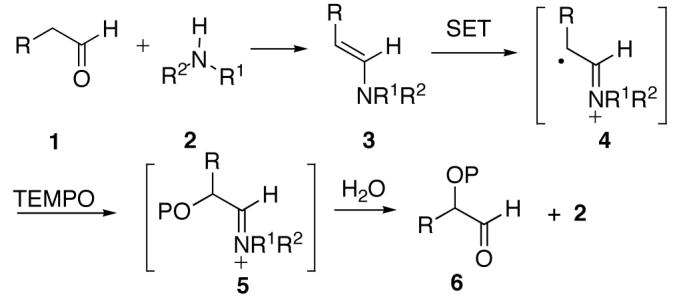
Chiral Lewis acids play a critical role in providing substrate activation and the necessary chirality for enantioselective transformations.1 Chiral Lewis acid mediated enantioselective radical reactions have been investigated extensively in many laboratories.2A complementary technique to chiral Lewis acid mediated processes is organocatalysis.3 Spectacular advances have been made in recent years using organocatalysts. However, only a few examples of radical reactions utilize organocatalysts.4 In this communication we report an enantioselective radical-mediated C-O bond forming reaction using organocatalysts.5 This new transformation further broadens the utility of organocatalysts in enantioselective transformations.
In general, radical species are produced using tin-mediated chain reactions. Alternatively, enolates6and enamines7 can be oxidized using a single electron transfer (SET) reagent, and the resultant radical species captured in useful bond forming processes (Scheme 1). We surmised that enamines prepared from aldehydes and chiral amines could be oxidized using a SET reagent and the intermediate radical trapped stereoselectively by TEMPO providing access to α-oxyaminated aldehydes, an important class of chiral building blocks.8 Herein, we describe a catalytic and highly enantioselective α-oxyamination process using chiral imidazolidinone catalysts.
Scheme 1.
We began our work to identify reaction conditions for the α-functionalization process and these results are tabulated in Table 1. Treatment of phenylpropanal 7 with a stoichiometric amount of ferrocenium terafluoroborate in the presence of TEMPO gave the α-oxygentated product in good yield (entry 1). This suggested that the aldehyde could undergo reaction through its enol form efficiently but at a slow rate. The product α-aminoxy aldehyde was reduced to the primary alcohol 9 to aid in analysis. An identical reaction as in entry 1 but using 1 equivalent of pyrrolidine gave 9 in high yield in 1 h (entry 2). This experiment indicated that the SET reagent could readily oxidize the pyrrolidine-derived enamine. Reaction using chiral imidazolidinone 10a gave the product in good yield and enantioselectivity (entry 3).9 Reaction with 20 mol% of 10a was also efficient indicating catalyst turnover (entry 4). Changing the catalyst to 10b, a tetrafluoroborate salt, led to an improvement in selectivity from 64 to 80% ee (entry 5). Proline was an efficient catalyst for the reaction but the enantioselectivity was very low (entry 6).
Table 1.
Identification of Reaction Conditions a
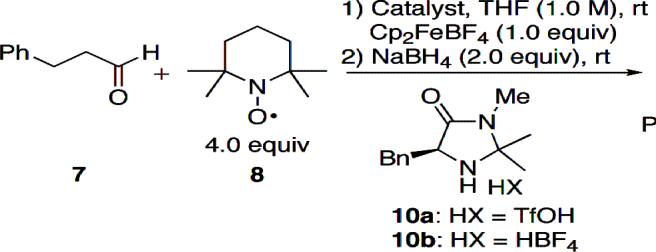 | ||||
|---|---|---|---|---|
| Ent | Catalyst, mol% | Time, h | Yld, %b | ee, %c |
| 1 | None | 24 | 79 | - |
| 2 | Pyrrolidine (100) | 1 | 61 | - |
| 3 | 10a (100) | 1 | 63 | 76 |
| 4 | 10a (20) | 1 | 78 | 64 |
| 5 | 10b (20) | 1 | 87 | 80 |
| 6 | L-Proline (20) | 1 | 71 | -3 |
For reaction conditions, see Supporting Information.
Isolated yield.
Determined by chiral HPLC.
