Abstract
Grapevine (Vitis vinifera) proanthocyanidins contribute to plant defense mechanisms against biotic stress and also play a critical role in organoleptic properties of wine. In grapevine berry, these compounds are mainly accumulated in exocarps and seeds in the very early stages of development. A previous study has already identified VvMybPA1 as the first transcription factor involved in the regulation of the proanthocyanidin pathway during seed development in grapevine. A novel Myb factor, VvMybPA2, which is described in this study, is in contrast mainly expressed in the exocarp of young berries and in the leaves. This transcription factor shows very high protein sequence homology with other plant Myb factors, which regulate flavonoid biosynthesis. Ectopic expression of either VvMybPA1 or VvMybPA2 in grapevine hairy roots induced qualitative and quantitative changes of the proanthocyanidin profiles. High-throughput transcriptomic analyses of transformed grapevine organs identified a large set of putative targets of the VvMybPA1 and VvMybPA2 transcription factors. Both genes significantly activated enzymes of the flavonoid pathway, including anthocyanidin reductase and leucoanthocyanidin reductase 1, the specific terminal steps in the biosynthesis of epicatechin and catechin, respectively, but not leucoanthocyanidin reductase 2. The functional annotation of the genes whose expression was modified revealed putative new actors of the proanthocyanidin pathway, such as glucosyltransferases and transporters.
Flavonoids are a family of plant secondary metabolites that comprise several groups of compounds (e.g. anthocyanins, flavonols, and flavan 3-ols) and accumulate in a wide variety of plant tissues, where they are involved in diverse functions. In particular, flavonols play a role in protection against UV radiation (Winkel-Shirley, 2002), proanthocyanidins (PAs; i.e. flavan 3-ol oligomers and polymers) protect plants against microbial attacks and fungal growth (Dixon et al., 2005), and anthocyanins of flowers and fruits attract pollinators and help to disseminate seeds (Grotewold, 2006).
Grapevine (Vitis vinifera) flavonoids are also of particular importance for wine quality: anthocyanins of red-skinned cultivars are responsible for the red wine color, while PAs or so-called “condensed tannins” confer its astringency to the wine.
In grapevine fruit, flavan 3-ols are present in skin and seed tissues (Kennedy et al., 2001) and to a lesser extent in the pulp (Mané et al., 2007; Verriès et al., 2008). In pericarp, flavan 3-ols accumulate mainly before véraison (i.e. induction of ripening; Kennedy et al., 2001; Verriès et al., 2008), whereas anthocyanin accumulation in skin is restricted to ripening. In vegetative organs, PA content constantly increases during leaf development, but the rate of synthesis decreases in old leaves (Bogs et al., 2005). Grapevine flavan 3-ols are found as monomers, oligomers, and polymers. The major flavan 3-ol monomers in grapes are (+)-catechin and its isomer, (−)-epicatechin, and to a lesser extent (−)-epicatechin 3-O-gallate, the gallic ester of (−)-epicatechin (Su and Singleton, 1969). Grapevine seed PAs are partly galloylated procyanidins, based on (+)-catechin and (−)-epicatechin units, with mean degrees of polymerization (mDP) lower than 10 (Prieur et al., 1994). Grapevine skin PAs have a higher mDP around 30. They contain (−)-epicatechin and (−)-epigallocatechin (trihydroxylated on the B ring) as their major extension units (EU) and are thus mixed procyanidin/prodelphinidin polymers with a few percentage of galloylated units (Souquet et al., 1996). Catechin is the major isomer found as free monomer or terminal unit (TU) in grape skin PAs (Souquet et al., 1996).
In plants, several structural genes of PA biosynthesis have already been identified. Genes encoding anthocyanidin reductase (ANR) and leucoanthocyanidin reductase (LAR) were shown to catalyze, respectively, the synthesis of epicatechin and catechin as monomer (Devic et al., 1999; Tanner et al., 2003). In grapevine, two isogenes of LAR (LAR1 and LAR2) and one gene of ANR have been identified (Bogs et al., 2005). Production of a LAR1 Escherichia coli recombinant protein confirmed its role in catechin synthesis (Bogs et al., 2005). A LAR2 yeast recombinant protein accepts the three leucoanthocyanidins (mono-, di-, and trihydroxylated on the B ring) as substrates (Pfeiffer et al., 2006). The expression of both ANR and LAR genes is high in young berries and then constantly decreases during fruit development. LAR1 expression was almost restricted to seeds, whereas LAR2 was expressed in both skin and seeds (Bogs et al., 2005). ANR transcripts and, to a lesser extent, LAR2 transcripts were also detected in the pulp of green berries (Verriès et al., 2008). In vegetative organs, ANR was expressed at high levels throughout leaf development, LAR2 expression was only detected in mature leaves, and LAR1 was hardly expressed in leaves (Bogs et al., 2005).
Despite this recent progress in the knowledge of PA unit synthesis, the mechanisms involved in either polymerization or galloylation of units and the fine regulation of the spatiotemporal PA composition remain to be elucidated. At least six transcription factors, belonging to Myb, bHLH, WD40, WRKY, zinc finger, and MADS box proteins, were found to regulate PA biosynthesis in Arabidopsis (Arabidopsis thaliana). TT2, TT8, and TTG1, encoding Myb, bHLH, and WD40 proteins, respectively, were found to be associated in a transcriptional complex driving the expression of flavonoid structural genes (Lepiniec et al., 2006).
In grapevine, several Myb transcription factors controlling different branches of the flavonoid pathway have been identified: Myb5a for the general flavonoid pathway (Deluc et al., 2006), MybA1 (Kobayashi et al., 2002, 2004) and MybA2 (Bogs et al., 2007; Walker et al., 2007) for the anthocyanin pathway, and MybPA1 (Bogs et al., 2007) for the PA pathway. VvMybPA1 was shown to be able to promote both VvANR and VvLAR1 and to complement Arabidopsis tt2 mutants (Bogs et al., 2007). However, in the absence of a definitive argument from homologous reverse genetics, these data are still speculative.
The important number of ESTs for grapevine and the recent release of the grapevine genome sequence (Jaillon et al., 2007; Velasco et al., 2007) pave the way for a more exhaustive screening of the additional factors involved in the regulation of PA accumulation. We present here the identification and functional validation of VvMybPA2 as a new transcription factor for the regulation of PA biosynthesis. VvMybPA2 presents very high protein sequence homology with other plant Myb factors regulating the flavonoid pathway, including AtTT2. Its expression pattern fits well with PA accumulation during the development of grapevine fruit tissues. Functional properties of VvMybPA2 were studied and compared with those of VvMybPA1 through their ectopic expression in a homologous system. The PA content of grapevine organs ectopically overexpressing either VvMybPA1 or VvMybPA2 was dramatically increased and showed noticeable composition changes. High-throughput comparative transcriptomic analysis revealed new putative targets of these Mybs in the PA pathway, possibly involved in gene regulation, PA modifications, and transport.
RESULTS
VvMybPA2 Encodes a Myb Domain Protein
Three ESTs, named EC939601, EC993595, and EC950762, were obtained by BLAST search in the Vitis database using AtTT2 as a query sequence. The sequences were assembled in a contig, and the full-length cDNA was amplified from cDNA of 3-week-postflowering Shiraz berries, a developmental stage corresponding to the maximum rate of PA biosynthesis. The sequence recovered, called VvMybPA2 (accession no. EU919682), encodes a protein of 284 amino acid residues (Fig. 1) with a predicted molecular mass of 31.8 kD and a calculated pI of 5.45. In the grapevine PN 40024 genome sequence, this gene is located on chromosome 11 (Jaillon et al., 2007). In this region, gene GSVIVT00016470001 was predicted. However, the in silico predicted coding region of GSVIVT00016470001 is shorter than the VvMybPA2 actual one experimentally observed. This difference is due to a failure in the automatic detection procedure of intron/exon boundaries used for the annotation of the whole genome.
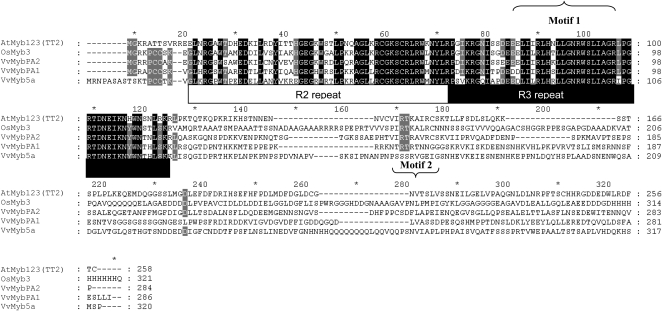
Figure 1.
Multialignment of VvMybPA2 with related MYB proteins by ClustalW: Arabidopsis AtMyb123 (TT2; Q9FJA2), rice (Oryza sativa) OsMYB3 (BAA23339), and grapevine VvMYB5b (AAX51291) and VvMybPA1 (CAJ90831). Identical amino acids are indicated in black, and similar amino acids are indicated in gray. The R2 and R3 repeats of the MYB domain, the motif participating in the interaction with bHLH proteins (motif 1), and the C-terminal conserved motif (motif 2) are indicated.
