Abstract
Background
The diagnosis of calpainopathy is obtained by identifying calpain‐3 protein deficiency or CAPN3 gene mutations. However, in many patients with limb girdle muscular dystrophy type 2A (LGMD2A), the calpain‐3 protein quantity is normal because loss‐of‐function mutations cause its enzymatic inactivation. The identification of such patients is difficult unless a functional test suggests pursuing a search for mutations.
Materials and methods
A functional in vitro assay, which was able to test calpain‐3 autolytic function, was used to screen a large series of muscle biopsy specimens from patients with unclassified LGMD/hyperCKaemia who have previously shown normal calpain‐3 protein quantity.
Results
Of 148 muscle biopsy specimens tested,17 samples (11%) had lost normal autolytic function. CAPN3 gene mutations were identified in 15 of 17 patients (88%), who account for about 20% of the total patients with LGMD2A diagnosed in our series.
Conclusions
The loss of calpain‐3 autolytic activity is highly predictive of primary calpainopathy, and the use of this test as part of calpainopathy diagnosis would improve the rate of disease detection markedly. This study provides the first evidence of the pathogenetic effect of specific CAPN3 gene mutations on the corresponding protein function in LGMD2A muscle and offers new insights into the structural–functional relationship of the gene and protein regions that are crucial for the autolytic activity of calpain‐3.
Limb girdle muscular dystrophies (LGMDs) comprise a clinically and genetically heterogeneous group of diseases usually characterised by progressive muscle weakness and wasting of pelvic and shoulder girdles. LGMD type 2A (LGMD2A, MIM 253600) was the first form of LGMD to be mapped and molecularly characterised, probably because it is the most frequent.1,2,3,4,5 LGMD2A is caused by mutations in the CAPN3 gene (MIM 114240) that encodes for a non‐structural protein, the enzyme called calpain‐3.1 Calpain‐3 is the muscle‐specific member of a family of Ca2+‐dependent proteases, which are supposed to play a part in many intracellular processes, including cell motility, apoptosis, differentiation and cell cycle regulation, by modulating the biological activity of their substrates through limited and strictly controlled proteolysis. Calpain‐3 is composed of four functional domains, and has three exclusive sequence inserts (NS, IS1 and IS2). The activation of calpain‐3 depends on phospholipids and Ca2+ ions,6,7 takes place after unknown stimuli, and results in partial autolytic degradation.8,9,10,11,12
The molecular diagnosis of calpainopathy is complex because of the variability of clinical phenotypes, the effort required to identify point mutations in a relatively large gene, and incomplete sensitivity and specificity of calpain‐3 protein analysis on muscle biopsy specimen.13,14,15,16,17,18,19
An increasing number of studies have reported patients with LGMD2A whose diagnosis had been obtained by mutation identification despite normal levels of calpain‐3 protein in their muscle biopsy specimen.4,15,20,21 As calpain‐3 is an enzyme, some mutations may not cause a reduction in protein content but its functional inactivation. Clinicians are increasingly aware that although the number of patients with LGMD2A showing normal calpain‐3 levels is marked, the identification of such cases is difficult. In patients with abnormal calpain‐3 protein levels, the effort required to search for mutations is justified because it will almost certainly confirm the LGMD2A diagnosis.16 Conversely, in patients with normal calpain‐3 protein content, an alternative diagnosis is usually pursued. For this reason, many patients with LGMD2A remain undiagnosed, unless a search is conducted for the CAPN3 gene mutation even if protein results are not indicative. To overcome this problem, we have developed a functional in vitro assay that is able to test the calpain‐3 autolytic activity in muscle samples,15 and showed that the loss of this function is associated with specific CAPN3 gene mutations.
We report the results of extensive use of this functional assay as a screening tool in muscle biopsy specimens from patients with unclassified LGMDs and normal calpain‐3 protein quantity. Besides identifying patients with loss‐of‐function mutations, which would improve the rate of detection of the disease, we aimed at designing a functional map of the mutations and the protein regions involved in the autolytic activity to determine how single mutations exert their deleterious effect on the mutant protein.
Materials and methods
Selection criteria of patients
The muscle biopsy bank at the Neuromuscular Centre, University of Padova (Padova, Italy), which contains over 6000 specimens, was surveyed for patients with an unidentified form of muscular dystrophy, proximal myopathy or asymptomatic hyperCKaemia, who fulfilled the following criteria:
normal protein levels of dystrophin, α‐sarcoglycan, dysferlin, emerin, caveolin‐3 and calpain‐3;
muscle biopsy histopathology consistent with a dystrophic or myopathic process;
increased serum creatine kinase concentrations (>500 U/l, normal values 0–190 U/l).
