Abstract
It is widely recognized that the next great challenge in the post-genomic period is to understand how the genome establishes the cell and tissue specific patterns of gene expression that underlie development. The β-globin genes are among the most extensively studied tissue specific and developmentally regulated genes. The onset of erythropoiesis in precursor cells and the progressive expression of different members of the β-globin family during development are accompanied by dramatic epigenetic changes in the locus. In this review, we will consider the relationship between histone and DNA modifications and the transcriptional activity of the β-globin genes, the dynamic changes in epigenetic modifications observed during erythroid development, and the potential these changes hold as new targets for therapy in human disease.
Keywords: β-globin, chromatin, epigenetics, histone modifications, locus control regions, insulators
1. INTRODUCTION
The human β-globin gene family on chromosome 11 encodes five genes, ε, Gγ, Aγ, δ and β (Figure 1) that are expressed sequentially during development [1]. Over this time course, β globin polypeptides combine with α globin polypeptides, encoded on chromosome 16, to produce a series of different hemoglobin tetramers that carry oxygen in red blood cells. One of the reasons for the intense interest in these genes is that the severe hemoglobinopathies β-thalassemia and Sickle Cell Disease together represent the most common single gene disorders of man [2].
Figure 1.
Hemoglobin switching and hemoglobins produced during development. The globin genes of the human α and β-globin loci are indicated by the colored block arrows that also represent the direction of transcription of the genes. The horizontal arrows above represent a switching event leading to expression of the next, downstream gene in the locus. Below the depiction of the loci, the various hemoglobin tetramers produced at each developmental stage in erythroid cells of humans are indicated along with their globin polypeptide composition.
The discrete stages of erythropoiesis during which different β-globin genes are expressed roughly correspond with the changing sites of erythropoiesis during development in mammals [3]. Primitive human embryonic erythropoiesis, during which ε-globin is expressed, first occurs in the large predominantly nucleated erythroid cells of the yolk sac blood islands. During the fetal stage of development, hematopoiesis takes place in the fetal liver and produces definitive circulating enucleated erythrocytes in which γ-globin predominates. Around the time of birth and post-natally, the bone marrow becomes the site of erythropoiesis and the β-globin gene becomes highly transcribed, along with a low level of δ-globin gene transcription. It is only at this time, after the switch from γ to β-globin synthesis, that β-thalassemia and Sickle Cell Disease are manifest since they are caused by regulatory or structural defects in the adult β-globin gene fueling interest in reversal of γ-globin silencing as a therapeutic measure.
1.1 DNA methylation
Methylation of cytosine in the context of a CpG di-nucleotide is the most common epigenetic modification affecting mammalian DNA [4]. CpG methylation is generally associated with repetitive genomic regions that must remain silent, while clustered CpGs (CpG islands) associated with gene promoters, primarily of housekeeping genes, are predominantly un-methylated. The β-globin locus has no CpG islands. Nevertheless, one of the earliest studies investigating DNA methylation and its correlation with transcription showed that CpGs in the promoters of the human β-globin genes tended to be un-methylated in erythroblasts at the developmental stages at which they are expressed and methylated in erythroblasts that do not express them or in other cell types [5]. Thus, interest remains in the possibility that DNA methylation may play a role in β-globin gene regulation (see below). DNA methylation may be inhibitory for transcription, either by direct interference with transcription factor binding or, more commonly, through binding by methyl cytosine-binding proteins (MCBPs) at methylated CpG; these MCBPs can then act to recruit histone modifying enzyme-containing co-repressor complexes [6, 7].
1.2 Histone modifications in chromatin
The histone N-terminal tails protruding from the surface of the nucleosome are subject to a variety of covalent modifications including acetylation, methylation and phosphorylation [8]. Acetylation of lysine residues in histone tails is typically associated with active genes. Acetylation has the potential to alter the chromatin fiber since it neutralizes the basic charge of lysine residues. Methylation of lysine residues can be associated with active or repressed genes, and lysine residues can be mono, di or tri-methylated. Histone methylation provides recognition sites for the recruitment of non-histone proteins and enzymes which affect downstream gene function.
Histone H3 K4 and K36 methylation mark active chromatin and in yeast the enzymes that carry out these modifications are associated with RNA polymerase II (pol II), establishing a link to transcription. H3 K4 methylation and H3 acetylation together mark extensive active chromatin domains of related genes in a tissue specific fashion [9]. Tri-methylation of H3 K9 and K27 and H4 K20 typically mark both heterochromatin and repressed genes in euchromatin [10], although recent studies also report detection of H3 K9 tri-methylation in the coding regions of active genes, including globin genes [11, 12]. The combinatorial power of these marks, and the influence of one modification on another, signal a histone code that serves as a dynamic framework coordinating transcription of the genome [13, 14].
1.3 Locus control region function
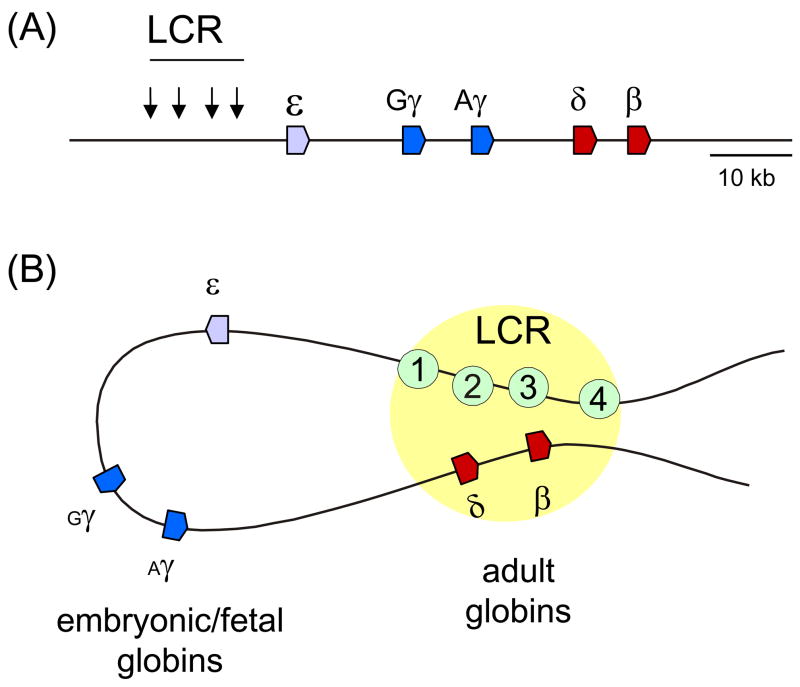
For high level expression, all β–globin genes rely on a locus control region (LCR) composed of four erythroid specific DNase I hypersensitive sites (DHS), HS1-4, that are made up of clusters of binding sites for transcription activators. The β-globin LCR lies almost 60 Kb from the β-globin gene (Figure 2A). A great deal of study over the years has been devoted to understanding how such distant enhancers exert their effect on promoter chromatin structure, with proposed models such as tracking across chromatin versus chromatin loop formation being actively tested [15]. The current view is that LCRs and the genes they activate come in close proximity through chromatin looping, which was first demonstrated for the β-globin LCR and gene [16, 17] (Figure 2B); a role for tracking is not ruled out and these processes need not be mutually exclusive.
Figure 2.
Long range chromatin looping in the β-globin locus. (A) The human β-globin locus is depicted with the LCR hypersensitive sites marked by downward arrows and the structural genes denoted by colored block arrow that indicate the direction of transcription of the genes. (B) The globin locus LCR and adult β-globin gene come in to close proximity in adult stage erythroid cells in which that gene is actively transcribed [16, 17]. The embryonic and fetal expressed genes that are silent at this stage of development are excluded from the close contacts. The looping interactions are developmentally regulated [48].
