Abstract
Post-embryonic neurogenesis is a fundamental feature of the vertebrate brain. However, the level of adult neurogenesis decreases significantly with phylogeny. In the first part of this review, a comparative analysis of adult neurogenesis and its putative roles in vertebrates are discussed. Adult neurogenesis in mammals is restricted to two telencephalic constitutively active zones. On the contrary, non-mammalian vertebrates display a considerable amount of adult neurogenesis in many brain regions. The phylogenetic differences in adult neurogenesis are poorly understood. However, a common feature of vertebrates (fish, amphibians and reptiles) that display a widespread adult neurogenesis is the substantial post-embryonic brain growth in contrast to birds and mammals. It is probable that the adult neurogenesis in fish, frogs and reptiles is related to the coordinated growth of sensory systems and corresponding sensory brain regions. Likewise, neurons are substantially added to the olfactory bulb in smell-oriented mammals in contrast to more visually oriented primates and songbirds, where much fewer neurons are added to the olfactory bulb. The second part of this review focuses on the differences in brain plasticity and regeneration in vertebrates. Interestingly, several recent studies show that neurogenesis is suppressed in the adult mammalian brain. In mammals, neurogenesis can be induced in the constitutively neurogenic brain regions as well as ectopically in response to injury, disease or experimental manipulations. Furthermore, multipotent progenitor cells can be isolated and differentiated in vitro from several otherwise silent regions of the mammalian brain. This indicates that the potential to recruit or generate neurons in non-neurogenic brain areas is not completely lost in mammals. The level of adult neurogenesis in vertebrates correlates with the capacity to regenerate injury, for example fish and amphibians exhibit the most widespread adult neurogenesis and also the greatest capacity to regenerate central nervous system injuries. Studying these phenomena in non-mammalian vertebrates may greatly increase our understanding of the mechanisms underlying regeneration and adult neurogenesis. Understanding mechanisms that regulate endogenous proliferation and neurogenic permissiveness in the adult brain is of great significance in therapeutical approaches for brain injury and disease.
Keywords: neural stem cell, adult neurogenesis, glia, zebrafish, CNS
1. Post-embryonic growth of the CNS in vertebrates
During the embryonic development of vertebrates, different classes of neurons, glia and ependymal cells are produced in a highly discrete spatio-temporal pattern (Alvarez-Buylla et al. 2001; Temple 2001).
Most neurons in the mammalian brain are generated embryonically during the restricted phases and the mature mammalian brain is characterized by a relatively constant number of neurons. There is still a low rate of constitutive gliogenesis and a more restricted neurogenesis in the adult mammalian central nervous system (CNS) (Jacobson 1991; Rakic 2002). The constitutive proliferation in adult mammals thus reflects glial and, to a lesser extent, neuronal turnover, rather than actual brain growth. Similarly, in birds, the brain has achieved its maximal mass and size within a month after birth. However, in songbirds a few brain nuclei involved in seasonal singing continue to grow post-natally for some months until they reach a maximum size (Bottjer et al. 1985; Alvarez-Buylla et al. 1992; Alvarez-Buylla & Kirn 1997; Ling et al. 1997).
In fish, amphibians and reptiles, morphometric studies have shown that the number of cells in the brain increases with age, body weight and length. However, it is not clear whether the brain grows uniformly or whether there are internal regional differences in brain growth (table 1).
Table 1.
A comparison of brain regions where constitutive adult neurogenesis occurs in vertebrates (see text for references). (+, regions with well-documented constitutive neurogenesis; (+), regions where neurogenesis have been reported occasionally or been induced experimentally; −, region lacking documented adult neurogenesis.)
| mammals | birds | reptiles | amphibians | teleost fish | |
|---|---|---|---|---|---|
| olfactory bulb | (+) | − | + | − | + |
| telencephalon | |||||
| ventral telencephalon/subpallium/SVZ | + | + | + | + | + |
| dorsal telencephalon/medial pallium/hippocampus | + | + | + | + | + |
| diencephalons | |||||
| preoptic region | − | − | − | + | + |
| epithalamus, habenula | − | − | − | − | + |
| thalamus | − | − | − | + | + |
| hypothalamus | − | (+) | − | + | + |
| pretectum | − | − | − | − | + |
| mesencephalon and rhombencephalon and spinal cord | |||||
| tectum | − | − | + | + | |
| cerebellum | (+) | − | + | + | + |
| midbrain | (+) | − | − | + | + |
| vagal complex | (+) | − | − | − | + |
| spinal cord | (+) | − | − | − | + |
Morphometric studies on post-natally growing reptiles demonstrated a significant age–size related increase in brain mass and number of cortical neurons (Platel 1974; Lopez-Garcia et al. 1984; Font et al. 2001). For example, the number of neurons in the medial cortex increases fourfold (Lopez-Garcia et al. 1984) during the normal lifespan of ca 5 years of the lizard, Podarcis hispanica, and the number of GABA-ergic and parvalbumin-containing interneurons in the cortex of lizards increases significantly with age (Martínez-Guijarro et al. 1994).
It is well known that the optic tectum and retina grow continuously throughout the lifespan of teleosts and amphibians (Straznicky & Gaze 1971, 1972; Johns & Easter 1977; Beach & Jacobson 1979; Raymond & Easter 1983; Marcus et al. 1999). Several morphometric studies have shown that the number of cells in the teleost fish brain increases with age, body weight and length (Leonard et al. 1978; Birse et al. 1980; Leyhausen et al. 1987; Brandstätter & Kotrschal 1989, 1990; Zupanc & Horschke 1995). Comparative allometric studies of the brain growth pattern in cyprinid teleosts show that the brain attains no definite final morphology. The general addition of neurons decreases with age in most brain areas. On the other hand, there are also lifelong differential growth-related shifts in relative sizes of primary sensory brain regions (Leonard et al. 1978; Birse et al. 1980; Brandstätter & Kotrschal 1989, 1990). The adult cyprinid and other teleost brain growth patterns all show a relative increase of cerebellar size, whereas the optic tectum decreases in relative size during growth. In general, species-specific growth patterns seem to predominantly affect primary sensory areas that relate to lifestyle. One example of this is the growth-related change of brain morphology in different species of cyprinids. The adult cyprinid brain morphology relates to the particular lifestyle of the species. For example, benthic feeding species show enlarged chemosensory brain regions on the expense of regions that receive input from the octavolateralis system, while surface feeding species show an inverse relationship (Brandstätter & Kotrschal 1989; Kotrschal & Palzenberger 1992). However, initially the larval and the early-juvenile brain morphologies of different species of cyprinids are highly similar. The species-specific brain structure starts to emerge during the late-larval and juvenile periods, a stage when differential brain growth is especially pronounced (Brandstätter & Kotrschal 1989, 1990).
The cellular and molecular mechanisms behind the differential brain growth in non-mammalian vertebrates are not yet known but may be related to those mechanisms that would be desirable to control in vertebrate brains. The great diversity of brain specialization among non-mammalian vertebrates and especially among teleost fish provides a unique platform to study region-specific allometric brain growth.
2. Adult neurogenesis is restricted to proliferation zones in the CNS of vertebrates
(a) Mammals
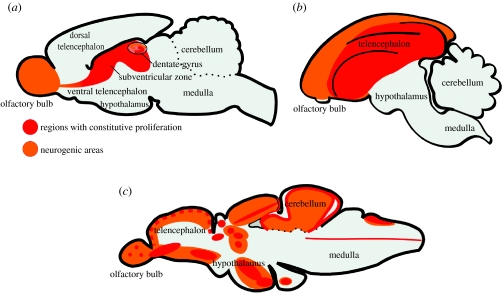
It has long been known that post-embryonic brain proliferation in vertebrates is restricted to defined zones, the so-called ‘matrix zones’ (Kirsche 1967). In the adult mammalian brain, only two restricted constitutively active neurogenic zones have been identified: the subependymal/subventricular zone (SVZ) of the telencephalic lateral ventricle and the subgranular zone (SGZ) of the dentate gyrus in the hippocampus (figure 1). Both of these zones are located in the telencephalon. Transient early post-natal neurogenesis has been reported in the cerebellum and the brain stem nuclei (Jacobson 1991).
Figure 1.
Parasagittal schematic overviews of the adult proliferation pattern and neurogenic regions in the brain of adult vertebrates. (a) Rodent (mouse), (b) Bird (songbird) and (c) Fish (zebrafish).
The most abundant site of adult neurogenesis in mammals resides in the SVZ of the telencephalic lateral ventricle (Altman 1969). In rodents and most other mammals studied, the cells generated in the SVZ migrate into the olfactory bulb where they differentiate into inhibitory GABA-ergic granule interneurons and periglomerular dopaminergic interneurons (Luskin 1993; Lois & Alvarez-Buylla 1994; Betarbet et al. 1996; Carleton et al. 2003). In rodents and some primates, the neuroblasts tangentially migrate from the SVZ to the olfactory bulb in long chains that are often enveloped by a tunnel of astrocytes (Lois et al. 1996; Pencea et al. 2001a,b). This stream of tangentially and rostrally migrating neuroblasts is often referred to as the rostral migratory stream (RMS). When cells reach the olfactory bulb, the neuroblasts migrate radially away from the RMS. The SVZ of humans seems to differ considerably morphologically and functionally from the SVZ of other studied mammals. The human SVZ is lined with a prominent ribbon of astrocytes, which is not found in other mammals except primates. Surprisingly, there is no evidence for a prominent RMS of neuroblasts into the olfactory bulb in adult humans (Sanai et al. 2004; Quinones-Hinojosa et al. 2006). However, proliferation and neurogenesis have been detected within the olfactory bulb in humans (Bedard & Parent 2004). Most of the neurons in the SVZ are generated in excess, and only a fraction of the neurons matures and integrates functionally into the circuitry of the olfactory bulb while the rest degenerates (Biebl et al. 2000; Winner et al. 2002). Interestingly, intraventricular brain injection of a caspase inhibitor decreases cell death of newly produced cells in the rodent brain but has no obvious influence on the number of newly generated neurons (Biebl et al. 2005).
In the hippocampus, a proliferating population of multipotent precursors is found in the innermost subgranular cell layer of the dentate gyrus (Altman & Das 1965). Newly born cells in the SGZ migrate a short distance as neuroblasts and are integrated as granule neurons in the granular cell layer of the dentate gyrus (Cameron et al. 1993; Eriksson et al. 1998; Seri et al. 2001; Kempermann et al. 2004a; Seri et al. 2004). It has been suggested that the neuroblasts start maturating rapidly as they extend processes already after 4 days (Hastings & Gould 1999). Most of the newly generated cells in the SGZ die, but the neurons that have survived the first two weeks will most probably mature and integrate into the functional circuitry (Kempermann et al. 2003). Granule neurons generated in the hippocampus of adult mice continue to mature for several weeks and they show functional electrophysiological properties after one month (van Praag et al. 2002).
Interestingly, adult proliferation and neurogenesis have also been reported in several other brain areas such as the neocortex (Gould 1999, 2001; Koketsu et al. 2003; Dayer et al. 2005), amygdala (Bernier et al. 2002), hypothalamus (Xu et al. 2005), midbrain (Zhao et al. 2003), dorsal vagal complex (Bauer et al. 2005) and spinal cord (Yamamoto et al. 2001). Although the results of these studies are very interesting, they still need further confirmation. The level of constitutive proliferation and neurogenesis in these areas under normal physiological conditions remain unclear, as well as the fate of the produced cells. Furthermore, progenitor cells have been isolated and differentiated in vitro into neurons and macroglia from several, otherwise non-neurogenic, regions of the mammalian brain such as the isocortex, optic nerve, spinal cord, hypothalamus and cerebellum (Kirschenbaum et al. 1994; Palmer et al. 1999; Kondo & Raff 2000; Laywell et al. 2000; Nunes et al. 2003; Markakis et al. 2004; Lee et al. 2005). These progenitors are thought to be responsible for the continuous low generation of astrocytes and oligodendrocytes in the mammalian brain (Dawson et al. 2000, 2003; Levine et al. 2001).
(b) Non-mammalian vertebrates, birds, reptiles, amphibians and fish
It is well established that proliferation occurs extensively in the brain and that neurons are continuously added to many brain regions of adult non-mammalian vertebrates (reviewed in: Doetsch & Scharff 2001; Zupanc 2001; Garcia-Verdugo et al. 2002). However, the understanding of post-embryonic neurogenesis, or in most cases more correctly, adult brain proliferation in non-mammalian vertebrates, is still scarce and very few detailed studies exist, especially in amphibians and fish. For example, only little is known about the origin, migratory routes and end targets of the produced cells, as well as the phenotype of the generated cells, long-term survival and functional integration.
(c) Birds
In birds, constitutive adult proliferation and neurogenesis are found dispersed along the telencephalic lateral ventricle (Goldman & Nottebohm 1983; Alvarez-Buylla et al. 1994; figure 1). However, focal proliferation centres, or ‘hot spots’, with a higher density of proliferating cells are found in the ventral and dorsal reaches of the lateral wall of the lateral ventricle (Alvarez-Buylla et al. 1990).
In adult songbirds, neuroblasts most probably migrate tangentially before they migrate radially along the glial scaffolds (Barami et al. 1994; Doetsch & Scharff 2001). However, radial glia do not span through the full width of the telencephalic parenchyma, and it is therefore unknown how the migrating neuroblasts find their end target. Cells with a neuronal morphology are seen 20 days after birth (Alvarez-Buylla & Nottebohm 1988). Neurons that are integrated into the high vocal centre (HVC) circuitry survive for a long time period (Kirn et al. 1991; Nottebohm et al. 1994). A substantial amount of the newly generated cells die and it has been reported that two-thirds of cells degenerate, and thus fail to differentiate and integrate into the pre-existing neuronal circuitry (Alvarez-Buylla & Nottebohm 1988; Alvarez-Buylla et al. 1990; Alvarez-Buylla & Kirn 1997). In the hippocampus of adult birds, the recruitment of neurons makes up for net loss with no gain in the total number of neurons (Barnea & Nottebohm 1996). The total number of neurons thus remains constant in the avian hippocampus.
In contrast to mammals, the major destination of tangentially migrating neuroblasts, the RMS in songbirds, is the lobus parolfactorius in the basal ganglia and not the olfactory bulb (Alvarez-Buylla et al. 1994). Several other telencephalic regions also receive a significant amount of newly produced neurons (Alvarez-Buylla et al. 1994; Alvarez-Buylla & Kirn 1997). In adult doves, new neurons are added to the forebrain without clustering in any particular nucleus (Ling et al. 1997). Interestingly, age-related decline in neurogenesis is seen at two different time points in doves. The first occurs around three months when the dove attains the adult physical size and the second occurs after 1 year, the time when the dove reaches sexual maturity (Ling et al. 1997). Even though neurogenesis is reported to be widespread and abundant in the telencephalon of birds, only little is known about the identity and function of the produced cells. The best-known area is the HVC where both interneurons and long projecting neurons are continuously incorporated during adulthood (Nottebohm 1985; Alvarez-Buylla et al. 1990; Kirn et al. 1991, 1999; Scharff et al. 2000).
(d) Reptiles
In reptiles, constitutive post-natal proliferation and neurogenesis have been found in several brain areas such as the olfactory bulb, striatum, dorsoventricular ridge, cortex, nucleus sphericus (lizards but not turtle) and cerebellum (López-García et al. 1988; García-Verdugo et al. 1989; Pérez-Sánchez et al. 1989; Marchioro et al. 2005; reviewed in Font et al. 2001).
The medial cortex of the lacertilian lizards is the most studied of these brain areas. Radial glia in contact with the ventricular lumen are the major, if not the only, progenitor cell types in the reptile telencephalon (Font et al. 2001). New cells are generated in the vicinity of the ventricle and some of the produced cells migrate along radial glia into the granule cell layer. There is no evidence for cell division of the migrating neuroblasts. Interestingly, the cells stop their migration into the cortex at random levels in the granule cell layer (López-García et al. 1988). However, there is a considerable species difference in the number of cells that migrate along radial glia. In the cortex of the tropical lizard Tropidurus, less than 30% of the proliferating cells migrate along radial glia in contrast to 90% in the European lizard Podacris (Lopez-Garcia et al. 1990; Marchioro et al. 2005). It takes 1–2 weeks for the cells to migrate and differentiate into neurons in the cortex of Podarcis and Tropidurus (Lopez-Garcia et al. 1990; Ramirez-Castillejo et al. 2002; Marchioro et al. 2005). Only a few or no degenerating cells have been found in the brain of reptiles and thus most of the cells produced seem to survive for long times (Lopez-Garcia et al. 1990; Font et al. 2001). This is supported by retrograde tracings that suggest emission of proper projection pathways and formation of synapses, key signs of functional integration into the neurocircuitry (Lopez-Garcia et al. 1990; Molowny et al. 1995). It has also been shown that the number of neurochemically identified interneurons and projecting neurons increases with age in the brain of reptiles (Lopez-Garcia et al. 1990; Martínez-Guijarro et al. 1994; Pérez-Canellas et al. 1997; Font et al. 2001). Altogether, this indicates that most of the generated cells in the medial cortex of lizards seem to be integrated into the existing neural circuitry.
