Abstract
Extracellular UDP-glucose is a natural purinergic receptor agonist, but its mechanisms of cellular release remain unclear. We studied these mechanisms in Saccharomyces cerevisiae, a simple model organism that releases ATP, another purinergic agonist. Similar to ATP, UDP-glucose was released by S. cerevisiae at a rate that was linear over time. However, unlike ATP release, UDP-glucose release was not dependent on glucose stimulation. This discrepancy was resolved by demonstrating the apparent glucose stimulation of ATP release reflected glucose-dependent changes in the intracellular pattern of adenine nucleotides, with AMP release dominating in the absence of glucose. Indeed, total adenine nucleotide release, like UDP-glucose release, did not vary with glucose concentration over the short term. The genetic basis of UDP-glucose release was explored through analysis of deletion mutants, aided by development of a novel bioassay for UDP-glucose based on signaling through heterologously expressed human P2Y14 receptors. Using this assay, an elevated rate of UDP-glucose release was demonstrated in mutants lacking the putative Golgi nucleotide sugar transporter YMD8. An increased rate of UDP-glucose release in ymd8Δ was reduced by deletion of the YEA4 UDP-N-acetylglucosamine or the HUT1 UDP-galactose transporters, and overexpression of YEA4 or HUT1 increased the rate of UDP-glucose release. These findings suggest an exocytotic release mechanism similar to that of ATP, a conclusion supported by decreased rates of ATP, AMP, and UDP-glucose release in response to the secretory inhibitor Brefeldin A. These studies demonstrate the involvement of the secretory pathway in nucleotide and nucleotide sugar efflux in yeast and offer a powerful model system for further investigation.
Purinergic signaling pathways are present in nearly all mammalian cells (1) and regulate numerous critical physiologic functions, including control of extracellular volume (2, 3) and inflammatory responses (4–7). The natural agonists of purinergic receptors are extracellular nucleosides, nucleotides, and nucleotide sugars such as adenosine triphosphate (ATP)1 and uridine diphosphate (UDP) glucose (UDP-glucose). The physiological actions mediated by purinergic pathways depend on cellular release of these agonists into the extracellular space. However, while vesicular release of ATP has been demonstrated in neuro-endocrine cells (1), the mechanisms by which ATP, UDP-glucose, and other purinergic agonists exit the cytoplasm in other cell types remain unclear.
Several mechanisms have been proposed to account for release of purinergic agonists in non-neuroendocrine cells. ATP release has been most intensively studied in this context, and conductive efflux through the cystic fibrosis conductance regulator (8, 9), connexins (10, 11), a maxi-anion channel (12), and a plasma membrane-localized splice variant of the mitochondrial voltage-dependent anion channel (13) has been described. Alternatively, ATP and other purinergic agonists may be released into the extracellular space via exocytosis, a hypothesis which has experimental support in both mammalian cells (14) and Xenopus oocytes (15). Similarly, the high concentration of UDP-glucose within the secretory apparatus (16) suggests that its release mechanism may also be exocytotic (17).
While critical to understanding signaling physiology, elucidation of release mechanisms of purinergic agonists has been hampered by the limitations of the genetic and biochemical tools available for studying mammalian and vertebrate cells. Extracellular nucleotide-mediated signaling has been described in evolutionarily diverse organisms, includinginsects(18,19),worms(20),andevenplants(21–23), suggesting that mechanisms for release of purinergic agonists may have early evolutionary origins. Indeed, ATP release has been demonstrated in a simple model system, the baker’s yeast Saccharomyces cerevisiae (24, 25). While yeast do not naturally express purinergic receptors, they release relatively large quantities of ATP into the extracellular space in response to glucose. This finding has facilitated exploration of release mechanisms, with recent determination that the product of the yeast gene MCD4 mediates the transport of ATP into the Golgi for release via exocytosis (24). These findings suggest that yeast could serve as a model system for studying the release of other nucleotides or nucleotide sugars.
This study utilized yeast to study the release mechanisms of UDP-glucose, the natural agonist of P2Y14 receptors in mammals. Efflux of UDP-glucose from yeast was studied in comparison to release of ATP and its metabolites (ADP, AMP, and adenosine). Both genetic and biochemical means were utilized, and these studies were facilitated by development of a UDP-glucose bioassay based on signaling through heterologous expression of a modified mammalian P2Y14 receptor (26). Using this bioassay, the effects of a biochemical blockade of exocytosis and the impact of mutants known to affect accumulation of nucleotide sugars within the ER and Golgi were examined. These studies helped elucidate the role of exocytosis and other potential release mechanisms of UDP-glucose and other naturally occurring purinergic agonists in yeast, providing insights that may facilitate studies in higher organisms.
EXPERIMENTAL PROCEDURES
Yeast Strain and Materials
The wild-type strain was BY4741 (MATa his3Δ leu2Δ met15Δ ura3Δ). Yeast knockout (YKO) mutants were purchased from Research Genetics (Huntsville, AL) and used BY4741 as the parent strain. The yeast strain used for the UDP-glucose bioassay was CY10981 (PFUS1-HIS3 can1-100 far1Δ1442 his3Δ200 leu2-3,112 lys2 sst2Δ2 ste14::trp1::LYS2 ste3Δ1156 trp1-1 ura3-52) carrying plasmid Cp1021 (PFUS1-LacZ 2 μm URA3) and P2Y14-2211 cloned into plasmid Cp1651 (PPGK1-hP2Y14 2 μm LEU2) as previously described (26). Brefeldin A was purchased from Sigma-Aldrich (St. Louis, MO) and 3-aminotriazole from Fisher Scientific (Pittsburgh, PA).
Extracellular and Intracellular Collections
Yeast were grown overnight in YPD or selective medium, recovered by centrifugation, and resuspended in 20 mM Tris and 1 mM KH2PO4 (pH 7.6) (TK buffer) with or without carbohydrate [typically 1% (w/v) glucose] as indicated in the text. Cells were incubated in TK buffer with or without carbohydrate for 15 min at 30 °C while being shaken, then centrifuged, and resuspended in TK buffer with or without carbohydrate. Extracellular collections were obtained at the indicated times and centrifuged for 5 min at 11000g, and supernatants were recovered and stored on ice. Intracellular collections were obtained by aliquoting 100 μL of cell suspension in TK buffer in a buffered ethanol solution [41 mL of ethanol and 2 mL of 1 M Tris (pH 7.6)] at 80 °C for 3 min and then transferred to ice. Intracellular collections were then diluted 1:10 in TK buffer. All collections were boiled for 2 min then stored at –20 °C until they were analyzed. All results from these samples derived from yeast are presented as the concentration of nucleotide, nucleoside, or nucleotide sugar divided by cell concentration as estimated by the optical density at 600 nm (OD600).
