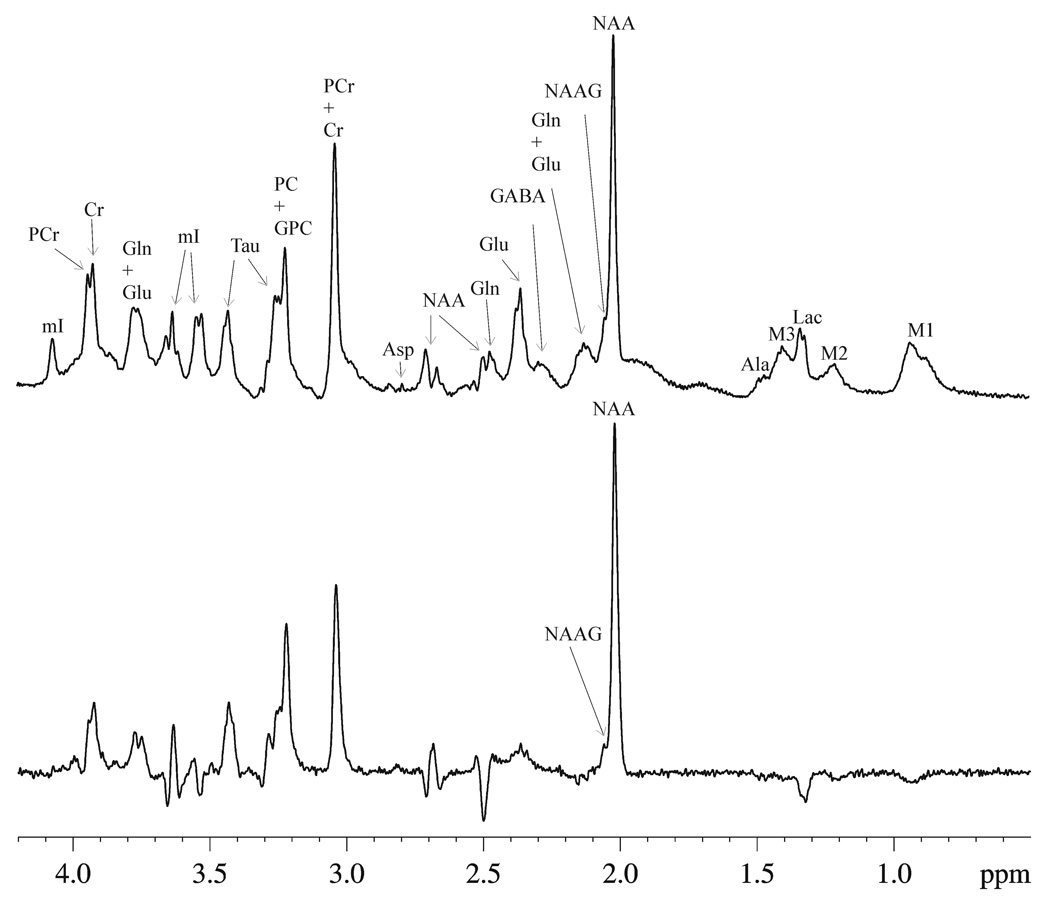
Fig. 2 shows examples of the localized (6 × 3 × 6 mm3) proton MRS spectra of a normal rat brain at short echo-time (Fig. 2a, TE = 15 ms) and at long echo-time (Fig. 2b, TE = 100 ms) without infusion of [U-13C]glucose. The accumulated FID of each data block was zero-filled to 16 K. Resolution-enhancing Lorentz-Gauss transformation (exponential broadening factor (lb) = −4 Hz, Gaussian broadening factor (gb) = 0.3) was applied before Fourier transform. The spectra were phased using zero-order phase only, without any baseline corrections. These in vivo spectra show high sensitivity and spectral resolution achieved at the high magnetic field strength of 11.7 T used in the current study. In Fig. 2, the spectral pattern of resonances between 0.8 and 4.0 ppm shows a strong dependence upon TE. The short echo-time spectrum (Fig. 2a) minimizes the signal-to-noise ratio (SNR) loss mostly by the spin-spin relaxation dephasing, phase and intensity abnormality due to evolution of homonuclear scalar couplings of weakly and strongly coupled resonances. However, the analysis of NAA satellite signals at short TE is complicated due to the broad baseline originating from macromolecules as well as overlapping resonances from glutamate and glutamine H3 methylene protons. As shown in Fig. 2b, at the longer TE of 100 ms interference from glutamate and glutamine H3 and macromolecules is minimized while the NAA resonance at 2.02 ppm still maintains a high SNR.
