Abstract
In the mammalian gastrointestinal tract, the cell fate decisions that specify the development of multiple, diverse lineages are governed in large part by interactions of stem and early lineage progenitor cells with their microenvironment, or niche. Here, we show that the gastric parietal cell (PC) is a key cellular component of the previously undescribed niche for the gastric epithelial neck cell, the progenitor of the digestive enzyme secreting zymogenic (chief) cell (ZC). Genetic ablation of PCs led to failed patterning of the entire zymogenic lineage: progenitors showed premature expression of differentiated cell markers, and fully differentiated ZCs failed to develop. We developed a separate mouse model in which PCs localized not only to the progenitor niche, but also ectopically to the gastric unit base, which is normally occupied by terminally differentiated ZCs. Surprisingly, these mislocalized PCs did not maintain adjacent zymogenic lineage cells in the progenitor state, demonstrating that PCs, though necessary, are not sufficient to define the progenitor niche. We induced this PC mislocalization by knocking out the cytoskeleton-regulating gene Cd2ap in Mist1−/− mice, which led to aberrant E-cadherin localization in ZCs, irregular ZC-ZC junctions, and disruption of the ZC monolayer by PCs. Thus, the characteristic histology of the gastric unit, with PCs in the middle and ZCs in the base, may depend on establishment of an ordered adherens junction network in ZCs as they migrate into the base.
Keywords: Bhlhb8, mucous neck cell, CD2AP, niche, chief cell
Introduction
Many of the hallmark properties of stem cells are regulated by their interactions with closely apposed supporting cells (Schofield, 1978; Xie and Li, 2007). These non-dividing, supporting cells, along with structural extracellular components including the basement membrane (BM), constitute the stem cell niche. The location of stem cells is relatively well established in the gastrointestinal tract (Huh et al., 2006). In the intestine, the components of the niche have been inferred, and molecular interactions within the niche have been studied (Mills and Gordon, 2001; Moore and Lemischka, 2006; Yen and Wright, 2006; Brown et al., 2007). The stomach epithelium also undergoes continuous renewal throughout adulthood, and this renewal is prone to disruption that can lead to gastric cancer, the second leading cause of cancer death worldwide (Parkin et al., 2005), yet the niche supporting gastric epithelial stem and progenitor cells has not been previously characterized.
The stem cell of the epithelium in the stomach antrum, which has an architecture that in many ways resembles that of the colon, resides in the base of the gastric unit and has been reported to express the putative intestinal stem cell marker Lgr5 (Barker et al., 2007). The stem cell in the body or corpus of the stomach, on the other hand, is unique along the gastrointestinal tract, as it resides in the upper middle (isthmus) of each repeating epithelial invagination, or gastric unit (Fig. 1A,B). The corpus stem cell gives rise to four functionally distinct cell lineages: parietal, surface mucous (pit), zymogenic, and enteroendocrine. The zymogenic lineage and its microenvironment are particularly amenable to developmental analysis, because zymogenic lineage differentiation proceeds in an orderly spatiotemporal pattern in which the degree of maturation correlates with distance migrated from the stem cell. The zymogenic progenitor cell, known as the mucous neck cell (NC), migrates through the neck, or middle, of the gastric unit towards the gastric unit base. These progenitors are long-lived (~14 days) and express markers that distinguish them from both the stem cell and the mature zymogenic cells (ZCs) to which they will ultimately give rise in the base (Karam and Leblond, 1993b; Karam and Leblond, 1993a; Hanby et al., 1999; Ramsey et al., 2007).
Figure 1. Parietal cells are a key cellular component of the gastric epithelial progenitor niche.