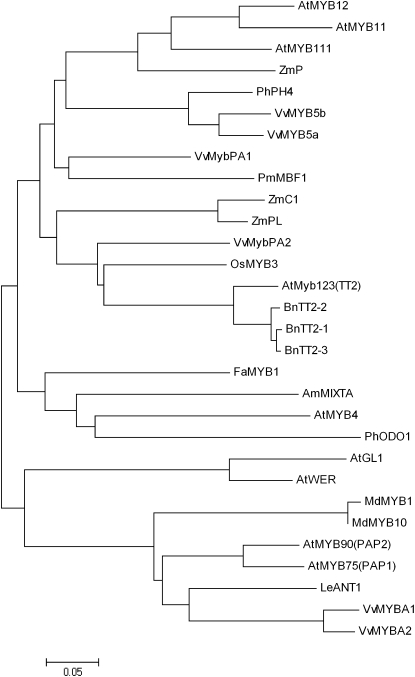
A phylogenetic tree was constructed using the neighbor-joining method with the protein sequences of other plant Myb factors involved in the regulation of the phenylpropanoid and flavonoid pathways (Fig. 2), like phenylpropanoid volatiles (PhODO1; Verdonk et al., 2005), flavonols (AtMyb112, AtMyb11, and AtMyb12; Stracke et al., 2007), anthocyanins (VvMybA1 and VvMybA2 [Kobayashi et al., 2002], AtPAP1 and AtPAP2 [Borevitz et al., 2000], LeANT1 [Mathews et al., 2003], MdMyb10 [Espley et al., 2007], and MdMyb1 [Takos et al., 2006]), PAs (VvMybPA1 [Bogs et al., 2007] and AtTT2 [Nesi et al., 2001]), vacuolar pH (PH4; Quattrocchio et al., 2006), or Myb whose function is still undetermined (OsMyb3, PmMBF1). This tree reveals that VvMybPA2 is very distinct from regulators of the anthocyanin pathway and appears to be more closely related to AtTT2 than the other grapevine Myb factors identified so far. Analyses of the primary structure (motif 1; Fig. 1) revealed that the R2R3 repeat region of VvMybPA2 is highly homologous to other plant MYB factors and contains the residues of a conserved signature sequence, [D/E]Lx2[R/K]x3Lx6Lx3R, for interaction with bHLH proteins (Grotewold et al., 2000). The nine-residue consensus sequence V(I/V)R(T/P)(K/R)A(I/L/V)(R/K)C is present in both AtTT2 and OsMYB3 in the highly variable C-terminal region (Nesi et al., 2001; Stracke et al., 2001). It is noticeably conserved in VvMybPA2 (motif 2; Fig. 1) while being absent from VvMybPA1 and VvMyb5a (Deluc et al., 2006; Bogs et al., 2007).
Figure 2.
Phylogenetic tree showing selected plant MYB transcription factors retrieved from public databases. The phylogenic tree was constructed from the Clustal alignment using the neighbor-joining method in the MEGA4 package. The scale bar represents 0.05 substitutions per site. The GenBank accession numbers of the MYB proteins are as follows: AtMYB12 (NP_182268), AtMYB11 (NP_191820), AtMYB111 (NP_199744), ZmP (P27898), PhPH4 (AAY52377), VvMyb5b (AAX51291), VvMYb5a (AAS68190), VvMybPA1 (CAJ90831), PmMBF1 (AAA82943), ZmC1 (AAA33482), ZmPl (AAA19821), VvMybPA2 (EU919682), OsMYB3 (BAA23339), AtTT2 (Q9FJA2), BnTT2-1(ABI13038), BnTT2-2 (ABI13039), BnTT2-3 (ABI13040), FaMYB1 (AAK84064), AmMIXTA (CAA55725), AtMyb4 (BAA21619), PhODO1 (AAV98200), AtGL1 (NP_189430), AtWER (AAF18939), MdMYB1 (ABK58136) MdMYB10 (ABB84753) AtPAP2 (NP_176813), AtPAP1 (NP_176057), LeANT1 (AAQ55181), VvMYBA1 (BAD18977), and VvMYBA2 (BAD18978).
Expression Profiling of VvMybPA2
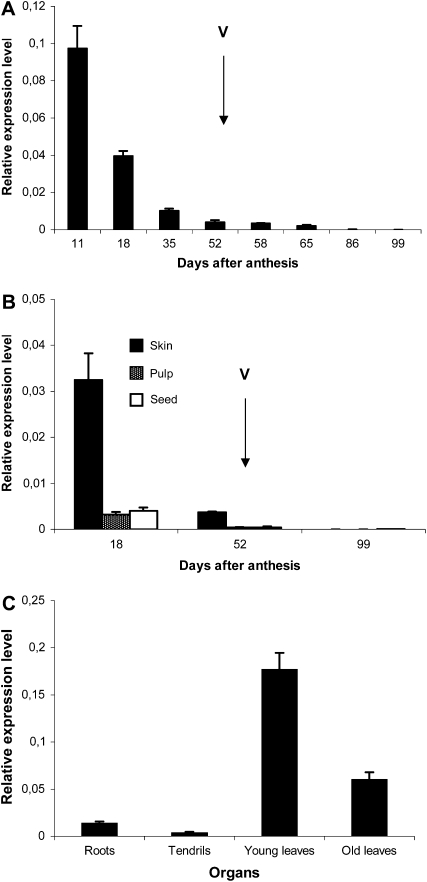
The spatiotemporal expression of VvMybPA2 was evaluated by real-time PCR with RNA isolated from several vegetative tissues and berries sampled at different stages of development. Figure 3A shows that VvMybPA2 was highly expressed in very young berries shortly after anthesis. Then, transcript abundance decreased to a very low level after véraison. When berries were divided into exocarp, mesocarp, and seed at three stages of development, VvMybPA2 expression was mostly restricted to the exocarp of very young berries (Fig. 3B). In vegetative organs, the maximum expression of VvMybPA2 was detected in leaves, especially in younger ones (Fig. 3C).
Figure 3.
Transcript levels of VvMybPA2 during berry pericarp development (A), in different berry tissues at three developmental stages (B), and in different organs of vine plants (C). Véraison (V) is marked with the arrows. Gene expression was determined by real-time PCR and normalized with the expression of EF1α. All data are means of three replicates, with error bars indicating sd.
Functional Characterization of VvMybPA1 and VvMybPA2
In order to establish the function of VvMybPA1 and VvMybPA2, each full-length cDNA driven by the 35S promoter was separately introduced into grapevine hairy roots. Hairy roots were screened by PCR for the presence of the hygromycin phosphotransferase gene from the pH2GW7 backbone, yielding seven positive independent transgenic lines from the 10 plants inoculated with each construct. Ectopic expression of VvMybPA1 and VvMybPA2 transcription factor led to PA accumulation. For each gene, the two lines with the highest PA content were selected as independent biological duplicates for more detailed phenotypic and transcriptomic analysis. Hairy roots without the VvMybPA1/2 transgene were used as a wild-type control.
Flavonoid and Lignin Content in Grapevine Organs Overexpressing VvMybPA1 and VvMybPA2
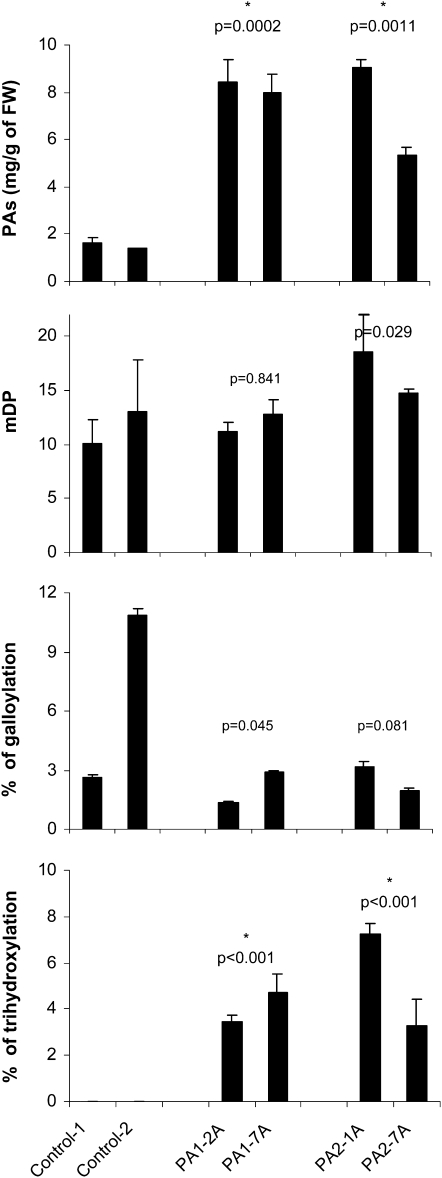
VvMybPA1- and VvMybPA2-expressing hairy roots contained around 8 mg PA g−1 fresh weight, which represents a 5-fold greater accumulation than the levels found in control lines (Fig. 4). Whereas PAs of wild-type roots do not contain any B-ring trihydroxylated units, the percentage of epigallocatechin reached 4% and 5% in roots expressing VvMybPA1 and VvMybPA2, respectively. The mDP exhibited a slight increase in VvMybPA2-expressing lines, with mean values of 16.7 (wild type = 11.6). The high heterogeneity between the two analyzed control lines prevents us from drawing any conclusion concerning the possible influence of the Myb factors on the galloylation level. Neither anthocyanin nor flavonol was detected.
Figure 4.
PA contents and mean characteristics (mDP, percentage galloylation, percentage trihydroxylation) in grapevine organs overexpressing VvMybPA1 (lines PA1-2A and PA1-7A) or VvMybPA2 (lines PA2-1A and PA2-7A) versus the wild type (lines control-1 and control-2). Data are means ± sd of triplicate extractions. Stars indicate that values are significantly different between control and transformed hairy roots (P < 0.01). FW, Fresh weight.
