Abstract
Kaposi's sarcoma (KS)-associated herpesvirus (KSHV), a human oncogenic gamma-2-herpesvirus, transforms human endothelial cells and establishes latent infection at a low efficiency in vitro. During latent infection, only a limited number of genes are expressed, and the circularized viral genome is maintained as a multicopy episome. Latency-associated nuclear antigen (LANA), exclusively expressed during latency, has been shown to have a multifunctional role in KS pathogenesis. LANA tethers the viral episome to the host chromosome, thus ensuring efficient persistence of the viral genome during successive rounds of cell division. Besides episome maintenance, LANA modulates the expression of genes of various cellular and viral pathways, including those of retinoblastoma protein and p53. Herpesvirus saimiri (HVS), another gamma-2-herpesvirus, primarily infects New World primates. Orf73, encoding the nuclear antigen of HVS, is the positional homolog of the LANA gene, and the ORF73 protein has some sequence homology to KSHV LANA. However, the function of ORF73 of HVS has not been thoroughly investigated. In this report, we show that HVS ORF73 may be important for episome persistence and colocalizes with the HVS genomic DNA on metaphase chromosomes. Furthermore, HVS terminal repeats (TRs) contain a cis-acting sequence similar to that in KSHV TRs, suggesting that the LANA binding sequence is conserved between these two viruses. This cis-acting element is sufficient to bind HVS ORF73 from strains C488 and A11, and plasmids containing the HVS C488 TR element are maintained and replicate in HVS C488 ORF73-expressing cells.
Kaposi's sarcoma (KS)-associated herpesvirus (KSHV), also known as human herpesvirus 8, is a gammaherpesvirus found in all forms of KS (3, 52, 64; C. Boshoff, D. Whitby, T. Hatziioannou, C. Fisher, J. van der Walt, A. Hatzakis, R. Weiss, and T. Schulz, Letter, Lancet 345:1043-1044, 1995), in AIDS-associated body cavity-based lymphomas or primary effusion lymphomas (11, 55), and in a plasmablastic variant of multicentric Castleman's disease (11, 12, 18). Herpesviruses establish in their natural host a latent infection which is characterized by the persistence of the viral genome as multicopy closed circular episomes and the expression of a limited number of viral genes (9, 19, 37). Latency-associated nuclear antigen (LANA), originally identified by an immunofluorescence assay with sera from KS patients (23, 38), is a latent protein encoded by ORF73 of the KSHV genome (60, 63). LANA is 1,162 amino acids (aa) long and has an apparent molecular mass of 222 to 234 kDa on sodium dodecyl sulfate (SDS)-polyacrylamide gels (60). LANA has no recognizable cellular homolog and has a multifunctional role in KSHV infection and pathogenesis.
LANA localizes to metaphase chromosomes in a punctate manner similar to that of Epstein-Barr virus (EBV) nuclear antigen 1 (EBNA-1) (31). EBNA-1 has been shown to bind multiple sites in the EBV genome and is important for replication and maintenance of the genome (43, 61, 72). There is no clear sequence homology between EBNA-1 and LANA. However, recent studies have suggested that LANA is functionally analogous to EBNA-1 (24, 25, 47, 58, 67). LANA is necessary for episome maintenance of viral DNA and plasmid DNA that contains a single terminal repeat (TR) region of the KSHV genome (7). Such plasmids have been shown to replicate in cells stably expressing LANA (6, 14, 33). Studies also have determined that the KSHV TR unit has multiple binding sites separated by a spacer sequence and that the dimeric unit of LANA binds these sites through its carboxy-terminal domain (7, 14, 27, 65). Simultaneously, LANA associates with mitotic chromosomes through its chromosome binding domain, located within aa 5 to 22 of its amino terminus, as well as the carboxy terminus (6, 56). These domains mediate chromosomal tethering potentially through interactions with cellular proteins, including the methyl CpG binding protein and DEK, respectively (43). The chromosome binding sequence (CBS) of the amino-terminal domain of LANA, when replaced with a fusion of chromatin-associated protein histone H1, maintains an artificial episome which contains the TRs of the KSHV genome (66). Thus, the interaction between histone H1 and LANA is likely to contribute to and be critical for the efficient maintenance of KSHV episomal DNA (14).
In addition to episome maintenance, LANA possesses various transcriptional modulatory activities that may play a role in KSHV-mediated oncogenesis. LANA interacts with a number of host cellular proteins, including the transcriptional corepressor mSin3 and the tumor suppressors pRb and p53 (22, 44) as well as the human immunodeficiency virus Tat protein, which enhances transcriptional activity at the human immunodeficiency virus type 1 long terminal repeat (34). The binding of LANA to pRb results in increased phosphorylation and degradation, thus upregulating E2F-dependent transcription (59).
Herpesvirus saimiri (HVS), a close primate relative of KSHV capable of infecting and transforming human T lymphocytes in vitro, is the prototype of the lymphotrophic gamma-2-herpesvirus subfamily (63). This virus was originally isolated from squirrel monkeys (Saimiri sciureus) and persists without causing any obvious disease symptoms (51). However, experimental transfer of this virus to other New World primates, including common marmosets or cottontop tamarins, leads to the development of a number of lymphoproliferative diseases within a few weeks of infection (2, 39).
HVS strains have been classified into three subgroups, A, B, and C, based on the pathogenic potential and sequence divergence at the left end of the long unique DNA region (48, 49). HVS subgroups A and C possess the ability to immortalize common marmoset T lymphocytes to interleukin-2-independent proliferation (68). However, only subgroup C viruses are capable of transforming human, rabbit, and rhesus monkey lymphocytes in vitro (8). HVS transduces T lymphocytes, unlike KSHV, which has been shown to be capable of infecting and potentially transforming human B lymphocytes, although the latter has not been demonstrated in vitro. HVS DNA is maintained in infected T lymphocytes as multiple nonintegrated circular episomes establishing latent replication (29). Transcriptional analysis of ORF73 expression showed that ORF71 to ORF73 are transcribed as a polycistronic mRNA produced from a common promoter upstream of ORF73 (29). KSHV and HVS both express polycistronic mRNA for ORF71 and ORF72 (17, 35, 53). These genes encode antiapoptotic protein v-FLIP and the v-cyclin D homolog, respectively (36; Y. Chang, P. S. Moore, S. J. Talbot, C. H. Boshoff, T. Zarkowska, K. Godden, H. Paterson, R. A. Weiss, and S. Mittnacht, Letter, Nature 382:410, 1996). There is limited homology between the ORF73 proteins of HVS strains and KSHV. However, studies have suggested that ORF73 is sufficient for the maintenance of plasmids containing HVS TRs, similar to the functions of LANA in KSHV (13).
In this study, we investigated ORF73 of HVS strain C488, the most potent strain of HVS, capable of mediating the transformation of human T lymphocytes in vitro. ORF73 and the genome of strain C488 have not been characterized, except for the transforming potential of the viral strain. We show that ORF73 of HVS strain C488 is strikingly distinct from that of HVS strain A11. To address the potential role of the ORF73 protein in episome maintenance, we determined the colocalization of viral DNA and protein on metaphase chromosomes. Furthermore, we show the binding of HVS ORF73 to cis-acting elements within the TRs as well as the maintenance and replication of HVS C488 DNA containing TRs. We propose that HVS ORF73 binds to cis-acting elements within the HVS TRs and mediates DNA replication, like LANA encoded by KSHV, and thus conserves viral episome maintenance.
MATERIALS AND METHODS
Cell lines and antibodies.
CJ-1 is a transformed Callithrix juccus T-cell line stably infected with HVS (C488) and was a generous gift from Jae Jung (Primate Research Center, HMS, Boston, Mass.). The cell line was cultured in RPMI medium supplemented with 20% fetal bovine serum, 2 mM l-glutamine, 5 U of penicillin/ml, 5 μg of streptomycin/ml, and 100 U of interleukin-2 per ml. HEK293T and HEK293 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 5 U of penicillin/ml, and 5 μg of streptomycin/ml. All cell lines were grown at 37°C in a humidified environment supplemented with 5% CO2. Antibody to C488 ORF73 was obtained from the serum of a squirrel monkey naturally infected with type strain C488 (Jae Jung, HMS). Anti-rabbit polyclonal antibody to A11 ORF73 was a kind gift from A. Whitehouse (30). Antibodies raised against LANA were obtained from Cocalico Inc. by inoculation of rabbits with a purified fusion of glutathione S-transferase to LANA (aa 762 to 1162). Ascites containing the Myc monoclonal antibody was produced at the University of Michigan Hybridoma Core Facility by using hybridoma cell line 9E10.
Cloning of HVS strain C488 ORF73.
Viral DNA from CJ-1 cells was isolated by a modified Hirt protocol (4). Primers complementary to the HVS A11 ORF73 coding region were used to amplify C488 ORF73 with viral DNA from CJ-1 cells as a template. Forward primers corresponded to sequence coordinates 107233 to 107210 and reverse primers corresponded to sequence coordinates 105900 to 106010 from HVS A11 (1). The amplified product was cloned in vector pA3M, which contains a carboxy-terminal Myc tag (5, 14). HVS A11 ORF73 was subcloned into pA3M from pCDNA3 (30). The open reading frame of KSHV LANA in pA3M was previously cloned and described (14).