Fig. 2.
The localized (6 × 3 × 6 mm3) proton MRS spectra acquired from an individual rat brain with (a) short TE (TE = 15 ms, NA = 440, TR = 3.2 s, lb = −4 Hz, gb = 0.3) and (b) long TE (TE = 100 ms, NA = 1760, all other parameters were the same as in (a)). The spectra were phased using zero-order phase only without any baseline corrections, and were plotted using the same absolute intensity scale. Ala = alanine, Asp= aspartate, Cr = creatine, GABA = γ-aminobutyric acid, Gln = glutamine, Glu = glutamate, GPC = glycerophosphorylcholine, Lac = lactate, mI = myo-Inositol, NAA = N-acetylaspartate, NAAG = N-acetylaspartyglutamate, PC = phosphocholine, PCr = phosphocreatine, Tau = taurine, M1 = macromolecule at 0.92 ppm, M2 = macromolecule at 1.21 ppm, M3 = macromolecule at 1.39 ppm.
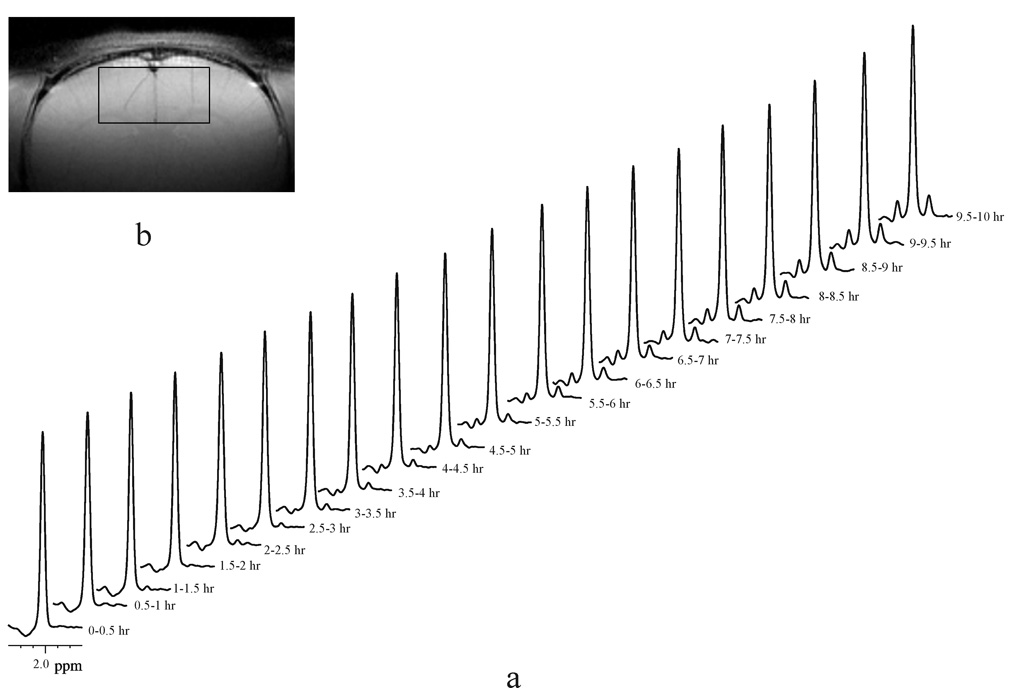
A typical time course of spectra showing dynamic 13C-label incorporation into the N-acetyl methyl group of NAA (1JCH = 128 Hz) during [U-13C]glucose infusion from the 6 × 3 × 6 mm3 (108 µL) voxel of an individual rat brain is illustrated in Fig. 3a (lb = −1Hz, gb= 0.005). The signal intensity of the NAA satellite peaks increased progressively as a function of time due to 13C-label incorporation from C1 and C6 of [U-13C]glucose into NAA C6. Both [U-13C]glucose and [1,6-13C2]glucose produce two acetyl-CoA with 13C labels at their C2 positions (and therefore at NAA C6), therefore doubling the SNR obtainable using [1-13C]glucose or [6-13C]glucose. Note that the simultaneous 13C-label incorporation from C2 and C5 of [U-13C]glucose into NAA C5, which has a heteronuclear coupling of ~5 Hz to the methyl protons of NAA, does not affect the measurement of VNAA. The use of [U-13C]glucose instead of [1,6-13C2]glucose is because the former is much less costly. The spectroscopy voxel used in this study is predominantly located in the rat neocortex as shown in a coronal anatomical image (Fig. 3b). Because the observed kinetics of 13C-label incorporation into the acetyl moiety of NAA was found to be approximately linear within the experimental time frame, the time course of 13C isotopic enrichment of the NAA methyl group, 13C-NAA (t), was modeled as a linear equation based on the work of Choi and Gruetter (2004):
| [1] |
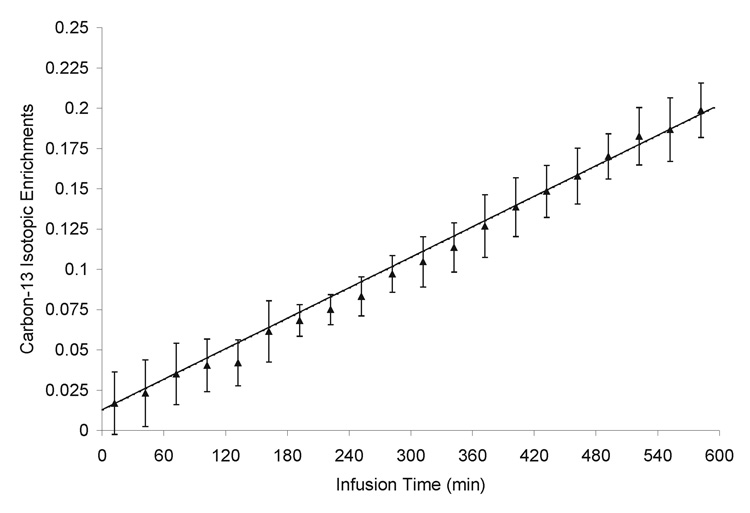
where NAA0 is the total concentration of NAA. The constant 0.011 accounts for the natural abundance signal of 13C-labeled NAA. NAA0 = 9.9 µmol/g was converted from our previously reported NAA concentration in the same brain region (Xu et al., 2005) based on the reported brain tissue specific gravity (DiResta et al., 1991). The NAA synthesis rate VNAA was subsequently determined using least-squares fitting of the measured time courses of the 13C isotopic enrichment of NAA to the above equation. The time courses of 13C isotopic enrichment from the twelve rats (expressed as mean and standard deviation) and the fit to the linear model are shown in Fig 4. The NAA synthesis rate was determined to be VNAA = 0.19 ± 0.02 µmol/g/h (mean ± standard deviation, n = 12).
Fig. 3.
Detection of dynamic 13C-label incorporation into the N-acetyl methyl group of NAA (1JCH = 128 Hz) resonance using a proton-only MRS method at 11.7 Tesla. (a) Each spectrum (lb = −1 Hz, gb = 0.005) was an accumulation of 24 minutes (NA = 440) during a 10-hour [U-13C]glucose infusion. The stack of spectra were acquired from a 6 × 3 × 6 mm3 (108 µL) voxel in an individual rat brain. (b) The coronal image shows the location of the measured spectroscopic volume (slice thickness = 1 mm, field of view = 2.56 cm, matrix size = 256 × 256, TR/TE = 1000/10 ms, number of averages = 2).
Fig. 4.
13C labeling time course of the N-acetyl methyl group of NAA and fitting to the linear model. Data were averaged from twelve rats. Error bars = ±1 standard deviation.
