SUMMARY
The function of the CLN3 protein, which is mutated in patients with the neurodegenerative lysosomal storage disorder Batten disease, has remained elusive since it was identified 13 years ago. Here, we exploited the Schizosaccharomyces pombe model to gain new insights into CLN3 function. We modelled all missense mutations of CLN3 in the orthologous protein Btn1p, as well as a series of targeted mutations, and assessed trafficking and the ability of the mutant proteins to rescue four distinct phenotypes of btn1Δ cells. Mutating the C-terminal cysteine residues of Btn1p caused it to be internalised into the vacuole, providing further evidence that this protein functions from pre-vacuole compartments. Mutations in the lumenal regions of the multi-spanning membrane protein, especially in the third lumenal domain which contains a predicted amphipathic helix, had the most significant impact on Btn1p function, indicating that these domains of CLN3 are functionally important. Only one mutant protein was able to rescue the cell curving phenotype (p.Glu295Lys), and since this mutation is associated with a very protracted disease progression, this phenotype could be used to predict the disease severity of novel mutations in CLN3. The ability to predict disease phenotypes in S. pombe confirms this yeast as an invaluable tool to understanding Batten disease.
INTRODUCTION
The Schizosaccharomyces pombe protein Btn1p has recently been shown to be required for multiple processes, including endocytic/vacuole homeostasis, cell wall structure and deposition, and polarised cell growth (Gachet et al., 2005; Codlin et al., 2008a; Codlin et al., 2008b). Btn1p is the orthologue of CLN3, mutations in which underlie the neurodegenerative disease juvenile neuronal ceroid lipofuscinosis (JNCL) (The International Batten Disease Consortium, 1995). Despite much effort since its identification in 1995, the function of CLN3 has remained elusive, although it too has been implicated in multiple roles, including lysosome homeostasis (Holopainen et al., 2001; Ramirez-Montealegre and Pearce, 2005; Pohl et al., 2007), autophagy (Cao et al., 2006), cytoskeletal organisation (Luiro et al., 2004; Luiro et al., 2006) and lipid synthesis or modification (Narayan et al., 2006; Hobert and Dawson, 2007). Work in other organisms, especially Saccharomyces cerevisae, also supports a role for CLN3/Btn1p in vacuole pH homeostasis (Pearce and Sherman, 1998; Pearce et al., 1999a; Pearce et al., 1999b; Pearce and Sherman, 1999; Chattopadhyay et al., 2003; Kim et al., 2003; Padilla-Lopez and Pearce, 2006). Significantly, expression of CLN3 restored phenotypes arising from deletion of btn1 in these organisms, indicating that these proteins exert a similar basic function. However, the molecular basis for the function of any of these proteins remains unknown.
JNCL, or Batten disease, is an autosomal recessive lysosomal storage disorder. Patients typically present with visual failure between 4-7 years of age, followed by epilepsy and progressive mental and physical deterioration, with premature death usually occurring in the third decade (Siintola et al., 2006). The most common mutation causing JNCL is an intragenic deletion of about 1 kb in CLN3 (which removes exons 7 and 8), which we have recently shown is not a null mutation, as had been assumed previously (Kitzmüller et al., 2008). Rather, this mutation permits some residual function that maintains lysosome size. Some patients carry a missense mutation in heterozygosity with this common deletion; these combinations have been linked with altered disease progression (Munroe et al., 1997b; Lauronen et al., 1999). All amino acid residues associated with disease-causing missense mutations are conserved across all species for which the sequence is available (for example, see supplementary material Fig. S1); this is consistent with these residues being crucial to protein function. The second most common mutation causing JNCL is another, larger, deletion of about 2.8 kb, which removes exons 10–13 (The International Batten Disease Consortium, 1995; Munroe et al., 1997a). The effect of this mutation on CLN3 function is not known.
A six transmembrane structure for CLN3 has been demonstrated experimentally and computationally (Ezaki et al., 2003; Mao et al., 2003; Kyttala et al., 2004), and recently we predicted a conserved amphipathic helix in the third lumenal loop (Nugent et al., 2008) that may be crucial for function, since four missense mutations are located in this region. The C-terminus of CLN3 has a CAAX farnesylation site (Cys435), which is of importance because farnesylation is required for correct trafficking (Pullarkat and Morris, 1997; Storch et al., 2007). This cysteine residue is conserved (supplementary material Fig. S1) but is only part of a CAAX motif (Zhang and Casey, 1996) in mammalian sequences. S. pombe Btn1p has an additional Cys residue at position 393 that might also be farnesylated.
Since mutating residues in human CLN3 results in disease and, therefore, a ‘faulty’ protein, we hypothesised that such residues might be crucial to CLN3 and Btn1p function. Previously, we modelled some disease-causing mutations of CLN3 in Btn1p and found that they altered the trafficking and/or function of Btn1p (Gachet et al., 2005; Codlin et al., 2008b). In this study, we modelled the full complement of disease-causing mutations in order to build up a complete picture of the impact of mutations on protein function. To exploit the S. pombe model effectively, we required distinct phenotypes associated with the deletion of btn1 that could be assayed easily. In the absence of btn1, S. pombe cells have larger vacuoles, which are also less acidic, than those in wild-type cells. Cells with the btn1 deletion are also delayed in the final stages of cytokinesis, and more cells are in the process of septation compared with wild-type cells (Codlin et al., 2008b). The cytokinesis delay is more severe at 37°C, when growth of btn1Δ cells is temperature-sensitive. Following 18 hours of growth at 37°C, btn1Δ cells are swollen or lysed owing to total loss of bipolar growth. Prior to this, after 7 hours of growth at 37°C, btn1Δ cells fail to initiate bipolar growth and remain monopolar for growth (Codlin et al., 2008a; Codlin et al., 2008b). Additionally, we report here that after 4 hours of growth at the non-permissive temperature, btn1Δ cells lose their rod-shaped morphology and become bent or curved. Therefore, we chose the following four readily-assayable marker phenotypes of btn1Δ cells: (1) increased vacuole size, (2) septation index during normal growth conditions, (3) cell curving and (4) monopolar growth at 37°C. Importantly, ectopic expression of Btn1p or CLN3 can rescue all of these phenotypes in btn1Δ cells. In these phenotypic assays, we carried out a comprehensive analysis of the impact of disease-causing and targeted mutations on the trafficking and function of Btn1p, in order to improve our understanding of the function of this elusive protein. Our results further establish this fission yeast as an excellent model system to understand the underlying role of CLN3.