Adenine Nucleotide and Nucleoside Detection
Luminometry for ATP was performed as previously described (27) using 30 μL of sample diluted 1:10 in water and loaded into the autosampler of a LB953 luminometer. One hundred microliters of the luciferin-luciferase reaction mix was injected and luminescence was recorded over 10 s and compared to an ATP standard curve performed in parallel. To measure all adenine nucleotides and nucleosides, etheno derivitization and HPLC were utilized (2). Briefly, 200 μL aliquots of sample were incubated for 30 min at 72 °C in the presence of 1.0 M chloroacetaldehyde and 25 mM Na2HPO4 (pH 4.0), transferred to ice, alkalinized with 50 μL of 0.5 M NH4HCO3, and analyzed by HPLC within 24 h. HPLC-based identification and quantification of ethenylated species were performed with an automated Waters HPLC apparatus equipped with a fluorescence detector by injection into a 250 mm, 10 μm Hamilton PRP-X100 anion exchange column using methanol and NH4HCO3 (pH 8.5) as the mobile phase. Because a component of the Tris buffer caused a fluorescent signal that interfered with etheno-derivatized adenosine detection, extracellular collections for adenosine assessment were performed in 10 mM KPO4 (pH 7.6) in lieu of TK buffer.
Invertase Expression
Invertase secretion was assessed in wild-type yeast grown overnight in YP and 2% (w/v) galactose and then rinsed and resuspended in TK glucose without sugar. Invertase activity was assessed by removing 20 μL of the cell suspension at various time points and incubating the samples at 37 °Cin100 μL of 50 mM NaOAc (pH 5.1), 0.1% Triton X-100, and 25 μL of 0.5 M sucrose. After 10 min, 60 μL of 0.5 M KH2PO4 was added to stop the reaction, the samples were centrifuged to remove cells, and the supernatant was boiled for 2 min. Liberated glucose was assessed using a glucose oxidase kit from Pointe Scientific (Canton, MI).
UDP-Glucose Biochemical Assay
The biochemical assay for UDP-glucose was performed as previously described (28). Samples (150 μL) were incubated in 25 mM HEPES (pH 7.4), 2 mM MgCl2, 1.6 mM CaCl2, 0.5 unit/mL UDP-glucose pyrophosphorylase from baker’s yeast (Sigma-Aldrich), and 100 nM [32P]PPi (200000 cpm). Incubations were terminated by addition of 0.3 mM PPi and heating of samples for 2 min at 95 °C. Conversion of UDP-glucose to [32P]UTP was assessed using HPLC (Shimadzu, Kyoto, Japan) via a 3.9 mm × 150 mm Nova-Pack C18 column (Waters, Milford, MA) with an ion pairing mobile phase (1 mL/min) consisting of 8 mM tetrabutylammonium hydrogen sulfate, 60 mM KH2PO4 (pH 5.3), and 10% methanol, and radioactivity was monitored online.
UDP-Glucose Bioassay
The bioassay was developed using yeast strain Cy10981 transformed with a modified P2Y14 receptor (P2Y14-2211) and a FUS1::LacZ construct in a high-copy expression vectors as previously described (26). The transformed Cy10981 was grown to an OD600 of ∼1.0, washed twice in sterile water, and resuspended in histidine free selective medium with 10 mM 3-AT at an OD600 of 0.1. A portion of this culture (1.8 mL) and 200 μL of sample were incubated for 20–24 h at 30 °C, and growth was measured as OD600.
Deletion Mutants
The YMD8 deletion mutant was created by cloning a genomic fragment containing the YMD8 gene by PCR (primers, 5′-AGCCAAAAATCTGGCCTATCA-3′ and 5′-TGGGTGGTATGTTGGAATAGA-3′) from the BY4741 parent strain into the pYES2.1/V5-His-TOPO vector (Invitrogen, Carlsbad, CA). The bulk of the YMD8 coding region was removed by restriction digestion with MfeI and BamHI and replaced with the MET15 gene isolated by PCR (primers, 5′-CGCGAATTCTATCTTCGGATGCAAGGGTT-3′ and 5′-CGCGGATCCTTTTTCTTCTCGACTGCGAAT-3′) from a genomic S. cerevisiae library in the YCp50 vector (ATCC, Manassas, VA) and digested with EcoRI and BamHI. The resulting deletion construct was isolated by PCR using the YMD8 primers described above and transformed using the TRAFO procedure into a BY4741 or YKO deletion strain as indicated in the text (29). Successful deletions were isolated by conversion from methionine auxotrophy and verified by PCR. The novel YEA4 deletion mutant was created by cloning the coding region of the YEA4 gene by PCR (5′-CGCTCTAGAATGTGGAACTCATAAAAGCA-3′ and 5′-CGCAAGCTTTCATTTACTTTTCTTTATCGC-3′) into the pYES2.1/V5-His-TOPO vector. The gene was disrupted by inserting a LEU2 gene isolated by PCR from pRS405 (5′-GCGGATCCTCCTCCTTTTTCTCCTTCTTG-3′ and 5′-GCGGATCCTTGTCCTGTACTTCCTTCTTCA-3′)into the BglII site in the YEA4 coding region. The deletion construct was isolated by PCR and transformed into YKO deletion strains as indicated in the text, with successful deletions selected by conversion from leucine auxotrophy and verified by PCR.
Overexpression of Nucleotide-Sugar Transporters
PCR was used to amplify the coding regions of HUT1 (5′CGCGGATCCATGGCGGGAAGTACATCCAGT-3′ and 5′-CGCGAATTCCTACGCAGATTTTGCCTTCG-3′) and YEA4 (primers as described above) which were then cloned into the pYES2.1/V5-His-TOPO vector. The plasmid was transformed into strain BY4741 by TRAFFO and selected on —ura selective medium. Successful transformants were grown to confluence in —ura selective medium with galactose as a carbon source to activate expression of the genes from the GAL1 promoter within the vector. Extracellular collections were performed as described above using TK buffer and 1% (w/v) galactose.
Statistical Analyses
All data are reported as means ± the standard deviation. Differences between groups were compared using a Student’s t test.
