Abstract
A variety of reactive oxygen species react readily with methionine residues in proteins to form methionine sulfoxide, thus scavenging the reactive species. Most cells contain methionine sulfoxide reductases, which catalyze a thioredoxin-dependent reduction of methionine sulfoxide back to methionine. Thus, methionine residues may act as catalytic antioxidants, protecting both the protein where they are located and other macromolecules. To test this hypothesis directly, we replaced 40% of the methionine residues in Escherichia coli with norleucine, the carbon-containing analog, in which the sulfur of methionine is substituted by a methylene group (-CH2-). The intracellular free methionine and S-adenosylmethionine pools were not altered, nor was the specific activity of the key enzyme, glutamine synthetase. When unstressed, both control and norleucine-substituted cells survived equally well at stationary phase for at least 32 h. However, oxidative stress was more damaging to the norleucine-substituted cells. They died more rapidly than control cells when challenged by hypochlorite, hydrogen peroxide, or ionizing radiation. One of the most abundant proteins in the cell, elongation factor Tu, was found to be more oxidatively modified in norleucine-substituted cells, consistent with loss of the antioxidant defense provided by methionine residues. The results of these studies support the hypothesis that methionine in protein acts as an endogenous antioxidant in cells.—Luo, S., Levine, R. L. Methionine in proteins defends against oxidative stress.
Keywords: norleucine, elongation factor Tu, hypochlorous acid
Methionine is one of the more readily oxidized residues in proteins and can be attacked by many of the reactive oxygen species (ROS) generated in biological systems (1, 2). α-2-Macroglobulin is a circulating protease inhibitor that must function at sites of inflammation where high levels of potentially damaging ROS may be present (3). In an earlier study, we found that each subunit of α-2-macroglobulin scavenged at least 10 mol of oxidizing species, with conversion of a stoichiometric number of Met residues to methionine sulfoxide (MetO) (4). Continued exposure to oxidant led to oxidation of a single tryptophan residue and inactivation of the antiprotease. The Met residues appeared to act as “molecular bodyguards,” protecting the critical tryptophan residue. In a subsequent study of glutamine synthetase (5), we found that 8 of the 16 Met residues in the protein scavenged hydrogen peroxide, and these 8 residues were shown to be surface- or solvent-exposed, while the other 8 were relatively buried. The Met residues were oxidized to MetO, but as with α-2-macroglobulin, the catalytic activity of glutamine synthetase was preserved. Topological mapping of the oxidized Met showed that they were arrayed along a bay that forms the entrance to the active site center of the enzyme. The ability of Met residues to scavenge ROS coupled with their nonrandom location again suggested that their function was to protect critical residues at the active site from oxidative modification, which in glutamine synthetase is known to cause inactivation (6).
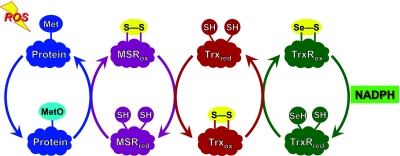
The oxidation of 8 strategically located Met residues per protein molecule without impairment of enzymatic function constitutes a substantive defense against oxidative damage to that specific protein molecule. However, oxidation of Met to MetO is reversible, providing a chemical basis for an even more efficient scavenging of reactive species and broadening protection from the specific molecule to the entire cell. Virtually all organisms from bacteria to mammals have several MetO reductases (msrs), which catalyze the reduction of MetO to Met (7). Reduction of Met residues in proteins allows them to react again with ROS, creating a system with catalytic efficiency in scavenging potentially damaging species (8) (Fig. 1). Reduction of the oxidized msr by thioredoxin readies the enzyme for another catalytic cycle. Oxidized thioredoxin is reduced by thioredoxin reductase at the expense of NADPH. The net result is the catalytic scavenging of reactive nitrogen and oxygen species by NADPH.
Figure 1.
Scheme of the antioxidant defense system created by cyclic oxidation and reduction of Met residues. Reduced forms of the proteins carry the subscript “red” and oxidized forms carry “ox.” Reading from left to right, ROS is intercepted by a Met residue, which is oxidized to MetO. MetO is reduced back to Met by msr, with the formation of a disulfide bond. The oxidized msr is reduced by thioredoxin, which now carries the disulfide bond. It is reduced by thioredoxin reductase, which, in mammals, contains a selenocysteine residue that is oxidized, forming a selenocysteine-cysteine bond. This disulfide analog is then reduced by NADPH. The net result is that ROS is reduced at the expense of NADPH.
Oxidation of Met to MetO creates also a chiral center, and the S- and R-epimers are substrates for specific msrs. In most organisms the S-epimer is reduced by msrA and the R-epimer by msrB, both using thioredoxin as the source of reducing equivalents. MsrA was described well before msrB, so that the majority of the studies in the literature were performed with msrA. These investigations provide strong evidence supporting the physiological importance of cyclic oxidation and reduction of Met residues outlined in Fig. 1. Knocking out msrA caused increased susceptibility to oxidative stress in mice (9), yeast (10), and bacteria (11,12,13). Conversely, overexpressing msrA conferred increased resistance to Drosophila (14), Saccharomyces (15), Arabidopsis, (16), PC-12 cells (17), and human T cells (15). Several studies demonstrated that modulation of msrA activity altered the level of ROS: overexpression lowered the level in PC-12 cells (17), while lens cells lacking msrA had an increased level (18). Increased expression of either msrA in WI-38 fibroblasts or msrB in MOLT-4 leukemia cells reduced the steady-state level of oxidized proteins after a hydrogen peroxide challenge (19, 20). Accumulation of oxidatively damaged proteins has been hypothesized to be an important mechanism of the aging process (21), and it is notable that overexpression of msrA in Drosophila doubled the life span of the flies (14).
Although these results demonstrate the importance of msr, they do not directly establish that Met residues in proteins participate in the mechanism. We elected to experimentally assess the importance of Met residues by substantially decreasing their content in all cellular proteins of an organism, a manipulation that is possible in E. coli. Norleucine (Nle) is the carbon analog of Met in which the sulfur of Met is replaced by a methylene group. In 1959, Cowie et al. (22) showed that Nle could substitute for Met and support normal bacterial protein synthesis. Barker and Bruton (23) followed up by demonstrating that Nle can be charged onto both tRNAm and tRNAf. Nle-tRNAf is then formylated and initiates protein synthesis. Notably, Nle incorporation did not produce misfolded or aberrant proteins, as is usually observed when amino acid analogues are incorporated into cellular proteins. Consistent with the production of normally folded proteins, Nle incorporation did not affect cell viability, even in early stationary phase. Thus, global replacement of a portion of Met residues by Nle in bacteria allows us to test whether the hypothesized antioxidant function of Met is important in vivo.