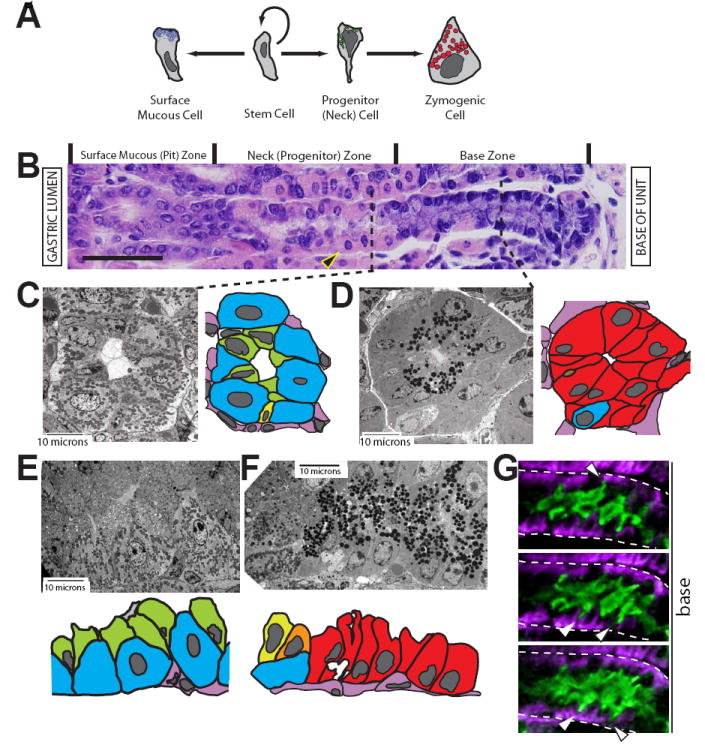
A, schematic of surface mucous and zymogenic lineage differentiation from a stem cell. The parietal and enteroendocrine lineages are not depicted. B, gastric unit stained with H&E. The unit opens out into the gastric lumen to the left; the lamina propria and muscle are to the right. Gastric unit zones are delineated above the image. The cells depicted in panel A are aligned with the zones in B in which they reside. The large eosinophilic (pink) cells are parietal cells (PCs, e.g., arrowhead). Scale bar = 50 μM. C-F, tEM images of gastric unit zones with accompanying cartoon traces of the EM image. PCs, blue; NCs, green; neck-to-zymogenic transitional (Ramsey et al., 2007), yellow/orange; ZCs, red; mesenchymal/endothelial cells, pink. C, cross section through the neck zone. Note how NCs (green) line the lumen apically but have scant basement membrane contact. D, cross section through the base zone. E-F, longitudinal sections through the progenitor (E) and base (F) zones. Note how the progression from NC to ZC involves not only a switch from electron lucent (mucous) to electron dense (zymogenic) granules but also substantial expansion of contact with the basement membrane. G, three serial slices from two-photon, 3-dimensional imaging of an entire gastric unit. The neck zone is shown, with NCs in green and PCs in purple. Note the thin NC projections (arrowheads) towards the basement membrane (dashed white line).
While many of the morphological features of the normal transition from NC to ZC have been described, the cellular and molecular events that regulate NC to ZC differentiation remain unclear. Previous results from our lab (Ramsey et al., 2007) identified the first gene involved in coordinating this conversion, Bhlhb8 (Mist1), which encodes the ZC-specific transcription factor Mist1 (Pin et al., 2000; Johnson et al., 2004). Mist1−/− ZCs exhibit defects in cell shape, organelle localization, and zymogenic granule homeostasis (Ramsey et al., 2007).
Additionally, parietal cells (PCs) are known to influence ZC differentiation. When PCs are lost, as can occur in the setting of chronic inflammation, mature ZCs do not form (Nomura et al., 2005; Nozaki et al., 2008). In humans, loss of PCs and associated loss of ZCs are thought to predispose the gastric epithelium to the development of gastric cancer (Merchant, 2005). The mechanisms by which PCs regulate zymogenic lineage differentiation, however, are not known.
The transition between NC and terminally differentiated ZC also correlates with a notable change in gastric unit architecture (Fig. 1C-F). Therefore, motility and cell shape change are likely to be important for the reorganization that occurs at this transition; however, the factors involved have not been elucidated. It has been demonstrated in vitro that the motility of gastric epithelial cells depends upon the multifunctional adaptor protein Cd2ap (Mustonen et al., 2005). This scaffolding molecule interacts with central cytoskeletal components involved in cell shape and motility; also, Cd2ap and its orthologues have been implicated in the control of cell migration and shape change in multiple systems (Dustin et al., 1998; Hutchings et al., 2003; Lehtonen et al., 2004; Welsch et al., 2005; Bruck et al., 2006; Johnson et al., 2008). A role for Cd2ap in vivo in gastrointestinal epithelial differentiation has not been shown.
