Abstract
Antigen-specific responses to chlamydiae have been demonstrated with lymphocytes isolated from the conjunctiva after primary ocular infection and after topical challenge of chlamydia-immune cynomolgus monkeys with noninfectious, Triton X-100-extracted antigen. Proliferative to viable elementary bodies homologous to the original infecting serovar were demonstrated. In addition, in vitro production of antichlamydial antibody by conjunctival B cells was demonstrated by enzyme-linked immunosorbent assay of culture supernatants collected after 7 to 21 days of culture. These findings demonstrate that antigen-specific lymphocytes appear in the conjunctiva as a result of ocular chlamydial infection and that a noninfectious chlamydial antigen stimulates their reappearance or expansion at the site of original infection.
Full text
PDF

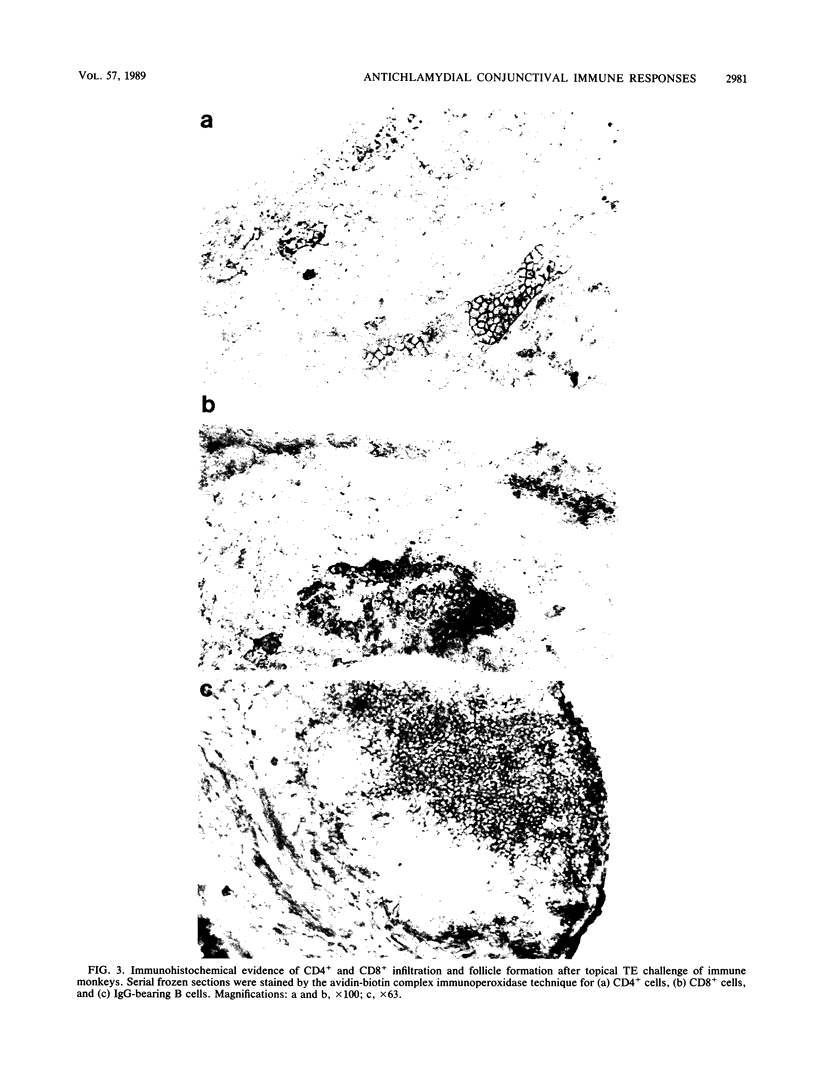


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Zhang Y. X., Joseph T., Su H., Nano F. E., Everett K. D., Caldwell H. D. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4000–4004. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard J., Levitt D. Chlamydia trachomatis stimulates human peripheral blood B lymphocytes to proliferate and secrete polyclonal immunoglobulins in vitro. Infect Immun. 1984 Jan;43(1):84–92. doi: 10.1128/iai.43.1.84-92.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham R. C., Martin D. H., Kuo C. C., Wang S. P., Stevens C. E., Hubbard T., Holmes K. K. Cellular immune response during uncomplicated genital infection with Chlamydia trachomatis in humans. Infect Immun. 1981 Oct;34(1):98–104. doi: 10.1128/iai.34.1.98-104.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham R. C., Peeling R., Maclean I., McDowell J., Persson K., Osser S. Postabortal Chlamydia trachomatis salpingitis: correlating risk with antigen-specific serological responses and with neutralization. J Infect Dis. 1987 Apr;155(4):749–755. doi: 10.1093/infdis/155.4.749. [DOI] [PubMed] [Google Scholar]
- Caldwell H. D., Perry L. J. Neutralization of Chlamydia trachomatis infectivity with antibodies to the major outer membrane protein. Infect Immun. 1982 Nov;38(2):745–754. doi: 10.1128/iai.38.2.745-754.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Stewart S., Johnson S., Taylor H. Tear and serum antibody response to Chlamydia trachomatis antigens during acute chlamydial conjunctivitis in monkeys as determined by immunoblotting. Infect Immun. 1987 Jan;55(1):93–98. doi: 10.1128/iai.55.1.93-98.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna L., Schmidt L., Sharp M., Stites D. P., Jawetz E. Human cell-mediated immune responses to chlamydial antigens. Infect Immun. 1979 Feb;23(2):412–417. doi: 10.1128/iai.23.2.412-417.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. P., Graeff A. S., Zeitz M., Kappus E., Quinn T. C. Cytotoxic and immunoregulatory function of intestinal lymphocytes in Chlamydia trachomatis proctitis of nonhuman primates. Infect Immun. 1987 May;55(5):1137–1143. doi: 10.1128/iai.55.5.1137-1143.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D., Danen R. Development and regulation of chlamydia-responsive murine B lymphocytes. J Immunol. 1987 May 15;138(10):3468–3474. [PubMed] [Google Scholar]
- Morrison R. P., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. Ocular hypersensitivity elicited by a genus-specific 57-kD protein. J Exp Med. 1989 Mar 1;169(3):663–675. doi: 10.1084/jem.169.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E. S., Charbonnet L. T., MacDonald A. B. Immunity to chlamydial infections of the eye. I. The role of circulatory and secretory antibodies in resistance to reinfection with guinea pig inclusion conjunctivitis. J Immunol. 1973 Jun;110(6):1518–1525. [PubMed] [Google Scholar]