The β-globin LCR was originally defined as a regulatory DNA sequence capable of conferring integration position independent and copy number dependent expression to an transgene [18]. It had been expected that the LCR was responsible for recruiting activities mediating promoter chromatin modification and that LCR loss would lead to loss of the modifications as has been seen at other loci [9]. To test this, the LCR was deleted from the mouse β-globin locus by homologous recombination and although transcription was only about 3% of the wild type level, confirming the strong enhancer activity of the LCR, there was little change in β-globin promoter acetylation [19]. Possibly, histone acetylase complexes can be recruited directly to this promoter under certain circumstances. These authors concluded that the primary effect of the LCR at its endogenous location in the mouse locus appears to be on transcription and pol II elongation [20]. On a human chromosome lacking the LCR, as well as a further 25 Kb upstream, chromatin throughout the locus was condensed and silent [21] but a comparable deletion in the endogenous mouse locus did not produce this result [22]. The reasons for this difference between the mouse and human loci remain unclear and may be related to actual differences in the regulation between these two loci [23].
2. ORGANIZATION AND FUNCTION OF VERTEBRATE β-GLOBIN LOCI
Salient features of the chicken, mouse and human β-globin loci and their overall structure are highly conserved, strongly suggesting conservation of function among the important components of the loci. Nevertheless, structural and functional differences and alternative epigenetic patterns of modification exist between the loci as highlighted below.
2.1 The chicken β-globin locus
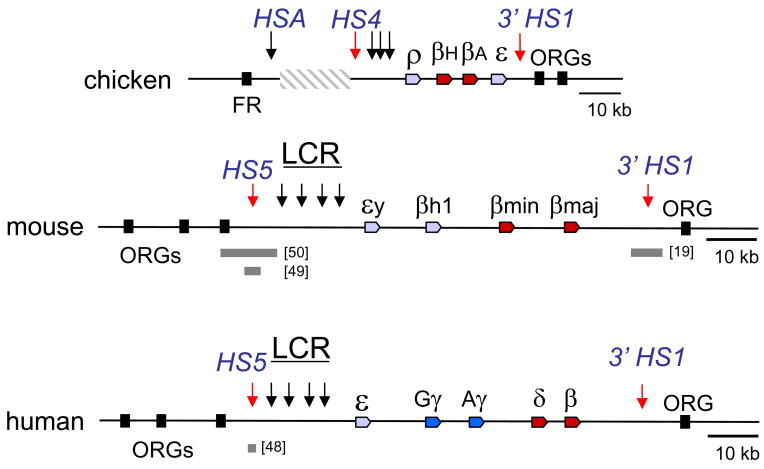
The chicken globin locus consists of four genes that are expressed at different stages of erythroid differentiation (Figure 3). The two adult genes, βH and βA, are flanked by the embryonic genes, ρ and ε. There is an intronic enhancer between βA and ε that is required for their expression and a series of DHSs upstream which functions as an LCR and activates all the genes to high levels [24, 25]. The LCR DHS are erythroid specific but two additional sites, HS4 and 3′HS1 border the locus and are present in non-erythroid tissues [26]. The upstream HS4 site (cHS4) is the most extensively described vertebrate insulator [27]. It separates the globin locus from a 16 kb region of heterochromatin [28]. Downstream 3′HS1 separates the globin locus from odorant receptor genes that are not active in erythroid cells [29].
Figure 3.
Structural organization of vertebrate β-globin genes. The β-globin genes of the chicken, mouse and human loci are represented by colored block arrows. The LCR DHSs are indicated by black arrows and the locus flanking, non-tissues specific DHSs are indicated by red arrows. FR, folate receptor gene, HSA, a DHS in the chicken locus associated with transcription of the FR, ORGs, odorant receptor genes not expressed in erythroid cells. The grey, striped rectangle represents a region of heterochromatin upstream of the LCR in chicken erythroid cells. The grey boxes under the loci represent the extents of HS5 or 3′HS1 deletions referred to in the text and are followed by the appropriate citation in brackets.
2.2 The mouse β-globin locus
The mouse globin locus has four genes of which the embryonic εy and βh1 genes are upstream of the adult βmaj and βmin genes, in contrast to the arrangement in the chicken locus (Figure 3). The mouse has a single switch from embryonic to adult β-type globin gene expression, but two switches in the site of hematopoiesis during development. Primitive erythrocytes from the embryonic yolk sac are gradually joined in the bloodstream by definitive erythrocytes from the fetal liver at around embryonic day 11.5 and then by definitive erythroid cells arising from the fetal bone marrow, late in fetal life [30]. Although repression of the embryonic genes, εy andβh1, and expression of the adult βmaj and βmin genes coincide with the switch from primitive to definitive erythropoiesis, respectively, primitive erythrocytes also exhibit a low level of adult gene expression [31]. This suggests that a programming switch could exist within an individual cell lineage that can change from an embryonic to an adult gene expression pattern.
It had long been thought that the mammalian globin genes were sequentially expressed in the order of their 5′ to 3′ chromosomal arrangement, in other words, with the LCR proximal gene expressed first. However, evidence now indicates that the first mouse globin gene to be expressed is βh1, followed by εy, indicating that proximity to the LCR is not the sole determinant of expression timing [30]. The LCR contains all the globin gene enhancer activity and consists of four erythroid DHSs in the 5′ end of the locus. The more widely detected HS5 and 3′HS1, orthologues of the chicken HS4 and 3′HS1 sites, border the mouse globin locus which is embedded in a cluster of silent odorant receptor genes and lacks the heterochromatic region found upstream of the chicken locus [32].
2.3 The human β-globin locus
In contrast to mice and chickens, humans undergo two major transitions in β-globin gene expression from the embryonic ε gene in primitive erythrocytes of the yolk sac to the fetal γ genes in definitive erythrocytes of the fetal liver and from the fetal gene expression to adult δ and β gene expression in erythrocytes from adult bone marrow after birth [3]. The human globin genes are expressed 5′ to 3′ in contrast to the mouse genes (Figure 3). Like the murine locus, the human locus contains an LCR far upstream of the β-globin genes, it is embedded in odorant receptor genes, and it is flanked by orthologous HS5 and 3′HS1 [32]. Human transgenes in mice adopt the murine expression pattern and undergo one globin switch, with human ε and γ globins expressed along with embryonic mouse εy and βh1, and human δ and β globins expressed along with adult mouse β globins [33]. Because of this, expression of human globin transgenes in mouse erythroid tissues is an imperfect reflection of regulation in human cells.
3. FUNCTION OF BORDERS/INSULATORS IN GLOBIN LOCI
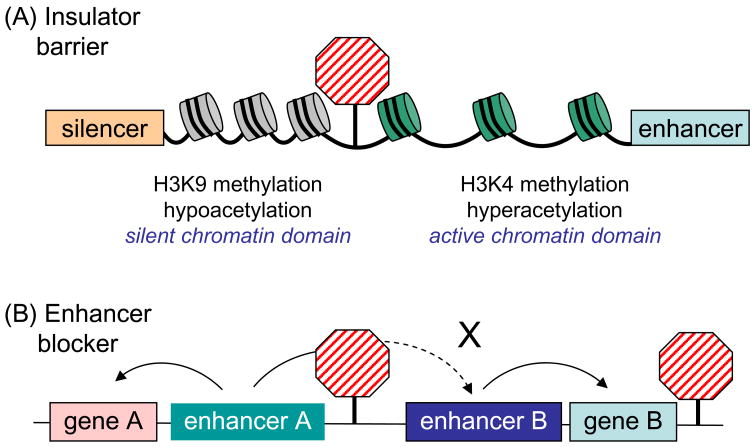
Insulators are DNA elements that were identified on the basis of their ability to protect genes from the influence of the surrounding chromatin environment, which could exert inappropriate activation or silencing effects. Insulators can have two types of activities: barrier function, which blocks the spread of heterochromatin into euchromatin and subsequent gene silencing (a frequent fate of transgenes), and enhancer-blocking, which prevents an enhancer from activating a promoter when placed between them (Figure 4) [34, 35]. At least some insulator functions involve epigenetic modification.
Figure 4.
Chromatin insulator function. (A) Insulator barrier. An insulator (red striped octagon) is depicted between a region of silent, hypo-acetylated chromatin featuring the H3K9 methylation mark and a region of active, hyper-acetylated chromatin marked by H3K4 methylation. The insulator is proposed to oppose the encroachment of the silent chromatin into the active region. Close-packed nucleosomes are depicted in grey and those that are loosely packed in green. (B) Enhancer blocker. Enhancer B can activate gene B but the insulator blocks enhancer A from activating gene B. Under these conditions, enhancer A can still activate gene A from which it is not blocked. The insulator is proposed to block a signal from an enhancer to a gene.