(e) Amphibians and fish
Proliferation and neurogenesis are not well known in the adult amphibian brain. There is only one recent detailed study that would describe the proliferative zones and the neurogenic areas in the amphibian brain (Raucci et al. 2006). Adult proliferation and putative neurogenesis have been detected in the telencephalon, preoptic region, thalamus, hypothalamus, midbrain and cerebellum (Kirsche 1967; Richter & Kranz 1981; Chetverukhin & Polenov 1993; Polenov & Chetverukhin 1993; Bernocchi et al. 1990; Dawley et al. 2000; Raucci et al. 2006).
Unfortunately, there is an almost complete lack of information about adult proliferation in the brain of adult cyclostomes and chondrichthyans. It has been reported that adult lampreys display proliferation in the rhombencephalon (Vidal Pizarro et al. 2004). The adult proliferation pattern in the teleost brain has been studied in several different species of fish. These studies show that many proliferative centres are found along the whole extent of the rostro-caudal brain axis (Kirsche 1967; Rahmann 1968; Meyer 1978; Zupanc & Horschke 1995; Zikopoulos et al. 2000; Ekström et al. 2001; Zupanc 2001, 2005; Grandel et al. 2006). However, the adult proliferation pattern has been studied in detail in only three species of teleost fish: the percomorph three-spined stickleback, Gasterosteus aculeatus; the gymnotiform weakly electric fish, Apteronotus leptorhynchus; and the cyprinid zebrafish, Danio rerio (Zupanc & Horschke 1995; Zupanc et al. 2005; Ekström et al. 2001; Grandel et al. 2006; figure 1). The distribution of proliferative centres in the brain of teleosts shows a remarkably high degree of similarity, despite the differences in ecological specialization and phylogenetic distance between these studied species. In these teleost fish, more than 10 distinct adult proliferative zones have been identified along the whole brain axis in areas such as: the olfactory bulb; telencephalon; thalamus; epithalamus; preoptic region; hypothalamus; tectum; cerebellum; rhombencephalon; and spinal cord. There is a massive ongoing proliferation and turnover of cells in the adult fish brain. In the cerebellum of the knifefish Apteronotus, quantitative analysis has shown that approximately 100 000 mitotic cells in the S-phase are found in the proliferative zone of the cerebellum of a teleost during any 2 h period (Zupanc & Horschke 1995). In the cerebellum of Apteronotus, approximately half of the produced cells die after the first weeks (Soutschek & Zupanc 1996; Zupanc et al. 1996). The rest seems to be integrated into the network as granule neurons presumably leading to a slow increase in cerebellar size.
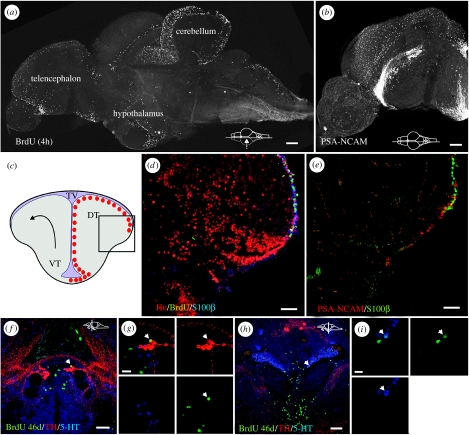
Recently, 16 distinct constitutively proliferating domains were identified along the whole anterior–posterior brain axis in adult zebrafish (Grandel et al. 2006). Furthermore, these proliferative domains contained ventricularly positioned label-retaining cells, indicating that these cells are self-renewing neural precursors (NPCs). Interestingly, the progenitors residing in the adult zebrafish telencephalon seem to express a different set of transcription factors compared with their embryonic counterparts (Adolf et al. 2006). Most of the generated cells from these zones seem to migrate slowly and differentiate into neurons (Zupanc et al. 2005; Grandel et al. 2006). In the telencephalon, some of the produced neurons differentiate into glutamic acid decarboxylase, tyrosine hydroxylase and parvalbumin neurons (figure 2f–i; Adolf et al. 2006; Grandel et al. 2006), while in the diencephalon both serotonin and tyrosine hydroxylase-containing neurons, of which some are long projecting, are generated (Grandel et al. 2006).
Figure 2.
(a) A parasagittal overview of the proliferation zones in the brain of an adult zebrafish. The zebrafish was pulsed for 4 h with BrdU and whole mount stained. (b) A parasagittal overview of the zebrafish telencephalon stained with PSA-NCAM showing a chain of cells along the telencephalic ventricle immunopositive for the PSA-NCAM. (c) Cross-section and overview of the proliferative zones (red dots) in the everted fish telencephalon (arrow). Boxed region shows the field of view in (d) and (e). (d) A cross-section through the dorsal telencephalon of an adult zebrafish showing BrdU-labelled cells (green) along the lateral margin, as well as Hu-positive neurons (red) and S100β-positive glia (blue). The fish was pulsed for 2 days with BrdU. (e) A cross-section from a similar level to (e) showing PSA-NCAM-positive cells (red) along the lateral margin as well as S100β-positive glia (green). (f, g) Cross-section of the pretectum of an adult zebrafish showing a newly generated tyrosine hydroxylase-positive neuron. (h, i) Cross-section of the hypothalamus showing newly generated serotonin neurons (Grandel et al. 2006). DT, dorsal telencephalon; VT, ventral telencephalon; TV, tectal ventricle. (f)–(i) is reprinted with kind permission from Grandel et al. (2006), © Elsevier.
(f) Highly conserved proliferation zones in the vertebrate brain
Tangentially migrating cells in the ventricular wall of the telencephalon have been found in all vertebrates studied (Doetsch & Scharff 2001). In rodents, most of these cells are migrating rostrally and are destined to the olfactory bulb (see above), while in other vertebrates the migratory route and final destination are less obvious. Although there is proliferation in the SVZ of humans, there is so far little evidence for a prominent RMS (Sanai et al. 2004; Quinones-Hinojosa et al. 2006), and instead proliferation and neurogenesis have been detected within the olfactory bulb (Bedard & Parent 2004).
In birds, tangentially migrating cells are found in the telencephalon. However, the migratory pattern is more diverse in songbirds and only a fraction of the produced cells ends up in the olfactory bulb (Alvarez-Buylla et al. 1994; Alvarez-Buylla & Kirn 1997). In mature doves, it has been reported that new neurons are dispersed into the forebrain without any particular clustering (Ling et al. 1997). Evidence for long-distance tangential migration from the telencephalic ventricular zone into the olfactory bulb has been found in reptiles, even though proliferation inherent to the olfactory bulb is also present (Penafiel et al. 1996; Pérez-Canellas et al. 1997; Font et al. 2001). In amphibians, there are no detailed studies of tangential migration in the telencephalon, though proliferation has been reported in the ventral telencephalon of salamanders and frogs. In adult zebrafish, there is evidence of migrating cells from the rostral end of the ventral telencephalon to the olfactory bulb (Byrd & Brunjes 2001; Adolf et al. 2006; Grandel et al. 2006). However, a substantial amount of cells generated in the ventral telencephalon do not migrate to the olfactory bulb, instead they disperse into ventral telencephalic nuclei. There is also an inherent population of proliferating cells in the olfactory bulb (Grandel et al. 2006). Interestingly, there is also a population of cells in this region that express the marker polysialylated neural cell adhesion molecule (PSA-NCAM), suggesting tangential chain migration of cells into the olfactory bulb (figure 2). PSA-NCAM has been used as a marker expressed by tangentially migrating cells in the RMS of other studied vertebrates (Rousselot et al. 1995; Doetsch & Scharff 2001).
Another conserved feature of vertebrates is the adult neurogenesis found in the pallium. Constitutive neurogenesis and turnover of cells is seen in the hippocampus of mammals and birds (Doetsch & Scharff 2001; Kempermann et al. 2004a). Similarly, active neurogenesis is seen in the dorsomedial cortex of reptiles, a structure that is thought to be homologous to the hippocampus of birds and mammals based on developmental, neuroanatomical and behavioural data (Lanuza et al. 2002; Rodriguez et al. 2002). Furthermore, recently generated neuroblasts in the medial cortex express PSA-NCAM as they migrate (Ramirez-Castillejo et al. 2002). PSA-NCAM is also observed in the recently generated neuroblasts in the mammalian hippocampus (Seki & Arai 1993). Proliferation and neurogenesis are found in lateral areas of the dorsal telencephalon in teleost fish (figure 2). The lateral areas of the dorsal telencephalon have been proposed to be equivalent to the medial pallium (hippocampus) of other vertebrates based on the ontogenical, neuroanatomical and behavioural data (Meek & Nieuwenhuys 1998; Rodriguez et al. 2002; Salas et al. 2003; Broglio et al. 2005). Interestingly, the newly generated cells in this area also express PSA-NCAM (figure 2).
Altogether, the highly conserved telencephalic proliferation patterns in the studied vertebrates so far suggest that they may be a plesiomorphic (ancestral) feature of the vertebrates.
3. Phylogenetic reduction of active proliferation zones
During evolution, it appears that the number of constitutively active adult proliferation zones was reduced or suppressed in the transition between anamniotes and amniotes and even more so in mammals in comparison with non-mammalian vertebrates. The cause for the phylogenetic reduction in active proliferation zones in vertebrates is unknown. It is not known whether the phylogenetic reduction in adult brain proliferation is caused by a selective loss of proliferative zones through adaptation and if active proliferation in mammals is suppressed by intrinsic or extrinsic factors in the brain. Understanding the mechanisms that regulate proliferation and neurogenic permissiveness is of course of great significance for biomedical research.
It is not clear why or how evolutionary selection would lead to a selective loss of proliferative zones in vertebrates. However, it has been proposed that ‘the systematic decrease in the extent of adult neurogenesis during vertebrate evolution, culminating in primates, may be the result of an adaptation to keep neuronal populations with their accumulated experience for an entire lifespan’ (Rakic 2004). If this primate- and mammalio-centric view holds true, non-mammalian vertebrates would have a much less complex neuronal network and/or it would be disadvantageous for non-mammalian vertebrates to be able to store experience for longer times. The first statement may be true if the total number of neurons and synapses are used as a key criterion, otherwise non-mammalian vertebrates display a high degree of neural complexity and specialization. It seems unlikely that it would be evolutionarily beneficial for non-primates to be not able to store experience for longer times. Birds have considerable cognitive abilities and a long lifespan that is comparable to primates and have, for example, a well-developed long-term memory and an episodic-like memory (Clayton et al. 2001, 2003). It should also be noted that several species of fish, reptiles and birds have a lifespan of several decades. This is accentuated by the turtle which lives the longest of all vertebrates.
The widespread post-embryonic brain neurogenesis in non-mammalian vertebrates may be related to brain growth in response to growth of the sensory systems. This is in contrast to mammals whose sensory systems are already fully grown in terms of number of sensory receptors shortly after birth. Recently hatched fish, amphibians and reptiles have a very small body size including sensory systems and brain compared with the size of their adult counterparts. This means that the CNS of these vertebrates needs to adapt to a considerable growing body size and increase in primary sensory input. It is therefore probable that the post-embryonic neurogenesis in fish, amphibians and reptiles is caused to a large extent by the need to enlarge CNS processing power for increasing primary sensory input. This is illustrated by an increasing differential growth of primary sensory brain areas in comparison with other brain regions. Examples of this are the growth of retina and optic tectum in amphibians and fish, and chemoreception and increase of vagal lobe in carps (Evans 1952; Brandstätter & Kotrschal 1990; Marcus et al. 1999). This is further supported by the lack of, or almost diminished, adult neurogenesis in the retina and the optic tectum of reptiles and birds (Beazley et al. 1998; Lang et al. 2002; Kubota et al. 2002). However, it is unknown whether all classes of neurons are added and integrated into the growing brain of fish, amphibians and reptiles. It is possible that the neurocircuitry in these vertebrates is rather fixed at the time of hatching and that only a few types of neurons actually are generated and integrated into the circuitry of the growing brain of these vertebrates. Likewise, there is a link between sensory input and neuronal turnover in mammals. Neurons are substantially added to the olfactory bulb in smell-oriented mammals like rodents in contrast to the more visually oriented primates and songbirds, where much fewer neurons are added. It is also known that sensory input affects the growth and the differentiation of specific brain regions during early and late embryogenesis, for example the specification of the visual cortex in mammals (Dehay et al. 2001 and references there in).
It has also been suggested that the poor capacity to add or replace neurons in the mammalian brain is caused by a resistance to integrate new cells into a mature neural network (Kempermann et al. 2004b; Rakic 2004). It is thought that adding new neurons to an already-existing circuitry may cause harmful effects to the whole network structure. Therefore, there seems to be a fine balance between adaptability, the benefits of adding new components and experience to a pre-existing network and, on the other hand, stability, not to compromise the network structure by the addition of new elements. It has also been proposed that the newly produced neurons are more plastic and readily adapt to new experiences in contrast to older ones (Song et al. 2005; Lledo et al. 2006 and references therein). For example, in the mammalian hippocampus, it has been proposed that the old neuronal population remain stable and preserve optimal encoding for learned known environments, while the newly added neurons are plastic and adapt to features that are new in the environment (Meltzer et al. 2005; Wiskott et al. 2006). Several recent findings show that the maturation and integration of new neurons into the neural circuitry of the olfactory bulb and hippocampus in mammals is a long and arduous process with many maturation steps on the way (recently reviewed in: Song et al. 2005; Lledo et al. 2006). It has also been shown that newly born granule neurons in the olfactory bulb have a greater immediate early genes response to novel odours in comparison with mature pre-existing neurons. Furthermore, the recently incorporated neuron population also show a long-lasting enhanced responsiveness to odours they were familiarized with (Magavi et al. 2005). Taken together, this suggests that the newly born cells in both hippocampus and olfactory bulb are more plastic than their older counterparts and more readily adapt to new experiences, and thus the adult neurogenesis would be mostly important for the processing and learning of novel experiences. This view also fits well together in the context of the non-mammalian vertebrates and the above-proposed hypothesis proposing that the post-embryonic neurogenesis is mostly related to the coordinated growth of sensory systems and sensory brain regions and thus used for processing of new experiences. It may be easier for the non-mammalian vertebrates to incorporate new cells into the pre-existing neural circuitry owing to the actual brain growth, which at least in some cases appears to be due to the addition of complete neural units instead of incorporation of single cells (e.g. in the optic tectum of frogs and fish).
It should also be taken into consideration that fish, amphibians and reptiles possess already relatively matured sensory and motor systems immediately after hatching. They are, for example, rapidly able to display complex and demanding behaviours like actively catching food and escaping from predators. This may indicate that some parts of the CNS in these vertebrates are primed before hatching in order to meet the immediate functional processing demands, while the later post-embryonic brain growth could be considered as a delayed development and more related to functions needed later on in life like social interactions and breeding. This would then imply that some brain parts of non-mammalians would remain in a neotene status long after embryogenesis. However, studies on fish and reptiles suggest little changes in the distribution of early post-embryonic proliferation zones versus the proliferation zones still detected near the end of the lifespan (Lopez-Garcia et al. 1984; Wullimann & Puelles 1999; Grandel et al. 2006). Currently, very little is known about the embryonic relationship with adult brain structures, behaviour and lifestyle in non-mammalian vertebrates in order to be able to draw any definitive conclusions about the neotene characteristics and the differential brain growth in non-mammalian vertebrates.
Several recent studies indicate that neurogenesis may be suppressed in adult mammalian brains, since it has been possible to induce cell proliferation and neurogenesis in otherwise non-neurogenic brain regions. Furthermore, progenitor cells have been isolated from otherwise non-neurogenic regions of the mammalian brain and differentiated in vitro to neurons, glia and oligodendrocytes (see below). It is possible that this pool of parenchymal progenitors can be activated by extrinsic signals released from, for example, the site of injury (see below). Intriguingly, the mammalian brain regions, where it is possible to induce neurogenesis or to isolate progenitors from, correspond well with the brain regions where constitutively active proliferation and neurogenesis take place in adult fish.
4. Compensatory neurogenesis and regeneration
Non-mammalian vertebrates have a remarkable capability to regenerate severe brain injuries such as stab wounds and ablation of whole brain parts, in striking contrast to mammals where neurons rarely if at all are replaced after injury. The cause for this remarkable plasticity is unknown but differences in the adult brain proliferation pattern are probably involved. Of the non-mammalian vertebrates, fish and amphibians exhibit the most widespread adult neurogenesis and also the greatest capacity to regenerate injuries to the CNS.
Compensatory proliferation and neurogenesis in response to injury of the CNS are the phenomena observed in all vertebrates including mammals. Different types of injuries to the CNS can induce ectopic proliferation and neurogenesis in proximity to the injury, as well as enhanced proliferation in constitutively active neurogenic domains followed by ectopic migration of neuronal precursors towards the site of injury. It should be noted that the origin and composition of cells contributing to the reconstitution are unclear in many studies. Currently, very little is known about the basics of compensatory neurogenesis and how it is possible to modulate and direct it. However, it is obvious that enhanced activation of inherent progenitors and recruitment of new neurons into the injury site could be a fundamental strategy in restorative therapies for brain injuries and neurodegenerative diseases.