RESULTS
Yeast Release UDP-Glucose
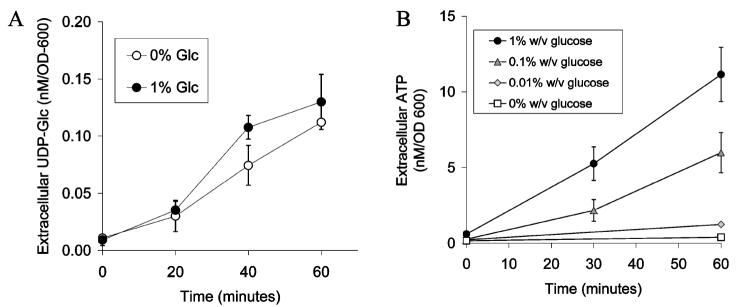
The previously demonstrated ability of yeast to release ATP suggests that this organism may release other purinergic agonists. To test this hypothesis, we examined the ability of yeast to release the nucleotidesugar UDP-glucose, the natural agonist of the P2Y14 receptor (30). We measured extracellular levels of UDP-glucose using a previously described biochemical assay (28) and compared these results to those for ATP release measured by luminometry. Yeast incubated in 1% (w/v) glucose released UDP-glucose at a rate that was approximately 1% of that of ATP under similar conditions (Figure 1A,B). Like that of ATP, release was linear over 1 h. However, in contrast to that of ATP, the rate of release of UDP-glucose was not significantly decreased in the absence of glucose (Figure 1A,B).
Figure 1.
Yeast release UDP-glucose. (A) Yeast were incubated in assay buffer with 0% (○) or 1% (w/v) glucose (◼) and UDP-glucose levels measured at the indicated times via a biochemical assay (n = 4). Yeast release UDP-glucose in a linear fashion in both the presence and absence of glucose. (B) ATP levels in yeast incubated with varying concentrations of glucose were measured at the indicated times (n = 4). Unlike that of UDP-glucose, the rate of release of ATP was greatly reduced in the absence of glucose.
While the significant reduction of the rate of ATP release in the absence of glucose has previously been interpreted as evidence of glucose-dependent stimulation of nucleotide release (24, 25), we noted yeast incubated in media without glucose are known to have decreased intracellular concentrations of ATP and increased intracellular concentrations of the lower-energy adenine nucleotides ADP and AMP (31). To determine if this glucose-dependent pattern of intracellular adenyl nucleotides impacted efflux, we measured extracellular and intracellular levels of all adenine nucleotides. As expected, yeast incubated in 1% (w/v) glucose released predominantly ATP, with very little ADP or AMP (Figure 2A). In contrast, cells incubated in the absence of glucose released significant quantities of nucleotides, but predominantly as AMP rather than ATP. Intermediate sugar concentrations [0.1% (w/v) glucose] supported release of intermediate levels of all three nucleotides. These findings were not likely influenced by extracellular nucleotide metabolism, since experimental conditions were chosen to minimize extracellular phosphatase activity (32), and exogenously added ATP (100 nM) did not undergo significant metabolism (122 ± 43% initial concentration after incubation for 1 h in TK buffer without glucose). Total adenine nucleotide release was similar at all sugar concentrations assayed over 1 h, suggesting that the net rate of release of adenine nucleotides was not influenced by glucose concentration over the relatively short time period of these experiments. These release patterns reflected the relative composition of intracellular nucleotide pools; e.g., yeast incubated without glucose accumulated higher levels of extracellular and intracellular AMP (Figure 2B). However, we also observed that yeast incubated without glucose retained a fraction of their intracellular nucleotide pool as ATP yet released AMP almost exclusively, suggesting that not all intracellular pools participate in release. Adenosine release was assessed independently and did not appear to be significantly different from zero (1.4 ± 2.1 nM/OD600, n = 4). Overall, these data suggest that neither ATP nor UDP-glucose release is stimulated by glucose, but that the pattern of nucleotide release reflects intracellular concentrations. Indeed, preservation of UDP-glucose release in the absence of extracellular glucose is consistent with previous data suggesting that yeast preserve intracellular nucleotide sugar concentrations in the absence of an energy source (31).
Figure 2.
Yeast release adenine nucleotides in a pattern dependent on intracellular adenine nucleotide concentations. (A) Yeast were incubated in varying concentrations of glucose, and net release of all adenyl nucleotides after 1 h was measured (n = 4). (B) Intracellular adenine nucleotide composition of yeast incubated in various glucose concentrations, showing accumulation of intracellular AMP in cells incubated without glucose (n = 2). (C) Yeast incubated without glucose continue to secrete over 1 h, as evidenced by increasing invertase activity. Addition of galactose did not significantly increase the initial secretion rate. Invertase activity actually stabilized after the 30 min time point, reflecting galactose-induced inhibition of invertase expression. (D) Yeast were grown in media containing glucose or galactose as indicated in the figure and then assayed after being incubated for 60 min in buffer containing 1% (w/v) galactose. Extracellular (black bars, left axis) and intracellular (white bars, right axis) ATP levels were measured (n = 4). Cells grown in glucose exhibited low extracellular and intracellular ATP levels in the presence of galactose, but cells grown in galactose have high intracellular ATP levels and release significant quantities of ATP. An asterisk indicates p < 0.05 vs control.
Yeast Maintain Exocytosis during Short-Term Incubation without Glucose
Exocytosis is a process that requires energy, yet our findings suggested that exocytosis accounted for adenine nucleotide and UDP-glucose release in the absence of glucose or other energy sources. We postulated that the yeast in our experiments retained sufficient energy stores to continue exocytosis over the relatively brief (1 h) period of our experiments, consistent with our previous observation that a fraction of intracellular ATP was retained after incubation in the absence of glucose. To test this hypothesis directly, we examined extracellular accumulation of the secreted enzyme invertase, commonly used as a marker of yeast secretion. Because glucose interferes with the invertase assay, we examined secretion in response to galactose in yeast preconditioned to utilize this sugar as an energy source. In these experiments, invertase secretion occurred in both the presence and absence of galactose (Figure 2C), indicating that exocytosis continued under our experimental conditions. It could be argued that these experiments are not relevant to nucleotide release mechanisms, since yeast were previously reported not to release ATP in response to galactose (24). However, on the basis of our previous results, we suspected that this finding reflected the known inability of yeast grown in glucose-containing media to immediately utilize galactose as an energy source, leading to low intracellular ATP levels available for release. Indeed, while we verified that yeast grown in glucose do not release ATP when incubated with galactose, we also demonstrated that intracellular ATP levels were low under these conditions (Figure 2D). In contrast, yeast preconditioned to utilize galactose by prior growth in galactose-containing media had intracellular ATP levels and extracellular release rates similar to those of yeast grown and incubated in glucose. These findings not only validate the use of galactose in the previous experiments but also provide further evidence that release of purinergic agonists does not require glucose specific stimulation.