Discussion
In this study, we demonstrated that proton MRS without the use of a 13C channel (Boumezbeur et al. 2004) could be utilized for measuring the NAA synthesis rate in vivo. We performed measurements of 13C-label incorporation into the acetyl moieties of NAA in an 108 µL volume in the rat brain with [U-13C]glucose infusion for ten hours. The proton MRS-only method takes advantage of the high sensitivity of proton detection and additional spectral resolution rendered by the 13C satellite peaks when no heteronuclear decoupling is applied. Compared to direct 13C MRS, in vivo proton MRS is a much more sensitive method due to its large gyromagnetic ratio (1H: 26.8 × 107 rads−1T−1 vs. 13C: 6.7 × 107 rads−1T−1). In 13C MRS studies, proton decoupling techniques are usually used for increasing spectral resolution and sensitivity. However, a double-tuned RF coil or a two-coil assembly and broadband amplifiers are required to perform direct 13C MRS with proton decoupling. In addition, two RF channels are needed for excitation at both 1H and 13C frequencies in direct 13C MRS methods. Therefore, the proton-only MRS technique significantly simplifies the hardware requirements for measuring the NAA synthesis rate in vivo.
Although NAA H3 signals at 2.50 and 2.70 ppm were detected in Fig. 2b, they are not useful for quantification of VNAA because of their substantially low intensity compared to the methyl proton signal of NAA at 2.02 ppm. No useful 13C satellite signals of NAA H3 were found after adding the last time point from the twelve animals (data not shown).
Compared with the activity of ANAT measured from rat brain homogenates, the smaller VNAA value determined in vivo from isoflurane-anesthetized rats in the current study suggests that NAA synthesis in vivo may be limited by substrate availability. The regulation of ANAT in vivo is still largely unknown (Arun et al., 2006). Since the Vmax-like activity of ANAT measured from brain homogenates does not necessarily reflect VNAA in vivo other explanations for the smaller in vivo VNAA measured in this study are also possible. It is unknown if anesthesia required for in vivo MRS study of animals affects VNAA. Current evidence suggests that depression of basal metabolism by anesthetics does not correlate with a reduced VNAA. A significantly higher VNAA was reported in the literature (0.7 ± 0.1 µmol/g/h, Choi and Gruetter, 2004) when using direct 13C MRS methods with α-chloralose-anesthetized rats, although α-chloralose is known to produce a larger attenuation of cerebral metabolism. The discrepancy between our study and that of Choi and Gruetter therefore may be due to other differences in experimental conditions. For example, the use of highly sensitive proton detection allowed us to place the spectroscopy voxel in mostly neocortical gray matter (Fig. 3b). In the Choi and Gruetter in vivo 13C MRS study, a much larger voxel (8.5 × 6 × 10 mm3 or 510 µl) which included a large portion of white matter and subcortical tissues was used to increase the sensitivity of 13C MRS. Since in several subcortical tissue types the activity of ANAT is substantially higher than that in the cortex (Truckenmiller et al., 1985) and the activity of ANAT in white matter is yet to be determined, the large difference in voxel size between the Choi and Gruetter study and our study is expected to have contributed significantly to this apparent discrepancy.
Eq. [1] intrinsically assumes that the 13C fractional enrichment of the precursor acetyl-CoA is 100%. If the presence of neuronal isotope dilution is significant, the 13C fractional enrichment of the precursor acetyl-CoA is expected to be less than 100%. The presence of endogenous unlabeled glucose will also reduce the 13C fractional enrichment of acetyl-CoA. However, based on our previous study using the same infusion protocol the 13C fractional enrichment of blood glucose is very close to 100% (96%, Xu and Shen, 2006). Isotope dilution of neuronal acetyl-CoA is also expected to be insignificant due to prolonged infusion of a large amount of fully and uniformly labeled glucose. The overall effect of overestimating the isotopic enrichment of acetyl-CoA leads to underestimating VNAA. Based on the above analysis, the underestimation of VNAA due to incomplete labeling of the precursor neuronal acetyl-CoA pool is expected to be very small. It should also be pointed out that one important advantage of using proton MRS without heteronuclear decoupling is that the total NAA signal remains unchanged during the course of spectroscopy measurement and can be used as a concentration reference. In contrast, direct 13C MRS lacks an internal concentration reference and requires a more complicated quantification procedure.