- Newhall W. J., Batteiger B., Jones R. B. Analysis of the human serological response to proteins of Chlamydia trachomatis. Infect Immun. 1982 Dec;38(3):1181–1189. doi: 10.1128/iai.38.3.1181-1189.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qvigstad E., Skaug K., Thorsby E. Proliferative human T cell responses to Chlamydia trachomatis in vitro. Acta Pathol Microbiol Immunol Scand C. 1983 Jun;91(3):203–209. [PubMed] [Google Scholar]
- Stephens R. S., Kuo C. C., Newport G., Agabian N. Molecular cloning and expression of Chlamydia trachomatis major outer membrane protein antigens in Escherichia coli. Infect Immun. 1985 Mar;47(3):713–718. doi: 10.1128/iai.47.3.713-718.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Wagar E. A., Schoolnik G. K. High-resolution mapping of serovar-specific and common antigenic determinants of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1988 Mar 1;167(3):817–831. doi: 10.1084/jem.167.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H. R., Johnson S. L., Prendergast R. A., Schachter J., Dawson C. R., Silverstein A. M. An animal model of trachoma II. The importance of repeated reinfection. Invest Ophthalmol Vis Sci. 1982 Oct;23(4):507–515. [PubMed] [Google Scholar]
- Taylor H. R., Johnson S. L., Schachter J., Caldwell H. D., Prendergast R. A. Pathogenesis of trachoma: the stimulus for inflammation. J Immunol. 1987 May 1;138(9):3023–3027. [PubMed] [Google Scholar]
- Taylor H. R., Prendergast R. A., Dawson C. R., Schachter J., Silverstein A. M. An animal model for cicatrizing trachoma. Invest Ophthalmol Vis Sci. 1981 Sep;21(3):422–433. [PubMed] [Google Scholar]
- Taylor H. R., Whittum-Hudson J., Schachter J., Caldwell H. D., Prendergast R. A. Oral immunization with chlamydial major outer membrane protein (MOMP). Invest Ophthalmol Vis Sci. 1988 Dec;29(12):1847–1853. [PubMed] [Google Scholar]
- Ward M. E., Treharne J. D., Murray A. Antigenic specificity of human antibody to chlamydia in trachoma and lymphogranuloma venereum. J Gen Microbiol. 1986 Jun;132(6):1599–1610. doi: 10.1099/00221287-132-6-1599. [DOI] [PubMed] [Google Scholar]
- Watkins N. G., Hadlow W. J., Moos A. B., Caldwell H. D. Ocular delayed hypersensitivity: a pathogenetic mechanism of chlamydial-conjunctivitis in guinea pigs. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7480–7484. doi: 10.1073/pnas.83.19.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. R., MacDonald A. B., Murray E. S., Modabber F. Z. Immunity to chlamydial infections of the eye. 3. Presence and duration of delayed hypersensitivity to guinea pig inclusion conjuctivitis. J Immunol. 1973 Aug;111(2):618–623. [PubMed] [Google Scholar]
- Whittum-Hudson J. A., Prendergast R. A., Taylor H. R. Changes in conjunctival lymphocyte populations induced by oral immunization with Chlamydia trachomatis. Curr Eye Res. 1986 Dec;5(12):973–979. doi: 10.3109/02713688608995179. [DOI] [PubMed] [Google Scholar]
- Whittum-Hudson J. A., Taylor H. R., Farazdaghi M., Prendergast R. A. Immunohistochemical study of the local inflammatory response to chlamydial ocular infection. Invest Ophthalmol Vis Sci. 1986 Jan;27(1):64–69. [PubMed] [Google Scholar]
- Young E., Taylor H. R. Immune mechanisms in chlamydial eye infection. Development of T suppressor cells. Invest Ophthalmol Vis Sci. 1986 Apr;27(4):615–619. [PubMed] [Google Scholar]
- Young E., Taylor H. R. Immune mechanisms in chlamydial eye infection: cellular immune responses in chronic and acute disease. J Infect Dis. 1984 Nov;150(5):745–751. doi: 10.1093/infdis/150.5.745. [DOI] [PubMed] [Google Scholar]