Sequencing and alignment.
Complete sequencing of HVS C488 ORF73 was done with primers for the sense and antisense strands at the Sequencing Core Facility of the University of Pennsylvania. Nucleotide and protein sequences for ORF73 of HVS A11 and KSHV were retrieved from the GenBank database under accession numbers X64346 and U52064, respectively. The nucleotide and protein sequences were aligned by using the Clustal W sequence alignment program (http://www.ebi.ac.uk/clustalw/) (70).
Transfection and Western blotting analysis of viral antigens.
A total of 106 HEK293T cells were used for transient transfection. Ten micrograms of expression construct containing ORF73 from the three viruses was transfected by electroporation. The efficiency of transfection was determined by cotransfecting an enhanced green fluorescent protein plasmid (pEGFP-C1; Clontech, Palo Alto, Calif.) and counting green fluorescent cells as a percentage of transfected cells. Cells were electroporated at 210 V and 975 μF in 400 μl of Dulbecco’s modified Eagle’s medium, resuspended in 15 ml of medium, and incubated for 36 h. Cells were collected for immunofluorescence and Western blot analyses. Approximately 106 cells were collected, washed once in phosphate-buffered saline (PBS), lysed by mixing with an equal amount of SDS loading buffer, and heated at 95°C for 5 min. Samples were subjected to SDS-8% polyacrylamide gel electrophoresis (PAGE) and transferred to 0.45-μm-pore-size nitrocellulose membranes as described previously (10). Membranes were incubated overnight with anti-Myc ascites, squirrel monkey polyclonal serum reactive to C488 ORF73, anti-rabbit polyclonal antibody reactive to A11 ORF73, and anti-rabbit polyclonal serum against KSHV LANA to detect specific viral antigens.
Immunofluorescence analysis of HEK293T cells.
HEK293T cells transfected with eukaryotic expression constructs were incubated for 24 h at 37°C with 5% CO2 in a humidified chamber. Cells were spread on a microscope slide and fixed in acetone-methanol (1:1). Nonspecific binding sites were blocked by incubation of the slides with 20% normal goat serum in PBS followed by three washes in PBS. The slides were then incubated with a primary antibody (squirrel monkey serum, anti-rabbit A11 ORF73 antibody, or LANA serum) at room temperature in a humidified chamber. The slides were washed three times for 5-min intervals and then incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit secondary antibodies at a dilution of 1:1,000 in PBS for 1 h at room temperature. The slides were washed four times in PBS and then mounted with antifade solution. The slides were examined by using an Olympus BX60 fluorescence microscope, and photographs were captured by using a PixelFly digital camera (Cooke Inc., Eugene, Oreg.).
Metaphase chromosome staining and immuno-FISH analysis.
Metaphase chromosome spreads were prepared as described before with some modifications (14). Cells (CJ-1) were arrested in metaphase by treatment with Colcemid (10 μg/ml; Invitrogen-Gibco, Carlsbad, Calif.) for 5 h at 37°C, treated with 75 mM KCl for 10 min at 37°C, and washed in methanol-acetic acid (3:1). Cells were fixed with fresh fixative solution (methanol-acetic acid, 3:1) at 4°C for 1 h. In order to preserve the chromosome-associated protein, cells were fixed for only 1 h, and the specificity for the signal was increased by modifying the stringency of the washes. Hybridization with the biotinylated HVS cosmid probe was done overnight at 37°C and then detected with a direct tyramide-rhodamine signal amplification system (Perkin-Elmer Life Sciences, Inc., Boston, Mass.) according to the manufacturer's instructions. This system increases the sensitivity of detection by amplifying the signal through the deposition of fluorescent dye at the site of the signal. Nonspecific signals were removed by repeated washing. The slides were then treated to determine the localization of ORF73 protein by immunofluorescence in situ hybridization (immuno-FISH) analysis as described above. The slides were washed in PBS, counterstained with 4′,6′-diamidino-2-phenylindole (DAPI), and mounted with antifade solution for fluorescence microscopy and photography.
Preparation of nuclear extracts and electrophoretic mobility shift assay (EMSA).
Nuclear extracts were prepared from transiently transfected HEK293T cells with different expression constructs and an empty vector as described previously (15). Briefly, transfected cells (108) were collected, washed with cold PBS, resuspended in buffer A (10 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 5 mM dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg of aprotinin/ml), incubated on ice for 1 h, and Dounce homogenized. Extracted nuclei were collected by centrifugation, washed once with buffer A, and then resuspended in buffer B (20 mM HEPES, 10% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 5 mM DTT, 0.5 mM PMSF, 10 μg of aprotinin/ml). After 30 min of incubation on ice, nuclear debris was removed by centrifugation at high speed. The supernatant containing the nuclear protein was added to an equal volume of buffer C (20 mM HEPES, 30% glycerol, 1.5 mM MgCl2, 0.2 mM EDTA, 5 mM DTT, 0.5 mM PMSF, 10 μg of aprotinin/ml), and the mixture was snap frozen in aliquots for storage at −80°C. The protein concentration in the nuclear extracts was determined by a standard Bradford assay, and the expression of transfected protein was confirmed by Western blotting with anti-Myc antibody.
Double-stranded DNA probes were prepared from synthetic oligonucleotides (IDT Inc., Coralville, Iowa) by annealing the complementary strand and purifying by 12% native PAGE. GATC 5′ overhang probes were end filled (16) with dGTP by using the Klenow fragment of DNA polymerase I. Labeled probes were purified from unincorporated nucleotides with a probe purification column (Stratagene, La Jolla, Calif.) according to the manufacturer's instructions. The EMSA binding reaction mixture (50 μl) contained 15 μg of nuclear extract, 50 ng of poly(dI-dC) nonspecific competitor, and 20,000 cpm of probe in binding buffer (20 mM HEPES [pH 7.5], 0.01% NP-40, 5.0% glycerol, 10 mM MgCl2, 100 μg of bovine serum albumin/ml, 2 mM DTT, 1 mM PMSF, 40 mM KCl) and was incubated at room temperature for 20 min. Cold competitor (200-fold) was added 5 min prior to the addition of radiolabeled probes. Anti-Myc ascites was added to the incubation mixture 10 min prior to the addition of radiolabeled probes to supershift protein bound to probes. Bound and unbound probes were resolved on 5.0% nondenaturating polyacrylamide gels in 0.5× Tris-borate-EDTA (TBE). The gels were dried, and the signals were detected by autoradiography.
Episome maintenance assay.
To detect the role of ORF73 in the maintenance of episomal DNA, 106 HEK293 cells were transfected with an HVS C488 TR cosmid (Cos331) (20), with an ORF73 expression vector, and with an empty vector (pA3M) separately. Transfected cells were selected in the presence of antibiotic G418 for 3 weeks, the generated clones were subjected to seven passages, and the cosmid was detected by gel analysis as described by Gardella et al. (26). Briefly, a large horizontal 0.75% agarose gel which contained a block of lysing agarose (0.8% agarose, 2.0% SDS in TBE, 1 mg of pronase/ml) was poured. A suspension of approximately 106 live cells was loaded in each well and electrophoresed in TBE at 15 V for 4 h followed by 100 V for 18 h in the cold. Slow electrophoresis enhanced lysis of the cells in the wells, liberating episomal DNA. Episomal DNA migrated into the gel, leaving behind most of the cellular DNA. DNA was transferred to a nylon membrane, and the cosmid (Cos331) was detected by Southern hybridization with a 32P-labeled probe. Cos331 was used to make the probe.
Cloning of HVS C488 TRs and transient replication assay.
pBSpuro was constructed by subcloning the puromycin resistance expression cassette from pBABEpuro (54) into the SalI and ClaI sites of pBS (Stratagene) containing multiple cloning sites. The complete TR unit of HVS C488 (1,458 bp) was excised from Cos331 with restriction endonuclease TaqI and ligated into pBSpuro at the ClaI site to obtain pBSpuroTR. The presence of a single TR unit was confirmed by restriction analysis, and the integrity of the sequence was confirmed by sequencing. Plasmid pBSpuroTR was used to transfect HEK293 cells with or without HVS ORF73 expression vectors. Puromycin was added 20 h after transfection, and the cells were allowed to grow for 96 h. Cells were trypsinized, collected, and washed three times with PBS containing EDTA. Low-molecular-weight DNA was isolated by a modified Hirt procedure (32). To evaluate the role of ORF73 in the replication of a TR-containing plasmid, isolated DNA was digested with restriction enzymes DpnI and EcoRI. Restriction enzyme DpnI digests the DNA replicated in dam methylase-positive Escherichia coli, while DNA replicated in mammalian cells is resistant to DpnI digestion. EcoRI, which digests once, was used to linearize the plasmid. After complete digestion, DNA was electrophoresed on an 0.8% agarose gel and transferred to a nitrocellulose membrane. Plasmids were detected with a randomly primed 32P-labeled pBSpuro backbone probe, and images were documented with a PhosphorImager (Molecular Dynamics).
Nucleotide sequence accession number.
The nucleotide sequence of HVS strain C488 was submitted to GenBank and assigned accession number AY274934.