Here, we show that PCs are abundant, non-dividing cells within the neck zone and are thus ideally situated to directly instruct NCs either by regulating access to circulating factors, by cell-cell interactions, or by secretion of paracrine factors. We find that genetic ablation of PCs led to abnormal gastric units characterized by 1) NCs that prematurely expressed differentiated (i.e., ZC) markers and 2) an almost complete absence of mature ZCs. Thus, PCs are required both for terminal ZC formation and for maintenance of NCs in an undifferentiated state. We also generated mice doubly deficient for Cd2ap and Mist1 and show that gastric units from these mice exhibited mislocalized E-cadherin in ZCs, leading in turn to irregular ZC-ZC junctional complexes that allowed substantial extension of PCs from the neck zone into the base. Surprisingly, despite greatly increased contact of PCs with these cytoskeletally aberrant cells, the cells still downregulated progenitor markers and upregulated terminally differentiated ZC markers in a pattern largely indistinguishable from wild-type. Therefore, we propose a model wherein PCs act indirectly or systemically to control proper development and patterning of the zymogenic lineage, from early progenitor to the terminally differentiated state.
Materials and Methods
Mice
All experiments involving animals were performed according to protocols approved by the Washington University School of Medicine Animal Studies Committee. Germline Mist1−/− mice were generated as described previously (Pin et al., 2001). Cd2ap−/− mice with a podocyte-specific Cd2ap transgene were generated as described (Grunkemeyer et al., 2005). Mist1−/−Cd2ap−/− double-deficient mice were generated by crossing Mist1+/−Cd2ap+/−tg+ or Mist1−/−Cd2ap+/−tg+ mice, and genotyping was performed by PCR. Both parental strains had been extensively (>10 times) backcrossed onto C57BL/6 genetic background. The tox176 mice and their wild-type littermates (used as controls for all tox176 experiments), gifts of Dr. Jeffrey Gordon (Washington University), were housed in a germ-free facility (Hooper et al., 2002). All other mice were maintained in a specific pathogen free barrier facility.
Electron and mutliphoton microscopy
For transmission electron microscopic studies, stomachs were fixed, sectioned, stained, and imaged as described (Ramsey et al., 2007). Cartoon traces of EM images were performed using Adobe Illustrator (Adobe Systems, San Jose, CA).
For multiphoton microscopic analysis, stomachs were fixed in formalin and <1 mm slices were blocked in 1% BSA, 0.5% Triton X-100. Slices were incubated with rabbit anti-H+/K+ ATPase and goat anti-gastric intrinsic factor (GIF) antibodies for 48 hours at 4°C, then incubated overnight with Alexa Fluor 488 conjugated Griffonia simplicifolia-II (GS-II) and Alexa Fluor 594 conjugated anti-rabbit and Alexa Fluor 350 conjugated anti-goat secondary antibodies (Invitrogen, Carlsbad, CA). Stomach tissue was then imaged with a custom built dual laser video-rate multiphoton microscope as previously described (Zinselmeyer et al., 2008). Samples were simultaneously excited at wavelengths of 780 nm and 820 nm. Fluorescence emission was separated by two dichroic mirrors (Semrock, Rochester, NY) with cutoff wavelengths of 505 nm and 560 nm.
Immunofluorescence
Stomachs were prepared and stained as described (Ramsey et al., 2007). Briefly, stomachs were inflated with freshly prepared methacarn fixative and suspended in fixative for 15-30 minutes at RT, followed by multiple rinses in 70% ETOH, arrangement in 2% agar in a tissue cassette, and routine paraffin processing. Sections (5 μm) were deparaffinized and rehydrated, then antigen retrieval was performed by boiling in 50 mM Tris-HCl, pH 9.0 or, for Mist1 staining, in Trilogy reagent (CellMarque, Rocklin, CA). Slides were blocked in 1% BSA, 0.3% Triton-X100 in PBS, then incubated in primary followed by secondary antibodies (see below) and/or with the fluorescently labeled lectins GS-II or Dolichos biflorus (DBA, parietal cells) (Alexa Fluor 488, 594 or 647, Invitrogen). Slides were incubated 5 minutes in 1 μg/ml bisbenzimide (Invitrogen) prior to mounting in 1:1 glycerol:PBS. Fluorescence microscopy and imaging were performed as described (Ramsey et al., 2007).