Mutants That Alter UDP-Glucose Release
To further explore the mechanisms underlying UDP-glucose release, we examined the effect of mutation of genes encoding the nucleotide sugar transporters known to concentrate these compounds with the secretory apparatus. We identified eight nucleotide sugar transporter homologues in S.cerevisiae(33,34) (Table 1). The products of three of these genes have been shown to transport nucleotide sugars: VRG4 (GDP-mannose) (34), HUT1 (UDP-galactose) (35), and YEA4 (UDP-N-acetylglucosamine) (36). HVG1 is very similar to VRG4 (92% protein similarity), although no function has been ascribed to its gene product. Two other genes, YMD8 and the hypothetical open reading frame YML0138C, have no known function but are ∼40% similar to VRG4. Through BLAST searches (37) with the known nucleotide sugar transporter homologues, another homologous gene was identified, the hypothetical open reading frame YDR438W. The last gene, MCH5, is homologous to VRG4 but was recently identified as a plasma membrane riboflavin transporter and is, therefore, unlikely to be a nucleotide sugar transporter (38).
Table 1.
Nucleotide Sugar Transporter Homologues in S. cerevisiae
| gene | function | gene | function |
|---|---|---|---|
| VRG4 | GDP-mannose transporter | YMD8 | unknown |
| HUT1 | UDP-galactose transporter | YML018C | unknown |
| YEA4 | UDP-N-acetylglucosamine transporter |
YDR438W | unknown |
| HVG1 | unknown | MCH5 | riboflavin transport |
UDP-glucose release after incubation in 1% (w/v) glucose was measured in the wild type and all seven available deletion mutants (VRG4 deletion mutants are not viable). Net UDP-glucose release after 1 h was not significantly impaired in any mutant; however, the rate of UDP-glucose release was increased significantly in the ymd8Δ mutant (Figure 3A). Extracellular ATP levels were similar to that of the wild type in the ymd8Δ strain and all other deletion mutants, suggesting that the increased rate of UDP-glucose release was not due to nonspecific changes in membrane permeability (Figure 3A), and no significant extracellular metabolism of UDP-glucose was detected in either the WT or ymd8Δ strain (the fraction of [3H]UDP-glucose recovered after incubation for 60 min was 97.9 ± 0.9% for WT and 99.7 ± 0.4% for ymd8Δ). Like that of the wild type, release of UDP-glucose from the ymd8Δ strain was linear over time (Figure 3B). To determine if an elevated rate of UDP-glucose release in the ymd8Δ strain reflected altered intracellular UDP-glucose concentrations, we measured the levels of extracellular and intracellular UDP-glucose in both the wild type and the mutant after incubation for 1 h. Interestingly, intracellular UDP-glucose concentrations were elevated in the ymd8Δ strain relative to that in the wild type, but the relative increase was less than the relative increase in the extracellular concentration of UDP-glucose (Figure 3C). In other words, the ymd8Δ strain released a proportionally greater percentage of its intracellular UDP-glucose over 1 h [3.2 ± 1.2% for ymd8Δ and 0.9 ± 0.4% for WT (p < 0.05)].
Figure 3.
Rate of UDP-glucose release increased in the ymd8Δ strain. (A) The net release of UDP-glucose (gray bars) and ATP (black bars) over 1 h was assessed in the wild-type strain and in deletion mutants of seven putative nucleotide sugar transporters (n = 4). UDP-glucose release was not significantly inhibited in any mutants but was accelerated significantly in the ymd8Δ mutant. (B) Extracellular UDP-glucose (gray symbols) and ATP (black symbols) levels were measured in both WT (circles) and YMD8 deletion (triangles) strains at the indicated time points (n = 2). The rate of release of UDP-glucose was linear in both WT and the ymd8Δ mutant but was much higher in the latter. ATP release was also linear and similar in both strains. (C) Extracellular (net release over 1 h, black bars) and intracellular (1 h time point, white bars) UDP-glucose concentrations were assessed in WT and the ymd8Δ mutant (n = 3). Both extracellular and intracellular UDP-glucose concentrations were elevated in the ymd8Δ mutant, though the relative increase over that in WT was greater for extracellular than intracellular UDP-glucose.
Bioassay for UDP-Glucose
The biochemical assay for UDP-glucose proved to be technically demanding, and to simplify the analysis, we developed a bioassay for UDP-glucose based on heterologous expression of the mammalian P2Y14 receptor in specialized yeast strains. For this bioassay, we utilized engineered yeast strains expressing a modified version of the human P2Y14 UDP-glucose receptor known to have increased sensitivity to nucleotide sugars (26). Receptor stimulation in this strain led to expression of the HIS3 gene in a his3Δ background, permitting detection of receptor activation as growth in histidine deficient media.
To determine the sensitivity and dynamic range of the bioassay, the P2Y14 receptor expression strain was incubated in histidine deficient media with various concentrations of UDP-glucose, and growth at 24 h was measured. UDP-glucose could be detected as growth above background with sensitivity in the low nanomolar range (Figure 4A). We carried out similar experiments using UDP-galactose and UDP-N-acetylglucosamine. These nucleotide sugars also stimulated concentration-dependent growth, consistent with their known ligand specificities at the P2Y14 receptor (26, 30).
Figure 4.
Development of a bioassay for UDP-glucose. A bioassay for UDP-glucose was developed using a yeast strain expressing a modified human P2Y14 receptor (P2Y14 —2211) (26). (A) The GPCR-sensing strain was incubated for 24 h in histidine deficient media in the presence of various concentrations of nucleotide sugars. Nanomolar concentrations of nucleotide sugars stimulate detectable growth, with the following rank potency at low concentrations: UDP-glucose (UDP-glc) > UDP-galactose (UDP-gal) > UDP-N-acetylglucosamine (UDP-GlcNAc). (B) Extracellular collections from WT and ymd8Δ mutants were tested in the bioassay with standards of 10 nM UDP-sugars as controls. The extracellular collection from the ymd8Δ strain stimulated significant growth in the bioassay, suggesting a high concentration of UDP-glucose in this sample. The rate of growth in the ymd8Δ extracellular collection and the UDP-glc and UDP-gal controls was significantly decreased by pretreatment for 30 min at 37 °C with 2 units/mL UDP-glucose pyrophosphorylase (UDP-Glc PP), demonstrating that the growth signal was due to the presence of UDP-glucose or UDP-galactose.