Although the acetyl methyl proton peak of NAAG overlaps with that of NAA (see Fig. 2), NAAG has a much lower concentration in the brain (<0.5–1 µmol/g, Robinson et al. 1987, Fuhrman et al., 1994). More importantly, the turnover of NAAG catalyzed by NAALADase (NAAG ↔ NAA + glutamate) is much more rapid, leading to an isotopic steady state between NAA and NAAG (Tyson and Sutherland, 1988). That is, NAA and NAAG have nearly the same 13C isotopic enrichment at the acetyl methyl carbon. Thus, Eq. [1] is valid when total NAA is used in lieu of NAA. Therefore, the presence of NAAG can be safely neglected during the measurement of VNAA.
The current study was performed to explore the possibility of measuring NAA synthesis in human subjects using proton MRS. Although we used the high field strength of 11.7 Tesla and a voxel size of 0.1 ml, the sensitivity of proton MRS at clinically accessible field strength has been shown to be sufficient to detect natural abundance 13C-labeled NAA from human brain at a spatial resolution of 12 ml (Chen W, et al., 1998). Therefore, the isotopically enriched NAA 13C satellite signal is well above the sensitivity limit of clinical scanners. The spectral resolution is generally reduced at lower field strength. However, at long echo time, proton MRS spectra of brain at low field strength are known to be spectrally simplified, which can be taken advantage of to spectrally resolve the NAA 13C satellite signals.
In addition to the steady-state concentrations of NAA, NAA turnover rates in the human brain measured by direct 13C MRS methods have also been reported recently, offering an opportunity to better understand the role of NAA in brain metabolism and disorders of metabolism. The NAA synthesis rate was found to be approximately 60% lower in Canavan patients (0.56 ± 0.23 µmol/g/h in controls versus 0.22 ± 0.01 µmol/g/h in Canavan disease) with a 50% increase in static NAA concentration (Moffett et al., 2007). In contrast to Canavan disease where NAA synthesis rate was reduced, NAA synthesis rates appeared to be modestly increased in patients with Alzheimer’s disease (Harris et al., 2006). Our proton-only MRS strategy for detection of NAA synthesis can be easily extended to lower field strength accessible to clinical research. Thus, with high sensitivity and easy implementation, the method proposed in this paper may provide potential applications to clinical studies of human brain disorders associated with abnormal NAA concentration and kinetics. It may also be used in measuring VNAA in white matter and deep brain structures using a resonator or phased-array coil and in determining tissue-type specific VNAA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arun P, Madhavarao CN, Moffett JR, Namboodiri MA. Regulation of N-acetylaspartate and N-acetylaspartylglutamate biosynthesis by protein kinase activators. J Neurochem. 2006;98:2034–2042. doi: 10.1111/j.1471-4159.2006.04068.x. [DOI] [PubMed] [Google Scholar]
- Barany M, Spigos DG, Mok E, Venkatasubramanian PN, Wilbur AC, Langer BG. High resolution proton magnetic resonance spectroscopy of human brain and liver. Magn Reson Imaging. 1987;5:393–398. doi: 10.1016/0730-725x(87)90128-7. [DOI] [PubMed] [Google Scholar]
- Baslow MH, Suckow RF, Sapirstein V, Hungund BL. Expression of aspartoacylase activity in cultured rat macroglial cells is limited to oligodendrocytes. J Mol Neurosci. 1999;13:47–53. doi: 10.1385/JMN:13:1-2:47. [DOI] [PubMed] [Google Scholar]
- Boumezbeur F, Besret L, Valette J, Vaufrey F, Henry P, Slavov V, Giacomini E, Hantraye P, Bloch G, Lebon V. NMR measurement of brain oxidative metabolism in monkeys using 13C-labeled glucose without a 13C radiofrequency channel. Magn Reson Med. 2004;52:33–40. doi: 10.1002/mrm.20129. [DOI] [PubMed] [Google Scholar]
