Abstract
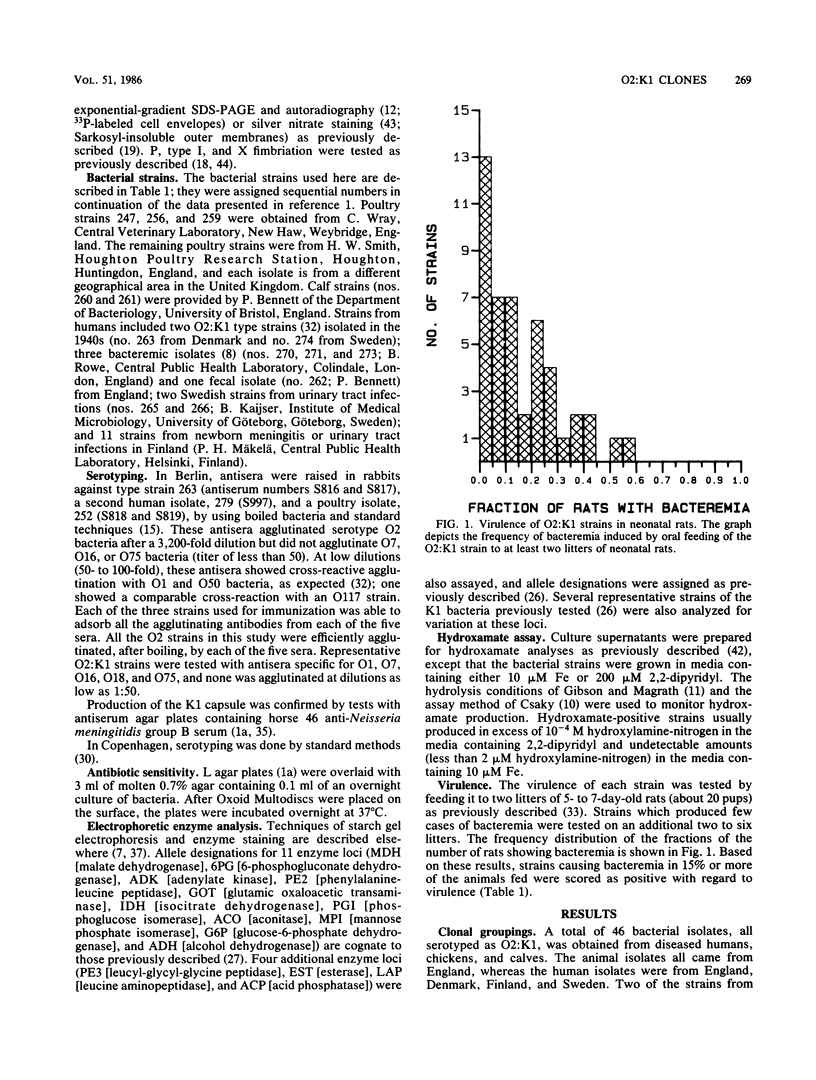
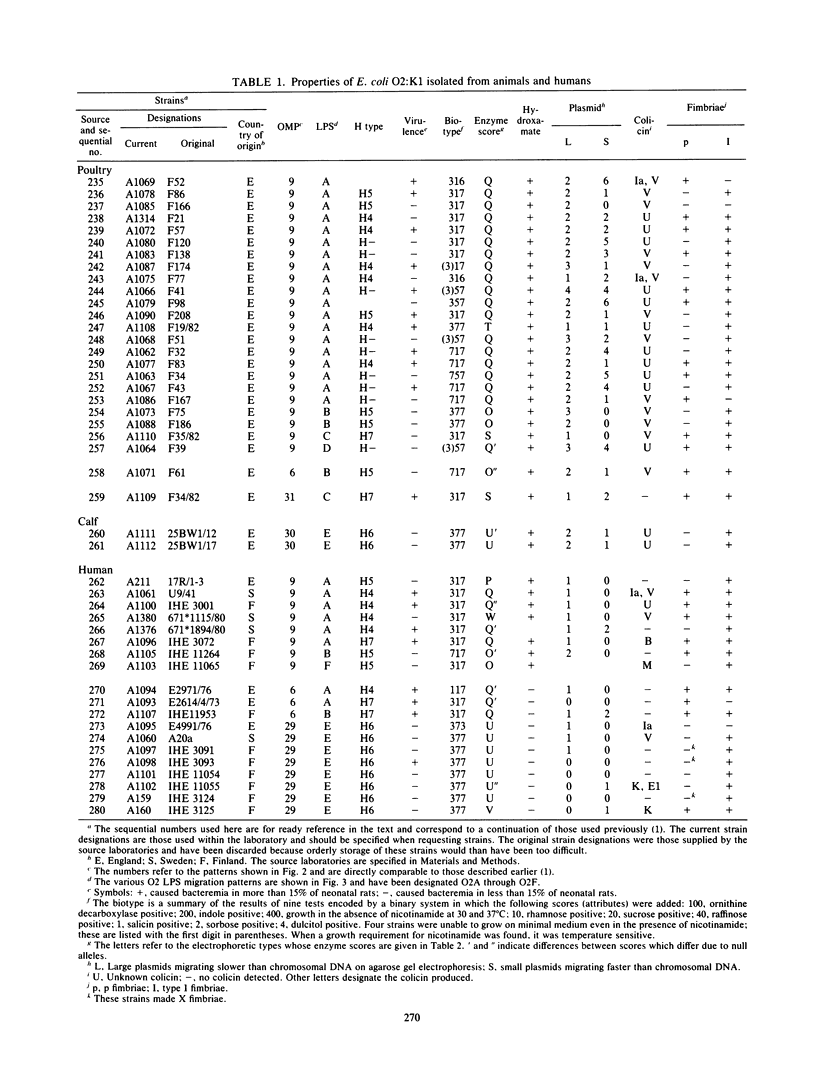
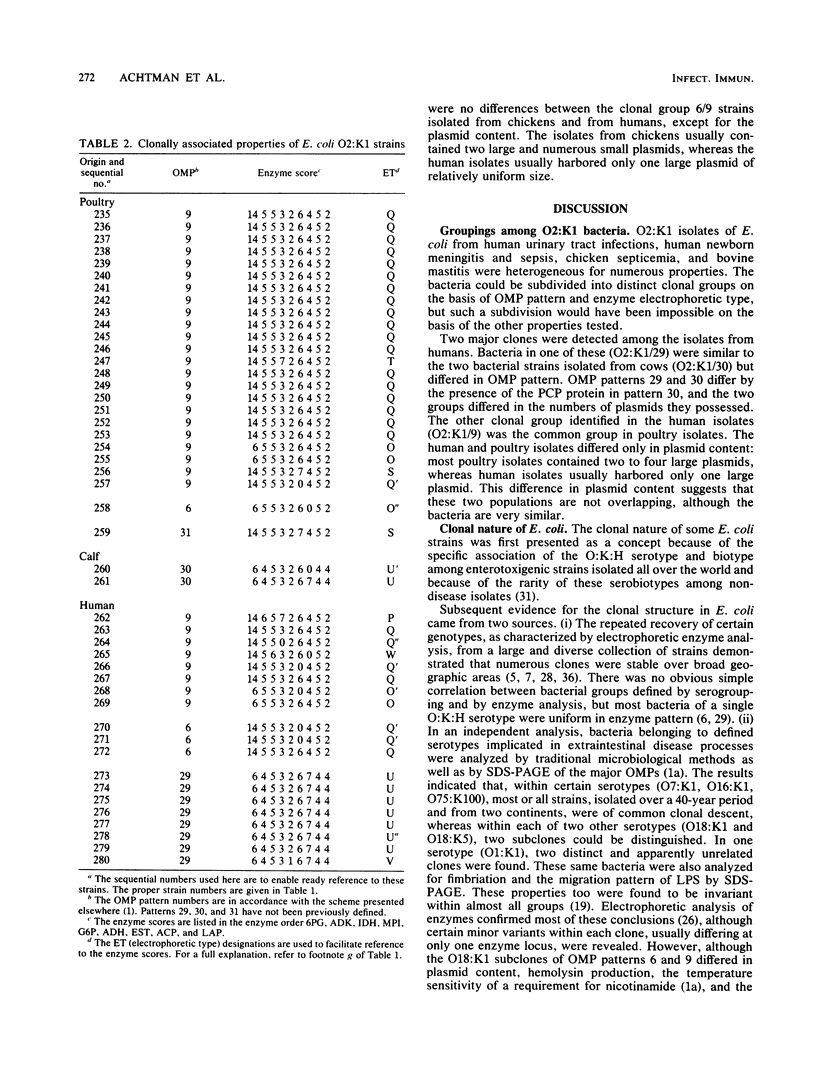
Forty-six Escherichia coli isolates of serotype O2:K1 from human urinary tract infections, chicken sepsis, and bovine mastitis were obtained from laboratories in England, Denmark, Sweden, and Finland. The bacteria were compared for outer membrane protein (OMP) pattern, lipopolysaccharide pattern, electrophoretic mobilities of enzymes, and flagellar serotype and were tested for fimbriation, biotype, hydroxamate production, hemolysin production, antibiotic resistance, plasmid content, colicin production, and virulence in neonatal rats. Isolates from humans were assigned to two clonal groups; poultry isolates belonged to one of these clonal groups, whereas bovine isolates belonged to the other. Poultry and human isolates of the same clonal group could be distinguished only by their plasmid content. Strains within this group were heterogeneous with respect to biotype, fimbriation, virulence, and flagellar serotype. Human and bovine isolates of the second clonal group were distinguished by a minor change in OMP pattern and by their plasmid content. It is concluded that meaningful clonal groupings are best recognized by the combination of OMP and electrophoretic enzyme patterns. The O:K serotype can aid in the recognition of important subclones, whereas the other microbiological properties tested can vary widely within clonal groupings. Furthermore, we conclude that certain O:K serotypes can contain very different clonal groupings having little genetic relatedness.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E. S. Drug resistance in Salmonella typhimurium and its implications. Br Med J. 1968 Aug 10;3(5614):333–339. doi: 10.1136/bmj.3.5614.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelheim K. A., Bushrod F. M., Chandler M. E., Cooke E. M., O'Farrell S., Shooter R. A. Escherichia coli serotype distribution in man and animals. J Hyg (Lond) 1974 Dec;73(3):467–471. [PMC free article] [PubMed] [Google Scholar]
