Abstract
Lysine-enriched analogs of the cecropin-mellitin hybrid peptide, CA1-7 M2-9 (designated CM15), designed with optimized amphipathicity, retained antimicrobial activities similar to that of wild-type CM15 and had substantially reduced levels of hemolytic activity and cytotoxicity toward cultured macrophages, resulting in enhanced selectivity. These lysine-enriched analogs provide templates for improved CM15 peptide or peptidomimetic antibiotics.
Antimicrobial peptides (AMPs) play an important role in the innate immune response (6, 13, 21) and have generated considerable interest as templates for the design of new antibiotics. Among the most promising AMPs are synthetic hybrids of cecropin and mellitin (1, 2, 5, 7, 10, 14, 18), and in particular, a 15-residue peptide composed of the first seven amino acids of cecropin A and residues 2 to 9 of mellitin, CA1-7 M2-9 (designated CM15 hereafter). In this study, we have examined the effects of introducing additional lysine residues into CM15 at positions designed to enhance the amphipathic distribution of amino acids. We find that these lysine-enriched peptides retain strong antimicrobial activity and, most importantly, have markedly reduced toxicity toward eukaryotic cells.
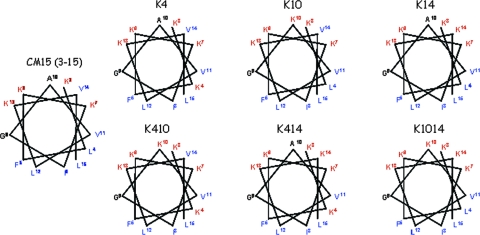
The results of previous studies have shown that CM15 is α-helical in the presence of helix-promoting solvents (15) or membranes (15, 16). In an α-helical configuration, CM15 has an almost-ideal amphipathic distribution of amino acid side chains (3, 16). Omitting the first two residues (Lys1 Trp2), which are not in a helical configuration in either full-length cecropins (12, 19) or a 26-residue cecropin-mellitin hybrid (20), the remaining four lysine residues lie along one surface of the helix, and the opposite face is composed entirely of nonpolar residues (Fig. 1). However, two sites on the polar face of the helix, alanine 10 and valine 14, are occupied by nonpolar amino acids. We replaced these two residues, individually and in combination, with lysines to yield analogs with enhanced amphipathicity (Fig. 1 and Table 1). In addition, we also examined analogs containing a leucine-to-lysine substitution in the N-terminal domain at residue 4. In contrast to alanine 10 and valine 14, this site is located on the hydrophobic face of the helix and the mutation therefore results in peptides with overall hydrophobicities similar to those ofthe other lysine-enriched peptides but with reduced amphipathicity (Table 1).
FIG. 1.
Helical wheel displays of wild-type CM15 with the first two residues (Lys1 Trp2) omitted and its lysine-enriched analogs. Hydrophilic residues are shown in red, hydrophobic residues in blue, and neutral residues in black.
TABLE 1.
Biophysical properties of CM15 and its lysine-enriched analogs
| Peptide | Sequencea | Mean residue hydrophobicityb | Hydrophobic momentb,c | Retention time (min)d |
|---|---|---|---|---|
| CM15 | KWKLFKKIGAVLKVL | 0.81 | 0.55 | 18.49 |
| K4 | KWKKFKKIGAVLKVL | −0.50 | 0.44 | 9.76 |
| K10 | KWKLFKKIGKVLKVL | 0.21 | 0.64 | 13.36 |
| K14 | KWKLFKKIGAVLKKL | −0.12 | 0.65 | 14.61 |
| K410 | KWKKFKKIGKVLKVL | −1.09 | 0.53 | 8.37 |
| K414 | KWKKFKKIGAVLKKL | −1.44 | 0.57 | 8.73 |
| K1014 | KWKLFKKIGKVLKKL | −0.72 | 0.75 | 12.05 |
Peptides were synthesized with acetylated N termini and amidated C termini and purified by semipreparative reverse-phase high-pressure liquid chromatography (RP-HPLC) as described previously (3). Analytical RP-HPLC and mass spectrometry were used to verify purity and composition. MICs were determined by standard methods (17). All of the peptides retained excellent activity against two gram-negative organisms, Escherichia coli and Pseudomonas aeruginosa (Table 2). In contrast, peptides containing a lysine substitution at position 4 had significantly diminished activity against each of two different strains (methicillin-sensitive 6538p and the methicillin-resistant BBA-41) of Staphyloccus aureus. The reason for this is still under investigation, but it suggests that the retention of the highly amphipathic character of the N-terminal domain is essential for activity against S. aureus. This loss of activity was not general for gram-positive organisms, as all of the lysine-enriched peptides displayed excellent activity against Staphylococcus epidermidis (Table 2).
TABLE 2.