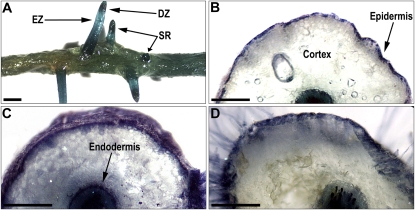
Coloration of grapevine root organs with dimethylaminocinnamaldehyde (DMACA), which reacts with PAs to form a blue stain, clearly shows that PAs were not uniformly located in the hairy root tissues (Fig. 5). PAs were quite abundant in the youngest part of the roots (i.e. in the cell division area below the apex). In the sectors accumulating high level of PAs, epidermis, endoderm, and vascular bundles were found to be the richest. Pericycle was less marked by the DMACA, and almost no coloration could be observed in the cortical parenchyma cells whatever the type of roots.
Figure 5.
Phenotype of grapevine hairy roots stained with DMACA. A, Wild-type hairy roots showing the distribution of blue dye formed with PA on main and secondary organs. Bar = 2 mm. B, Wild-type hairy root section. Bar = 250 μm. C, VvMybPA1-expressing hairy root. Bar = 250 μm. D, VvMybPA2-expressing hairy root. Bar = 250 μm. DZ, Division zone; EZ, elongation zone; SR, secondary roots.
With the objective to evaluate whether the transformation induced some redirection in the phenolic metabolism, preliminary analyses of lignin content and composition of the hairy root samples were performed by thioacidolysis (Lapierre et al., 1995) and are presented in Supplemental Table S1. The total yields of lignin-derived thioacidolysis guaiacyl and syringyl monomers were similarly low, whatever the samples (ranging between 8 and 14 μmol g−1 dry weight), and the nonmethoxylated H monomers were recovered as trace components. This yield suggests that all of the hairy root samples have a similarly low lignin content. By contrast, the molar frequency of the monomethoxylated guaiacyl thioacidolysis monomers was found to be increased, particularly in the hairy roots of the VvMybPA2-expressing lines. This result suggests that the formation of the guaiacyl lignin units might be affected by the transformation, whereas the total lignin content is not changed.
Expression Analysis of Putative Targets
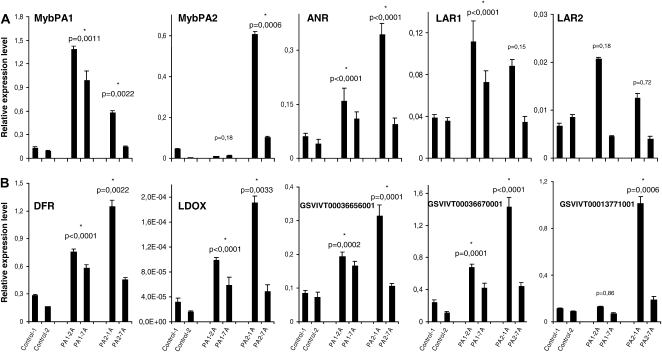
In order to decipher the mechanisms that underlie the phenotypic changes observed in grapevine ectopically expressing VvMybPA1 and VvMybA2, we first performed real-time PCR experiments on previously known genes of grapevine PA metabolism (Fig. 6A). Overexpression of VvMybPA1 and VvMybPA2 transgenes was confirmed in the respective transformed lines. Some direct or indirect activation was found, as endogenous VvMybPA1 was slightly induced by 35S∷VvMybPA2, while the converse was not true concerning VvMybPA2.
Figure 6.
Transcript levels of putative targets (A) and validation of microarray results (B) in grapevine organs overexpressing VvMybPA1 (lines PA1-2A and PA1-7A) or VvMybPA2 (lines PA2-1A and PA2-7A) versus the wild type (lines control-1 and control-2). Gene expression was determined by real-time PCR and normalized with the expression of EF1α. All data are means ± sd of three replicates. Stars indicate that expression levels are significantly different between wild-type and transformed hairy roots (P < 0.01).
ANR transcripts were significantly increased by VvMybPA1 and VvMybPA2 overexpression. Similar results were observed with LAR1, except that its induction by VvMybPA2 was slightly lower (+65% compared with wild-type organs) and not significant. LAR2 expression was not significantly modified upon overexpression of the VvMybPA factors when compared with controls.
Global Transcriptome Response Analysis Induced by VvMybPA1/2 Overexpression
Transcriptome analysis was performed with a 14 K microarray to identify new genes involved in PA biosynthesis and particularly the putative targets of VvMybPA1 and VvMybPA2. The design corresponded to a comparison analysis of two couples of independent biological replicates of wild-type/overexpressing organs for both VvMybPA1 and VvMybPA2 (comprehensive data are available as Supplemental Materials and Methods S1).
A t test (P < 0.01 based on permutation) revealed that only 2% to 3% of the total number of oligonucleotides spotted on the array presented significant variations due to VvMybPA1 and VvMybPA2 overexpression (510 and 371, respectively). For VvMybPA1, 305 oligonucleotides presented an increase in their hybridization signal and 205 presented a decrease. In the case of VvMybPA2, the respective increase and decrease were 158 and 213 (Supplemental Materials and Methods S1). Among the transcripts whose abundance increased, 55 are common to the VvMybPA1- and VvMybPA2-overexpressing organs (Table I). Further analysis revealed that this set of 55 genes corresponds to only 51 unigenes, as four couples of oligonucleotides may hybridize the same transcript. Although significant, the induction ratios in organs ectopically expressing VvMybPA were rather low, as they ranged from 1.1 to 3.7.
Table I.
List of transcripts whose expression is induced after both VvMybPA1 and VvMybPA2 overexpression
Magnitudes of induction relative to wild-type controls are given. The Vitis transcript number refers to the genome model (Jaillon et al., 2007; http://www.genoscope.cns.fr/externe/GenomeBrowser/Vitis/) or to the DFCI tentative contig (http://compbio.dfci.harvard.edu/tgi/cgi-bin/tgi/gimain.pl?gudb=grape). The four right columns present best annotated matches after BLASTX analysis against the nr protein public database, species, putative protein functions, and match e values.
| PA2/Wild-Type Ratio | PA1/Wild-Type Ratio | Probe Set Identifier | Vitis Transcript | Best Match | Species | Putative Function | E Value |
|---|---|---|---|---|---|---|---|
| 3.6 | 2.21 | Vv_10003904 | GSVIVT00014419001 | ABM67589 | Vitis vinifera | Flavanone-3-hydroxylase 2 | 0.0 |
| 3.3 | 2.15 | Vv_10003855 | GSVIVT00036784001 | P41090 | Vitis vinifera | Flavonone-3-hydroxylase 1 | 0.0 |
| 2.82 | 1.68 | Vv_10000003 | GSVIVT00030258001 | None | |||
| 2.8 | 3.74 | Vv_10004449 | GSVIVT00024561001 | ACC63891 | Populus trichocarpa | Phe ammonia lyase | 0.0 |
| 2.8 | 2.51 | Vv_10012881 | TC64176 | None | |||
| 2.76 | 1.98 | Vv_10013426 | GSVIVT00036670001 | ABH03018 | Vitis labrusca | Resveratrol/hydroxycinnamic acid O-glucosyltransferase | 0.0 |
| 2.66 | 2.17 | Vv_10000485 | GSVIVT00014584001 | P51110 | Vitis vinifera | Dihydroflavonol-4-reductase | 2e-157 |
| 2.63 | 2.81 | Vv_10004167 | GSVIVT00006341001 | BAB84111 | Vitis vinifera | Chalcone synthase 3 | 0.0 |
| 2.6 | 2.56 | Vv_10009527 | GSVIVT00014031001 | P31687 | Glycine max | 4-Coumarate-CoA ligase 2 | 2e-173 |
| 2.37 | 2.04 | Vv_10000352 | GSVIVT00001063001 | ABV82967 | Vitis vinifera | Leucoanthocyanidin dioxygenase | 3e-170 |
| 2.31 | 1.71 | Vv_10000110 | GSVIVT00012660001 | BAE47626 | Lactuca sativa | Cytochrome b6/f complex subunit 4 | 3e-61 |
| 2.23 | 1.76 | Vv_10004634 | GSVIVT00036656001 | ABH03018 | Vitis labrusca | Resveratrol/hydroxycinnamic acid O-glucosyltransferase | 0.0 |
| 2.15 | 1.47 | Vv_10003799 | GSVIVT00001815001 | BAF03494 | Populus nigra | XRCC4 homolog | 9e-44 |
| 2.08 | 1.98 | Vv_10004484 | GSVIVT00015738001 | AAL67601 | Oryza sativa | Cinnamoyl-CoA reductase | 8e-77 |
| 2.07 | 2.72 | Vv_10000926 | GSVIVT00038153001 | NP_196974 | Arabidopsis thaliana | Cinnamoyl-CoA reductase-related | 3e-59 |
| 2.03 | 2.27 | Vv_10003778 | GSVIVT00005344001 | CAD91911 | Vitis vinifera | Anthocyanidin reductase | 0.0 |
| 2.03 | 2.61 | Vv_10002321 | GSVIVT00018839001 | NP_191462 | Arabidopsis thaliana | TT12, MATE-type transporter | 4e-169 |
| 2 | 2.26 | Vv_10001009 | GSVIVT00025894001 | AAD29806 | Arabidopsis thaliana | Disease resistance response protein | 2e-16 |