To determine if the bioassay could detect UDP-glucose in biological samples, we evaluated the growth response to extracellular media collected from the wild type (slow UDP-glucose release) and ymd8Δ strain (fast UDP-glucose release) with UDP-sugar controls. As expected, the extracellular media from the ymd8Δ strain supported significantly more growth than that collected from the wild-type strain (Figure 4B). To test specificity, the UDP-sugar standard solutions and extracellular media were treated with UDP-glucose pyrophosphorylase prior to the bioassay, which is known to metabolize both UDP-glucose and UDP-galactose but not UDP-N-acetylglucosamine (39). Treatment with the pyrophosphorylase diminished the bioassay signals from UDP-glucose and UDP-galactose controls and from extracellular media, confirming that the bioassay signal was mediated by nucleotide sugars (Figure 4B). As expected, the bioassay signal from the UDP-N-acetylglucosamine control was not affected by enzymatic treatment.
UDP-Glucose Release Is Mediated by Nucleotide Sugar Transporters
To determine whether other nucleotide sugar transporters were involved in UDP-glucose release in the ymd8Δ strain, we deleted the YMD8 gene in each nucleotide sugar transporter deletion strain to create double deletion mutants and in the wild-type background as a control. UDP-glucose release after incubation for 60 min in 1% (w/v) glucose was assayed in all using both the biochemical assay (Figure 5A) and bioassay (Figure 5B). As expected, the new ymd8Δ mutant exhibited an elevated rate of release of extracellular UDP-glucose, similar to the YKO YMD8 deletion mutant. The results were similar for the two assays, further confirming the validity of the bioassay. Compared to that of the ymd8Δ strain, the rate of UDP-glucose release was decreased modestly in the ymd8Δ/hut1Δ mutant and more significantly in the ymd8Δ/yea4Δ mutant, though both double deletion mutants exhibited significantly more UDP-glucose release than wild-type yeast. The other double deletion mutants had elevated rates of UDP-glucose release, similar to that of the single ymd8Δ mutant. These results suggest that YEA4, and to a lesser extent HUT1, support the increased rate of release of UDP-glucose in the ymd8Δ background.
Figure 5.

Nucleotide sugar transporters encoded by HUT1 and YEA4 mediate UDP-glucose release in the ymd8Δ mutant. (A) The biochemical UDP-glucose assay was used to measure the rate of release of extracellular UDP-glucose in single and double deletion mutants after incubation for 1 h in 1% (w/v) glucose. An elevated rate of release of UDP-glucose was observed in the ymd8Δ mutant, while the ymd8Δ/hut1Δ and ymd8Δ/yea4Δ double mutants had rates of release that were decreased from that of the single ymd8Δ mutant although higher than that of the wild type (n = 2). (B) The bioassay was used to measure UDP-glucose concentrations in single, double, and triple deletion mutants after incubation for 1 h in 1% (w/v) glucose. Results were overall similar to those of the biochemical assay, although the ymd8Δ/hut1Δ mutant was not significantly different from the ymd8Δ mutant. UDP-glucose release in a ymd8Δ/ hut1Δ/yea4Δ triple was similar to that of the ymd8Δ/yea4Δ double mutant (n = 3). An asterisk indicates p < 0.05 vs WT. A dagger indicates p < 0.05 vs ymd8Δ.
To further examine the contribution of HUT1- and YEA4-encoded transporters to UDP-glucose release, a hut1Δ/yea4Δ double mutant and a ymd8Δ/hut1Δ/yea4Δ triple mutant were created. Extracellular UDP-glucose levels in the ymd8Δ/hut1Δ/yea4Δ triple mutant were similar to that of the ymd8Δ/yea4Δ double mutant, i.e., significantly lower than that of the ymd8Δ single mutant but higher than that of the wild type (Figure 5B). The hut1Δ/yea4Δ double mutant had extracellular UDP-glucose release similar to that of the wild type. We therefore conclude that the YEA4 gene product mediates most of the excess UDP-glucose release in the ymd8Δ strain, with the HUT1 gene product playing a lesser role.
The roles of YMD8, YEA4, and HUT1 in UDP-glucose release were further assessed by overexpressing these genes and measuring the rate of release of UDP-glucose with the bioassay. Overexpression of YMD8 had little effect on UDP-glucose release in a wild-type strain but did partially correct the increased rate of release in the ymd8Δ strain (Figure 6A). In contrast, overexpression of either HUT1 or YEA4 in the wild type led to an increased rate of UDP-glucose release (Figure 6B).
Figure 6.
Overexpression of HUT1 and YEA4 nucleotide sugar transporters increases the rate of UDP-glucose release. (A) A wild-type copy of the YMD8 gene was cloned by PCR into a GAL1 overexpression vector to form the pYMD8 plasmid, which was then transformed into wild-type and ymd8Δ strains. Cells for the extracellular collection were grown to confluence in selective media with galactose to induce gene expression. The bioassay was used to detect UDP-glucose in extracellular collections from 1 h incubations in 1% (w/v) galactose. Overexpression of YMD8 has no impact on UDP-glucose release in WT strains but did significantly decrease the rate of release in a ymd8Δ strain (n = 3). (B) Wild-type copies of the HUT1 and YEA4 genes were cloned into the overexpression vector to form plasmids pHUT1 and pYEA4, which were transformed into wild-type strains. The bioassay was used to detect extracelluar UDP-glucose in extracellular collections. Both HUT1 and YEA4 overexpression led to significant increases in the rate of UDP-glucose release (n = 3). An asterisk indicates p < 0.05 vs vector alone.