RESULTS
Sequence analysis of ORF73 from HVS strain C488 reveals a glycine- and glutamic acid-rich region containing 94 aa residues distinct from those of strain A11.
PCR amplification of HVS C488 ORF73 from DNA isolated from CJ-1 cells (HVS-transformed C. juccus T lymphocytes) produced an amplicon of approximately 1.5 kbp, a size considerably larger than that expected from a comparison with HVS A11. Cloning of the amplicon followed by complete sequencing of the amplicon revealed an open reading frame of 1,506 bp, making ORF73 of HVS C488 282 bp larger than ORF73 of HVS A11. Additionally, the sequence analysis did not reveal any obvious splicing donor or acceptor signal motif in the cloned ORF73 sequence, suggesting that it was an intact open reading frame.
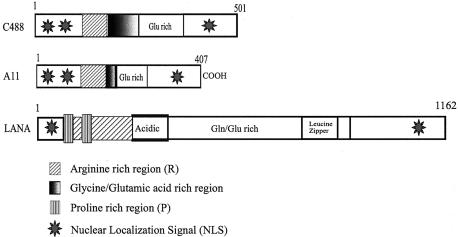
In order to determine the conserved region of ORF73 in these two HVS strains, the nucleotide and translated amino acid sequences of C488 were aligned with the A11 sequences retrieved from GenBank (accession number X64346) (Fig. 1). The alignment showed that the overall structure of the gene was preserved, with the exception of a 94-aa central domain of acidic amino acid residues in the C488 molecule. The amino-terminal (70-aa) domains of both strains had 85% conserved amino acid residues, and the carboxy-terminal (165-aa) regions had 90% conserved residues. The major distinguishing factor between these two HVS strains was that the central domain of glycine and glutamic acid residues in strain A11 was shorter than that in strain C488. Secondary structure and motif searches showed that there are two potential nuclear localization sequences in the amino-terminal region and one in the carboxy-terminal region (Fig. 1B). A number of phosphorylation sites recognized by different kinases are present. In addition, there are myristoylation and amidation sites, which were also seen in KSHV LANA. The schematic diagram of ORF73 of HVS strains C488 and A11 and KSHV in Fig. 2 suggests that KSHV LANA has distinct domains, which include a proline-rich region in the amino-terminal domain, an acidic amino acid-rich domain, and a conserved leucine zipper at the carboxy domain (Fig. 2). These domains were not evident by sequence comparison in HVS ORF73 of either strain A11 or strain C488. Strikingly, the central region of C488 was 94 aa longer than that of A11. Interestingly, this region was over 300 aa long in KSHV LANA.
FIG. 1.
Sequence comparison of the ORF73 genomic regions of HVS strains C488 and A11. (A) The nucleotide sequence of HVS C488 was aligned with the nucleotide sequence of HVS A11 retrieved from GenBank by using the Clustal W multiple-sequence-alignment program (http://www.ebi.ac.uk/clustalw/). Identical nucleotides are enclosed in boxes. (B) An alignment of the deduced amino acid sequences for ORF73 from both strains shows a high degree of conserved amino acids in their amino- and carboxy-terminal domains and an additional 94 aa in the central region. Identical amino acids are enclosed in boxes.The nuclear localization sequences of both strains are shaded.
FIG. 2.
Schematic representations of the ORF73 polypeptides of both HVS strains (C488 and A11) and KSHV LANA showing the similarity in the structural motifs. HVS C488 ORF73 had a larger glycine- and glutamic acid-containing domain than did HVS A11 ORF73. The nuclear localization signals in the N- and C-terminal domains of these two molecules are conserved. Distinct proline-rich, acidic, and leucine zipper domains that were seen in KSHV LANA were not detected in the HVS ORF73 secondary structure by motif searches.
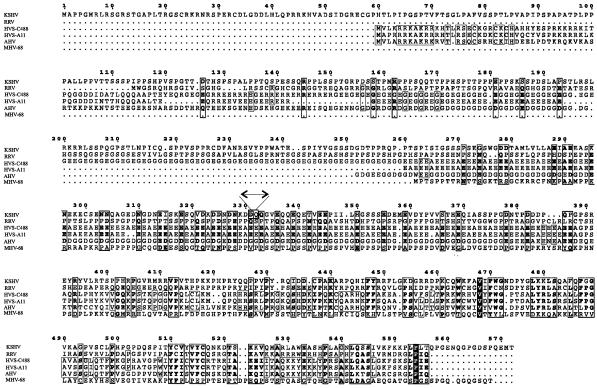
Most gammaherpesviruses carry an ORF73 protein; LANA carried by KSHV is the largest, with over 1,100 aa, essentially due to expansion of the central acidic domain (57). To determine the degree of amino acid sequence conservation of ORF73 molecules carried by the different gammaherpesviruses, the amino acid sequence of KSHV LANA (with the central repeat domain deleted for alignment simplification) was aligned with the ORF73 amino acid sequences of both strains of HVS (A11 and C488), rhesus rhadinovirus, Ateles herpesvirus (AHV), and murine herpesvirus 68. The results showed that the overall degree of conservation in amino acid sequence was low, with less than 20% of the amino acid sequence being conserved in the six ORF73 sequences compared. Although the overall amino acid sequence similarity was low for the ORF73 molecules, the C terminus of ORF73 was somewhat more conserved, at approximately 30% (Fig. 3). Among the sequences aligned, AHV ORF73 showed the highest degree of conserved amino acid residues with ORF73 from the HVS strains (approximately 50%).
FIG. 3.
Amino acid sequence alignment of ORF73 positional homologs among members of the gammaherpesviruses. Amino acid sequences of ORF73 polypeptides of KSHV, rhesus rhadinovirus (RRV), HVS A11, AHV, and murine herpesvirus 68 (MHV-68) were aligned with that of HVS C488. The central repeat region (aa 332 to 921) of KSHV LANA was deleted from the alignment for simplification (↔). The carboxy-terminal domains in the polypeptides of all of the members have conserved amino acid residues, in contrast to the amino termini. Boxes indicate that residues are either identical or positive for any motif. Black shading shows that the given residue is conserved in all viral molecules investigated.
Expression and detection of HVS strain C488 ORF73 in human cells.
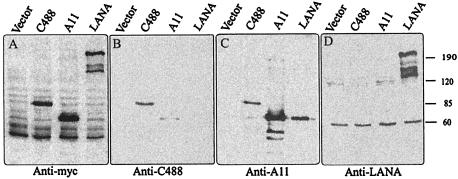
A eukaryotic expression vector containing the full-length coding region of ORF73 from C488 cloned downstream of the cytomegalovirus immediate-early promoter and Myc tagged at its C terminus was used to transiently transfect human embryonic kidney (HEK293T) cells. Cells were harvested after 24 h, and protein extracts were resolved by SDS-8% PAGE. The fractionated proteins were transferred to nitrocellulose membranes and detected with an anti-Myc monoclonal antibody. As expected, immunodetection analysis indicated that HVS C488 ORF73 was approximately 85 kDa (Fig. 4), larger than that previously seen for HVS A11 ORF73 (30). Similarly, the HVS A11 and KSHV LANA proteins were also detected with the anti-Myc monoclonal antibody, as they contained a Myc tag at their C termini (Fig. 4). It should be noted, however, that the predicted size of C488 ORF73 based strictly on amino acid composition is approximately 56 kDa.
FIG. 4.
Western blot detection of ORF73 protein with anti-Myc ascites and sera raised against ORF73 polypeptides. A eukaryotic expression vector (pA3M) containing ORF73 of both strains of HVS and KSHV was used to transiently transfect HEK293T cells. At 24 h after transfection, 106 cells were harvested, lysed in SDS sample buffer, and resolved by SDS-8% PAGE. The resolved protein was transferred to a nitrocellulose membrane electronically, and the blot was used for the detection of ORF73 polypeptides. (A) Detection of proteins by anti-Myc monoclonal antibody. Lane Vector contained lysates from HEK293T cells transfected with empty vector pA3M. Lanes C488, A11, and LANA contained lysates from HEK293T cells transfected with expression constructs containing ORF73 of HVS C488, HVS A11, and KSHV, respectively. (B) The blot was stripped and reprobed with anti-C488 polyclonal serum from a squirrel monkey and showed cross-reactivity with C488 and A11 proteins. (C and D) Immunoblots showing cross-reactivity with anti-A11 and anti-LANA sera, respectively. Numbers at right indicate kilodaltons.
Antibodies against ORF73 from HVS strain A11 recognize ORF73 from strain C488 but not KSHV LANA.