We excluded from the study patients who, after clinical and muscle biopsy specimen evaluation, had congenital muscular dystrophy (neonatal muscle weakness with dystrophic muscle histopathology), congenital myopathy (peculiar structural muscle pathological changes), distal myopathy (overt weakness in distal leg muscles), dominant muscular dystrophy, and metabolic or inflammatory myopathy.
We also excluded from the study, patients who showed quantitative calpain‐3 protein defect (absent or reduced) on western blot, as the constitutive protein deficiency prevents the assessment of any biochemical protein function, such as autolysis.
A total of 148 patients matched our selection criteria and were selected for the study of calpain‐3 autocatalytic activity in their muscle biopsy specimens: 136 had the LGMD phenotype either with pelvic onset (classical LGMD) or with shoulder onset (Erb), and 12 were asymptomatic with hyperCKaemia. According to the age at onset of muscle weakness, we defined our patients as follows: “early‐onset” LGMD when onset was before 12 years of age, classic LGMD phenotype when onset was between 12 and 30 years of age, and “late‐onset” LGMD when onset was after 30 years of age.
As normal controls we used muscle biopsy specimens from patients who were free of any muscle disease and had normal creatine kinase levels; as neuromuscular controls we used 20 muscle biopsy specimens from patients with various molecularly defined muscular dystrophies (Duchenne, Becker, LGMD2D, LGMD2B, LGMD1C and facio‐scapulo‐humeral dystrophy).
Muscle biopsies
At the time of diagnosis, an open biopsy of quadriceps femoris or biceps brachii muscle was obtained after written informed consent. Immediately after surgery, muscle specimens were frozen in liquid nitrogen and stored at −80°C. The possibility that in some of the samples in this study a spontaneous calpain‐3 protein degradation had occurred as a consequence of long‐term conservation or poor tissue preservation conditions13 was ruled out by the preliminary demonstration of normal protein expression on conventional calpain‐3 immunoblot (see inclusion criteria).
Semiquantitative immunoblot analysis of calpain‐3
Two parallel sets of 20 cryostat sections (10 μm thick) from patients and control muscle biopsy specimens were collected in tubes chilled in liquid nitrogen. One set of sections was used for total protein determination by Bradford's method. The second set of sections was quickly dissolved in Laemmli loading buffer (0.05 M DTT, 0.1 M EDTA, 0.125 M TRIS, 4% sodium dodecyl sulphate, 10% glycerol, 0.005% bromophenol blue, pH = 8), boiled for 3 min and centrifuged. A volume corresponding to 40 μg of protein was loaded in duplicated 1.5‐mm‐thick 3.5–12% polyacrylamide gradient gels. Proteins were resolved by overnight sodium dodecyl sulphate‐poly‐acrylamide gel electrophoresis (110 V constant), and electroblotted (1.6 A constant) to nitrocellulose membrane for 5 h with cooling. Post‐transfer gels were stained with Coomassie blue. Nitrocellulose membranes were air dried, blocked for 1 h with 3% bovine serum albumin in Tris‐buffered saline with Tween‐20 (TTBS) and incubated for 1 h with Calp‐12A2 anti‐calpain‐3 antibody (Novocastra Laboratories, Newcastle upon Tyne, UK; http://www.novocastra.co.uk) diluted 1:800 in TTBS.
Immunoreactive bands were detected using anti‐mouse biotinylated Ig and streptavidin–horseradish peroxidase complex (Amersham, Little Chalfont, UK; http://www.amersham.com), diluted 1:1000 in TTBS for 1 h each, and the ECL chemioluminescence system (Amersham). Visualisation of specific bands was obtained by exposure of blots to photographic films. The amount of calpain‐3 protein band (molecular weight 94 kDa) was normalised to the amount of tissue loaded, as determined by the skeletal myosin bands in the post‐transfer Coomassie blue‐stained gels, and quantitated by densitometry using ImageJ software V.1.34.