ATP, AMP, and UDP-Glucose Efflux Are Inhibited by a Biochemical Blockade of Secretion
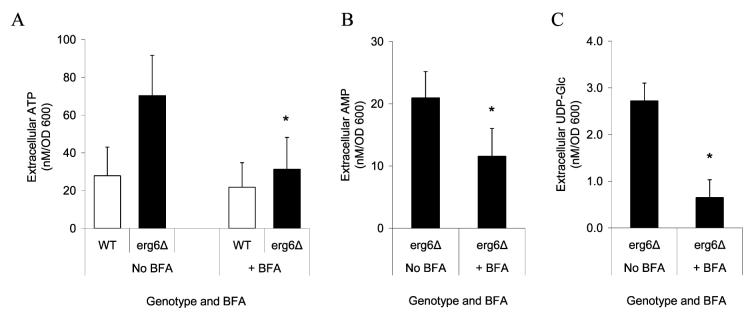
Previous studies have demonstrated that the rate of release of ATP is decreased by pharmacological blockade of secretion (24), suggesting that such blockade could alter efflux of other adenine nucleotides and UDP-glucose. To test this hypothesis, we first verified previous studies by incubating yeast with the inhibitor of secretion, Brefeldin A (BFA). BFA does not penetrate the cell membrane of wild-type yeast, and incubation with BFA had no effect on ATP efflux in wild-type yeast (Figure 7A). Therefore, we used erg6Δ mutants in which BFA can penetrate the cell and inhibit secretion (40, 41). In the erg6Δ mutant, incubation with BFA caused a substantial decrease in the rate of ATP release in cells incubated with glucose (Figure 7A) and in the rate of AMP release when cells were incubated in the absence of glucose (Figure 7B). Similarly, BFA inhibited UDP-glucose release as measured by the bioassay (Figure 7C). These results indicate that pharmacological blockade of secretion inhibits release of both nucleotides and nucleotide sugars, consistent with an exocytotic release mechanism.
Figure 7.
Brefeldin A inhibits ATP, AMP, and UDP-glucose release in susceptible strains. Yeast were incubated in the presence or absence of 150 μg/mL Brefeldin A (BFA) beginning 2 h prior to the assay, and net rates of ATP, AMP, or UDP-glucose release were determined after incubation for 1 h. (A) In cells incubated in 1% (w/v) glucose, incubation with BFA had little impact on wild-type cells but caused a significant reduction in ATP levels in the sensitive erg6Δ deletion strain (n = 3). (B) In erg6Δ cells incubated without glucose, BFA inhibited AMP release (n = 5). (C) Incubation with BFA significantly reduced the rate of release of UDP-glucose in the erg6Δ strain (n = 4). An asterisk indicates p < 0.05.
DISCUSSION
This study demonstrates that yeast release both adenine nucleotides (ATP and its metabolites) and a nucleotide sugar (UDP-glucose) into the extracellular medium, representing the first evidence of UDP-glucose efflux by yeast. Release of UDP-glucose was similar to that of adenine nucleotides: linear over time and reduced by the secretory inhibitor Brefeldin A. These similarities suggest that UDP-glucose may share the exocytotic release mechanisms previously identified for ATP (24). The fact that UDP-glucose release was perturbed by manipulation of the genes encoding nucleotide sugar transporters lends further support to the involvement of secretory pathways, since these transporters concentrate nucleotide sugars into the ER or Golgi compartments (35, 36) where they would be available for exocytotic release (28, 42). Such compartmentalization could also explain the discrepancies between intracellular and extracellular concentrations of adenine nucleotides or UDP-glucose observed under various experimental conditions.
Our studies suggest that UDP-glucose release is mediated through the Hut1p UDP-galactose transporter and the Yea4p UDP-N-acetylglucosamine transporter. Although previous reports indicated that Hut1p and Yea4p transport other nucleotide sugars, our results suggest that these transporters can also transport UDP-glucose, an activity consistent with broad substrate specificity observed in nucleotide sugar transporters in other species (43). The HUT1 and YEA4 gene products are likely not the only UDP-glucose transporters in yeast, since deletion of both genes did not eliminate efflux of UDP-glucose from either the wild-type or ymd8Δ strain.
It is not clear why deletion of the YMD8 gene resulted in an increase in the rate of UDP-glucose release. YMD8 is homologous to known nucleotide sugar transporters (34) and, like other members of the class, is localized to the Golgi apparatus (44) and interacts with other Golgi proteins (45). Therefore, YMD8 almost certainly encodes a nucleotide sugar transporter, although our data suggest that it is unlikely to transport UDP-glucose. However, its activity could influence the transport rates of other nucleotide sugar transporters that do transport UDP-glucose. All known UDP-sugar transporters are UMP antiporters, and transport rates depend on the intravesicular concentrations of UMP. Loss of YMD8-mediated transport activity in the ymd8Δ strain could lead to elevated intravesicular UMP levels and increased transport activities of other UDP-sugar transporters, including those encoded by HUT1 and YEA4. While this would result in higher intravesicular concentrations of multiple nucleotide sugars, excess UDP-galactose and UDP-N-acetylglucosamine could be consumed through enzymatic reactions. In contrast, there are no known intravesicular reactions that utilize UDP-glucose in S. cerevisiae (16), and even modest increases in transport rates could result in significant accumulation and release of this nucleotide sugar. This mechanism would also explain why overexpression of HUT1 or YEA4 causes only a moderate increase in the rate of UDP-glucose release, since these transporters would have to compete with others for UMP.
UDP-Glucose Bioassay
The studies of UDP-glucose release were greatly facilitated by development of a simple, sensitive bioassay based on signaling through the P2Y14 receptor. While functional purinergic receptors have previously been expressed in yeast, this is the first report using a purinergic receptor bioassay to analyze biologic samples. The assay proved reliable and generated data comparable to those of a previously established biochemical assay for UDP-glucose. The utility of this assay will permit future investigations involving large-scale analysis of mutants that were not feasible with the biochemical assay.
Because yeast are not known to express purinergic receptors, it is not clear why they would release nucleotides or nucleotide sugars into their environment. Nonreceptor nucleotide-mediated signaling could occur, and indeed, nucleotide secretion, reuptake, and utilization have been proposed as a basis for cell-to-cell communication in sporulating yeast (46). Alternatively, extracellular nucleotides could provide a source of inorganic phosphate for extracellular enzymatic reactions, which has been observed in Arabidopsis (47). Regardless, these findings suggest that the mechanisms that support release of nucleotides and nucleotide sugars that occurred early in evolution are likely found in many cell types. While more complex mechanisms for regulated release of nucleotides may have evolved later, the efflux pathway identified here offers the opportunity for genetic and biochemical studies that are not possible in mammalian cells.