Based on the amino acid sequence alignment of the HVS ORF73 molecules and KSHV LANA, we have shown that these sequences are not highly conserved. Therefore, we wanted to determine whether the serum raised against HVS ORF73 cross-reacted with KSHV LANA and whether the function of LANA is conserved in the HVS ORF73 proteins. To address the issue of conserved epitopes, polyclonal serum obtained from a squirrel monkey naturally infected with HVS C488 was incubated with a nitrocellulose membrane containing polypeptides from the three ORF73 proteins from HVS and KSHV. C488 and A11 ORF73 polypeptides were recognized by the squirrel monkey polyclonal serum, but KSHV LANA polypeptides were not (Fig. 4B). Similarly, the rabbit polyclonal serum raised against A11 ORF73 recognized C488 and A11 ORF73 polypeptides but not KSHV LANA polypeptides (Fig. 4C). However, the polyclonal serum raised against the carboxy-terminal 400 aa of KSHV LANA cross-reacted with only LANA polypeptides (cf. Fig. 4B to D). Furthermore, human serum from KSHV-positive patients showed cross-reactivity with only LANA polypeptides and not with any of the HVS ORF73 polypeptides (data not shown). These observations indicated that the ORF73 protein of strains C488 and A11 had conserved epitopes or motifs that are highly antigenic in the two strains and that these motifs are not likely to be conserved across viruses of other species, including the human virus KSHV.
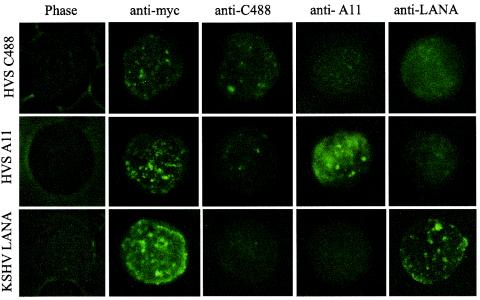
HVS C488 ORF73 localizes to the nucleus and produces a speckled pattern similar to that of KSHV LANA.
HVS A11 ORF73 has been shown to localize in the nucleus with a pattern similar to that of KSHV LANA (30). Since the nuclear localization signal sequence of HVS C488 ORF73 was conserved, it was important to determine whether it would localize in a pattern similar to that of HVS A11 ORF73 and KSHV LANA. 293 cells transfected with the pA3M expression constructs were subjected to immunofluorescence analysis with an anti-Myc primary antibody, which recognizes the Myc epitope, and an FITC-conjugated anti-mouse secondary antibody. The results showed that HVS C488 ORF73 had a speckled pattern in the nucleus similar to that of ORF73 from HVS A11 and LANA from KSHV (Fig. 5). Detection of C488 ORF73 with the squirrel monkey serum revealed an intensity of speckled signals in the nucleus comparable to that of A11 ORF73. Similarly, the rabbit polyclonal serum raised against A11 detected the speckled nuclear pattern of C488 ORF73 as well as that of A11 ORF73. However, the rabbit polyclonal serum raised against LANA detected only KSHV LANA, similar to the results of the Western blot analysis (Fig. 5). These results corroborate the data reported above in that the polyclonal serum raised against HVS ORF73 from either strain A11 or strain C488 can recognize polypeptides from either strain but not from the human virus KSHV. Additionally, serum raised against KSHV LANA does not detect either HVS A11 or HVS C488 ORF73 polypeptides. Therefore, these epitopes may be evolutionarily distinct and likely are not conserved across humans and higher primates.
FIG. 5.
HVS C488 ORF73 is expressed in human cells and shows nuclear speckled localization. Human embryonic kidney cells (HEK293T) transfected with pA3M containing ORF73 were harvested after 24 h. Cells were spread uniformly on Teflon-coated slides. Cells adhering to the slides were fixed in methanol-acetone and incubated with anti-Myc monoclonal antibody, anti-C488 squirrel monkey polyclonal serum, anti-A11 rabbit polyclonal serum, and anti-LANA rabbit polyclonal serum. Signals were detected with FITC-conjugated goat anti-mouse (anti-Myc monoclonal antibody) and goat anti-rabbit (squirrel monkey and rabbit sera) antibodies. Serum from a squirrel monkey (anti-C488) and rabbit serum (anti-A11) both detected the nuclear speckled localization of C488 ORF73, but anti-LANA rabbit polyclonal serum did not show distinct localization of the protein. HVS A11 ORF73 and KSHV LANA were detected by anti-Myc monoclonal antibody as positive controls. The cross-reactivities of the anti-C488, anti-A11, and anti-LANA antibodies are shown.
HVS C488 ORF73 truncated at amino and carboxy termini localizes to the nucleus.
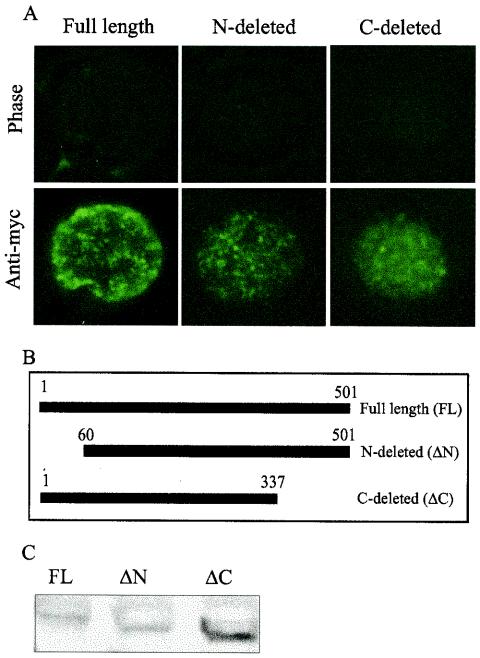
To characterize the functional domains and nuclear localization of C488 ORF73, amino- and carboxy-terminal deletions of the ORF73 coding region were amplified by PCR. Each deletion mutant was amplified with BamHI and EcoRI restriction endonuclease sites incorporated at the termini to facilitate cloning into the expression vector. The integrity of the DNA sequence was confirmed by sequencing. These deletion expression constructs were used to transiently transfect HEK293 cells, which were then subjected to immunofluorescence analysis with an anti-Myc monoclonal antibody. This analysis showed that both constructs had a distinctive nuclear speckling pattern similar to that observed with the wild-type protein (Fig. 6). These results suggest that nuclear localization signals present in both carboxy- and amino-terminal domains are sufficient to mediate translocation of the proteins to the nucleus.
FIG. 6.
Carboxy-terminally truncated (ΔC) and amino-terminally truncated (ΔN) polypeptides of HVS C488 ORF73 localize to the nucleus. (A and B) A deletion mutant (B) of ORF73 generated by PCR was cloned in expression vector pA3M, which was used to transfect HEK293T cells. At 24 h after transfection, cells were subjected to immunofluorescence analysis with anti-Myc monoclonal antibody and FITC-conjugated goat anti-mouse secondary antibody (A). The bottom row of panel A shows the distinct nuclear speckled localization of the truncated C488 ORF73 polypeptides. (C) Western blot detection of full-length (FL), ΔN, and ΔC polypeptides of C488 ORF73.
Immuno-FISH analysis demonstrates that HVS ORF73 and the HVS genome colocalize to metaphase chromosomes in HVS-transformed marmoset (C. juccus) T cells.
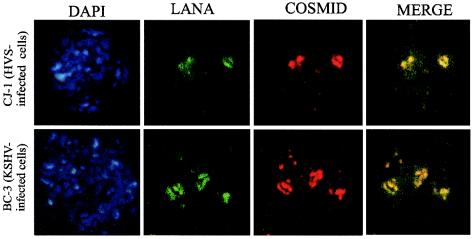
KSHV LANA has been shown to bind within the TR elements of the KSHV genome and to associate with the chromatin protein histone H1, thus tethering the genome to the host chromosome (14, 66). Since the ORF73 proteins of KSHV and HVS share limited amino acid sequence conservation, we were interested in determining whether HVS ORF73, like KSHV LANA, colocalizes to viral DNA on chromosome spreads. Chromosome spreads of CJ-1, an HVS-transformed marmoset T-cell line, were prepared by quick fixation in methanol-acetic acid (3:1) to preserve the chromosome-associated proteins. The specificity of detection was maintained by modifying the stringency of the wash steps. These spreads were probed with HVS cosmid DNA, and signals were amplified with a tyramide-based fluorochrome for increased sensitivity. An immunofluorescence assay with anti-C488 ORF73 was used to colocalize ORF73 and viral DNA on metaphase chromosomes. Our results showed that viral DNA and ORF73 both were detected on metaphase chromosome spreads (Fig. 7). These signals, when superimposed, showed colocalization at the same position on metaphase chromosomes in HVS-transformed CJ-1 marmoset T cells (Fig. 7). Spreads of an HVS-negative cell line did not show any specific signals for either ORF73 or HVS DNA (data not shown). Therefore, the signals seen from CJ-1 metaphase spreads were specific for HVS ORF73 protein and HVS genomic DNA. These data strongly suggest that C488 ORF73 and HVS genomic DNA colocalize to host metaphase chromosomes in HVS-infected marmoset T lymphocytes. BC-3 (KSHV-positive) cells were analyzed as a positive control. The results showed that KSHV LANA protein and KSHV genomic DNA colocalized on metaphase spreads of cell line BC-3 (Fig. 7).
FIG. 7.
HVS C488 DNA colocalizes with ORF73 on the host chromosomes of HVS-transformed human T lymphocytes (CJ-1 cells). Metaphase chromosome spreads of CJ-1 cells were probed with Cos331 (HVS cosmid)-labeled probes and anti-ORF73 serum for the specific localization of signals. DAPI panels show staining of the chromosomes with DAPI counterstain. The LANA panels show the detection of ORF73 protein with anti-C488 serum by immunofluorescence. The COSMID panels show hybridization of the metaphase chromosome spreads with the Cos331-specific HVS DNA probe. The MERGE panels show the specific signal from the HVS probe and anti-C488 serum colocalizing on the host chromosomes of HVS-transformed marmoset T lymphocytes (CJ-1 cells).