RESULTS
Construction of a panel of mutant proteins
We generated a set of plasmids in which btn1 was mutated to model all reported disease-causing missense mutations of CLN3. In order to study the localisation and function of the mutant proteins, they were placed under the transcriptional control of the thiamine-repressible nmt promoter in the pREP42GFP plasmid. We also generated a series of targeted mutations in Btn1p: Cys382 (the conserved cysteine residue that is equivalent to the farnesylation site in CLN3, Cys435) and Cys393 (the putative farnesylation site in Btn1p) were both changed to serines, and Asn23, equivalent to Asn48 in CLN3, was changed to phenylalanine in order to observe the effect of disrupting a conserved region of the protein that is not known to be mutated in disease. We had previously modelled the common 1 kb deletion (Kitzmüller et al., 2008) and, in addition, we modelled the second most common mutation of CLN3, a 2.8 kb deletion which results in the loss of exons 10–15, by inserting a base after base 621, thereby generating a frameshift and a premature stop codon (GFP-Btn1p207fsX6). In summary, these expression constructs cover residues that correspond to most of the significant conservation regions between Btn1p, CLN3 and orthologues in other species (supplementary material Fig. S1).
Establishing a set of marker phenotypes
We selected four distinct marker phenotypes to assess the impact of the various mutations on Btn1p function. Three of these, increased vacuole size, cytokinesis delay at the permissive temperature and loss of bipolar growth after 7 hours of growth at 37°C, have been reported previously (Fig. 1B–D; Table 1) (Gachet et al., 2005; Codlin et al., 2008a). The fourth marker phenotype, cell curving, we report here for the first time. During growth at high temperature, btn1Δ cells lose their rod-shaped morphology and become bent or curved, with 60% being curved by 4 hours at 37°C (Fig. 1E), before the additional loss of bipolar growth. All four phenotypes can be fully rescued by ectopic expression of green fluorescent protein (GFP)-tagged Btn1p (GFP-Btn1p), and fully or partially rescued by expression of GFP-CLN3 (Fig. 1B–E), consistent with the conserved function of these proteins. We used these assays to investigate the phenotype of btn1Δ cells that ectopically expressed the mutant constructs described above, compared with btn1Δ cells expressing GFP alone. Since the location of Btn1p is tightly controlled (Gachet et al., 2005; Codlin et al., 2008a), we also investigated the location of the GFP-tagged mutant proteins in live cells at steady state and following promoter repression. We had shown previously that GFP-Btn1p expressed in this way is present in pre-vacuolar compartments at steady state, and traffics to the vacuole within 3 hours of promoter repression (Fig. 1F) (Gachet et al., 2005).
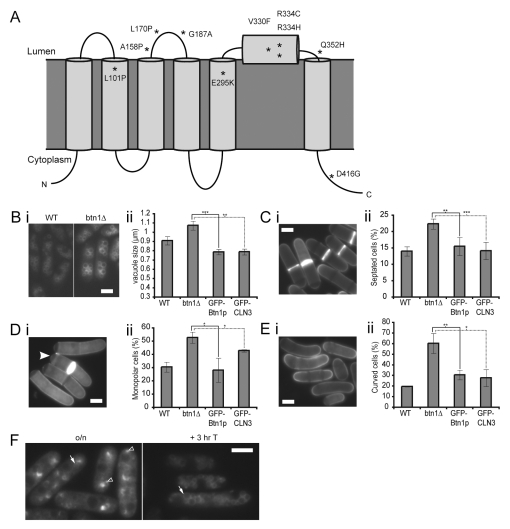
Fig. 1.
btn1Δ cells have multiple phenotypes which can be rescued by ectopic expression of Btn1p or CLN3.(A) Predicted topology of CLN3, based on Nugent et al., 2008. Missense mutations are marked with asterisks. (B) btn1Δ cells have larger vacuoles than wild-type cells: (i) vacuoles of wild-type (left panel) and btn1Δ (right panel) cells stained with FM4-64, (ii) bar chart of the mean vacuole size of the indicated cells. (C) btn1Δ cells have a higher septation index than wild-type cells: (i) btn1Δ cells stained with calcofluor to visualise cell walls and septa, (ii) bar chart of the percentage of the indicated cells with a visible septum. (D) btn1Δ cells have a defect in the initiation of bipolar growth after 7 hours at 37°C: (i) btn1Δ cells stained with calcofluor to show growing ends, the filled arrowhead indicates a monopolar cell with calcofluor staining absent at one pole, (ii) bar chart of septated cells with monopolar calcofluor staining. (E) btn1Δ cells are curved by 4 hours at 37°C: (i) btn1Δ cells stained with calcofluor, (ii) bar chart of the percentage of bent or curved cells. (F) Localisation of GFP-Btn1p in btn1Δ cells. Localisation of GFP-Btn1p after overnight expression (left panel) and 3 hours after promoter repression (right panel). Arrow=vacuole, filled arrowhead=endoplasmic reticulum (ER), unfilled arrowhead=pre-vacuolar compartment. (Data shown is the mean±s.d. of at least three independent experiments; ***P<0.001, **P<0.01, *P<0.05.) Bars, 5 μm.
Table 1.
Summary of mutants and phenotypes
| Human CLN3 | S. pombe Btn1p | Vacuole size | Septation | Monopolarity | Curving |
|---|---|---|---|---|---|
| – | Btn1p | +++ | +++ | +++ | +++ |
| CLN3 | – | +++ | +++ | + | +++ |
| Asn48Phe | Asn23Phe (N23F) | +++ | +++ | +++ | +++ |
| Leu101Pro | Leu48Pro (L48P) | ++ | +++ | +++ | − |
| Ala158Pro | Ala106Pro (A106P) | − | + | ++ | − |
| Leu170Pro | Leu118Pro (L118P) | + | + | − | − |
| Gly187Ala | Gly136Ala (G136A) | − | − | +/− | − |
| Glu295Lys | Glu240Lys (E240K) | − | − | − | +++ |
| Val330Phe | Val278Phe (V278F) | − | ++ | − | − |
| Arg334Cys | Arg282Cys (R282C) | − | +++ | +/− | − |
| Arg334His | Arg282His (R282H) | − | ++ | +++ | − |
| Gln352His | Gln300His (Q300H) | − | − | ++ | − |
| Asp416Gly | Asp363Gly (D363G) | +++ | ++ | +++ | − |
| Cys435Ser | Cys382Ser (C382S) | +++ | ++ | +++ | + |
| – | Cys393Ser (C393S) | +++ | ++ | +++ | +++ |
| 1 kb deletion | Btn1p102fsX5 | +++ | − | − | − |
| 1 kb alt. splice | Btn1p102-208del | +++ | +/− | − | − |
| 2.8 kb deletion | Btn1p207fsX6 | +++ | + | − | − |
This table includes all human CLN3 and corresponding S. pombe Btn1p residues mutated in this study; those in bold are associated with JNCL. The ability of these tagged mutant proteins to rescue phenotypes of btn1Δ cells is summarised.