| 1.93 | 1.89 | Vv_10001107 | GSVIVT00038626001 | AAF64227 | Solanum pennellii | Glc acyltransferase | 2e-114 |
| 1.91 | 1.57 | Vv_10013578 | GSVIVT00025888001 | NP_187420 | Arabidopsis thaliana | Arogenate dehydratase/prephenate dehydratase | 1e-116 |
| 1.89 | 1.92 | Vv_10007208 | GSVIVT00016217001 | CAI54278 | Vitis vinifera | Flavonoid-3′-hydroxylase | 0.0 |
| 1.85 | 1.42 | Vv_10003899 | TC59772 | None | |||
| 1.81 | 1.56 | Vv_10001147 | GSVIVT00015196001 | ABA54865 | Fagus sylvatica | 3-Deoxy-d-arabino-heptulosonate 7-phosphate synthase | 0.0 |
| 1.74 | 2.02 | Vv_10011954 | GSVIVT00023340001 | ABK94803 | Populus trichocarpa | Unknown protein | 3e-112 |
| 1.74 | 2.21 | Vv_10013105 | GSVIVT00029573001 | AAF02155 | Arabidopsis thaliana | Unknown protein | 4e-17 |
| 1.69 | 1.67 | Vv_10008665 | GSVIVT00008901001 | ABA54867 | Fagus sylvatica | 3-Dehydroquinate dehydratase/shikimate 5-dehydrogenase | 4e-159 |
| 1.68 | 2.02 | Vv_10003316 | GSVIVT00006090001 | ABN09011 | Medicago truncatula | Sugar transporter superfamily | 2e-131 |
| 1.66 | 1.77 | Vv_10004589 | GSVIVT00012147001 | NP_176734 | Arabidopsis thaliana | Allyl alcohol dehydrogenase, putative | 4e-143 |
| 1.58 | 1.59 | Vv_10004860 | GSVIVT00013718001 | NP_565423 | Arabidopsis thaliana | Unknown protein | 7e-21 |
| 1.57 | 1.94 | Vv_10010085 | GSVIVT00005432001 | ABA54868 | Fagus sylvatica | Shikimate kinase | 6e-69 |
| 1.56 | 1.51 | Vv_10002822 | GSVIVT00017259001 | BAE71196 | Trifolium pratense | Hydroxymethylglutaryl-CoA lyase | 2e-176 |
| 1.48 | 1.52 | Vv_10002309 | GSVIVT00005204001 | AAL36369 | Arabidopsis thaliana | Receptor kinase | 0.0 |
| 1.46 | 1.96 | Vv_10002140 | GSVIVT00015239001 | AAT77693 | Vitis vinifera | Hexose transporter HT2 | 0.0 |
| 1.46 | 1.42 | Vv_10000937 | GSVIVT00032373001 | NP_567869 | Arabidopsis thaliana | Electron carrier | 2e-145 |
| 1.44 | 1.48 | Vv_10000117 | TC68399 | CAL07988 | Platanus × acerifolia | Translation elongation factor 1α | 9e-5 |
| 1.38 | 1.22 | Vv_10002123 | GSVIVT00005969001 | AAB37756 | Solanum tuberosum | Kinesin heavy chain-like protein | 0.0 |
| 1.37 | 1.48 | Vv_10004956 | GSVIVT00017469001 | NP_563818 | Arabidopsis thaliana | Strictosidine synthase family protein | 8e-146 |
| 1.37 | 1.46 | Vv_10000360 | GSVIVT00030469001 | ABW34393 | Vitis vinifera | R2R3 Myb transcription factor C2 repressor motif protein | 4e-130 |
| 1.36 | 1.42 | Vv_10009241 | GSVIVT00024556001 | NP_565668 | Arabidopsis thaliana | Unknown protein | 4e-95 |
| 1.31 | 1.52 | Vv_10008052 | GSVIVT00020463001 | NP_193106 | Arabidopsis thaliana | Purple acid phosphatase | 0.0 |
| 1.31 | 1.3 | Vv_10009804 | GSVIVT00024607001 | ABN08215 | Medicago truncatula | Zinc finger (CCCH-type) family protein | 0.0 |
| 1.31 | 1.32 | Vv_10000047 | GSVIVT00033143001 | NP_199757| | Arabidopsis thaliana | ATP-citrate lyase B-2 | 0.0 |
| 1.28 | 1.15 | Vv_10004398 | GSVIVT00034002001 | AAK55470 | Oryza sativa | Phosphoinositide kinase | 3e-150 |
| 1.26 | 1.2 | Vv_10004784 | GSVIVT00014475001 | ABC68397 | Glycine max | Cytochrome P450 monooxygenase CYP83E8 | 6e-156 |
| 1.26 | 1.32 | Vv_10013455 | GSVIVT00026033001 | AAR88099 | Arabidopsis thaliana | Glutamate receptor ion channel | 0.0 |
| 1.26 | 1.24 | Vv_10000645 | GSVIVT00031453001 | NP_568860 | Arabidopsis thaliana | CBL-interacting protein kinase | 1e-144 |
| 1.25 | 1.42 | Vv_10004922 | GSVIVT00031779001 | ABW38332 | Fragaria × ananassa | Cytosolic Fru-1,6-bisphosphatase | 1e-176 |
| 1.24 | 1.24 | Vv_10010519 | GSVIVT00029310001 | AAF29605 | Pisum sativum | Gibberellin c20-oxidase | 6e-06 |
| 1.20 | 1.08 | Vv_10004613 | GSVIVT00001461001 | NP_179081 | Arabidopsis thaliana | Vacuolar sorting receptor | 0.0 |
| 1.16 | 1.23 | Vv_10009243 | GSVIVT00018590001 | NP_179660 | Arabidopsis thaliana | Glycosyl hydrolase family 5 protein | 1e-180 |
| 1.14 | 1.14 | Vv_10010645 | GSVIVT00034434001 | NP_001117624 | Arabidopsis thaliana | Coatomer protein complex, subunit β2 | 0.0 |
Among the genes induced in common by VvMybPA1 and VvMybPA2, 10 are linked to the PA pathway, including nine genes previously identified in grapevine: Phe ammonia lyase, 4-coumarate:CoA ligase, chalcone synthase, two genes of flavanone-3-hydroxylase, flavonoid-3′-hydroxylase (F3′H), dihydroflavonol-4-reductase, leucoanthocyanidin dioxygenase (LDOX), ANR, and a multidrug and toxic compound extrusion (MATE) transporter (GSVIVP00018839001) exhibiting homology with TT12 (Debeaujon et al., 2001) that represents a new putative actor in the PA pathway in grapevine. Two isogenes encoding cinnamoyl-CoA reductase of the phenylpropanoid pathway were also identified. Both transcription factors led to the induction of four genes linked to the metabolism of aromatic amino acids and shikimate (shikimate kinase, shikimate dehydrogenase, 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase, and prephenate dehydratase), precursors of the flavonoid pathway. In addition to genes encoding enzymes already annotated as actors in the flavonoid pathway, the experiments revealed several genes linked with sugar metabolism: two glucosyltransferases (GSVIVT00036656001 and GSVIVT00036670001), one Glc acyltransferase (GSVIVT00038626001), two sugar transporters, and a Fru-1,6-bisphosphatase. Another striking category contains seven genes related to signaling mechanisms, including a Myb factor, a calcineurin B-like (CBL) protein, and a CBL-interacting protein kinase. All of these genes represent the most induced genes, and for some of them this induction was confirmed by real-time PCR (Fig. 6B). Genes induced to a lower extent have miscellaneous or unknown functions, and some of them do not even exhibit homologies with other genes of the plant kingdom.
A total of 828 genes were found significantly differentially expressed between hairy roots overexpressing VvMybPA1 and VvMybPA2. Among them, 59 are specifically induced in 35S∷VvMybPA1 transformants, such as a chalcone synthase isogene, another MATE transporter, while 21 are more specific for VvMybPA2, such as another glucosyltransferase, GSVIVT00013771001 (Fig. 6B; Supplemental Materials and Methods S1).
DISCUSSION
VvMybPA1 and VvMybPA2 Regulate the PA Pathway in Grapevine Berries
PA synthesis in grapevine berries is restricted to the early stages of development in skin and seed and in pulp to a certain extent (Verriès et al., 2008). Moreover, PAs of these different tissues exhibit noticeable structural differences whose biological determinism is unknown, although it is probably linked to the differential regulation of F3′H, flavonoid 3′,5′-hydroxylase (F3′5′H), ANR, LAR1, and LAR2 isogenes in a precise time- and tissue-specific manner. The data presented here indicate that at least two transcription factors belonging to the Myb family are involved in the control of PA content in grapevine.
VvMybPA1 was previously identified by Bogs et al. (2007). VvMybPA2, which was identified here, appears to be the closest ortholog of AtTT2 identified so far in grapevine. R2R3 Myb factors share very limited homology in their C-terminal regions, which could be related to their target specificity. A sequence conserved between AtTT2 and OsMYB3 and more recently confirmed in Brassica napus putative orthologs of TT2 was identified in this highly variable C-terminal region (Nesi et al., 2001; Stracke et al., 2001; Wei et al., 2007). This motif is also present in VvMybPA2 but is not strictly conserved in the VvMybPA1 sequence (Bogs et al., 2007).