HVS TRs have a potential cis-acting ORF73 binding sequence.
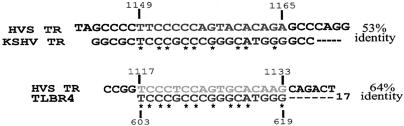
KSHV LANA had been shown previously to colocalize with viral DNA on metaphase chromosomes and tether the viral episome to the host chromosome (14). Studies have shown that plasmids containing at least one copy of the TR element can persist in LANA-expressing cells, suggesting that a single TR element is sufficient for LANA binding. Mapping of the LANA binding sequence in the TR element by a number of strategies identified a 17-bp cognate sequence in the TR element which was sufficient for LANA binding (7, 15). The sequence, identified as an imperfect palindromic sequence, lies at positions 603 to 619 in the TR element (7, 15). The HVS TR element also was recently shown to be important for efficient long-term maintenance of plasmids in ORF73-expressing cells (13). However, the binding sequence within the TR element for HVS ORF73 has not been mapped. We hypothesized that some level of conservation in nucleotide sequence may be required for binding and maintenance of the viral genome during latent infection. We aligned the TR elements from KSHV and HVS and identified an HVS sequence which aligned with the 17-bp KSHV LANA binding sequence at approximately 53% nucleotide sequence identity. However, alignment of only the 17-bp KSHV LANA binding sequence with the HVS TR element sequence showed 64% sequence identity approximately 22 bp upstream from the sequence identified when the complete TR elements were aligned. These results suggest that there could be two LANA binding sequences separated by a 22-bp sequence (Fig. 8).
FIG. 8.
KSHV LANA binding sequence (TLBR4) aligned at two sites in the HVS TR unit. The complete TR units of HVS and KSHV were aligned and examined for the 17-bp imperfect palindromic sequence (TLBR4) previously identified in the HVS TR unit. The sequence corresponding to TLBR4 lies at positions 1149 to 1165 in the HVS TR unit. However, a separate alignment of the TLBR4 sequence with the complete HVS TR unit revealed another site lying at positions 1117 to 1133 and having 64% identical nucleotides, compared to the 53% identity for the other alignment.
HVS ORF73 binds to the cognate KSHV LANA binding sequence.
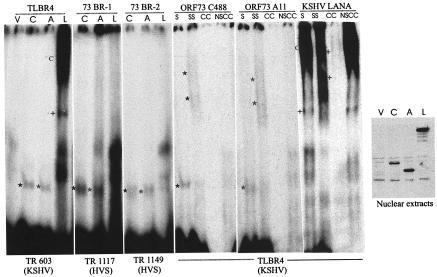
Sequence alignment showed two putative LANA binding sequences within the TR element of HVS (Fig. 8). 32P-radiolabeled double-stranded DNA probes corresponding to these two sequences were analyzed by an EMSA to determine whether ORF73 from HVS strains C488 and A11 binds to either probe. Nuclear extracts were prepared from HEK293T cells transiently transfected with expression constructs for HVS ORF73 from C488 and A11 and for KSHV LANA. An EMSA of TLBR4 (KSHV LANA binding sequence) showed that nuclear extracts prepared from HVS ORF73-transfected cells formed a complex with the probe, as indicated by a shift in the mobility of the probe. This shift was likely to be specific, as the shifted complex was not seen in nuclear extracts prepared from cells transfected with the empty vector control (Fig. 9). As expected, nuclear extracts prepared from KSHV LANA-transfected cells showed the formation of a multimeric complex and a specific shift (Fig. 9). The HVS probes (TR 1117 and TR 1149) showed binding with ORF73 from both strains C488 and A11, similar to that seen for TLBR4 and KSHV LANA. However, an EMSA of the two putative HVS probes (TR 1117 and TR 1149) did not show any specific shift in mobility with the KSHV LANA protein (Fig. 9).
FIG. 9.
HVS ORF73 binds to two putative ORF73 binding sequences in the TRs and the KSHV 17-bp cognate LANA binding sequence. A 32P-labeled probe for the TLBR4 sequence was incubated with nuclear extracts from HEK293T cells transfected with pA3M expression constructs for C488 ORF73 (lanes C), A11 ORF73 (lanes A), and KSHV LANA (lanes L). Nuclear extracts from cells transfected with empty vector pA3M were used as controls. Lanes C and A in the TR 603 (KSHV) panel show the shift in the mobility of the TLBR4 probe (asterisks), and lane L shows the specific shift (+) and complex formation (C) reported previously (15, 25). The TR 1117 (HVS) and TR 1149 (HVS) panels show the specific shifts in the mobilities of probe 73BR-1 (HVS TR at positions 1117 to 1133) and probe 73BR-2 (HVS TR at positions 1149 to 1165) sequences for both C488 and A11 ORF73 proteins (asterisks). The specificity of the shifts (lanes S) in TLBR4 mobility for the C488 and A11 ORF73 proteins was shown by incubation of the probe-protein complex with anti-Myc antibody targeted to ORF73 protein in order to supershift (lanes SS) the complex. In the presence of 200-fold cold competitor probe (lanes CC), the shift was abolished, but this was not the case with a nonspecific cold competitor probe (lanes NSCC). The mobility of the TLBR4 probe-LANA complex was supershifted and abolished in the presence of anti-Myc antibody and 200-fold cold probe as a positive control (25). The separate panel at the right shows Western blot detection of ORF73 protein in nuclear extracts from HEK293T cells transfected with pA3M containing C488 (lane C), pA3M containing A11 (lane A), and pA3M containing LANA (lane L) proteins by use of the same anti-Myc monoclonal antibody as that used for the supershift. Lanes V show control protein nuclear extracts made from HEK293T cells transfected with empty vector pA3M.
To confirm the specificity of the shifted complexes, the HVS ORF73 probe complex was incubated with an anti-Myc antibody in order to supershift the complex containing the specific protein. The anti-Myc antibody was used for this assay because all polypeptides were tagged with a Myc epitope. The anti-Myc antibody generated a specific supershift of probe TLBR4 with both of the HVS ORF73 polypeptides as well as with KSHV LANA polypeptides (Fig. 9). The shift in the mobility of the probes with these proteins was abolished by a 200-fold excess of cold competitor probe but was not affected in the presence of the same amount of a nonspecific cold probe. These results suggest that the shift seen with these proteins was specific and that the HVS TR element is the putative binding site of HVS ORF73 required for tethering the viral genome to the host chromosome.
HVS C488 ORF73 acts in trans to mediate long-term episome maintenance.
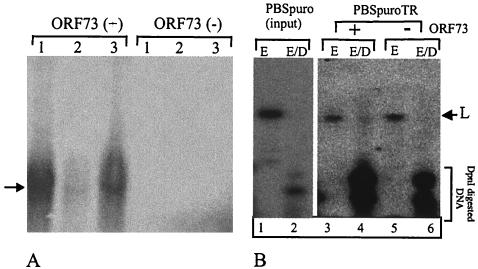
Since KSHV LANA has been shown to mediate the long-term maintenance of the KSHV cosmid containing TR DNA (7, 33), we analyzed the ability of HVS C488 ORF73 to support the maintenance of HVS TR-containing cosmid (Cos331) in HEK293 cells by gel analysis as described by Gardella et al. (26). In this analysis, approximately 106 live cells are loaded into wells and undergo lysis with SDS and proteases. Episomal DNA migrates into the gel, but chromosomal DNA cannot enter the gel because of its large size. A total of 106 HEK293 cells were transfected with a TR-containing HVS cosmid (Cos331) and with or without HVS C488 ORF73 expression vectors. G418 selection of the transfected cells for 3 weeks produced a significantly large number of resistant clones in HVS C488 ORF73-expressing cells compared to cells lacking ORF73. Three clones from each set of ORF73-expressing and non-ORF73-expressing HEK293 cells were subjected to gel analysis as described above. Figure 10A shows that the clones expressing ORF73 were able to retain the cosmid, as detected by Southern hybridization, whereas the clones lacking ORF73 expression did not show any detectable level of the cosmid (Cos331). These results suggest that ORF73 is required for the maintenance of episomal DNA and support the existing hypothesis that the ORF73 binding sequence identified in previous experiments acts as an anchoring site for HVS C488 ORF73 tethering cosmid DNA to the cell chromosome.
FIG. 10.
