Abstract
The diversification of chemical production in glandular trichomes is important in the development of resistance against pathogens and pests in two species of tomato. We have used genetic and genomic approaches to uncover some of the biochemical and molecular mechanisms that underlie the divergence in trichome metabolism between the wild species Solanum habrochaites LA1777 and its cultivated relative, Solanum lycopersicum. LA1777 produces high amounts of insecticidal sesquiterpene carboxylic acids (SCAs), whereas cultivated tomatoes lack SCAs and are more susceptible to pests. We show that trichomes of the two species have nearly opposite terpenoid profiles, consisting mainly of monoterpenes and low levels of sesquiterpenes in S. lycopersicum and mainly of SCAs and very low monoterpene levels in LA1777. The accumulation patterns of these terpenoids are different during development, in contrast to the developmental expression profiles of terpenoid pathway genes, which are similar in the two species, but they do not correlate in either case with terpenoid accumulation. However, our data suggest that the accumulation of monoterpenes in S. lycopersicum and major sesquiterpenes in LA1777 are linked both genetically and biochemically. Metabolite analyses after targeted gene silencing, inhibitor treatments, and precursor feeding all show that sesquiterpene biosynthesis relies mainly on products from the plastidic 2-C-methyl-d-erythritol-4-phosphate pathway in LA1777 but less so in the cultivated species. Furthermore, two classes of sesquiterpenes produced by the wild species may be synthesized from distinct pools of precursors via cytosolic and plastidial cyclases. However, highly trichome-expressed sesquiterpene cyclase-like enzymes were ruled out as being involved in the production of major LA1777 sesquiterpenes.
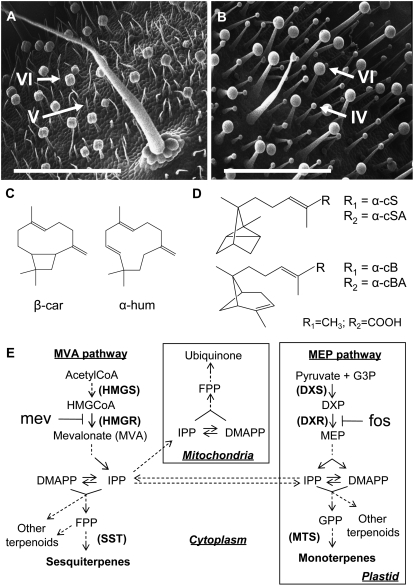
Plants collectively produce a large diversity of secondary metabolites as part of a defense strategy against pests, diseases, and different forms of abiotic stress. Many of these compounds are produced at the surface of the plant by epidermal secretory structures called glandular trichomes. These hair- or gland-like structures typically consist of a unicellular or multicellular stalk capped by one or more secretory cells that accumulate or exude chemicals (McCaskill and Croteau, 1999; Werker, 2000). In two species of tomato, the glandular type VI trichomes are particularly abundant on the leaves and stems of the cultivated species Solanum lycopersicum (formerly Lycopersicon esculentum) and its wild relative Solanum habrochaites (formerly Lycopersicon hirsutum) f. typicum LA1777 (Luckwill, 1943; Gianfagna et al., 1992; Fig. 1, A and B). They have been shown to accumulate monoterpenes in S. lycopersicum and very high levels of sesquiterpenes, mostly in the form of insecticidal carboxylic acid derivatives, in LA1777 (Coates et al., 1988, Frelichowski and Juvik, 2001; Li et al., 2004; Fig. 1D).
Figure 1.
Sesquiterpene production in the glandular trichomes of S. lycopersicum and S. habrochaites f. typicum LA1777. A and B, Scanning electron microscopy images of stem surfaces of S. lycopersicum (cv M82; A) and LA1777 (B). The most abundant trichomes (types IV, V ,and VI) are indicated by arrows. Bars = 1 mm. C and D, Major class I (C) and class II (D) sesquiterpene derivatives found in the trichomes of S. lycopersicum and LA1777, respectively. β-car, β-Caryophyllene; α-hum, α-humulene; α-cS, α-cis santalene; α-cSA, α-cis santalenoic acid; α-cB, α-cis bergamotene; α-cBA, α-cis bergamotenoic acid. E, Current model for terpenoid biosynthesis in plants. DXP, Deoxyxylulose phosphate; fos, fosmidomycin, inhibitor of DXR; G3P, glyceraldehyde-3-phosphate; GPP, geranyl diphosphate; MTS, monoterpene synthases; SST, sesquiterpene synthases.
On the basis of genetic evidence and heterologous expression, it has been proposed that enzymes related to germacrene C synthase, corresponding to a locus on chromosome 6, mediate the accumulation of a group of structurally similar compounds termed class I sesquiterpenes (cI-Ss) in LA1777 and S. lycopersicum (Colby et al., 1998; van der Hoeven et al., 2000; Fig. 1C). This group comprises germacrenes as well as α-humulene and β-caryophyllene. A distinct but so far unidentified enzyme or group of enzymes, associated with a locus on chromosome 8, has been hypothesized to catalyze the formation of class II sesquiterpenes (cII-Ss) in LA1777 but not in S. lycopersicum (van der Hoeven et al., 2000). These compounds are also structurally different from cI-Ss and include α-santalene, α-bergamotene, and β-bergamotene, which are thought to be further modified to form the sesquiterpene carboxylic acids (SCAs) α-santalenoic and α- and β-bergamotenoic acid, which have strong insecticidal properties (Coates et al., 1988; Frelichowski and Juvik, 2001; Fig. 1D).
Terpenoid production in plants is largely dependent on flux through two pathways that provide isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). Initially, it was suggested that the mevalonate (MVA) pathway, which operates in the cytosol, supplies acetyl CoA-derived precursors for the production of sesquiterpenes and triterpenes, whereas the plastid-localized 2-C-methyl-d-erythritol-4-phosphate (MEP) pathway provides pyruvate-derived IPP and DMAPP for the biosynthesis of hemiterpenes, monoterpenes, diterpenes, and tetraterpenes (Lichtenthaler, 1999; Newman and Chappell, 1999; Fig. 1E). However, this might be an oversimplification, and the contribution of the pathways to specific compounds may be much more flexible and variable. In lima bean (Phaseolus lunatus), for example, different stresses can differentially allocate precursors to the synthesis of induced terpenoids, and limiting conditions in one pathway can be overcome using precursors supplied by the other pathway (Piel et al., 1998; Jux et al., 2001; Bartram et al., 2006). The sesquiterpenes from mint (Mentha spp.) essential oils have been found to derive from the MEP pathway, like the emitted floral sesquiterpenes from snapdragon (Antirrhinum majus) flowers, due to inactive MVA pathways (McCaskill and Croteau, 1995; Lange et al., 2000; Dudareva et al., 2005). Similarly, it has been shown that some monoterpenes in strawberry (Fragaria spp.) have an exclusive MVA origin (Hampel et al., 2006). Sesquiterpene precursors in carrot (Daucus carota), chamomile (Matricaria chamomilla), and Solidago have been shown to be derived in part from both the MEP and MVA pathways (Adam et al., 1999; Steliopoulos et al., 2002; Hampel et al., 2005). This metabolic cross talk between cytosolic and chloroplastic compartments mediated by precursors such as IPP seems to have a preferred direction of flux from plastids to the cytoplasm (Estévez et al., 2000; Bick and Lange, 2003; Hemmerlin et al., 2003; Laule et al., 2003; Flügge and Gao, 2005).
The study of metabolic pathway control in glandular trichomes has been hindered by the lack of tools for the rapid evaluation of gene function. Recently, virus-induced gene silencing (VIGS) has emerged as a useful tool for the study of gene function in the Solanaceae species and other species (Burch-Smith et al., 2004; Robertson, 2004). However, there are still few examples of its use in metabolic studies, and there is no evidence that the method can be used for studying trichome metabolism in cultivated and wild tomato species (Chen et al., 2004; Page et al., 2004; Park et al., 2005; van Schie et al., 2007).
To investigate further the divergence in terpenoid production between S. lycopersicum and S. habrochaites, we have undertaken a comparative study based on a combination of genetic, genomic, and physiological approaches. We show that there is a close relationship between monoterpene and cII-S metabolism at both the genetic and biochemical levels. We also show that cII-Ss in LA1777 are mostly derived from MEP precursors, whereas the MVA pathway contributes more significantly to sesquiterpene production in S. lycopersicum. Our results further suggest that S. habrochaites produces its two classes of sesquiterpenes from distinct precursor pools derived from the MVA and MEP pathways, respectively, which implies the existence of a mechanism for partitioning sesquiterpene production, most likely by a different subcellular localization of the corresponding cyclases.
RESULTS
Divergence in Terpenoid Quantity and Quality in Trichomes of S. lycopersicum and S. habrochaites LA1777
To date, the profiles of monoterpenes, sesquiterpenes, and their carboxylic acids (SCAs) in S. lycopersicum and LA1777 have been obtained in separate experiments (Coates et al., 1988; van der Hoeven et al., 2000; Fridman et al., 2005). To obtain a complete picture of terpenoid accumulation in the two species, we profiled surface extracts of S. lycopersicum (cv M82) and LA1777 from comparable stem internodes. Monoterpenes were prevalent in S. lycopersicum, with sesquiterpenes as only minor components. The major monoterpenes represented 90.5% of all terpenoids measured, whereas known cI-Ss represented 0.5% of the total and cII-Ss were completely absent (Table I). In LA1777, sesquiterpenes and especially cII-S derivatives were the major components, with monoterpenes either present in small amounts or absent (Table I). The cII-SCAs represented 93.5%, and their presumed precursors, the cII-Ss α-santalene, α-cis-bergamotene, and α-trans-bergamotene, together formed 0.7% of the total terpenoids, whereas the cI-Ss constituted 0.6% of the terpenoids measured (Table I).
Table I.