(a) Mammals
It has long been thought that neurons generally are not replaced in the mammalian brain after injury or during the course of a disease. Interestingly, several recent studies have reported compensatory proliferation and neurogenesis in response to injury or disease in the mammalian brain (recently reviewed in Goldman 2005; Emsley et al. 2005; Zhang et al. 2005).
General increases in proliferation and limited neurogenesis have been found in the constitutively neurogenic brain regions of patients with Alzheimer's and Huntington's disease (Curtis et al. 2003; Jin et al. 2004a,b). Chemically or electrically induced seizures stimulate proliferation and neurogenesis in the hippocampus of rodents (Bengzon et al. 1997; Parent et al. 1997, 2002a, 2006). Focal ischemia in rodents leads to enhanced proliferation in the SVZ and SGZ and ectopic recruitment of neuronal precursors into the injured brain regions from the constitutively active neurogenic domains (Arvidsson et al. 2002; Nakatomi et al. 2002; Parent et al. 2002b; Jin et al. 2003; Zhang et al. 2004).
Interestingly, compensatory neurogenesis from inherent progenitors takes place ectopically in the neocortex after selective ablation and apoptosis of cortical interneurons (Magavi et al. 2000) or long projecting corticospinal neurons (Chen et al. 2004). Other types of brain damage such as stab wounds, stroke and chronic injury by the accumulation of amyloid plaques also induce ectopic proliferation of various cell types such as microglia, astrocytes and endogenous precursors (Levine et al. 2001). However, normally these precursors fail to generate neurons and instead contribute to the generation of glial scarring. Some of these endogenous precursors show the potential to generate neurons in vitro but somehow fail to do so upon injury in vivo. Recently, it was shown that the basic helix–loop–helix transcription factor oligodendrocytes factor 2 (Olig2) is greatly upregulated in endogenous precursors after injury (Buffo et al. 2005). Interestingly, repression of Olig2 in proliferating precursors leads to the production of immature neurons indicating that Olig2 acts as a suppressive non-neurogenic signal in vivo and thus one of the first non-permissiveness signals that has been found to act in vivo in the mammalian brain (Buffo et al. 2005).
These results clearly indicate that a limited compensatory neurogenesis can take place in the adult mammalian brain and, more importantly, that it may be possible to evoke endogenous neuronal repair in normally non-neurogenic brain regions. Although the compensatory proliferation and neurogenesis in mammals are very interesting and promising, the yields are so far modest and therapeutically insignificant.
(b) Non-mammalian vertebrates
The origin, fate and function of the reconstituted cells are not known in most of the regeneration studies performed in non-mammalian species. Therefore, the term compensatory proliferation is more appropriate than compensatory neurogenesis.
(c) Birds
Compensatory proliferation and neurogenesis as well as functional integration of interneurons and long projecting neurons are seen in the adult zebra finch after selective ablation of neurons (Scharff et al. 2000; Dawley et al. 2000). Three types of neurons, interneurons, HVC-archistriatum(RA) and HVC-X long projecting neurons, reside in the HVC of the adult zebra finch. Interneurons and long projecting HVC-RA neurons both show a low turnover rate, while the number of long projecting HVC-X neurons remains constant (Kirn et al. 1991, 1999; Scharff et al. 2000). Selective lesions of HVC-RA neurons induce deterioration of song. Shortly after the lesioning, enhanced compensatory neurogenesis is seen in HVC-RA neurons and interneurons. New projections from HVC-RA neurons are formed and birds are eventually able to recover singing to variable degrees. The ability to produce song coincides with the time when the HVC-RA neuronal projections reach the RA area. This suggests that neuronal replacement can restore function and learned behaviour in birds (Scharff et al. 2000). Surprisingly, the HVC-X neurons are not regenerated after selective ablation indicating that only the neuronal types that normally display turnover are able to respond to the compensatory neurogenesis. The mechanism underlying this selective restoration is not yet known. Similarly, electrolytic lesions to the hypothalamus of adult doves induce compensatory neurogenesis and recruitment of new neurons into the lesion site (Cao et al. 2002).
(d) Reptiles
The medial cortex of lizards shows a remarkable capacity to morphologically and functionally regenerate severe chemical and physical lesioning (reviewed in: Font et al. 2001; López-García et al. 2002; Romero-Aleman et al. 2004). Specific lesioning with the neurotoxin 3-acetylpyridine primarily affects the medial cortex and induces rapid degeneration of neurons. Already, degeneration is detectable only after 12 h of administration. The degeneration process progresses for 4–10 days, after which over 90% of the nuclei in the dorsomedial cortex display pyknotic nuclei in severely affected specimens (Font et al. 1997). One to two weeks after the lesion, there is a wave of compensatory neurogenesis directed towards the site of injury. The newly produced cells originate from the pool of ventricular progenitors that constitutively proliferate in the cortex of adult lizards. The compensatory neurogenesis morphologically and most probably functionally restores the damaged areas within 4–8 weeks after the lesion (Font et al. 1991; Molowny et al. 1995; Font et al. 1997). Compensatory gliogenesis and neurogenesis play a key role in the structural repair after physical lesioning of the cortex of adult lizards (Romero-Aleman et al. 2004). Interestingly, neuronal regeneration after injury is abolished in lizards during wintertime. The low temperature seems to prevent migration of newly generated neurons (Ramirez et al. 1997).
(e) Amphibians
Regenerative studies in amphibians have mostly been performed in urodele newts and Xenopus and are mainly concentrated on regeneration of the peripheral nervous system, tail and limbs (reviewed in: Brockes & Kumar 2002; Ferretti 2004; Slack et al. 2004). In general, the regenerative capacity of urodele newts is much higher than that in other amphibians. However, both urodeles and anurans have the capability to regenerate severe injury to most brain parts during juvenile stages but not as adults (Sibbing 1953; Srebro 1965; Filoni & Gibertini 1969, 1971). The urodele newt Triturus cristatus carnifex morphologically regenerates a lesion to the optic tectum or telencephalon after approximately three months. There seems to be a direct relationship in the compensatory response between the number of proliferating cells in the injured optic tectum and the extent of the lesion (Del Grande et al. 1990; Minelli et al. 1990). Interestingly, lesions to the telencephalon or the optic tectum induce a response in several proliferative zones indicating that signals mediated from the lesion site are able to elicit a broad proliferative response. The clawed frog Xenopus laevis can regenerate severe lesions to the telencephalon, optic tectum and cerebellum during larval stages but not after metamorphosis (Srebro 1965; Filoni & Gibertini 1969, 1971; Filoni et al. 1995). The reconstitution of ablated parts is rapid in juvenile frogs, for example the telencephalon is reconstituted already one month after lesion. Although morphological alterations are still seen, correct connections are formed from the regenerated telencephalon (Yoshino & Tochinai 2004). Recently, it was shown that juvenile frogs can regenerate severe telencephalic lesions both morphologically and functionally (Yoshino & Tochinai 2006). The differences in the decrease in regenerative capacity of various brain regions of larval and metamorphosed X. laevis are related to differences of the undifferentiated cell populations (Filoni et al. 1995). In the early-larval stages, the populations of proliferating cells are very widespread in the brain, while in late-larval stages and after metamorphosis the cells are discretely restricted to proliferation zones. The restriction of proliferating cells occurs later in the telencephalon than in the rhombencephalon and mesencephalon, and correlates with the regenerative capacity of the respective brain region (Filoni et al. 1995). However, more recently, it was shown that neither the lack of proliferative response nor an intrinsic deficiency in organizational capacity is the main reason to why post-metamorphic frogs cannot regenerate severe telencephalic lesions. Instead, slow and imperfect sealing of the wound by ependymal cells seems to be a contributing factor for the poor regeneration capability (Yoshino & Tochinai 2004).
(f) Fish
It has been shown that different teleost fish species have a tremendous capability for regeneration of the CNS, spinal cord and retina (Kirsche 1965; Zupanc 2001). This comprises regeneration after incisions in the brain or after removal of whole brain parts, as well as regeneration of the spinal cord after transection. It is also evident that the regeneration can take place throughout the whole length of the rostro-caudal brain axis. Neural regeneration has been demonstrated in many different teleost lineages suggesting that it is a common feature of teleost fish in general (Kirsche 1950, 1960, 1965; Richter 1965, 1969; Segaar 1965; Zupanc 2001).
Different types of physical lesions have been carried out to test the regenerative abilities of post-embryonic teleost brains, ranging from stab wounds to removal of whole brain parts. Compensatory proliferation and morphological restoration are detected in the dorsal telencephalon of juvenile and adult guppies, Poecilia reticulata (Lebistes reticulatus) in response to a stab wound (Richter 1969). The stab wound first induces degeneration after which mitotic cells are detected in the constitutively active proliferation zones of the dorsal and ventral telencephalon. Later, the produced cells show a directional migration towards the lesion site and participate in the restorative process. Sprouting of capillaries into the injured area is also detected (Richter 1969). Interestingly, the regenerative capacity of the brain in the adult guppy is diminished. The reduction in regenerative capacity may be related to a reduction of proliferation in the adult brain (Richter 1969). Similarly, the architecture of the optic tectum is restored after a stab wound in juveniles of the cyprinid minnow, Leucaspius delineatus (Richter 1965).
The necessity of proliferative zones for regenerative success in the teleost brain was elegantly demonstrated in a series of experiments by Kirsche (1960). Kirsche performed a series of experiments in which he removed parts or the whole lobes of optic tectum in juvenile and adult carps, Carassius carassius. Only when the proliferative zones (‘Matrixzonen’) remained, the regenerative process could take place. In the juvenile fish, he carried out four different types of experiments: first, unilateral ablation of the optic tectum, keeping the three proliferative zones (dorsal, basal and caudal) intact. Second, unilateral ablation of the optic tectum and ablation of the rostral part of the dorsal proliferative zone. Third, unilateral ablation of the optic tectum together with the removal of the dorsal and caudal proliferative zones. Fourth, deletion of the whole optic tectum and removal of all proliferative zones. In the first case, the optic tectum regenerated, although the macro architecture did not recover to its original form. The regenerated tectum stayed smaller and the architecture of the layers was not completely restored due to the irregularly distributed proliferation zones. Nevertheless, new neurons and glia were produced and the fibre layers of the optic tectum were reconstituted, indicating that the micro arrangement was correctly restored. Increased removal of the proliferative zones lead to successively diminished regenerative capacity of the lesioned optic tectum. When the front part of the dorsal proliferative zone was removed completely, there was no regeneration in that area. Furthermore, when all the proliferative areas were removed, regeneration did not take place at all. In the adult C. carassius, however, the first type of experiment also did not lead to any regenerative success, which Kirsche explains with an exhaustion of the proliferative zones. Taken together, these experiments illustrate the importance of the constitutively active proliferation zones in relation to successful histogenesis in the post-embryonic teleost brain. These experiments also illustrate that there are differences in the regenerative capacity between the juvenile and adult teleost brains. In a more recent study, the cerebellum of the weakly electric knifefish, A. leptorhynchus, regenerates completely within weeks after a stab wound (Zupanc 1999; reviewed in Zupanc 2001). There is normally a significant amount of constitutive proliferation in the cerebellum of adult Apteronotus (Zupanc & Horschke 1995; Zupanc & Ott 1999). The stab wound induces enhanced proliferation in both the constitutively active zones and in ectopic locations at the lesion site. There is a substantial increase of glial fibrillary acidic protein (GFAP)-positive radial fibres at the lesion site as well as the migration of newly produced cells from the proliferation zones (Clint & Zupanc 2001). At least some of the generated cells are differentiating into granule neurons that emit projections (Zupanc & Ott 1999).
5. If some vertebrate can regenerate CNS injuries, why cannot we?
The fact that non-mammalian vertebrates are much more able to regenerate and restore brain function after injury naturally leads to the question, what are the differences that make them able to regenerate while mammalians cannot?
It has been argued that selective evolutionary pressure led mammals to loose the ability to regenerate neural tissue (Rakic 2002). Although tissue regeneration is observed in many metazoan species, it is not clear if and how any selective pressures would be exerted that favour various forms of regeneration (reviewed in: Goss 1992; Alvarado 2000). It is therefore difficult to understand and dissect out mechanisms related to regeneration from an evolutionary perspective. There is a correlation between development and regeneration, for example regeneration of tissue seems to recapitulate some aspects of the developmental programmes used in the creation of the injured tissue (reviewed in: Brockes 1997; Brockes & Kumar 2002). Although it is evident that many developmental signals are active and important in the adult organism, the relationship between embryonic tissue formation and regeneration has not yet been systematically analysed to any greater extent.
Of the non-mammalian vertebrates, fish and amphibians exhibit the most widespread adult neurogenesis and also the greatest capacity to regenerate injuries to the CNS. The correlation between post-embryonically continued proliferation and regenerative capability may explain why teleost fish and amphibians in comparison with mammals are so successful in regenerating brain injuries. Interestingly, mammals are also able to regenerate the brain parts, the olfactory nerve and olfactory epithelium, that are still proliferating in the adult (Monti Graziadei & Morrison 1988; Calof et al. 1998; Emsley et al. 2004). It is probable that more widespread constitutive proliferation and neurogenesis in the non-mammalian brain contribute to the capability to regenerate brain injuries in several ways. The enhanced constitutive proliferation and neurogenesis in non-mammalian vertebrates may facilitate neuronal repair by simply being able to quickly provide building blocks, undifferentiated cells, to the site of injury. The widespread adult neurogenesis in non-mammalian brains may also lead to the fact that the non-neurogenic brain regions are kept in a more permissive state in order to facilitate the survival and the integration of undifferentiated cells. This would of course also greatly improve the restorative capacity. However, what this permissive state actually means in terms of involved mechanisms is not yet known.
Interestingly, several recent studies indicate that neurogenesis is suppressed in adult mammalian brains, for example, it has been possible to induce cell proliferation and neurogenesis in otherwise non-neurogenic brain regions. Furthermore, progenitor cells have been isolated from otherwise non-neurogenic regions of the mammalian brain and differentiated in vitro to multiple lineages. These parenchymal progenitors are thought to be oligodendrocyte precursors responsible for the continuous low generation of astrocytes and oligodendrocytes in the adult mammalian brain (Dawson et al. 2000, 2003; Levine et al. 2001). At least some of the parenchymal progenitors can be activated by extrinsic signals released from, for example, the injured cells. The number of these precursors is greatly increased after various types of injury and in line with the notion that these progenitors normally generate various glia and oligodendrocytes, the precursors fail to generate neurons and instead contribute to the glial scarring (Levine et al. 2001; Hampton et al. 2004). Recently, the transcription factor Olig2 was found to be a repressor of the neurogenic potential of endogenous precursors reacting to brain injury (Buffo et al. 2005). Antagonizing Olig2 function in vivo leads to the generation of immature neurons showing that it is possible to reverse the non-neurogenic signals, which opens up novel strategies in neuronal repair. Injury to the mammalian CNS often induces formation of a glial scar that can complicate and stop healing (reviewed in: Fawcett & Asher 1999; Qiu et al. 2000). The glial scar may function as a mechanical and molecular barrier to prevent regeneration. This phenomenon is much less prominent or even absent in non-mammalian vertebrates and may be one of the major contributing factors for the difference in regenerative potential between mammals and non-mammalian vertebrates.
It has been suggested that the poor capacity to add or replace neurons in the mammalian brain is caused by a resistance to integrate new cells into a mature neural network (Kempermann et al. 2004b; Rakic 2004; see above). If the non-mammalian brain, in general, is more permissive for maintenance and integration of new neurons into the circuitry, this would be a contributing factor that facilitates regeneration. Unfortunately, experimental data proving or disproving a more efficient incorporation of newborn neurons into the non-mammalian brain are currently lacking.
6. Induced proliferation and neurogenesis in the adult CNS
Adult brain proliferation and neurogenesis can be modulated by diverse signals. Interestingly, adult proliferation and neurogenesis recapitulate some of the molecular and cellular mechanisms used during embryonic development of the CNS (Temple 2001). Therefore, it is not surprising that many growth factors, morphogens, hormones and signalling molecules, which play a role in embryonic development, also have been shown to regulate the expansion and fate of neural progenitors in vivo in the adult mammalian brain. There are many recent excellent reviews on this topic (Panchision & McKay 2002; Alvarez-Buylla & Lim 2004; Ma et al. 2005; Vergara et al. 2005; Lledo et al. 2006) and we will therefore only summarize some of the recent key findings. Unfortunately, so far very little is known about the effect of growth factors and signalling molecules in the adult brains of non-mammalian vertebrates.