- Caugant D. A., Levin B. R., Lidin-Janson G., Whittam T. S., Svanborg Edén C., Selander R. K. Genetic diversity and relationships among strains of Escherichia coli in the intestine and those causing urinary tract infections. Prog Allergy. 1983;33:203–227. doi: 10.1159/000318331. [DOI] [PubMed] [Google Scholar]
- Caugant D. A., Levin B. R., Orskov I., Orskov F., Svanborg Eden C., Selander R. K. Genetic diversity in relation to serotype in Escherichia coli. Infect Immun. 1985 Aug;49(2):407–413. doi: 10.1128/iai.49.2.407-413.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caugant D. A., Levin B. R., Selander R. K. Genetic diversity and temporal variation in the E. coli population of a human host. Genetics. 1981 Jul;98(3):467–490. doi: 10.1093/genetics/98.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheasty T., Gross R. J., Rowe B. Incidence of K1 antigen in Escherichia coli isolated from blood and cerebrospinal fluid of patients in the United Kingdom. J Clin Pathol. 1977 Oct;30(10):945–947. doi: 10.1136/jcp.30.10.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke E. M., Hettiaratchy I. G., Buck A. C. Fate of ingested Escherichia coli in normal persons. J Med Microbiol. 1972 Aug;5(3):361–369. doi: 10.1099/00222615-5-3-361. [DOI] [PubMed] [Google Scholar]
- Gibson F., Magrath D. I. The isolation and characterization of a hydroxamic acid (aerobactin) formed by Aerobacter aerogenes 62-I. Biochim Biophys Acta. 1969 Nov 18;192(2):175–184. doi: 10.1016/0304-4165(69)90353-5. [DOI] [PubMed] [Google Scholar]
- Glynn A. A., Brumfitt W., Howard C. J. K antigens of Escherichia coli and renal involvement in urinary-tract infections. Lancet. 1971 Mar 13;1(7698):514–516. [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Heller E. D., Smith H. W. The incidence of antibiotic resistance and other characteristics amongst Escherichia coli strains causing fatal infection in chickens: the utilization of these characteristics to study the epidemiology of the infection. J Hyg (Lond) 1973 Dec;71(4):771–781. doi: 10.1017/s0022172400023032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukes T. H. Public health significance of feeding low levels of antibiotics to animals. Adv Appl Microbiol. 1973;16:1–54. doi: 10.1016/s0065-2164(08)70021-2. [DOI] [PubMed] [Google Scholar]