Composition of trichome-produced terpenoids in S. lycopersicum cv M82 and S. habrochaites f. typicum LA1777
Terpenoids were from surface extracts of the fifth youngest stem internode of 3-month-old greenhouse-grown plants. Values are averages and se (in parentheses) for nine plants. Only a selection of terpenoids is shown.
| Compound | LA1777 | cv M82 | ||
|---|---|---|---|---|
| ng mm−2 | % of totala | ng mm−2 | % of totala | |
| Monoterpenes | ||||
| Limonene | 2.2 (0.1)b | ND | ||
| α-Pinene | ND | 19.8 (3.0) | ||
| Verbenene | ND | 20.5 (3.0) | ||
| δ-2-Carene | ND | 150.1 (24.4) | ||
| β-Phellandrene | ND | 458.4 (63.7) | ||
| Total | 2.2 (0.1)b | 0.04 (0.007) | 648.7 (94.1) | 90.48 (1.49) |
| cI-Ss | ||||
| β-Elemene | 6.4 (0.6) | 1.1 (0.1) | ||
| γ-Elemene | 4.8 (1.4) | ND | ||
| Germacrene D | 6.0 (0.5) | ND | ||
| Germacrene B | 2.4 (0.5) | ND | ||
| β-Caryophyllene | 4.1 (0.3) | 1.2 (0.1) | ||
| α-Humulene | 3.7 (0.2) | 1.1 (0.1) | ||
| Total | 27.4 (3.5) | 0.55 (0.09) | 3.3 (0.24) | 0.52 (0.06) |
| cII-S hydrocarbons | ||||
| α-cis-Bergamotene | 8.6 (0.8) | ND | ||
| α-Santalene | 21.8 (2.2) | ND | ||
| α-trans-Bergamotene | 5.3 (0.4) | ND | ||
| Total | 35.7 (3.4) | 0.71 (0.08) | 0.0 | 0.00 |
| Carboxylic acids | ||||
| α-cis-Bergamotenoic acid | 713.8 (103.1) | ND | ||
| α-Santalenoic acid | 2,340.6 (359.3) | ND | ||
| α-trans-Bergamotenoic acid | 174.9 (26.3) | ND | ||
| β-cis-Bergamotenoic acid | 1,968.9 (303.6) | ND | ||
| Total | 5,198.2 (792.3) | 93.49 (1.27) | 0.0 | 0.00 |
| Otherc | ||||
| β-Cubebene | 9.7 (1.1) | ND | ||
| Unknown | 15.2 (1.6) | ND | ||
| Total | 24.9 (2.7) | 0.49 (0.06) | 0.0 | 0.00 |
| Total terpenoids | 5,289.0 (802.8) | 95.28 (1.50) | 652.0 (94.3) | 91.00 (1.55) |
Calculation based on the quantification of peak areas of all terpenoids in chromatograms from chloroform extracts as described in “Materials and Methods.”
Could not be consistently detected throughout this study.
Has not been allocated to a specific class of sesquiterpenes. ND, Not detected.
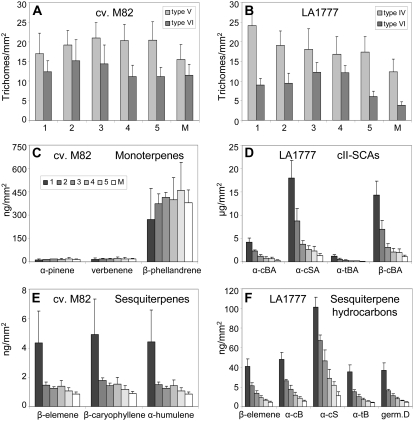
As shown in Figure 1B, there are two abundant types of glandular trichomes present on stems of LA1777. The type VI trichomes are the second most abundant trichome type after glandular type IV in LA1777 and after nonglandular type V in S. lycopersicum (Figs. 1, A and B, and 2, A and B). Careful sampling of metabolites from individual glands with a capillary revealed that the composition of terpenoids in type VI trichome heads was nearly identical to that of extracts derived from whole tissue after immersion in chloroform, which suggested that all surface terpenoids measured are accumulated in type VI trichomes in LA1777 (Supplemental Fig. S1). The same result was obtained by sampling type VI glands from S. lycopersicum (data not shown). Although type VI trichome densities on equivalent internodes were slightly lower in LA1777 (Figs. 1, A and B, and 2, A and B), the trichomes of LA1777 accumulated 8-fold higher levels of free terpenoids per surface area of stems of greenhouse-grown plants than those of S. lycopersicum (Table I).
Figure 2.
Developmental profiles of terpenoid accumulation and trichome density in S. lycopersicum and LA1777. A and B, Trichome density of the most abundant types on equivalent stem internodes of S. lycopersicum ‘M82’ type V and VI trichomes (A) and of S. habrochaites LA1777 type IV and VI trichomes (B). C to F, Accumulation of terpenoids in trichomes of equivalent stem internodes of cv M82 monoterpenes (C) and sesquiterpenes (E) and of LA1777 cII-SCAs (D) and cI-S and cII-S (F) hydrocarbons. Columns 1 to 5, First to fifth internodes from the apex; column M, internodes from the midsection of the stem. B, Bergamotene; BA, bergamotenoic acid; c, cis; germ, germacrene; S, santalene; SA, santalenoic acid; t, trans. Values represent averages and sd from nine plants. For metabolite analysis, material was extracted in three pools of three plants each. A repeat experiment gave similar results.
The Developmental Profiles of Terpenoid Accumulation Are Different in S. lycopersicum and LA1777
To determine whether fundamental differences in the developmental pattern of terpenoid accumulation could contribute to the highly divergent metabolite profiles between the wild and cultivated species, terpenoid accumulation was measured in successive internodes of 3-month-old S. lycopersicum and LA1777 plants.
Monoterpene accumulation remained fairly constant on an area basis in successive internodes of S. lycopersicum (Fig. 2C). In contrast, sesquiterpene levels were much higher in younger tissue and decreased from one internode to the next in LA1777 (Fig. 2, D and F). As trichome density in the uppermost internodes did not change significantly despite the increase in internode length (Fig. 2, A and B), terpenoid accumulation in trichomes was lower in older stem segments in the wild but not in the cultivated species. Interestingly, sesquiterpene levels likewise decreased from younger to older internodes in S. lycopersicum, especially in the most apical internodes (Fig. 2E). Therefore sesquiterpene and monoterpene accumulation appear to be differentially regulated in tomato, although the accumulation of each compound class may be similarly controlled in LA1777 and S. lycopersicum.
Genomic Analysis of S. habrochaites Glandular Trichomes
To shed more light on the molecular basis of terpenoid production in the wild species, we examined a collection of 2,435 public ESTs produced from LA1777 trichomes (R.S. van der Hoeven, unpublished data). Cluster analysis produced a total of 1,641 unigenes, suggesting a relatively low level of redundancy among the expressed sequences. Some of the ESTs, however, were disproportionately represented, and interestingly, many of these corresponded to genes that could be associated with terpenoid production. In particular, ESTs for SSTLH1, which has been predicted to encode a cI-S cyclase (van der Hoeven et al., 2000), represented over 5% of all ESTs in the collection. Additional pathway enzymes were also represented by multiple ESTs of the corresponding genes, for example 3-hydroxy-3-methylglutaryl-CoA synthase (HMGS; eight ESTs), 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR; seven ESTs), 1-deoxy-d-xylulose-5-phosphate synthase (DXS; six ESTs), and 1-deoxy-d-xylulose-5-phosphate reductoisomerase (DXR; five ESTs; Supplemental Table S1). In total, 9% of the ESTs of the 100 most highly represented genes could be associated with terpenoid metabolism, representing the highest proportion for a single functional class (Supplemental Fig. S2). Based on the predicted functions of the encoded proteins, many more of the 100 most highly represented genes could be associated with other aspects of metabolism, in particular lipid and cell wall metabolism (6% and 5% of the ESTs, respectively). A significant proportion of the EST collection also appeared to represent genes involved in stress responses (8%) or defense (4%; Supplemental Fig. S2). These predicted functions were generally consistent with the role of trichomes as epidermal defensive structures actively secreting secondary metabolites.
Regulation of Terpenoid Pathway Gene Expression in Tomato Trichomes
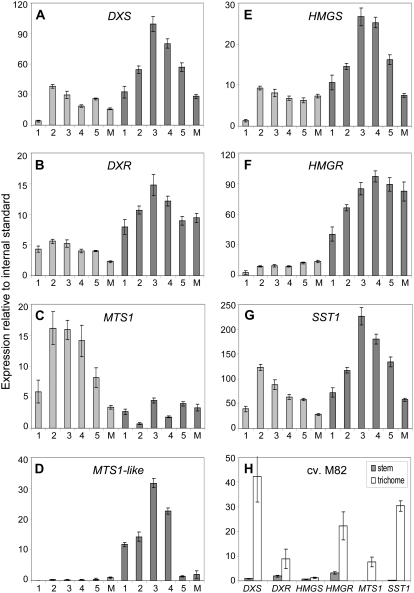
To determine whether transcriptional regulation is likely to play a role in controlling terpenoid production, we examined the expression of genes encoding different pathway enzymes in trichomes from successive stem internodes. For this experiment, DXS, DXR, HMGS, and HMGR were selected to represent the MEP and MVA pathways, as well as several genes encoding terpenoid cyclases that are represented in the EST collection (MTS1, MTS1-like, and SSTLH1).