MATERIALS AND METHODS
E. coli growth and viability assay
The E. coli K-12 Met auxotroph DL41 strain (24) was CGSC#7177 (F-, λ-, metA28), a derivative of the wild-type MG1655. The seed cells were grown overnight at 37°C in minimal M9 medium (25) supplemented with 50 μM l-Met, 0.2% glucose, 1 mg/L thiamine, 1 mM MgSO4, 1 mg/L FeSO4 · 7H2O, and trace elements (Cu2+, Mn2+, Zn2+, and MoO42−; 1 μg/L). The seed culture was then diluted 100-fold into either the same medium for control cells or into medium supplemented with 500 μM dl-Nle (N1398; Sigma, St. Louis, MO, USA) to produce Nle-containing cells. Cultures were grown to stationary phase by incubation for 24 h at 37°C at 250 rpm in 250-ml Erlenmeyer flasks (#431144; Corning, Corning, NY, USA). Oxidative stress challenges were performed in the same medium except that glucose, Met, and Nle were omitted (M9-G). The 24-h-grown cells were harvested, washed twice, and resuspended in M9-G medium at room temperature to a density of 2–3 × 108 colony-forming units (cfu)/ml (OD600nm=0.28–0.3). Cells were then incubated at 37°C, 250 rpm for 2 h before challenge. Hydrogen peroxide, hypochlorite (NaOCl), or paraquat were then added to the medium. Cells were harvested at 4°C, washed twice with 20 mM sodium phosphate buffer (pH 7.2), and stored at −20°C until analyzed.
To determine viability, cultures were diluted into M9-G medium and then plated in duplicate on Luria-Bertani (LB) agar plates. After overnight incubation at 37°C, colonies were counted manually, and the results were expressed as colony forming units per milliliter of the original growth.
Irradiation
For the higher doses (100–1200 Gy), 1 ml was placed in a 1.5-ml tube and irradiated on ice at 86 Gy/min (60Co, Model 109; J. L. Shepard and Associates, San Fernanado, CA, USA). Low-dose (10–40 Gy) irradiation was performed at 44 Gy/h in a 137Cs Gammacell 40 irradiation unit (Atomic Energy of Canada Limited, Ottowa, ON, Canada). Three tubes containing 50 ml culture each were incubated at 37°C in a water bath during irradiation. A tube was transferred to a separate 37°C water bath located outside the irradiator after 13.6, 27.3, and 54.5 min exposure. Then all three tubes were placed on ice until they were assessed for viability as described above.
Amino acid analyses
Quantitation of MetO requires that the protein be treated with cyanogen bromide before acid hydrolysis (26). Cell pellets were disrupted with 70% formic acid, and subsequent analyses were performed in duplicate. Before hydrolysis and amino acid analysis, one aliquot of the extract was treated with cyanogen bromide, while another was not (26). The MetO level is expressed as the fraction of total Met + MetO.
To determine the pool size of free and total Met, cell pellets were disrupted in 70% trifluoroacetic acid (Pierce, Rockford, IL, USA) on ice for 30 min. To measure the protein content of the extracts, 1 μl was spotted on a polyvinylidene fluoride membrane and then stained with Fast Green. Using bovine serum albumin as a standard, protein was quantitated from the Fast Green fluorescence in an Odyssey infrared fluorescence detection system (Li-Cor, Omaha, NE, USA) (27). Extracts were dried in a vacuum centrifuge (Savant Instruments, Holbrook, NY, USA), resuspended in water, and centrifuged. The supernatant was assayed for free Met and S-adenosylmethionine (28), while the pellet was hydrolyzed and then subjected to amino acid analysis. The sum of the Met content of the supernatant and pellet gave the total cellular Met.
Amino acid analysis, including Nle, was performed on the acid hydrolysate of the whole cell pellet by HPLC following precolumn derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (29) and by o-phthaldialdehyde (30). The C18 reversed-phase column used for the first method and for S-adenosylmethionine analysis was a WAT052885 (Waters, Milford, MA, USA) and that for the second method was a Jones Apex 4M15310 (Jones Chromatography, Englewood, CO, USA). The second method was used to check for possible changes in amino acids other than Met and Nle because it provides more equal fluorimetric response for each amino acid than the first method. However, it does not separate Nle from Leu, which is accomplished by the first method. No differences in amino acid content were observed between control and Nle-grown cells except in their content of Met and Nle.
Glutamine synthetase activity
Cells were sonicated on ice in 20 mM phosphate buffer, pH 7.2, containing protease inhibitors (leupeptin 0.5 mg/L, pepstatin 0.7 mg/L, aprotinin 0.5 mg/L, and phenylmethylsulfonyl fluoride 40 mg/L). After centrifugation, the supernatant was assayed for protein with bicinchoninic acid and for glutamine synthetase activity by γ-glutamyltransferase assay (31).
Protein carbonyl assay
Cell pellets were disrupted by French press for spectrophotometric carbonyl determination (32). They were disrupted in 6% SDS for quantitative immunodetection of protein carbonyls, performed as described previously (33) with modification (27). SDS-PAGE was run on 15-lane 4–20% gradient gels (EC60255; Invitrogen, Carlsbad, CA, USA) and proteins were transferred onto 0.2-μm polyvinylidene fluoride (Invitrogen LC2002). Both the rabbit anti-dinitrophenyl primary antibody (D-9656; Sigma) and the goat anti-rabbit secondary antibody labeled with infrared fluorescent IRDye800CW (611-131-122; Rockland Immunochemicals, Rockland, IL, USA) were diluted 1:10,000.
Purified E. coli glutamine synthetase was oxidatively modified as described previously (27). Its carbonyl content was determined spectrophotometrically and then used as a quantitative standard on Western blots. Gels were loaded with either 50 ng of glutamine synthetase or 2.25 μg protein from cell extracts. When assaying with the spectrophotometric method, cell extracts were treated with 1% streptomycin on ice for 30 min to precipitate nucleic acids (32), because they cause a marked, artifactual increase in the measured carbonyl content.