HVS C488 ORF73 is essential for episome maintenance and DNA replication. (A) HVS cosmid Cos331 was used to transfect HEK293 cells with or without ORF73 expression vectors. Transfected cells were selected in the presence of G418 for 3 weeks. Clones generated after G418 selection were passaged seven times, and three clones from each set were analyzed for the presence of episomal DNA as described by Gardella et al. (26). Episomal DNA migrated into the gel and was detected with a 32P-labeled cosmid probe. Clones expressing ORF73 showed the presence of cosmid DNA (arrow), but cells lacking the expression of ORF73 did not show detectable amounts of cosmid DNA. (B) Replication of TR-containing plasmids in ORF73-expressing cells. HVS C488 TRs were excised from Cos331 with TaqI and ligated into pBSpuro at the ClaI restriction endonuclease site. The resulting plasmid was used to transfect HEK293 cells, with selection in the presence of puromycin for 96 h. Low-molecular-weight DNA extracted by a modified Hirt procedure (32) was digested with either EcoRI (which cuts only once in the plasmid backbone) (E) or EcoRI and DpnI (E/D). Digested products were resolved on an 0.8% agarose gel and analyzed by Southern hybridization with a 32P-labeled pBSpuro backbone probe. Lanes 1 and 2 show the digestion of input plasmid pBSpuro. The absence of a DpnI-resistant band in lane 2 indicates the complete digestion of DNA (lighter exposure than that in other lanes). The presence of a DpnI-resistant band in lane 4 suggests the replication of DNA containing HVS TRs in ORF73-expressing cells (L). In contrast, cells lacking ORF73 expression did not show a detectable DpnI-resistant band (lane 6), suggesting that ORF73 is important for DNA replication.
Transient replication of a plasmid containing the TR unit of HVS C488 was also demonstrated in vitro. To evaluate the role of HVS C488 ORF73 in replication, pBSpuroTR plasmids were used to transfect HEK293 cells with or without ORF73 expression vectors. Transfected cells were passaged and selected in the presence of puromycin for 96 h. Low-molecular-weight DNA was isolated by a modified Hirt procedure (32); 10% of the total extract was digested with EcoRI to linearize the plasmid, and the rest was digested with EcoRI and DpnI to detect the replicated copies of the input plasmid. EcoRI and EcoRI-DpnI completely digested pBSpuro DNA amplified in a dam methylase-positive E. coli strain (Fig. 10B, lanes 1 and 2). The absence of a detectable amount of DpnI-resistant DNA in lane 2 of Fig. 10B suggested complete digestion with DpnI. Lanes 3 and 4 of Fig. 10B show digestion with EcoRI and EcoRI-DpnI of the low-molecular-weight plasmid isolated from HEK293 cells expressing ORF73. The presence of a DpnI-resistant band in lane 4 of Fig. 10B (at a level similar to that in lane 3) suggested that some copies of this plasmid replicated in mammalian cells and thus rendered them resistant to DpnI digestion. In contrast, 293 cells lacking ORF73 expression failed to mediate plasmid replication. These data suggest that HVS C488 ORF73 is important for mediating the replication and episome maintenance of the viral genome, similar to what has been shown for HVS A11 ORF73 and KSHV LANA (6, 7, 13, 28, 33).
DISCUSSION
HVS strains are classified into three subgroups, A, B, and C, on the basis of their transforming potential and differences in the terminal long unique DNA sequence (49, 50). Among these subgroups, only subgroups A and C have been shown to immortalize T lymphocytes of marmosets and other New World primates (21). However, only subgroup C has been shown to immortalize human T lymphocytes in vitro (8). Previously, it was suggested that there was little or no significant sequence difference between strains of these subgroups other than that seen in the transformation-associated genes (40, 42). Recently, however, it was shown that there is pronounced sequence divergence between other genes, including the immediate-early transactivator gene, ORF50, of HVS strains A11 and C488 (71).
We analyzed the ORF73 sequences of HVS strains A11 and C488 and showed that the C488 ORF73 sequence is 94 aa larger (501 aa) than the A11 ORF73 sequence (407 aa). The amino acid sequences at the N and C termini were approximately 85 to 90% identical. However, the C488 ORF73 encoded a large central domain of acidic residues. Although this observation has not been shown to correlate with transforming potential, these residues may have a potential role in pathogenesis, as KSHV LANA, now shown to be a multifunctional protein, contains a long central acidic domain (22, 41, 44-46, 59, 62). The functional role of the additional central acidic amino acid residues in HVS C488 is under investigation to determine their association with cellular molecules and for a comparison with HVS A11 and KSHV LANA. Nuclear localization signals are conserved in ORF73 from both HVS strains and in KSHV LANA. However, HVS ORF73 did not show any distinctive proline-rich or leucine zipper domain like that seen in KSHV LANA.
A sequence comparison of ORF73 in other gammaherpesviruses indicated that ORF73 was poorly conserved across the subgroups; interestingly, one member, equine herpesvirus 2, was found to lack any positional or genetic homolog of ORF73 in an analysis of genomic sequences (69). An amino acid sequence alignment of ORF73 proteins from the six known members of the gammaherpesvirus subfamily showed that the C-terminal region of the protein has more conserved specific amino acid residues than does the N-terminal region. This finding suggests that the function of ORF73 in all members of this subfamily may be conserved in the context of the C-terminal domain. The C termini of these proteins have been shown to be involved in dimerization, transcriptional repression, binding to cis-acting elements, gene targeting, and targeting to nuclear bodies (7, 25, 45, 46, 65).
Western blot analysis of HVS C488 ORF73 showed a size of ∼85 kDa, as detected with an anti-Myc monoclonal antibody. In contrast to the molecular mass of 56 kDa predicted from the protein sequence, HVS C488 ORF73 migrated at an apparent mass of 85 kDa on SDS-PAGE, presumably due to the high degree of negatively charged amino acid residues present within the internal repeat as well as potential posttranslational modifications resulting in a mobility slower than the predicted molecular mass (30, 60). A similar observation was made for KSHV LANA, which has a sequence of 1,162 aa (strain BC-1) and a predicted molecular mass of 128 kDa; however, the actual size observed by SDS-PAGE analysis indicated a much larger molecule, of approximately 224 kDa (60).
To test the conservation of epitopes of these proteins, antibodies raised against the ORF73 proteins of HVS A11 and C488 and KSHV LANA were tested for cross-reactivity with each protein. Immunodetection of these proteins suggested that the immunogenic site of the ORF73 proteins of A11 and C488 most likely is conserved but that this epitope might have been lost due to divergent evolution in the human virus (KSHV) LANA molecule, as serum from an HVS C488-infected squirrel monkey and KSHV-infected humans did not cross-react with the viral proteins in Western blots. These suggestions were confirmed by immunolocalization of the transiently transfected proteins. Localization with the anti-Myc monoclonal antibody showed very distinct nuclear speckled patterns with the three proteins. Moreover, serum from the C488-infected squirrel monkey showed a nuclear speckled pattern with the C488 protein similar to that seen with the A11 protein but not with KSHV LANA. This finding strongly indicates that serum raised against HVS ORF73 lacks the ability to recognize similar antigenic sites on KSHV LANA.
HVS ORF73 has nuclear localization signals in its N- and C-terminal domains, and the truncated proteins localize to the nucleus (30). C488 ORF73 truncated proteins lacking either the N or the C terminus showed a nuclear speckled pattern, suggesting that the nuclear localization signals are conserved in the amino- and carboxy-terminal domains.
Colocalization of HVS C488 ORF73 and viral genomic DNA on metaphase chromosome spreads of a stably transformed cell line (CJ-1) suggested that ORF73 anchors the viral DNA to the chromosome by binding in a manner similar to that seen with KSHV LANA (14, 25, 43). These initial data show that HVS ORF73 binds to chromosomes most likely through the binding of a CBS at the N terminus to histone H1; however, the N terminus in HVS ORF73 is highly divergent and has no CBS similar to that mapped in KSHV LANA (14, 56). Therefore, experiments are warranted to confirm the mechanism of tethering of HVS episomes to host chromosomes. Recently, it was reported that HVS TRs can be efficiently maintained long term in ORF73-expressing cells, suggesting that the ORF73 binding sequence is located within the TR unit (13). In an attempt to identify the possible HVS ORF73 binding sequence, the 17-bp imperfect palindromic sequence of the KSHV TR unit was aligned to the sequence of the HVS TR unit, revealing two putative binding sites. These two sites show different degrees of homology to the nucleotide sequences of the KSHV TRs (LBS-1 and LBS-2) required for binding to KSHV LANA (25). Like LBS-1 and LBS-2 of KSHV, which are separated by 21 bp in a single TR unit, the two putative binding sites (73BR-1 and 73BR-2) in HVS TRs are separated by 22 bp (24). These two putative HVS ORF73 binding sites potentially bind to a dimeric or multimeric unit of ORF73, forming the origin replication complex. These results showed considerable organizational similarities between the dyad symmetry elements of EBV oriP bound by EBNA-1 and the TR sequences bound by KSHV LANA (25).