A similar pattern of transcript accumulation was observed for most genes in the two species, with a strong increase in elongating internodes followed by a gradual decrease in more mature sections of the stem (Fig. 3, A–G; Supplemental Fig. S3, A–E), which was less pronounced in S. lycopersicum (HMGR, MTS1-like; Fig. 3, D and F). The expression patterns of DXS, DXR, HMGS, and HMGR also closely matched those of the genes encoding the SSTLE1 and SSTLH1 sesquiterpene cyclases (SST1) as well as known (MTS1) and putative (MTS1-like) monoterpene cyclases. This indicated that genes encoding terpenoid pathway enzymes synthesizing isoprenoid precursors and acting farther downstream may be similarly regulated (Fig. 3, A–G). Interestingly, both monoterpene cyclase genes represented in the EST collection were differentially regulated in the two species, a situation that may be relevant to the divergence in monoterpene production between S. lycopersicum and LA1777 (Fig. 3, C and D; Table I).
Figure 3.
Developmental profiles of terpenoid pathway gene expression in trichomes of S. lycopersicum and LA1777. A to G, Expression of genes involved in terpenoid biosynthesis in trichomes of stem internodes in S. lycopersicum ‘M82’ (light gray bars) and LA1777 (dark gray bars). Columns 1 to 5, First to fifth internodes from the apex; column M, internodes from the midsection of the stem. H, Expression of selected genes in stem trichomes and underlying tissues in S. lycopersicum ‘M82’. For abbreviations of gene names, see Figure 1 legend. SST1, SSTLE1/SSTLH1. Gene expression was measured by real-time PCR relative to an internal standard (actin); see Supplemental Table S2 for respective Ct values. Each bar represents a pool of trichome RNA derived from three to six plants and sd for three technical replicates. A repeat experiment gave similar results (Supplemental Fig. S3).
There was no major difference in the expression of genes encoding MVA versus MEP pathway enzymes in the two species, suggesting that both pathways may be similarly active in S. lycopersicum and LA1777 (Fig. 3, A, B, E, and F). While the selected genes were generally more expressed in trichomes than in underlying tissues in S. lycopersicum (Fig. 3H; Supplemental Fig. S3F) and LA1777 (data not shown), the expression levels of MVA and MEP pathway genes were generally higher in LA1777 in comparison with S. lycopersicum, with a maximum (10-fold) difference for HMGR. Furthermore, the onset of terpenoid accumulation preceded the observed increase in pathway gene expression in both species, and the drop in sesquiterpene production in the first three internodes was not accompanied by a decrease in transcript levels (Figs. 2, C–F, and 3, A–G). Thus, a correlation between transcript and metabolite levels, especially in successive internodes, could not be detected.
cII-Ss Derive from MEP Pathway Precursors in LA1777
Early MEP pathway genes such as DXS and DXR were expressed in LA1777 at comparable levels to those in S. lycopersicum trichomes (Fig. 3, A and B; Supplemental Fig. S3, A and B). As LA1777 in contrast to S. lycopersicum does not accumulate significant levels of monoterpenes, which are classically associated with the MEP pathway, we investigated the roles of the two IPP pathways in the trichome terpenoid metabolism of this species.
VIGS has been used successfully to investigate gene function in tomato (Liu et al., 2002; van Schie et al., 2007), but its efficiency has not been tested in trichomes or in any of the wild species. We found that tobacco rattle virus (TRV)-based vectors could be used to silence the gene encoding phytoene desaturase (PDS) in LA1777 as efficiently as in S. lycopersicum, as shown by extensive photobleaching of PDS-silenced plants (Supplemental Fig. S4, A and B). We also found that VIGS was effective in trichomes, as silencing the trichome-specific gene SSTLE1 in S. lycopersicum caused a significant reduction (t test, P ≤ 0.05) in the levels of α-humulene, β-caryophyllene, and β-elemene (representing the thermal decomposition products of germacrenes; Fig. 3H; Supplemental Fig. S4, C and D). This is consistent with the expected but so far unconfirmed activity of the SSTLE1 enzyme (Colby et al., 1998; van der Hoeven et al., 2000). Monoterpene accumulation was unaffected by the knockdown of SSTLE1 (see β-phellandrene, Supplemental Fig. S4C).
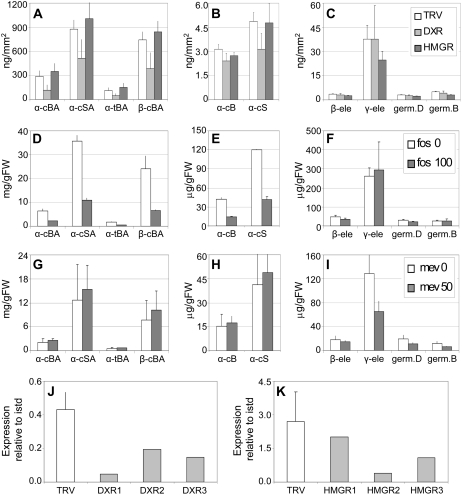
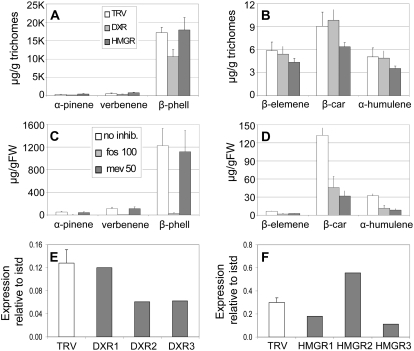
Targeting the rate-limiting enzyme of the MEP pathway by silencing DXR to down-regulate the synthesis of MEP-derived IPP had a significant impact on trichome terpenoid metabolism in LA1777. Specifically, it led to a strong reduction in the accumulation of SCAs and their cII-S hydrocarbon precursors (t test, P ≤ 0.01; Fig. 4, A and B). In contrast, silencing DXR did not reduce the accumulation of the cI-Ss germacrene B, germacrene D, or β- and γ-elemene, which represent their thermal decomposition products (Fig. 4C). Interestingly, silencing HMGR, which encodes the enzyme catalyzing the rate-limiting step in the MVA pathway, to reduce the MVA-derived IPP pool had a nearly opposite effect: accumulation of cI-Ss was significantly reduced (t test, P ≤ 0.01), whereas cII-Ss and carboxylic acids were unaffected, if not slightly more abundant (Fig. 4, A–C).
Figure 4.
Variations in MEP and MVA pathway activity affect terpenoid accumulation in LA1777 trichomes. A to C, Effects of silencing DXR (light gray bars) and HMGR (dark gray bars) compared with empty vector controls (TRV; white bars) on the accumulation of cII-SCA (A), cII-S (B), or cI-S (C) hydrocarbons in LA1777 trichomes. B, Bergamotene; BA, bergamotenoic acid; c, cis; ele, elemene; germ, germacrene; S, santalene; SA, santalenoic acid; t, trans. Values represent averages and sd of six samples taken from three plants. D to I, Effects of inhibitor applications on the accumulation of cII-SCA (D and G), cII-S (E and H), or cI-S (F and I) hydrocarbons in the trichomes of LA1777 cuttings treated with fosmidomycin (D–F, gray bars) or mevinolin (G–I, gray bars) compared with mock-treated controls (white bars). fos 0 and fos 100, 0 and 100 μm fosmidomycin applied, respectively; FW, fresh weight; mev 0 and mev 50, 0 and 50 μm mevinolin applied, respectively. Values are averages of two samples of one to three pooled plants each. Repeat experiments gave similar results. J and K, Expression levels of DXR (J) and HMGR (K) in trichomes of control and silenced LA1777 plants (same plants as in A–C). Gene expression was measured by real-time PCR relative to an internal standard (istd; actin). TRV, Empty vector controls; DXR1 to -3 and HMGR1 to -3, three DXR- and HMGR-silenced plants, respectively. Bars represent averages and sd for three controls and single values for individual plants.
To verify the above results, we measured the effects of mevinolin (an inhibitor of HMGR) and fosmidomycin (an inhibitor of DXR) on trichome metabolism in LA1777 cuttings by profiling trichome metabolites from tissue that had expanded after inhibitor application (Alberts et al., 1980; Mueller et al., 2000). The phenotypes of inhibitor-treated plants were as expected (i.e. leaves were chlorotic with fosmidomycin and darker green with mevinolin; data not shown). In agreement with the DXR-silencing data, fosmidomycin had little effect on the accumulation of the cI-Ss but caused a significant decrease in the accumulation of cII-S species (both SCAs and their hydrocarbon precursors; t test, P ≤ 0.05 and P ≤ 0.01, respectively; Fig. 4, D–F). Fosmidomycin application to cuttings of the cII-S hydrocarbon-producing introgression line LA3935 also caused the near disappearance of the cII-S compounds, such as α-cis-bergamotene, α-santalene, and α-trans-bergamotene. However, accumulation of major cI-S β-caryophyllene was less affected, as was that of other more minor class I compounds (Supplemental Fig. S5A). Consistent with the results of HMGR silencing, mevinolin application had no impact on the accumulation of class II terpenoids in LA1777 but caused a strong decrease in cI-S accumulation (Fig. 4, G–I).
To confirm that silencing and inhibitor applications truly target the MEP and MVA pathways, we supplied 14C-labeled pyruvate (a MEP precursor) and [14C]MVA to LA1777 cuttings in the presence or absence of inhibitors. After incubation, trichome metabolites were extracted and separated on thin-layer chromatography (TLC) plates (Supplemental Fig. S6), and label incorporation into sesquiterpene acids and hydrocarbons was measured. Whereas SCAs were strongly labeled at 3 h after [14C]pyruvate feeding, only low levels of incorporation (about 50 times less) were detected from [14C]MVA (Table II). The cI-S and cII-S hydrocarbons were not resolved under our TLC conditions. However, as a group, sesquiterpene hydrocarbons were strongly labeled by [14C]pyruvate, in agreement with cII-S hydrocarbons acting as intermediates in SCA biosynthesis. Hydrocarbons were also labeled from [14C]MVA, although less strongly, consistent with the low abundance of cI-S accumulation in LA1777 trichomes.
Table II.
