Abstract
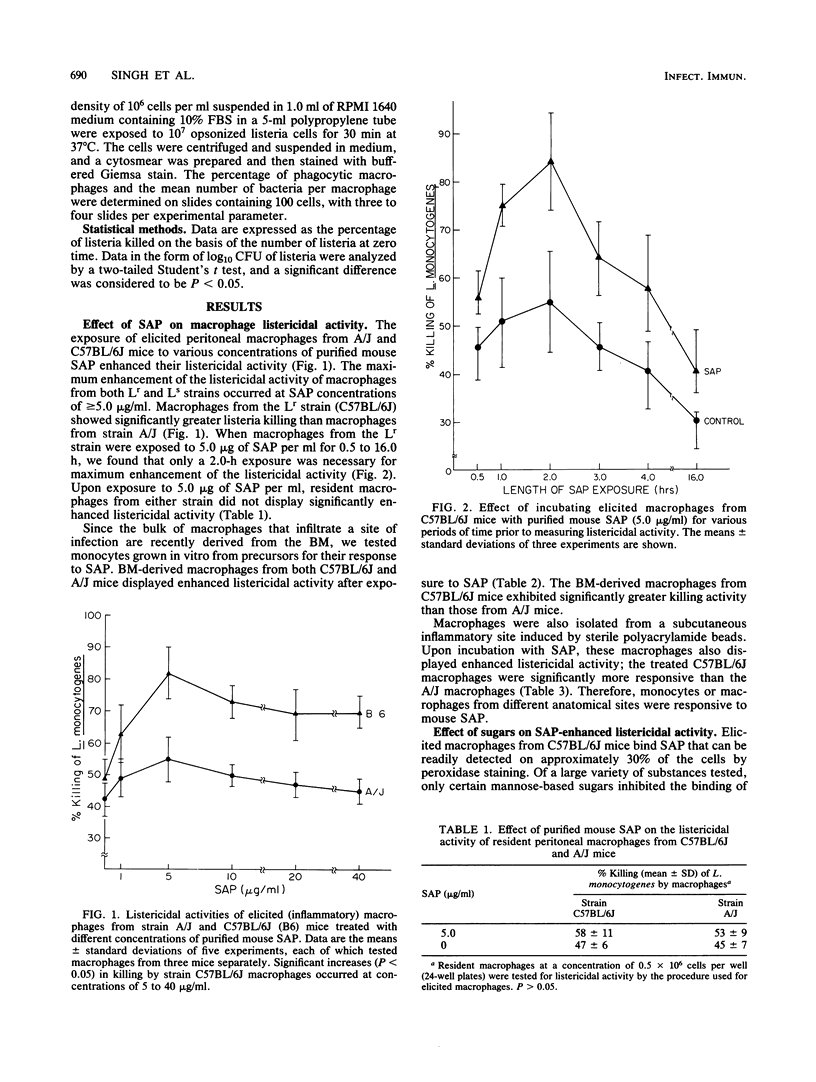
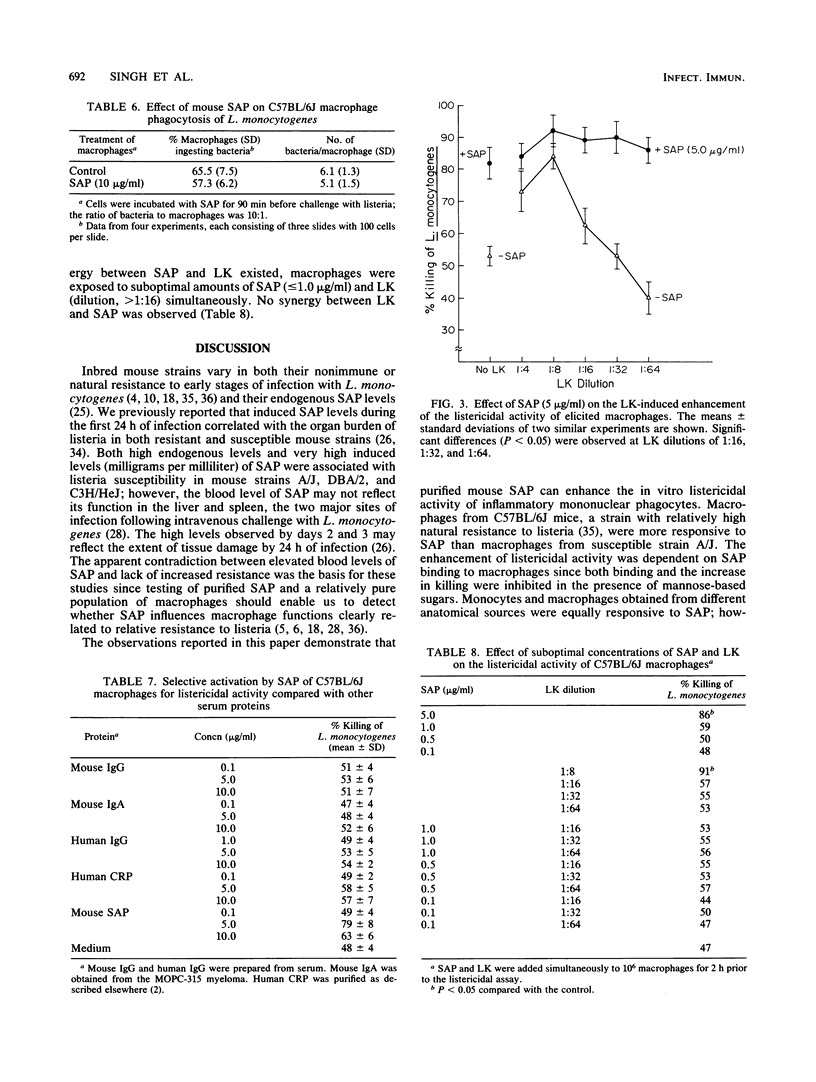
Purified serum amyloid P component (SAP), the major acute-phase reactant of mice, augmented the in vitro listericidal activity of inflammatory (elicited) macrophages, bone marrow-derived monocytes, and macrophages from a subcutaneous site of inflammation. Monocytes and macrophages from C57BL/B6 mice, which are relatively resistant to Listeria monocytogenes, exhibited a significantly greater enhanced killing capacity for listeria than macrophages from listeria-susceptible A/J mice. SAP did not alter the extent of phagocytosis by macrophages of opsonized L. monocytogenes, nor was SAP opsonic for listeria. Mannose-derived simple sugars inhibited the binding of SAP to macrophages and consequently prevented the enhanced SAP-dependent listericidal activity. Macrophages from lipopolysaccharide-hyporesponsive mice also had increased microbicidal activity following incubation with SAP. SAP activated macrophages independently of lymphokine. Therefore, SAP may serve as a mediator of the heightened nonspecific host defense response that is associated with the acute phase of the systemic inflammatory response.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barna B. P., Deodhar S. D., Gautam S., Yen-Lieberman B., Roberts D. Macrophage activation and generation of tumoricidal activity by liposome-associated human C-reactive protein. Cancer Res. 1984 Jan;44(1):305–310. [PubMed] [Google Scholar]
- Benson M. D., Skinner M., Shirahama T., Cohen A. S. P-component of amyloid. Isolation from human serum by affinity chromatography. Arthritis Rheum. 1976 Jul-Aug;19(4):749–754. doi: 10.1002/1529-0131(197607/08)19:4<749::aid-art1780190415>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F., Pavlov H., Waid C., York J. Resistance and susceptibility of mice to bacterial infection: course of listeriosis in resistant or susceptible mice. Infect Immun. 1978 Mar;19(3):763–770. doi: 10.1128/iai.19.3.763-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Henson P. M., Campbell P. A. Killing of Listeria monocytogenes by inflammatory neutrophils and mononuclear phagocytes from immune and nonimmune mice. J Leukoc Biol. 1984 Feb;35(2):193–208. doi: 10.1002/jlb.35.2.193. [DOI] [PubMed] [Google Scholar]
- Deodhar S. D., James K., Chiang T., Edinger M., Barna B. P. Inhibition of lung metastases in mice bearing a malignant fibrosarcoma by treatment with liposomes containing human C-reactive protein. Cancer Res. 1982 Dec;42(12):5084–5088. [PubMed] [Google Scholar]