3.1 The chicken HS4 insulator
The chicken β-globin 5′HS4, flanking the upstream border of the locus (see Figure 3), is the best characterized vertebrate insulator and has both enhancer-blocking and barrier functions which are separable in ectopic assays [27, 36]. The protein CTCF is required for the enhancer blocking activity of cHS4 [37] and CTCF also interacts at 3′HS1 which forms the downstream border of the locus. These sites represent strong transitions in chromatin structure consistent with a role in defining a domain encompassing the globin genes and their distant regulatory elements [38–40]. In particular, the heterochromatic region upstream of cHS4 is enriched in H3 K9 di-methylation while this silencing mark is not otherwise detected within the globin locus. In erythroid cells, 3′HS1 also has enhancer-blocking activity although it is considerably weaker than cHS4 [41].
The same 250-bp “core” element from within cHS4 that has enhancer blocking activity is sufficient to protect against silencing of transgenes caused by chromosome position effects [42]. This barrier activity of cHS4 is associated with maintenance of a high level of active histone marks at the insulator [43]. The histone acetyl transferases (HAT) CBP and p300 are enriched at cHS4 and are thought to maintain histone acetylation in cHS4 [44]. H3 K4 methylation at cHS4 is also actively maintained at a high level [40, 45]. In contrast, 3′HS1 is not enriched for the acH3 and H3 K4 methyl modifications and does not function as a barrier element.
The protein USF1 is required to recruit histone modifying complexes to cHS4, which include, the histone methyl transferase (HMT) SET7/9 and the HAT PCAF [45]. In addition, arginine 3 methylation on histone H4 was observed in the cHS4 insulator and LCR in early developmental stages, before globin gene expression, and appears to spread from the LCR to the active gene promoters in cells expressing globin genes [46]. Importantly, H3 arginine methylation at cHS4 is required for acetylation of H3 and H4 and it is possible that this mark might play a role in protecting the active locus from encroachment by heterochromatic marks, such as H3 K9 di-methylation, present in adjacent heterochromatin. The cHS4 CTCF site can be deleted from cHS4 with expected loss of enhancer blocking but with retention of histone acetylation, at least in an ectopic location [47]. This suggests a strong association between epigenetic marks and barrier activity at cHS4, but not with enhancer blocking (which might function through another mechanism).
3.2 Mouse and human 5′HS5 and 3′HS1
The mouse and human β-globin loci are embedded within an array of odorant receptor genes that are expressed in olfactory epithelium and they lack the domain of heterochromatin upstream of cHS4 (see Figure 3). However, flanking the murine and human loci are HS5 and 3′HS1 that are orthologous to the chicken 5′HS4 and 3′HS1. The endogenous functions of these flanking elements in mouse and human β-globin loci are controversial, but the evolutionary conservation suggests a possible role for these sites in ensuring appropriate control of globin gene expression by the LCR, while preventing inappropriate activation of odorant receptor genes expression in erythroid tissues or vice versa. The mammalian HS5 and 3′HS1 sites loop together in erythroid cells in a chromatin hub before the globin genes are active [16, 48] consistent with delineation of a globin chromatin domain. Furthermore, the 5′ HS5 and 3′HS1 sites of mouse and human β-globin loci have been identified as CTCF binding sites in vivo and all possess varying enhancer-blocking activity in vitro or in vivo [41, 49].
However, the mouse and human HS5 and 3′HS1 sites do not mark strong transitions in chromatin structure, unlike the orthologous chicken HS4 site [12, 50]. Deletion of human HS5 in an erythroid background has only a small effect on transcription of the β-globin genes [51] and targeted deletion of HS5 or 3′HS1 in the mouse locus was similarly unremarkable [22, 52, 53]. In addition, mutation of the 3′HS1 CTCF site or knock down of CTCF does not perturb globin gene expression or affect expression of the locus flanking odorant receptor genes, although these manipulations compromise looping between the sites [54]. These authors found some evidence for increased H3 K9/K27 di-methylation within 3′HS1 after deletion of the CTCF site, but not for spreading of this mark. Taken together, these findings suggest that HS5 and 3′HS1 of the mouse and human β-globin LCR are neither necessary for maintaining the β-globin locus in an active configuration nor for protecting it from a surrounding repressed chromatin. This is consistent with the idea that in mammals the LCR/globin gene interaction is the primary determinant of regulation in the locus [55].
Several groups have recently profiled histone modifications across the human and mouse β-globin loci in erythroid cells [50, 56, 57] and in erythroid cell lines [12], representing different stages of development. In general, the active histone marks acH3 and H3 K4 di-methylation are not present at 3′HS1 but are enriched at HS5 although at lower levels than are seen in the chicken locus [39]. The differences in histone modification at cHS4 versus the orthologous mouse and human HS5 may lie in the upstream chromatin environment of each. In chicken, the heterochromatic region upstream of HS4 may make insulator activity of cHS4 essential to oppose the spread of repressive H3 K9 methylation into the globin gene locus. This may be achieved by USF1 binding, and HAT and HMT recruitment, to cHS4 with consequent maintenance of high levels of H3 acetylation and K4 methylation. The odorant receptor genes flanking the human and mouse globin loci, although silent, lack H3 K9 methylation [12], and may not require the same ‘blocking’.
4. EPIGENETICS IN DEVELOPMENT AND DIFFERENTIATION OF ERYTHROID CELLS
4.1 DNA methylation
Methylation of CpGs found in the 5′ regions of β-globin genes at particular stages of development is inversely correlated with the transcriptional activity of the gene at that time, regardless of the number of CpGs in the region. For example, the embryonic chicken ρ-globin gene contains a CpG dense region that spans the promoter and proximal transcribed region. Although un-methylated in embryonic development when the gene is highly expressed, methylation gradually accumulates and spreads bi-directionally until the entire gene becomes completely methylated when ρ-globin is silenced in adult erythroid cells [58].
In primitive erythroid cells of the mouse, the small number of CpGs found in the embryonic gene promoters as well as HS2 of the LCR are hypo-methylated, while the βmin promoter and βmaj coding region are hyper-methylated, again consistent with the globin expression pattern of these cells [59]. Conversely, in definitive mouse erythroid cells, HS2 and the β major promoter are hypo-methylated and the embryonic genes become hyper-methylated. Interestingly, retrotransposons within the embryonic domain are also hypo-methylated in primitive erythroid cells although they remain transcriptionally silent.
The same inverse relationship between DNA methylation and active transcription was observed in human primary fetal liver cells, where the fetal γ genes were expressed and the promoters hypo-methylated, and in adult bone marrow cells, where the adult β gene was expressed and the promoter hypo-methylated [60]. Furthermore, a low transient expression of the γ globin genes in early differentiating adult bone marrow cells was associated with concurrent hypo-methylation of that gene even though silencing and hyper-methylation of the fetal genes was the end result of differentiation. In other studies using a YAC transgenic mouse system that allowed control of Aγ methylation, it was observed that methylation worked as a dominant repressor of Aγ expression [61]. The authors speculate that this effect may be mediated through recruitment of HDACs by the methylation mark, loss of histone acetylation and interference with transcription activator binding. All these observations are consistent with a mechanistic relationship during development between DNA methylation and silencing of globin expression and between loss of methylation and gene activation upon switching. This difference has been exploited in the search for therapies for hemoglobinopathies (discussed below).
4.2. Progressive accumulation of histone modifications in the chicken β-globin locus
A domain of histone H3 and H4 acetylation was first described for the chicken β-globin locus in erythroid cells delineated by cHS4 and 3′HS1 [38]. All the genes reside in this domain, whether they are active or not, indicating that histone acetylation is not sufficient for transcription activation; subsequent studies showed the progressive accumulation of these marks. In HD24 and 6C2 cells, representing two early stages of erythropoiesis when globin genes are not expressed, low levels of acetylation increased with developmental time at cHS4, LCR HS2 and the adult β-globin promoter [39]. In 10 day old embryonic erythrocytes, high levels of acetylation were observed across the locus, consistent with earlier data. Histone H3 K4 di-methylation generally coincided with the changing pattern of acetylation observed in each of these cells lines and inversely correlated with di-methylation of lysine 9, which was observed at constitutive heterochromatin and at developmentally inactive globin genes [40].