Incorporation of radiolabeled MVA and MEP pathway-derived precursors into sesquiterpenes in LA1777
Values are averages and se (in parentheses) for four replicates. * Significant and ns no significant difference of treatment versus control in t test at P ≤ 0.05. fos, 100 μm fosmidomycin; mev, 100 μm mevinolin.
| Substrate | Inhibitor | Sesquiterpene Hydrocarbonsa | SCAs |
|---|---|---|---|
| pmol g−1 fresh weight h−1 | |||
| [14C]MVA (75 nmol, 2.5 μCi) | 1.2 (0.3) | 1.7 (0.3) | |
| [14C]Pyruvate (152.5 nmol, 2.5 μCi) | Control | 21.4 (4.8) | 67.5 (13.2) |
| fos | 6.8* (0.9) | 20.2* (2.4) | |
| Control | 13.5 (3.8) | 41.5 (5.5) | |
| mev | 16.5ns (3.7) | 71.9ns (14.5) | |
Class I and II sesquiterpene hydrocarbons were not resolved in this experiment.
The above data suggest that the MEP pathway directly contributes to cII-S formation and that the contribution of the MVA pathway, if it exists, is negligible. In contrast, the incorporation of 14C label from MVA into sesquiterpene hydrocarbons indicated that other noncarboxylic sesquiterpenes are likely derived from the MVA pathway.
To show that 14C incorporation into SCAs from pyruvate depends on DXR and not on HMGR activity, LA1777 cuttings were treated with fosmidomycin or mevinolin prior to labeling. Fosmidomycin significantly inhibited incorporation, whereas mevinolin had little or no effect (Table II).
Taken together, the results from VIGS experiments, inhibitor applications, and labeled precursor feeding studies were consistent and suggested that pyruvate, but not MVA, is a direct precursor in the biosynthesis of cII-Ss in LA1777. On the other hand, these data also indicate that cI-Ss are produced mainly from MVA via the MVA pathway in LA1777.
The MVA and MEP Pathways Contribute to Sesquiterpene Biosynthesis in S. lycopersicum
To evaluate the relative contributions of the MVA and MEP pathways to terpenoid biosynthesis in S. lycopersicum trichomes, we performed VIGS and inhibitor studies in cv M82 plants.
Silencing of DXR, leading to the reduction of MEP-derived precursor accumulation, caused a significant drop in monoterpene accumulation (t test, P ≤ 0.01) but had no effect on sesquiterpene levels (Fig. 5, A and B). In contrast to the effects of DXR silencing, down-regulation of HMGR and MVA-derived precursor synthesis did not affect monoterpene production (Fig. 5A) but caused a small and consistent decrease in cI-S accumulation (Fig. 5B). A similar effect was observed when DXR and HMGR were down-regulated in the cv MicroTom plants (Supplemental Fig. S7, A–D). However, the down-regulation of transcript levels of DXR and HMGR was variable among silenced plants (Fig. 5, E and F).
Figure 5.
Effects of variations in MEP and MVA pathway activity on terpenoid accumulation in S. lycopersicum trichomes. A and B, Effects of silencing DXR (light gray bars) and HMGR (dark gray bars) compared with empty vector controls (TRV; white bars) on the accumulation of monoterpenes (A) and sesquiterpenes (B) in S. lycopersicum trichomes (cv M82). β-car, β-Caryophyllene; β-phell, β-phellandrene. Values represent averages of triplicate determinations and sd. Repeat experiments gave similar results. C and D, Effects of inhibitor applications on the accumulation of monoterpenes (C) and sesquiterpenes (D) in trichomes of cv M82 cuttings treated with fosmidomycin (light gray bars) or mevinolin (dark gray bars) as compared with mock-treated controls (white bars). fos 100, 100 μm fosmidomycin applied; FW, fresh weight; mev 50, 50 μm mevinolin applied; no inhib, no inhibitor applied. Values represent averages of triplicate determinations of three to four pooled plants each and sd. Repeat experiments gave similar results. E and F, Expression levels of DXR (E) and HMGR (F) in control and silenced cv M82 plants (same plants as in A and B). Gene expression was measured by real-time PCR relative to an internal standard (istd; actin). TRV, Empty vector controls; DXR1 to -3 and HMGR1 to -3, three DXR- and HMGR-silenced plants, respectively. Bars represent averages and sd for three controls and single values for individual plants.
To assess further the contributions of the MVA and MEP pathways to the production of different trichome terpenoid classes in S. lycopersicum, cv M82 cuttings were treated independently with mevinolin (MVA) and fosmidomycin (MEP) and trichome metabolites were profiled from the youngest tissue. The phenotypes of inhibitor-treated plants were as observed for LA1777. Consistent with the results of VIGS, fosmidomycin nearly abolished monoterpene accumulation (Fig. 5C) but also caused a strong decline in the production of sesquiterpenes (t test, P ≤ 0.01; Fig. 5D). This effect was not obvious from VIGS experiments, which generally show a weaker phenotype than inhibitor applications, as they reduce rather than eliminate transcript levels and show some “patchiness” due to nonuniform spread of the virus (Fig. 5, E and F; Supplemental Fig. S4A). Applying different concentrations of fosmidomycin (from 10 to 100 μm) showed that even the lowest concentration caused the same reduction in monoterpene and sesquiterpene accumulation as described before, although there was no visible bleaching phenotype (Supplemental Fig. S5B). This indicated that the observed changes were not due to secondary effects of the inhibitor on plant health. Application of mevinolin, on the other hand, caused a significant decrease in sesquiterpene (t test, P ≤ 0.01) but did not affect monoterpene accumulation, consistent with the HMGR-silencing data (Fig. 5, C and D).
Together, the silencing and inhibitor results indicated that the MEP pathway is central to monoterpene metabolism in S. lycopersicum and suggested that the biosynthesis of cI-Ss, in contrast to cII-Ss in LA1777, is dependent on both MVA and MEP pathway-derived precursors.
Genetic Association between Monoterpene and cII-S Production in Tomato Trichomes
The coincidence of low monoterpene accumulation with high cII-S production in LA1777 led us to investigate the genetic relationship between the two metabolic traits. For this experiment, we took advantage of nearly isogenic lines (NILs) containing genomic introgressions from LA1777 in a S. lycopersicum background (Monforte and Tanksley, 2000) and used them to obtain backcross progeny segregating for the accumulation of cII-Ss.
One cII-S-accumulating NIL (TA517 = LA3935) had previously been identified (van der Hoeven et al., 2000), although monoterpene production was not evaluated in this screen. To obtain a more complete picture, we measured sesquiterpene and monoterpene levels in a total of 57 NILs and identified five new cII-S-accumulating lines. Some of these lines, however, segregated for terpenoid content. Interestingly, among those that appeared to be stable, cII-S accumulation was accompanied by a nearly complete lack of monoterpene production (Supplemental Table S3). This observation suggested that the loci controlling cII-S and monoterpene accumulation (or the lack thereof) are linked, or that most cII-S-producing NILs contain one or more additional introgression(s) that negatively affect(s) monoterpene production. To distinguish between these two possibilities, we generated two BC1S1 segregating populations by selfing backcross lines obtained from crosses between two cII-S-producing NILs (LA3935 and LA3940) with the cultivated parent cv E6203. About 40 progeny for each BC1S1 population were grown and analyzed for terpenoid production.
Three classes of progeny, which shared similar terpenoid profiles, were identified in each population. The first class, which comprised the larger number of progeny in each population, produced monoterpenes that were similar to those found in cv E6203 plants and also accumulated cII-Ss. The second class accumulated cII-Ss but little or no monoterpenes, and the third class produced monoterpenes but no cII-Ss. The proportions of plants in each phenotypic category were similar in the two BC1S1 populations and suggested that cII-S and E6203 monoterpene accumulation are both under the control of single dominant loci (Table III). The sizes of the phenotypic groups and the lack of a fourth class of progeny, which produced neither cII-Ss nor E6203-type monoterpenes, were more compatible with linkage between these two loci than with independent assortment (Table III).
Table III.
Genetic analysis of S. lycopersicum monoterpene and LA1777 cII-S accumulation
Observed and expected numbers of BC1S1 progeny from isogenic lines accumulating S. lycopersicum monoterpenes (M) or LA1777 cII-Ss under four different hypotheses: monoterpene (1) and cII-S accumulation (2) are under the control of single dominant loci; (3) the loci are completely linked; (4) the loci are unlinked. The progeny are the F2s of two backcrosses to S. lycopersicum E6203, involving LA3935 (39 plants) and LA3940 (40 plants). The data from both populations were pooled.
| Phenotype | Observed No. of Individuals | Expected No. of Individuals
|
Monoterpenes | cII-Ss | |||
|---|---|---|---|---|---|---|---|
| M One Locus (1) | cII-S One Locus (2) | M/cII-S Linked (3) | M/cII-S Unlinked (4) | ||||
| ng cm−2 | |||||||
| M/− | 19 | 19.75 | 19.75 | 14.81 | 2,034.5 | 0.0 | |
| All M | 62 | 59.25 | |||||
| M/cII-S | 43 | 39.50 | 44.44 | 1,457.8 | 272.8 | ||
| All cII-S | 60 | 59.25 | |||||
| −/cII-S | 17 | 19.75 | 19.75 | 14.81 | 13.5 | 486.9 | |
| −/− | 0 | 0.00 | 4.94 | ||||
| χ2 probability | 0.48 | 0.85 | 0.70 | 0.09 | |||
All plants producing E6203-type monoterpenes accumulated α-pinene, verbenene, δ-2-carene, and β-phellandrene, and these compounds always accumulated in nearly identical proportions (approximately 3%, 5%, 20%, and 72%, respectively). This finding and the segregation ratios of monoterpene accumulation strongly suggest that the accumulation of the different monoterpenes is controlled either by a single gene or by closely linked genes.