Optimization with respect to the catalyst, SET reagent, and solvent was investigated next and results from these experiments are tabulated in Table 2. Reducing the amount of SET reagent from 100 mol% to 50 mol% in reaction using 10b as a catalyst gave lower chemical efficiency (compare entry 1 with 2). We then explored a cheaper SET reagent, FeCl3. The oxygenation reaction catalyzed by 10b did not proceed in the presence of a stoichiometric amount of FeCl3 with THF as the solvent (entry 3). However, DMF as a solvent proved to be effective and gave the product in high yield and ee (entry 4). A catalytic amount of the SET reagent could be used when a cooxidant (NaNO2/O2)10was employed without compromising yield or selectivity (entries 5 and 6). We then explored alternative chiral amines with the hope of improving selectivity (entries 7-10).11 However, these reactions were not very rewarding.
Table 2.
Effect of SET Reagent, Ligand, and Solventa
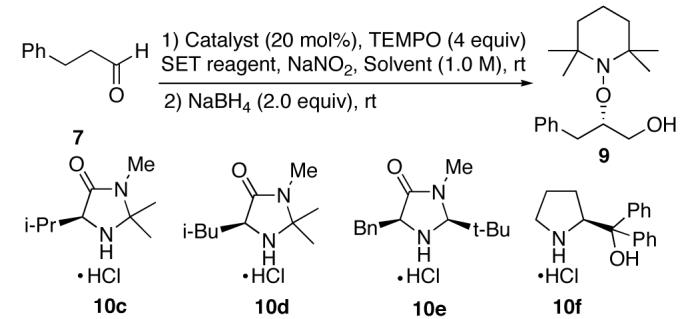 | ||||||
|---|---|---|---|---|---|---|
| Ent | SET Reagent (mol%) |
Ligand | NaNO2 (equiv) |
Solv | Yld (%)b |
ee (%)c |
| 1 | Cp2FeBF4 (100) | 10b | 0 | THF | 87 | 80 |
| 2 | Cp2FeBF4 (50) | 10b | 0 | THF | 40 | 74 |
| 3 | FeCl3 (100) | 10b | 0 | THF | 4 | ndd |
| 4 | FeCl3 (100) | 10b | 0 | DMF | 74 | 72 |
| 5e | FeCl3 (30) | 10b | 0.3 | DMF | 82 | 75 |
| 6e | FeCl3 (10) | 10b | 0.3 | DMF | 83 | 72 |
| 7e | FeCl3 (10) | 10c | 0.3 | DMF | 75 | 5 |
| 8e | FeCl3 (10) | 10d | 0.3 | DMF | 64 | 46 |
| 9e | FeCl3 (10) | 10e | 0.3 | DMF | 26 | 0 |
| 10e | FeCl3 (10) | 10f | 0.3 | DMF | 33 | 17 |
For reaction conditions, see Supporting Information.
Isolated yield.
Determined by chiral HPLC.
Not determined.
2 equiv of TEMPO was used.
Having identified a reasonable set of conditions, we then investigated reactions with a variety of aldehydes and these results are presented in Table 3. The reactions were carried out at two different temperatures (rt and -10 °C) using 10b as the catalyst and FeCl3 as the SET reagent. Reaction with phenylacetaldehyde provided modest selectivity due to relatively rapid background reaction and partial racemization of the product (entry 1). In contrast, compound 7 gave good yield and selectivity at rt (entry 2). The selectivity could be improved by conducting the reaction at -10 °C (compare entry 2 with 3). However the reaction was less efficient and took longer to complete. Reaction with phenylbutanal 12 was efficient and selectivity could be improved by cooling the reaction to -10 °C (entries 4 and 5). A variety of aryl substituted aldehydes underwent α-oxygenation with high selectivity (entries 6-11). Heterocycle containing aldehydes were also competent substrates in the oxygenation reaction (entries 12-15). Reaction with 4-pentene-1-al was also successful with selectivity reaching 90% for reaction at -10 °C (entries 16 and 17). Isovaleraldehyde, a simple aliphatic aldehyde gave the oxidation product in good yield but with no selectivity (entry 18). Overall, the data in Table 3. demonstrates that there is broad substrate scope in these α-oxygenation reactions.12
Table 3.