The following primary antibodies were used for immunostaining in this study: rabbit (1:10,000) and goat (1:2000) anti-human gastric intrinsic factor (GIF) (gifts of Dr. David Alpers, Washington University), monoclonal mouse anti-Tff2 IgM (1:10, gift of Sir Nicholas Wright, London Research Institute), rabbit anti-Mist1 (1:500, described previously (Pin et al., 2001), gift of Dr. Steve Konieczny, Purdue University), rabbit anti-H+/K+ ATPase α subunit (1:1000, gift of Dr. Michael Caplan (Blostein et al., 1993), Yale University); goat anti-VEGF-B (1:100, sc-1876, Santa Cruz Biotechnology, Santa Cruz, CA), goat anti-bromodeoxyuridine (BrdU, (1:10,000, gift of Dr. Jeff Gordon, Washington University), rabbit anti-Cd2ap (1:5000, (Grunkemeyer et al., 2005)), monoclonal mouse anti-E-cadherin (1:150, BD Biosciences), and rabbit anti-actin (1:200, gift of Dr. Thaddeus Stappenbeck, clone AC-40; Sigma, St Louis, MO). Secondary antibodies used were: Alexa Fluor 488, 594 or 647 conjugated donkey anti-goat, anti-rabbit, or anti-mouse (1:500, Invitrogen). Cartoon traces of immunofluorescent images were performed using Adobe Illustrator (Adobe Systems, San Jose, CA).
BrdU counts
Four tox176 mice and four nontransgenic littermate controls, ~9 weeks old, were injected with a BrdU solution (8mg/ml BrdU, 0.8 mg/ml FDU) 90 minutes before sacrificing. The corpora of these mice were prepared and immunostained as previously described (Ramsey et al., 2007 and above). Stomach sections were costained with anti-BrdU and anti-GIF antibodies as well as GS-II and bisbenzimide. The numbers of BrdU+ cells and BrdU+ GIF+ cells were scored for every well oriented unit (an average of 440 units per wild-type mouse and 200 units per tox176 mouse). The mean numbers of BrdU+ cells and BrdU+ GIF+ cells per unit were calculated for each mouse. GraphPad Prism 3.02 (GraphPad Software, San Diego, CA) was used for statistical analysis and graph plotting.
Antral RNA extraction and quantitative PCR
Four tox176 mice and six nontransgenic littermate controls were sacrificed at ~9 weeks of age. Antra were removed immediately and snap frozen in liquid nitrogen, then crushed to a fine powder using mortar and pestle under liquid nitrogen. The powdered tissue was solubilized and RNA was isolated using an RNAeasy kit (Qiagen, Valencia, CA). cDNA was synthesized using Superscript III (Invitrogen) with random primers. Quantitative real-time PCR (qRT-PCR) was performed using SYBR Green Mix (ABgene, Rochester, NY) and gene-specific primers on an Mx3000P (Stratagene, La Jolla, CA). The following primers were used to quantify Gastrin mRNA: forward ACA CAA CAG CCA ACT ATT C, reverse CAA AGT CCA TCC ATC CGT AG (Jain et al., 2008).
For all qRT-PCR analysis, we used the following methods to analyze and plot data. In each PCR run, we used, as an internal control for every sample, 18s rRNA primers: forward CAT TCG AAC GTC TGC CCT ATC, reverse CCT GTG CCT TCC TTG GA. Differences in 18s RNA Ct between each sample were used to normalize each gene-specific Ct for overall starting RNA concentration. To establish a background Ct at which primer pairs amplified non-specific message, we also ran a no cDNA (“water”) control for each set of gene-specific primers. The background Ct was usually between 37 and 40 (which was the maximum number of cycles run). To plot “PCR cycles above background” (as in Fig. 3), the Ct from each gene-specific primer for each sample was normalized to all other samples by relative 18s Ct and then subtracted from the background Ct. In this way, plots are essentially Log2 scale (because each cycle means ~2-fold increase in amplicon), and higher numbers = higher amounts of starting RNA.