We demonstrated here that the spatiotemporal expression of VvMybPA2, restricted to the skin of young berries and leaves, is compatible with a putative role in the regulation of PA biosynthesis. The VvMybPA2 expression pattern argues in favor of a preferential role in PA synthesis and accumulation in berry skins when compared with VvMybPA1. VvMybPA1 is expressed before véraison and actually correlates with PA accumulation in seeds (Bogs et al., 2007). Its involvement in the accumulation of PA accumulation in skin was ruled out, on the basis of its very low expression level in this tissue. Moreover, according to some authors, VvMybPA1 showed an unexpected maximum of expression 2 weeks after véraison when PA accumulation was stopped (Bogs et al., 2007; Deluc et al., 2007). Conversely, the profile of VvMybPA2 is consistent with a critical function in the accumulation of PAs in berry skin. It was hypothesized that the different branches of the flavonoid and phenylpropanoid pathways are specified by Myb proteins inside the activating complex (Zhang et al., 2003). However, Deluc et al. (2006), expressing VvMyb5a transcription factor in tobacco (Nicotiana tabacum), showed that the activation of diverse branches of the flavonoid and phenylpropanoid pathways in the different parts of the transgenic plants could also be modulated by bHLH and WD40 as members of the transcriptional machinery. Nevertheless, this transcription factor did not trigger anthocyanin accumulation in natural circumstances, being expressed in berries at green stage, and its actual function in grapevine remains to be clarified. We show here that both VvMybPA1 and the new VvMybPA2 actually act as specific positive regulators of PA biosynthesis when stably expressed in grapevine, alleviating the hazard of artifacts upon ectopic expression in heterologous plants, as suspected for VvMyb5a (Deluc et al., 2006). Both transcription factors described here appeared as extremely specific to the PA pathway, since no shift toward lignin, anthocyanin, and flavonol production appeared in long-term experiments.
VvMybPA1 and VvMybPA2 Overexpression Give Similar Results
In order to clarify the specific roles of VvMybPA1 and VvMybPA2, we observed the respective phenotypic and transcriptomic variations induced by their ectopic overexpression in grapevine. Both genes were able to modify PA contents and their subunit composition. When overexpressed in hairy roots, VvMybPA1 and VvMybPA2 actually triggered de novo B ring trihydroxylation, and a slightly increased mDP was observed in the case of VvMybPA2-expressing lines, which is consistent with the composition of skin PAs. The level of trihydroxylation of transgenic hairy root PAs was similar to that of Maccabeo berry skin (3.5%) and higher than in seeds (0%; Souquet et al., 2006). In this respect, the increase of trihydroxylated units observed here upon the overexpression of VvMybPA1 is consistent with its ability to activate the promoter of F3′5′H (Bogs et al., 2007). However grapevine seed PAs do not contain any (epi)gallocatechin unit, which may suggest a pretranscriptional or posttranscriptional negative regulation of F3′5′H in the seeds. Despite exhibiting an increase upon ectopic expression of VvMybPA2, the PA chain length remained three times lower than the mDP of approximately 50 encountered inside the skin of Maccabeo plants from the field (Souquet et al., 2006). More generally, we did not succeed in reproducing the large structural differences among seed and skin tannins upon differential ectopic expression of VvMybPA1 and VvMybPA2.
This PA accumulation was found restricted to some tissues despite the use of a 35S promoter. At the transcription level, several causes could explain the deficit in PA accumulation in some tissues and cells: (1) lack of members of the activating complex, (2) absence of transcription factors in the role of activators, or (3) expression of inhibitors. In Arabidopsis, Nesi et al. (2001), observing that ectopic expression of TT2 activated TT8, BAN, or TT12 without subsequent PA accumulation, hypothesized the lack of activation of earlier genes of the pathway. In grapevine cell suspensions, cotransformation with a bHLH factor was found mandatory for the transient activation of the LDOX promoter by VvMybPA1 (Bogs et al., 2007). In our experiments, the sole overexpression of VvMybPA1 or VvMybPA2 proved to be sufficient to enhance the LDOX expression. This means that either the partners forming the PA regulatory complex were already present in the tissues of the roots accumulating PAs or that long-term overexpression is necessary to induce all partners required for full activation of the PA pathway. The latter hypothesis appears rather unlikely, since no induction of any bHLH or WD40 transcription factors was found associated with the ectopic accumulation of PAs in grapevine. However, it must be kept in mind that the 14 K oligoarray used in this study may probe only half of the Vitis transcriptome, according to Jaillon et al. (2007).
As the phenotypes of transgenic grapevine organs expressing VvMybPA1 or VvMybPA2 are quite similar, the reason for the existence of two distinct transcription factors with apparent redundant function can be questioned. A similar functional redundancy was described in Arabidopsis, in which three different Myb factors controlling the flavonol pathway were identified, each of them exhibiting a specific tissue expression pattern (Stracke et al., 2007). Recently, Matus et al. (2008) classified the 108 members of the grape R2R3 Myb family in terms of their genomic structures and similarity to their putative Arabidopsis orthologues. Eight genes, including VvMybPA2 but neither VvMybPA1 nor VvMyb5a, were designated as putative candidates for PA pathway regulators. Further work is needed to better understand the putative functions of these regulators in the tissue-specific regulation of the PA pathway.
Despite the phenotype similarities, the high-throughput transcriptomic analysis presented here revealed that several targets were specifically induced by each Myb factor. However, either the genes identified were isogenes of already identified targets, like the identified glucosyltransferase specifically induced in VvMybPA2 transgenic lines, suggesting here again functional redundancy (or very subtle differences in their catalytic properties), or the level of induction for these genes was remarkably low.
This experiment also raises the question of the interactions between transcription factors. A first result emerged from this study, where overexpression of VvMybPA2 resulted in the accumulation of VvMybPA1 transcripts, suggesting that VvMybPA2 signal acts upstream to VvMybPA1.
Expected and Unexpected Targets, and Absence of Expected Targets
Enzymes of the Flavonoid Pathway
Being the final result of a transcriptomic screening after overexpression of two different transcription factors inducing PA accumulation, the list of the 51 induced transcripts is very likely to contain specific isogenes involved in the PA pathway. Microarray analysis revealed the induction of genes from Phe ammonia lyase to ANR. Rather low expression ratios were observed after overexpression of both Myb factors. This could result from the preactivation of the PA pathway in wild-type hairy roots, which already contain significant amounts of PAs. In addition, we observed that despite the use of a constitutive promoter, only specific tissues were able to ectopically accumulate PAs. Consequently, the sensibility of the analysis is probably hampered by the low number of cells inside the organs affected by PA accumulation. Some genes belonging to this pathway were not detected. For example, the only copy of chalcone isomerase in the genome is induced in both types of transformants, but its induction reaches a significant threshold only under VvMybPA1 overexpression. In addition, some genes could not be monitored due to the absence of a probe, like F3′5′H. The induction of F3′5′H seems necessary to synthesize the trihydroxylated units observed in transgenic roots. Unfortunately, the grapevine genome contain 10 genes coding for putative F3′5′H (Jaillon et al., 2007; Velasco et al., 2007), which precluded the design of isogene-specific 70-mer-long probes.
Neither the overexpression of VvMybPA1 nor that of VvMybPA2 resulted in a significant induction of LAR2. Bogs et al. (2007) reported that VvMybPA1 was able to activate the promoters of LAR1, as confirmed here, but they did not address LAR2 activation. The proportion of catechin as TU or free monomer was not impaired when compared with the wild type, probably due to the activity of LAR1 (data not shown). Disturbing results concerning particular LAR isogenes are reported in the literature on other plants (Pang et al., 2007; Paolocci et al., 2007). Similarly, ectopic expression of the maize (Zea mays) bHLH flavonoid regulator Sn in Lotus corniculatus resulted in an increase of PA content and induction of ANR and LAR1 transcripts, while LAR2 transcription remained unaffected (Paolocci et al., 2007). They failed to detect any catechin synthesis after the heterologous production of the LAR2 enzyme (Paolocci et al., 2007). PAs of Medicago truncatula seed coat contain insignificant amounts of catechin as TU or EU, despite the presence of LAR transcript in this tissue and the ability of the heterologous LAR protein to synthesize catechin in vitro (Pang et al., 2007). These authors also reported that transgenic tobacco plants overexpressing MtLAR did not exhibit changes in their PA or catechin content. Taken together, these results indicate that LAR isogenes occupy a particular place in the PA pathway, from the functional and transcriptional regulation points of view, and the precise role of LAR2 remains to be elucidated.
In addition to the induction of genes previously shown to be involved in sensu stricto PA biosynthesis, screenings revealed the induction of one (GSVIVT00018839001) of the 65 MATE-type transporters of the grapevine genome (C. Gomez, personal communication). With 70% amino acid homology, this gene appears to be the closest homolog of TT12, which was shown to be critical for PA accumulation in the Arabidopsis seed testa (Debeaujon et al., 2001). The protein encoded by TT12 was found located on the tonoplast, and in vitro experiments showed that TT12 transports the anthocyanin cyanidin-3-O-glucoside (Marinova et al., 2007). A glutathione S-transferase (Kitamura et al., 2004) and a AHA10-like H+-ATPase (Baxter et al., 2005) have been reported to be involved in PA storage, but no grapevine homolog of these transcripts has been identified in our microarray screening.
Several genes were described as systematically expressed concomitantly with anthocyanin accumulation, like UDP-Glc:flavonoid 3-O-glucosyltransferase (Boss et al., 1996) or a putative methyl transferase and a particular isogene of glutathione S-transferase (Ageorges et al., 2006). Other experiments were performed by our group with the aim to ectopically activate anthocyanin accumulation in hairy roots with the VlMybA1 gene identified by Kobayashi et al. (2002). It resulted in anthocyanin accumulation and the induction of a set of genes specific for the anthocyanin pathway, without any modification of their PA content or of the expression level of the PA biosynthetic genes, ANR, LAR1, and LAR2 (Cutanda-Perez et al., 2009). On the other hand, neither the anthocyanin biosynthetic genes nor flavonol synthase were induced in the hairy roots overexpressing VvMybPA1 and VvMybPA2 genes, confirming their specific role in the regulation of the PA pathway and an activation spectrum different from that of VlMybA1.