In summary, this study demonstrates that yeast release nucleotides and nucleotide sugars into the extracellular space via what is most likely an exocytotic release mechanism. The constitutive efflux may model basal nucleotide and nucleotide sugar release that occurs in many cell types (2, 27), and the genes identified in this study have homologues in mammalian cells (24, 42). These observations suggest that yeast is a model system for studies of the release mechanisms of compounds that serve as purinergic agonists in vertebrate systems.
ACKNOWLEDGMENT
We thank Catja van Heusden for her technical assistance with nucleotide analysis.
Footnotes
This work was supported by National Institutes of Health Grants NIH/NHLBI 5 P01 34322-18, HL34322, HL60280, HL074158, SCOR, and MTCC (R.C.B. and E.R.L.), NIH Grant RR17667-02, the Parker B. Francis Families Foundation, and the Cystic Fibrosis Foundation (C.R.E.).
- ADP
- adenosine diphosphate
- AMP
- adenosine monophosphate
- ATP
- adenosine triphosphate
- UDP
- uridine diphosphate
REFERENCES
- 1.Burnstock G. Purinergic signalling. Br. J. Pharmacol. 2006;147(Suppl 1):S172–S181. doi: 10.1038/sj.bjp.0706429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J. Biol. Chem. 2004;279:36855–36864. doi: 10.1074/jbc.M405367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boucher RC. Regulation of airway surface liquid volume by human airway epithelia. Pfluegers Arch. 2003;445:495–498. doi: 10.1007/s00424-002-0955-1. [DOI] [PubMed] [Google Scholar]
- 4.Mohsenin A, Blackburn MR. Adenosine signaling in asthma and chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 2006;12:54–59. doi: 10.1097/01.mcp.0000199002.46038.cb. [DOI] [PubMed] [Google Scholar]
- 5.Ma B, Blackburn MR, Lee CG, Homer RJ, Liu W, Flavell RA, Boyden L, Lifton RP, Sun CX, Young HW, Elias JA. Adenosine metabolism and murine strain-specific IL-4-induced inflammation, emphysema, and fibrosis. J. Clin. Invest. 2006;116:1274–1283. doi: 10.1172/JCI26372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: An emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- 7.Scrivens M, Dickenson JM. Functional expression of the P2Y14 receptor in human neutrophils. Eur. J. Pharmacol. 2006;543:166–173. doi: 10.1016/j.ejphar.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 8.Schwiebert EM, Egan ME, Hwang TH, Fulmer SB, Allen SS, Cutting GR, Guggino WB. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell. 1995;81:1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- 9.Prat AG, Reisin IL, Ausiello DA, Cantiello HF. Cellular ATP release by the cystic fibrosis transmembrane conductance regulator. Am. J. Physiol. 1996;270:C538–C545. doi: 10.1152/ajpcell.1996.270.2.C538. [DOI] [PubMed] [Google Scholar]
- 10.Bahima L, Aleu J, Elias M, Martin-Satue M, Muhaisen A, Blasi J, Marsal J, Solsona C. Endogenous hemichannels play a role in the release of ATP from Xenopus oocytes. J. Cell. Physiol. 2006;206:95–102. doi: 10.1002/jcp.20440. [DOI] [PubMed] [Google Scholar]
- 11.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabirov RZ, Dutta AK, Okada Y. Volume-dependent ATP-conductive large-conductance anion channel as a pathway for swelling-induced ATP release. J. Gen. Physiol. 2001;118:251–266. doi: 10.1085/jgp.118.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okada SF, O’Neal WK, Huang P, Nicholas RA, Ostrowski LE, Craigen WJ, Lazarowski ER, Boucher RC. Voltage-dependent anion channel-1 (VDAC-1) contributes to ATP release and cell volume regulation in murine cells. J. Gen. Physiol. 2004;124:513–526. doi: 10.1085/jgp.200409154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad S, Ahmad A, McConville G, Schneider BK, Allen CB, Manzer R, Mason RJ, White CW. Lung epithelial cells release ATP during ozone exposure: Signaling for cell survival. Free Radical Biol. Med. 2005;39:213–226. doi: 10.1016/j.freeradbiomed.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Maroto R, Hamill OP. Brefeldin A block of integrin-dependent mechanosensitive ATP release from Xenopus oocytes reveals a novel mechanism of mechanotransduction. J. Biol. Chem. 2001;276:23867–23872. doi: 10.1074/jbc.M101500200. [DOI] [PubMed] [Google Scholar]
- 16.Castro O, Chen LY, Parodi AJ, Abeijon C. Uridine diphosphate-glucose transport into the endoplasmic reticulum of Saccharomyces cerevisiae: In vivo and in vitro evidence. Mol. Biol. Cell. 1999;10:1019–1030. doi: 10.1091/mbc.10.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol. Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 18.Galun R, Friend WG, Nudelman S. Purinergic reception by culicine mosquitoes. J. Comp. Physiol., A. 1988;163:665–670. doi: 10.1007/BF00603850. [DOI] [PubMed] [Google Scholar]
- 19.Dolezal T, Dolezelova E, Zurovec M, Bryant PJ. A role for adenosine deaminase in Drosophila larval development. PLoS Biol. 2005;3:e201. doi: 10.1371/journal.pbio.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raouf R, Blais D, Seguela P. High zinc sensitivity and pore formation in an invertebrate P2X receptor. Biochim. Biophys. Acta. 2005;1669:135–141. doi: 10.1016/j.bbamem.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Chivasa S, Ndimba BK, Simon WJ, Lindsey K, Slabas AR. Extracellular ATP functions as an endogenous external metabolite regulating plant cell viability. Plant Cell. 2005;17:3019–3034. doi: 10.1105/tpc.105.036806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeter CR, Tang W, Henaff E, Butterfield T, Roux SJ. Evidence of a novel cell signaling role for extracellular adenosine triphosphates and diphosphates in Arabidopsis. Plant Cell. 2004;16:2652–2664. doi: 10.1105/tpc.104.023945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song CJ, Steinebrunner I, Wang X, Stout SC, Roux SJ. Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiol. 2006;140:1222–1232. doi: 10.1104/pp.105.073072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong X, Malhotra R, Guidotti G. ATP uptake in the Golgi and extracellular release require Mcd4 protein and the vacuolar H+-ATPase. J. Biol. Chem. 2003;278:33436–33444. doi: 10.1074/jbc.M305785200. [DOI] [PubMed] [Google Scholar]