An EMSA demonstrating the binding abilities of KSHV and HVS ORF73 proteins suggested that there is some level of conservation of the fundamental mechanisms involved in DNA replication and episome maintenance. The ORF73 proteins of both strains of HVS showed detectable binding to the LANA binding sequence of KSHV, suggesting that the conservation of this nucleotide sequence is essential for binding. However, KSHV LANA showed little or no detectable binding to the HVS putative binding sequences. This result may have been due to the acquisition of specific changes in the KSHV TR sequence during divergent evolution, as required for the more recently diverged KSHV LANA. Maintenance of the HVS C488 cosmid (Cos331) in HEK293 cells expressing HVS C488 ORF73 suggested its requirement for episome maintenance. A previous report indicated that high-G-+-C DNA from HVS strain A11 was not detected in a maintenance assay in cells lacking ORF73 expression (13). Using a short-term replication assay, we showed that HVS C488 ORF73 is essential for the replication of a plasmid containing viral TR DNA, suggesting ORF73-dependent replication of the HVS genome. It was previously reported that HVS strain A11 requires ORF73 for maintenance, but here we show, for the first time, that ORF73 of HVS strain C488 is also essential for replication. These observations suggest that HVS ORF73 is similar to other DNA viral replication proteins, such simian virus 40 T antigen, EBNA-1, E1 and E2 of human papillomavirus, and KSHV LANA (24, 25, 33). The identification in HVS TRs of ORF73 binding sequences similar to LANA binding sequences (LBS-1 and LBS-2) in KSHV TRs suggests that these two viruses use the same strategy for latent DNA replication. To assess the conservation of functional roles of HVS C488 ORF73 and KSHV LANA in binding and maintenance in these two viruses, short-term maintenance and transient replication assays with plasmids containing TRs in the presence or absence of KSHV or HVS ORF73 proteins would be needed. Such assays also would determine whether the core elements of latent genome replication have been conserved in these viruses through evolution.
Acknowledgments
This work was supported by grants from the Leukemia and Lymphoma Society of America and Public Health Service grants from NCI (CA072510 and CA091792) and from NIDCR (DE01436) (to E.S.R.). E.S.R. is a scholar of the Leukemia and Lymphoma Society of America.
REFERENCES
- 1.Albrecht, J. C., J. Nicholas, D. Biller, K. R. Cameron, B. Biesinger, C. Newman, S. Wittmann, M. A. Craxton, H. Coleman, B. Fleckenstein, et al. 1992. Primary structure of the herpesvirus saimiri genome. J. Virol. 66:5047-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander, L., Z. Du, M. Rosenzweig, J. U. Jung, and R. C. Desrosiers. 1997. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 nef alleles in lymphocyte activation. J. Virol. 71:6094-6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambroziak, J. A., D. J. Blackbourn, B. G. Herndier, R. G. Glogau, J. H. Gullett, A. R. McDonald, E. T. Lennette, and J. A. Levy. 1995. Herpes-like sequences in HIV-infected and uninfected Kaposi's sarcoma patients. Science 268:582-583. [DOI] [PubMed] [Google Scholar]
- 4.Arad, U. 1998. Modified Hirt procedure for rapid purification of extrachromosomal DNA from mammalian cells. BioTechniques 24:760-762. [DOI] [PubMed] [Google Scholar]
- 5.Aster, J. C., E. S. Robertson, R. P. Hasserjian, J. R. Turner, E. Kieff, and J. Sklar. 1997. Oncogenic forms of NOTCH1 lacking either the primary binding site for RBP-Jkappa or nuclear localization sequences retain the ability to associate with RBP-Jkappa and activate transcription. J. Biol. Chem. 272:11336-11343. [DOI] [PubMed] [Google Scholar]
- 6.Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641-644. [DOI] [PubMed] [Google Scholar]
- 7.Ballestas, M. E., and K. M. Kaye. 2001. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mediates episome persistence through cis-acting terminal repeat (TR) sequence and specifically binds TR DNA. J. Virol. 75:3250-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biesinger, B., I. Muller-Fleckenstein, B. Simmer, G. Lang, S. Wittmann, E. Platzer, R. C. Desrosiers, and B. Fleckenstein. 1992. Stable growth transformation of human T lymphocytes by herpesvirus saimiri. Proc. Natl. Acad. Sci. USA 89:3116-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boshoff, C., T. F. Schulz, M. M. Kennedy, A. K. Graham, C. Fisher, A. Thomas, J. O. McGee, R. A. Weiss, and J. J. O'Leary. 1995. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat. Med. 1:1274-1278. [DOI] [PubMed] [Google Scholar]
- 10.Callahan, J., S. Pai, M. Cotter, and E. S. Robertson. 1999. Distinct patterns of viral antigen expression in Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus coinfected body-cavity-based lymphoma cell lines: potential switches in latent gene expression due to coinfection. Virology 262:18-30. [DOI] [PubMed] [Google Scholar]
- 11.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 12.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 13.Collins, C. M., M. M. Medveczky, T. Lund, and P. G. Medveczky. 2002. The terminal repeats and latency-associated nuclear antigen of herpesvirus saimiri are essential for episomal persistence of the viral genome. J. Gen. Virol. 83:2269-2278. [DOI] [PubMed] [Google Scholar]
- 14.Cotter, M. A., II, and E. S. Robertson. 1999. The latency-associated nuclear antigen tethers the Kaposi's sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology 264:254-264. [DOI] [PubMed] [Google Scholar]
- 15.Cotter, M. A., II, C. Subramanian, and E. S. Robertson. 2001. The Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen binds to specific sequences at the left end of the viral genome through its carboxy-terminus. Virology 291:241-259. [DOI] [PubMed] [Google Scholar]
- 16.Desrosiers, R. C. 1981. Herpesvirus saimiri DNA in tumor cells—deleted sequences and sequence rearrangements. J. Virol. 39:497-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dittmer, D., M. Lagunoff, R. Renne, K. Staskus, A. Haase, and D. Ganem. 1998. A cluster of latently expressed genes in Kaposi's sarcoma-associated herpesvirus. J. Virol. 72:8309-8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dupin, N., T. L. Diss, P. Kellam, M. Tulliez, M. Q. Du, D. Sicard, R. A. Weiss, P. G. Isaacson, and C. Boshoff. 2000. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood 95:1406-1412. [PubMed] [Google Scholar]
- 19.Dupin, N., C. Fisher, P. Kellam, S. Ariad, M. Tulliez, N. Franck, E. van Marck, D. Salmon, I. Gorin, J. P. Escande, R. A. Weiss, K. Alitalo, and C. Boshoff. 1999. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc. Natl. Acad. Sci. USA 96:4546-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ensser, A., R. Pflanz, and B. Fleckenstein. 1997. Primary structure of the alcelaphine herpesvirus 1 genome. J. Virol. 71:6517-6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fickenscher, H., B. Biesinger, A. Knappe, S. Wittmann, and B. Fleckenstein. 1996. Regulation of the herpesvirus saimiri oncogene stpC, similar to that of T-cell activation genes, in growth-transformed human T lymphocytes. J. Virol. 70:6012-6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friborg, J., Jr., W. Kong, M. O. Hottiger, and G. J. Nabel. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402:889-894. [DOI] [PubMed] [Google Scholar]
- 23.Gao, S. J., L. Kingsley, M. Li, W. Zheng, C. Parravicini, J. Ziegler, R. Newton, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, Y. Chang, and P. S. Moore. 1996. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nat. Med. 2:925-928. [DOI] [PubMed] [Google Scholar]
- 24.Garber, A. C., J. Hu, and R. Renne. 2002. Latency-associated nuclear antigen (LANA) cooperatively binds to two sites within the terminal repeat, and both sites contribute to the ability of LANA to suppress transcription and to facilitate DNA replication. J. Biol. Chem. 277:27401-27411. [DOI] [PubMed] [Google Scholar]
- 25.Garber, A. C., M. A. Shu, J. Hu, and R. Renne. 2001. DNA binding and modulation of gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:7882-7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardella, T., P. Medveczky, T. Sairenji, and C. Mulder. 1984. Detection of circular and linear herpesvirus DNA molecules in mammalian cells by gel electrophoresis. J. Virol. 50:248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godden-Kent, D., S. J. Talbot, C. Boshoff, Y. Chang, P. Moore, R. A. Weiss, and S. Mittnacht. 1997. The cyclin encoded by Kaposi's sarcoma-associated herpesvirus stimulates cdk6 to phosphorylate the retinoblastoma protein and histone H1. J. Virol. 71:4193-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grundhoff, A., and D. Ganem. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus permits replication of terminal repeat-containing plasmids. J. Virol. 77:2779-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall, K. T., M. S. Giles, D. J. Goodwin, M. A. Calderwood, I. M. Carr, A. J. Stevenson, A. F. Markham, and A. Whitehouse. 2000. Analysis of gene expression in a human cell line stably transduced with herpesvirus saimiri. J. Virol. 74:7331-7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall, K. T., M. S. Giles, D. J. Goodwin, M. A. Calderwood, A. F. Markham, and A. Whitehouse. 2000. Characterization of the herpesvirus saimiri ORF73 gene product. J. Gen. Virol. 81:2653-2658. [DOI] [PubMed] [Google Scholar]