The cII-S production was lower in plants that produced monoterpenes than in plants that did not. In both populations, cII-S production was only half when accompanied by monoterpenes than that observed in their absence, and monoterpene production appeared to be inversely affected (Table III). This suggests either an effect of semidominant alleles or competition for precursors such as IPP between the cII-S and the monoterpene synthases.
Taken together, the above results suggested strong genetic and metabolic associations between cII-S and monoterpene production in the wild and cultivated species.
The Role of Sesquiterpene Cyclases in cII-S Accumulation in LA1777
The molecular mechanisms controlling cII-S accumulation in LA1777 have not been defined, although genetic analysis has ruled out the involvement of the cI-S synthase genes SSTLH1 and SSTLH2 or any gene that is highly similar in sequence to them (van der Hoeven et al., 2000).
To examine the possible role of other, more diverged sesquiterpene cyclases in this process, we screened the EST collection and identified eight unigenes with similarity to known terpenoid synthases. Among these, four had highest similarity to sesquiterpene cyclases (Supplemental Table S4). The most highly expressed of these sequences corresponded to SSTLH1 (92 ESTs), and the sequences of two other unigenes matched nonoverlapping sections of SSTLH2 (five and one EST, respectively). A fourth sequence, represented by only one EST (corresponding to GenBank accession no. AW616373) with little to no DNA sequence similarity to either SSTLH1 or SSTLH2, was associated with a predicted product most similar to sesquiterpene synthases, which we termed SSTLH3 (Supplemental Table S4).
Using a primer pair that could amplify an insertion in the LA1777 allele of SSTLH3, a smaller fragment diagnostic of the S. lycopersicum allele was found to be present in each introgression line tested, whereas none of the lines contained the fragment for the LA1777 allele (Supplemental Fig. S8A). This pattern is incompatible with the presence of an introgressed fragment at the SSTLH3 locus. In addition, the expression level of SSTLH3 was much higher in S. lycopersicum than in LA1777 trichomes, and its developmental expression pattern was different from those of the other terpenoid pathway genes tested in LA1777 (Fig. 3, A–G; Supplemental Fig. S8, B and C).
On the basis of these and previously reported results, we conclude that cII-S production is unlikely to involve a highly trichome-expressed gene encoding a typical sesquiterpene cyclase.
DISCUSSION
In this report, we have investigated the molecular basis of terpenoid accumulation and the relative contributions of precursor pathways in the glandular trichomes of two related tomato species, S. lycopersicum and its wild relative S. habrochaites LA1777. Both species have greatly diverged in the types and amounts of terpenoids they accumulate in trichomes: S. lycopersicum mainly produces monoterpenes and traces of cI-Ss, whereas LA1777 produces mainly cII-S carboxylic acids, small amounts of cI-Ss, and traces of monoterpenes (Table I). The monoterpenes and cI-Ss and cII-Ss analyzed in this study all derive from type VI trichomes (Supplemental Fig. S1; Frelichowski and Juvik, 2001; Li et al., 2004). Despite a similar density of type VI trichomes in both species, LA1777 accumulated much higher levels of measured terpenoids per surface area compared with S. lycopersicum. This could be related to the difference in volatility between monoterpenes and SCAs and/or to differences in the chemical forms in which terpenoids accumulate in the two species (glycosylated, etc.). The different terpenoid levels measured in both species could also result from differences in trichome productivity. Determining which is the most likely scenario would require further analysis. The regulation of monoterpene and sesquiterpene accumulation differs during development, as decreasing levels of sequiterpenes and constant levels of monoterpenes were measured in aging internodes. This might reflect different functions of these terpenoids in the plant. However, the regulation patterns of terpenoid synthesis seem to be conserved in both species.
Developmental Regulation of Terpenoid Metabolism Does Not Occur at the Transcript Level
Analysis of a public EST collection from LA1777 trichomes showed that most ESTs represent gene products associated with metabolism, and about 9% of the ESTs representing the 100 most highly expressed sequences represent genes involved in terpenoid metabolism. In comparison with the representation of other metabolic pathways in the trichomes of LA1777 (e.g. flavonoid [3%] or lipid [6%] metabolism), the relatively higher EST representation of the terpenoid metabolism reflects its high activity in the glandular trichomes and is consistent with the high level of terpenoid accumulation in LA1777 trichomes. It has been shown that up to 1.2% of leaf fresh weight consists of SCAs in LA1777 (Frelichowski and Juvik, 2001), when trichome exudate levels of 3% to 30% of leaf dry weight are considered to indicate high-level accumulators (Wagner, 1991).
The comparable EST representation of MEP to MVA pathway genes in the LA1777 trichome library as well as similar (to higher) expression levels of MEP pathway genes in LA1777 compared with S. lycopersicum may hint at the involvement of the MEP pathway in the production of terpenoids in LA1777. Transcript levels of MVA pathway genes appeared to be up to 10-fold higher in LA1777 than in S. lycopersicum, and the cI-S synthase encoding SSTLH1 was the most highly represented gene in the EST collection. This may reflect the clearly higher levels of cI-S accumulation in LA1777 compared with S. lycopersicum but does not correlate with the main components of the terpenoid profile in LA1777, namely the cII-SCAs. This discrepancy could be due to differences in turnover of cI-Ss and cII-Ss and/or in the activity and stability of the enzymes involved in their synthesis.
The expression levels of most of the genes of the MVA or MEP pathway and monoterpene or sesquiterpene synthesis follow the same profile during development, suggesting a coordinated regulation of terpenoid biosynthesis at the gene level. However, although expression of these genes is relatively trichome specific, the expression profiles do not mirror metabolite accumulation during development, suggesting that terpenoid synthesis is not regulated at the transcript level in tomato trichomes but rather involves other (posttranscriptional) regulatory mechanisms. Similarly, only a loose correlation between terpenoid pathway gene expression and enzyme activity has been found in basil (Ocimum basilicum) terpenoid metabolism (Iijima et al., 2004). This is in contrast to the regulation of monoterpene synthesis in mint, in which transcript, protein, and metabolite accumulation have been shown to follow the same developmental pattern (McConkey et al., 2000). Therefore, terpenoid metabolism in glandular trichomes seems to be regulated at different levels depending on the species involved.
A Functional Genomics Strategy for Dissecting Terpenoid Metabolism in Trichomes of Wild and Cultivated Tomato Species
Our results show that TRV-mediated VIGS can be used as an effective approach to silence trichome-expressed genes in cultivated as well as wild tomato species. By silencing SSTLE1, we have demonstrated, to our knowledge for the first time, the function of this gene, confirming its expected activity in vivo (van der Hoeven et al., 2000).
The effectiveness of VIGS in tomato trichomes will facilitate future comparative genomic studies that would otherwise be impractical using stable transformation. The validity of candidate gene approaches has been demonstrated for studying the regulation of epidermal metabolism in Arabidopsis (Broun et al., 2004), and VIGS should enable similar strategies in tomato. However, since TRV does not induce complete and uniform silencing in tomato plants (including trichomes), VIGS will likely be most suitable for the study of processes that are particularly sensitive to relatively small variations in target gene expression.
Precursor Origin Influences Terpenoid Accumulation Patterns in Trichomes of Wild and Cultivated Tomato
Using a combination of strategies from VIGS to inhibitor application and labeled precursor feeding, we have demonstrated that the production of cII-Ss in LA1777 is, similar to monoterpene biosynthesis in S. lycopersicum, very sensitive to fluctuations in the MEP pathway but seemingly insensitive to variations in the supply of MVA. cI-S production, in contrast, is strongly influenced by changes in MVA pathway activity. The large difference in the levels of cI-Ss and cII-Ss in LA1777, therefore, may be as much due to differences in precursor supply through the MEP and MVA pathways as to differences in catalytic activities of cI-S and cII-S cyclases and/or turnover of the compounds. In S. lycopersicum, however, the biosynthesis of cI-Ss appears to rely partially also on non-MVA precursors, since inhibition of DXR activity by fosmidomycin is detrimental to their accumulation. This implies an exchange of precursors between compartments from plastids to the cytosol (Adam et al., 1999; Hemmerlin et al., 2003; Laule et al., 2003; Dudareva et al., 2005), which is more pronounced in S. lycopersicum than in LA1777.
Our findings suggest the existence, in the wild species, of a mechanism controlling the production of two distinct groups of sesquiterpenes from different precursor pools. Such a partition in sesquiterpene biosynthesis could be the result of metabolite channeling, for example through two distinct farnesyl diphosphate (FPP) synthases in the cytoplasm that are associated either with cI-S synthases or with plastidial transporters and the cII-S synthases. However, it seems unlikely that MVA precursors are available for the biosynthesis of other terpenoid classes, such as sterols, but not for cII-Ss, or that MEP precursors exported into the cytoplasm are only available for cII-S synthases but not for other cytosolic enzymes. A physical separation of cI-S and cII-S synthases in different subcellular compartments would be a simpler explanation.