Immunoblot analysis of calpain‐3 autolytic activity
To test calpain‐3 autolytic activity, we used an in vitro assay that was developed by modifying the conventional immunoblot.15 Muscle cryostat sections from controls and patients were quickly dissolved in 50 μl of saline solution (0.9% NaCl in distilled water) and incubated at room temperature for 5 min, before blocking the reaction by adding a further 50 μl of the loading buffer containing EDTA (the reaction depends on trace amounts of Ca2+ ions). All samples were then processed as described for the conventional calpain‐3 immunoblot.
CAPN3 gene mutation analysis
Genomic DNA from patients with loss of autolytic activity was extracted from blood leucocytes or muscle tissue using a commercially available DNA extraction kit and method (Sigma, St Louis, Missouri, USA; http://pubs.acs.org/pin/sigma/sigma.html). Screening for CAPN3 gene mutations was performed by single‐strand conformational polymorphism (SSCP) technique using primers and conditions described previously.15,16 The coding sequences of exons 1, 4, 5, 10, 11, 13, 17 and 21 (which are most commonly involved in mutations) were amplified by PCR; the PCR products containing aberrant migration bands after SSCP analysis were purified by enzyme reaction (ExoSap‐I, Amersham) and directly sequenced using the BigDye di‐deoxy‐terminator cycle sequencing kit (Applied Biosystems, Foster City, California, USA; http://www.appliedbiosystems.com). Extension products were run on an ABI‐PRISM 3700 automated sequencer (Applied Biosystems) at the CRIBI Biotechnology Centre, University of Padova, Padova, Italy. Sequence analysis was conducted using Chromas software with the human CAPN3 gene sequence as reference (GenBank accession #AF209502.1). Each mutation found was confirmed using allele‐specific tests.
Calpain‐3 structure modelling
The macromolecular 3D structure of calpain‐3 protein domains and the localisation of the amino acids involved in mutations affecting autolytic activity were analysed using the open source version 2.6 of RasMol software (www.openrasmol.org/). Because a molecular model of calpain‐3 is not available owing to its rapid autolysis and extremely short half‐life, we used the crystal structure generated by the superimposition of the calpain‐3 sequence on the human m‐calpain large subunit (which has high sequence homology with calpain‐3). The coordinates of the reported protein structure were obtained from the Protein Data Bank (www.pdb.org/).
Results
Screening of calpain‐3 autolytic activity
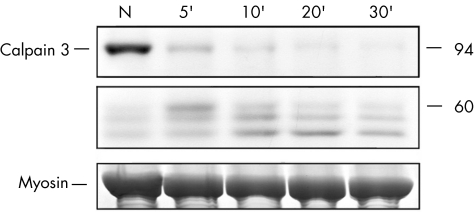
All muscle biopsy specimens included in this study, which previously showed normal calpain‐3 protein content under standard immunoblot conditions, were subjected to further analysis of calpain‐3 autolytic activity. This assay is based on the observation that normal muscle usually presents physiological calpain‐3 autolytic activity, which leads to almost complete protein degradation of the calpain‐3 band at 94 kDa after 5 min of incubation with saline solution, and to the progressive appearance of degradation products after prolonged incubation times (fig 1).
Figure 1 Functional assay of calpain‐3 autolytic activity on a muscle biopsy specimen from a normal control. The muscle sample is loaded after conventional treatment for calpain‐3 immunoblot analysis (N) or under experimental conditions that induce spontaneous protein autolysis (incubation in saline solution for different times: 5, 10, 20, and 30 min). The upper panel shows the bands corresponding to the full‐length calpain‐3 protein at 94 kDa molecular weight and the middle panel shows the triplet of calpain‐3 bands at lower molecular weights (60, 58 and 55 kDa) resulting from protein degradation after autolysis. Physiological calpain‐3 autolytic activity leads to almost complete disappearance of the full‐length protein after 5 min and to the production of small‐sized degradation bands after prolonged incubation times. The myosin content in the post‐transfer Coomassie blue‐stained gel (lower panel) is used to normalise the amount of calpain‐3 in each lane.
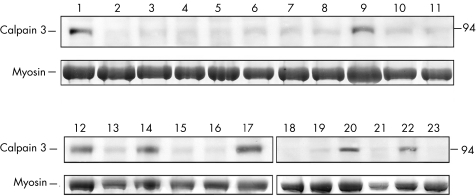
The screening of calpain‐3 autolytic activity showed that in 131 of the 148 (88%) muscle biopsy specimens analysed there was full protein degradation, which corresponds to normal function. The same result was observed in all the samples from disease controls (data not shown). Conversely, after autolysis the muscle biopsy specimens from 17 (11%) patients with unclassified LGMD or hyperCKaemia showed lack of protein degradation and a considerable amount of residual non‐degraded protein (fig 2). This abnormal finding suggests that the normal autolytic function has been lost in such cases.