Epidermal growth factor (EGF), fibroblast growth factor 2 (FGF2) and transforming growth factor α (TGF-α) have been shown to have a dramatic mitogenic effect on adult neural progenitors in vivo. Subcutaneous administration or infusion of FGF2 leads to increased proliferation and recruitment of neurons to the olfactory bulb (Craig et al. 1996; Tao et al. 1996; Kuhn et al. 1997). FGF2 regulates the number of slow-proliferating progenitors in the SVZ as fgf2 knockout mice have a significantly lower amount of progenitors in the SVZ resulting in smaller olfactory bulbs due to decreased output (Zheng et al. 2004). Infusion of EGF leads to significantly increased proliferation of subependymal progenitor cells and directs the produced cells towards a glial fate (Craig et al. 1996). FGF2 or EGF has little effect on the progenitor cells in the SGZ, which indicates a heterogeneous response of progenitor cells to growth factors (Kuhn et al. 1997). Whether this is caused by intrinsic properties of the progenitors or extrinsic differences in the niche is unclear. Interestingly, intracerebral injections of TGF-α leads to rapid proliferation in the SVZ, migration and neuronal differentiation towards the infusion site, though the lasting effects and significance of the generated cells are unclear (Fallon et al. 2000).
The ephrin/Eph family of signalling molecules seems to control cell proliferation, migration and survival of neural progenitors. Intraventricular administration of the ectodomain of either EphB2 or ephrin-B2 disrupts migration of neuroblasts and increases cell proliferation in the SVZ (Conover et al. 2000). During embryonic development, ephrin/Eph family signalling alters the cortical size and shape by regulating the survival of neural progenitors (Depaepe et al. 2005; Holmberg et al. 2005).
Bone morphogenetic protein (BMP) signalling inhibits neurogenesis and promotes glial differentiation. Noggin, a BMP antagonist, is produced by ependymal cells and inhibits BMP signalling in the SVZ progenitor cells and thereby promotes neuronal differentiation (Lim et al. 2000). At low concentration, BMPs promote proliferation, while at high concentration BMPs induce glial differentiation (Panchision et al. 2001). Sonic hedgehog (Shh) is required for the maintenance and proliferation of progenitor cells in the mouse SVZ and SGZ (Lai et al. 2003; Machold et al. 2003; Palma et al. 2005). Astrocyte-derived Wnt signalling may be an important regulator of adult neurogenesis. Wnt3 promotes proliferation of adult progenitors and neurogenesis in the SGZ of mice, while expression of a Wnt inhibitor decreases neurogenesis. Furthermore, overexpression of Wnt3 leads to enhanced neurogenesis (Lie et al. 2005). Taken together, it appears that BMP signalling, Shh and Wnt signalling are involved in maintaining the gliogenic versus neurogenic balance in the niche in the adult rodent brain.
Interestingly, intraventricular administration of brain-derived neurotrophic factor (BDNF) induces enhanced neurogenesis, ectopic migration and long-term survival of neurons in otherwise non-neurogenic brain regions (Rasika et al. 1999; Benraiss et al. 2001; Pencea et al. 2001b). However, it is not clear whether the ectopically recruited neurons also display a correct phenotype of the surrounding area or whether they are functionally integrated. Furthermore, administration of BDNF during a limited time window increases the lifetime expectancy of neurons in the HVC of adult canaries (Alvarez-Borda et al. 2004).
Peripheral infusion of insulin-like growth factor-I (IGF-I) leads to increased proliferation of progenitors and neurogenesis selective to the SGZ of rodents (Aberg et al. 2000). Interestingly, enhanced neurogenesis induced by exercise in rodents is mediated by brain parenchyma uptake of serum IGF-I (Anderson et al. 2002). This shows that systemic signals can modulate proliferation and neurogenesis in the adult brain. Long-term exposure of mice to retinoic acid leads to decreased proliferation of progenitors in both SVZ and SGZ. Furthermore, treatment with retinoic acid and the resulting diminished neurogenesis in the SGZ lead to impaired hippocampal-dependent learning (Crandall et al. 2004). However, one cannot exclude effects other than reduced neurogenesis in the SGZ of retinoic acid on the hippocampal-dependent learning paradigm. Vascular endothelial growth factor (VEGF) leads to increased proliferation of cells both in the SVZ and the SGZ (Jin et al. 2002). Interestingly, enriched environment and cognitive performance increases hippocampal expression of VEGF and neurogenesis (Cao et al. 2004). This indicates that hippocampal plasticity and neurogenesis are mediated by VEGF.
It is now clear that there is a multitude of factors in the adult brain, which can regulate the production of new cell, steer migration, control differentiation and maintenance. However, few of these factors and signalling molecules are able to recruit or induce neurogenesis in non-neurogenic areas of the mammalian brain. Only little is also known about the long-term consequences of induced neurogenesis, including long-term survival and functional integration, as well as harmful side effects.
7. Environmental cues that modulate adult proliferation and neurogenesis in non-mammalian vertebrates
It is well known that the hippocampal neurogenesis in mammals is highly responsive to diverse physiological and behavioural inputs. Hippocampal neurogenesis has been altered by subjecting rodents to stress, exercise, learning, enriched environment, antidepressants and ageing (recently reviewed in: Kempermann et al. 2004a; Doetsch & Hen 2005; Emsley et al. 2005). It has been shown that hippocampal plasticity in response to behavioural or physiological input is mediated through IGF, VEGF and BDNF (see §6).
Several studies link adult neurogenesis to cyclic/seasonal behaviour and thus it is probably hormonally controlled. The most studied example of this is the extensive neurogenesis in the sexual dimorphic telencephalic nuclei in songbirds that are seasonally and hormonally regulated (Nottebohm 1980). In the brain of songbirds, seasonal changes in the size of telencephalic nuclei that control song show seasonal changes in neuron numbers. New neurons are replacing older dying ones and this phenomenon thus reflects turnover of neurons. Interestingly, the turnover is seasonally regulated. During the breeding season, there is a decrease in the turnover and an increase of survival of new neurons resulting in a net gain and increase in size (Tramontin & Brenowitz 2000). This is thought to be controlled by light-induced increase in circulating testosterone levels. The effect of testosterone on the neuronal turnover is at least partially mediated through BDNF (Rasika et al. 1999). Neurogenesis is also seasonally regulated in the hippocampus of birds. During autumn, there is a peak in proliferation but no net neurogenesis is detected, which suggests that the turnover of cells is also higher during this time of the year (Barnea & Nottebohm 1994; Healy & Krebs 1996).
Seasonal variations in temperature and photoperiod influence proliferation in lizards. In lizards, more neurons are produced during spring and summer. Long photoperiods increase mitotic activity, while short photoperiods decrease proliferation. Low temperature seems to decrease or prevent radial migration and may even hinder regeneration (Ramirez et al. 1997). Proliferation and neurogenesis in the hypothalamus of frogs show a seasonal variation that is related to breeding. There is a substantial increase in proliferation and addition of new neurosecretory neurons in the preoptic area of Rana prior to breeding in the spring and a reduction in proliferation and degeneration of neurons after breeding in autumn (Chetverukhin & Polenov 1993; Polenov & Chetverukhin 1993). Similarly, during spring, proliferation is upregulated in the chemosensory epithelium and telencephalon in salamanders (Dawley et al. 2000).
In the weakly electric fish Apteronotus, sexual maturation is induced by the onset of the rainy season. Sexually mature Apteronotus males display a special set of transient electric discharges that is controlled by the posterior/prepacemaker nucleus (Zupanc & Maler 1993). Interestingly, sexual maturation in male Apteronotus leads to a decrease in size and number of cells in the posterior/prepacemaker nucleus. Furthermore, the decrease in the size of the posterior/prepacemaker nucleus correlates with the increase in gonadal weight in males (Zupanc & Zupanc 1992; Zupanc & Horschke 1995; Zupanc & Clint 2003). The cyclostome lamprey displays a peak of proliferation in the rhombencephalon during spring, which may be related to spawning behaviour (Vidal Pizarro et al. 2004). Changes in the adult brain proliferation pattern have been found in the sex-changing sequential hermaphroditic teleost Sparus aurata. Sparus forms mature testes at the end of the first year and start to undergo sex change at the end of the second year. A significantly higher number of mitotic cells were found in the periventricular hypothalamus of female fish (Zikopoulos et al. 2001). Interestingly, there is a link between hormone biosynthesis and glia in teleost fish. The fish brain contains extraordinarily high levels of cytrochrome P450 aromatase (CYP19), the final enzyme in oestrogen biosynthesis (Callard et al. 1978). In contrast to other studied vertebrates, teleosts have two structurally and functionally distinct aromatase proteins (Pellegrini et al. 2005). Aromatase B, the predominant form in the brain of teleosts, is found in periventricular radial glia (Forlano et al. 2001; Menuet et al. 2003, 2005) and colocalizes with the glial markers, GFAP and vimentin. However, not all radial glia express aromatase B. Aromatase B colocalizes with glial markers in periventricular radial glia in the telencephalon and diencephalon but not in the tectum (Forlano et al. 2001). Intriguingly, the location of the aromatase B-containing radial glia corresponds well with the reported proliferative/neurogenic zones in the adult teleost brain.
Taken together, this demonstrates that adult neurogenesis can be specifically modulated by systemic signals induced by diverse environmental cues such as light, temperature and enriched environment. Interestingly, this modulation is very region specific, indicating a sophisticated interaction between the proliferative zones, sensory input and systemic signals. Sexual hormones are potent regulators of neuronal turnover in many species of vertebrates.
8. Adult stem cells and radial glia
Stem cells persist throughout life in many tissues of vertebrates and are important in tissue replacement and homeostasis. Adult stem cells and more restricted neuronal and glial progenitors are dispersed throughout the vertebrate brain. Multipotent neural stem cells are found along the ventricles or subventricularly in the adult CNS of vertebrates. Cells with glial characteristics seem to function both as stem cells and progenitors in the adult brain of vertebrates (Alvarez-Buylla et al. 2001; Garcia-Verdugo et al. 2002; Doetsch 2003; Goldman 2003; Götz & Barde 2005). Glia with a stellate shape seem to function as neurogenic stem cells in adult mammals, while radial glia function as adult neural stem cells in non-mammalian vertebrates (Garcia-Verdugo et al. 2002).
Radial glia and basal progenitor cells function as neurogenic progenitors during the development of brain in vertebrates (Alvarez-Buylla et al. 2001; Ever & Gaiano 2005; Götz & Barde 2005). The link between embryonic radial glia and adult neural stem cells in mammals is not clear, but recent evidence suggests that adult neural stem cells in mammals originate from the embryonic radial glia (Götz et al. 2002; Doetsch 2003; Goldman 2003). During embryogenesis, neuroepithelial cells may turn into radial glia, and a small portion of these cells persists as stem cells in specialized niches in the adult brain (Doetsch 2003; Alvarez-Buylla & Lim 2004). Interestingly, radial glia persist in regions where constitutive adult neurogenesis is found in non-mammalian vertebrates. In birds and mammals, astrocytes are abundant and the dominant form of macroglia in the adult CNS. The abundance of free macroglia, except radial glia, in the brain parenchyma is low in fish, amphibians and reptiles (Jacobson 1991; Kalman 2002). It is well known that radial glia persist in the adult CNS of non-mammalian vertebrates (Alvarez-Buylla et al. 2002; Kalman 2002; Garcia-Verdugo et al. 2002).
In mammals, radial glia disappear or become astrocytes shortly after birth, while the radial morphology is retained in the CNS of non-mammalian vertebrates. In regions where constitutive adult neurogenesis is lost during adulthood, radial glia lose their radial morphology and are transformed into astrocytes (Voigt 1989). The morphological transformation of radial glia into astrocytes also correlates with molecular changes (Hunter & Hatten 1995; Heins et al. 2002). However, not all radial glia are transformed into astrocytes. In adult mice, a few cells with a radial morphology are found in the ventral most part of the lateral ventricle, which are also positive for the glial markers, vimentin and GFAP (Sundholm-Peters et al. 2004). Recently, it was shown that ependymal cells are derived from radial glia and are post-mitotic in the adult rodent brain (Spassky et al. 2005). Therefore, it is unlikely that ependymal cells would function as neural stem cells in the adult mammalian brain as proposed earlier (Johansson et al. 1999).
In adult birds, vimentin-containing radial glia are found primarily in the neurogenic regions of the telencephalon (Alvarez-Buylla et al. 1988). However, the radial glia in adult birds do not span through the full width of the telencephalic parenchyma. Radial glia are concentrated in the mitotic hot spots and they continue to divide in the brain of the adult bird (Alvarez-Buylla et al. 1990; Goldman et al. 1996). This strongly suggests that the radial glia serve as neurogenic progenitor cells in the brain of the adult bird. In reptiles, ventricularly located GFAP-positive radial glia seem to be the major, if not the only, progenitor cell type in the adult reptile telencephalon (Font et al. 2001). GFAP-positive radial glia are the predominant macroglia type in the lizard telencephalon (Font et al. 1995) in contrast to turtles, where other macroglia are also found (Pérez-Canellas et al. 1997). It is unknown whether radial glia serve as neuronal precursors in teleost fish, even though vimentin and GFAP-positive radial glia are abundant in the adult brain (Cerda et al. 1998; Kalman 2002; Zupanc & Clint 2003; Arochena et al. 2004).
The structural composition of the proliferative zones has been described in reptiles, birds and mammals (Doetsch & Scharff 2001; Garcia-Verdugo et al. 2002). In mammals, the NPC cells are found in a subventricular position (SVZ) or deep in the brain (SGZ), while in birds, reptiles, amphibians and fish the NPC are dispersed in close proximity to the ventricle. A common feature for the ventricularly located NPC in mammals (SVZ), birds and reptiles is that they extend a single cilium into the ventricle, while the surrounding differentiated ependymal cells extend several cilia (Garcia-Verdugo et al. 2002). In the adult fish and amphibian brain, the structural composition of the proliferative zones is poorly known. However, studies in fish and frogs report that proliferating cells display both ependymal (tanycyte) and glia-like characteristics (Chetverukhin & Polenov 1993; Zupanc & Clint 2003).
The intermediate filament composition in glia may be related to their potential to generate neurons. Changes in the composition of intermediate filaments in glia are seen during development, phylogeny of vertebrates and during regeneration (Margotta & Morelli 1997; Götz et al. 2002; Pekny & Pekna 2004). In mammalian glia, there is a shift of expression of vimentin to GFAP during embryonic development. Vimentin is principally found in radial glia during the embryonic stages, while the glia in the mature brain express GFAP (Pixley & de Vellis 1984; Götz et al. 2002). Similarly, there is a sequential transition of vimentin expression towards GFAP in radial glia in the shift between embryonic and juvenile stages in reptiles and teleost fish (Yanes et al. 1990; Arochena et al. 2004). It has been shown that the lack of GFAP, but not of vimentin, promotes neuronal survival and growth of processes (Menet et al. 2000; Menet et al. 2001). It has also been demonstrated that lack of intermediate filaments promotes cellular migration and integration of CNS transplants (reviewed in Pekny & Pekna 2004). However, the presence of vimentin in the neural progenitors in birds, in contrast to GFAP in the neural progenitors in lizards and mammals, suggests that the relationship between intermediate filament composition, phylogeny, regeneration and neurogenic potential is complex. How the differences in composition of intermediate filaments affect the differentiation, survival and integration of new cells in the adult brain is still unknown. It may well be that the presence of different intermediate filament components could be used as a means to identify diversity among radial glia and their respective potential.
9. Conclusions
Adult brain proliferation and neurogenesis is a conserved characteristic of all vertebrates. In mammals, adult neurogenesis occurs in two restricted brain regions of the lateral telencephalic ventricle and hippocampus. Similarly, non-mammalian vertebrates also display adult neurogenesis in equivalent and additional telencephalic brain areas. Although it has long been known that non-mammalian vertebrates display a considerable amount of adult neurogenesis in many brain regions, only little is known about the origin and fate of the generated cells.
A common feature of vertebrates (fish, amphibians and reptiles) that display a widespread adult neurogenesis is the substantial post-embryonic brain growth in contrast to birds and mammals. It is probable that the adult neurogenesis in fish, frogs and reptiles is related to the coordinated growth of sensory systems and corresponding sensory brain regions. Likewise, neurons are substantially added to the olfactory bulb in smell-oriented mammals such as rodents in contrast to the more visually oriented primates and songbirds, where much fewer neurons are added. This indicates that adult neurogenesis may also be related to modulation of sensory processing in mammals and birds.
Only little is known about the differences between constitutively neurogenic and non-neurogenic brain regions. However, adult neurogenesis in mammals can be modulated by injury, disease or administration of growth factors and signalling molecules indicating a previously unappreciated level of plasticity. Although limited neurogenesis can be induced ectopically in non-neurogenic areas of the brain, the modulation of neurogenesis has still principally been restricted to the constitutively neurogenic regions of the brain. Understanding how to manipulate the endogenous progenitors efficiently in order to generate diverse neuron types to the right place at the right time in the adult brain is of great importance in therapeutical approaches for brain injuries and diseases.
Neuronal turnover is another feature that has been found in the adult brain of all vertebrates. The reason why new neurons need to be replaced in the adult vertebrate brain is still unknown. The turnover of neurons in the adult brain shows surprising plasticity and is modulated by sensory input and systemic signals elicited from diverse environmental cues and behavioural responses.