+++=phenotype indistinguishable from Btn1p; ++=significant rescue, but phenotype remains different from Btn1p, + =significant rescue, but less than ++; +/–=small reduction in severity compared with btn1Δ cells plus GFP alone, but still significantly different to Btn1p; –=no rescue.
Altering residues in the transmembrane domains has varying effects on function
Three of the mutations modelled for this study are located in the predicted transmembrane segments 1, 2 and 5: GFP-Btn1pN23F, GFP-Btn1pL48P and GFP-Btn1pE240K (corresponding to Asn48, Leu101 and Glu295, respectively, in CLN3) (Fig. 1A) (Nugent et al., 2008). Expression of these mutants in btn1Δ cells revealed that they localised to pre-vacuolar compartments at steady state, and that all are slightly delayed in trafficking compared with GFP-Btn1p (Fig. 2A). In particular, GFP-Btn1pL48P still shows some staining of the endoplasmic reticulum (ER) at 3 hours after promoter repression. The GFP-Btn1pN23F mutant, a targeted mutation not associated with disease, is otherwise indistinguishable in function from the wild-type protein (Fig. 2B–E).
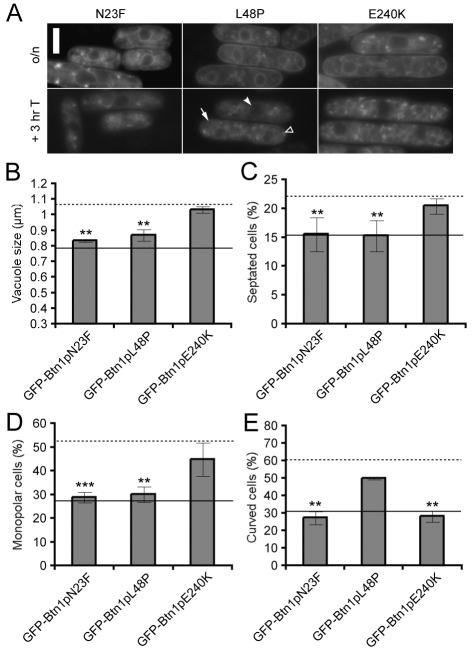
Fig. 2.
Mutation of residues in predicted transmembrane domains has varying effects on function.(A) Localisation of GFP-tagged mutants at steady state (upper panels) and after promoter repression (lower panels). Arrow=vacuole, filled arrowhead=ER, unfilled arrowhead=pre-vacuolar compartment. (B-E) Phenotypes of btn1Δ cells expressing the indicated mutant GFP-Btn1p proteins: (B) mean vacuole diameter (μm); (C) mean septation index (% of total cells); (D) mean percentage of monopolar cells (% of total septated cells after 7 hours at 37°C); and (E) mean percentage of curved or bent cells (% of total cells after 4 hours at 37°C). Dotted line=mean value for btn1Δ cells, unbroken line=mean value for btn1Δ cells + GFP-Btn1p. (Data shown is the mean±s.d. of at least three independent experiments; ***P<0.001, **P<0.01.) Bar, 5 μm.
GFP-Btn1pL48P and GFP-Btn1pE240K, which model disease-causing missense mutations of CLN3, had opposing phenotypes in all assays. Expression of GFP-Btn1pL48P rescued the increased vacuole size (Fig. 2B), the septation index (Fig. 2C) and the bipolar growth defect at 37°C (Fig. 2D), but did not significantly reduce the number of cells that were bent or curved by 4 hours at the non-permissive temperature (Fig. 2E). In contrast, btn1Δ cells expressing GFP-Btn1pE240K had large vacuoles and did not have significantly fewer septated or monopolar cells (Fig. 2B–D). However, expression of this mutant did rescue the curving defect of btn1Δ cells – the only disease-causing missense mutant to do so (Fig. 2E). We have reported previously that btn1Δ cells are longer than wild-type cells (Gachet et al., 2005), although this phenotype was not a focus for this study. Patients carrying the mutation that corresponds to S. pombe Btn1pE240K (p.Glu295Lys), in heterozygosity with the 1 kb deletion, have a very mild phenotype and protracted disease progression (Åberg et al., 2008), so the ability of this mutant to rescue certain phenotypes might reflect significant retention of function.
The second lumenal loop is crucial for CLN3 function
The second lumenal region of CLN3 contains a highly conserved sequence of amino acids (supplementary material Fig. S1), which includes three disease-causing mutations, p.Ala158Pro, p.Leu170Pro and p.Gly187Ala (Fig. 1A). For two of the mutant proteins modelling these mutations, GFP-Btn1pA106P and GFP-Btn1pG136A, exit of the GFP-tagged protein from the ER was prevented (Fig. 3A), suggesting that these mutations disrupt protein folding, perhaps resulting in degradation. The other mutant protein, GFP-Btn1pL118P, showed some ER staining at steady state, but it did reach the vacuole, although pre-vacuole and ER staining was still visible 3 hours after promoter repression.
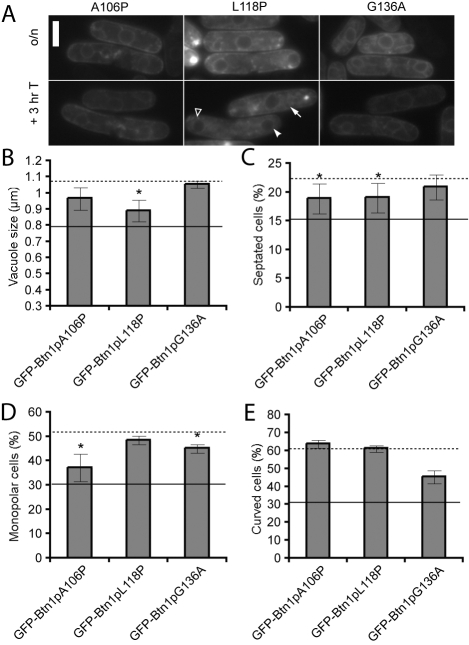
Fig. 3.
Mutations in the second lumenal region impair the trafficking and function of Btn1p.(A) Localisation of GFP-tagged mutants at steady state (upper panels) and after promoter repression (lower panels). Arrow=vacuole, filled arrowhead=ER, unfilled arrowhead=pre-vacuolar compartment. (B-E) Phenotypes of btn1Δ cells expressing the indicated mutant GFP-Btn1p proteins: (B) mean vacuole diameter (μm); (C) mean septation index (% of total cells); (D) mean percentage of monopolar cells (% of total septated cells after 7 hours at 37°C); and (E) mean percentage of curved or bent cells (% of total cells after 4 hours at 37°C). Dotted line=mean value for btn1Δ cells, unbroken line=mean value for btn1Δ cells + GFP-Btn1p. (Data shown is the mean±s.d. of at least three independent experiments; *P<0.05.) Bar, 5 μm.