4.3 Changes in mouse β-globin histone modification during development
Globin gene switching in the mouse β-globin locus depends on a switch from primitive to definitive erythroid lineages and a non-linear (non-5′ to 3′) activation of embryonic gene expression during primitive erythrocyte maturation in the bloodstream [30]. These investigators described a domain of acH3, acH4 and H3 K4 di-methylation extending from the LCR through εy and βh1 in primitive erythrocytes that did not significantly change as primitive cells transition from βh1 to εy expression during maturation. A less significant enrichment of acetylation in the adult βmaj and βmin genes increases substantially during primitive erythrocyte maturation, similar to that in definitive erythrocytes and correlating with transcriptional activation during these stages.
Definitive erythroid cells from fetal liver that predominantly express the adult βmaj and βmin genes have smaller, discontinuous sub-domains of acetylation and H3 K4 di-methylation localized over the LCR and the actively transcribed adult genes [50, 62]. Importantly, fetal liver cells treated with the HDAC inhibitor butyrate had increased histone acetylation at the developmentally silenced εy promoter, suggesting that de-acetylation contributes to silencing [62]. However, butyrate treatment did not induce εy expression, indicating that additional factors are required for reactivation. The histone acetylation pattern of the β-globin locus in murine erythroleukemia (MEL) cells induced to transcribe high levels of the adult globins, studied earlier, recapitulated that of definitive erythroid cells in fetal liver [62]. Unlike in the chicken locus, the silent embryonic genes are excluded from the modified domains at the adult stage. Also unlike the chicken locus, the HS5 and 3′HS1 DHS do not precisely define the extent of acetylation across the locus [50].
4.4 Human β-globin locus during development
In the context of a large human globin transgene, sub-domains of about 20 kilobases that are highly enriched for histone acetylation and H3 K4 di-, and tri-methylation were observed. These correspond to the LCR, active at all developmental stages, and to the regions surrounding each active gene at each developmental stage. [57]. A extensive analysis of epigenetic modification across this locus in K562 cells that express the fetal γ-globin genes reveals that the domain of acetylation and H3 K4 di-methylation extends from the LCR through the embryonic and fetal genes [12]. The embryonic ε-globin gene which is active at a low level in K562 cells, shows lower levels of modification, suggesting a gradual loss or gain of these marks as transcription levels change. There was a reciprocal absence of H3 acetylation and presence of H3 K9 methylation over the silent adult genes in these cells. Surprisingly, in K562 cells, and in induced MEL and G1E cells, the transcribed region of the active globin gene (γ-globin in K562 and β-globin in MEL and G1E) was enriched for H3 K4 and K36 tri-methylation, as expected, but also for H3 K9 tri-methylation, which is generally associated with silent heterochromatin [11, 12]. H3 K9 tri-methylation has been found at other actively transcribed genes, suggesting a new role for this modification that remains to be further explored [63].
A study directly comparing human fetal liver cells and differentiated adult CD34+ cells from peripheral blood, observed acetylation patterns very similar to those seen in K562 cells and in the human transgene [56]. Other work, using single cell RT-qPCR on bone marrow hematopoietic progenitor cells (HPCs) containing a human transgene or primary human CD34+ HPCs indicated that histone acetylation at the LCR occurs before a high level of globin transcription is established [23]. These studies suggest that epigenetic marking of the LCR is an early step in erythroid differentiation, consistent with studies showing that the LCR DHS form before the globin genes are activated [64].
Taken together, the data are consistent with the idea that the mammalian β-globin loci acquire positive histone modifications in the LCR and at active globin genes during differentiation, whereas silencing histone modifications mark the genes before they are activated and after they are silenced (Figure 5). Although there are significant differences in the levels of acetylation at any particular stage of development between the endogenous mouse globin promoters and globin promoters on a human transgene [23], in general, acetylation patterns in human transgenes are strikingly similar to those observed in human erythroid cells at similar developmental stages, despite the adoption of the murine switching pattern by the human transgenic globin genes.
Figure 5.
Changes in epigenetic modification of the human β-globin locus during development. The β-globin genes of the human locus are represented by colored block arrows. The LCR DHSs are indicated by black arrows and the locus flanking, non-tissues specific DHSs are indicated by red arrows. Domains of histone acetylation and H3K4 di-methylation are depicted by colored shapes (magnitude not drawn to scale) below the locus at different stages of development. The patterns are consistent with or implied by data from several laboratories [12, 23, 57]. Epigenetic modifications in the mouse locus are, overall, similar [50, 70]. Some data support the reciprocal presence of H3 K9 methylation at the adult genes before they are activated [12, 73]. ORGs, odorant receptor genes
4.5 Intergenic transcription and globin locus modification
Non-coding transcripts have been detected spanning across the human β-globin LCR and intergenic regions of the locus in erythroid cell lines and erythroid tissues including those of transgenic mice [12, 57, 65, 66]. When the human locus was studied during development in human erythroid cells or as a transgene, a very good correlation was observed between the intergenic transcription at different stages and the acetylated domains depicted in Figure 5 suggesting the transcripts are developmentally regulated. Since RNA pol II can associate with histone methyl transferases, the question arises whether histone modification and intergenic transcription in the globin locus are related mechanistically.
Deletion of an endogenous intergenic transcript promoter between the γ- and δ-globin genes affects chromatin accessibility and transcription of the downstream β-globin gene [66]. Other studies used a plasmid based assay with insertion of a transcription terminator between LCR HS2 and a test globin gene promoter suggested that intergenic transcription from the LCR may be important for globin gene expression [67]. Regardless, the precise role endogenous intergenic transcription may play in globin gene transcriptional regulation or the establishment of active chromatin domains remains largely unclear.
4.6 Erythroid transcription factors and epigenetic modification
GATA-1, NF-E2 and EKLF are key erythroid specific transcription factors with roles in hematopoiesis and globin gene regulation and these activators occupy LCR DHS and globin gene promoters [3]. Cell lines or mice lacking one or another critical erythroid transcription factor have been useful in defining steps in globin gene activation. For example, G1E cells lack GATA-1 and do not produce globins unless GATA-1 is re-supplied [68]. Studies of G1E cells along with CB3 cells, a MEL cell line lacking NF-E2, have revealed much about the roles GATA-1 and NF-E2 play in the acquisition of epigenetic modifications during erythroid differentiation [69–72]. Although there is some variation among the reports, it appears that both GATA-1 and NF-E2 induce histone acetylation and H3 K4 di-, and tri-methylation at the β-globin gene but have little or no effect on these modifications at the LCR, consistent with an early potentiation of the LCR chromatin structure. Although less extensively studied, in one report the acquisition of H3 K9 acetylation in the globin locus in differentiated G1E cells corresponded with a loss of the K9 methylation mark [73].
Other studies showed that GATA-1 and NF-E2 play important and differing roles in recruitment of the HAT CBP and the MLL histone methyl transferase associated Ash2L to the LCR [71, 74–76]. These data, taken together with the observation of close proximity between the LCR and β-globin gene during transcription activation [16], suggest that histone modifying activities recruited to the LCR are subsequently transferred to the promoter. However, it is difficult to reconcile this view with continued promoter acetylation after deletion of the LCR, discussed earlier.
5. EPIGENETICS, DEVELOPMENT, AND DISEASE
βglobin gene disorders, such as sickle cell disease (SCD) and β-thalassemia are the result of genetic mutations in the β-globin gene, and were the first disorders to be approached with epigenetic-specific therapy [77, 78]. Strategies for epigenetic therapy focused on the reactivation of fetal γ-globin expression, fueled by the observation that SCD and β-thalassemia patients with more than moderate increases in HbF levels had significantly reduced clinical symptoms [79]. A number of therapeutic agents with the potential to relieve the developmental epigenetic silencing of fetal γ-globin have been investigated with mixed clinical results and a limited understanding of their true mechanism of action; questions remain about whether the epigenetic modifications resulting from these treatments are primarily the cause of transcriptional up-regulation, or the effect of transcriptional up-regulation mediated by other actions of these pleiotropic agents, such as cytotoxicity and possible post-translational effects [80, 81]. These agents fall into two main classes: DNA methyl-transferase inhibitors and histone deacetylase inhibitors.