Another feature of post-embryonic neurogenesis in vertebrates is the relatively low amount of produced neurons that actually survive a longer time and are functionally integrated into the neural circuitry. This mimics the process during late development when an excess of neurons is produced, while only a fraction maturates and survives. How the long-term survival and functional integration into the pre-existing neural network is controlled in adult neurogenesis is still to a large extent unknown. This is also a fundamental issue in any restorative therapy. Studies of non-mammalian vertebrates, in which a considerable number of new neurons are apparently added to the circuitry of the adult brain, may facilitate the revelation of how such therapies can be achieved.
Adult proliferation and neurogenesis seem to recapitulate some of the molecular and cellular mechanisms used during embryonic development. It would therefore be beneficial to study adult neurogenesis in model organisms used in developmental biology like Xenopus and zebrafish vertebrates that also display abundant adult neurogenesis and a great capacity to repair brain injuries.
Acknowledgments
We thank Heiner Grandel and Voelker Kroehne for their helpful discussions and important comments on the manuscript. We also thank anonymous reviewers for their helpful suggestions. Our work is supported by the Sigrid Juselius Foundation and the Deutsche Forschungsgemeinschaft (SFB 655) and Centre for Regenerative Therapies, Dresden, CRTD/Cluster of excellence .
Footnotes
One contribution of 14 to a Theme Issue ‘Stem cells and brain repair’.
References
- Aberg M.A, Aberg N.D, Hedbacker H, Oscarsson J, Eriksson P.S. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J. Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolf B, Chapouton P, Lam C.S, Topp S, Tannhäuser B, Strähle U, Götz M, Bally-Cuif L. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev. Biol. 2006;295:278–293. doi: 10.1016/j.ydbio.2006.03.023. doi:10.1016/j.ydbio.2006.03.023 [DOI] [PubMed] [Google Scholar]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J. Comp. Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. doi:10.1002/cne.901370404 [DOI] [PubMed] [Google Scholar]
- Altman J, Das G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. doi:10.1002/cne.901240303 [DOI] [PubMed] [Google Scholar]
- Alvarado A.S. Regeneration in the metazoans: why does it happen? Bioessays. 2000;22:578–590. doi: 10.1002/(SICI)1521-1878(200006)22:6<578::AID-BIES11>3.0.CO;2-#. doi:10.1002/(SICI)1521-1878(200006)22:6<578::AID-BIES11>3.0.CO;2-# [DOI] [PubMed] [Google Scholar]
- Alvarez-Borda B, Haripal B, Nottebohm F. Timing of brain-derived neurotrophic factor exposure affects life expectancy of new neurons. Proc. Natl Acad. Sci. USA. 2004;101:3957–3956. doi: 10.1073/pnas.0308118101. doi:10.1073/pnas.0308118101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kirn J.R. Birth, migration, incorporation, and death of vocal control neurons in adult songbirds. J. Neurobiol. 1997;33:585–601. doi:10.1002/(SICI)1097-4695(19971105)33:5<585::AID-NEU7>3.0.CO;2-0 [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim D.A. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. doi:10.1016/S0896-6273(04)00111-4 [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Nottebohm F. Migration of young neurons in adult avian brain. Nature. 1988;335:353–354. doi: 10.1038/335353a0. doi:10.1038/335353a0 [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Theelen M, Nottebohm F. Mapping of radial glia and of a new cell type in adult canary brain. J. Neurosci. 1988;8:2707–2712. doi: 10.1523/JNEUROSCI.08-08-02707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Theelen M, Nottebohm F. Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron. 1990;5:101–109. doi: 10.1016/0896-6273(90)90038-h. doi:10.1016/0896-6273(90)90038-H [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Ling C.Y, Nottebohm F. High vocal center growth and its relation to neurogenesis, neuronal replacement and song acquisition in juvenile canaries. J. Neurobiol. 1992;23:396–406. doi: 10.1002/neu.480230406. doi:10.1002/neu.480230406 [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Ling C.Y, Yu W.S. Contribution of neurons born during embryonic, juvenile, and adult life to the brain of adult canaries: regional specificity and delayed birth of neurons in the song-control nuclei. J. Comp. Neurol. 1994;347:233–248. doi: 10.1002/cne.903470207. doi:10.1002/cne.903470207 [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo J.M, Tramontin A.D. A unified hypothesis on the lineage of neural stem cells. Nat. Rev. Neurosci. 2001;2:287–293. doi: 10.1038/35067582. doi:10.1038/35067582 [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Seri B, Doetsch F. Identification of neural stem cells in the adult vertebrate brain. Brain Res. Bull. 2002;57:751–758. doi: 10.1016/s0361-9230(01)00770-5. doi:10.1016/S0361-9230(01)00770-5 [DOI] [PubMed] [Google Scholar]
- Anderson M.F, Aberg M.A, Nilsson M, Eriksson P.S. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Brain Res. Dev. Brain Res. 2002;134:115–122. doi: 10.1016/s0165-3806(02)00277-8. doi:10.1016/S0165-3806(02)00277-8 [DOI] [PubMed] [Google Scholar]
- Arochena M, Anadon R, Diaz-Regueira S.M. Development of vimentin and glial fibrillary acidic protein immunoreactivities in the brain of gray mullet (Chelon labrosus), an advanced teleost. J. Comp. Neurol. 2004;469:413–436. doi: 10.1002/cne.11021. doi:10.1002/cne.11021 [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002;8:963–970. doi: 10.1038/nm747. doi:10.1038/nm747 [DOI] [PubMed] [Google Scholar]
- Barami K, Kirschenbaum B, Lemmon V, Goldman S.A. N-Cadherin and Ng-CAM/8D9 are involved serially in the migration of newly generated neurons into the adult songbird brain. Neuron. 1994;13:567–582. doi: 10.1016/0896-6273(94)90026-4. doi:10.1016/0896-6273(94)90026-4 [DOI] [PubMed] [Google Scholar]
- Barnea A, Nottebohm F. Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proc. Natl Acad. Sci. USA. 1994;91:11 217–11 221. doi: 10.1073/pnas.91.23.11217. doi:10.1073/pnas.91.23.11217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea A, Nottebohm F. Recruitment and replacement of hippocampal neurons in young and adult chickadees: an addition to the theory of hippocampal learning. Proc. Natl Acad. Sci. USA. 1996;93:714–718. doi: 10.1073/pnas.93.2.714. doi:10.1073/pnas.93.2.714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S, Hay M, Amilhon B, Jean A, Moyse E. In vivo neurogenesis in the dorsal vagal complex of the adult rat brainstem. Neuroscience. 2005;130:75–90. doi: 10.1016/j.neuroscience.2004.08.047. doi:10.1016/j.neuroscience.2004.08.047 [DOI] [PubMed] [Google Scholar]
- Beach D.H, Jacobson M. Patterns of cell proliferation in the retina of the clawed frog during development. J. Comp. Neurol. 1979;183:603–613. doi: 10.1002/cne.901830308. doi:10.1002/cne.901830308 [DOI] [PubMed] [Google Scholar]
- Beazley L.D, Tennant M, Stewart T.M, Anstee S.D. The primary visual system of adult lizards demonstrates that neurogenesis is not obligatorily linked to central nerve regeneration but may be a prerequisite for the restoration of maps in the brain. Vis. Res. 1998;38:789–793. doi: 10.1016/s0042-6989(97)00212-5. doi:10.1016/S0042-6989(97)00212-5 [DOI] [PubMed] [Google Scholar]
- Bedard A, Parent A. Evidence of newly generated neurons in the human olfactory bulb. Brain Res. Dev. Brain Res. 2004;151:159–168. doi: 10.1016/j.devbrainres.2004.03.021. doi:10.1016/j.devbrainres.2004.03.021 [DOI] [PubMed] [Google Scholar]
- Bengzon J, Kokaia Z, Elmer E, Nanobashvili A, Kokaia M, Lindvall O. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc. Natl Acad. Sci. USA. 1997;94:10 432–10 437. doi: 10.1073/pnas.94.19.10432. doi:10.1073/pnas.94.19.10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman S.A. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J. Neurosci. 2001;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier P.J, Bedard A, Vinet J, Levesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc. Natl Acad. Sci. USA. 2002;99:11 464–11 469. doi: 10.1073/pnas.172403999. doi:10.1073/pnas.172403999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernocchi G, Scherini E, Giacometti S, Mares V. Premitotic DNA synthesis in the brain of the adult frog (Rana esculenta L.): an autoradiographic 3H-thymidine study. Anat. Rec. 1990;228:461–470. doi: 10.1002/ar.1092280413. doi:10.1002/ar.1092280413 [DOI] [PubMed] [Google Scholar]
- Betarbet R, Zigova T, Bakay R.A, Luskin M.B. Dopaminergic and GABAergic interneurons of the olfactory bulb are derived from the neonatal subventricular zone. Int. J. Dev. Neurosci. 1996;14:921–930. doi: 10.1016/s0736-5748(96)00066-4. doi:10.1016/S0736-5748(96)00066-4 [DOI] [PubMed] [Google Scholar]
- Biebl M, Cooper C.M, Winkler J, Kuhn H.G. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci. Lett. 2000;291:17–20. doi: 10.1016/s0304-3940(00)01368-9. doi:10.1016/S0304-3940(00)01368-9 [DOI] [PubMed] [Google Scholar]
- Biebl M, Winner B, Winkler J. Caspase inhibition decreases cell death in regions of adult neurogenesis. Neuroreport. 2005;16:1147–1150. doi: 10.1097/00001756-200508010-00003. doi:10.1097/00001756-200508010-00003 [DOI] [PubMed] [Google Scholar]
- Birse S.C, Leonard R.B, Coggeshall R.E. Neuronal increase in various areas of the nervous system of the guppy, Lebistes. J. Comp. Neurol. 1980;194:291–301. doi: 10.1002/cne.901940202. doi:10.1002/cne.901940202 [DOI] [PubMed] [Google Scholar]
- Bottjer S.W, Glaessner S.L, Arnold A.P. Ontogeny of brain nuclei controlling song learning and behavior in zebra finches. J. Neurosci. 1985;5:1556–1562. doi: 10.1523/JNEUROSCI.05-06-01556.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstatter R, Kotrschal K. Life history of roach, Rutilus rutilus (Cyprinidae, Teleostei). A qualitative and quantitative study on the development of sensory brain areas. Brain Behav. Evol. 1989;34:35–42. doi: 10.1159/000116489. [DOI] [PubMed] [Google Scholar]
- Brandstatter R, Kotrschal K. Brain growth patterns in four European cyprinid fish species (Cyprinidae, Teleostei): roach (Rutilus rutilus), bream (Abramis brama), common carp (Cyprinus carpio) and sabre carp (Pelecus cultratus) Brain Behav. Evol. 1990;35:195–211. doi: 10.1159/000115867. [DOI] [PubMed] [Google Scholar]
- Brockes J.P. Amphibian limb regeneration: rebuilding a complex structure. Science. 1997;276:81–87. doi: 10.1126/science.276.5309.81. doi:10.1126/science.276.5309.81 [DOI] [PubMed] [Google Scholar]
- Brockes J.P, Kumar A. Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat. Rev. Mol. Cell Biol. 2002;3:566–574. doi: 10.1038/nrm881. doi:10.1038/nrm881 [DOI] [PubMed] [Google Scholar]
- Broglio C, Gomez A, Duran E, Ocana F.M, Jimenez-Moya F, Rodriguez F, Salas C. Hallmarks of a common forebrain vertebrate plan: specialized pallial areas for spatial, temporal and emotional memory in actinopterygian fish. Brain Res. Bull. 2005;66:277–281. doi: 10.1016/j.brainresbull.2005.03.021. doi:10.1016/j.brainresbull.2005.03.021 [DOI] [PubMed] [Google Scholar]
- Buffo A, Vosko M.R, Erturk D, Hamann G.F, Jucker M, Rowitch D, Gotz M. Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair. Proc. Natl Acad. Sci. USA. 2005;102:18 183–18 188. doi: 10.1073/pnas.0506535102. doi:10.1073/pnas.0506535102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd C.A, Brunjes P.C. Neurogenesis in the olfactory bulb of adult zebrafish. Neuroscience. 2001;105:793–801. doi: 10.1016/s0306-4522(01)00215-9. doi:10.1016/S0306-4522(01)00215-9 [DOI] [PubMed] [Google Scholar]
- Callard G.V, Petro Z, Ryan K.J. Phylogenetic distribution of aromatase and other androgen-converting enzymes in the central nervous system. Endocrinology. 1978;103:2283–2290. doi: 10.1210/endo-103-6-2283. [DOI] [PubMed] [Google Scholar]
- Calof A.L, Mumm J.S, Rim P.C, Shou J. The neuronal stem cell of the olfactory epithelium. J. Neurobiol. 1998;36:190–205. doi: 10.1002/(sici)1097-4695(199808)36:2<190::aid-neu7>3.0.co;2-x. doi:10.1002/(SICI)1097-4695(199808)36:2<190::AID-NEU7>3.0.CO;2-X [DOI] [PubMed] [Google Scholar]
- Cameron H.A, Woolley C.S, McEwen B.S, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. doi:10.1016/0306-4522(93)90335-D [DOI] [PubMed] [Google Scholar]
- Cao J, Wenberg K, Cheng M.F. Lesion induced new neuron incorporation in the adult hypothalamus of the avian brain. Brain Res. 2002;943:80–92. doi: 10.1016/s0006-8993(02)02537-4. doi:10.1016/S0006-8993(02)02537-4 [DOI] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga D.S, Liu Y, Fong D.M, Young D, During M.J. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat. Genet. 2004;36:827–835. doi: 10.1038/ng1395. doi:10.1038/ng1395 [DOI] [PubMed] [Google Scholar]
- Carleton A, Petreanu L.T, Lansford R, Alvarez-Buylla A, Lledo P.M. Becoming a new neuron in the adult olfactory bulb. Nat. Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- Cerda J, Conrad M, Markl J, Brand M, Herrmann H. Zebrafish vimentin: molecular characterization, assembly properties and developmental expression. Eur. J. Cell Biol. 1998;77:175–187. doi: 10.1016/S0171-9335(98)80105-2. [DOI] [PubMed] [Google Scholar]
- Chen J, Magavi S.S, Macklis J.D. Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proc. Natl Acad. Sci. USA. 2004;101:16 357–16 362. doi: 10.1073/pnas.0406795101. doi:10.1073/pnas.0406795101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetverukhin V.K, Polenov A.L. Ultrastructural radioautographic analysis of neurogenesis in the hypothalamus of the adult frog, Rana temporaria, with special reference to physiological regeneration of the preoptic nucleus. I. Ventricular zone cell proliferation. Cell Tissue Res. 1993;271:341–350. doi: 10.1007/BF00318621. doi:10.1007/BF00318621 [DOI] [PubMed] [Google Scholar]
- Clayton N.S, Griffiths D.P, Emery N.J, Dickinson A. Elements of episodic-like memory in animals. Phil. Trans. R. Soc. B. 2001;356:1483–1491. doi: 10.1098/rstb.2001.0947. doi:10.1098/rstb.2001.0947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton N.S, Bussey T.J, Dickinson A. Can animals recall the past and plan for the future? Nat. Rev. Neurosci. 2003;4:685–691. doi: 10.1038/nrn1180. doi:10.1038/nrn1180 [DOI] [PubMed] [Google Scholar]
- Clint S.C, Zupanc G.K. Neuronal regeneration in the cerebellum of adult teleost fish, Apteronotus leptorhynchus: guidance of migrating young cells by radial glia. Brain Res. Dev. Brain Res. 2001;130:15–23. doi: 10.1016/s0165-3806(01)00193-6. doi:10.1016/S0165-3806(01)00193-6 [DOI] [PubMed] [Google Scholar]
- Conover J.C, Doetsch F, Garcia-Verdugo J.M, Gale N.W, Yancopoulos G.D, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat. Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. doi:10.1038/80606 [DOI] [PubMed] [Google Scholar]