MICs and hemolytic activities
| Peptide | MIC (μM) rangea for:
|
% Hemolysisb | ||||
|---|---|---|---|---|---|---|
| E. coli BL21 | P. aeruginosa PAO1 | S. aureus 6538p | S. aureus BBA-41 | S. epidermidis | ||
| CM15 | 0.5-1 | 2-4 | 0.5-2 | 1-2 | 0.5-1 | 45 |
| K4 | 0.25-0.5 | 0.5-1 | 16 | >16 | 0.5 | 21 |
| K10 | 0.5-1 | 1-2 | 1-2 | 4 | 0.5 | 67 |
| K14 | 1-4 | 2-4 | 4-8 | 8 | 0.25-0.5 | 4 |
| K410 | 0.5 | 0.5-1 | 16 | >16 | 0.25 | 9 |
| K414 | 1-2 | 1-2 | >16 | >16 | 0.5 | 3 |
| K1014 | 1 | 1-2 | 4-8 | 16 | 0.25 | 8 |
Entries represent the range of values observed in at least three independent experiments.
Percent hemolysis observed with 64 μM peptide. Means of values from multiple independent experiments are reported.
The peptide-induced hemolysis was tested by incubating peptides with a 1% solution of fresh human erythrocytes at 37°C for 1 h, followed by measurement of the hemoglobin released. High concentrations of peptide (32 to 256 times the MIC for E. coli) were required in order to observe appreciable hemolysis, consistent with the results of previous studies showing that CM hybrid peptides are relatively nonhemolytic (2, 14). All of the lysine-enriched peptides except K10 (lysine substitution at residue 10) showed decreased hemolysis relative to that of wild-type CM15 (Fig. 2 and Table 2). In particular, the levels of hemolysis for the peptides containing two additional lysines (K410, K414, and K1014 [lysine substitutions at residues 4 and 10, 4 and 14, and 10 and 14, respectively]), along with the level of hemolysis for K14 (lysine substitution at residue 14), were very low, with less than 10% hemolysis observed at all peptide concentrations tested (Fig. 2).
FIG. 2.
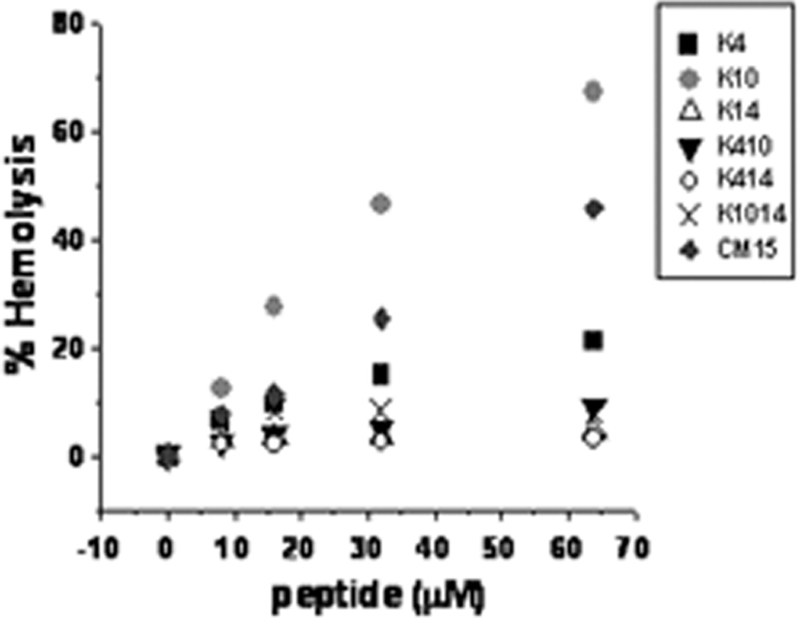
Hemolysis of fresh human erythrocytes as a function of peptide concentration. Release of hemoglobin by peptides was determined compared to that of erythrocytes lysed with 1% Triton X-100. Results from one of several independent experiments are shown.
As a measure of relative efficacy, we calculated a selectivity index, defined as the inverse of the product of the MIC and the fraction of hemolysis observed at a standard peptide concentration of 64 μM. Thus, a lower MIC and decreased hemolysis both contribute to a higher index. K4 (with a lysine substitution at residue 4), K14, K410, K414, and K1014 all had improved selectivity indices relative to that of CM15 against the two gram-negative strains. K410 displayed the highest degree of selectivity, with selectivity indices against E. coli and P. aeruginosa that were 10 to 20 times greater, respectively, than that of CM15 (Table 3). Several of the analogs had extremely high selectivity indices for S. epidermidis, as much as 30 times greater than that of CM15, owing to a combination of good antimicrobial activity and low hemolytic activity. In contrast, only K14 had an appreciably improved selectivity index against S. aureus (Table 3).
TABLE 3.