Identification of the major oxidatively modified protein
The ∼50-kDa band was cut from a Coomassie-stained gel, subjected to ingel digestion with endoproteinase Lys-C, and analyzed by HPLC mass spectrometry as described previously (34). The protein was identified by both Mascot (Matrix Science, Boston, MA, USA) and Spectrum Mill (Agilent Technologies, Santa Clara, CA, USA).
RESULTS
Replacement of Met by Nle in E. coli
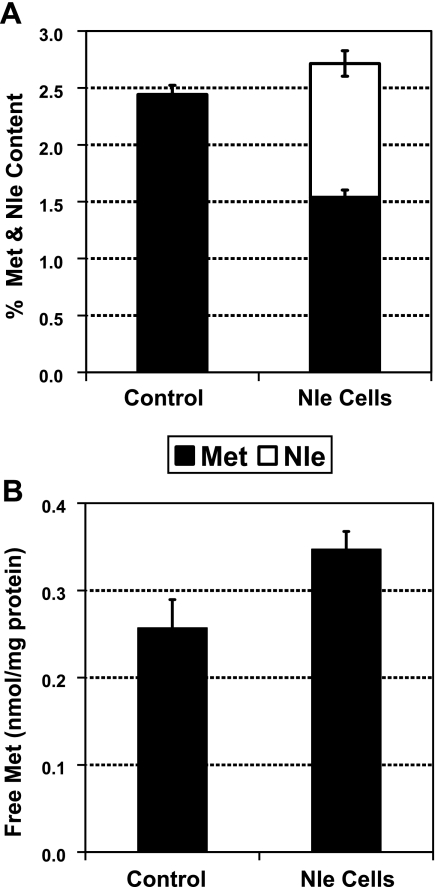
Barker and Bruton (23) found that Met auxotrophs incorporated substantial Nle into their proteins in late log phase as Met was exhausted from the medium and that the cells had normal viability even after 24 h in the Nle-enriched medium. We confirmed their findings and thus settled on a 24-h growth period to generate cells for susequent studies. Compared with control cells, Nle-grown cells showed ∼40% reduction of total Met content and an equivalent increase in Nle (Fig. 2A). No significant change was found in any other amino acid, including cysteine. The content of Met residues in proteins and peptides was 161 ± 18.7 nmol/mg protein in control cells, and decreased to 109 ± 9.5 nmol/mg protein in Nle cells. The intracellular free Met pool size was not reduced after Nle incorporation and was less than 0.4% of the Met content in protein (Fig. 2B). Thus, growth in Nle-enriched medium produced the desired decrease in Met residues in proteins.
Figure 2.
Growth in Nle-containing medium reduces protein Met content without affecting the free Met pool. A) Met and Nle content of cells. B) Free Met content.
Substitution of Met by Nle did not alter S-adenosylmethionine pool size nor the activity of a key enzyme
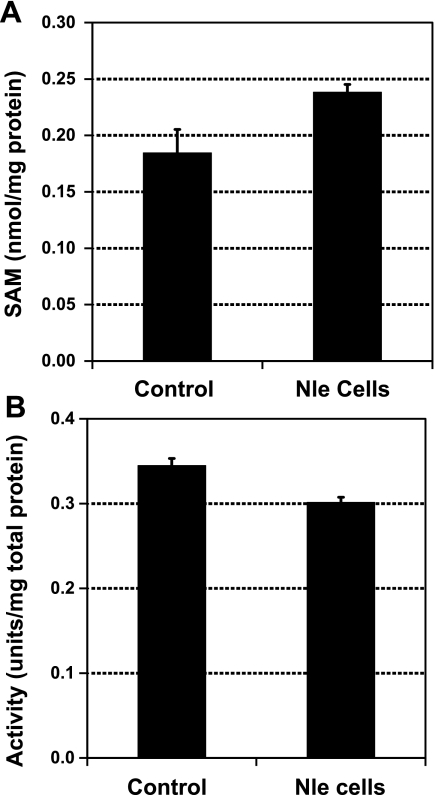
S-adenosylmethionine is an important molecule in intermediary metabolism, functioning as the major donor of methyl groups. If growth in Nle affected the concentration of S-adenosylmethionine, then interpretation of the cellular response to oxidative stress could be difficult. However, its pool size was unchanged in the Nle-grown cells (Fig. 3A).
Figure 3.
Growth in Nle-containing medium does not affect S-adenosylmethionine pool size nor the activity of glutamine synthetase. A) S-adenosylmethionine content. B) Glutamine synthetase activity.
As noted above, replacement of Met by Nle did not produce misfolded proteins (23). Nle replacement did not affect the enzymatic activity of staphylococcal nuclease (23, 35), β-galactosidase (36), adenylate kinase (37), or human recombinant macrophage colony stimulating factor (38). We also examined the enzyme glutamine synthetase, which has 16 Met residues on each subunit and plays a key role in controlling bacterial metabolism by regulating the flow of nitrogen. Again, no significant variation of activity was observed after 40% replacement of Met residues in the cells (Fig. 3B).
Nle-substituted cells are more sensitive to oxidative stress
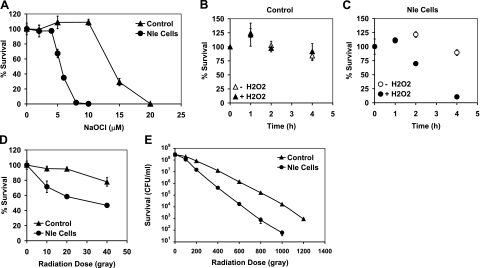
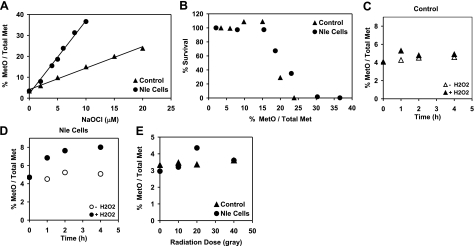
Both control and Nle-containing cells survived well during a 32-h glucose starvation, allowing comparison of their susceptibilities to different oxidative stresses. Hypochlorite (HOCl) is membrane permeable and preferentially attacks thioethers and thiols, that is, Met and Cys (39). One hour exposure to 5 μM hypochlorite killed half of the Nle cells but none of the control cells. Extending the treatment time up to 4 h caused no additional change, presumably because of the short half-life of hypochlorite (1). We, therefore, determined the concentration dependence of cell killing after 1-hour exposure. Control cells were not killed by10 μM hypochlorite, while Nle cells were rapidly killed by concentrations above 4 μM (Fig. 4A).