Figure 2 Screening of calpain‐3 autolytic activity in muscle biopsy specimens from patients with unclassified LGMD or hyperCKaemia. Lanes numbered 1–23 correspond to muscle samples from 23 different patients loaded after 5 min of incubation in saline solution, to promote calpain‐3 autolysis. The upper panel shows bands corresponding to the full‐length calpain‐3 protein at 94 kDa molecular weight, and the lower panel shows bands corresponding to myosin content in the post‐transfer Coomassie blue‐stained gel used to normalise the amount of calpain‐3 in each lane. In most muscle biopsy specimens analysed, there was full protein degradation, corresponding to normal function. Conversely, muscle samples numbered 1, 9, 12, 14, 17, 20 and 22 show the lack of protein degradation, resulting in a considerable amount of non‐degraded protein, which suggests the loss of normal autolytic function.
CAPN3 gene mutations
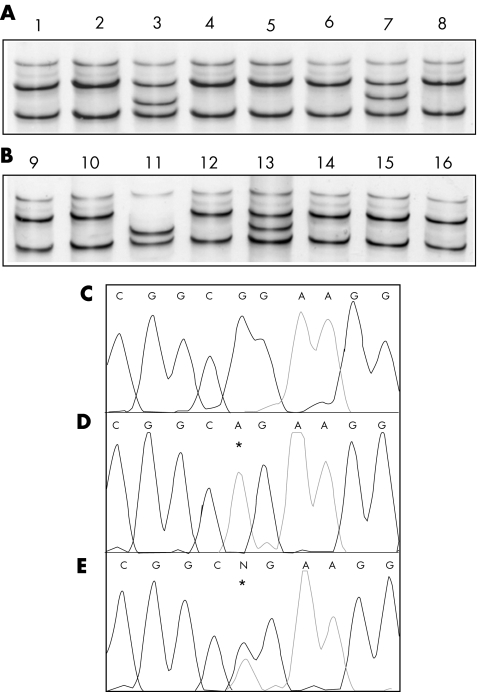
The screening of mutations in eight exons of the CAPN3 gene, which was conducted in all 17 patients with loss of calpain‐3 autolytic activity, allowed us to identify 15 (88%) patients with mutations (fig 3, table 1). This result indicates that the loss of autolytic activity is highly predictive of primary calpainopathy. We have calculated that these 15 patients with mutations account for about 20% of the total number of patients with LGMD2A so far diagnosed in our series (15/79), which suggests that the use of this diagnostic assay would help to significantly increase the detection rate of the disease.
Figure 3 CAPN3 gene mutation analysis in DNA samples from patients with loss of calpain‐3 autolytic activity. Example of SSCP analysis (A, B) showing the aberrant migration pattern (conformer) of samples numbered 3, 7, 11 and 13 as compared with control (number 1). Example of direct DNA sequencing of exon 11 in a normal control (C), in a patient homozygotic for 1469G→A, R490Q missense mutation (D), and in a patient heterozygote for 1469G→A, R490Q missense mutation (E). Asterisks indicate the localisation of mutant nucleotides.
Table 1 Loss of calpain‐3 autolytic activity in patients with LGMD2A.
| Pt no, sex | Clinical phenotype | Nucleotide change | Amino acid change, zygosity | Exon |
|---|---|---|---|---|
| 1, M | Early‐onset LGMD | 1469G→A | R490Q, homozygote | 11 |
| 2, M | LGMD | 1469G→A | R490Q, homozygote | 11 |
| 3, M | High CK at 11 years | 1469G→A | R490Q, heterozygote | 11 |
| 1466G→A | R489Q, heterozygote | 11 | ||
| 4, M | Early‐onset LGMD | 1486G→A | G496R, heterozygote | 11 |
| 984C→T | C328X, heterozygote | 7 | ||
| 5, M | LGMD | 1468C→T | R490W, heterozygote | 11 |
| 6, F | Erb | 1469G→A | R490Q, homozygote | 11 |
| 7, M | Late‐onset Erb | 1469G→A | R490Q, homozygote | 11 |
| 8, M | LGMD | 1469G→A | R490Q, heterozygote | 11 |
| 550delA | T184fsX219, heterozygote | 4 | ||
| 9, F | Late‐onset LGMD | 1984G→T | A662S, heterozygote | 17 |
| 10, M | Early‐onset LGMD | 550delA | T184fsX219, heterozygote | 4 |
| 1468C→T | R490W, heterozygote | 11 | ||
| 11, F | LGMD | 575C→T | T192I, heterozygote | 4 |
| 1611C→A | Y537X, heterozygote | 13 | ||
| 12, F | Late‐onset LGMD | 1486G→A | G496R, heterozygote | 11 |
| 13, F | Early‐onset LGMD | 328C→T | R110X, heterozygote | 2 |
| 664G→A | G222R, heterozygote | 5 | ||
| 14, F | Late‐onset LGMD | 1469G→A | R490Q, homozygote | 11 |
| 15, F | Early‐onset LGMD | 1468C→T | R490W, heterozygote | 11 |
| 2242C→T | R748X, heterozygote | 21 |
CK, creatine kinase; F, female; LGMD, limb girdle muscular dystrophy; M, male.