Signal Transduction
Overexpression of both VvMybPA1 and VvMybPA2 also induced the expression of a set of genes associated with signaling. A homolog of Arabidopsis Myb4 was ectopically induced in grapevine organs. AtMyb4 was shown to act as a repressor of the phenylpropanoid pathway (Jin et al., 2000). Its induction as a consequence of VvMybPA1 and VvMybPA2 overexpression could be considered as a way to counterbalance their attempt to avoid the bolting of the PA pathway.
CBL proteins and their target kinases, CBL-interacting protein kinases, have often been described as functioning in complex in the response to abiotic stresses (Batistic and Kudla, 2004). The simultaneous induction of these genes may result from the stress experienced by the plant after ectopic expression of the VvMybPA or from a sudden increase of intracellular PA concentration.
Genes Linked to Sugar Metabolism
Several genes related to sugar metabolism appeared as potentially driven by VvMybPA factors. Among them, the induction of a hexose transporter called VvHT2 (Fillion et al., 1999) was unexpected. Fillion et al. (1999) described its expression maximum 1 week after véraison, whereas Hayes et al. (2007) reported a constant decrease of expression during berry development. However, the in planta role of VvHT2 remains to be characterized. Incidentally, a protein from Lotus japonica annotated as “Suc transporter” and located at the tonoplast proved to be capable of transporting phenyl glucosides (Reinders et al., 2008). A possible involvement of genes annotated as “sugar transporter” in the transport of a glucosylated intermediate of the pathway cannot be excluded.
The two closest identified homologs of GSVIVT00038626001, a putative Glc acyltransferase, are (1) DkSCPL1, a gene of persimmon (Diospyros kaki) identified through a suppression subtractive hybridization between fruits differing in their PA content (Ikegami et al., 2007), and (2) a Solanum pennellii acyltransferase catalyzing the formation of diacylglucose (Li and Steffens, 2000). These proteins could act as Glc acyltransferases that use 1-O-β-acetyl Glc esters as acyl donors (Milkowski and Strack, 2004).
Several glucosyltransferases were also identified. Two of them (GSVIVT00036670001 and GSVIVT00036656001) are induced by both transcription factors, whereas another one (GTGSVIVT00013771001) appears more specifically driven by VvMybPA2. GSVIVT00036656001 was already identified as a bifunctional resveratrol/hydroxycinnamic acid glucosyltransferase in Vitis labrusca (Hall and DeLuca, 2007). This protein forms Glc esters with phenolic acids at low pH (5–7) and O-glucosides of resveratrol or flavonols at higher pH (7–9) at a much lower rate. Here, only trace amounts of transresveratrol and of the O-glucosides of cis- and trans-resveratrol were detected without any difference between wild-type and VvMybPA-overexpressing organs (data not shown). Recently, a transcriptomic screening after ectopic expression of TT2 in M. truncatula hairy roots identified a glucosyltransferase able to catalyze the formation of epicatechin 3′-glucoside (Pang et al., 2008). However, this protein is quite divergent from the glucosyltransferases identified in this work, with only 26% to 28% identity. The occurrence of glycosylated PAs was described in other species (Xie and Dixon, 2005), but they were never encountered in grapevine or in the hairy roots described here. The absence of glucosylated PA in VvMybPA-overexpressing hairy roots makes it unlikely that these GT isogenes are involved in PA glucosylation. The actual biological functions of Glc acyltransferase and/or glucosyltransferases in grapevine PA biosynthesis remain to be determined and should be further investigated.
Original PA composition with respect to model plants, recent developments of new genomic tools, and the availability of reverse genetics technologies allow grapevine to be considered as a valuable model for the study of PA biosynthesis. We demonstrated here that the identification of a new specific transcription factor associated with homologous transformation allowed the determination of a specific network of genes associated with the activation of PA accumulation. This network was shown to involve several already annotated genes and revealed new putative actors.
MATERIALS AND METHODS
Plant Material and Nucleic Acid Extraction
Organs (berries, leaves, tendrils, and roots) from grapevine (Vitis vinifera ‘Shiraz’) plants grown in the SupAgro-INRA vineyard in Montpellier, France, were collected at several developmental stages. Young leaves corresponded to leaves explanted from the third node below the shoot tips, with a mean weight of 0.3 g. Old leaves corresponded to fully expanded leaves sampled from the mature shoot part, with a mean weight of 2.8 g. Eight- to 10-week-old plantlets of cv Maccabeu grapevine propagated onto half-strength Murashige and Skoog medium were used for Agrobacterium tumefaciens transformation procedures.
After sampling, hairy roots and plant organs were rapidly frozen in liquid nitrogen and then ground to a fine powder with a Dangoumau blender (Dangoumill 300) and stored at −80°C until use.
DNA was extracted from 50 mg of frozen tissue using the DNA Plant Mini kit (Qiagen). Total RNA was extracted using the RNeasy Plant Mini kit (Qiagen) following the manufacturer's instructions, starting from 200 mg of tissue.
Cloning and Vectors
The coding regions of VvMybPA1 and VvMybPA2 were amplified from young Shiraz pericarp cDNA with high-fidelity Taq polymerase (Advantage-HF 2 PCR kit; Clontech) using the forward primers 5′-CACCATGGGCAGAGCACCTTGTTGT-3′ and 5′-CACCATGGGAAGAAGACCTTGCTG-3′ and the reverse primers 5′-TTAAATGAGTAGTGATTCGGC-3′ and 5′-CTATGGGACTTGATTATTTTC-3′ for VvMybPA1 and VvMybPA2, respectively. The amplicons were directionally cloned in pENTR/d-TOPO (Invitrogen Life Technologies) according to the manufacturer's instructions. The sequences of the positive clones were confirmed following transformation of One Shot competent Escherichia coli (Invitrogen Life Technologies) and LR recombination in the binary vector pH2GW7 (Karimi et al., 2002) to yield the 35S VvMybPAs constructs. The sequence of VvMybPA2 was deposited in GenBank under accession number EU919682.
Recombinant plasmids were electroporated into Agrobacterium rhizogenes strain A4 introduced from Collection Française de Bactéries Phytopathogènes (http://www-intranet.angers.inra.fr/cfbp/).
Sequence Analysis
Full-length amino acid sequences of Myb factors from several species were retrieved from public databases. Alignments were performed with the ClustalW2 algorithm with default parameters (Thompson et al., 1994). The phylogenic tree was constructed from the Clustal alignment using the neighbor-joining method in the MEGA4 package (Kumar et al., 2004).
Real-Time PCR
The RNA was accurately quantified with Ribogreen reagent (Molecular Probes). A triplicate reverse transcription was performed on 500 ng of total RNA from each developmental stage using the SuperScript II reverse transcription kit (Invitrogen) according to the manufacturer's instructions. Triplicate reverse transcriptions for PCR were pooled to minimize the heterogeneity of the reverse transcription reaction efficiency. Specific oligonucleotide primer pairs were designed with Primer3 software except for LDOX (Bogs et al., 2005) and for dihydroflavonol-4-reductase (Jeong et al., 2004) and are listed in Supplemental Table S2. Specific annealing of the oligonucleotides was controlled on dissociation kinetics performed at the end of each PCR run. The efficiency of each primer pair was measured on a PCR product or plasmid serial dilutions. PCR was performed in triplicate on 1 μL of cDNA from each sample using the model 7300 Sequence Detection System (Applied Biosystems) and the Power SYBR-Green PCR Master kit (Applied Biosystems Applera France). EF1α was used as a reference for normalization of gene expression (Terrier et al., 2005; Reid et al., 2006). The difference between the cycle threshold (Ct) of the target gene and EF1α was used to obtain the normalized expression of the target gene, calculated as 2 exp − (Cttarget − CtEF1alpha).
Genetic Transformation Procedures
Induction and culture of transgenic hairy roots in grapevine were performed as described by Torregrosa and Bouquet (1997) with the following modifications: (1) A. rhizogenes strain A4 was used as vector; (2) bacterial cultures were grown on semisolid MGL/B (5 g L−1 tryptone, 5 g L−1 mannitol, 2.5 g L−1 yeast extract, 1 g L−1 Glu, 250 mg L−1 K2HPO4, 100 mg L−1 NaCl, 100 mg L−1 MgSO4.7H2O, and 5 μg L−1 biotin at pH 7) medium (Torregrosa et al., 2002) supplemented with 50 μg mL−1 spectinomycin and then suspended with half-strength Murashige and Skoog liquid medium (optical density at 600 nm = 0.3) in the presence of 100 μm acetosyringone for inoculation; and (3) roots tips extracted from explants were cultured on modified LG0 medium (LG0 as described by Torregrosa and Bouquet [1997] added with 250 mg L−1 casein hydrolysate and solidified 5 g L−1 Phytagel [Sigma] with 200 μg mL−1 both augmentin and cefotaxime [Duchefa] to prevent bacterial growth). Stable hairy root lines were subcultured every 4 to 5 weeks on the same medium until use.