- Kim T. K., Stephens J. F. Drug resistance and transferable drug resistance of Escherichia coli isolated from "ready-to-cook" broilers. Poult Sci. 1972 Jul;51(4):1165–1170. doi: 10.3382/ps.0511165. [DOI] [PubMed] [Google Scholar]
- Kiser J. S. A perspective on the use of antibiotics in animal feeds. J Anim Sci. 1976 Apr;42(4):1058–1072. doi: 10.2527/jas1976.4241058x. [DOI] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen V., Saxén H., Hultberg H., Svenson S. B. P-antigen-recognizing fimbriae from human uropathogenic Escherichia coli strains. Infect Immun. 1982 Jul;37(1):286–291. doi: 10.1128/iai.37.1.286-291.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusecek B., Wloch H., Mercer A., Vaisänen V., Pluschke G., Korhonen T., Achtman M. Lipopolysaccharide, capsule, and fimbriae as virulence factors among O1, O7, O16, O18, or O75 and K1, K5, or K100 Escherichia coli. Infect Immun. 1984 Jan;43(1):368–379. doi: 10.1128/iai.43.1.368-379.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton A. H., Howe K., Bennett P. M., Richmond M. H., Whiteside E. J. The colonization of the human gut by antibiotic resistant Escherichia coli from chickens. J Appl Bacteriol. 1977 Dec;43(3):465–469. doi: 10.1111/j.1365-2672.1977.tb00773.x. [DOI] [PubMed] [Google Scholar]
- Linton A. H., Howe K., Hartley C. L., Clements H. M., Richmond M. H., Osborne A. D. Antibiotic resistance among Escherichia coli O-serotypes from the gut and carcases of commercially slaughtered broiler chickens: a potential public health hazard. J Appl Bacteriol. 1977 Jun;42(3):365–378. doi: 10.1111/j.1365-2672.1977.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Linton A. H., Howe K., Sojka W. J., Wray C. A note on the range of Escherichia coli O-serotypes causing clinical bovine mastitis and their antibiotic resistance spectra. J Appl Bacteriol. 1979 Jun;46(3):585–590. doi: 10.1111/j.1365-2672.1979.tb00859.x. [DOI] [PubMed] [Google Scholar]
- Lyons R. W., Samples C. L., DeSilva H. N., Ross K. A., Julian E. M., Checko P. J. An epidemic of resistant Salmonella in a nursery. Animal-to-human spread. JAMA. 1980 Feb 8;243(6):546–547. [PubMed] [Google Scholar]