- Craig C.G, Tropepe V, Morshead C.M, Reynolds B.A, Weiss S, van der Kooy D. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J. Neurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall J, Sakai Y, Zhang J, Koul O, Mineur Y, Crusio W.E, McCaffery P. 13-cis-retinoic acid suppresses hippocampal cell division and hippocampal-dependent learning in mice. Proc. Natl Acad. Sci. USA. 2004;101:5111–5116. doi: 10.1073/pnas.0306336101. doi:10.1073/pnas.0306336101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis M.A, Penney E.B, Pearson A.G, van Roon-Mom W.M, Butterworth N.J, Dragunow M, Connor B, Faull R.L. Increased cell proliferation and neurogenesis in the adult human Huntington's disease brain. Proc. Natl Acad. Sci. USA. 2003;100:9023–9027. doi: 10.1073/pnas.1532244100. doi:10.1073/pnas.1532244100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawley E.M, Fingerlin A, Hwang D, John S.S, Stankiewicz C.A. Seasonal cell proliferation in the chemosensory epithelium and brain of red-backed salamanders, Plethodon cinereus. Brain Behav. Evol. 2000;56:1–13. doi: 10.1159/000006673. doi:10.1159/000006673 [DOI] [PubMed] [Google Scholar]
- Dawson M.R, Levine J.M, Reynolds R. NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J. Neurosci. Res. 2000;61:471–479. doi: 10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.0.CO;2-N. doi:10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.0.CO;2-N [DOI] [PubMed] [Google Scholar]
- Dawson M.R, Polito A, Levine J.M, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. doi:10.1016/S1044-7431(03)00210-0 [DOI] [PubMed] [Google Scholar]
- Dayer A.G, Cleaver K.M, Abouantoun T, Cameron H.A. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J. Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. doi:10.1083/jcb.200407053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehay C, Savatier P, Cortay V, Kennedy H. Cell-cycle kinetics of neocortical precursors are influenced by embryonic thalamic axons. J. Neurosci. 2001;21:201–214. doi: 10.1523/JNEUROSCI.21-01-00201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Grande P, Franceschini V, Minelli G, Ciani F. Mitotic activity of the telencephalic matrix areas following optic tectum or pallial cortex lesion in newt. Z. Mikrosk Anat. Forsch. 1990;104:617–624. [PubMed] [Google Scholar]
- Depaepe V, Suarez-Gonzalez N, Dufour A, Passante L, Gorski J.A, Jones K.R, Ledent C, Vanderhaeghen P. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature. 2005;435:1244–1250. doi: 10.1038/nature03651. doi:10.1038/nature03651 [DOI] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat. Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. doi:10.1038/nn1144 [DOI] [PubMed] [Google Scholar]
- Doetsch F, Hen R. Young and excitable: the function of new neurons in the adult mammalian brain. Curr. Opin. Neurobiol. 2005;15:121–128. doi: 10.1016/j.conb.2005.01.018. doi:10.1016/j.conb.2005.01.018 [DOI] [PubMed] [Google Scholar]
- Doetsch F, Scharff C. Challenges for brain repair: insights from adult neurogenesis in birds and mammals. Brain Behav. Evol. 2001;58:306–322. doi: 10.1159/000057572. doi:10.1159/000057572 [DOI] [PubMed] [Google Scholar]
- Ekstrom P, Johnsson C.M, Ohlin L.M. Ventricular proliferation zones in the brain of an adult teleost fish and their relation to neuromeres and migration (secondary matrix) zones. J. Comp. Neurol. 2001;436:92–110. [PubMed] [Google Scholar]
- Emsley J.G, Mitchell B.D, Magavi S.S, Arlotta P, Macklis J.D. The repair of complex neuronal circuitry by transplanted and endogenous precursors. NeuroRx. 2004;1:452–471. doi: 10.1602/neurorx.1.4.452. doi:10.1602/neurorx.1.4.452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley J.G, Mitchell B.D, Kempermann G, Macklis J.D. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog. Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. doi:10.1016/j.pneurobio.2005.04.002 [DOI] [PubMed] [Google Scholar]
- Eriksson P.S, Perfilieva E, Bjork-Eriksson T, Alborn A.M, Nordborg C, Peterson D.A, Gage F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. doi:10.1038/3305 [DOI] [PubMed] [Google Scholar]
- Evans H.E. The correlation of brain pattern and feeding habits in four species of cyprinid fishes. J. Comp. Neurol. 1952;97:133–142. doi: 10.1002/cne.900970108. doi:10.1002/cne.900970108 [DOI] [PubMed] [Google Scholar]
- Ever L, Gaiano N. Radial ‘glial’ progenitors: neurogenesis and signaling. Curr. Opin. Neurobiol. 2005;15:29–33. doi: 10.1016/j.conb.2005.01.005. doi:10.1016/j.conb.2005.01.005 [DOI] [PubMed] [Google Scholar]
- Fallon J, et al. In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain. Proc. Natl Acad. Sci. USA. 2000;97:14 686–14 691. doi: 10.1073/pnas.97.26.14686. doi:10.1073/pnas.97.26.14686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J.W, Asher R.A. The glial scar and central nervous system repair. Brain Res. Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. doi:10.1016/S0361-9230(99)00072-6 [DOI] [PubMed] [Google Scholar]
- Ferretti P. Neural stem cell plasticity: recruitment of endogenous populations for regeneration. Curr. Neurovasc. Res. 2004;1:215–229. doi: 10.2174/1567202043362397. doi:10.2174/1567202043362397 [DOI] [PubMed] [Google Scholar]
- Filoni S, Gibertini G. A study of the regenerative capacity of the central nervous system of anuran amphibia in relation to their stage of development. I. Observations on the regeneration of the optic lobe of Xenopus laevis (Daudin) in the larval stages. Arch. Biol. 1969;80:369–411. [PubMed] [Google Scholar]
- Filoni S, Gibertini G. A study of the regeneration of the cerebellum of Xenopus leavis (Daudin) in the larval stages and after metamorphosis. Arch. Biol. 1971;82:433–470. [PubMed] [Google Scholar]
- Filoni S, Bernardini S, Cannata S.M. Differences in the decrease in regenerative capacity of various brain regions of Xenopus laevis are related to differences in the undifferentiated cell populations. J. Hirnforsch. 1995;36:523–529. [PubMed] [Google Scholar]
- Font E, Garcia-Verdugo J.M, Alcantara S, Lopez-Garcia C. Neuron regeneration reverses 3-acetylpyridine-induced cell loss in the cerebral cortex of adult lizards. Brain Res. 1991;551:230–235. doi: 10.1016/0006-8993(91)90937-q. doi:10.1016/0006-8993(91)90937-Q [DOI] [PubMed] [Google Scholar]
- Font C, Hoogland P.V, Vermeulen van der Zee E, Perez-Clausell J, Martinez-Garcia F. The septal complex of the telencephalon of the lizard Podarcis hispanica. I. Chemoarchitectonical organization. J. Comp. Neurol. 1995;359:117–130. doi: 10.1002/cne.903590108. doi:10.1002/cne.903590108 [DOI] [PubMed] [Google Scholar]
- Font E, Desfilis E, Perez-Canellas M, Alcantara S, Garcia-Verdugo J.M. 3-Acetylpyridine-induced degeneration and regeneration in the adult lizard brain: a qualitative and quantitative analysis. Brain Res. 1997;754:245–259. doi: 10.1016/s0006-8993(97)00085-1. doi:10.1016/S0006-8993(97)00085-1 [DOI] [PubMed] [Google Scholar]
- Font E, Desfilis E, Perez-Canellas M.M, Garcia-Verdugo J.M. Neurogenesis and neuronal regeneration in the adult reptilian brain. Brain Behav. Evol. 2001;58:276–295. doi: 10.1159/000057570. doi:10.1159/000057570 [DOI] [PubMed] [Google Scholar]
- Forlano P.M, Deitcher D.L, Myers D.A, Bass A.H. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: aromatase enzyme and mRNA expression identify glia as source. J. Neurosci. 2001;21:8943–8955. doi: 10.1523/JNEUROSCI.21-22-08943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Verdugo J.M, Llahi S, Ferrer I, Lopez-Garcia C. Postnatal neurogenesis in the olfactory bulbs of a lizard. a tritiated thymidine autoradiographic study. Neurosci. Lett. 1989;98:247–252. doi: 10.1016/0304-3940(89)90408-4. doi:10.1016/0304-3940(89)90408-4 [DOI] [PubMed] [Google Scholar]
- Garcia-Verdugo J.M, Ferron S, Flames N, Collado L, Desfilis E, Font E. The proliferative ventricular zone in adult vertebrates: a comparative study using reptiles, birds, and mammals. Brain Res. Bull. 2002;57:765–775. doi: 10.1016/s0361-9230(01)00769-9. doi:10.1016/S0361-9230(01)00769-9 [DOI] [PubMed] [Google Scholar]
- Goldman S. Glia as neural progenitor cells. Trends Neurosci. 2003;26:590–596. doi: 10.1016/j.tins.2003.09.011. doi:10.1016/j.tins.2003.09.011 [DOI] [PubMed] [Google Scholar]
- Goldman S. Stem and progenitor cell-based therapy of the human central nervous system. Nat. Biotechnol. 2005;23:862–871. doi: 10.1038/nbt1119. doi:10.1038/nbt1119 [DOI] [PubMed] [Google Scholar]
- Goldman S.A, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc. Natl Acad. Sci. USA. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. doi:10.1073/pnas.80.8.2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S.A, Zukhar A, Barami K, Mikawa T, Niedzwiecki D. Ependymal/subependymal zone cells of postnatal and adult songbird brain generate both neurons and nonneuronal siblings in vitro and in vivo. J. Neurobiol. 1996;30:505–520. doi: 10.1002/(SICI)1097-4695(199608)30:4<505::AID-NEU6>3.0.CO;2-7. doi:10.1002/(SICI)1097-4695(199608)30:4<505::AID-NEU6>3.0.CO;2-7 [DOI] [PubMed] [Google Scholar]
- Goss R.J. The evolution of regeneration: adaptive or inherent? J. Theor. Biol. 1992;159:241–260. doi: 10.1016/s0022-5193(05)80704-0. doi:10.1016/S0022-5193(05)80704-0 [DOI] [PubMed] [Google Scholar]
- Götz M, Barde Y.A. Radial glial cells defined and major intermediates between embryonic stem cells and CNS neurons. Neuron. 2005;46:369–372. doi: 10.1016/j.neuron.2005.04.012. doi:10.1016/j.neuron.2005.04.012 [DOI] [PubMed] [Google Scholar]
- Götz M, Hartfuss E, Malatesta P. Radial glial cells as neuronal precursors: a new perspective on the correlation of morphology and lineage restriction in the developing cerebral cortex of mice. Brain Res. Bull. 2002;57:777–788. doi: 10.1016/s0361-9230(01)00777-8. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves A.J, Graziano M.S, Gross C.G. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. doi:10.1126/science.286.5439.548 [DOI] [PubMed] [Google Scholar]
- Gould E, Vail N, Wagers M, Gross C.G. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc. Natl Acad. Sci. USA. 2001;98:10 910–10 917. doi: 10.1073/pnas.181354698. doi:10.1073/pnas.181354698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandel H, Kaslin J, Ganz J, Wenzel I, Brand M. Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Dev. Biol. 2006;295:263–277. doi: 10.1016/j.ydbio.2006.03.040. doi:10.1016/j.ydbio.2006.03.040 [DOI] [PubMed] [Google Scholar]
- Hampton D.W, Rhodes K.E, Zhao C, Franklin R.J, Fawcett J.W. The responses of oligodendrocyte precursor cells, astrocytes and microglia to a cortical stab injury, in the brain. Neuroscience. 2004;127:813–820. doi: 10.1016/j.neuroscience.2004.05.028. doi:10.1016/j.neuroscience.2004.05.028 [DOI] [PubMed] [Google Scholar]
- Hastings N.B, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J. Comp. Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. doi:10.1002/(SICI)1096-9861(19991011)413:1<146::AID-CNE10>3.0.CO;2-B [DOI] [PubMed] [Google Scholar]
- Healy S.D, Krebs J.R. Food storing and the hippocampus in Paridae. Brain Behav. Evol. 1996;47:195–199. doi: 10.1159/000113239. [DOI] [PubMed] [Google Scholar]
- Heins N, Malatesta P, Cecconi F, Nakafuku M, Tucker K.L, Hack M.A, Chapouton P, Barde Y.A, Gotz M. Glial cells generate neurons: the role of the transcription factor Pax6. Nat. Neurosci. 2002;5:308–315. doi: 10.1038/nn828. doi:10.1038/nn828 [DOI] [PubMed] [Google Scholar]
- Holmberg J, Armulik A, Senti K.A, Edoff K, Spalding K, Momma S, Cassidy R, Flanagan J.G, Frisen J. Ephrin-A2 reverse signaling negatively regulates neural progenitor proliferation and neurogenesis. Genes Dev. 2005;19:462–471. doi: 10.1101/gad.326905. doi:10.1101/gad.326905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K.E, Hatten M.E. Radial glial cell transformation to astrocytes is bidirectional: regulation by a diffusible factor in embryonic forebrain. Proc. Natl Acad. Sci. USA. 1995;92:2061–2065. doi: 10.1073/pnas.92.6.2061. doi:10.1073/pnas.92.6.2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. 3rd edn. Plenum Press; New York, NY: 1991. Developmental neurobiology. [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao X.O, Xie L, Greenberg D.A. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl Acad. Sci. USA. 2002;99:11 946–11 950. doi: 10.1073/pnas.182296499. doi:10.1073/pnas.182296499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Peel A, Mao X.O, Batteur S, Greenberg D.A. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol. Cell. Neurosci. 2003;24:171–189. doi: 10.1016/s1044-7431(03)00159-3. doi:10.1016/S1044-7431(03)00159-3 [DOI] [PubMed] [Google Scholar]
- Jin K, Peel A.L, Mao X.O, Xie L, Cottrell B.A, Henshall D.C, Greenberg D.A. Increased hippocampal neurogenesis in Alzheimer's disease. Proc. Natl Acad. Sci. USA. 2004a;101:343–347. doi: 10.1073/pnas.2634794100. doi:10.1073/pnas.2634794100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Galvan V, Xie L, Mao X.O, Gorostiza O.F, Bredesen D.E, Greenberg D.A. Enhanced neurogenesis in Alzheimer's disease transgenic (PDGF-APPSw,Ind) mice. Proc. Natl Acad. Sci. USA. 2004b;101:13 363–13 367. doi: 10.1073/pnas.0403678101. doi:10.1073/pnas.0403678101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C.B, Momma S, Clarke D.L, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. doi:10.1016/S0092-8674(00)80956-3 [DOI] [PubMed] [Google Scholar]
- Johns P.R, Easter S.S., Jr Growth of the adult goldfish eye. II. Increase in retinal cell number. J. Comp. Neurol. 1977;176:331–341. doi: 10.1002/cne.901760303. doi:10.1002/cne.901760303 [DOI] [PubMed] [Google Scholar]
- Kalman M. GFAP expression withdraws—a trend of glial evolution? Brain Res. Bull. 2002;57:509–511. doi: 10.1016/s0361-9230(01)00713-4. doi:10.1016/S0361-9230(01)00713-4 [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage F.H. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. doi:10.1242/dev.00203 [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004a;27:447–452. doi: 10.1016/j.tins.2004.05.013. doi:10.1016/j.tins.2004.05.013 [DOI] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage F.H. Functional significance of adult neurogenesis. Curr. Opin. Neurobiol. 2004b;14:186–191. doi: 10.1016/j.conb.2004.03.001. doi:10.1016/j.conb.2004.03.001 [DOI] [PubMed] [Google Scholar]
- Kirn J.R, Alvarez-Buylla A, Nottebohm F. Production and survival of projection neurons in a forebrain vocal center of adult male canaries. J. Neurosci. 1991;11:1756–1762. doi: 10.1523/JNEUROSCI.11-06-01756.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn J.R, Fishman Y, Sasportas K, Alvarez-Buylla A, Nottebohm F. Fate of new neurons in adult canary high vocal center during the first 30 days after their formation. J. Comp. Neurol. 1999;411:487–494. doi:10.1002/(SICI)1096-9861(19990830)411:3<487::AID-CNE10>3.0.CO;2-M [PubMed] [Google Scholar]
- Kirsche W. Die regenerativen Vorgänge am Rückenmark erwaschsener Teleostier nach operativer kontinuitätstrennung. Z. Mikrosk Anat. Forsch. 1950;56:190–265. [Google Scholar]
- Kirsche W. Experimentelle untersuchungen zur frage der regeneration und function des tectum opticum con Carassius carassius L. Zeittschr.f.mikro.-anat. Forschung. 1960;67:140–182. [PubMed] [Google Scholar]
- Kirsche W. Regenerative processes in the brain and spinal cord. Ergeb Anat. Entwicklungsgesch. 1965;38:143–194. [PubMed] [Google Scholar]
- Kirsche W. On postembryonic matrix zones in the brain of various vertebrates and their relationship to the study of the brain structure. Z. Mikrosk Anat. Forsch. 1967;77:313–406. [PubMed] [Google Scholar]
- Kirschenbaum B, Nedergaard M, Preuss A, Barami K, Fraser R.A, Goldman S.A. In vitro neuronal production and differentiation by precursor cells derived from the adult human forebrain. Cereb. Cortex. 1994;4:576–589. doi: 10.1093/cercor/4.6.576. doi:10.1093/cercor/4.6.576 [DOI] [PubMed] [Google Scholar]