We have identified both mutant alleles in 12 of the 15 patients with mutations (27/30 = 90% alleles). In three cases, only one mutant allele was found (table 1). The possibility that these three patients were true heterozygotes instead of compound heterozygotes seems unlikely for several reasons: they are clinically affected by LGMD; the calpain‐3 autolytic activity in their muscle biopsy was lost, and this finding seems to be specific for primary calpainopathy; the hypothetical occurrence of three isolated heterozygotes among a total of 15 cases cannot be explained by chance because the heterozygote frequency in the population is estimated to be 1:120–1:1505,22; and the incomplete sensitivity of the SSCP method and the fact that the entire coding sequence of the CAPN3 gene has not been analysed suggest that the second mutant allele has most likely escaped identification in such cases. This hypothesis is also the most likely explanation for failing to identify mutations in the remaining two patients (2/16) with loss of autolytic activity.
We identified a total of 12 different CAPN3 gene mutations (R490Q, R489Q, G496R, C328X, R490W, 550delA, A662S, T192I, Y537X, R110X, G222R and R748X), seven of which are of missense type (58%) and five are of null type (42%) (ie, nonsense or frame shifting). Except for the T192I mutation in exon 4, which has been identified for the first time in this study, the other mutant alleles have previously been reported in patients with LGMD2A from different countries, according to the Leiden Muscular Dystrophy Database (www.dmd.nl).
Genotype–phenotype correlations
At the clinical level, only one out of 15 patients in this study was asymptomatic at age 11 years but showed hyperCKaemia, whereas 14 patients had LGMD with widely varying age at onset, clinical severity and distribution of muscle involvement (table 1). Most patients with early‐onset LGMD and rapid progression of the disease were compound heterozygotes for one null and one missense mutant allele. Conversely, most patients who were homozygous for one missense allele or compound heterozygotes for two missense alleles had a milder LGMD course with adult or late onset (table 1). It is worth noting that the five patients who were sharing the same homozygotic mutation (R490Q) had different degrees of muscle impairment, which suggests the important role of as yet unidentified modulating factors in the individual genetic background in determining both the clinical phenotype and progress of the disease.
Genotype–protein correlations
As expected from the study of muscle biopsy specimens with normal protein content, we did not find any patient with two null mutant alleles. Five patients in our series (no 1, 2, 6, 7 and 14) had a homozygotic mutation and 10 patients were compound heterozygotes either for two different missense alleles or for one missense and one null allele.
Whereas in five patients (no 1, 2, 6, 7, 14) the loss of autolytic activity could be easily attributed to the deleterious effect of the homozygotic mutant allele (R490Q), in the remaining 10 patients with compound heterozygotic mutations the protein phenotype should be determined by the allele with the less severe effect, so that one missense mutant allele is sufficient for the patient to have a normal amount of calpain‐3 even when the second allele is null (no 4, 8, 10, 11, 13 and 15).
The effect of the A662S mutation on protein function could not be determined in this study, as it occurred in heterozygotic state in only one patient (no 9) in whom the second mutant allele was not identified. Conversely, the loss of calpain‐3 autolytic activity in our study could be attributed to six missense alleles—R490Q, R489Q, G496R, R490W, T192I and G222R.