In addition to the altered location of these mutants, none was able to rescue fully any of the marker phenotypes. GFP-Btn1pL118P partially rescued the vacuole size defect (Fig. 3B) and the number of septated cells (Fig. 3C), whereas GFP-Btn1pA106P reduced the number of septated cells and monopolar cells at 7 hours at 37°C (Fig. 3D). None of these mutants had any impact on the number of curved cells at 37°C (Fig. 3E). Taken together, these results indicate that the second lumenal region of CLN3 is crucial for function.
Mutating the amphipathic helix has a significant effect on Btn1p function
Four missense mutations are located in the third lumenal region, which contains a putative amphipathic helix (Nugent et al., 2008). Three of these mutations, p.Val330Phe, p.Arg334Cys and p.Arg334His, are on the predicted lumenal face of this helix, whereas the fourth, p.Gln352His, is situated at the boundary between the lumenal region and the transmembrane domain (Fig. 1A). All of the mutant proteins modelling these mutations trafficked to the vacuole (Fig. 4A), although in the case of GFP-Btn1pQ300H some GFP signal was still visible in the ER after 3 hours.
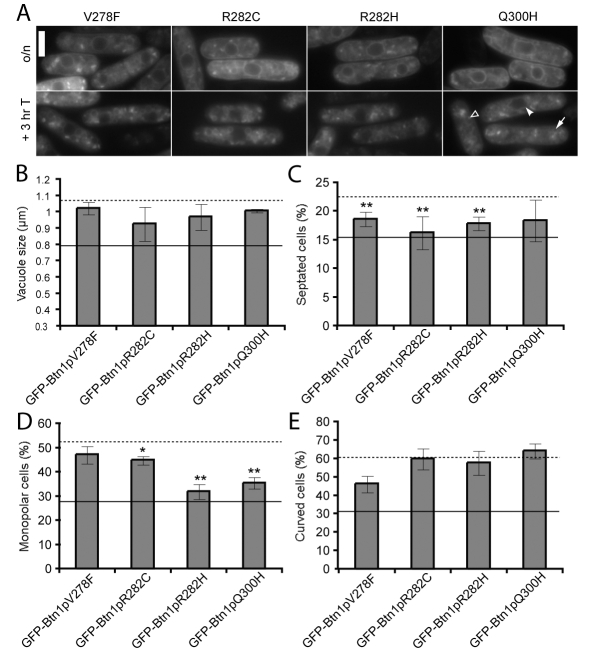
Fig. 4.
Mutations in the third lumenal loop, including the predicted amphipathic helix, impair most functions of Btn1p. (A) Localisation of GFP-tagged mutants at steady state (upper panels) and after promoter repression (lower panels). Arrow=vacuole, filled arrowhead=ER, unfilled arrowhead=pre-vacuolar compartment. (B-E) Phenotypes of btn1Δ cells expressing the indicated mutant GFP-Btn1p proteins: (B) mean vacuole diameter (μm); (C) mean septation index (% of total cells); (D) mean percentage of monopolar cells (% of total septated cells after 7 hours at 37°C); and (E) mean percentage of curved or bent cells (% of total cells after 4 hours at 37°C). Dotted line=mean value for btn1Δ cells, unbroken line=mean value for btn1Δ cells + GFP-Btn1p. (Data shown is the mean±s.d. of at least three independent experiments; ** P<0.01, *P<0.05.) Bar, 5 μm.
All proteins mutated in this region were defective in function at both 25°C and 37°C, since none rescued the vacuole size defect (Fig. 4B) or the number of curved cells (Fig. 4E). However, the GFP-Btn1pV278F, GFP-Btn1pR282C and GFP-Btn1pR282H mutant proteins (i.e. the three proteins with mutated residues in the predicted amphipathic helix) partially rescued the septation defect of btn1Δ cells, whereas GFP-Btn1pQ300H, which is outside the amphipathic helix, did not significantly reduce the number of septated cells (Fig. 4C). There were also differences in the function of these mutants at the restrictive temperature. Expression of GFP-Btn1pR282H or GFP-Btn1pQ300H in btn1Δ cells rescued the bipolar growth defect. In contrast, GFP-Btn1pV278F and GFP-Btn1pR282C mutants did not cause a significant reduction in monopolar cells compared with GFP alone (Fig. 4D). Therefore, mutating the putative amphipathic helix, or the adjacent region, has a significant impact on function.
The C-terminal residues of CLN3/Btn1p are crucial for correct trafficking and function
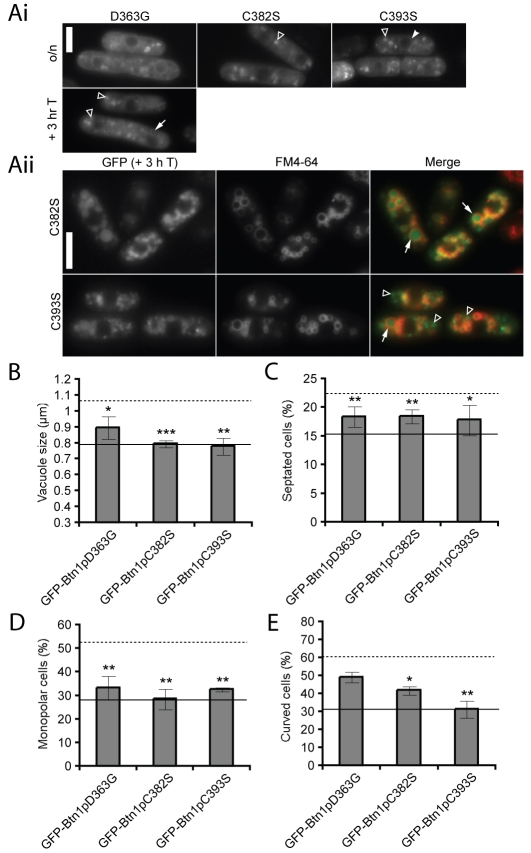
Three of the mutations are in the cytoplasmic C-terminal tail of Btn1p: the disease-causing missense mutation p.Asp363Gly (p.Asp416Gly in CLN3) and two targeted cysteine residues, p.Cys382Ser (p.Cys435Ser in CLN3) and p.Cys393Ser (the additional cysteine in Btn1p that could be farnesylated) (supplementary material Fig. S1). The location of the p.Asp416Gly mutation in CLN3, in the middle of the atypical lysosomal targeting motif 409Met(X)9Gly419 (Kyttala et al., 2004), suggests that it might affect the location or trafficking of the protein. However, the previously identified lysosomal targeting motifs in CLN3 are not conserved in Btn1p (supplementary material Fig. S1). The equivalent mutant, GFP-Btn1pD363G, did traffic to the vacuole, albeit slightly more slowly than the wild-type protein, with GFP fluorescence remaining in pre-vacuole structures, even 3 hours after promoter repression (Fig. 5A). The trafficking of the GFP-Btn1pC382S and GFP-Btn1pC393S mutants was indistinguishable from GFP-Btn1p at steady state, but was strikingly altered 3 hours after promoter shut-off, with the GFP signal inside the vacuole (Fig. 5A) rather than on the perimeter membrane. This was particularly apparent for GFP-Btn1pC382S, since almost all the GFP signal was within the vacuole, whereas at least some pre-vacuolar staining was still visible for GFP-Btn1pC393S at this time point.