- 31.Harris, A., B. D. Young, and B. E. Griffin. 1985. Random association of Epstein-Barr virus genomes with host cell metaphase chromosomes in Burkitt's lymphoma-derived cell lines. J. Virol. 56:328-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirt, B. 1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 26:365-369. [DOI] [PubMed] [Google Scholar]
- 33.Hu, J., A. C. Garber, and R. Renne. 2002. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus supports latent DNA replication in dividing cells. J. Virol. 76:11677-11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyun, T. S., C. Subramanian, M. A. Cotter II, R. A. Thomas, and E. S. Robertson. 2001. Latency-associated nuclear antigen encoded by Kaposi's sarcoma-associated herpesvirus interacts with Tat and activates the long terminal repeat of human immunodeficiency virus type 1 in human cells. J. Virol. 75:8761-8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeong, J., J. Papin, and D. Dittmer. 2001. Differential regulation of the overlapping Kaposi's sarcoma-associated herpesvirus vGCR (orf74) and LANA (orf73) promoters. J. Virol. 75:1798-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung, J. U., M. Stager, and R. C. Desrosiers. 1994. Virus-encoded cyclin. Mol. Cell. Biol. 14:7235-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katano, H., Y. Sato, T. Kurata, S. Mori, and T. Sata. 1999. High expression of HHV-8-encoded ORF73 protein in spindle-shaped cells of Kaposi's sarcoma. Am. J. Pathol. 155:47-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kedes, D. H., E. Operskalski, M. Busch, R. Kohn, J. Flood, and D. Ganem. 1996. The seroepidemiology of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat. Med. 2:918-924. (Erratum, 2:1041.) [DOI] [PubMed] [Google Scholar]
- 39.Knappe, A., G. Feldmann, U. Dittmer, E. Meinl, T. Nisslein, S. Wittmann, K. Matz-Rensing, T. Kirchner, W. Bodemer, and H. Fickenscher. 2000. Herpesvirus saimiri-transformed macaque T cells are tolerated and do not cause lymphoma after autologous reinfusion. Blood 95:3256-3261. [PubMed] [Google Scholar]
- 40.Knappe, A., C. Hiller, M. Thurau, S. Wittmann, H. Hofmann, B. Fleckenstein, and H. Fickenscher. 1997. The superantigen-homologous viral immediate-early gene ie14/vsag in herpesvirus saimiri-transformed human T cells. J. Virol. 71:9124-9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knight, J. S., M. A. Cotter II, and E. S. Robertson. 2001. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus transactivates the telomerase reverse transcriptase promoter. J. Biol. Chem. 276:22971-22978. [DOI] [PubMed] [Google Scholar]
- 42.Kraft, M. S., G. Henning, H. Fickenscher, D. Lengenfelder, J. Tschopp, B. Fleckenstein, and E. Meinl. 1998. Herpesvirus saimiri transforms human T-cell clones to stable growth without inducing resistance to apoptosis. J. Virol. 72:3138-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krithivas, A., M. Fujimuro, M. Weidner, D. B. Young, and S. D. Hayward. 2002. Protein interactions targeting the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus to cell chromosomes. J. Virol. 76:11596-11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krithivas, A., D. B. Young, G. Liao, D. Greene, and S. D. Hayward. 2000. Human herpesvirus 8 LANA interacts with proteins of the mSin3 corepressor complex and negatively regulates Epstein-Barr virus gene expression in dually infected PEL cells. J. Virol. 74:9637-9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lim, C., Y. Gwack, S. Hwang, S. Kim, and J. Choe. 2001. The transcriptional activity of cAMP response element-binding protein-binding protein is modulated by the latency associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Biol. Chem. 276:31016-31022. [DOI] [PubMed] [Google Scholar]
- 46.Lim, C., H. Sohn, Y. Gwack, and J. Choe. 2000. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) binds ATF4/CREB2 and inhibits its transcriptional activation activity. J. Gen. Virol. 81:2645-2652. [DOI] [PubMed] [Google Scholar]
- 47.Lim, C., H. Sohn, D. Lee, Y. Gwack, and J. Choe. 2002. Functional dissection of latency-associated nuclear antigen 1 of Kaposi's sarcoma-associated herpesvirus involved in latent DNA replication and transcription of terminal repeats of the viral genome. J. Virol. 76:10320-10331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Medveczky, M. M., E. Szomolanyi, R. Hesselton, D. DeGrand, P. Geck, and P. G. Medveczky. 1989. Herpesvirus saimiri strains from three DNA subgroups have different oncogenic potentials in New Zealand White rabbits. J. Virol. 63:3601-3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medveczky, P., E. Szomolanyi, R. C. Desrosiers, and C. Mulder. 1984. Classification of herpesvirus saimiri into three groups based on extreme variation in a DNA region required for oncogenicity. J. Virol. 52:938-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Medveczky, P. G., and M. M. Medveczky. 1989. Expression of interleukin 2 receptors in T cells transformed by strains of Herpesvirus saimiri representing three DNA subgroups. Intervirology 30:213-226. [DOI] [PubMed] [Google Scholar]
- 51.Melendez, L. V., M. D. Daniel, R. D. Hunt, and F. G. Garcia. 1968. An apparently new herpesvirus from primary kidney cultures of the squirrel monkey (Saimiri sciureus). Lab. Anim. Care 18:374-381. [PubMed] [Google Scholar]
- 52.Moore, P., and Y. Chang. 1995. Detection of herpes-like virus DNA sequences in Kaposi's sarcoma in patients with and without HIV infection. N. Engl. J. Med. 332:1181-1185. [DOI] [PubMed] [Google Scholar]
- 53.Moore, P. S., L. A. Kingsley, S. D. Holmberg, T. Spira, P. Gupta, D. R. Hoover, J. P. Parry, L. J. Conley, H. W. Jaffe, and Y. Chang. 1996. Kaposi's sarcoma-associated herpesvirus infection prior to onset of Kaposi's sarcoma. AIDS 10:175-180. [DOI] [PubMed] [Google Scholar]
- 54.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nador, R. G., E. Cesarman, A. Chadburn, D. B. Dawson, M. Q. Ansari, J. Sald, and D. M. Knowles. 1996. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood 88:645-656. [PubMed] [Google Scholar]
- 56.Piolot, T., M. Tramier, M. Coppey, J. C. Nicolas, and V. Marechal. 2001. Close but distinct regions of human herpesvirus 8 latency-associated nuclear antigen 1 are responsible for nuclear targeting and binding to human mitotic chromosomes. J. Virol. 75:3948-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Platt, G. M., G. R. Simpson, S. Mittnacht, and T. F. Schulz. 1999. Latent nuclear antigen of Kaposi's sarcoma-associated herpesvirus interacts with RING3, a homolog of the Drosophila female sterile homeotic (fsh) gene. J. Virol. 73:9789-9795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Polvino-Bodnar, M., and P. A. Schaffer. 1992. DNA binding activity is required for EBNA 1-dependent transcriptional activation and DNA replication. Virology 187:591-603. [DOI] [PubMed] [Google Scholar]
- 59.Radkov, S. A., P. Kellam, and C. Boshoff. 2000. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat. Med. 6:1121-1127. [DOI] [PubMed] [Google Scholar]
- 60.Rainbow, L., G. M. Platt, G. R. Simpson, R. Sarid, S.-J. Gao, H. Stoiber, C. S. Herrington, P. S. Moore, and T. F. Schulz. 1997. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J. Virol. 71:5915-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rawlins, D. R., G. Milman, S. D. Hayward, and G. S. Hayward. 1985. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance region. Cell 42:859-868. [DOI] [PubMed] [Google Scholar]
- 62.Renne, R., C. Barry, D. Dittmer, N. Compitello, P. O. Brown, and D. Ganem. 2001. Modulation of cellular and viral gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:458-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schalling, M., M. Ekman, E. E. Kaaya, A. Linde, and P. Biberfeld. 1995. A role for a new herpes virus (KSHV) in different forms of Kaposi's sarcoma. Nat. Med. 1:707-708. [DOI] [PubMed] [Google Scholar]
- 65.Schwam, D. R., R. L. Luciano, S. S. Mahajan, L. Wong, and A. C. Wilson. 2000. Carboxy terminus of human herpesvirus 8 latency-associated nuclear antigen mediates dimerization, transcriptional repression, and targeting to nuclear bodies. J. Virol. 74:8532-8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shinohara, H., M. Fukushi, M. Higuchi, M. Oie, O. Hoshi, T. Ushiki, J. Hayashi, and M. Fujii. 2002. Chromosome binding site of latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus is essential for persistent episome maintenance and is functionally replaced by histone H1. J. Virol. 76:12917-12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shirakata, M., and K. Hirai. 1998. Identification of minimal oriP of Epstein-Barr virus required for DNA replication. J. Biochem. (Tokyo) 123:175-181. [DOI] [PubMed] [Google Scholar]
- 68.Talbot, S. J., R. A. Weiss, P. Kellam, and C. Boshoff. 1999. Transcriptional analysis of human herpesvirus-8 open reading frames 71, 72, 73, K14, and 74 in a primary effusion lymphoma cell line. Virology 257:84-94. [DOI] [PubMed] [Google Scholar]
- 69.Telford, E. A., M. S. Watson, H. C. Aird, J. Perry, and A. J. Davison. 1995. The DNA sequence of equine herpesvirus 2. J. Mol. Biol. 249:520-528. [DOI] [PubMed] [Google Scholar]
- 70.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thurau, M., A. Whitehouse, S. Wittmann, D. Meredith, and H. Fickenscher. 2000. Distinct transcriptional and functional properties of the R transactivator gene orf50 of the transforming herpesvirus saimiri strain C488. Virology 268:167-177. [DOI] [PubMed] [Google Scholar]
- 72.Yates, J. L., N. Warren, and B. Sugden. 1985. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature 313:812-815. [DOI] [PubMed] [Google Scholar]