Figure 4.
Nle-containing cells are more susceptible to killing by oxidative stresses. A) Viability after 1 h exposure to hypocholorite. B, C) Viability after exposure to 5 mM hydrogen peroxide. D) Viability after 10–40 Gy irradiation exposure at 37°C. E) Viability after 100–1200 Gy irradiation exposure at 0°C.
As mentioned above, growth in Nle had no effect on free Met or S-adenosylmethionine pool size, so that the observed differences in killing by hypochlorite can be attributed to Met residues in proteins. As an additional check on this conclusion, we allowed the cells to replenish their low-molecular-weight Met-containing pools before challenge with hypochlorite. After the usual 24-h growth in Met- or Nle-containing medium, the cells were harvested and resuspended in carbon starvation medium (M9-G) supplemented with 25 μM Met for 2 h. This allowed for replenishment of low-molecular-weight Met pools without significant additional protein synthesis. The cells were then harvested and given the usual 2-h recovery in fresh M9-G medium followed by hypochlorite challenge. The results were unchanged from those observed for cells not exposed to the 25-μM Met supplementation, supporting the conclusion that the differences in viability are due to the decrease in Met residues in Nle-grown cells.
Nle cells were also more susceptible to killing by hydrogen peroxide. Control cells were unaffected by the addition of 5 mM H2O2 to the medium, while Nle cells began to die after 1 h of exposure (Fig. 4B, C). At 4 h, 90% of the Nle cells had died, while almost all of the control cells were still alive.
Ionizing radiation generates a variety of reactive species, including the potent oxidizing agent, hydroxyl radical, which reacts with most biological molecules (39, 40). We exposed the cells to a range of radiation doses and determined their survival. At 37°C and lower doses (10–40 Gy), control cells could tolerate 20 Gy, but 40% of Nle cells died (Fig. 4D). At higher doses of 100–1200 Gy, the cells were kept on ice during irradiation to avoid heating. Under these conditions, we expect enzymatic antioxidant systems to be ineffective, providing a relatively direct test of the importance of Met residues as antioxidants. Nle cells were again killed faster than control cells (Fig. 4E).
We also challenged the cells with 10 mM paraquat for up to 24 h and found no significant difference between Nle cells and control cells. There was almost no killing after 4 h exposure, but by 24 h, the viability of both control and Nle cells decreased to ∼50% viability.
Protein carbonyl levels
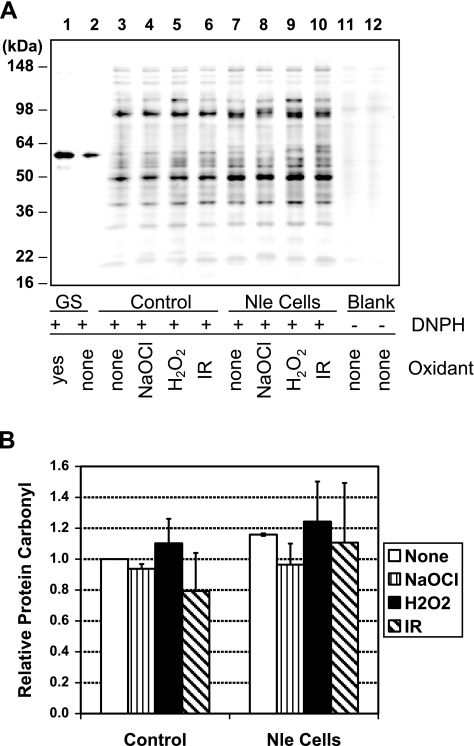
Protein carbonyl level has been widely used as an indicator of oxidative damage in cells. We quantitated the total protein carbonyl levels in SDS lysates of cells exposed to hypochlorite, peroxide, or irradiation. For each of the 3 stresses, we sampled cells at the point at which decreased viability was observed in the Nle cells in order to make measurements on living cells and not those which were already nonviable or dead (4 μM hypochlorite, 1 h peroxide exposure, or 10 Gy irradiation) (Fig. 5). Little difference in total protein carbonyl was observed at this point. However, proteins differ in their susceptibility to oxidative modification (41, 42), and inspection of the carbonyl immunoblot revealed an ∼50-kDa band with distinctly increased carbonylation in Nle cells exposed to all 3 stresses (Fig. 5A). We identified this protein as elongation factor Tu (EF-Tu), a very abundant protein in E. coli and one which has 10 Met residues. Some or all of these are presumably important in protecting this important protein from oxidative damage.
Figure 5.
Protein carbonylation. A) Western blot. Lanes 1–2: oxidized and nonoxidized glutamine synthetase standards. Lanes 3–10: whole-cell SDS extracts. Cells were sampled after 1 h at 37°C in the absence or presence of 5 μM (control cells) or 4 μM (Nle cells) hypochlorite, 5 mM hydrogen peroxide, or 10 Gy irradiation. Lanes 11–12: Duplicates of lanes 3 and 7 but without 2,4-dintriophenylhydrazine exposure as a control for specificity of carbonyl labeling. B) Quantitation of the results. Bars show average and sd of 2 independent experiments.
MetO levels
MetO levels in proteins might serve as a measure of oxidative damage in cells, although there is little experimental evidence to validate its use for this purpose (43). We found that hypochlorite exposure caused a concentration-dependent linear increase of MetO level in both control and Nle cells, but the increase was much faster in Nle cells (Fig. 6A). A combined analysis of the survival results (Fig. 4A) with the MetO data (Fig. 6A) revealed that both cells lost viability when the MetO content exceeded 15% (Fig. 6B). Peroxide exposure caused a time-dependent increase of MetO level in Nle cells but not in control cells (Fig. 6C, D), again consistent with the survival results. Low-dose radiation did not alter the MetO levels in either control or Nle cells (Fig. 6E), perhaps because MetO is only one of the products expected from the radiolysis of Met residues (44, 45).
Figure 6.
MetO content, shown as the percentage of the sum of Met + MetO. A) MetO content in hypochlorite-exposed cells. B) Cell viability as a function of MetO content in hypochlorite-exposed cells (from A and Fig. 4A). C, D) MetO content in control cells (C) and cells exposed to hydrogen peroxide (D). E) MetO content in irradiated cells.