Selectivity indices
| Peptide | Selectivity indexa for:
|
|||
|---|---|---|---|---|
| E. coli BL21 | P. aeruginosa PAO1 | S. aureus 6538p | S. epidermidis | |
| CM15 | 2.2 | 0.56 | 1.1 | 2.2 |
| K4 | 9.5 | 4.8 | 0.3 | 9.5 |
| K10 | 1.5 | 0.4 | 0.8 | 3 |
| K14 | 6.25 | 6.25 | 3 | 48 |
| K410 | 22 | 11 | 0.7 | 44 |
| K414 | 16.7 | 8.3 | <0.5 | 68 |
| K1014 | 12.5 | 6.25 | 1.5 | 50 |
The selectivity index is [MIC × (fraction of hemolysis at 64 μM peptide)]−1. The values were calculated using the highest MIC observed in any independent experiment (see Table 2). For samples where the MIC was >16 μM, a value of 64 μM was assigned.
The selectivity indices against E. coli and P. aeruginosa correlated well with the mean residue hydrophobicities (r2 values of 0.887 and 0.895, respectively), with the selectivity index increasing as the peptides became more hydrophilic. There was no correlation between the selectivity indices and calculated hydrophobic moments (r2 values, ∼0.25). The efficacy did not simply correlate with the peptide charge: K4, with a charge of +6, had higher selectivity indices than K1014, with a charge of +7; K14 had indices equal to those of K1014; and K10 had lower selectivity than CM15.
To further examine the interaction of these peptides with eukaryotic cells, we examined cytotoxicity to cultured murine macrophages. Each of the lysine-enriched peptides was significantly less toxic than CM15 (P values of <0.05 to <0.001 when compared at a single concentration) (Table 4). In general, the cytotoxicity of AMPs to macrophages correlated with the data for erythrocytes, except in the case of K1014.
TABLE 4.
Percent survival of cultured murine macrophages following treatment with CM15 and its lysine-enriched analogs
| Peptide | Mean (SEM) % survival of macrophagea
|
|
|---|---|---|
| RAW 264.7 | J744 A.1 | |
| CM15 | 21 (9) | 16 (1) |
| K4 | 73 (21) | 81 (1) |
| K10 | 56 (16)† | 43 (13)† |
| K14 | 88 (1)§ | 74 (15) |
| K410 | 86 (22) | 88 (18) |
| K414 | 104 (1)§ | 94 (4)§ |
| K1014 | 57 (16)† | 45 (4) |
Percent survival in comparison to the survival of buffer-treated controls, based on the conversion of MTT [3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide] to formazan. Values are the mean and standard error of the mean of the results from at least three independent experiments. All peptides were at a final concentration of 30 μM, with the exception of wild-type CM15, which was at 10 μM. †, P < 0.05; §, P < 0.001. All other samples had a P value of <0.01 compared to the results for wild-type CM15. P values are based on Student's t test.
The ability of AMPs to interact with membranes is thought to be governed by a relative balance of hydrophobic and hydrophilic amino acids, as well as their amphipathic distribution (4, 8, 9, 11, 22). In this study, all of the peptides tested contained a sufficient number of nonpolar residues (8 to 10 out of 15 total) to retain antimicrobial activity. There was a strong inverse correlation between peptide hydrophobicity and hemolytic activity, and this general trend held for toxicity to murine macrophages. To our knowledge, this is the first report of CM15 analogs with improved selectivity. Our results suggest that optimization of amphipathicity should be incorporated when using CM15 in the design of peptide or peptidomimetic antibiotics. The lysine-enriched analogs of CM15 described in this study may therefore serve as templates for even further improvements in selectivity.
Acknowledgments
This work was supported by Public Health Service grant GM068829 from the U.S. National Institutes of Health.
Footnotes
Published ahead of print on 13 October 2008.