Functional map of calpain‐3 autolytic activity
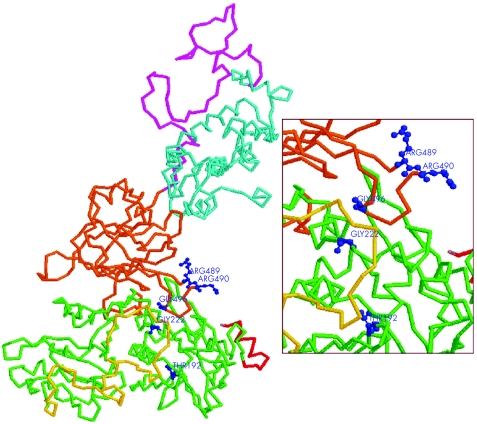
We observed that none of the missense mutations associated with the loss of calpain‐3 autolytic activity has been localised in the insert sequences NS (exon 1), IS1 (exon 6) or IS2 (exons 15, 16); conversely, four of six missense mutant alleles were clustered in exon 11 of the gene (protein domain III) and two were localised in exons 4 and 5 (protein domain II). The tridimensional view of the calpain‐3 protein showed that the amino acids involved in such mutations (except T192) were grouped together and localised at the external surface of the protein, at the contact region between domain II and domain III (fig 4).
Figure 4 Tridimensional model of calpain‐3 protein showing the functional domains and the localisation of mutant amino acids identified in patients with loss of calpain‐3 autolytic activity. The protein structure is displayed as backbone graphic mode (domain I: red; domain II: green; IS1: yellow; domain III: orange; IS2: magenta; domain IV: cyan), whereas the mutant amino acids are depicted in ball‐and‐stick graphic mode (blue). The enlarged window shows that the amino acids involved in mutations (except T192) are grouped together and localised both at the external surface of the protein and at the contact region between domain II and domain III.
Discussion
It is common experience that most patients who show calpain‐3 protein deficiency are indeed affected by primary calpainopathy,13,16,17,19 indicating that the detection of calpain‐3 protein defect helps to target CAPN3 gene mutation screening in selected patient populations.
On the other hand, several studies have shown that a sizeable proportion of patients with LGMD2A show a normal quantity of calpain‐3 protein,4,13,16,20,21 despite carrying pathogenetic mutations that presumably impair one of the calpain‐3 enzyme functions. Diagnosis in such patients has been obtained by the identification of CAPN3 gene mutations, using laborious and expensive methods to screen widespread point mutations in this relatively large gene.4,5,13,14,15,18 Sensitive methods, such as denaturing high performance liquid chromatography, could be used to screen the exons preferably involved in mutations (eg, exons 4 and 11). Furthermore, mutations in the promoter region, in the introns or large deletions18 may escape identification, thus providing false negative results. The major drawback in the diagnostic approach of searching directly for CAPN3 gene mutations in patients with an unclassified form of LGMD is the large genetic heterogeneity. Although LGMD2A is considered the most frequent form in European countries, direct mutation search is often inefficient in patients with unclassified LGMD, highlighting the role of protein analysis in addressing mutation analysis. Protein testing however, could be unsuitable for identifying patients with mutations and a normal amount of non‐functional protein, and emphasises the need for a muscle marker to identify this group of patients who otherwise would remain undiagnosed. A simple assay that tests calpain‐3 autolytic activity in muscle15 has been used in this study as a screening tool to select patients with loss of this function for subsequent mutation analysis.
Screening of calpain‐3 autolytic function on muscle biopsy specimens
We used this assay in a retrospective analysis of a large series of muscle biopsy specimens from patients with unclassified LGMD or hyperCKaemia and normal calpain‐3 protein quantity. Although the sensitivity of this method could hardly be shown, its specificity seems very high, because CAPN3 gene mutations have been found in 88% of positive cases (some mutations could have escaped identification because of the technical limitations of the SSCP method).
We have shown that the loss of normal calpain‐3 autolytic activity is highly predictive of primary calpainopathy, and that the use of an autolytic test followed by screening of CAPN3 gene mutations has resulted in identification of 20% of the total number of patients with LGMD2A in our series. This relatively high proportion of cases can partly be attributed to the recurrence of the R490Q mutation in a population from northeastern Italy.22 However, the R490Q mutation is the second most frequent mutant allele in patients with LGMD2A from Turkey,23 and it has also been reported in other countries according to the Leiden Muscular Dystrophy database; furthermore, even if we exclude the five cases with homozygotic R490Q mutation, the proportion of mutant patients with loss of autolytic function remains high (12.3%) and probably reflects the real estimate in populations where there are no genetic isolates. Such cases would probably not have been diagnosed following the finding of normal protein quantity on conventional immunoblotting, unless there also was thorough CAPN3 gene mutation screening which, however, is currently available only on a research basis.