DISCUSSION
A global decrease in protein Met content provides an experimental approach to testing the hypothesis that Met residues in proteins function in antioxidant defense. While we had considered utilizing site-specific mutagensis to alter the Met content of specific proteins, we concluded that the data from such experiments could not be unambiguously interpreted. Mutation of Met to other hydrophobic residues could alter the folding and 3-dimensional structure of the protein, particularly if multiple Mets were altered. Moreover, there was no rational basis for selecting the one or several proteins that would be mutated. However, global substitution by Nle would affect all proteins, and as summarized in the introduction, there is good evidence that folding and function of the proteins would be unaffected. We chose survival as our endpoint measurement to avoid ambiguities with surrogate markers of oxidative stress.
Cells with decreased protein Met could not survive oxidative stress as well as control cells. Exposure to hypochlorite, peroxide, and ionizing radiation killed Nle-grown cells more readily than control cells. Had this not been the case, we would have argued that the hypothesis of Met residues as antioxidants should be rejected. The observed results are consistent with and support the hypothesis, although, of course, they cannot prove it. Along with the many references cited in the introduction, they also provide support for the scheme outlined in Fig. 1. As always, alternative explanations cannot be entirely ruled out, even if they are less likely to be correct. For example, a phenotype of increased sensitivity to oxidative stress could occur if substitution of Met by Nle adversely affected the function of any of the proteins involved in the catalytic cycle shown in Fig. 1. We also cannot rule out the possibility that oxidatively modified proteins are degraded, releasing MetO as the free amino acid that is then reduced by msr and reincorporated into protein.
Paraquat exposure gave results different from the other 3 stresses in that loss of viability occurred much later and did not differ between control and Nle cells. One can readily demonstrate in vitro that paraquat is a redox cycling agent that generates superoxide radical in the presence of oxygen and a reducing agent. Its in vivo toxicity has reasonably been attributed to generation of superoxide, although the large literature on the subject is complicated, and the exact mechanisms of toxicity are not certain (46). There is evidence that metal-dependent Fenton chemistry is important in paraquat toxicity (47). However, under our conditions, hydroxyl radical and peroxide are unlikely to be important mediators of paraquat toxicity because Nle cells were more susceptibile to irradiation and peroxide but not to paraquat. Some strains of E. coli are not killed by paraquat during the initial exposure but instead die on the plates that are used to determine viability (48), and this is one of the mechanisms that could explain the lack of difference in viability between the Nle and control cells.
The total protein carbonyl in stressed Nle cells was not significantly elevated, although an increase in carbonylation of a specific protein, EF-Tu, was easily observed. One possible reason we did not detect an increase in total carbonylated protein is that the stationary-phase cells under study already had a high level of carbonylated proteins. The studies of Nyström and colleagues established that the stationary phase itself is an oxidative stress (49, 50). Consistent with this point, we found that the protein carbonyl content of exponentially growing cells was about half of that of the cells in stationary phase. When exponentially growing E. coli were exposed to various oxidants, their protein carbonyl levels rose, but only 2-fold (41). The protein carbonyl content of tissues from a variety of animals increases with age, but again it approximately doubles in the oldest animals (51). Thus, a doubling of protein carbonyl may be the maximal increase that can be observed in cells, either because higher levels are cleared or because the cells die above that level.
The protection provided by the Met system may be important in a variety of stresses because their damaging effects result from oxidative damage. Both prokaryotes and eukaroytes that are resistant to one type of stress are often resistant to other stresses. For example, the bacterium Deinococcus radiodurans withstands a dose of ionizing radiation 1000 times greater than that which kills E. coli, and it is also very resistant to ultraviolet radiation, dessication, and exposure to hydrogen peroxide (52). Similarly, yeast rendered resistant to heat shock also acquired resistance to ultraviolet radiation and to ethanol exposure (53). Cross-resistance is not limited to microorganisms, and it can be induced. Animals, including mammals, exposed to a sublethal stress develop resistance to that stress and to others, a process sometimes termed hormesis. The transcription factors and proteins induced in such cross-resistance have been studied in detail by many investigators working with a variety of organisms and cells. Stresses that seem unrelated to oxidative stress may induce a secondary oxidative stress which directly damages cellular macromolecules. For example, hypertonic stress in the kidney or in cultured kidney cells causes production of reactive oxygen species which oxidize both DNA and protein (54). Oxidative stress is also implicated in diseases whose primary defect may seem unrelated to oxidative stress. For example, Werner syndrome is clinically notable for its phenotype of premature aging. Cells from patients with Werner syndrome were shown to carry an increased burden of oxidatively damaged proteins well before the protein mutated in Werner syndrome was recognized to be a DNA helicase and exonuclease (55,56,57). Ataxia-telangiectasia is an inherited neurodegenerative disease caused by mutation of the ATM kinase, a protein kinase that becomes activated following the formation of double-stranded DNA breaks. Tissues from mice lacking the ATM kinase are also under oxidative stress (58), and as in Werner syndrome, the precise pathways by which a defective response to DNA damage leads to oxidative stress are unknown. Nevertheless, interventions that modulate the oxidative damage can improve outcome (58, 59). Stimulation of the Met-based antioxidant system or pharmacological mimics could do so as well.
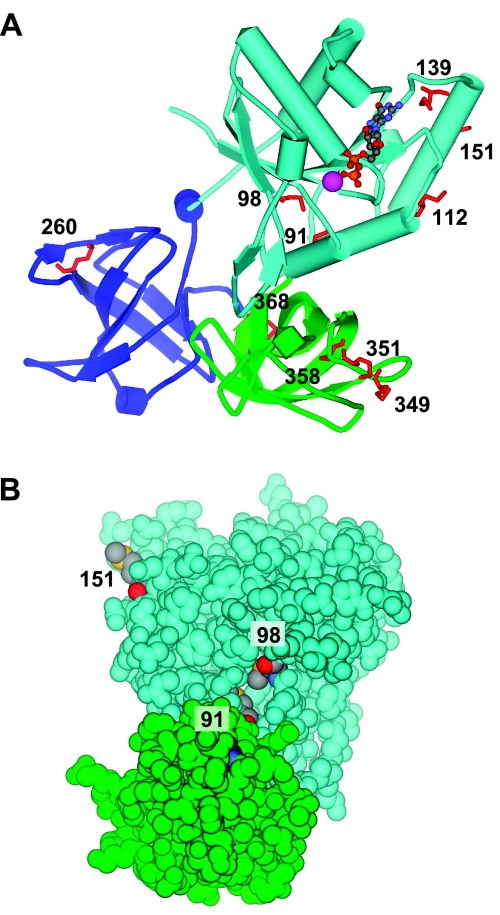
EF-Tu is known to be susceptible in vivo to oxidative modification with carbonylation (59). Our finding of increased carbonylation of EF-Tu in the Nle cells suggests that the protein contains Met residues that serve to protect against oxidative modification of other residues. Of the 10 Met residues in EF-Tu, 6 are surface exposed (Fig. 7A), and another 2 are solvent accessible (Fig. 7B). Each of the 6 surface-exposed residues are situated near functionally important domains (60). Three are near the Mg-GTP binding site in domain-1; one is in domain-2 near the binding site for the aminoacylated region of tRNA; and two are in domain-3 near the binding site for the T stem of the tRNA. EF-Tu is the most plentiful protein in bacteria, constituting a remarkable 5–10% of their total protein. Thus, the oxidant-accessible Met residues of EF-Tu may provide not only an antioxidant defense for itself but also for the entire cell.