5.1 DNA methyl-transferase inhibition
The DNA methyl transferase inhibitor 5-azacytidine was the first pharmacological agent knowingly targeted to epigenetic modifications. It was used to re-activate HbF, initially in a baboon model [82] and later in clinical studies in patients with SCD and β-thalassemia [77, 78]. Although none of the β-like globin genes contain significant numbers of CpG di-nucleotides, there is an inverse correlation between DNA methylation at these sites and transcriptional activity. In spite of promising preliminary results with regard to HbF induction, 5-azacytidine was set aside clinically due to questions about toxicity and potential carcinogenic effects of the drug [83].
Tens of patients with SCD have been treated, intermittently for up to a year, with the 5-azacytidine analog decitabine (5-aza 2-deoxycytidine). Most have shown clear-cut, and likely clinically meaningful, increases in HbF and total hemoglobin levels [84–86], and there may be clinical utility in high-risk patients. However, DNA methylating agents are bone marrow toxic, and concerns about carcinogenesis from these compounds, when used chronically for low-risk disease, have not been systematically allayed.
Decitabine treatment in a primate model, in which the β-globin gene locus is similar to humans, resulted in a marked diminution in DNA methylation of individual CpGs in the ε- and γ-globin promoters in erythroid precursor cells, with transcriptional up-regulation of the γ-globin gene; these embryonic/fetal promoter sites are normally fully methylated in anemic adult baboons [87]. Histone acetylation of H3 and H4 and RNA polymerase II occupancy, were reduced at the β-globin promoter and increased at γ-globin promoters after treatment. The mechanistic relationship between DNA methylation and histone modifications is an evolving story, but it is not surprising that drugs, such as decitabine, which have a single epigenetic target, i.e. DNA methylation that is linked with transcriptional activation, will have additional secondary epigenetic effects, as on histone modification. Experimentally, 5-azacytidine and decitabine consistently show hypo-methylation at, and increased expression from, the γ-globin promoter, and decreased expression from the β-globin promoter. However, the role of DNA hypo-methylation as a primary cause of γ-globin gene activation has been put into question by experiments in which silencing of DNMT, and resultant ‘pure’ hypo-methylation, of the β-globin gene locus in human erythroid progenitors did not increase γ-globin expression [80].
5.2 Histone deacetylase inhibition
HDAC inhibitors, such as short-chain fatty acids (e.g. butyrate), also hold promise as therapeutic agents in SCD and β-thalassemia patients through sustained elevations of γ-globin expression. Increased HbF levels and total hemoglobin levels have been seen in SCD patients given pulse butyrate therapy [88], but technical problems with an inconvenient dosing route and schedule persist. Mechanistic studies in cultured CD34+ blood cells have shown that butyrate activation of γ globin gene expression is associated with an increase in histone acetylation and decreased DNA methylation at γ-globin genes with opposite changes at the β-globin gene [89]. Using computational modeling and screening of a chemical library a novel, highly potent short chain fatty acid derivative was identified that caused displacement of HDAC3 and associated co-repressor NCoR from the γ-globin promoter and resulted in an increase in promoter hyper-acetylation [90]. Efforts are underway to find oral butyrate-like agents.
Again, the relationship between epigenetic modifications at the globin locus, following treatment with hypo-methylating agents or HDAC inhibitors remains unclear, since work in which HDAC inhibition failed to activate avian erythroid sequences that had been silenced by in vitro methylation suggest that methylation dominates over histone acetylation in methylation-mediated silencing at CpG rich sites [91]. It is likely that butyrate exerts other effects, beyond only HDAC inhibition, which may increase transcription at the βglobin locus and which will have additional secondary epigenetic effects. Other studies have attributed elevated γ-globin production to an increase in the efficiency of γ-globin translation or activation of the guanylate cyclase and p38 MAPK signaling pathways; how this signaling may result in γ-globin re-activation has not been elucidated [92]
The HDAC inhibitor apicidin, was associated with significant HbF synthesis in K562 cells and similar to butyrate was reported to result in (and also require) the activation of the p38 MAPK pathway for HbF induction [93]. At the chromatin level, apicidin treatment of K562 cells results in an overall increase in H3 and H4 hyper-acetylation spanning from the LCR to regions flanking the γ-globin promoter and this acetylation is dependent on the p38 signaling pathway [94]. Apicidin had little effect on methylation of histone H3 lysine 4, but enhanced recruitment of GATA-1 to the LCR and, to a lesser extent, γ-globin promoters was observed. Surprisingly, inhibition of the p38 signaling pathway did not affect this recruitment, suggesting that the increase is not directly related to a p38-dependent γ-globin induction by apicidin.
It is possible that butyrate’s effects on erythropoiesis may require integration of epigenetically and non-epigenetically directed signals. Relevant parallels may be evident in the molecular mechanisms underlying cardiac hypertrophy, in which stress-signaled egress of HDACs from the nucleus is coupled with additional stress responses, resulting in the activation of a, in this case pathological, fetal transcriptional program [95]. Indeed, it is possible that epigenetic modulation by DNA de-methylation or HDAC inhibition alone, without other as yet uncharacterized signals from these therapeutic agents, may be insufficient to reactivate fetal globin expression.
6. PERSPECTIVE
Studies from numerous laboratories using different systems are consistent, overall, with the idea that the mammalian β-globin loci acquire positive histone modifications in the LCR and at active globin genes during differentiation, whereas silencing histone modifications mark the genes before they are activated and after they are silenced. The studies are also consistent with a mechanistic relationship during development between DNA methylation and silencing of globin expression and between loss of methylation and gene activation upon switching. The question of whether these states can be manipulated, safely, for therapeutic benefit still remains before us.
The goal of the epigenetic manipulation strategies discussed above is to facilitate the reactivation of fetal globin genes in SCD and β-thalassemia patients who lack a functional adult globin gene, and, in at least some cases, promising results have been achieved albeit with little or no understanding of the mechanism of this induction. Soluble small molecules with the potential to reactivate γ-globin genes, possibly through epigenetic alterations, hold promise, but it will be essential to carry out further mechanistic studies since HDACs and DNA methyl transferases affect transcription genome-wide and in some cases are critical for survival.