- Mercer A. A., Morelli G., Heuzenroeder M., Kamke M., Achtman M. Conservation of plasmids among Escherichia coli K1 isolates of diverse origins. Infect Immun. 1984 Dec;46(3):649–657. doi: 10.1128/iai.46.3.649-657.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H., Selander R. K. Evidence for clonal population structure in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jan;81(1):198–201. doi: 10.1073/pnas.81.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H., Selander R. K. Standard reference strains of Escherichia coli from natural populations. J Bacteriol. 1984 Feb;157(2):690–693. doi: 10.1128/jb.157.2.690-693.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H., Whittam T. S., Caugant D. A., Selander R. K. Enzyme polymorphism and genetic population structure in Escherichia coli and Shigella. J Gen Microbiol. 1983 Sep;129(9):2715–2726. doi: 10.1099/00221287-129-9-2715. [DOI] [PubMed] [Google Scholar]
- Orskov F., Orskov I., Evans D. J., Jr, Sack R. B., Sack D. A., Wadström T. Special Escherichia coli serotypes among enterotoxigenic strains from diarrhoea in adults and children. Med Microbiol Immunol. 1976 Jun 1;162(2):73–80. doi: 10.1007/BF02121318. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Mercer A., Kusećek B., Pohl A., Achtman M. Induction of bacteremia in newborn rats by Escherichia coli K1 is correlated with only certain O (lipopolysaccharide) antigen types. Infect Immun. 1983 Feb;39(2):599–608. doi: 10.1128/iai.39.2.599-608.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H. Some environmental consequences of the use of antibiotics: or 'what goes up must come down'. J Appl Bacteriol. 1972 Jun;35(2):155–176. doi: 10.1111/j.1365-2672.1972.tb03687.x. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Bradshaw M., Whisnant J. K., Myerowitz R. L., Parke J. C., Jr, Robbins J. B. An Escherichia coli antigen cross-reactive with the capsular polysaccharide of Haemophilus influenzae type b: occurrence among known serotypes, and immunochemical and biologic properties of E. coli antisera toward H. influenzae type b. J Immunol. 1972 Jun;108(6):1551–1562. [PubMed] [Google Scholar]
- Selander R. K., Levin B. R. Genetic diversity and structure in Escherichia coli populations. Science. 1980 Oct 31;210(4469):545–547. doi: 10.1126/science.6999623. [DOI] [PubMed] [Google Scholar]