Figure 7.
Topography of Met in elongation factor Tu. A) Schematic diagram of EF-Tu. Stick model shows the 10 Met residues in red with their sequence numbers. Six (112, 139, 151, 260, 349, and 351) are surface exposed. Domain 1 is light blue, domain 2 dark blue, and domain 3 green. Nucleotide GDP is rendered as a ball-and-stick model; Mg2+ is shown as a purple sphere. B) CPK diagram of EF-Tu. For clarity, only domains 1 and 3 are shown; colors as in A. Within Met residues, sulfur atoms are yellow, carbon atoms are gray, and oxygen atoms are red. An additional 2 Met residues (91 and 98) are solvent accessible. Figures were generated with DSViewerPro (Accelrys, San Diego, CA, USA) from the coordinates of Song et al. (61), taken from the Protein Data Bank entry 1EFC.
We conclude that Met residues in proteins can provide antioxidant defense, directly for the molecule in which they reside and for the entire cell through the recycling pathway shown in Fig. 1.
Acknowledgments
The authors thank Elena K. Gaidamakova and Alexander Vasilenko for performing the irradiation of the E. coli. This research was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute, National Institutes of Health.
References
- Vogt W. Oxidation of methionine residues in proteins: Tools, targets, and reversal. Free Rad Biol Med. 1995;18:93–105. doi: 10.1016/0891-5849(94)00158-g. [DOI] [PubMed] [Google Scholar]
- Stadtman E R, Moskovitz J, Berlett B S, Levine R L. Cyclic oxidation and reduction of protein methionine residues is an important antioxidant mechanism. Mol Cell Biochem. 2002;234–235:3–9. [PubMed] [Google Scholar]
- Reddy V Y, Pizzo S V, Weiss S J. Functional inactivation and structural disruption of human alpha 2-macroglobulin by neutrophils and eosinophils. J Biol Chem. 1989;264:13801–13809. [PubMed] [Google Scholar]
- Reddy V Y, Desrochers P E, Pizzo S V, Gonias S L, Sahakian J A, Levine R L, Weiss S J. Oxidative dissociation of human α2macroglobulin tetramers into dysfunctional dimers. J Biol Chem. 1994;269:4683–4691. [PubMed] [Google Scholar]
- Levine R L, Mosoni L, Berlett B S, Stadtman E R. Methionine residues as endogenous antioxidants in proteins. Proc Natl Acad Sci U S A. 1996;93:15036–15040. doi: 10.1073/pnas.93.26.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber J M, Levine R L. Sequence of a peptide susceptible to mixed function oxidation: probable cation binding site in glutamine synthetase. J Biol Chem. 1986;261:4574–4578. [PubMed] [Google Scholar]
- Weissbach H, Resnick L, Brot N. Methionine sulfoxide reductases: history and cellular role in protecting against oxidative damage. Biochim Biophys Acta. 2005;1703:203–212. doi: 10.1016/j.bbapap.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Levine R L, Moskovitz J, Stadtman E R. Oxidation of methionine in proteins: roles in antioxidant defense and cellular regulation. IUBMB Life. 2000;50:301–307. doi: 10.1080/713803735. [DOI] [PubMed] [Google Scholar]
- Moskovitz J, Bar-Noy S, Williams W M, Requena J, Berlett B S, Stadtman E R. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci U S A. 2001;98:12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz J, Berlett B S, Poston J M, Stadtman E R. The yeast peptide-methionine sulfoxide reductase functions as an antioxidant in vivo. Proc Natl Acad Sci U S A. 1997;94:9585–9589. doi: 10.1073/pnas.94.18.9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz J, Rahman M A, Strassman J, Yancey S O, Kushner S R, Brot N, Weissbach H. Escherichia coli peptide methionine sulfoxide reductase gene: regulation of expression and role in protecting against oxidative damage. J Bacteriol. 1995;177:502–507. doi: 10.1128/jb.177.3.502-507.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas T, Daniel D S, Parida B K, Jagannath C, Dhandayuthapani S. Methionine sulfoxide reductase A (MsrA) deficiency affects the survival of Mycobacterium smegmatis within macrophages. J Bacteriol. 2004;186:3590–3598. doi: 10.1128/JB.186.11.3590-3598.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John G, Brot N, Ruan J, Erdjument-Bromage H, Tempst P, Weissbach H, Nathan C. Peptide methionine sulfoxide reductase from Escherichia coli and Mycobacterium tuberculosis protects bacteria against oxidative damage from reactive nitrogen intermediates. Proc Natl Acad Sci U S A. 2001;98:9901–9906. doi: 10.1073/pnas.161295398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan H, Tang X D, Chen M L, Joiner M L, Sun G, Brot N, Weissbach H, Heinemann S H, Iverson L, Wu C F, Hoshi T, Chen M L, Joiner M A, Heinemann S H. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc Natl Acad Sci U S A. 2002;99:2748–2753. doi: 10.1073/pnas.032671199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz J, Flescher E, Berlett B S, Azare J, Poston J M, Stadtman E R. Overexpression of peptide-methionine sulfoxide reductase in Saccharomyces cerevisiae and human T cells provides them with high resistance to oxidative stress. Proc Natl Acad Sci U S A. 1998;95:14071–14075. doi: 10.1073/pnas.95.24.14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero H M, Berlett B S, Jensen P J, Pell E J, Tien M. Investigations into the role of the plastidial peptide methionine sulfoxide reductase in response to oxidative stress in Arabidopsis. Plant Physiol. 