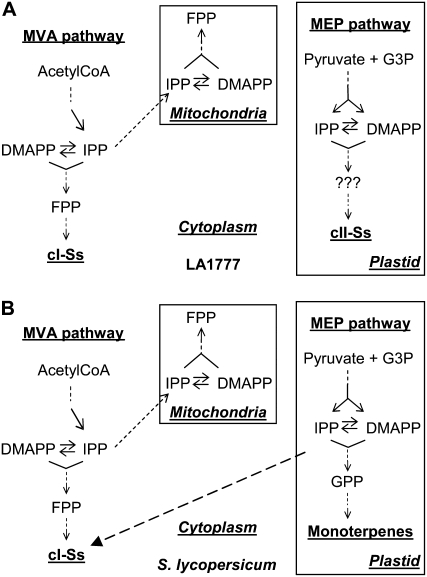
Since terpenoids in the mitochondria are known to derive from the MVA pathway (Disch et al., 1998), a plastidial site of synthesis would be most logical (Fig. 6). Such localization would be highly unusual, as sesquiterpene production is thought to be limited to the cytoplasmic or mitochondrial compartments in plant cells. There are a few examples of sesquiterpenes deriving solely from MEP pathway precursors. For example, the biosynthesis of the phytohormone abscisic acid ultimately involves MEP precursors and is initiated in chloroplasts. Interestingly, however, as abscisic acid derives from carotenoids, it is not produced from FPP but from gernaylgeranyl diphosphate (Cutler and Krochko, 1999). Two other irregular sesquiterpenes, anthecotuloide from Anthemis cotula and hodgsonox from a liverwort (Marchantia polymorpha), are formed exclusively via the MEP pathway (Barlow et al., 2003; van Klink et al., 2003). Anthecotuloide has been found to derive from a non-FPP precursor, which has been proposed to be synthesized via a “head-to-head” linkage of geranyl diphosphate to DMAPP (van Klink et al., 2003). Another irregular sesquiterpene, artemone from Artemisia pallens, has been reported to originate from an unusual non-FPP precursor, produced by condensation of IPP and dimethylvinylcarbinylpyrophosphate (Akhila et al., 1986). These reports led us to speculate that if the cII-S synthase is localized in the plastids, MEP-derived IPP and DMAPP might be used to produce a non-FPP precursor that is then utilized to form the cII-Ss, as there are no reports describing a substantial FPP synthase activity in plastids (Fig. 6). A localization in plastids would also easily explain the metabolic linkage between monoterpene and cII-S accumulation in the NILs containing S. habrochaites introgressions. MEP-derived precursors that are available for monoterpene cyclases in the plastids of S. lycopersicum are also used by the cII-S cyclase in cII-S-producing introgression lines, leading to a competition for precursors and a reduction in S. lycopersicum-type monoterpene synthesis. However, the ultimate discrimination between cytoplasmic and plastidial localization requires the identification of the cII-S cyclase and its subcellular location.
Figure 6.
Proposed terpenoid pathway in the trichomes of S. habrochaites f. typicum LA1777 and S. lycopersicum.
cII-S Cyclase
The nature of the cII-S cyclase remains unclear, as cII-S and cI-S cyclases are thought to have substantially diverged in sequence (van der Hoeven et al., 2000). We confirm in this study that none of the highly expressed trichome-specific typical sesquiterpene cyclases are likely to produce cII-Ss. Our analysis of cII-S-accumulating NILs suggests that LA1777 cII-S and E2603 monoterpene accumulation are both inherited as single-locus dominant traits. The backcross data suggest that these two traits represent either alleles at the same locus or two closely linked loci. Recent work by Kampranis et al. (2007) supports the possible evolutionarily simple interconversion of terpene synthases that are involved in the biosynthesis of different size classes of isoprenoids. Especially synthesis of monoterpenes and sesquiterpenes, including the cII-S trans-α-bergamotene, has been shown to be interconverted by a single amino acid alteration of a terpene synthase. If the cII-S synthase has evolved from a monoterpene synthase in tomato, it would be likely that it is still localized in the plastids, corroborating our results on precursor origin. It remains to be shown if sufficient FPP supply is available in the plastids or if another non-FPP precursor is used as a starting point for cII-S synthesis.
There are two highly expressed terpene synthase homologs other than monoterpene or sesquiterpene synthases represented in the trichome EST database, an ent-kaurene synthase and a prenyl transferase (Supplemental Table S1; TC116365, now TC174632, and TC124549). The close relationship of ent-kaurene synthases with linalool synthases, which appear to have evolved from a recombination event between monoterpene and ent-kaurene synthases (Cseke et al., 1998; Aubourg et al., 2002), suggests this homolog as a candidate monoterpene/sesquiterpene synthase gene. Further investigation is necessary to show that the major accumulated monoterpenes in S. lycopersicum and the cII-Ss in S. habrochaites are derived from this gene product. The prenyl transferase homolog may represent a candidate gene for non-FPP precursor production in the chloroplasts, but its function and putative involvement in the synthesis of cII-Ss in the wild tomato species has to be shown.
The extensive differences in the terpenoid profiles of LA1777 and S. lycopersicum trichomes underline the remarkable metabolic flexibility of glandular trichomes, the highly productive cellular factories that provide the plants with a huge variety of chemicals. Such metabolic shifts also reflect the staggering capacity of plants to evolve in their production of defense-related compounds in a relatively short evolutionary time. Our data suggest that the metabolic divergence from a common ancestor required few evolutionary steps affecting catalytic mechanisms and precursor availability.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Tomatoes (Solanum lycopersicum ‘M82’, ‘Moneymaker’, ‘MicroTom’, and ‘E6203’ and Solanum habrochaites f. typicum accession LA1777 and backcross recombinant inbred lines [introgression lines] representing the genome of LA1777 in the background of S. lycopersicum E6203 [a core set of 57 NILs, LA3913–LA3969; Monforte and Tanksley, 2000]) were obtained from the Tomato Genetics Resource Center. Additional LA1777 seeds were generously provided by Steven Tanksley (Cornell University). Prior to germination, seeds were soaked in 10% (w/v) trisodium phosphate dodecahydrate for 30 min and then for another 2 h at room temperature in fresh solution. After washing five times in sterile water, seeds were incubated at 50°C in water (30 min), placed in 2.7% sodium hypochlorite (30 min), and washed a further five times in sterile water before being placed in soil. Plants were grown in the greenhouse (16-h/8-h photoperiod) or in a growth chamber for silencing experiments (16-h/8-h photoperiod, 20°C day, 18°C night) in 3.5-inch pots (one plant per pot) using F2+S Levington modular compost.
VIGS
Conditions for VIGS were adapted from Liu et al. (2002). Plants were grown in the greenhouse to the two-leaf stage, at which time they were submerged upside down in Agrobacterium tumefaciens suspensions (GV3101; optical density at 600 nm of approximately 0.4) containing the TRV RNA1- and RNA2-derived pTRV1 and pTRV2 VIGS vectors (ratio 1:1) for vacuum infiltration. Bacterial suspensions were obtained by inoculating Luria-Bertani medium (50 mL, pH 7, with 10 mm MES, pH 5.5, 20 μm acetosyringone, and antibiotic selection) with precultures (5 mL) that were incubated at 28°C overnight. Cells were washed with 10 mm MgCl2 solution, resuspended in 5 mL of infiltration medium (10 mm MgCl2, 10 mm MES, pH 5.5, and 200 μm acetosyringone), and incubated for 2 h at room temperature before TRV1 and TRV2 cultures were mixed and used for vacuum infiltration with Silwet L-77 (0.004%). The plants were then transferred to a growth chamber for 4 to 5 weeks prior to harvest.
For silencing experiments, gene-specific fragments were PCR amplified from S. lycopersicum or S. habrochaites cDNA and verified by sequencing before being cloned in antisense orientation into pTRV2 (pYL156; Liu et al., 2002) by restriction digest. The following primers were designed from sequences in the Tomato Gene Index databases (http://compbio.dfci.harvard.edu/tgi/): SSTLE1 (TC162858): 5′-ATGTGTTCAAGCAATTCA-3′ and 5′-ATCCAAATCTTTCCACCA-3′; DXR (TC163170): 5′-TCCATTTGTCCTTCCTCCTG-3′ and 5′-TGAATCCTGTGTTTCCACCA-3′; HMGR (TC155027): 5′-CCTACACGCTTCCAAACACA-3′ and 5′-AACCACCACATCGTCCTCTA-3′. A pTRV2-PDS construct containing a genomic fragment of tomato PDS (X71023; 1,545 bp spanning exon 8 to exon 11; kindly provided by Sophia K. Ekengren, Cornell University) was used as a control for silencing experiments.
RNA Extraction, Real-Time Reverse Transcription-PCR, and Genomic PCR Analyses
Trichome glands were harvested by abrasion of frozen stem tissue (three to four internodes) and represented a mixture of all trichome types. Other tissues (approximately 100 mg) were ground in liquid nitrogen. The resulting powders were dispersed in RNA extraction buffer (50 mm Tris base, pH 9, 0.8 mm NaCl, 10 mm EDTA, 0.5% [w/v] cetyl-trimethyl-ammonium bromide, 2% [w/v] polyvinylpolypyrrolidone, and 1% [v/v] β-mercaptoethanol). Following extractions with phenol:chloroform (1:1, v/v) and chloroform, the RNA was recovered by ethanol precipitation and centrifugation in the cold, before being resuspended in diethyl pyrocarbonate-treated water. After treatment with DNase I (DNA-free; Ambion), cDNA was prepared using SuperScript II reverse transcriptase (Invitrogen) according to the manufacturer's protocol. Three microliters of a diluted reverse transcriptase reaction was used for real-time PCR analysis. Amplification was performed in triplicate reactions using the fluorescent dye SYBR Green (Applied Biosystems) in a thermocycler (AB7000 Sequence Detection System; Applied Biosystems). Expression of a tomato LeACTIN gene (TC116322; http://compbio.dfci.harvard.edu/tgi/) was used for normalization. Calibration curves were produced for each of the primer pairs, and quantification was performed using the AB7000 Sequence Detection Software version 1.2.3 (Applied Biosystems).