Fig. 5.
Mutations in the C-terminal domain alter the trafficking of Btn1p, but have varying effects on protein function. (A) (i) Localisation of GFP-tagged mutants at steady state (upper panels) and after promoter repression for GFP-Btn1pD363G (lower panel); (ii) GFP-Btn1pC382S and GFP-Btn1pC393S were present inside the vacuole after 3 hours of promoter repression. Cells were co-labelled with FM4-64 to stain the vacuole perimeter. GFP-Btn1pC393S was also visible in pre-vacuolar compartments that were not labelled with FM4-64. Arrow=vacuole, filled arrowhead=ER, unfilled arrowhead=pre-vacuolar compartment. (B-E) Phenotypes of btn1Δ cells expressing the indicated mutant GFP-Btn1p proteins: (B) mean vacuole diameter (μm); (C) mean septation index (% of total cells); (D) mean percentage of monopolar cells (% of total septated cells after 7 hours at 37°C); and (E) mean percentage of curved or bent cells (% of total cells after 4 hours at 37°C). Dotted line=mean value for btn1Δ cells, unbroken line=mean value for btn1Δ cells + GFP-Btn1p. (Data shown is the mean±s.d. of at least three independent experiments; ***P<0.001, **P<0.01, *P<0.05.) Bar, 5 μm.
All three of these mutants rescued the vacuole size defect of btn1Δ cells as effectively as GFP-Btn1p (Fig. 5B). In addition, expression of any of these mutants in btn1Δ cells resulted in significant rescue of the septation defect (Fig. 5C) and of the bipolar growth defect observed after 7 hours at 37°C (Fig. 5D). However, btn1Δ cells expressing GFP-Btn1pD363G still exhibited a curving defect after 4 hours of growth at 37°C (Fig. 5E). Significantly, we observed differences in the function of the GFP-Btn1pC382S and GFP-Btn1pC393S (non-disease causing) mutants: expression of GFP-Btn1pC393S reduced the number of curved cells to a greater degree than GFP-Btn1pC382S (Fig. 5E). This suggests that the conserved cysteine Cys382 (supplementary material Fig. S1) is crucial for function, as well as trafficking, and further investigation of the importance of this residue, particularly with regard to its reported post-translation modification, is necessary.
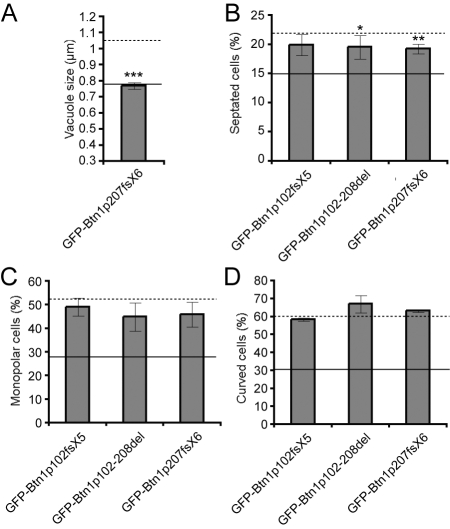
The most common CLN3 mutant proteins retain some function
Expression of Btn1p mutants modelling the CLN3 gene transcripts that are present in patients who are homozygous for the 1 kb deletion (GFP-Btn1p102fsX5 and GFP-Btn1p102-208del) was sufficient to rescue the vacuole size defect observed in btn1Δ cells (Kitzmüller et al., 2008). Expression of GFP-Btn1p207fsX6, which models the transcript predicted from the 2.8 kb deletion, rescued the vacuole size defect as effectively as the full-length protein (Fig. 6A). However, none of these mutants was sufficient to completely rescue the other phenotypes (Fig. 6B–D), although both GFP-Btn1p102-208del and GFP-Btn1p207fsX6 reduced the number of septated cells at 25°C. This suggests that these constructs retained some additional function compared with GFP-Btn1p102fsX5.
Fig. 6.
The most common mutations of CLN3 can rescue the vacuole size defect, but have minimal effects on other phenotypes. (A-D) Phenotypes of btn1Δ cells expressing the indicated mutant GFP-Btn1p proteins: (A) mean vacuole diameter (μm); (B) mean septation index (% of total cells); (C) mean percentage of monopolar cells (% of total septated cells after 7 hours at 37°C); (D) mean percentage of curved or bent cells (% of total cells after 4 hours at 37°C). Dotted line=mean value for btn1Δ cells, unbroken line=mean value for btn1Δ cells + GFP-Btn1p. (Data shown is the mean±s.d. of at least three independent experiments; ***P<0.001, **P<0.01, *P<0.05.)
DISCUSSION
The multiple phenotypes of cells with the btn1 deletion suggest that Btn1p, and CLN3, have a complex function, affecting several cellular pathways. We used sequence homology between CLN3 and Btn1p, as well as information from disease-causing mutations of CLN3, to suggest residues and regions that could be important for function. This study showed that disease-causing mutations can have quite different effects on Btn1p function or location, and confirmed fission yeast as an accurate model for predicting the severity of disease caused by mutations in CLN3. Mutations in the lumenal regions, including those in the conserved and recently described amphipathic helix (Nugent et al., 2008), had the most significant impact on function, suggesting a lumenal function for CLN3. Furthermore, evidence that Btn1p requires a pre-vacuolar location for correct function was provided by mutating C-terminal cysteine residues. These mutants further demonstrated the importance of these residues to protein function. Finally we identified a phenotype, cell curving, that is rescued by a mutation associated with very protracted disease progression, p.Glu295Lys. This phenotype could be used to predict the disease severity of novel mutations in CLN3.
It is clear that the subcellular location of Btn1p is functionally important. Previous work has shown that Btn1p traffics slowly to the vacuole through the endomembrane system, as does CLN3 (Storch et al., 2007), and can rescue some phenotypes from pre-vacuolar compartments (Gachet et al., 2005; Codlin et al., 2008a) (S.C. and S.E.M., unpublished). Mutation of either of the C-terminal cysteines of Btn1p (Cys382, which is conserved in CLN3, or Cys393) caused the GFP signal to be internalised into the vacuole, probably by inward vesiculation. The continued presence of some GFP-Btn1pC393S in pre-vacuolar compartments correlated with greater retention of function by this mutant compared with GFP-Btn1pC382S, confirming previous results which suggested that a pre-vacuolar location for Btn1p is important for function (Gachet et al., 2005). Furthermore, this result suggests that the C-terminal cysteine residue(s) might be involved in controlling Btn1p activity, with the internalisation of Btn1p into the vacuole being a method of ‘switching off’ the protein. Studies on CLN3 had also shown that residues in the C-terminal tail were crucial for correct trafficking (Storch et al., 2004; Kyttala et al., 2005; Storch et al., 2007). In particular, inhibition of farnesylation of Cys435 causes more CLN3 to be located on the plasma membrane (Storch et al., 2007). This fission yeast study has confirmed the importance of the conserved cysteine residue in both the localisation and function of these proteins, and revealed a potential mechanism by which post-translational modification of this residue might control protein function.