- Ezekowitz R. A., Gordon S. Down-regulation of mannosyl receptor-mediated endocytosis and antigen F4/80 in bacillus Calmette-Guérin-activated mouse macrophages. Role of T lymphocytes and lymphokines. J Exp Med. 1982 Jun 1;155(6):1623–1637. doi: 10.1084/jem.155.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauve R. M., Jusforgues H., Hevin B. Maintenance of granuloma macrophages in serum-free medium. J Immunol Methods. 1983 Nov 25;64(3):345–351. doi: 10.1016/0022-1759(83)90442-8. [DOI] [PubMed] [Google Scholar]
- Gervais F., Stevenson M., Skamene E. Genetic control of resistance to Listeria monocytogenes: regulation of leukocyte inflammatory responses by the Hc locus. J Immunol. 1984 Apr;132(4):2078–2083. [PubMed] [Google Scholar]
- Godfrey R. W., Horton P. G., Wilder M. S. Time course of antilisterial activity by immunologically activated murine peritoneal macrophages. Infect Immun. 1983 Feb;39(2):532–539. doi: 10.1128/iai.39.2.532-539.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hind C. R., Collins P. M., Renn D., Cook R. B., Caspi D., Baltz M. L., Pepys M. B. Binding specificity of serum amyloid P component for the pyruvate acetal of galactose. J Exp Med. 1984 Apr 1;159(4):1058–1069. doi: 10.1084/jem.159.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Invest. 1980 Jan;65(1):82–94. doi: 10.1172/JCI109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T., Youmans G. P. The in vitro inhibition of growth of intracellular Listeria monocytogenes by lymphocyte products. Cell Immunol. 1973 Dec;9(3):353–362. doi: 10.1016/0008-8749(73)90050-6. [DOI] [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Kilpatrick J. M., Volanakis J. E. Opsonic properties of C-reactive protein. Stimulation by phorbol myristate acetate enables human neutrophils to phagocytize C-reactive protein-coated cells. J Immunol. 1985 May;134(5):3364–3370. [PubMed] [Google Scholar]
- Kushner I., Gewurz H., Benson M. D. C-reactive protein and the acute-phase response. J Lab Clin Med. 1981 Jun;97(6):739–749. [PubMed] [Google Scholar]
- Le P. T., Mortensen R. F. In vitro induction of hepatocyte synthesis of the acute phase reactant mouse serum amyloid P-component by macrophages and IL 1. J Leukoc Biol. 1984 Jun;35(6):587–603. doi: 10.1002/jlb.35.6.587. [DOI] [PubMed] [Google Scholar]
- Le P. T., Muller M. T., Mortensen R. F. Acute phase reactants of mice. I. Isolation of serum amyloid P-component (SAP) and its induction by a monokine. J Immunol. 1982 Aug;129(2):665–672. [PubMed] [Google Scholar]