Breadth and Scope of the Aldehyde Substratesa
 | |||||
|---|---|---|---|---|---|
| Ent | R | Temp, °C |
Time h |
Yield (%)b |
ee (%)c |
| 1 | C6H5 11 | rt | 2 | 74 | 32 |
| 2 | C6H5CH2 7 | rt | 2 | 80 | 71 |
| 3d | -10 | 24 | 68 | 82 | |
| 4 | C6H5CH2CH2 12 | rt | 2 | 78 | 60 |
| 5 | -10 | 24 | 64 | 84 | |
| 6 | 4-MeOC6H4CH2CH2 13 | rt | 2 | 77 | 81 |
| 7 | -10 | 24 | 64 | 86 | |
| 8 | 3,4-(MeO)2C6H3CH2 14 | rt | 2 | 76 | 72 |
| 9d | -10 | 24 | 68 | 84 | |
| 10 | 4-NO2C6H4CH2CH2 15 | rt | 2 | 74 | 75 |
| 11 | -10 | 24 | 75 | 82 | |
| 12 |  |
rt | 2 | 65 | 84 |
| 13 | -10 | 24 | 66 | 90 | |
| 14 | rt | 2 | 49 | 56 | |
| 15 | -10 | 24 | 50 | 85 | |
| 16 | Allyl 18 | rt | 2 | 49 | 80 |
| 17 | -10 | 24 | 58 | 90 | |
| 18 | (CH3)2CH 19 | rt | 24 | 74 | 0 |
For reaction conditions, see Supporting Information.
Isolated yield.
Determined by chiral HPLC.
4.0 equiv of TEMPO was used.
The N-O bond of product 9 was cleaved using Zn/AcOH to produce 3-phenyl-1,2-propanediol. Comparison of its sign of optical rotation with that reported in the literature established the absolute stereochemistry as S.5a A model consistent with the observed product stereochemistry is shown in Figure 1. This is also consistent with models proposed in the literature for reactions of aldehydes using imidazolidinone catalysts.2, 13
Figure 1.
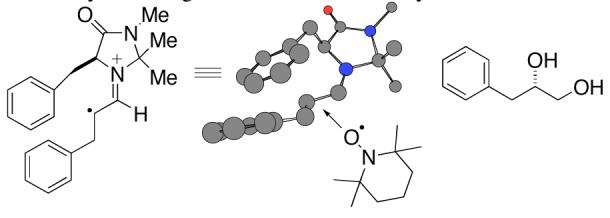
Stereochemical model
In conclusion we have developed an efficient radical α-oxygenation reaction using organocatalysts. The reactions proceed with good to excellent enantioselectivity. The methodology reported in this work adds to the repertoire of reactions that can be conducted using organocatalysts. Work is underway to utilize organocatalysts in radical-mediated C-C bond forming processes.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the National Institutes of Health (NIGMS-54656).
Footnotes
Supporting Information Available: Characterization data for compounds 9, 15, 17, 20-28 and experimental procedures. See any current masthead page for ordering information and Web access instructions.
REFERENCES
- (1).Yamamoto H, editor. Lewis Acids in Organic Synthesis. 1 and 2. Wiley-VCH; New York: 2000. [Google Scholar]
- (2)(a).Sibi MP, Manyem S, Zimmerman J. Chem. Rev. 2003;103:3263. doi: 10.1021/cr020044l. [DOI] [PubMed] [Google Scholar]; (b) Sibi MP, Porter NA. Acc. Chem. Res. 1999;32:163. [Google Scholar]; (c) Sibi MP, Manyem S. Tetrahedron. 2000;56:8033. [Google Scholar]
- (3)(a).Berkessel A, Groger H. Asymmetric Organocatalysis. Wiley-VCH; Weinheim: 2005. [Google Scholar]; (b) Dalko PI, Moisan L. Angew. Chem. Int. Ed. 2004;43:5138. doi: 10.1002/anie.200400650. [DOI] [PubMed] [Google Scholar]; (c) Lelais G, MacMillan DWC. Aldrichimica Acta. 2006;39:79. [Google Scholar]; (d) Taylor MS, Jacobsen EN. Angew. Chem. Int. Ed. 2006;45:1520. doi: 10.1002/anie.200503132. [DOI] [PubMed] [Google Scholar]; (e) List B, Yang JW. Science. 2006;313:1584. doi: 10.1126/science.1131945. [DOI] [PubMed] [Google Scholar]; (f) List B. Chem. Commun. 2006:819. doi: 10.1039/b514296m. [DOI] [PubMed] [Google Scholar]