The following primers were designed from sequences in the Tomato Gene Index databases (http://compbio.dfci.harvard.edu/tgi/) or from GenBank and were used for measuring transcript levels by real-time reverse transcription-PCR: DXR (TC163170): 5′-ACTACCTTTTCGGAGCTGAGTATGA-3′ and 5′-CCACCCTAGCTGTGCCAATACT-3′; DXS (TC154799): 5′-CAGAACTAAGAGCAGAAATTGTGTATTCA-3′ and 5′-CCACAGTTAAATCCACAACACCTAAA-3′; HMGR (TC155027): 5′-TCCGGTGGCGCTACGA-3′ and 5′-TGCGCTGCCGAACCTAA-3′; HMGS (TC153567): 5′-CCTCTTCCCGAACTTGTAGGATT-3′ and 5′-CACCGACGACGTTTATTTCCTT-3′; SSTLE1 (TC162858): 5′-AGCAAACCTTAGAACAAACAAGCAA-3′ and 5′-CCAAACAGATGGGTGAAAATTAGC-3′; SSTLH1 (TC162148): 5′-GCAAACCTTAGAACAAACAAGCAATGG-3′ and 5′-TAATTGTCTCTTTGTACTCATCAACTTCAAC-3′; SSTLH3 (AW616373): 5′-GAACTCATCAACACAATCCAATGTC-3′ and 5′-ATAGCATGAAGATCACCAATCGAA-3′; MTS1 (TC166486): 5′-GTAACATAGGGATGATGATTGTCACCTT-3′ and 5′-CTGAACGCCTTGTGGTGGAAAT-3′; MTS1-like (AW617523): 5′-CCTCTCCACTGGACAGCCACTT-3′ and 5′-CCACATGGTAGGCTCGTAATTCC-3′; ACTIN (TC116322): 5′-AAATTGTCAGGGACGTGAAAGAA-3′ and 5′-TCTCAACAGAAGAGCTGGTCTTTG-3′. Each amplicon derived using these primers was cloned from S. lycopersicum and S. habrochaites cDNA and sequenced to confirm amplification of the target sequence.
The following primers were used to PCR amplify a diagnostic sequence for SSTLH3 (AW616373) from genomic DNA, which was extracted using standard methods: 5′-CTCCTTGTGAAAATGGAGTTGTGTA-3′ and 5′-CCTTGAAATTTCCTTGGTCATTAGT-3′. Amplicons derived from both tomato species were cloned and sequenced to verify amplification of the target.
Extraction and Analysis of Trichome Essential Oils
Trichome essential oils were extracted from target tissues by immersion of the tissue in chloroform for 2 to 3 h at room temperature. Alternatively, the trichomes were first separated by abrasion of frozen tissue before being added to the solvent (200 μL), which gave comparable terpenoid profiles to the previous method (data not shown). The resulting extracts were dried over Na2SO4 and filtered through Whatman paper before being concentrated, if necessary, to approximately 200 μL under nitrogen (a 5-fold concentration). The content of individual type VI glands was collected from leaflets with a capillary containing a small volume of solvent (50 μL) and compared with leaf extracts from the opposite leaflet of the same leaf. Tetradecane (10 ng μL−1; Fluka) was added to the solvent as an internal standard before extractions. For gas chromatography-mass spectrometry analyses of sesquiterpene carboxylic acids, a separate fraction of the chloroform extracts was derivatized in BSTFA-TMCS (99:1; Supelco) at 70°C for 1 h after complete drying of the original extracts under nitrogen. The amount of input material was determined either by weighing or, in the case of stems, by measuring their surface areas.
One to 2 μL of extract was used for gas chromatography-mass spectrometry (GCQplus; ThermoQuest Finnigan) or gas chromatography-flame ionization detection (GC8000top; ThermoQuest Finnigan). The essential oil constituents were separated on a ZB-1 column (30 m, 0.25 mm i.d., 0.50-μm film thickness; Zebron-Phenomenex) using the following temperature profile: 50°C to 250°C, 5°C min−1; 250°C to 320°C, 70°C min−1; 320°C, 3 min; 320°C to 50°C, 70°C min−1. Temperature of the injector was 250°C, that of the transfer line was 320°C, and that of the ion source was 200°C.
The essential oil constituents were identified by comparing their mass spectra with those of true standards whenever possible. When standards were not available, the mass spectra of the compounds were matched to published information (Coates et al., 1988; van der Hoeven et al., 2000). In the latter case, the Kovats index of the compounds was used in combination with spectral information (Adams, 2004). Sesquiterpene carboxylic acids were identified on the basis of their mass spectra, relative abundance, and similarity with cII-S hydrocarbons (Coates et al., 1988). Standard curves of α-pinene and α-humulene were used as a reference for the quantification of monoterpenes and sesquiterpenes, respectively, after normalization of peak areas to the internal standard and surface area or weight of input material.
Scanning Electron Microscopy Analysis
Stem segments were mounted on stubs and immersed in liquid nitrogen before examination on the cold stage of a Hitachi S-5000 scanning electron microscope.
Inhibitor Treatments and Radioactive Precursor Feeding
Treatments with fosmidomycin (Molecular Probes) and mevinolin (Sigma-Aldrich) were performed on 3-week-old S. lycopersicum seedlings or side shoots of mature S. habrochaites plants after severing them with a razor blade under water. Inhibitor or control solutions were replaced weekly. After 19 to 20 d, the youngest shoot tissue was harvested for metabolite analysis.
For radiolabeled precursor feeding experiments, cuttings were placed in 50 mm phosphate buffer (pH 7.2) to which 2.5 μCi of RS-[2-14C]mevalonic acid (dibenzyl ethylenediamine salt, specific activity of 67 mCi mmol−1; Amersham Bioscience) or [2-14C]pyruvic acid (sodium salt, specific activity of 16.4 mCi mmol−1; Perkin-Elmer) had been added, until the solution was taken up. The cuttings were then transferred to water (or inhibitor solutions) and incubated for 3 to 5.5 h at room temperature. When appropriate, cuttings were grown for 3 d in the presence of inhibitor (100 μm) before being fed radiolabeled substrates.
Labeled terpenoids were concentrated and run on TLC plates (SILGUR-25 UV254; Macherey-Nagel) using hexane:diethylether:formic acid (80:20:2, v/v/v) as the mobile phase. The plates were then exposed to phosphorimager screens, and incorporation into the different terpenoids was measured using Quantity One software (Bio-Rad).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Terpenoid accumulation in type VI trichomes of S. habrochaites LA1777.
Supplemental Figure S2. Functional classification of the 100 most highly represented genes in an EST collection derived from LA1777 trichomes.
Supplemental Figure S3. Developmental profile of terpenoid pathway gene expression in trichomes of S. lycopersicum and LA1777: repeat experiment.
Supplemental Figure S4. VIGS as a tool for the analysis of gene function in tomato species and trichomes.
Supplemental Figure S5. Effects of fosmidomycin applications on terpenoid profiles from cII-S-accumulating introgression line LA3935 (TA517) and S. lycopersicum ‘M82’.
Supplemental Figure S6. TLC analysis of LA1777 sesquiterpenes.
Supplemental Figure S7. Effects of variations in MEP and MVA pathway activity on terpenoid accumulation in S. lycopersicum ‘MicroTom’ trichomes.
Supplemental Figure S8. Evaluation of a putative role for SSTLH3 in cII-S production by genetic and gene expression analyses.
Supplemental Table S1. Unigenes represented in the LA1777 trichome EST library annotated according to the results of BLASTX searches against protein sequences deposited in GenBank.
Supplemental Table S2. Ct values for quantitative PCR amplifications for developmental profiles of terpenoid pathway gene expression in trichomes of S. lycopersicum (L) and S. habrochaites (H).
Supplemental Table S3. Terpenoid accumulation of cII-S-producing NILs of LA1777 introgressions in cv E6203 background.
Supplemental Table S4. Terpene synthase-like genes represented in the LA1777 trichome EST library.
Supplementary Material
Acknowledgments
We thank Meg Stark for help with scanning electron microscopy analysis, Tony Larson and Stuart Graham for assistance with metabolite profiling, and Savithramma Dinesh-Kumar and Sophia Ekengren for providing pTRV1 and pTRV2 vectors.