- 25.Boyum R, Guidotti G. Glucose-dependent, cAMP-mediated ATP efflux from Saccharomyces cerevisiae. Microbiology. 1997;143(Part 6):1901–1908. doi: 10.1099/00221287-143-6-1901. [DOI] [PubMed] [Google Scholar]
- 26.Ault AD, Broach JR. Creation of GPCR-based chemical sensors by directed evolution in yeast. Protein Eng., Des. Sel. 2006;19:1–8. doi: 10.1093/protein/gzi069. [DOI] [PubMed] [Google Scholar]
- 27.Lazarowski ER, Boucher RC, Harden TK. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J. Biol. Chem. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- 28.Lazarowski ER, Shea DA, Boucher RC, Harden TK. Release of cellular UDP-glucose as a potential extracellular signaling molecule. Mol. Pharmacol. 2003;63:1190–1197. doi: 10.1124/mol.63.5.1190. [DOI] [PubMed] [Google Scholar]
- 29.Gietz RD, Woods RA. Yeast transformation by the LiAc/SS Carrier DNA/PEG method. Methods Mol. Biol. 2006;313:107–120. doi: 10.1385/1-59259-958-3:107. [DOI] [PubMed] [Google Scholar]
- 30.Chambers JK, Macdonald LE, Sarau HM, Ames RS, Freeman K, Foley JJ, Zhu Y, McLaughlin MM, Murdock P, McMillan L, Trill J, Swift A, Aiyar N, Taylor P, Vawter L, Naheed S, Szekeres P, Hervieu G, Scott C, Watson JM, Murphy AJ, Duzic E, Klein C, Bergsma DJ, Wilson S, Livi GP. A G protein-coupled receptor for UDP-glucose. J. Biol. Chem. 2000;275:10767–10771. doi: 10.1074/jbc.275.15.10767. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson A, Pahlman IL, Jovall PA, Blomberg A, Larsson C, Gustafsson L. The catabolic capacity of Saccharomyces cerevisiae is preserved to a higher extent during carbon compared to nitrogen starvation. Yeast. 2001;18:1371–1381. doi: 10.1002/yea.786. [DOI] [PubMed] [Google Scholar]
- 32.Vogel K, Hinnen A. The yeast phosphatase system. Mol. Microbiol. 1990;4:2013–2017. doi: 10.1111/j.1365-2958.1990.tb00560.x. [DOI] [PubMed] [Google Scholar]
- 33.Poster JB, Dean N. The yeast VRG4 gene is required for normal Golgi functions and defines a new family of related genes. J. Biol. Chem. 1996;271:3837–3845. doi: 10.1074/jbc.271.7.3837. [DOI] [PubMed] [Google Scholar]
- 34.Dean N, Zhang YB, Poster JB. The VRG4 gene is required for GDP-mannose transport into the lumen of the Golgi in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 1997;272:31908–31914. doi: 10.1074/jbc.272.50.31908. [DOI] [PubMed] [Google Scholar]
- 35.Kainuma M, Chiba Y, Takeuchi M, Jigami Y. Overexpression of HUT1 gene stimulates in vivo galactosylation by enhancing UDP-galactose transport activity in Saccharomyces cerevisiae. Yeast. 2001;18:533–541. doi: 10.1002/yea.708. [DOI] [PubMed] [Google Scholar]
- 36.Roy SK, Chiba Y, Takeuchi M, Jigami Y. Characterization of Yeast Yea4p, a uridine diphosphate-N-acetylglucosamine transporter localized in the endoplasmic reticulum and required for chitin synthesis. J. Biol. Chem. 2000;275:13580–13587. doi: 10.1074/jbc.275.18.13580. [DOI] [PubMed] [Google Scholar]
- 37.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reihl P, Stolz J. The monocarboxylate transporter homolog Mch5p catalyzes riboflavin (vitamin B2) uptake in Saccharomyces cerevisiae. J. Biol. Chem. 2005;280:39809–39817. doi: 10.1074/jbc.M505002200. [DOI] [PubMed] [Google Scholar]
- 39.Lai K, Elsas LJ. Overexpression of human UDP-glucose pyrophosphorylase rescues galactose-1-phosphate uridyltransferase-deficient yeast. Biochem. Biophys. Res. Commun. 2000;271:392–400. doi: 10.1006/bbrc.2000.2629. [DOI] [PubMed] [Google Scholar]
- 40.Shah N, Klausner RD. Brefeldin A reversibly inhibits secretion in Saccharomyces cerevisiae. J. Biol. Chem. 1993;268:5345–5348. [PubMed] [Google Scholar]
- 41.Vogel JP, Lee JN, Kirsch DR, Rose MD, Sztul ES. Brefeldin A causes a defect in secretion in Saccharomyces cerevisiae. J. Biol. Chem. 1993;268:3040–3043. [PubMed] [Google Scholar]
- 42.Ishida N, Kawakita M. Molecular physiology and pathology of the nucleotide sugar transporter family (SLC35) Pfluegers Arch. 2004;447:768–775. doi: 10.1007/s00424-003-1093-0. [DOI] [PubMed] [Google Scholar]
- 43.Reyes F, Marchant L, Norambuena L, Nilo R, Silva H, Orellana A. AtUTr1, a UDP-glucose/UDP-galactose transporter from Arabidopsis thaliana, is located in the endoplasmic reticulum and up-regulated by the unfolded protein response. J. Biol. Chem. 2006;281:9145–9151. doi: 10.1074/jbc.M512210200. [DOI] [PubMed] [Google Scholar]
- 44.Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O’Shea EK. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- 45.Schuldiner M, Collins SR, Thompson NJ, Denic V, Bhamidipati A, Punna T, Ihmels J, Andrews B, Boone C, Greenblatt JF, Weissman JS, Krogan NJ. Exploration of the function and organization of the yeast early secretory pathway through an epistatic miniarray profile. Cell. 2005;123:507–519. doi: 10.1016/j.cell.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 46.Jakubowski H, Goldman E. Evidence for cooperation between cells during sporulation of the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 1988;8:5166–5178. doi: 10.1128/mcb.8.12.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas C, Sun Y, Naus K, Lloyd A, Roux S. Apyrase functions in plant phosphate nutrition and mobilizes phosphate from extracellular ATP. Plant Physiol. 1999;119:543–552. doi: 10.1104/pp.119.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]