- (4)(a).Dressel M, Aechtner T, Bach T. Synthesis. 2006:2206. [Google Scholar]; (b) Bauer A, Westkaemper F, Grimme S, Bach T. Nature. 2005;436:1139. doi: 10.1038/nature03955. [DOI] [PubMed] [Google Scholar]; (c) Aechtner T, Dressel M, Bach T. Angew. Chem. Int. Ed. 2004;43:5849. doi: 10.1002/anie.200461222. [DOI] [PubMed] [Google Scholar]; (d) Cho DH, Jang DO. Chem. Commun. 2006:5045. doi: 10.1039/b613045c. [DOI] [PubMed] [Google Scholar]
- (5)(a).(b) Zhong G. Angew. Chem. Int. Ed. 2003;42:4247. doi: 10.1002/anie.200352097. [DOI] [PubMed] [Google Scholar]; (c) Hayashi Y, Yamaguchi J, Hibino K, Shoji M. Tetrahedron Lett. 2003;44:8293. [Google Scholar]; (d) Córdova A, Sundén H, Engqvist M, Ibrahem I, Casas J. J. Am. Chem. Soc. 2004;126:8914. doi: 10.1021/ja047930t. [DOI] [PubMed] [Google Scholar]; (e) Kim S-G, Park T-H. Tetrahedron Lett. 2006;47:9067. [Google Scholar]; (g) Hayashi M, Yamaguchi J, Sumaiya T, Shoji M. Angew. Chem., Int. Ed. 2004;43:1112. doi: 10.1002/anie.200353085. [DOI] [PubMed] [Google Scholar]; (h) Bøgevig A, Sunden H, Córdova A. Angew Chem. Int. Ed. 2004;43:1109. doi: 10.1002/anie.200353018. [DOI] [PubMed] [Google Scholar]; (i) Sundén H, Engqvist M, Casas J, Ibrahem I, Córdova A. Angew Chem. Int. Ed. 2004;43:6532. doi: 10.1002/anie.200460295. [DOI] [PubMed] [Google Scholar]; For the use of organocatalysts in α-functionalization of carbonyl compounds; Aldehydes: Brown SP, Brochu MP, Sinz CJ, MacMillan DWC. J. Am. Chem. Soc. 2003;125:10808. doi: 10.1021/ja037096s.; Guo H-M, Cheng L, Cun L-F, Gong L-Z, Mi A-Q, Jiang Y-Z. Chem. Commun. 2006:429. doi: 10.1039/b514194j. [DOI] [PubMed] [Google Scholar]
- (6)(a).Jahn U, Müller M, Aussieker S. J. Am. Chem. Soc. 2000;122:5212. [Google Scholar]; (b) Jahn U, Rudakov D. Org. Lett. 2006;8:4481. doi: 10.1021/ol061666m. [DOI] [PubMed] [Google Scholar]; (c) Jahn U, Hartmann P, Kaasalainen E. Org. Lett. 2004;6:257. doi: 10.1021/ol036233n. [DOI] [PubMed] [Google Scholar]; (d) Jahn U. J. Org. Chem. 1998;63:7130. doi: 10.1021/jo981180m. [DOI] [PubMed] [Google Scholar]
- (7)(a).Cossy J, Bouzide A. J. Chem. Soc., Chem. Commun. 1993:1218. [Google Scholar]; (b) Narasaka K, Okauchi T, Tanaka K, Murakami M. Chem. Lett. 1992:2099. [Google Scholar]; (c) Russell GA, Yao C-F, Rajaratnam R, Kim BH. J. Am. Chem. Soc. 1991;113:373. [Google Scholar]; (d) Barletta G, Chung AC, Rios CB, Jordan F, Schlegel JM. J. Am. Chem. Soc. 1990;112:8144. [Google Scholar]
- (8)(a).(b) Guillena G, Ramon DJ. Tetrahedron: Asymmetry. 2006;17:1465. [Google Scholar]; (c) Marigo M, Jørgensen KA. Chem. Commun. 2006:2001. doi: 10.1039/b517090g. [DOI] [PubMed] [Google Scholar]; (d) Janey JM. Angew. Chem. Int. Ed. 2005;44:4292. doi: 10.1002/anie.200462314. [DOI] [PubMed] [Google Scholar]; For reviews on α-hetero functionalization of carbonyl compounds see Chen B-C, Zhou P, Davis FA, Ciganek E. Org. React. 2003;62:1.
- (9).For details on reaction conditions and workup see Supporting Information.
- (10)(a).Epstein IR, Kustin K, Warshaw LJ. J. Am. Chem. Soc. 1980;102:3751. [Google Scholar]; (b) Wang N, Liu R, Chen J, Liang X. Chem. Commun. 2005:5322. doi: 10.1039/b509167e. [DOI] [PubMed] [Google Scholar]
- (11).We have screened a large number of organocatalysts in an effort to improve selectivity. Of these, compound 10b was optimal.
- (12).Reaction with cyclohexanone gave the oxygenated product as a racemate in 86% yield.
- (13).Austin JF, MacMillan DWC. J. Am. Chem. Soc. 2002;124:1172. doi: 10.1021/ja017255c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