Patients carrying p.Glu295Lys have the mildest phenotype associated with a CLN3 mutation, with onset of blindness at the normal age, but few or no other symptoms until the third or fourth decade of life (Munroe et al., 1997b; Wisniewski et al., 1998; Åberg et al., 2008). GFP-Btn1pE240K was the only disease-associated mutant that rescued the curving defect. Understanding the molecular basis of this defect might reveal a novel target for therapies to delay onset of symptoms in patients with classic JNCL. This approach would augment more conventional strategies for therapy development using mouse models (Cooper, 2008; Kovacs and Pearce, 2008).
Recently, we reported that classic JNCL is a mutation-specific phenotype, and that the most common mutation, a 1 kb intragenic deletion, retains significant function (Kitzmüller et al., 2008). Expression of Btn1p truncation mutants that modelled the common deletion of CLN3 was sufficient to rescue the vacuole size defect, but not the other defects assayed here (Table 1). As most disease-causing missense mutations (with the exception of p.Asp416Gly) occur in heterozygosity with the 1 kb deletion, these missense mutations are functioning against a background of partial CLN3 function in patients. Future therapeutic strategies may need to take into account the CLN3 genotype of individual patients so that the appropriate molecular pathways can be targeted. To predict this combinatorial effect, it will be important to model the phenotypic effects of all CLN3 mutations.
In conclusion, we used a fission yeast model to assay the involvement of Btn1p in multiple pathways, and demonstrated the use of this yeast as an accurate model for predicting disease severity. The importance of the conserved cysteine (Cys435 in CLN3) was confirmed and extended, suggesting a potential role in regulating protein function. This study also showed that the lumenal face of CLN3 is functionally important, and future investigation of this protein should consider its lumenal role. Significantly, we highlighted that strategies to rescue the curving defect of btn1Δ cells might ultimately provide therapy to significantly slow down the cognitive and motor defects associated with JNCL.
METHODS
S. pombe strains and cell growth
Strains used in this study are listed in Table 2. The btn1Δ strain used in this study is SC2A (Gachet et al., 2005). Media, growth and maintenance of strains were as described (Moreno et al., 1991; Gachet et al., 2005). Cells were grown in minimal medium (MM) containing appropriate supplements. For protein expression experiments, cells were grown overnight in MM plus thiamine (4 μM), which inhibits expression from the nmt promoter. Cells were then washed three times in MM lacking thiamine and then grown in the same medium to allow expression, and visualised after 18 hours. For experiments at 37°C, cells were prepared as above (i.e. grown in media without thiamine for 18 hours), then transferred to 37°C for the appropriate period of time. For gene repression studies, following overnight expression, plasmids were repressed by the addition of thiamine (final concentration of 4 μm) and the cells were then grown for a further 3 hours before visualisation.
Table 2.
Strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| 972 (WT) | h– | Laboratory stock |
| SC2A (YG660 +pREP42GFP) | h+, btn1::leu2, ura4-D18, leu1-32, his2 | Laboratory stock |
| SC5A (YG660 +pREP42GFPBtn1) | h+, btn1::leu2, ura4-D18, leu1-32, his2 | Laboratory stock* |
| SC522D (YG660+pREP42GFPCLN3) | h+, btn1::leu2, ura4-D18, leu1-32, his2 | Laboratory stock* |
| SC8A (YG660+pREP42GFPBtn1N23F) | h+, btn1::leu2, ura4-D18, leu1-32, his2 | This study |
| RH405D (YG660+pREP42GFPBtn1L48P) | h+, btn1::leu2, ura4-D18, leu1-32, his2 | This study |
| RH414D (YG660+pREP42GFPBtn1A106P) | h+, btn1::leu2, ura4-D18, leu1-32, his2 | This study |
| RH456D (YG660+pREP42GFPBtn1L118P) | h+, btn1::leu2, ura4-D18, leu1-32, his2 | This study |
| SC11A (YG660+pREP42GFPBtn1G136A) | h+, btn1::leu2, ura4-D18, leu1-32, his2 | Laboratory stock* |
| SC14A (YG660+pREP42GFPBtn1E240K) | h+, btn1::leu2, ura4-D18, leu1-32, his2 | Laboratory stock* |
| SC17A (YG660+pREP42GFPBtn1V278F) | h+, btn1::leu2, ura4-D18, leu1-32, his2 | Laboratory stock* |
| RH408D (YG660+pREP42GFPBtn1R282C) | h+, btn1::leu2, ura4-D18, leu1-32, his2 | This study |
| RH411D (YG660+pREP42GFPBtn1R282H) | h+, btn1::leu2, ura4-D18, leu1-32, his2 | This study |
| RH535D (YG660+pREP42GFPBtn1Q300H) | h+, btn1::leu2, ura4-D18, leu1-32, his2 | This study |
| RH609D (YG660+pREP42GFPBtn1D363G) | h+, btn1::leu2, ura4-D18, leu1-32, his2 | This study |
| SC20A (YG660+pREP42GFPBtn1C382S) | h+, btn1::leu2, ura4-D18, leu1-32, his2 | This study |
| SC23A (YG660+pREP42GFPBtn1C393S) | h+, btn1::leu2, ura4-D18, leu1-32, his2 | This study |
| RH561D (YG660+pREP42GFPBtn1102fsX5) | h+, btn1::leu2, ura4-D18, leu1-32, his2 | Laboratory stock† |
| RH453D (YG660+pREP42GFPBtn1102-208del) | h+, btn1::leu2, ura4-D18, leu1-32, his2 | Laboratory stock† |
| RH573D (YG660+pREP42GFPBtn1207fsX6) | h+, btn1::leu2, ura4-D18, leu1-32, his2 | This study |
Construction of yeast expression plasmids
Mutations were introduced into the clone pREP42GFPBtn1, which contains GFP fused to the N terminus of Btn1p (Gachet et al., 2005), using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). The forward and reverse primers that were used are listed in Table 3. The construction of pREP42GFPBtn1G136A, pREP42 -GFPBtn1E240K, pREP42GFPBtn1V278F, pREP42GFPBtn1102fsX5 and pREP42GFPBtn1102-208del has been described previously (Gachet et al., 2005; Kitzmüller et al., 2008). All constructs were verified by sequence analysis and transformed into cells in which btn1 had been deleted (YG660) (Gachet et al., 2005).