- Shooter R. A., Cooke E. M., Faiers M. C., Breaden A. L., O'Farrell S. M. Isolation of Escherichia coli, Pseudomonas aeruginosa, and Klebsiella from food in hospitals, canteens, and schools. Lancet. 1971 Aug 21;2(7721):390–392. doi: 10.1016/s0140-6736(71)90111-5. [DOI] [PubMed] [Google Scholar]
- Shooter R. A., Cooke E. M., Rousseau S. A., Breaden A. L. Animal sources of common serotypes of Escherichia coli in the food of hospital patients. Possible significance in urinary-tract infections. Lancet. 1970 Aug 1;2(7666):226–228. doi: 10.1016/s0140-6736(70)92583-3. [DOI] [PubMed] [Google Scholar]
- Smith H. W. Transfer of antibiotic resistance from animal and human strains of Escherichia coli to resident E. coli in the alimentary tract of man. Lancet. 1969 Jun 14;1(7607):1174–1176. doi: 10.1016/s0140-6736(69)92164-3. [DOI] [PubMed] [Google Scholar]
- Stenderup J., Orskov F. The clonal nature of enteropathogenic Escherichia coli strains. J Infect Dis. 1983 Dec;148(6):1019–1024. doi: 10.1093/infdis/148.6.1019. [DOI] [PubMed] [Google Scholar]
- Stuart S. J., Greenwood K. T., Luke R. K. Iron-suppressible production of hydroxamate by Escherichia coli isolates. Infect Immun. 1982 Jun;36(3):870–875. doi: 10.1128/iai.36.3.870-875.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Väisänen-Rhen V., Elo J., Väisänen E., Siitonen A., Orskov I., Orskov F., Svenson S. B., Mäkelä P. H., Korhonen T. K. P-fimbriated clones among uropathogenic Escherichia coli strains. Infect Immun. 1984 Jan;43(1):149–155. doi: 10.1128/iai.43.1.149-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väisänen V., Elo J., Tallgren L. G., Siitonen A., Mäkelä P. H., Svanborg-Edén C., Källenius G., Svenson S. B., Hultberg H., Korhonen T. Mannose-resistant haemagglutination and P antigen recognition are characteristic of Escherichia coli causing primary pyelonephritis. Lancet. 1981 Dec 19;2(8260-61):1366–1369. doi: 10.1016/s0140-6736(81)92796-3. [DOI] [PubMed] [Google Scholar]
- Walton J. R. The public health implications of drug-resistant bacteria in farm animals. Ann N Y Acad Sci. 1971 Jun 11;182:358–361. doi: 10.1111/j.1749-6632.1971.tb30671.x. [DOI] [PubMed] [Google Scholar]