- Koketsu D, Mikami A, Miyamoto Y, Hisatsune T. Nonrenewal of neurons in the cerebral neocortex of adult macaque monkeys. J. Neurosci. 2003;23:937–942. doi: 10.1523/JNEUROSCI.23-03-00937.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. doi:10.1126/science.289.5485.1754 [DOI] [PubMed] [Google Scholar]
- Kotrschal K, Palzenberger M. Neuroecology of cyprinids: comparative, quantitative histology reveals diverse brain patterns. Environ. Biol. Fishes. 1992;33:135–152. doi:10.1007/BF00002560 [Google Scholar]
- Kubota R, Hokoc J.N, Moshiri A, McGuire C, Reh T.A. A comparative study of neurogenesis in the retinal ciliary marginal zone of homeothermic vertebrates. Brain Res. Dev. Brain Res. 2002;134:31–41. doi: 10.1016/s0165-3806(01)00287-5. doi:10.1016/S0165-3806(01)00287-5 [DOI] [PubMed] [Google Scholar]
- Kuhn H.G, Winkler J, Kempermann G, Thal L.J, Gage F.H. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J. Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K, Kaspar B.K, Gage F.H, Schaffer D.V. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat. Neurosci. 2003;6:21–27. doi: 10.1038/nn983. doi:10.1038/nn983 [DOI] [PubMed] [Google Scholar]
- Lang D.M, del Mar Romero-Aleman M, Arbelo-Galvan J.F, Stuermer C.A, Monzon-Mayor M. Regeneration of retinal axons in the lizard Gallotia galloti is not linked to generation of new retinal ganglion cells. J. Neurobiol. 2002;52:322–335. doi: 10.1002/neu.10099. doi:10.1002/neu.10099 [DOI] [PubMed] [Google Scholar]
- Lanuza E, Novejarque A, Moncho-Bogani J, Hernandez A, Martinez-Garcia F. Understanding the basic circuitry of the cerebral hemispheres: the case of lizards and its implications in the evolution of the telencephalon. Brain Res. Bull. 2002;57:471–473. doi: 10.1016/s0361-9230(01)00710-9. doi:10.1016/S0361-9230(01)00710-9 [DOI] [PubMed] [Google Scholar]
- Laywell E.D, Rakic P, Kukekov V.G, Holland E.C, Steindler D.A. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc. Natl Acad. Sci. USA. 2000;97:13 883–13 888. doi: 10.1073/pnas.250471697. doi:10.1073/pnas.250471697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Kessler J.D, Read T.A, Kaiser C, Corbeil D, Huttner W.B, Johnson J.E, Wechsler-Reya R.J. Isolation of neural stem cells from the postnatal cerebellum. Nat. Neurosci. 2005;8:723–729. doi: 10.1038/nn1473. doi:10.1038/nn1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R.B, Coggeshall R.E, Willis W.D. A documentation of an age related increase in neuronal and axonal numbers in the stingray, Dasyatis sabina, Leseuer. J. Comp. Neurol. 1978;179:13–21. doi: 10.1002/cne.901790103. doi:10.1002/cne.901790103 [DOI] [PubMed] [Google Scholar]
- Levine J.M, Reynolds R, Fawcett J.W. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. doi:10.1016/S0166-2236(00)01691-X [DOI] [PubMed] [Google Scholar]
- Leyhausen C, Kirschbaum F, Szabo T, Erdelen M. Differential Growth in the brain of the weakly electric fish, Apteronotus leptorhynchus (Gymnotiformes), during ontogenesis. Brain Behav. Evol. 1987;30:230–248. doi: 10.1159/000118648. [DOI] [PubMed] [Google Scholar]
- Lie D.C, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. doi:10.1038/nature04108 [DOI] [PubMed] [Google Scholar]
- Lim D.A, Tramontin A.D, Trevejo J.M, Herrera D.G, Garcia-Verdugo J.M, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. doi:10.1016/S0896-6273(00)00148-3 [DOI] [PubMed] [Google Scholar]
- Ling C, Zuo M, Alvarez-Buylla A, Cheng M.F. Neurogenesis in juvenile and adult ring doves. J. Comp. Neurol. 1997;379:300–312. doi:10.1002/(SICI)1096-9861(19970310)379:2<300::AID-CNE10>3.0.CO;2-T [PubMed] [Google Scholar]
- Lledo P.M, Alonso M, Grubb M.S. Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. doi:10.1038/nrn1867 [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. doi:10.1126/science.8178174 [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo J.M, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. doi:10.1126/science.271.5251.978 [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia C, Tineo P.L, Del Corral J. Increase of the neuron number in some cerebral cortical areas of a lizard, Podarcis hispanica, (Steind., 1870), during postnatal periods of life. J. Hirnforsch. 1984;25:255–259. [PubMed] [Google Scholar]
- Lopez-Garcia C, Molowny A, Garcia-Verdugo J.M, Ferrer I. Delayed postnatal neurogenesis in the cerebral cortex of lizards. Brain Res. 1988;471:167–174. doi: 10.1016/0165-3806(88)90096-x. [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia C, Molowny A, Garcia-Verdugo J.M, Martinez-Guijarro F.J, Bernabeu A. Late generated neurons in the medial cortex of adult lizards send axons that reach the Timm-reactive zones. Brain Res. Dev. Brain Res. 1990;57:249–254. doi: 10.1016/0165-3806(90)90050-9. doi:10.1016/0165-3806(90)90050-9 [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia C, Molowny A, Nacher J, Ponsoda X, Sancho-Bielsa F, Alonso-Llosa G. The lizard cerebral cortex as a model to study neuronal regeneration. An. Acad. Bras. Cienc. 2002;74:85–104. doi: 10.1590/s0001-37652002000100006. [DOI] [PubMed] [Google Scholar]
- Luskin M.B. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. doi:10.1016/0896-6273(93)90281-U [DOI] [PubMed] [Google Scholar]
- Ma D.K, Ming G.L, Song H. Glial influences on neural stem cell development: cellular niches for adult neurogenesis. Curr. Opin. Neurobiol. 2005;15:514–520. doi: 10.1016/j.conb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Machold R, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. doi:10.1016/S0896-6273(03)00561-0 [DOI] [PubMed] [Google Scholar]
- Magavi S.S, Leavitt B.R, Macklis J.D. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. doi:10.1038/35016083 [DOI] [PubMed] [Google Scholar]
- Magavi S.S, Mitchell B.D, Szentirmai O, Carter B.S, Macklis J.D. Adult-born and preexisting olfactory granule neurons undergo distinct experience-dependent modifications of their olfactory responses in vivo. J. Neurosci. 2005;25:10 729–10 739. doi: 10.1523/JNEUROSCI.2250-05.2005. doi:10.1523/JNEUROSCI.2250-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchioro M, de Azevedo Mota Nunes J.M, Rabelo Ramalho A.M, Molowny A, Perez-Martinez E, Ponsoda X, Lopez-Garcia C. Postnatal neurogenesis in the medial cortex of the tropical lizard Tropidurus hispidus. Neuroscience. 2005;134:407–413. doi: 10.1016/j.neuroscience.2005.04.014. doi:10.1016/j.neuroscience.2005.04.014 [DOI] [PubMed] [Google Scholar]
- Marcus R.C, Delaney C.L, Easter S.S., Jr Neurogenesis in the visual system of embryonic and adult zebrafish (Danio rerio) Vis. Neurosci. 1999;16:417–424. doi: 10.1017/s095252389916303x. doi:10.1017/S095252389916303X [DOI] [PubMed] [Google Scholar]
- Margotta V, Morelli A. Contribution of radial glial cells to neurogenesis and plasticity of central nervous system in adult vertebrates. Anim. Biol. 1997;6:101–108. [Google Scholar]
- Markakis E.A, Palmer T.D, Randolph-Moore L, Rakic P, Gage F.H. Novel neuronal phenotypes from neural progenitor cells. J. Neurosci. 2004;24:2886–2897. doi: 10.1523/JNEUROSCI.4161-03.2004. doi:10.1523/JNEUROSCI.4161-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Guijarro F.J, Blasco-Ibanez J.M, Lopez-Garcia C. Postnatal increase of GABA- and PV-IR cells in the cerebral cortex of the lizard Podarcis hispanica. Brain Res. 1994;634:168–172. doi: 10.1016/0006-8993(94)90272-0. doi:10.1016/0006-8993(94)90272-0 [DOI] [PubMed] [Google Scholar]
- Meek J, Nieuwenhuys R. Holosteans and Teleosts. In: Nieuwenhuys R, ten Donkelaar H.J, Nicholson C, editors. The central nervous system of vertebrates. Springer; Berlin, Germany: 1998. pp. 759–939. [Google Scholar]
- Meltzer L.A, Yabaluri R, Deisseroth K. A role for circuit homeostasis in adult neurogenesis. Trends Neurosci. 2005;28:653–660. doi: 10.1016/j.tins.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Menet V, Gimenez Y Ribotta M, Sandillon F, Privat A. GFAP null astrocytes are a favorable substrate for neuronal survival and neurite growth. Glia. 2000;31:267–272. doi: 10.1002/1098-1136(200009)31:3<267::aid-glia80>3.0.co;2-n. doi:10.1002/1098-1136(200009)31:3<267::AID-GLIA80>3.0.CO;2-N [DOI] [PubMed] [Google Scholar]
- Menet V, Gimenez y Ribotta M, Chauvet N, Drian M.J, Lannoy J, Colucci-Guyon E, Privat A. Inactivation of the glial fibrillary acidic protein gene, but not that of vimentin, improves neuronal survival and neurite growth by modifying adhesion molecule expression. J. Neurosci. 2001;21:6147–6158. doi: 10.1523/JNEUROSCI.21-16-06147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menuet A, Anglade I, Le Guevel R, Pellegrini E, Pakdel F, Kah O. Distribution of aromatase mRNA and protein in the brain and pituitary of female rainbow trout: comparison with estrogen receptor alpha. J. Comp. Neurol. 2003;462:180–193. doi: 10.1002/cne.10726. doi:10.1002/cne.10726 [DOI] [PubMed] [Google Scholar]
- Menuet A, Pellegrini E, Brion F, Gueguen M.M, Anglade I, Pakdel F, Kah O. Expression and estrogen-dependent regulation of the zebrafish brain aromatase gene. J. Comp. Neurol. 2005;485:304–320. doi: 10.1002/cne.20497. doi:10.1002/cne.20497 [DOI] [PubMed] [Google Scholar]
- Meyer R.L. Evidence from thymidine labeling for continuing growth of retina and tectum in juvenile goldfish. Exp. Neurol. 1978;59:99–111. doi: 10.1016/0014-4886(78)90204-2. doi:10.1016/0014-4886(78)90204-2 [DOI] [PubMed] [Google Scholar]
- Minelli G, del Grande P, Franceschini V, Ciani F. Proliferative response of the mesencephalic matrix areas in the reparation of the optic tectum of Triturus cristatus carnifex. Z. Mikrosk. Anat. Forsch. 1990;104:17–25. [PubMed] [Google Scholar]
- Molowny A, Nacher J, Lopez-Garcia C. Reactive neurogenesis during regeneration of the lesioned medial cerebral cortex of lizards. Neuroscience. 1995;68:823–836. doi: 10.1016/0306-4522(95)00201-s. doi:10.1016/0306-4522(95)00201-S [DOI] [PubMed] [Google Scholar]
- Monti Graziadei A.G, Morrison E.E. Experimental studies on the olfactory marker protein. IV. Olfactory marker protein in the olfactory neurons transplanted within the brain. Brain Res. 1988;455:401–406. doi: 10.1016/0006-8993(88)90103-5. doi:10.1016/0006-8993(88)90103-5 [DOI] [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. doi:10.1016/S0092-8674(02)00862-0 [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Testosterone triggers growth of brain vocal control nuclei in adult female canaries. Brain Res. 1980;189:429–436. doi: 10.1016/0006-8993(80)90102-x. doi:10.1016/0006-8993(80)90102-X [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Neuronal replacement in adulthood. Ann. N. Y. Acad. Sci. 1985;457:143–161. doi: 10.1111/j.1749-6632.1985.tb20803.x. doi:10.1111/j.1749-6632.1985.tb20803.x [DOI] [PubMed] [Google Scholar]
- Nottebohm F, O'Loughlin B, Gould K, Yohay K, Alvarez-Buylla A. The life span of new neurons in a song control nucleus of the adult canary brain depends on time of year when these cells are born. Proc. Natl Acad. Sci. USA. 1994;91:7849–7853. doi: 10.1073/pnas.91.17.7849. doi:10.1073/pnas.91.17.7849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes M.C, Roy N.S, Keyoung H.M, Goodman R.R, McKhann G, II, Jiang L, Kang J, Nedergaard M, Goldman S.A. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat. Med. 2003;9:439–447. doi: 10.1038/nm837. doi:10.1038/nm837 [DOI] [PubMed] [Google Scholar]
- Palma V, et al. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. doi: 10.1242/dev.01567. doi:10.1242/dev.01567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer T.D, Markakis E.A, Willhoite A.R, Safar F, Gage F.H. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J. Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchision D.M, McKay R.D. The control of neural stem cells by morphogenic signals. Curr. Opin. Genet. Dev. 2002;12:478–487. doi: 10.1016/s0959-437x(02)00329-5. doi:10.1016/S0959-437X(02)00329-5 [DOI] [PubMed] [Google Scholar]
- Panchision D.M, Pickel J.M, Studer L, Lee S.H, Turner P.A, Hazel T.G, McKay R.D. Sequential actions of BMP receptors control neural precursor cell production and fate. Genes Dev. 2001;15:2094–2110. doi: 10.1101/gad.894701. doi:10.1101/gad.894701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent J.M, Yu T.W, Leibowitz R.T, Geschwind D.H, Sloviter R.S, Lowenstein D.H. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent J.M, Valentin V.V, Lowenstein D.H. Prolonged seizures increase proliferating neuroblasts in the adult rat subventricular zone-olfactory bulb pathway. J. Neurosci. 2002a;22:3174–3188. doi: 10.1523/JNEUROSCI.22-08-03174.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent J.M, Vexler Z.S, Gong C, Derugin N, Ferriero D.M. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann. Neurol. 2002b;52:802–813. doi: 10.1002/ana.10393. doi:10.1002/ana.10393 [DOI] [PubMed] [Google Scholar]
- Parent J.M, von dem Bussche N, Lowenstein D.H. Prolonged seizures recruit caudal subventricular zone glial progenitors into the injured hippocampus. Hippocampus. 2006;16:321–328. doi: 10.1002/hipo.20166. doi:10.1002/hipo.20166 [DOI] [PubMed] [Google Scholar]
- Pekny M, Pekna M. Astrocyte intermediate filaments in CNS pathologies and regeneration. J. Pathol. 2004;204:428–437. doi: 10.1002/path.1645. doi:10.1002/path.1645 [DOI] [PubMed] [Google Scholar]
- Pellegrini E, Menuet A, Lethimonier C, Adrio F, Gueguen M.M, Tascon C, Anglade I, Pakdel F, Kah O. Relationships between aromatase and estrogen receptors in the brain of teleost fish. Gen. Comp. Endocrinol. 2005;142:60–66. doi: 10.1016/j.ygcen.2004.12.003. doi:10.1016/j.ygcen.2004.12.003 [DOI] [PubMed] [Google Scholar]
- Penafiel A, Gutierrez A, Martin R, Mar Perez-Canellas M, de la Calle A. A tangential neuronal migration in the olfactory bulbs of adult lizards. Neuroreport. 1996;7:1257–1260. doi: 10.1097/00001756-199605170-00007. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman K.D, Freedman L.J, Luskin M.B. Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Exp. Neurol. 2001a;172:1–16. doi: 10.1006/exnr.2001.7768. doi:10.1006/exnr.2001.7768 [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman K.D, Wiegand S.J, Luskin M.B. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J. Neurosci. 2001b;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Canellas M.M, Font E, Garcia-Verdugo J.M. Postnatal neurogenesis in the telencephalon of turtles: evidence for nonradial migration of new neurons from distant proliferative ventricular zones to the olfactory bulbs. Brain Res. Dev. Brain Res. 1997;101:125–137. doi: 10.1016/s0165-3806(97)00058-8. doi:10.1016/S0165-3806(97)00058-8 [DOI] [PubMed] [Google Scholar]
- Perez-Sanchez F, Molowny A, Garcia-Verdugo J.M, Lopez-Garcia C. Postnatal neurogenesis in the nucleus sphericus of the lizard, Podarcis hispanica. Neurosci. Lett. 1989;106:71–75. doi: 10.1016/0304-3940(89)90204-8. doi:10.1016/0304-3940(89)90204-8 [DOI] [PubMed] [Google Scholar]
- Pixley S.K, de Vellis J. Transition between immature radial glia and mature astrocytes studied with a monoclonal antibody to vimentin. Brain Res. 1984;317:201–209. doi: 10.1016/0165-3806(84)90097-x. [DOI] [PubMed] [Google Scholar]
- Platel R. Poids encéphalique et indice d'encéphalisation chez les reptiles sauriens. Zool. Anz. 1974;192:332–382. [Google Scholar]