- Lepay D. A., Steinman R. M., Nathan C. F., Murray H. W., Cohn Z. A. Liver macrophages in murine listeriosis. Cell-mediated immunity is correlated with an influx of macrophages capable of generating reactive oxygen intermediates. J Exp Med. 1985 Jun 1;161(6):1503–1512. doi: 10.1084/jem.161.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer M. S., Occhionero M., Ruco L. P. Macrophage activation for tumor cytotoxicity: regulatory mechanisms for induction and control of cytotoxic activity. Fed Proc. 1982 Apr;41(6):2198–2205. [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- Mortensen R. F., Beisel K., Zeleznik N. J., Le P. T. Acute-phase reactants of mice. II. Strain dependence of serum amyloid P-component (SAP) levels and response to inflammation. J Immunol. 1983 Feb;130(2):885–889. [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmand A. P., Friedenson B., Gewurz H., Painter R. H., Hofmann T., Shelton E. Characterization of C-reactive protein and the complement subcomponent C1t as homologous proteins displaying cyclic pentameric symmetry (pentraxins). Proc Natl Acad Sci U S A. 1977 Feb;74(2):739–743. doi: 10.1073/pnas.74.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B., Baltz M. L. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Baltz M., Gomer K., Davies A. J., Doenhoff M. Serum amyloid P-component is an acute-phase reactant in the mouse. Nature. 1979 Mar 15;278(5701):259–261. doi: 10.1038/278259a0. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Dash A. C., Fletcher T. C., Richardson N., Munn E. A., Feinstein A. Analogues in other mammals and in fish of human plasma proteins, C-reactive protein and amyloid P component. Nature. 1978 May 11;273(5658):168–170. doi: 10.1038/273168a0. [DOI] [PubMed] [Google Scholar]
- Sarlo K. T., Mortensen R. F. Enhanced interleukin 1 (IL-1) production mediated by mouse serum amyloid P component. Cell Immunol. 1985 Jul;93(2):398–405. doi: 10.1016/0008-8749(85)90144-3. [DOI] [PubMed] [Google Scholar]
- Skamene E. Genetic regulation of host resistance to bacterial infection. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S823–S832. doi: 10.1093/clinids/5.supplement_4.s823. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Kongshavn P. A., Skamene E. Genetic linkage of resistance to Listeria monocytogenes with macrophage inflammatory responses. J Immunol. 1981 Aug;127(2):402–407. [PubMed] [Google Scholar]
- Warren M. K., Vogel S. N. Bone marrow-derived macrophages: development and regulation of differentiation markers by colony-stimulating factor and interferons. J Immunol. 1985 Feb;134(2):982–989. [PubMed] [Google Scholar]
- Wing E. J., Gardner I. D., Ryning F. W., Remington J. S. Dissociation of effector functions in populations of activated macrophages. Nature. 1977 Aug 18;268(5621):642–644. doi: 10.1038/268642a0. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Craigmyle L. S., Silverstein S. C. Fibronectin and serum amyloid P component stimulate C3b- and C3bi-mediated phagocytosis in cultured human monocytes. J Exp Med. 1983 Oct 1;158(4):1338–1343. doi: 10.1084/jem.158.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]