2004;136:3784–3794. doi: 10.1104/pp.104.046656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yermolaieva O, Xu R, Schinstock C, Brot N, Weissbach H, Heinemann S H, Hoshi T. Methionine sulfoxide reductase A protects neuronal cells against brief hypoxia/reoxygenation. Proc Natl Acad Sci U S A. 2004;101:1159–1164. doi: 10.1073/pnas.0308215100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti M A, Lee W, Cowell T L, Wells T M, Weissbach H, Kantorow M. Silencing of the methionine sulfoxide reductase A gene results in loss of mitochondrial membrane potential and increased ROS production in human lens cells. Exp Eye Res. 2006;83:1281–1286. doi: 10.1016/j.exer.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabreiro F, Picot C R, Perichon M, Mary J, Friguet B, Petropoulos I. Identification of proteins undergoing expression level modifications in WI-38 SV40 fibroblasts overexpressing methionine sulfoxide reductase A. Biochimie (Paris) 2007;89:1388–1395. doi: 10.1016/j.biochi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Cabreiro F, Picot C R, Perichon M, Castel J, Friguet B, Petropoulos I. Overexpression of mitochondrial methionine sulfoxide reductase B2 protects leukemia cells from oxidative stress-induced cell death and protein damage. J Biol Chem. 2008;283:16673–16681. doi: 10.1074/jbc.M708580200. [DOI] [PubMed] [Google Scholar]
- Levine R L, Stadtman E R. Oxidative modification of proteins during aging. Exp Gerontol. 2001;36:1495–1502. doi: 10.1016/s0531-5565(01)00135-8. [DOI] [PubMed] [Google Scholar]
- Cowie D B, Cohen G N, Bolton E T, De Robichon-Szulmajster H. Amino acid analog incorporation into bacterial proteins. Biochim Biophys Acta. 1959;34:39–46. doi: 10.1016/0006-3002(59)90230-6. [DOI] [PubMed] [Google Scholar]
- Barker D G, Bruton C J. The fate of norleucine as a replacement for methionine in protein synthesis. J Mol Biol. 1979;133:217–231. doi: 10.1016/0022-2836(79)90531-x. [DOI] [PubMed] [Google Scholar]
- Hendrickson W A, Horton J R, LeMaster D M. Selenomethionyl proteins produced for analysis by multiwavelength anomalous diffraction (MAD): a vehicle for direct determination of three-dimensional structure. EMBO J. 1990;9:1665–1672. doi: 10.1002/j.1460-2075.1990.tb08287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J H. Plainview, NY, USA: Cold Spring Harbor Laboratory Press; A Short Course in Bacterial GeneticsA Laboratory Manual and Handbook for Escherichia coli and Related Bacteria. 1992 [Google Scholar]
- Taggart C, Cervantes-Laurean D, Kim G, McElvaney N G, Wehr N, Moss J, Levine R L. Oxidation of either methionine 351 or methionine 358 in alpha 1-antitrypsin causes loss of anti-neutrophil elastase activity. J Biol Chem. 2000;275:27258–27265. doi: 10.1074/jbc.M004850200. [DOI] [PubMed] [Google Scholar]
- Luo S, Wehr N B, Levine R L. Quantitation of protein on gels and blots by infrared fluorescence of Coomassie blue and Fast Green. Anal Biochem. 2006;350:233–238. doi: 10.1016/j.ab.2005.10.048. [DOI] [PubMed] [Google Scholar]
- Wang Y, Oberley L W, Murhammer D W. Evidence of oxidative stress following the viral infection of two lepidopteran insect cell lines. Free Radic Biol Med. 2001;31:1448–1455. doi: 10.1016/s0891-5849(01)00728-6. [DOI] [PubMed] [Google Scholar]
- Cohen S A, Michaud D P. Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal Biochem. 1993;211:279–287. doi: 10.1006/abio.1993.1270. [DOI] [PubMed] [Google Scholar]
- Jones B N, Gilligan J P. o-Phthaldialdehyde precolumn derivatization and reversed-phase high-performance liquid chromatography of oplypeptide hydrolysates and physiological fluids. J Chromatogr. 1983;266:471–482. doi: 10.1016/s0021-9673(01)90918-5. [DOI] [PubMed] [Google Scholar]
- Stadtman E R, Smyrniotis P Z, Davis J N, Wittenberger M E. Enzymic procedures for determining the average state of adenylylation of Escherichia coli glutamine synthetase. Anal Biochem. 1979;95:275–285. doi: 10.1016/0003-2697(79)90217-3. [DOI] [PubMed] [Google Scholar]
- Levine R L, Garland D, Oliver C N, Amici A, Climent I, Lenz A G, Ahn B W, Shaltiel S, Stadtman E R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- Levine R L, Wehr N, Williams J A, Stadtman E R, Shacter E. Determination of carbonyl groups in oxidized proteins. Methods Mol Biol. 2000;99:15–24. doi: 10.1385/1-59259-054-3:15. [DOI] [PubMed] [Google Scholar]
- Liu X, Shu S, Hong M S, Levine R L, Korn E D. Phosphorylation of actin Tyr-53 inhibits filament nucleation and elongation and destabilizes filaments. Proc Natl Acad Sci U S A. 2006;103:13694–13699. doi: 10.1073/pnas.0606321103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anfinsen C B, Corley L G. An active variant of staphylococcal nuclease containing norleucine in place of methionine. J Biol Chem. 1969;244:5149–5152. [PubMed] [Google Scholar]
- Naider F, Bohak Z, Yariv J. Reversible alkylation of a methionyl residue near the active site of β-galactosidase. Biochemistry. 1972;11:3202–3208. doi: 10.1021/bi00767a010. [DOI] [PubMed] [Google Scholar]
- Gilles A M, Marliere P, Rose T, Sarfati R, Longin R, Meier A, Fermandjian S, Monnot M, Cohen G N, Barzu O. Conservative replacement of methionine by norleucine in Escherichia coli adenylate kinase. J Biol Chem. 1988;263:8204–8209. [PubMed] [Google Scholar]
- Randhawa Z I, Witkowska H E, Cone J, Wilkins J A, Hughes P, Yamanishi K, Yasuda S, Masui Y, Arthur P, Kletke C, Bitsch F, Shackleton C H L. Incorporation of norleucine at methionine positions in recombinant human macrophage-colony-stimulating factor (M-Csf, 4-153) expressed in Escherichia coli: structural analysis. Biochemistry. 1994;33:4352–4362. doi: 10.1021/bi00180a032. [DOI] [PubMed] [Google Scholar]