REFERENCES
- 1.Alberola, J., A. Rodriguez, O. Francino, X. Roura, L. Rivas, and D. Andreu. 2004. Safety and efficacy of antimicrobial peptides against naturally acquired leishmaniasis. Antimicrob. Agents Chemother. 48:641-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreu, D., J. Ubach, A. Boman, B. Wahlin, D. Wade, R. B. Merrifield, and H. G. Boman. 1992. Shortened cecropin A-melittin hybrids. Significant size reduction retains potent antibiotic activity. FEBS Lett. 296:190-194. [DOI] [PubMed] [Google Scholar]
- 3.Bhargava, K., and J. B. Feix. 2004. Membrane binding, structure, and localization of cecropin-mellitin hybrid peptides: a site-directed spin-labeling study. Biophys. J. 86:329-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blondelle, S. E., J. M. Ostresh, R. A. Houghten, and E. Perez-Paya. 1995. Induced conformational states of amphipathic peptides in aqueous/lipid environments. Biophys. J. 68:351-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boman, H. G., D. Wade, I. A. Boman, B. Wahlin, and R. B. Merrifield. 1989. Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett. 259:103-106. [DOI] [PubMed] [Google Scholar]
- 6.Brogden, K. A., J. M. Guthmiller, M. Salzet, and M. Zasloff. 2005. The nervous system and innate immunity: the neuropeptide connection. Nat. Immunol. 6:558-564. [DOI] [PubMed] [Google Scholar]
- 7.Chicharro, C., C. Granata, R. Lozano, D. Andreu, and L. Rivas. 2001. N-terminal fatty acid substitution increases the leishmanicidal activity of CA(1-7)M(2-9), a cecropin-melittin hybrid peptide. Antimicrob. Agents Chemother. 45:2441-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dathe, M., and T. Wieprecht. 1999. Structural features of helical antimicrobial peptides. Biochim. Biophys. Acta 1462:71-87. [DOI] [PubMed] [Google Scholar]
- 9.Epand, R. M., and H. J. Vogel. 1999. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta 1462:11-28. [DOI] [PubMed] [Google Scholar]
- 10.Giacometti, A., O. Cirioni, W. Kamysz, G. D'Amato, C. Silvestri, M. Simona Del Prete, J. Lukasiak, and G. Scalise. 2004. In vitro activity and killing effect of the synthetic hybrid cecropin A-melittin peptide CA(1-7)M(2-9)NH(2) on methicillin-resistant nosocomial isolates of Staphylococcus aureus and interactions with clinically used antibiotics. Diagn. Microbiol. Infect. Dis. 49:197-200. [DOI] [PubMed] [Google Scholar]
- 11.Giangaspero, A., L. Sandri, and A. Tossi. 2001. Amphipathic alpha-helical antimicrobial peptides. Eur. J. Biochem. 268:5589-5600. [DOI] [PubMed] [Google Scholar]
- 12.Holak, T. A., A. Engstrom, P. J. Kraulis, G. Lindeberg, H. Bennich, T. A. Jones, A. M. Gronenborn, and G. M. Clore. 1988. The solution conformation of the antibacterial peptide cecropin A: a nuclear magnetic resonance and dynamical simulated annealing study. Biochemistry 27:7620-7629. [DOI] [PubMed] [Google Scholar]
- 13.Jenssen, H., P. Hamill, and R. E. W. Hancock. 2006. Peptide antimicrobial agents. Clin. Microbiol. Rev. 19:491-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juvvadi, P., S. Vunnam, E. L. Merrifield, H. G. Boman, and R. B. Merrifield. 1996. Hydrophobic effects on antibacterial and channel-forming properties of cecropin A-melittin hybrids. J. Pep. Sci. 2:223-232. [DOI] [PubMed] [Google Scholar]
- 15.Pistolesi, S., R. Pogni, and J. B. Feix. 2007. Membrane insertion and bilayer perturbation by antimicrobial peptide CM15. Biophys. J. 93:1651-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato, H., and J. B. Feix. 2006. Peptide-membrane interactions and mechanisms of membrane destruction by amphipathic alpha-helical antimicrobial peptides. Biochim. Biophys. Acta 1758:1245-1256. [DOI] [PubMed] [Google Scholar]
- 17.Sato, H., and J. B. Feix. 2006. Osmoprotection of bacterial cells from toxicity caused by antimicrobial hybrid peptide CM15. Biochemistry 45:9997-10007. [DOI] [PubMed] [Google Scholar]
- 18.Saugar, J. M., M. J. Rodriguez-Hernandez, B. G. de la Torre, M. E. Pachon-Ibanez, M. Fernandez-Reyes, D. Andreu, J. Pachon, and L. Rivas. 2006. Activity of cecropin A-melittin hybrid peptides against colistin-resistant clinical strains of Acinetobacter baumannii: molecular basis for the differential mechanisms of action. Antimicrob. Agents Chemother. 50:1251-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sipos, D., M. Andersson, and A. Ehrenberg. 1992. The structure of the mammalian antibacterial peptide cecropin P1 in solution, determined by proton-NMR. Eur. J. Biochem. 209:163-169. [DOI] [PubMed] [Google Scholar]
- 20.Sipos, D., K. Chandrasekhar, K. Arvidsson, A. Engstrom, and A. Ehrenberg. 1991. Two-dimensional proton-NMR studies on a hybrid peptide between cecropin A and melittin. Resonance assignments and secondary structure. Eur. J. Biochem. 199:285-291. [DOI] [PubMed] [Google Scholar]
- 21.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]
- 22.Zelezetsky, I., and A. Tossi. 2006. Alpha-helical antimicrobial peptides: using a sequence template to guide structure-activity relationship studies. Biochim. Biophys. Acta 1758:1436-1449. [DOI] [PubMed] [Google Scholar]