Our results have shown that the assay of calpain‐3 autolytic activity is a powerful diagnostic tool, as it helps in selecting cases for the laborious CAPN3 gene mutation analysis. This new approach would improve the rate of detection of this disease markedly and it should be recommended as part of the diagnostic examination of calpainopathy.
Functional map of calpain‐3 autolytic activity
Investigations of the physiological functions of calpain‐3 protein have been complicated because of the failure to recover intact protein from cell extracts owing to its rapid autolysis and extremely short half life. The integrity of IS1 and IS2 calpain‐3‐specific regions seemed to be crucial for autolytic function. Indeed, rapid autolysis was found to be prevented in mutant COS cells expressing a deletion of IS1 and IS2.6,8 Furthermore, several missense mutant alleles have been associated with impaired calpain‐3 autolytic function by site‐directed mutagenesis on COS cells,8,9 and some of them have also been reported to be causative mutations in patients with LGMD2A.
Our study provides the first functional characterisation of CAPN3 gene mutations in the muscle from patients with LGMD2A. Indeed, the availability of a functional assay that is able to test the autolytic activity in muscle biopsy specimens allowed us to show that several missense mutations in the CAPN3 gene (T192I, G222R, R489Q, R490Q, R490W and G496R) exert their pathogenetic effect by abolishing the autolytic capability of this enzyme. These data are of crucial importance because they could be used to build up structural–functional relationships and to draw a “genetic map” of the autolytic function. Although further studies and mutation data will be necessary to identify the entire protein region involved in this function, the preliminary results obtained in this study suggest that, despite the amino acids implicated in the autolytic function being localised in different and distant exons of the CAPN3 gene, the tridimensional view of the protein has shown that they are grouped together, localised at the external surface of the protein, and confined to a small area at the contact region between domains II and III. These observations seem to agree with previous studies, which suggested that most missense alleles in the CAPN3 gene are clustered in three regions of domains II and III that were supposed to affect the intramolecular domain interactions and may impair the assembly and activation of the protein. In particular, domain III has been suggested to play an important part in Ca2+‐induced activation of calpain‐3 involving electrostatic interactions (salt bridges) with domain II.24
Our study provides novel evidence that missense mutations localised in calpain‐3 domains II and III would impair its autolytic activity, possibly because of the charge variation in the residues involved in internal salt bridges; this would finally result in a reduced sensitivity to Ca2+ ions. The pathogenetic effect of these mutations may be understood in terms of impaired communications between protein interdomains.
Acknowledgments
We acknowledge support from AFM‐Association Francaise contre le Myopathies (2004.0957/10615 to MF), Telethon‐Italy (GTF06001 to CA) and the Eurobiobank network (QLRT2001‐027769 toCA).
Abbreviations
LGMD - limb girdle muscular dystrophy
LGMD2A - limb girdle muscular dystrophy type 2A
SSCP - single‐strand conformational polymorphism
TTBS - Tris‐buffered saline with Tween‐20
Footnotes
The research in this study was funded by a grant from Telethon‐Italy (GUP030516 to MF).
Competing interests: None declared.