Phenotyping the Transformed Hairy Roots
Extraction and HPLC Methods
PA extractions were performed as described by Verriès et al. (2008). During 1 h, 100 mg of frozen and powdered sample was mixed with 750 μL of the extraction solution (acetone:water [70:30, v/v] containing 0.05% trifluoroacetic acid) and 50 μL of an internal standard solution (p-hydroxy methyl ester, 3 g L−1 in methanol). p-Hydroxy methyl ester was chosen because of its stability during extraction and phloroglucinolysis reactions, its retention time being quite different from those of PA units in our HPLC conditions, and its absorbance spectrum. Samples were centrifuged (13,000 g, 15 min, 4°C), and the supernatants were recovered. Immediately, 200 μL of each supernatant was dried under vacuum at 35°C for 2 h (Genevac) and resuspended in 100 μL of reagent solution (0.25 g of phloroglucinol, 0.05 g of ascorbic acid, and 5 mL of acidified methanol [0.2 n HCl]) for acid-catalyzed degradation in the presence of excess phloroglucinol. After incubation (50°C, 20 min), the reaction was stopped by adding 100 μL of sodium acetate buffer (200 mm, pH 7.5). Samples were then centrifuged before injection into the HPLC system. HPLC analyses were performed as described by Verriès et al. (2008). Results were expressed in milligrams per gram fresh weight. Polymer length was estimated by the mDP, calculated as the ratio between the sum of EU (phloroglucinol adducts) and TU and the sum of TU, including free monomers. The mDP, the percentage of galloylation, and the percentage of epigallocatechin units (or trihydroxylation) were calculated on a molar basis.
The lignin content and composition of the hairy root samples were evaluated by thioacidolysis, as described previously, on 5 to 10 mg of freeze-dried sample (Lapierre et al., 1995; Nakashima et al., 2008).
Coloration with DMACA
DMACA reacts quite specifically with flavan 3-ol monomers and PAs to form a blue chromophore (Feucht et al., 2004). The presence and localization of PAs in hairy roots were detected by staining the tissues with the DMACA solution (1% DMACA and 1% 6 n HCl in methanol) for 15 min. The roots were then rinsed with distilled water, and blue staining was visualized with a microscope and captured using a digital camera. Figures were formatted and assembled with Adobe Photoshop 7.0.
Microarray Experiments
We used the Qiagen Operon Array-Ready Oligo Set for the Grape Genome Version 1.0 containing 14,562 70-mer probes representing 14,562 transcripts from The Institute for Genomic Research (TIGR) Grape Gene Index, release 3. Oligonucleotides were reannotated using the DFCI Grape Gene Index, release 5.0 (June 21, 2006; http://compbio.dfci.harvard.edu/tgi/cgi-bin/tgi/gimain.pl?gudb=grape). Oligonucleotides were spotted on mirror slides, and the probes were labeled with Cy3 and Cy5 dyes with the Amino Allyl MessageAmp II aRNA kit (Ambion). The experiment was performed using eight slides (two biological replicates for each condition and a dye swap, detailed in Supplemental Materials and Methods S1). Hybridized microarrays were simultaneously scanned for Cy3- and Cy5-labeled probes with an Axon Genepix 4000B scanner.
Statistical Treatments
Regarding microarray experiments, data from both channels corresponding to Cy3- and Cy5-labeled probes were normalized using the Lowess algorithm in the Microarray Data Analysis System at TIGR. Data from all of the slides were log2 transformed and normalized (centered on 0, variance equalized to 1); those data are available in Supplemental Materials and Methods S1. The significance was calculated at the 0.01 level by permutation t test in Multiexperiment Viewer from TIGR.
The significance (P < 0.01) of the results from biochemical analysis and real-time PCR was statistically assessed with a permutation t test in the Past software.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number EU919682.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Lignin content, subunit composition, and proportion (guaiacyl and syringyl lignin-derived monomers) in grapevine organs overexpressing VvMybPA1 or VvMybPA2 compared with controls.
Supplemental Table S2. Primers used for real-time PCR and expected sizes of the amplified fragments.
Supplemental Materials and Methods S1. terriersupS2.xls contains experimental design (“design”; sheet 1), complete hybridization results log2 transformed and normalized (“totlognorm”; sheet 2), list of genes for which transcript accumulation is significantly affected by ectopic overexpression of VvMybPA1 (“PA1signif”; sheet 3) or VvMybPA2 (“PA2signif”; sheet 4), list of genes for which transcript accumulation is significantly different between ectopic overexpression of VvMybPA1 and VvMybPA2 (“PA2diffPA1”; sheet 5), list of genes specifically induced by ectopic overexpression of VvMybPA1 (“PA1spec”; sheet 6), and list of genes specifically induced by ectopic overexpression of VvMybPA2 (“PA2spec”; sheet 6).
Supplementary Material
Acknowledgments
The assistance of Prof. Catherine Lapierre and of Mrs. Brigitte Pollet (UMR 206 Chimie Biologique, AgroParisTech-INRA, Agroparistech Centre de Grignon) is sincerely acknowledged for the thioacidolysis experiments and their interpretation.
This work was supported by the European Community (STREP project FLAVO–FOOD–CT–2004–513960).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Nancy Terrier (terrier@supagro.inra.fr).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Ageorges A, Fernandez L, Vialet S, Merdinoglu D, Terrier N, Romieu C (2006) Four specific isogenes of the anthocyanin metabolic pathway are systematically co-expressed with the red colour of grape berries. Plant Sci 170 372–383 [Google Scholar]
- Batistic O, Kudla J (2004) Integration and channeling of calcium signaling through the CBL calcium sensor/CIPK protein kinase network. Planta 219 915–924 [DOI] [PubMed] [Google Scholar]
- Baxter IR, Young JC, Armstrong G, Foster N, Bogenschutz N, Cordova T, Peer WA, Hazen SP, Murphy AS, Harper JF (2005) A plasma membrane H+-ATPase is required for the formation of proanthocyanidins in the seed coat endothelium of Arabidopsis thaliana. Proc Natl Acad Sci USA 102 2649–2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogs J, Downey M, Harvey J, Ashton A, Tanner G, Robinson S (2005) Proanthocyanidin synthesis and expression of genes encoding leuanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant Physiol 139 652–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogs J, Jaffe FW, Takos AM, Walker AR, Robinson SP (2007) The grapevine transcription factor VvMYBPA1 regulates proanthocyanidin synthesis during fruit development. Plant Physiol 143 1347–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borevitz J, Xia Y, Blount J, Dixon R, Lamb C (2000) Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 12 2383–2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss PK, Davies C, Robinson SP (1996) Analysis of the expression of anthocyanin pathway genes in developing Vitis vinifera L. cv Shiraz grape berries and the implications for pathway regulation. Plant Physiol 111 1059–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutanda-Perez M, Ageorges A, Gomez C, Vialet S, Terrier N, Romieu C, Torregrosa L (2009) Ectopic expression of VlmybA1 in grapevine activates a narrow set of genes involved in anthocyanin synthesis and transport. Plant Mol Biol (in press) [DOI] [PubMed]
- Debeaujon I, Peeters AJ, Léon-Kloosterziel KM, Koornneef M (2001) The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell 13 853–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluc L, Barrieu F, Marchive C, Lauvergeat V, Decendit A, Richard T, Carde JP, Merillon JM, Hamdi S (2006) Characterization of a grapevine R2R3-MYB transcription factor that regulates the phenylpropanoid pathway. Plant Physiol 140 499–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluc LG, Grimplet J, Wheatley MD, Tillett RL, Quilici DR, Osborne C, Schooley DA, Schlauch KA, Cushman JC, Cramer GR (2007) Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development. BMC Genomics 8 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devic M, Guilleminot J, Debeaujon I, Bechtold N, Bensaude E, Koornneef M, Pelletier G, Delseny M (1999) The BANYULS gene encodes a DFR-like protein and is a marker of early seed coat development. Plant J 19 387–398 [DOI] [PubMed] [Google Scholar]
- Dixon RA, Xie DY, Sharma SB (2005) Proanthocyanidins: a final frontier in flavonoid research? New Phytol 165 9–28 [DOI] [PubMed] [Google Scholar]
- Espley R, Hellens R, Putterill J, Stevenson D, Kutty-Amma S, Allan A (2007) Red coloration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J 49 414–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feucht W, Treutter D, Polster J (2004) Flavanol binding of nuclei from tree species. Plant Cell Rep 22 430–436 [DOI] [PubMed] [Google Scholar]
- Fillion L, Ageorges A, Picaud S, Coutos-Thévenot P, Lemoine R, Romieu C, Delrot S (1999) Cloning and expression of a hexose transporter gene expressed during the ripening of grape berry. Plant Physiol 120 1083–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewold E (2006) The genetics and biochemistry of floral pigments. Annu Rev Plant Biol 57 761–780 [DOI] [PubMed] [Google Scholar]
- Grotewold E, Sainz MB, Tagliani L, Hernandez JM, Bowen B, Chandler VL (2000) Identification of the residues in the Myb domain of maize C1 that specify the interaction with the bHLH cofactor R. Proc Natl Acad Sci USA 97 13579–13584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D, De Luca V (2007) Mesocarp localization of a bi-functional resveratrol/hydroxycinnamic acid glucosyltransferase of Concord grape (Vitis labrusca). Plant J 49 579–591 [DOI] [PubMed] [Google Scholar]
- Hayes MA, Davies C, Dry IB (2007) Isolation, functional characterization, and expression analysis of grapevine (Vitis vinifera L.) hexose transporters: differential roles in sink and source tissues. J Exp Bot 58 1985–1997 [DOI] [PubMed] [Google Scholar]