Acknowledgments
We would like to acknowledge support from the Intramural Program of the NIDDK, NIH.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stamatoyannopoulos G, Grosveld F. Hemoglobin Switching. In: Stamatoyannopoulos G, Majerus PW, Perlmutter RM, Varmus H, editors. The Molecular Basis of Blood Diseases. W. B. Saunders; Philadelphia: 2001. pp. 135–182. [Google Scholar]
- 2.Weatherall DJ. The global problem of genetic disease. Ann Hum Biol. 2005;32:117–122. doi: 10.1080/03014460500075480. [DOI] [PubMed] [Google Scholar]
- 3.Stamatoyannopoulos G. Control of globin gene expression during development and erythroid differentiation. Exp Hematol. 2005;33:259–271. doi: 10.1016/j.exphem.2004.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–610. doi: 10.1038/nrg1655. [DOI] [PubMed] [Google Scholar]
- 5.van der Ploeg LH, Flavell RA. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980;19:947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]
- 6.Fuks F. DNA methylation and histone modifications: teaming up to silence genes. Curr Opin Genet Dev. 2005;15:490–495. doi: 10.1016/j.gde.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Ginder GD, Gnanapragasam MN, Mian OY. The role of the epigenetic signal, DNA methylation, in gene regulation during erythroid development. Curr Top Dev Biol. 2008;82:85–116. doi: 10.1016/S0070-2153(07)00004-X. [DOI] [PubMed] [Google Scholar]
- 8.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Dean A. On a chromosome far, far away: LCRs and gene regulation. Trends Genet. 2006;22:38–45. doi: 10.1016/j.tig.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Lachner M, O’Sullivan RJ, Jenuwein T. An epigenetic road map for histone lysine methylation. J Cell Sci. 2003;116:2117–2124. doi: 10.1242/jcs.00493. [DOI] [PubMed] [Google Scholar]
- 11.Vakoc CR, Mandat SA, Olenchock BA, Blobel GA. Histone H3 lysine 9 methylation and HP1gamma are associated with transcription elongation through mammalian chromatin. Mol Cell. 2005;19:381–391. doi: 10.1016/j.molcel.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Kim A, Kiefer CM, Dean A. Distinctive signatures of histone methylation in transcribed coding and noncoding human β-globin sequences. Mol Cell Biol. 2007;27:1271–1279. doi: 10.1128/MCB.01684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 14.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 15.Bulger M, Groudine M. Looping versus linking: toward a model for long-distance gene activation. Genes Dev. 1999;13:2465–2477. doi: 10.1101/gad.13.19.2465. [DOI] [PubMed] [Google Scholar]
- 16.Tolhuis B, Palstra RJ, Splinter E, Grosveld F, de Laat W. Looping and interaction between hypersensitive sites in the active β-globin locus. Mol Cell. 2002;10:1453–1465. doi: 10.1016/s1097-2765(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 17.Carter D, Chakalova L, Osborne CS, Dai Y, Fraser P. Long-range chromatin regulatory interactions in vivo. Nat Genet. 2002;32:623–626. doi: 10.1038/ng1051. [DOI] [PubMed] [Google Scholar]
- 18.Grosveld F, van Assendelft GB, Greaves DR, Kollias G. Position-independent, high-level expression of the human β-globin gene in transgenic mice. Cell. 1987;51:975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- 19.Bender MA, Bulger M, Close J, Groudine M. β-globin gene switching and DNase I sensitivity of the endogenous β-globin locus in mice do not require the locus control region. Mol Cell. 2000;5:387–393. doi: 10.1016/s1097-2765(00)80433-5. [DOI] [PubMed] [Google Scholar]
- 20.Sawado T, Halow J, Bender MA, Groudine M. The β-globin locus control region (LCR) functions primarily by enhancing the transition from transcription initiation to elongation. Genes Dev. 2003;17:1009–1018. doi: 10.1101/gad.1072303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forrester WC, Epner E, Driscoll MC, Enver T, Brice M, Papayannopoulou T, Groudine M. A deletion of the human β-globin locus activation region causes a major alteration in chromatin structure and replication across the entire β-globin locus. Genes and Development. 1990;4:1637–1649. doi: 10.1101/gad.4.10.1637. [DOI] [PubMed] [Google Scholar]
- 22.Bender MA, Byron R, Ragoczy T, Telling A, Bulger M, Groudine M. Flanking HS-62.5 and 3′ HS1, and regions upstream of the LCR, are not required for beta-globin transcription. Blood. 2006;108:1395–1401. doi: 10.1182/blood-2006-04-014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bottardi S, Aumont A, Grosveld F, Milot E. Developmental stage-specific epigenetic control of human beta-globin gene expression is potentiated in hematopoietic progenitor cells prior to their transcriptional activation. Blood. 2003;102:3989–3997. doi: 10.1182/blood-2003-05-1540. [DOI] [PubMed] [Google Scholar]
- 24.Nickol JM, Felsenfeld G. Bidirectional control of the chicken β- and Σ-globin genes by a shared enhancer. Proc Natl Acad Sci USA. 1988;85:2548–2552. doi: 10.1073/pnas.85.8.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reitman M, Felsenfeld G. Mutational analysis of the chicken β-globin enhancer reveals two positive-acting domains. Proc Natl Acad Sci USA. 1988;85:6267–6271. doi: 10.1073/pnas.85.17.6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reitman M, Felsenfeld G. Developmental regulation of topoisomerase II sites and DNase I- hypersensitive sites in the chickenβ-globin locus. Mol Cell Biol. 1990;10:2774–2786. doi: 10.1128/mcb.10.6.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung JH, Bell AC, Felsenfeld G. Characterization of the chicken beta-globin insulator. Proc Natl Acad Sci U S A. 1997;94:575–580. doi: 10.1073/pnas.94.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prioleau MN, Nony P, Simpson M, Felsenfeld G. An insulator element and condensed chromatin region separate the chicken beta-globin locus from an independently regulated erythroid-specific folate receptor gene. EMBO J. 1999;18:4035–4048. doi: 10.1093/emboj/18.14.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saitoh N, Bell AC, Recillas-Targa F, West AG, Simpson M, Pikaart M, Felsenfeld G. Structural and functional conservation at the boundaries of the chicken beta-globin domain. EMBO J. 2000;19:2315–2322. doi: 10.1093/emboj/19.10.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kingsley PD, Malik J, Emerson RL, Bushnell TP, McGrath KE, Bloedorn LA, Bulger M, Palis J. “Maturational” globin switching in primary primitive erythroid cells. Blood. 2006;107:1665–1672. doi: 10.1182/blood-2005-08-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trimborn T, Gribnau J, Grosveld F, Fraser P. Mechanisms of developmental control of transcription in the murine α- and β-globin loci. Genes Dev. 1999;13:112–124. doi: 10.1101/gad.13.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bulger M, van Doorninck JH, Saitoh N, Telling A, Farrell C, Bender MA, Felsenfeld G, Axel R, Groudine M, von Doorninck JH. Conservation of sequence and structure flanking the mouse and human β-globin loci: the β-globin genes are embedded within an array of odorant receptor genes. Proc Natl Acad Sci U S A. 1999;96:5129–5134. doi: 10.1073/pnas.96.9.5129. [published erratum appears in Proc Natl Acad Sci U S A 1999 Jul 6;96(14):8307] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaensler KM, Kitamura M, Kan YW. Germ-line transmission and developmental regulation of a 150-kb yeast artificial chromosome containing the human beta-globin locus in transgenic mice. Proc Natl Acad Sci U S A. 1993;90:11381–11385. doi: 10.1073/pnas.90.23.11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgess-Beusse B, Farrell C, Gaszner M, Litt M, Mutskov V, Recillas-Targa F, Simpson M, West A, Felsenfeld G. The insulation of genes from external enhancers and silencing chromatin. Proc Natl Acad Sci U S A. 2002;99(Suppl 4):16433–16437. doi: 10.1073/pnas.162342499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallace JA, Felsenfeld G. We gather together: insulators and genome organization. Curr Opin Genet Dev. 2007;17:400–407. doi: 10.1016/j.gde.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Recillas-Targa F, Pikaart MJ, Burgess-Beusse B, Bell AC, Litt MD, West AG, Gaszner M, Felsenfeld G. Position-effect protection and enhancer blocking by the chicken beta-globin insulator are separable activities. Proc Natl Acad Sci U S A. 2002;99:6883–6888. doi: 10.1073/pnas.102179399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bell AC, West AG, Felsenfeld G. The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell. 1999;98:387–396. doi: 10.1016/s0092-8674(00)81967-4. [DOI] [PubMed] [Google Scholar]