Table 3.
Primers for mutagenesis of pREP42GFPBtn1
| Mutation | Forward primer (5′3′) | Reverse primer (5′-3′) |
|---|---|---|
| Asn23Phe | tttttggattgttattcaaccttctctacg | cgtagagaaggttgaataacaatccaaaaa |
| Leu48Pro | tctaagggtgtggtaccgctttctaatattgttcc | ggaacaatattagaaagcggtaccacacccttaga |
| Ala106Pro | ttggtgtatctctgccggccatttcatcgagttttgg | ccaaaactcgatgaaatggccggcagagatacaccaa |
| Leu118Pro | ttggcgaaatctctttcccgcatctatcaagtcgttatc | gataacgacttgatagatgcgggaaagagatttcgccaa |
| Arg282Cys | tgttttcctatcgtgctcctccatttcattttttac | gtaaaaaatgaaatggaggagcacgataggaaaaca |
| Arg282His | tgttttcctatcgcactcctccatttcattttttac | gtaaaaaatgaaatggaggagtgcgataggaaaaca |
| Gln300His | gcgcacattggcaataacacatttcattatccttc | gaaggataatgaaatgtgttattgccaatgtgcgc |
| Asp363Gly | ctgttggctcttcaggtagctctggaatttttttagc | gctaaaaaaattccagagctacctgaagagccaacag |
| Cys382Ser | aaccctccttgtcccatttccaagctg | cagcttggaaatgggacaaggagggtt |
| Cys393Ser | ggccgagattggagtgccttaacttg | caagttaaggcactccaatctcggcc |
| Btn1p207fsX6 | cttcgagcagggcacgttAtcattcaatttcgttaattc | gaattaacgaaattgaatgaTaacgtgccctgctcgaag |
Mutated bases are in red; inserted bases are in upper case.
Microscopy
Images of yeast cells were obtained as described previously (Codlin et al., 2008a).
Vacuole size
Vacuoles were visualised using the lipophilic dye FM4-64 according to our previously described protocol (Kitzmüller et al., 2008). Only data that is above the resolving limit of the microscope used is presented. Over 500 vacuoles per experiment were measured using OpenLab 3.5.1 software and downloaded to Microsoft Excel for analysis.
Septation
For measurement of the septation index, cells in log phase growth were fixed in 10% formaldehyde for 15 minutes, then washed three times in 1×PBS and stored at 4°C, and then stained with calcofluor to visualise septa (Moreno et al., 1991). Over 300 cells per experiment were counted.
Curving and monopolar growth
Cells were grown overnight at 25°C then transferred to 37°C and fixed after 4 or 7 hours. For assessment of cell curving, at least 300 cells were counted and the percentage of curved cells was recorded. For assessment of monopolar growth, over 50 cells with a septum were counted and monopolar cells were then designated as those lacking calcofluor stain at one tip.
Data processing and statistics
For each assay, the data presented is the mean±s.d. of three independent experiments with every strain. GraphPad Prism 4.0 software was used to perform Student’s unpaired t-tests (99% CI) for each mutant versus btn1Δ cells expressing pREP42GFP alone.
Supplementary Material
Acknowledgments
We thank C. Kitzmüller for helpful discussion. This work was supported by the Medical Research Council, UK (studentship to R.L.H., core support to the LMCB); the European Commission (503051); the Wellcome Trust, UK (067991 and Value in People Award with UCL); and UCL.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/content/2/1-2/84/suppl/DC1
REFERENCES
- Åberg L., Lauronen L., Hämäläinen J., Mole S. E., Autii T. (2008). A 30-year follow-up of a patient with mutations in CLN3 with a markedly protracted disease course. Ped. Neurol. (in press). [DOI] [PubMed]
- Cao Y., Espinola J. A., Fossale E., Massey A. C., Cuervo A. M., MacDonald M. E., Cotman S. L. (2006). Autophagy is disrupted in a knock-in mouse model of juvenile neuronal ceroid lipofuscinosis. J. Biol. Chem. 281, 20483–20493 [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S., Roberts P. M., Pearce D. A. (2003). The yeast model for Batten disease: a role for Btn2p in the trafficking of the Golgi-associated vesicular targeting protein, Yif1p. Biochem. Biophys. Res. Commun. 302, 534–538 [DOI] [PubMed] [Google Scholar]
- Codlin S., Haines R. L., Mole S. E. (2008a). btn1 affects endocytosis, polarisation of sterol-rich membrane domains and polarised growth in Schizosaccharomyces pombe. Traffic 9, 936–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codlin S., Haines R. L., Burden J. J. E., Mole S. E. (2008b). btn1 affects cytokinesis and cell wall deposition by independent mechanisms, one of which is linked to vacuole pH dysregulation. J. Cell. Sci. 121, 2860–2870 [DOI] [PubMed] [Google Scholar]
- Cooper J. D. (2008). Moving towards therapies for juvenile Batten disease? Exp. Neurol. 211, 329–331 [DOI] [PubMed] [Google Scholar]
- Ezaki J., Takeda-Ezaki M., Koike M., Ohsawa Y., Taka H., Mineki R., Murayama K., Uchiyama Y., Ueno T., Kominami E. (2003). Characterization of Cln3p, the gene product responsible for juvenile neuronal ceroid lipofuscinosis, as a lysosomal integral membrane glycoprotein. J. Neurochem. 87, 1296–1308 [DOI] [PubMed] [Google Scholar]
- Gachet Y., Codlin S., Hyams J. S., Mole S. E. (2005). btn1, the Schizosaccharomyces pombe homologue of the human Batten disease gene CLN3, regulates vacuole homeostasis. J. Cell. Sci. 118, 5525–5536 [DOI] [PubMed] [Google Scholar]
- Hobert J. A., Dawson G. (2007). A novel role of the Batten disease gene CLN3: association with BMP synthesis. Biochem. Biophys. Res. Commun. 358, 111–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holopainen J. M., Saarikoski J., Kinnunen P. K., Jarvela I. (2001). Elevated lysosomal pH in neuronal ceroid lipofuscinoses (NCLs). Eur. J. Biochem. 268, 5851–5856 [DOI] [PubMed] [Google Scholar]
- Kim Y., Ramirez-Montealegre D., Pearce D. A. (2003). A role in vacuolar arginine transport for yeast Btn1p and for human CLN3, the protein defective in Batten disease. Proc. Natl. Acad. Sci. USA 100, 15458–15462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzmüller C., Haines R. L., Codlin S., Cutler D. F., Mole S. E. (2008). A function retained by the common mutant CLN3 protein is responsible for the late onset of juvenile neuronal ceroid lipofuscinosis. Hum. Mol. Genet. 17, 303–312 [DOI] [PubMed] [Google Scholar]