References
- 1.Richard I, Broux O, Allamand V.et al Mutations in the proteolytic enzyme calpain 3 cause limb‐girdle muscular dystrophy type 2A. Cell 19958127–40. [DOI] [PubMed] [Google Scholar]
- 2.Richard I, Brenguier L, Dincer P.et al Multiple independent molecular etiology for limb‐girdle muscular dystrophy type 2A patients from various geographical origins. Am J Hum Genet 1997601128–1138. [PMC free article] [PubMed] [Google Scholar]
- 3.Dincer P, Leturcq F, Richard I.et al A biochemical, genetic, and clinical survey of autosomal recessive limb girdle muscular dystrophies in Turkey. Ann Neurol 199742222–229. [DOI] [PubMed] [Google Scholar]
- 4.De Paula F, Vainzof M, Passos‐Bueno M R.et al Clinical variability in calpainopathy: what makes the difference? Eur J Hum Genet 200210825–832. [DOI] [PubMed] [Google Scholar]
- 5.Piluso G, Politano L, Aurino S.et al The extensive scanning of the calpain‐3 gene broadens the spectrum of LGMD2A phenotypes. J Med Genet 200542686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorimachi H, Toyama‐Sorimachi M, Saido T.et al Muscle specific calpain, p94, is degraded by autolysis immediately after translation, resulting in disappearance from muscle. J Biol Chem 199326810593–10605. [PubMed] [Google Scholar]
- 7.Rey M A, Davies P L. The protease core of the muscle specific calpain, p94, undergoes Ca++‐dependent intramolecular autolysis. FEBS Lett 2002532401–406. [DOI] [PubMed] [Google Scholar]
- 8.Ono Y, Shimada H, Sorimachi H.et al Functional defects of a muscle specific calpain, p94, caused by mutations associated with limb girdle muscular dystrophy type 2A. J Biol Chem 199827317073–17078. [DOI] [PubMed] [Google Scholar]
- 9.Ono Y, Torii F, Ojima K.et al Suppressed disassembly of autolyzing p94/CAPN3 by N2A connectin/titin in a genetic reporter system. J Biol Chem 200628118519–18531. [DOI] [PubMed] [Google Scholar]
- 10.Diaz B G, Moldoveanu T, Kuiper M J.et al Insertion sequence 1 of muscle‐specific calpain, p94, acts as an internal propeptide. J Biol Chem 200427927656–27666. [DOI] [PubMed] [Google Scholar]
- 11.Diaz B E G, Gauthier S, Davies P L. Ca++ dependency of calpain‐3 (p94) activation. Biochemistry 2006453714–3722. [DOI] [PubMed] [Google Scholar]
- 12.Ojima K, Ono Y, Hata S.et al Possible functions of p94 in connectin‐mediated signaling pathways in skeletal muscle cells. J Muscle Res Cell Motil 200526409–417. [DOI] [PubMed] [Google Scholar]
- 13.Anderson L V B, Davison K, Moss J A.et al Characterisation of monoclonal antibodies to calpain‐3 and protein expression in muscle from patients with limb girdle muscular dystrophy type 2A. Am J Pathol 19981531169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou F L, Angelini C, Daentl D.et al Calpain‐3 mutation analysis of a heterogeneous limb‐girdle muscular dystrophy population. Neurology 1999521015–1020. [DOI] [PubMed] [Google Scholar]
- 15.Fanin M, Nascimbeni A, Fulizio L.et al Loss of calpain‐3 autocatalytic activity in LGMD2A patients with normal protein expression. Am J Pathol 20031631929–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fanin M, Fulizio L, Nascimbeni A C.et al Molecular diagnosis in LGMD2A: mutation analysis or protein testing? Hum Mut 20042452–62. [DOI] [PubMed] [Google Scholar]
- 17.Pollitt C, Anderson L V B, Pogue R.et al The phenotype of calpainopathy: diagnosis based on a multidisciplinary approach. Neuromusc Disord 200111287–296. [DOI] [PubMed] [Google Scholar]
- 18.Richard I, Roudaut C, Saenz A.et al Calpainopathy. A survey of mutations and polymorphisms. Am J Hum Genet 1999641524–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saenz A, Leturcq F, Cobo A M.et al LGMD2A: genotype‐phenotype correlations based on a large mutational survey on the calpain 3 gene. Brain 2005128732–742. [DOI] [PubMed] [Google Scholar]
- 20.Talim B, Ognibene A, Mattioli E.et al Normal calpain expression in genetically confirmed limb‐girdle muscular dystrophy type 2A. Neurology 200156692–693. [DOI] [PubMed] [Google Scholar]
- 21.Lanzillo R, Aurino S, Fanin M.et al Early onset calpainopathy with normal non‐functional calpain‐3 level. Dev Med Child Neurol 200648304–306. [DOI] [PubMed] [Google Scholar]
- 22.Fanin M, Nascimbeni A C, Fulizio L.et al The frequency of limb girdle muscular dystrophy 2A in northeastern Italy. Neuromusc Disord 200515218–224. [DOI] [PubMed] [Google Scholar]
- 23.Balci B, Aurino S, Haliloglu G.et al Calpain‐3 mutations in Turkey. Eur J Pediatr 2006165293–298. [DOI] [PubMed] [Google Scholar]
- 24.Jia Z, Petrounevitch V, Wong A.et al Mutations in calpain‐3 associated with limb girdle muscular dystrophy: analysis by molecular modeling and by mutation in m‐calpain. Biophys J 2001802590–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]