- 38.Hebbes TR, Clayton AL, Thorne AW, Crane-Robinson C. Core histone hyperacetylation co-maps with generalized DNase I sensitivity in the chicken β-globin chromosomal domain. EMBO J. 1994;13:1823–1830. doi: 10.1002/j.1460-2075.1994.tb06451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Litt MD, Simpson M, Recillas-Targa F, Prioleau MN, Felsenfeld G. Transitions in histone acetylation reveal boundaries of three separately regulated neighboring loci. EMBO J. 2001;20:2224–2235. doi: 10.1093/emboj/20.9.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litt MD, Simpson M, Gaszner M, Allis CD, Felsenfeld G. Correlation between histone lysine methylation and developmental changes at the chicken beta-globin locus. Science. 2001;293:2453–2455. doi: 10.1126/science.1064413. [DOI] [PubMed] [Google Scholar]
- 41.Farrell CM, West AG, Felsenfeld G. Conserved CTCF insulator elements flank the mouse and human β-globin loci. Mol Cell Biol. 2002;22:3820–3831. doi: 10.1128/MCB.22.11.3820-3831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pikaart MJ, Recillas-Targa F, Felsenfeld G. Loss of transcriptional activity of a transgene is accompanied by DNA methylation and histone deacetylation and is prevented by insulators. Genes Dev. 1998;12:2852–2862. doi: 10.1101/gad.12.18.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mutskov VJ, Farrell CM, Wade PA, Wolffe AP, Felsenfeld G. The barrier function of an insulator couples high histone acetylation levels with specific protection of promoter DNA from methylation. Genes Dev. 2002;16:1540–1554. doi: 10.1101/gad.988502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.West AG, Huang S, Gaszner M, Litt MD, Felsenfeld G. Recruitment of histone modifications by USF proteins at a vertebrate barrier element. Mol Cell. 2004;16:453–463. doi: 10.1016/j.molcel.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Huang S, Li X, Yusufzai TM, Qiu Y, Felsenfeld G. USF1 recruits histone modification complexes and is critical for maintenance of a chromatin barrier. Mol Cell Biol. 2007;27:7991–8002. doi: 10.1128/MCB.01326-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang S, Litt M, Felsenfeld G. Methylation of histone H4 by arginine methyltransferase PRMT1 is essential in vivo for many subsequent histone modifications. Genes Dev. 2005;19:1885–1893. doi: 10.1101/gad.1333905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao H, Dean A. An insulator blocks spreading of histone acetylation and interferes with RNA polymerase II transfer between an enhancer and gene. Nucleic Acids Res. 2004;32:4903–4919. doi: 10.1093/nar/gkh832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palstra RJ, Tolhuis B, Splinter E, Nijmeijer R, Grosveld F, de Laat W. The β-globin nuclear compartment in development and erythroid differentiation. Nat Genet. 2003;35:190–194. doi: 10.1038/ng1244. [DOI] [PubMed] [Google Scholar]
- 49.Tanimoto K, Sugiura A, Omori A, Felsenfeld G, Engel JD, Fukamizu A. Human beta-globin locus control region HS5 contains CTCF- and developmental stage-dependent enhancer-blocking activity in erythroid cells. Mol Cell Biol. 2003;23:8946–8952. doi: 10.1128/MCB.23.24.8946-8952.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bulger M, Schubeler D, Bender MA, Hamilton J, Farrell CM, Hardison RC, Groudine M. A complex chromatin landscape revealed by patterns of nuclease sensitivity and histone modification within the mouse β-globin locus. Mol Cell Biol. 2003;23:5234–5244. doi: 10.1128/MCB.23.15.5234-5244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reik A, Telling A, Zitnik G, Cimbora D, Epner E, Groudine M. The locus control region is necessary for gene expression in the human β-globin locus but not the maintenance of an open chromatin structure in erythroid cells. Mol Cell Biol. 1998;18:5992–6000. doi: 10.1128/mcb.18.10.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bender MA, Reik A, Close J, Telling A, Epner E, Fiering S, Hardison R, Groudine M. Description and targeted deletion of 5′ hypersensitive site 5 and 6 of the mouse β-globin locus control region. Blood. 1998;92:4394–4403. [PubMed] [Google Scholar]
- 53.Farrell CM, Grinberg A, Huang SP, Chen D, Pichel JG, Westphal H, Felsenfeld G. A large upstream region is not necessary for gene expression or hypersensitive site formation at the mouse β-globin locus. Proc Natl Acad Sci U S A. 2000;97:14554–14559. doi: 10.1073/pnas.97.26.14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Splinter E, Heath H, Kooren J, Palstra RJ, Klous P, Grosveld F, Galjart N, de Laat W. CTCF mediates long-range chromatin looping and local histone modification in the beta-globin locus. Genes Dev. 2006;20:2349–2354. doi: 10.1101/gad.399506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dillon N, Sabbattini P. Functional gene expression domains: defining the functional unit of eukaryotic gene regulation. Bioessays. 2000;22:657–665. doi: 10.1002/1521-1878(200007)22:7<657::AID-BIES8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 56.Yin W, Barkess G, Fang X, Xiang P, Cao H, Stamatoyannopoulos G, Li Q. Histone acetylation at the human beta-globin locus changes with developmental age. Blood. 2007;110:4101–4107. doi: 10.1182/blood-2007-05-091256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miles J, Mitchell JA, Chakalova L, Goyenechea B, Osborne CS, O’Neill L, Tanimoto K, Engel JD, Fraser P. Intergenic transcription, cell-cycle and the developmentally regulated epigenetic profile of the human beta-globin locus. PLoS ONE. 2007;2:e630. doi: 10.1371/journal.pone.0000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singal R, Ferris R, Little JA, Wang SZ, Ginder GD. Methylation of the minimal promoter of an embryonic globin gene silences transcription in primary erythroid cells. Proc Natl Acad Sci U S A. 1997;94:13724–13729. doi: 10.1073/pnas.94.25.13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hsu M, Mabaera R, Lowrey CH, Martin DI, Fiering S. CpG hypomethylation in a large domain encompassing the embryonic beta-like globin genes in primitive erythrocytes. Mol Cell Biol. 2007;27:5047–5054. doi: 10.1128/MCB.02234-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mabaera R, Richardson CA, Johnson K, Hsu M, Fiering S, Lowrey CH. Developmental- and differentiation-specific patterns of human gamma- and beta-globin promoter DNA methylation. Blood. 2007;110:1343–1352. doi: 10.1182/blood-2007-01-068635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goren A, Simchen G, Fibach E, Szabo PE, Tanimoto K, Chakalova L, Pfeifer GP, Fraser PJ, Engel JD, Cedar H. Fine tuning of globin gene expression by DNA methylation. PLoS ONE. 2006;1:e46. doi: 10.1371/journal.pone.0000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forsberg EC, Downs KM, Christensen HM, Im H, Nuzzi PA, Bresnick EH. Developmentally dynamic histone acetylation pattern of a tissue- specific chromatin domain. Proc Natl Acad Sci U S A. 2000;97:14494–14499. doi: 10.1073/pnas.97.26.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vakoc CR, Sachdeva MM, Wang H, Blobel GA. A profile of histone lysine methylation across transcribed mammalian chromatin. Mol Cell Biol. 2006 doi: 10.1128/MCB.01529-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jimenez G, Griffiths SD, Ford AM, Greaves MF, Enver T. Activation of the β-globin locus control region precedes commitment to the erythroid lineage. Proc Natl Acad Sci U S A. 1992;89:10618–10622. doi: 10.1073/pnas.89.22.10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ashe HL, Monks J, Wijgerde M, Fraser P, Proudfoot NJ. Intergenic transcription and transinduction of the human β-globin locus. Genes Dev. 1997;11:2494–2509. doi: 10.1101/gad.11.19.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gribnau J, Diderich K, Pruzina S, Calzolari R, Fraser P. Intergenic transcription and developmental remodeling of chromatin subdomains in the human β-globin locus. Mol Cell. 2000;5:377–386. doi: 10.1016/s1097-2765(00)80432-3. [DOI] [PubMed] [Google Scholar]