- Kovacs A. D., Pearce D. A. (2008). Attenuation of AMPA receptor activity improves motor skills in a mouse model of juvenile Batten disease. Exp. Neurol. 209, 288–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyttala A., Ihrke G., Vesa J., Schell M. J., Luzio J. P. (2004). Two motifs target Batten disease protein CLN3 to lysosomes in transfected nonneuronal and neuronal cells. Mol. Biol. Cell 15, 1313–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyttala A., Yliannala K., Schu P., Jalanko A., Luzio J. P. (2005). AP-1 and AP-3 facilitate lysosomal targeting of batten disease protein CLN3 via its dileucine motif. J. Biol. Chem. 280, 10277–10283 [DOI] [PubMed] [Google Scholar]
- Lauronen L., Munroe P. B., Jarvela I., Autti T., Mitchison H. M., O’Rawe A. M., Gardiner R. M., Mole S. E., Puranen J., Hakkinen A. M., et al. (1999). Delayed classic and protracted phenotypes of compound heterozygous juvenile neuronal ceroid lipofuscinosis. Neurology 52, 360–365 [DOI] [PubMed] [Google Scholar]
- Luiro K., Yliannala K., Ahtiainen L., Maunu H., Jarvela I., Kyttala A., Jalanko A. (2004). I nterconnections of CLN3, Hook1 and Rab proteins link Batten disease to defects in the endocytic pathway. Hum. Mol. Genet. 13, 3017–3027 [DOI] [PubMed] [Google Scholar]
- Luiro K., Kopra O., Blom T., Gentile M., Mitchison H. M., Hovatta I., Tornquist K., Jalanko A. (2006). Batten disease (JNCL) is linked to disturbances in mitochondrial, cytoskeletal, and synaptic compartments. J. Neurosci. Res. 84, 1124–1138 [DOI] [PubMed] [Google Scholar]
- Mao Q., Foster B. J., Xia H., Davidson B. L. (2003). Membrane topology of CLN3, the protein underlying Batten disease. FEBS Lett. 541, 40–46 [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. (1991). Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795–823 [DOI] [PubMed] [Google Scholar]
- Munroe P. B., O’Rawe A. M., Mitchison H. M., Jarvela I. E., Santavuori P., Lerner T. J., Taschner P. E., Gardiner R. M., Mole S. E. (1997a). Strategy for mutation detection in CLN3: characterisation of two Finnish mutations. Neuropediatrics 28, 15–17 [DOI] [PubMed] [Google Scholar]
- Munroe P. B., Mitchison H. M., O’Rawe A. M., Anderson J. W., Boustany R. M., Lerner T. J., Taschner P. E., de Vos N., Breuning M. H., Gardiner R. M., et al. (1997b). Spectrum of mutations in the Batten disease gene, CLN3. Am. J. Hum. Genet. 61, 310–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan S. B., Rakheja D., Tan L., Pastor J. V., Bennett M. J. (2006). CLN3P, the Batten’s disease protein, is a novel palmitoyl-protein Delta-9 desaturase. Ann. Neurol. 60, 570–577 [DOI] [PubMed] [Google Scholar]
- Nugent T., Mole S. E., Jones D. T. (2008). The transmembrane topology of Batten disease protein CLN3 determined by consensus computational prediction constrained by experimental data. FEBS Lett. 582, 1019–1024 [DOI] [PubMed] [Google Scholar]
- Padilla-Lopez S., Pearce D. A. (2006). Saccharomyces cerevisiae lacking Btn1p modulate vacuolar ATPase activity to regulate pH imbalance in the vacuole. J. Biol. Chem. 281, 10273–10280 [DOI] [PubMed] [Google Scholar]
- Pearce D. A., Sherman F. (1998). A yeast model for the study of Batten disease. Proc. Natl. Acad. Sci. USA 95, 6915–6918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce D. A., Sherman F. (1999). Investigation of Batten disease with the yeast Saccharomyces cerevisiae. Mol. Genet. Metab. 66, 314–319 [DOI] [PubMed] [Google Scholar]
- Pearce D. A., Ferea T., Nosel S., Das B., Sherman F. (1999a). Action of BTN1, the yeast orthologue of the gene mutated in Batten disease. Nat. Genet. 22, 55–58 [DOI] [PubMed] [Google Scholar]
- Pearce D. A., Nosel S. A., Sherman F. (1999b). Studies of pH regulation by Btn1p, the yeast homolog of human Cln3p. Mol. Genet. Metab. 66, 320–323 [DOI] [PubMed] [Google Scholar]
- Pohl S., Mitchison H. M., Kohlschutter A., Van Diggelen O. P., Braulke T., Storch S. (2007). Increased expression of lysosomal acid phosphatase in CLN3-defective cells and mouse brain tissue. J. Neurochem. 103, 2177–2188 [DOI] [PubMed] [Google Scholar]
- Pullarkat R. K., Morris G. N. (1997). Farnesylation of Batten disease CLN3 protein. Neuropediatrics 28, 42–44 [DOI] [PubMed] [Google Scholar]
- Ramirez-Montealegre D., Pearce D. A. (2005). Defective lysosomal arginine transport in juvenile Batten disease. Hum. Mol. Genet. 14, 3759–3773 [DOI] [PubMed] [Google Scholar]
- Siintola E., Lehesjoki A. E., Mole S. E. (2006). Molecular genetics of the NCLs –status and perspectives. Biochim. Biophys. Acta. 1762, 857–864 [DOI] [PubMed] [Google Scholar]
- Storch S., Pohl S., Braulke T. (2004). A dileucine motif and a cluster of acidic amino acids in the second cytoplasmic domain of the batten disease-related CLN3 Protein are required for efficient lysosomal targeting. J. Biol. Chem. 279, 53625–53634 [DOI] [PubMed] [Google Scholar]
- Storch S., Pohl S., Quitsch A., Falley K., Braulke T. (2007). C-Terminal prenylation of the CLN3 membrane glycoprotein Is required for efficient endosomal sorting to lysosomes. Traffic 8, 431–444 [DOI] [PubMed] [Google Scholar]
- The International Batten Disease Consortium (1995). Isolation of a novel gene underlying Batten disease, CLN3. Cell 82, 949–957 [DOI] [PubMed] [Google Scholar]
- Wisniewski K. E., Zhong N., Kaczmarski W., Kaczmarski A., Kida E., Brown W. T., Schwarz K. O., Lazzarini A. M., Rubin A. J., Stenroos E. S., et al. (1998). Compound heterozygous genotype is associated with protracted juvenile neuronal ceroid lipofuscinosis. Ann. Neurol. 43, 106–110 [DOI] [PubMed] [Google Scholar]
- Zhang F. L., Casey P. J. (1996). Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 65, 241–269 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.