This work was supported by a grant from the Samuel Roberts Noble Foundation.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Pierre Broun (pierre.broun@rdto.nestle.com).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Adam KP, Thiel R, Zapp J (1999) Incorporation of 1-[1-(13)C]deoxy-D-xylulose in chamomile sesquiterpenes. Arch Biochem Biophys 369 127–132 [DOI] [PubMed] [Google Scholar]
- Adams RP (2004) Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Allured Publishing, Carol Stream, IL
- Akhila A, Sharma PK, Thakur RS (1986) A novel biosynthesis of irregular sesquiterpene artemone in Artemisia pallens. Tetrahedron Lett 27 5885–5888 [Google Scholar]
- Alberts AW, Chen J, Kuron G, Hunt V, Huff J, Hoffman C, Rothrock J, Lopez M, Joshua H, Harris E, et al (1980) Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci USA 77 3957–3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubourg S, Lecharny A, Bohlmann J (2002) Genomic analysis of the terpenoid synthase (AtTPS) gene family of Arabidopsis thaliana. Mol Genet Genomics 267 730–745 [DOI] [PubMed] [Google Scholar]
- Barlow AJ, Lorimer SD, Morgan ER, Weavers RT (2003) Biosynthesis of the sesquiterpene hodgsonox from the New Zealand liverwort Lepidolaena hodgsoniae. Phytochemistry 63 25–29 [DOI] [PubMed] [Google Scholar]
- Bartram S, Jux A, Gleixner G, Boland W (2006) Dynamic pathway allocation in early terpenoid biosynthesis of stress-induced lima bean leaves. Phytochemistry 67 1661–1672 [DOI] [PubMed] [Google Scholar]
- Bick JA, Lange BM (2003) Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: unidirectional transport of intermediates across the chloroplast envelope membrane. Arch Biochem Biophys 415 146–154 [DOI] [PubMed] [Google Scholar]
- Broun P, Poindexter P, Osborne E, Jiang CZ, Riechmann JL (2004) WIN1, a transcriptional activator of epidermal wax accumulation in Arabidopsis. Proc Natl Acad Sci USA 101 4706–4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch-Smith TM, Anderson JC, Martin GB, Dinesh-Kumar SP (2004) Applications and advantages of virus-induced gene silencing for gene function studies in plants. Plant J 39 734–746 [DOI] [PubMed] [Google Scholar]
- Chen JC, Jiang CZ, Gookin TE, Hunter DA, Clark DG, Reid MS (2004) Chalcone synthase as a reporter in virus-induced gene silencing studies of flower senescence. Plant Mol Biol 55 521–530 [DOI] [PubMed] [Google Scholar]
- Coates RM, Denissen JF, Juvik JA, Babka BA (1988) Identification of alpha-santalenoic and endo-beta bergamotenoic acids as moth oviposition stimulants from wild tomato leaves. J Org Chem 53 2186–2192 [Google Scholar]
- Colby SM, Crock J, Dowdle-Rizzo B, Lemaux PG, Croteau R (1998) Germacrene C synthase from Lycopersicon esculentum cv. VFNT cherry tomato: cDNA isolation, characterization, and bacterial expression of the multiple product sesquiterpene cyclase. Proc Natl Acad Sci USA 95 2216–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cseke L, Dudareva N, Pichersky E (1998) Structure and evolution of linalool synthase. Mol Biol Evol 15 1491–1498 [DOI] [PubMed] [Google Scholar]
- Cutler AJ, Krochko JE (1999) Formation and breakdown of ABA. Trends Plant Sci 4 472–478 [DOI] [PubMed] [Google Scholar]
- Disch A, Hemmerlin A, Bach TJ, Rohmer M (1998) Mevalonate-derived isopentenyl diphosphate is the biosynthetic precursor of ubiquinone prenyl side chain in tobacco BY-2 cells. Biochem J 331 615–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Andersson S, Orlova I, Gatto N, Reichelt M, Rhodes D, Boland W, Gershenzon J (2005) The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc Natl Acad Sci USA 102 933–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estévez JM, Cantero A, Romero C, Kawaide H, Jimenez LF, Kuzuyama T, Seto H, Kamiya Y, Leon P (2000) Analysis of the expression of CLA1, a gene that encodes the 1-deoxyxylulose 5-phosphate synthase of the 2-C-methyl-D-erythritol-4-phosphate pathway in Arabidopsis. Plant Physiol 124 95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügge U-I, Gao W (2005) Transport of isoprenoid intermediates across chloroplast envelope membranes. Plant Biol 7 91–97 [DOI] [PubMed] [Google Scholar]
- Frelichowski JE, Juvik JA (2001) Sesquiterpene carboxylic acids from a wild tomato species affect larval feeding behavior and survival of Helicoverpa zea and Spodoptera exigua (Lepidoptera: Noctuidae). J Econ Entomol 94 1249–1259 [DOI] [PubMed] [Google Scholar]
- Fridman E, Wang J, Iijima Y, Froehlich JE, Gang DR, Ohlrogge J, Pichersky E (2005) Metabolic, genomic, and biochemical analyses of glandular trichomes from the wild tomato species Lycopersicon hirsutum identify a key enzyme in the biosynthesis of methylketones. Plant Cell 17 1252–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfagna TJ, Carter CD, Sacalis JN (1992) Temperature and photoperiod influence trichome density and sesquiterpene content of Lycopersicon hirsutum f. hirsutum. Plant Physiol 100 1403–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel D, Mosandl A, Wust M (2005) Biosynthesis of mono- and sesquiterpenes in carrot roots and leaves (Daucus carota L.): metabolic cross talk of cytosolic mevalonate and plastidial methylerythritol phosphate pathways. Phytochemistry 66 305–311 [DOI] [PubMed] [Google Scholar]
- Hampel D, Mosandl A, Wust M (2006) Biosynthesis of mono- and sesquiterpenes in strawberry fruits and foliage: 2H labeling studies. J Agric Food Chem 54 1473–1478 [DOI] [PubMed] [Google Scholar]
- Hemmerlin A, Hoeffler JF, Meyer O, Tritsch D, Kagan IA, Grosdemange-Billiard C, Rohmer M, Bach TJ (2003) Cross-talk between the cytosolic mevalonate and the plastidial methylerythritol phosphate pathways in tobacco Bright Yellow-2 cells. J Biol Chem 278 26666–26676 [DOI] [PubMed] [Google Scholar]
- Iijima Y, Davidovich-Rikanati R, Fridman E, Gang DR, Bar E, Lewinsohn E, Pichersky E (2004) The biochemical and molecular basis for the divergent patterns in the biosynthesis of terpenes and phenylpropenes in the peltate glands of three cultivars of basil. Plant Physiol 136 3724–3736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jux A, Gleixner G, Boland W (2001) Classification of terpenoids according to the methylerythritolphosphate or the mevalonate pathway with natural 12C/13C isotope ratios: dynamic allocation of resources in induced plants. Angew Chem Int Ed 40 2091–2093 [DOI] [PubMed] [Google Scholar]
- Kampranis SC, Ioannidis D, Purvis A, Mahrez W, Ninga E, Katerelos NA, Anssour S, Dunwell JM, Degenhardt J, Makris AM, et al (2007) Rational conversion of substrate and product specificity in a Salvia monoterpene synthase: structural insights into the evolution of terpene synthase function. Plant Cell 19 1994–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange BM, Wildung MR, Stauber EJ, Sanchez C, Pouchnik D, Croteau R (2000) Probing essential oil biosynthesis and secretion by functional evaluation of expressed sequence tags from mint glandular trichomes. Proc Natl Acad Sci USA 97 2934–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laule O, Furholz A, Chang HS, Zhu T, Wang X, Heifetz PB, Gruissem W, Lange M (2003) Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 100 6866–6871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhao Y, McCaig BC, Wingerd BA, Wang J, Whalon ME, Pichersky E, Howe GA (2004) The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell 16 126–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK (1999) The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 50 47–65 [DOI] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Dinesh-Kumar SP (2002) Virus-induced gene silencing in tomato. Plant J 31 777–786 [DOI] [PubMed] [Google Scholar]
- Luckwill LC (1943) The genus Lycopersicon. Aberdeen University Studies 120 5–44 [Google Scholar]
- McCaskill D, Croteau R (1995) Monoterpene and sesquiterpene biosynthesis in glandular trichomes of peppermint (Mentha x piperita) rely exclusively on plastid-derived isopentenyl diphosphate. Planta 197 49–56 [Google Scholar]
- McCaskill D, Croteau R (1999) Strategies for bioengineering the development and metabolism of glandular tissues in plants. Nat Biotechnol 17 31–36 [DOI] [PubMed] [Google Scholar]
- McConkey ME, Gershenzon J, Croteau RB (2000) Developmental regulation of monoterpene biosynthesis in the glandular trichomes of peppermint. Plant Physiol 122 215–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monforte AJ, Tanksley SD (2000) Development of a set of near isogenic and backcross recombinant inbred lines containing most of the Lycopersicon hirsutum genome in a L. esculentum genetic background: a tool for gene mapping and gene discovery. Genome 43 803–813 [PubMed] [Google Scholar]
- Mueller C, Schwender J, Zeidler J, Lichtenthaler HK (2000) Properties and inhibition of the first two enzymes of the non-mevalonate pathway of isoprenoid biosynthesis. Biochem Soc Trans 28 792–793 [PubMed] [Google Scholar]
- Newman JD, Chappell J (1999) Isoprenoid biosynthesis in plants: carbon partitioning within the cytoplasmic pathway. Crit Rev Biochem Mol Biol 34 95–106 [DOI] [PubMed] [Google Scholar]
- Page JE, Hause G, Raschke M, Gao W, Schmidt J, Zenk MH, Kutchan TM (2004) Functional analysis of the final steps of the 1-deoxy-D-xylulose 5-phosphate (DXP) pathway to isoprenoids in plants using virus-induced gene silencing. Plant Physiol 134 1401–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JA, Kim TW, Kim SK, Kim WT, Pai HS (2005) Silencing of NbECR encoding a putative enoyl-CoA reductase results in disorganized membrane structures and epidermal cell ablation in Nicotiana benthamiana. FEBS Lett 579 4459–4464 [DOI] [PubMed] [Google Scholar]
- Piel J, Donath J, Bandemer K, Boland W (1998) Mevalonate-independent biosynthesis of terpenoid volatiles in plants: induced and constitutive emission of volatiles. Angew Chem Int Ed 37 2478–2481 [DOI] [PubMed] [Google Scholar]
- Robertson D (2004) VIGS vectors for gene silencing: many targets, many tools. Annu Rev Plant Biol 55 495–519 [DOI] [PubMed] [Google Scholar]
- Steliopoulos P, Wüst M, Adam K-P, Mosandl A (2002) Biosynthesis of sesquiterpene germacrene D in Solidago canadensis: 13C and 2H labeling studies. Phytochemistry 60 13–20 [DOI] [PubMed] [Google Scholar]
- van der Hoeven RS, Monforte AJ, Breeden D, Tanksley SD, Steffens JC (2000) Genetic control and evolution of sesquiterpene biosynthesis in Lycopersicon esculentum and L. hirsutum. Plant Cell 12 2283–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Klink J, Becker H, Andersson S, Boland W (2003) Biosynthesis of anthecotuloide, an irregular sesquiterpene lactone from Anthemis cotula L. (Asteraceae) via a non-farnesyl diphosphate route. Org Biomol Chem 1 1503–1508 [DOI] [PubMed] [Google Scholar]
- van Schie CCN, Ament K, Schmidt A, Lange T, Haring MA, Schuurink RC (2007) Geranyl diphosphate synthase is required for biosynthesis of gibberellins. Plant J 52 752–762 [DOI] [PubMed] [Google Scholar]
- Wagner GJ (1991) Secreting glandular trichomes: more than just hairs. Plant Physiol 96 675–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker E (2000) Trichome diversity and development. In DL Hallahan, JC Gray, eds, Advances in Botanical Research. Elsevier Science, Amsterdam, pp 1–35
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.