- Hampton M B, Kettle A J, Winterbourn C C. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- Daly M J, Gaidamakova E K, Matrosova V Y, Vasilenko A, Zhai M, Leapman R D, Lai B, Ravel B, Li S M, Kemner K M, Fredrickson J K. Protein oxidation implicated as the primary determinant of bacterial radioresistance. PLoS Biol. 2007;5:e92. doi: 10.1371/journal.pbio.0050092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamarit J, Cabiscol E, Ros J. Identification of the major oxidatively damaged proteins in Escherichia coli cells exposed to oxidative stress. J Biol Chem. 1998;273:3027–3032. doi: 10.1074/jbc.273.5.3027. [DOI] [PubMed] [Google Scholar]
- Reverter-Branch, Cabiscol E, Tamarit J, Ros J. Oxidative damage to specific proteins in replicative and chronological-aged Saccharomyces cerevisiae: common targets and prevention by calorie restriction. J Biol Chem. 2004;279:31983–31989. doi: 10.1074/jbc.M404849200. [DOI] [PubMed] [Google Scholar]
- Stadtman E R, Van R H, Richardson A, Wehr N B, Levine R L. Methionine oxidation and aging. Biochim Biophys Acta. 2005;1703:135–140. doi: 10.1016/j.bbapap.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Kopoldova J, Liebster J, Gross E. Radiation chemical reactions in aqueous solutions of methionine and its peptides. Radiat Res. 1967;30:261–274. [PubMed] [Google Scholar]
- Schoneich C. Methionine oxidation by reactive oxygen species: reaction mechanisms and relevance to Alzheimer’s disease. Biochim Biophys Acta. 2005;1703:111–119. doi: 10.1016/j.bbapap.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Minakami H, Fridovich I. Effects of paraquat on cultures of Escherichia coli: turbidity versus enumeration. Free Radic Biol Med. 1990;8:387–391. doi: 10.1016/0891-5849(90)90105-r. [DOI] [PubMed] [Google Scholar]
- Kohen R, Chevion M. Transition metals potentiate paraquat toxicity. Free Radic Res Commun. 1985;1:79–88. doi: 10.3109/10715768509056540. [DOI] [PubMed] [Google Scholar]
- Kitzler J W, Minakami H, Fridovich I. Effects of paraquat on Escherichia coli: differences between B and K-12 strains. J Bacteriol. 1990;172:686–690. doi: 10.1128/jb.172.2.686-690.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukan S, Nystrom T. Oxidative stress defense and deterioration of growth-arrested Escherichia coli cells. J Biol Chem. 1999;274:26027–26032. doi: 10.1074/jbc.274.37.26027. [DOI] [PubMed] [Google Scholar]
- Nystrom T. Oxidation of bacterial proteome in response to starvation. Methods Biochem Anal. 2006;49:89–95. doi: 10.1002/0471973165.ch7. [DOI] [PubMed] [Google Scholar]
- Levine R L. Carbonyl modified proteins in cellular regulation, aging, and disease. Free Rad Biol Med. 2002;32:790–796. doi: 10.1016/s0891-5849(02)00765-7. [DOI] [PubMed] [Google Scholar]
- Makarova K S, Aravind L, Wolf Y I, Tatusov R L, Minton K W, Koonin E V, Daly M J. Genome of the extremely radiation-resistant bacterium Deinococcus radiodurans viewed from the perspective of comparative genomics. Microbiol Mol Biol Rev. 2001;65:44–79. doi: 10.1128/MMBR.65.1.44-79.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida H. Multistress resistance of Saccharomyces cerevisiae is generated by insertion of retrotransposon Ty into the 5′ coding region of the adenylate cyclase gene. Mol Cell Biol. 1988;8:5555–5560. doi: 10.1128/mcb.8.12.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Dmitrieva N I, Park J H, Levine R L, Burg M B. High urea and NaCl carbonylate proteins in renal cells in culture and in vivo, and high urea causes 8-oxoguanine lesions in their DNA. Proc Natl Acad Sci U S A. 2004;101:9491–9496. doi: 10.1073/pnas.0402961101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver C N, Ahn B W, Moerman E J, Goldstein S, Stadtman E R. Age-related changes in oxidized proteins. J Biol Chem. 1987;262:5488–5491. [PubMed] [Google Scholar]
- Gray M D, Shen J C, Kamath-Loeb A S, Blank A, Sopher B L, Martin G M, Oshima J, Loeb L A. The Werner syndrome protein is a DNA helicase. Nat Genet. 1997;17:100–103. doi: 10.1038/ng0997-100. [DOI] [PubMed] [Google Scholar]
- Shen J C, Gray M D, Oshima J, Kamath-Loeb A S, Fry M, Loeb L A. Werner syndrome protein. I. DNA helicase and dna exonuclease reside on the same polypeptide. J Biol Chem. 1998;273:34139–34144. doi: 10.1074/jbc.273.51.34139. [DOI] [PubMed] [Google Scholar]
- Barlow C, Dennery P A, Shigenaga M K, Smith M A, Morrow J D, Roberts L J, Wynshaw-Boris A, Levine R L. Loss of the ataxia-telangiectasia gene product causes oxidative damage in target organs. Proc Natl Acad Sci U S A. 1999;96:9915–9919. doi: 10.1073/pnas.96.17.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukan S, Nystrom T. Bacterial senescence: stasis results in increased and differential oxidation of cytoplasmic proteins leading to developmental induction of the heat shock regulon. Genes Dev. 1998;12:3431–3441. doi: 10.1101/gad.12.21.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen P, Kjeldgaard M, Thirup S, Polekhina G, Reshetnikova L, Clark B F, Nyborg J. Crystal structure of the ternary complex of Phe-tRNAPhe, EF-Tu, and a GTP analog. Science. 1995;270:1464–1472. doi: 10.1126/science.270.5241.1464. [DOI] [PubMed] [Google Scholar]
- Song H, Parsons M R, Rowsell S, Leonard G, Phillips S E. Crystal structure of intact elongation factor EF-Tu from Escherichia coli in GDP conformation at 2.05 A resolution. J Mol Biol. 1999;285:1245–1256. doi: 10.1006/jmbi.1998.2387. [DOI] [PubMed] [Google Scholar]