- Polenov A.L, Chetverukhin V.K. Ultrastructural radioautographic analysis of neurogenesis in the hypothalamus of the adult frog, Rana temporaria, with special reference to physiological regeneration of the preoptic nucleus. II. Types of neuronal cells produced. Cell Tissue Res. 1993;271:351–362. doi: 10.1007/BF00318622. doi:10.1007/BF00318622 [DOI] [PubMed] [Google Scholar]
- Qiu J, Cai D, Filbin M.T. Glial inhibition of nerve regeneration in the mature mammalian CNS. Glia. 2000;29:166–174. doi:10.1002/(SICI)1098-1136(20000115)29:2<166::AID-GLIA10>3.0.CO;2-G [PubMed] [Google Scholar]
- Quinones-Hinojosa A, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J. Comp. Neurol. 2006;494:415–434. doi: 10.1002/cne.20798. doi:10.1002/cne.20798 [DOI] [PubMed] [Google Scholar]
- Rahmann H. Autoradiographische Untersuchungen zum DNSstoffwechsel (Mitose-Haüfigkeit) im ZNS von Brachydanio rerio HAM. BUCH. (Cyprinidae, Pisces) J. Hirnforsch. 1968;10:279–284. [PubMed] [Google Scholar]
- Rakic P. Neurogenesis in adult primate neocortex: an evaluation of the evidence. Nat. Rev. Neurosci. 2002;3:65–71. doi: 10.1038/nrn700. doi:10.1038/nrn700 [DOI] [PubMed] [Google Scholar]
- Rakic P. Neuroscience: immigration denied. Nature. 2004;427:685–686. doi: 10.1038/427685a. doi:10.1038/427685a [DOI] [PubMed] [Google Scholar]
- Ramirez C, Nacher J, Molowny A, Sanchez-Sanchez F, Irurzun A, Lopez-Garcia C. Photoperiod-temperature and neuroblast proliferation-migration in the adult lizard cortex. Neuroreport. 1997;8:2337–2342. doi: 10.1097/00001756-199707070-00047. doi:10.1097/00001756-199707070-00047 [DOI] [PubMed] [Google Scholar]
- Ramirez-Castillejo C, Nacher J, Molowny A, Ponsoda X, Lopez-Garcia C. PSA-NCAM immunocytochemistry in the cerebral cortex and other telencephalic areas of the lizard Podarcis hispanica: differential expression during medial cortex neuronal regeneration. J. Comp. Neurol. 2002;453:145–156. doi: 10.1002/cne.10390. doi:10.1002/cne.10390 [DOI] [PubMed] [Google Scholar]
- Rasika S, Alvarez-Buylla A, Nottebohm F. BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999;22:53–62. doi: 10.1016/s0896-6273(00)80678-9. doi:10.1016/S0896-6273(00)80678-9 [DOI] [PubMed] [Google Scholar]
- Raucci F, Di Fiore M.M, Pinelli C, D'Aniello B, Luongo L, Polese G, Rastogi R.K. Proliferative activity in the frog brain: a PCNA-immunohistochemistry analysis. J. Chem. Neuroanat. 2006;32:127–142. doi: 10.1016/j.jchemneu.2006.08.001. doi:10.1016/j.jchemneu.2006.08.001 [DOI] [PubMed] [Google Scholar]
- Raymond P.A, Easter S.S., Jr Postembryonic growth of the optic tectum in goldfish. I. Location of germinal cells and numbers of neurons produced. J. Neurosci. 1983;3:1077–1091. doi: 10.1523/JNEUROSCI.03-05-01077.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter W. Regeneration in the tectum opticum of Leucaspius delineatus (Heckel 1843) Z. Mikrosk. Anat. Forsch. 1965;74:46–68. [PubMed] [Google Scholar]
- Richter W. Regeneration im telencephalon von juvenilen und adulten Lebistes reticulates (Teleostei) Zeittschr.f.mikro.-anat. Forschung. 1969;81:345–358. [PubMed] [Google Scholar]
- Richter W, Kranz D. Autoradiographische Untersuchungen der postnatalen Proliferationsaktivität der Matrixzonen des Gehirns der Forelle (Salmo irideus) Z. Mikrosk. Anat. Forsch. 1981;95:491–520. [PubMed] [Google Scholar]
- Rodriguez F, Lopez J.C, Vargas J.P, Gomez Y, Broglio C, Salas C. Conservation of spatial memory function in the pallial forebrain of reptiles and ray-finned fishes. J. Neurosci. 2002;22:2894–2903. doi: 10.1523/JNEUROSCI.22-07-02894.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Aleman M.M, Monzon-Mayor M, Yanes C, Lang D. Radial glial cells, proliferating periventricular cells, and microglia might contribute to successful structural repair in the cerebral cortex of the lizard Gallotia galloti. Exp. Neurol. 2004;188:74–85. doi: 10.1016/j.expneurol.2004.03.014. doi:10.1016/j.expneurol.2004.03.014 [DOI] [PubMed] [Google Scholar]
- Rousselot P, Lois C, Alvarez-Buylla A. Embryonic (PSA) N-CAM reveals chains of migrating neuroblasts between the lateral ventricle and the olfactory bulb of adult mice. J. Comp. Neurol. 1995;351:51–61. doi: 10.1002/cne.903510106. doi:10.1002/cne.903510106 [DOI] [PubMed] [Google Scholar]
- Salas C, Broglio C, Rodriguez F. Evolution of forebrain and spatial cognition in vertebrates: conservation across diversity. Brain Behav. Evol. 2003;62:72–82. doi: 10.1159/000072438. doi:10.1159/000072438 [DOI] [PubMed] [Google Scholar]
- Sanai N, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. doi:10.1038/nature02301 [DOI] [PubMed] [Google Scholar]
- Scharff C, Kirn J.R, Grossman M, Macklis J.D, Nottebohm F. Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds. Neuron. 2000;25:481–492. doi: 10.1016/s0896-6273(00)80910-1. doi:10.1016/S0896-6273(00)80910-1 [DOI] [PubMed] [Google Scholar]
- Segaar J. Behavioural aspects of degeneration and regeneration in fish brain: a comparison with higher vertebrates. Prog. Brain Res. 1965;14:143–231. doi: 10.1016/s0079-6123(08)63743-7. [DOI] [PubMed] [Google Scholar]
- Seki T, Arai Y. Highly polysialylated neural cell adhesion molecule (NCAM-H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J. Neurosci. 1993;13:2351–2358. doi: 10.1523/JNEUROSCI.13-06-02351.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo J.M, McEwen B.S, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo J.M, Collado-Morente L, McEwen B.S, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J. Comp. Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. doi:10.1002/cne.20288 [DOI] [PubMed] [Google Scholar]
- Sibbing W. Postnatale regeneration der verschiedenen Hirnabschnitte bei Urodelen. Arch. Entwmech. 1953;146:433–486. doi: 10.1007/BF00576578. doi:10.1007/BF00576578 [DOI] [PubMed] [Google Scholar]
- Slack J.M, Beck C.W, Gargioli C, Christen B. Cellular and molecular mechanisms of regeneration in Xenopus. Phil. Trans. R. Soc. B. 2004;359:745–751. doi: 10.1098/rstb.2004.1463. doi:10.1098/rstb.2004.1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Kempermann G, Overstreet Wadiche L, Zhao C, Schinder A.F, Bischofberger J. New neurons in the adult mammalian brain: synaptogenesis and functional integration. J. Neurosci. 2005;25:10 366–10 368. doi: 10.1523/JNEUROSCI.3452-05.2005. doi:10.1523/JNEUROSCI.3452-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutschek J, Zupanc G.K. Apoptosis in the cerebellum of adult teleost fish, Apteronotus leptorhynchus. Brain Res. Dev. Brain Res. 1996;97:279–286. doi: 10.1016/s0165-3806(96)00145-9. doi:10.1016/S0165-3806(96)00145-9 [DOI] [PubMed] [Google Scholar]
- Spassky N, Merkle F.T, Flames N, Tramontin A.D, Garcia-Verdugo J.M, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J. Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. doi:10.1523/JNEUROSCI.1108-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srebro Z. Endbrain regeneration in adult Xenopus laevis. Folia Biol. 1965;13:269–280. [PubMed] [Google Scholar]
- Straznicky K, Gaze R.M. Growth of the retina in Xenopus laevis: an autoradiographic study. J. Embryol. Exp. Morphol. 1971;26:67–79. [PubMed] [Google Scholar]
- Straznicky K, Gaze R.M. The development of the tectum in Xenopus laevis: an autoradiographic study. J. Embryol. Exp. Morphol. 1972;28:87–115. [PubMed] [Google Scholar]
- Sundholm-Peters N.L, Yang H.K, Goings G.E, Walker A.S, Szele F.G. Radial glia-like cells at the base of the lateral ventricles in adult mice. J. Neurocytol. 2004;33:153–164. doi: 10.1023/B:NEUR.0000029654.70632.3a. doi:10.1023/B:NEUR.0000029654.70632.3a [DOI] [PubMed] [Google Scholar]
- Tao Y, Black I.B, DiCicco-Bloom E. Neurogenesis in neonatal rat brain is regulated by peripheral injection of basic fibroblast growth factor (bFGF) J. Comp. Neurol. 1996;376:653–663. doi: 10.1002/(SICI)1096-9861(19961223)376:4<653::AID-CNE11>3.0.CO;2-N. doi:10.1002/(SICI)1096-9861(19961223)376:4<653::AID-CNE11>3.0.CO;2-N [DOI] [PubMed] [Google Scholar]
- Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. doi:10.1038/35102174 [DOI] [PubMed] [Google Scholar]
- Tramontin A.D, Brenowitz E.A. Seasonal plasticity in the adult brain. Trends Neurosci. 2000;23:251–258. doi: 10.1016/s0166-2236(00)01558-7. doi:10.1016/S0166-2236(00)01558-7 [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder A.F, Christie B.R, Toni N, Palmer T.D, Gage F.H. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. doi:10.1038/4151030a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara M.N, Arsenijevic Y, Del Rio-Tsonis K. CNS regeneration: a morphogen's tale. J. Neurobiol. 2005;64:491–507. doi: 10.1002/neu.20158. doi:10.1002/neu.20158 [DOI] [PubMed] [Google Scholar]
- Vidal Pizarro I, Swain G.P, Selzer M.E. Cell proliferation in the lamprey central nervous system. J. Comp. Neurol. 2004;469:298–310. doi: 10.1002/cne.11013. doi:10.1002/cne.11013 [DOI] [PubMed] [Google Scholar]
- Voigt T. Development of glial cells in the cerebral wall of ferrets: direct tracing of their transformation from radial glia into astrocytes. J. Comp. Neurol. 1989;289:74–88. doi: 10.1002/cne.902890106. doi:10.1002/cne.902890106 [DOI] [PubMed] [Google Scholar]
- Winner B, Cooper-Kuhn C.M, Aigner R, Winkler J, Kuhn H.G. Long-term survival and cell death of newly generated neurons in the adult rat olfactory bulb. Eur. J. Neurosci. 2002;16:1681–1689. doi: 10.1046/j.1460-9568.2002.02238.x. doi:10.1046/j.1460-9568.2002.02238.x [DOI] [PubMed] [Google Scholar]
- Wiskott L, Rasch M.J, Kempermann G. A functional hypothesis for adult hippocampal neurogenesis: avoidance of catastrophic interference in the dentate gyrus. Hippocampus. 2006;16:329–343. doi: 10.1002/hipo.20167. doi:10.1002/hipo.20167 [DOI] [PubMed] [Google Scholar]
- Wullimann M.F, Puelles L. Postembryonic neural proliferation in the zebrafish forebrain and its relationship to prosomeric domains. Anat. Embryol. 1999;199:329–348. doi: 10.1007/s004290050232. doi:10.1007/s004290050232 [DOI] [PubMed] [Google Scholar]
- Xu Y, Tamamaki N, Noda T, Kimura K, Itokazu Y, Matsumoto N, Dezawa M, Ide C. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp. Neurol. 2005;192:251–264. doi: 10.1016/j.expneurol.2004.12.021. doi:10.1016/j.expneurol.2004.12.021 [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Yamamoto N, Kitamura T, Nakamura K, Nakafuku M. Proliferation of parenchymal neural progenitors in response to injury in the adult rat spinal cord. Exp. Neurol. 2001;172:115–127. doi: 10.1006/exnr.2001.7798. doi:10.1006/exnr.2001.7798 [DOI] [PubMed] [Google Scholar]
- Yanes C, Monzon-Mayor M, Ghandour M.S, de Barry J, Gombos G. Radial glia and astrocytes in developing and adult telencephalon of the lizard Gallotia galloti as revealed by immunohistochemistry with anti-GFAP and anti-vimentin antibodies. J. Comp. Neurol. 1990;295:559–568. doi: 10.1002/cne.902950405. doi:10.1002/cne.902950405 [DOI] [PubMed] [Google Scholar]
- Yoshino J, Tochinai S. Successful reconstitution of the non-regenerating adult telencephalon by cell transplantation in Xenopus laevis. Dev. Growth Differ. 2004;46:523–534. doi: 10.1111/j.1440-169x.2004.00767.x. doi:10.1111/j.1440-169x.2004.00767.x [DOI] [PubMed] [Google Scholar]
- Yoshino J, Tochinai S. Functional regeneration of the olfactory bulb requires reconnection to the olfactory nerve in Xenopus larvae. Dev. Growth Differ. 2006;48:15–24. doi: 10.1111/j.1440-169X.2006.00840.x. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Zhang C, Zhang L, Robin A, Wang Y, Lu M, Chopp M. Stroke transiently increases subventricular zone cell division from asymmetric to symmetric and increases neuronal differentiation in the adult rat. J. Neurosci. 2004;24:5810–5815. doi: 10.1523/JNEUROSCI.1109-04.2004. doi:10.1523/JNEUROSCI.1109-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R.L, Zhang Z.G, Chopp M. Neurogenesis in the adult ischemic brain: generation, migration, survival, and restorative therapy. Neuroscientist. 2005;11:408–416. doi: 10.1177/1073858405278865. doi:10.1177/1073858405278865 [DOI] [PubMed] [Google Scholar]
- Zhao M, et al. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc. Natl Acad. Sci. USA. 2003;100:7925–7930. doi: 10.1073/pnas.1131955100. doi:10.1073/pnas.1131955100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Nowakowski R.S, Vaccarino F.M. Fibroblast growth factor 2 is required for maintaining the neural stem cell pool in the mouse brain subventricular zone. Dev. Neurosci. 2004;26:181–196. doi: 10.1159/000082136. doi:10.1159/000082136 [DOI] [PubMed] [Google Scholar]
- Zikopoulos B, Kentouri M, Dermon C.R. Proliferation zones in the adult brain of a sequential hermaphrodite teleost species (Sparus aurata) Brain Behav. Evol. 2000;56:310–322. doi: 10.1159/000047215. doi:10.1159/000047215 [DOI] [PubMed] [Google Scholar]
- Zikopoulos B, Kentouri M, Dermon C.R. Cell genesis in the hypothalamus is associated to the sexual phase of a hermaphrodite teleost. Neuroreport. 2001;12:2477–2481. doi: 10.1097/00001756-200108080-00038. doi:10.1097/00001756-200108080-00038 [DOI] [PubMed] [Google Scholar]
- Zupanc G.K. Neurogenesis, cell death and regeneration in the adult gymnotiform brain. J. Exp. Biol. 1999;202:1435–1446. doi: 10.1242/jeb.202.10.1435. [DOI] [PubMed] [Google Scholar]
- Zupanc G.K. Adult neurogenesis and neuronal regeneration in the central nervous system of teleost fish. Brain Behav. Evol. 2001;58:250–275. doi: 10.1159/000057569. doi:10.1159/000057569 [DOI] [PubMed] [Google Scholar]
- Zupanc G.K, Clint S.C. Potential role of radial glia in adult neurogenesis of teleost fish. Glia. 2003;43:77–86. doi: 10.1002/glia.10236. doi:10.1002/glia.10236 [DOI] [PubMed] [Google Scholar]
- Zupanc G.K, Horschke I. Proliferation zones in the brain of adult gymnotiform fish: a quantitative mapping study. J. Comp. Neurol. 1995;353:213–233. doi: 10.1002/cne.903530205. doi:10.1002/cne.903530205 [DOI] [PubMed] [Google Scholar]
- Zupanc G.K.H, Maler L. Evoked chirping in the weakly electric fish Apteronotus leptorhynchus: a quantitative biophysical analysis. Can. J. Zool. 1993;71:2301–2310. [Google Scholar]
- Zupanc G.K, Ott R. Cell proliferation after lesions in the cerebellum of adult teleost fish: time course, origin, and type of new cells produced. Exp. Neurol. 1999;160:78–87. doi: 10.1006/exnr.1999.7182. doi:10.1006/exnr.1999.7182 [DOI] [PubMed] [Google Scholar]
- Zupanc G.K, Zupanc M.M. Birth and migration of neurons in the central posterior/prepacemaker nucleus during adulthood in weakly electric knifefish (Eigenmannia sp.) Proc. Natl Acad. Sci. USA. 1992;89:9539–9543. doi: 10.1073/pnas.89.20.9539. doi:10.1073/pnas.89.20.9539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupanc G.K, Horschke I, Ott R, Rascher G.B. Postembryonic development of the cerebellum in gymnotiform fish. J. Comp. Neurol. 1996;370:443–464. doi: 10.1002/(SICI)1096-9861(19960708)370:4<443::AID-CNE3>3.0.CO;2-4. doi:10.1002/(SICI)1096-9861(19960708)370:4<443::AID-CNE3>3.0.CO;2-4 [DOI] [PubMed] [Google Scholar]
- Zupanc G.K, Hinsch K, Gage F.H. Proliferation, migration, neuronal differentiation, and long-term survival of new cells in the adult zebrafish brain. J. Comp. Neurol. 2005;488:290–319. doi: 10.1002/cne.20571. doi:10.1002/cne.20571 [DOI] [PubMed] [Google Scholar]