- Ikegami A, Eguchi S, Kitajima A, Inoue K, Yonemori K (2007) Identification of genes involved in proanthocyanidin biosynthesis of persimmon (Diospyros kaki) fruit. Plant Sci 172 1037–1047 [Google Scholar]
- Jaillon O, Aury JM, Noel B, Policriti A, Clepet C, Casagrande A, Choisne N, Aubourg S, Vitulo N, Jubin C, et al (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449 463–467 [DOI] [PubMed] [Google Scholar]
- Jeong ST, Goto-Yamamoto N, Kobayashi S, Esaka M (2004) Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Sci 167 247–252 [Google Scholar]
- Jin H, Cominelli E, Bailey P, Parr A, Mehrtens F, Jones J, Tonelli C, Weisshaar B, Martin C (2000) Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis. EMBO J 19 6150–6161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Inze D, Depicker A (2002) Gateway vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7 193–195 [DOI] [PubMed] [Google Scholar]
- Kennedy JA, Hayasaka Y, Vidal S, Waters EJ, Jones GP (2001) Composition of grape skin proanthocyanidins at different stages of berry development. J Agric Food Chem 49 5348–5355 [DOI] [PubMed] [Google Scholar]
- Kitamura S, Shikazono N, Tanaka A (2004) TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. Plant J 37 104–114 [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Goto-Yamamoto N, Hirochika H (2004) Retrotransposon-induced mutations in grape skin color. Science 304 982. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Ishimaru M, Hiraoka K, Honda C (2002) Myb related genes of the Kyoho grape (Vitis labruscana) regulate anthocyanin biosynthesis. Planta 215 924–933 [DOI] [PubMed] [Google Scholar]
- Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5 150–163 [DOI] [PubMed] [Google Scholar]
- Lapierre C, Pollet B, Rolando R (1995) New insights into the molecular architecture of hardwood lignins by chemical degradation methods. Res Chem Intermediat 21 397–412 [Google Scholar]
- Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, Caboche M (2006) Genetics and biochemistry of seed flavonoids. Annu Rev Plant Biol 57 405–430 [DOI] [PubMed] [Google Scholar]
- Li AX, Steffens JC (2000) An acyltransferase catalyzing the formation of diacylglucose is a serine carboxypeptidase-like protein. Proc Natl Acad Sci USA 97 6902–6907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mané C, Souquet JM, Olle D, Verriès C, Veran F, Mazerolles G, Cheynier V, Fulcrand H (2007) Optimization of simultaneous flavanol, phenolic acid, and anthocyanin extraction from grapes using an experimental design: application to the characterization of champagne grape varieties. J Agric Food Chem 55 7224–7233 [DOI] [PubMed] [Google Scholar]
- Marinova K, Pourcel L, Weder B, Schwarz M, Barron D, Routaboul JM, Debeaujon I, Klein M (2007) The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell 19 2023–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews H, Clendennen S, Caldwell C, Liu XL, Connors K, Matheis N, Schuster D, Menasco D, Wagoner W, Lightner J, Ry Wagner D (2003) Activation tagging in tomato identifies a transcriptional regulator of anthocyanin biosynthesis, modification, and transport. Plant Cell 15 1689–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus JT, Aquea F, Arce-Johnson P (2008) Analysis of the grape MYB R2R3 subfamily reveals expanded wine quality-related clades and conserved gene structure organization across Vitis and Arabidopsis genomes. BMC Plant Biol 8 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milkowski C, Strack D (2004) Serine carboxypeptidase-like acyltransferases. Phytochemistry 65 517–524 [DOI] [PubMed] [Google Scholar]
- Nakashima J, Chen F, Jackson L, Shadle G, Dixon RA (2008) Multi-site genetic modification of monolignol biosynthesis in alfalfa (Medicago sativa): effects on lignin composition in specific cell types. New Phytol 179 738–750 [DOI] [PubMed] [Google Scholar]
- Nesi N, Jond C, Debeaujon I, Caboche M, Lepiniec L (2001) The Arabidopsis TT2 gene encodes an R2R3 MYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed. Plant Cell 13 2099–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Peel GJ, Sharma SB, Tang Y, Dixon RA (2008) A transcript profiling approach reveals an epicatechin-specific glucosyltransferase expressed in the seed coat of Medicago truncatula. Proc Natl Acad Sci USA 105 14210–14215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Peel GJ, Wright E, Wang Z, Dixon RA (2007) Early steps in proanthocyanidin biosynthesis in the model legume Medicago truncatula. Plant Physiol 145 601–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolocci F, Robbins MP, Madeo L, Arcioni S, Martens S, Damiani F (2007) Ectopic expression of a basic helix-loop-helix gene transactivates parallel pathways of proanthocyanidin biosynthesis: structure, expression analysis, and genetic control of leucoanthocyanidin 4-reductase and anthocyanidin reductase genes in Lotus corniculatus. Plant Physiol 143 504–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer J, Kuhnel C, Brandt J, Duy D, Punyasiri P, Forkmann G, Fischer T (2006) Biosynthesis of flavan 3-ols by leucoanthocyanidin 4-reductases and anthocyanidin reductases in leaves of grape (Vitis vinifera L.), apple (Malus domestica Borkh.) and other crops. Plant Physiol Biochem 44 323–334 [DOI] [PubMed] [Google Scholar]
- Prieur C, Rigaud J, Cheynier V, Moutounet M (1994) Oligomeric and polymeric procyanidins from grape seeds. Phytochemistry 36 781–784 [Google Scholar]
- Quattrocchio F, Verweij W, Kroon A, Spelt C, Mol J, Koes R (2006) PH4 of Petunia is an R2R3 MYB protein that activates vacuolar acidification through interactions with basic-helix-loop-helix transcription factors of the anthocyanin pathway. Plant Cell 18 1274–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid KE, Olsson N, Schlosser J, Peng F, Lund ST (2006) An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol 14 6–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders A, Sivitz AB, Starker CG, Gantt JS, Ward JM (2008) Functional analysis of LjSUT4, a vacuolar sucrose transporter from Lotus japonicus. Plant Mol Biol 68 289–299 [DOI] [PubMed] [Google Scholar]
- Souquet JM, Cheynier V, Brossaud F, Moutounet M (1996) Polymeric proanthocyanidins from grape skins. Phytochemistry 43 509–512 [Google Scholar]
- Souquet JM, Veran F, Mané C, Cheynier V (2006) Optimization of extraction conditions on phenolic yields from the different parts of grape clusters: quantitative distribution of their proanthocyanidins. In F Daayf, A El Hadrami, L Adam, GM Ballance, eds, XXIII International Conference on Polyphenols, Winnipeg, Manitoba, Canada. Groupe Polyphénols, Bordeaux, France, pp 245–246
- Stracke R, Ishihara H, Huep G, Barsch A, Mehrtens F, Niehaus K, Weisshaar B (2007) Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J 50 660–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R, Werber M, Weisshaar B (2001) The R2R3-MYB gene family in Arabidopsis thaliana. Curr Opin Plant Biol 4 447–456 [DOI] [PubMed] [Google Scholar]
- Su C, Singleton V (1969) Identification of three flavan-3-ols from grapes. Phytochemistry 8 1553–1558 [Google Scholar]
- Takos A, Jaffe F, Jacob S, Bogs J, Robinson S, Walker A (2006) Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol 142 1216–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner GJ, Francki KT, Abrahams S, Watson JM, Larkin PJ, Ashton AR (2003) Proanthocyanidin biosynthesis in plants: purification of legume leucoanthocyanidin reductase and molecular cloning of its cDNA. J Biol Chem 278 31647–31656 [DOI] [PubMed] [Google Scholar]
- Terrier N, Glissant D, Grimplet J, Barrieu B, Abbal A, Couture C, Ageorges A, Atanassova R, Léon C, Renaudin JP, et al (2005) Isogene specific oligo arrays reveal multifaceted changes in gene expression during grape berry (Vitis vinifera L.) development. Planta 222 832–847 [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrosa L, Bouquet A (1997) Agrobacterium tumefaciens and Agrobacterium rhizogenes co-transformation to obtain grapevine hairy roots producing the coat protein of grapevine chrome mosaic nepovirus. Plant Cell Tissue Organ Cult 49 53–62 [Google Scholar]
- Torregrosa L, Verriès C, Tesniere C (2002) Grapevine (Vitis vinifera L.) promoter analysis by biolistic mediated transient transformation of cell suspensions. Vitis 41 27–32 [Google Scholar]
- Velasco R, Zharkikh A, Troggio M, Cartwright DA, Cestaro A, Pruss D, Pindo M, Fitzgerald LM, Vezzulli S, Reid J, et al (2007) A high quality draft consensus sequence of the genome of a heterozygous grapevine variety. PLoS One 19 e1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdonk JC, Haring MA, van Tunen AJ, Schuurink RC (2005) ODORANT1 regulates fragrance biosynthesis in petunia flowers. Plant Cell 17 1612–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verriès C, Guiraud JL, Souquet JM, Vialet S, Terrier N, Ollé D (2008) Validation of an extraction method on whole pericarp of grape berry (Vitis vinifera L. cv. Shiraz) to study biochemical and molecular aspects of flavan-3-ol synthesis during berry development. J Agric Food Chem 56 5896–5904 [DOI] [PubMed] [Google Scholar]
- Walker AR, Lee E, Bogs J, McDavid DAJ, Thomas MR, Robinson SP (2007) White grapes arose through the mutation of two similar and adjacent regulatory genes. Plant J 49 772–785 [DOI] [PubMed] [Google Scholar]
- Wei YL, Li JN, Lu J, Tang ZL, Pu DC, Chai YR (2007) Molecular cloning of Brassica napus TRANSPARENT TESTA 2 gene family encoding potential MYB regulatory proteins of proanthocyanidin biosynthesis. Mol Biol Rep 34 105–120 [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley B (2002) Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol 5 218–223 [DOI] [PubMed] [Google Scholar]
- Xie DY, Dixon RA (2005) Proanthocyanidin biosynthesis: still more questions than answers? Phytochemistry 66 2127–2144 [DOI] [PubMed] [Google Scholar]
- Zhang F, Gonzalez A, Zhao M, Payne CT, Lloyd A (2003) A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development 130 4859–4869 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.