- Burns S, Chalmers R, West R, Iles R. Measurement of human brain aspartate N-acetyl transferase flux in vivo. Biochem Soc Trans. 1992;20:107S. doi: 10.1042/bst020107s. [DOI] [PubMed] [Google Scholar]
- Burri R, Stefeen C, Herschkowitz N. N-acetyl-L-aspartate is a major source of acetyl groups for lipid synthesis during rat brain development. Dev Neurosci. 1991;13:403–411. doi: 10.1159/000112191. [DOI] [PubMed] [Google Scholar]
- Bzdega T, Turi T, Wroblewska B, She D, Chung HS, Kim H, Neale JH. Molecular cloning of a peptidase against N-acetylaspartylglutamate from a rat hippocampal cDNA library. J Neurochem. 1997;69:2270–2277. doi: 10.1046/j.1471-4159.1997.69062270.x. [DOI] [PubMed] [Google Scholar]
- Chen W, Adriany G, Zhu XH, Gruetter R, Ugurbil K. Detecting natural abundance carbon signal of NAA metabolite within 12-cm3 localized volume of human brain using 1H-[13C] NMR spectroscopy. Magn Reson Med. 1998;40:180–184. doi: 10.1002/mrm.1910400203. [DOI] [PubMed] [Google Scholar]
- Chen Z, Li SS, Yang J, Letizia D, Shen J. Measurement and automatic correction of high-order B0 inhomogeneity in the rat brain at 11.7 Tesla. Mag Reson Imag. 2004;2:835–842. doi: 10.1016/j.mri.2004.01.062. [DOI] [PubMed] [Google Scholar]
- Choi I, Gruetter R. Dynamic or inert metabolism? Turnover of N-acetyl aspartate and glutathione from D-[1-13C]glucose in the rat brain in vivo. J Neurochem. 2004;91:778–787. doi: 10.1111/j.1471-4159.2004.02716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JB. N-acetyl aspartate: a marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci. 1998;20:271–276. doi: 10.1159/000017321. [DOI] [PubMed] [Google Scholar]
- Conolly S, Glover G, Nishimura D, Macovski A. A reduced power selective adiabatic spin-echo pulse sequence. Magn Reson Med. 1991;18:28–38. doi: 10.1002/mrm.1910180105. [DOI] [PubMed] [Google Scholar]
- DiResta GR, Lee JB, Arbit E. Measurement of brain tissue specific gravity using pycnometry. J Neurosci Methods. 1991;39:245–251. doi: 10.1016/0165-0270(91)90103-7. [DOI] [PubMed] [Google Scholar]
- Fan TW, Higashi RM, Lane AN, Jardetzky O. Combined use of 1H-NMR and GC-MS for metabolite monitoring and in vivo1H-NMR assignments. Biochim Biophys Acta. 1986;882:154–167. doi: 10.1016/0304-4165(86)90150-9. [DOI] [PubMed] [Google Scholar]
- Fuhrman S, Palkovits M, Cassidy M, Neale JH. The Regional Distribution of N-Acetylaspartylglutamate (NAAG) and Peptidase Activity Against NAAG in the Rat Nervous System. J Neurochem. 1994;62:275–281. doi: 10.1046/j.1471-4159.1994.62010275.x. [DOI] [PubMed] [Google Scholar]
- Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med. 1993;29:804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- Gueron M, Plateau P, Decorps M. Solvent signal suppression in NMR. Progr NMR Spectrosc. 1991;23:135–209. [Google Scholar]
- Harris K, Lin A, Bhattacharya P, Tran T, Wong W, Ross BD. Regulation of NAA-synthesis in the human grain in vivo: Canavan’s disease, Alzheimer’s desease and schizophrenia. In: Moffett JR, Tieman SS, Weinberger DR, Coyle JT, MA Namboodiri MA, editors. N-Acetylaspartate: A Unique Naeronal Molecule in the Central Nervous Ststem. New York: Springer Science + Business Media; 2006. pp. 263–273. [Google Scholar]
- Kaul R, Casanova J, Johnson AB, Tang P, Matalon R. Purification, characterization, and localization of aspartoacylase from bovine brain. J Neurochem. 1991;56:129–135. doi: 10.1111/j.1471-4159.1991.tb02571.x. [DOI] [PubMed] [Google Scholar]
- Koller KJ, Zaczek R, Coyle JT. N-acetyl-aspartyl-glutamate: regional levels in rat brain and the effects of brain lesions as determined by a new HPLC method. J Neurochem. 1984;43:1136–1142. doi: 10.1111/j.1471-4159.1984.tb12854.x. [DOI] [PubMed] [Google Scholar]