- 67.Ling J, Ainol L, Zhang L, Yu X, Pi W, Tuan D. HS2 enhancer function is blocked by a transcriptional terminator inserted between the enhancer and the promoter. J Biol Chem. 2004;279:51704–51713. doi: 10.1074/jbc.M404039200. [DOI] [PubMed] [Google Scholar]
- 68.Weiss MJ, Yu C, Orkin SH. Erythroid-cell-specific properties of transcription factor GATA-1 revealed by phenotypic rescue of a gene-targeted cell line. Mol Cell Biol. 1997;17:1642–1651. doi: 10.1128/mcb.17.3.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson KD, Christensen HM, Zhao B, Bresnick EH. Distinct mechanisms control RNA polymerase II recruitment to a tissue- specific locus control region and a downstream promoter. Mol Cell. 2001;8:465–471. doi: 10.1016/s1097-2765(01)00309-4. [DOI] [PubMed] [Google Scholar]
- 70.Kiekhaefer CM, Grass JA, Johnson KD, Boyer ME, Bresnick EH. Hematopoietic-specific activators establish an overlapping pattern of histone acetylation and methylation within a mammalian chromatin domain. Proc Natl Acad Sci U S A. 2002;99:14309–14314. doi: 10.1073/pnas.212389499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Letting DL, Rakowski C, Weiss MJ, Blobel GA. Formation of a tissue-specific histone acetylation pattern by the hematopoietic transcription factor GATA-1. Mol Cell Biol. 2003;23:1334–1340. doi: 10.1128/MCB.23.4.1334-1340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Im H, Grass JA, Johnson KD, Kim SI, Boyer ME, Imbalzano AN, Bieker JJ, Bresnick EH. Chromatin domain activation via GATA-1 utilization of a small subset of dispersed GATA motifs within a broad chromosomal region. Proc Natl Acad Sci U S A. 2005;102:17065–17070. doi: 10.1073/pnas.0506164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stopka T, Amanatullah DF, Papetti M, Skoultchi AI. PU.1 inhibits the erythroid program by binding to GATA-1 on DNA and creating a repressive chromatin structure. EMBO J. 2005;24:3712–3723. doi: 10.1038/sj.emboj.7600834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim SI, Bultman SJ, Jing H, Blobel GA, Bresnick EH. Dissecting Molecular Steps in Chromatin Domain Activation during Hematopoietic Differentiation. Mol Cell Biol. 2007;27:4551–4565. doi: 10.1128/MCB.00235-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Demers C, Chaturvedi CP, Ranish JA, Juban G, Lai P, Morle F, Aebersold R, Dilworth FJ, Groudine M, Brand M. Activator-mediated recruitment of the MLL2 methyltransferase complex to the beta-globin locus. Mol Cell. 2007;27:573–584. doi: 10.1016/j.molcel.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim A, Song SH, Brand M, Dean A. Nucleosome and transcription activator antagonism at human beta-globin locus control region DNase I hypersensitive sites. Nucleic Acids Res. 2007;35:5831–5838. doi: 10.1093/nar/gkm620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ley TJ, DeSimone J, Noguchi CT, Turner PH, Schechter AN, Heller P, Nienhuis AW. 5-Azacytidine increases gamma-globin synthesis and reduces the proportion of dense cells in patients with sickle cell anemia. Blood. 1983;62:370–380. [PubMed] [Google Scholar]
- 78.Charache S, Dover G, Smith K, Talbot CC, Jr, Moyer M, Boyer S. Treatment of sickle cell anemia with 5-azacytidine results in increased fetal hemoglobin production and is associated with nonrandom hypomethylation of DNA around the gamma-delta-beta-globin gene complex. Proc Natl Acad Sci U S A. 1983;80:4842–4846. doi: 10.1073/pnas.80.15.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fathallah H, Atweh GF. Induction of fetal hemoglobin in the treatment of sickle cell disease. Hematology Am Soc Hematol Educ Program. 2006:58–62. doi: 10.1182/asheducation-2006.1.58. [DOI] [PubMed] [Google Scholar]
- 80.Mabaera R, Greene MR, Richardson CA, Conine SJ, Kozul CD, Lowrey CH. Neither DNA hypomethylation nor changes in the kinetics of erythroid differentiation explain 5-azacytidine’s ability to induce human fetal hemoglobin. Blood. 2008;111:411–420. doi: 10.1182/blood-2007-06-093948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weinberg RS, Ji X, Sutton M, Perrine S, Galperin Y, Li Q, Liebhaber SA, Stamatoyannopoulos G, Atweh GF. Butyrate increases the efficiency of translation of gamma-globin mRNA. Blood. 2005;105:1807–1809. doi: 10.1182/blood-2004-02-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.DeSimone J, Heller P, Hall L, Zwiers D. 5-Azacytidine stimulates fetal hemoglobin synthesis in anemic baboons. Proc Natl Acad Sci U S A. 1982;79:4428–4431. doi: 10.1073/pnas.79.14.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carr BI, Rahbar S, Asmeron Y, Riggs A, Winberg CD. Carcinogenicity and haemoglobin synthesis induction by cytidine analogues. Br J Cancer. 1988;57:395–402. doi: 10.1038/bjc.1988.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koshy M, Dorn L, Bressler L, Molokie R, Lavelle D, Talischy N, Hoffman ROW, van DeSimone J. 2-deoxy 5-azacytidine and fetal hemoglobin induction in sickle cell anemia. Blood. 2000;96:2379–2384. [PubMed] [Google Scholar]
- 85.DeSimone J, Koshy M, Dorn L, Lavelle D, Bressler L, Molokie R, Talischy N. Maintenance of elevated fetal hemoglobin levels by decitabine during dose interval treatment of sickle cell anemia. Blood. 2002;99:3905–3908. doi: 10.1182/blood.v99.11.3905. [DOI] [PubMed] [Google Scholar]
- 86.Saunthararajah Y, Hillery CA, Lavelle D, Molokie R, Dorn L, Bressler L, Gavazova S, Chen YH, Hoffman R, DeSimone J. Effects of 5-aza-2′-deoxycytidine on fetal hemoglobin levels, red cell adhesion, and hematopoietic differentiation in patients with sickle cell disease. Blood. 2003;102:3865–3870. doi: 10.1182/blood-2003-05-1738. [DOI] [PubMed] [Google Scholar]
- 87.Lavelle D, Vaitkus K, Hankewych M, Singh M, DeSimone J. Effect of 5-aza-2′-deoxycytidine (Dacogen) on covalent histone modifications of chromatin associated with the epsilon-, gamma-, and beta-globin promoters in Papio anubis. Exp Hematol. 2006;34:339–347. doi: 10.1016/j.exphem.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 88.Atweh GF, Sutton M, Nassif I, Boosalis V, Dover GJ, Wallenstein S, Wright E, McMahon L, Stamatoyannopoulos G, Faller DV, Perrine SP. Sustained induction of fetal hemoglobin by pulse butyrate therapy in sickle cell disease. Blood. 1999;93:1790–1797. [PMC free article] [PubMed] [Google Scholar]
- 89.Fathallah H, Weinberg RS, Galperin Y, Sutton M, Atweh GF. Role of epigenetic modifications in normal globin gene regulation and butyrate-mediated induction of fetal hemoglobin. Blood. 2007;110:3391–3397. doi: 10.1182/blood-2007-02-076091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mankidy R, Faller DV, Mabaera R, Lowrey CH, Boosalis MS, White GL, Castaneda SA, Perrine SP. Short-chain fatty acids induce gamma-globin gene expression by displacement of a HDAC3-NCoR repressor complex. Blood. 2006;108:3179–3186. doi: 10.1182/blood-2005-12-010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singal R, Wang SZ, Sargent T, Zhu SZ, Ginder GD. Methylation of promoter proximal-transcribed sequences of an embryonic globin gene inhibits transcription in primary erythroid cells and promotes formation of a cell type-specific methyl cytosine binding complex. J Biol Chem. 2002;277:1897–1905. doi: 10.1074/jbc.M105580200. [DOI] [PubMed] [Google Scholar]
- 92.Fathallah H, Portnoy G, Atweh GF. Epigenetic analysis of the human alpha- and beta-globin gene clusters. Blood Cells Mol Dis. 2008;40:166–173. doi: 10.1016/j.bcmd.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Witt O, Monkemeyer S, Ronndahl G, Erdlenbruch B, Reinhardt D, Kanbach K, Pekrun A. Induction of fetal hemoglobin expression by the histone deacetylase inhibitor apicidin. Blood. 2003;101:2001–2007. doi: 10.1182/blood-2002-08-2617. [DOI] [PubMed] [Google Scholar]
- 94.Wei GH, Zhao GW, Song W, Hao DL, Lv X, Liu DP, Liang CC. Mechanisms of human gamma-globin transcriptional induction by apicidin involves p38 signaling to chromatin. Biochem Biophys Res Commun. 2007;363:889–894. doi: 10.1016/j.bbrc.2007.06.191. [DOI] [PubMed] [Google Scholar]
- 95.Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA, Olson EN. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell. 2002;110:479–488. doi: 10.1016/s0092-8674(02)00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]