- Kvittingen E, Guldal G, Borsting S, Skalpe I, Stokke O, Jellum E. N-acetylaspartic aciduria in a child with a progressive cerebral atrophy. Clin Chim Acta. 1986;158:217–227. doi: 10.1016/0009-8981(86)90285-8. [DOI] [PubMed] [Google Scholar]
- Luthi-Carter R, Berger UV, Barczak AK, Enna M, Coyle JT. Isolation and expression of a rat brain cDNA encoding glutamate carboxypeptidase II. Proc Natl Acad Sci U S A. 1998;95:3215–3220. doi: 10.1073/pnas.95.6.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten PR, den Hollander JA. Observation of metabolites in the human brain by MR spectroscopy. Radiology. 1986;161:795–798. doi: 10.1148/radiology.161.3.3786735. [DOI] [PubMed] [Google Scholar]
- Matalon R, Michals K, Sebesta D, Deanching M, Gashkoff P, Casanova J. Aspartoacylase deficiency and N-acetylaspartic aciduria in patients with Canavan disease. Am J Med Genet. 1988;29:463–471. doi: 10.1002/ajmg.1320290234. [DOI] [PubMed] [Google Scholar]
- Miyake M, Kakimoto Y, Sorimachi M. A gas chromatographic method for the determination of N-acetyl-L-aspartic acid, N-acetyl-alpha-aspartylglutamic acid and beta-citryl-L-glutamic acid and their distributions in the brain and other organs of various species of animals. J Neurochem. 1981;36:804–810. doi: 10.1111/j.1471-4159.1981.tb01665.x. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AMA. N- Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno A, Ross BD, Blüml S. Direct determination of the N-acetyl-L-aspartate synthesis rate in the human brain by 13C MRS and [1-13C]glucose infusion. J Neurochem. 2001;77:347–350. doi: 10.1046/j.1471-4159.2001.t01-1-00282.x. [DOI] [PubMed] [Google Scholar]
- Pan JW, Takahashi K. Interdependence of N-acetyl aspartate and high-energy phosphates in healthy human brain. Ann Neurol. 2005;57:92–97. doi: 10.1002/ana.20317. [DOI] [PubMed] [Google Scholar]
- Patel TB, Clark JB. Synthesis of N-acetyl-L-aspartate by rat brain mitochondria and its involvement in mitochondrial/cytosolic carbon transport. Biochem J. 1979;184:539–546. doi: 10.1042/bj1840539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MB, Blakely RD, Couto R, Coyle JT. Hydrolysis of the brain dipeptide N-acetyl-L-aspartyl-L-glutamate. Identification and characterization of a novel N-acetylated alpha-linked acidic dipeptidase activity from rat brain. J Biol Chem. 1987;262:14498–14506. [PubMed] [Google Scholar]
- Slotboom J, Mehlkopf AF, Bovee WMMJ. A single-shot localization pulse sequence suited for coils with inhomogeneous RF field using adiabatic slice-selective RF pulses. J Magn Reson. 1991;95:396–404. [Google Scholar]
- Tallan HH. Studies on the distribution of N-acetyl-L-aspartic acid in brain. J Boil Chem. 1957;224:41–45. [PubMed] [Google Scholar]
- Truckenmiller ME, Namboodiri MAA, Brownstein MJ, Neale JH. N-Acetylation of L-aspartate in the nervous system: differential distribution of a specific enzyme. Neurochemistry. 1985;45:1658–1662. doi: 10.1111/j.1471-4159.1985.tb07240.x. [DOI] [PubMed] [Google Scholar]
- Tyson RL, Sutherland GR. Labeling of N-acetylaspartate and N-acetylaspartylglutamate in rat neocortex, hippocampus and cerebellum from [1-13C]glucose. Neurosci Lett. 1998;251:181–184. doi: 10.1016/s0304-3940(98)00527-8. [DOI] [PubMed] [Google Scholar]
- Xu S, Yang J, Li CQ, Zhu W, Shen J. Metabolic alterations in focally activated primary somatosensory cortex of α-chloralose-anesthetized rats measured by 1H MRS at 11.7 T. NeuroImage. 2005;28:401–409. doi: 10.1016/j.neuroimage.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Xu S, Shen J. In vivo dynamic turnover of cerebral 13C isotopomers from [U-13C]glucose. J Magn Reson. 2006;182:221–228. doi: 10.1016/j.jmr.2006.07.003. [DOI] [PubMed] [Google Scholar]