Figure 3. Cellular proliferation is increased in tox176 gastric units.
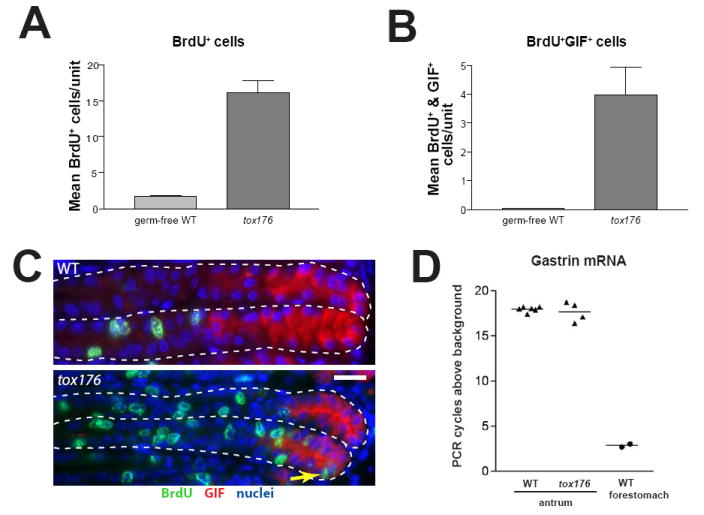
A, quantification of mean (±SEM) bromodeoxyuridine (BrdU) positive cells per gastric unit in corpus (n=4 mice/genotype). B, quantification of mean (±SEM) BrdU and GIF double-positive cells per gastric unit. C, WT (top) and tox176 (bottom) gastric units immunostained with anti-BrdU (green); anti-GIF (red); and bisbenzimide (blue). The presence of BrdU+/GIF+ cells in the base of the tox176 gastric units (arrow) highlights the rapid turnover and altered differentiation pattern of the zymogenic lineage in the absence of PCs. Scale bar = 20μm. D, WT and tox176 Gastrin mRNA levels in whole antrum resections, measured by qRT-PCR. Each data point represents a single mouse. Shown is the number of PCR cycles required to detect the Gastrin amplicon in a given mouse subtracted from the number of cycles required for non-specific amplification with Gastrin primers. (See Methods) Therefore, higher numbers represent more abundant message. PCR products from forestomach mRNA, which contains negligible numbers of Gastrin producing cells, are identifiable only a few cycles above baseline. Gastrin messages in tox176 and WT mice are abundant but virtually equal.
Parietal cell (PC) counts
Stomach sections were costained with GS-II, DBA, anti-GIF antibody, and bisbenzimide. The number of PCs basal to the last GS-II+ cell was scored for every tenth well-oriented unit; 10 or more units were counted from each of 3-4 mice for all genotypes (n = 4 for Mist1−/−Cd2ap−/−). All graphing and statistical analysis was performed using GraphPad Prism 3.02 (GraphPad Software, San Diego, CA).
Immunofluorescence quantification
All quantification was performed on fluorescence images of well-oriented gastric units (longitudinally sectioned) using Adobe Photoshop software (Adobe Systems). For PC density calculations, stomach sections were costained with GS-II and antibodies against gastric intrinsic factor (GIF) and VEGF-B (which is parietal cell-specific in the stomach (Mills et al., 2001)). PCs of the base were defined as VEGF-B+ cells beneath the neck zone that were not adjacent to GS-II+ cells. The base zone VEGF-B+ cells of a single unit were individually lassoed to calculate the total PC area in the base. This was divided by the total area of the base in the section (defined as all cells beneath the last PC adjacent to a GS-II+ cell) to yield the PC occupation percentage of the base. At least 30 bases were quantified per animal, and 2-3 animals were analyzed for each genotype.
Quantification of the zymogenic lineage differentiation markers GS-II and GIF along the gastric unit was performed as described (Ramsey et al., 2007). Briefly, stomach sections were costained with GS-II and anti-GIF antibody. On fluorescence images, individual neck and zymogenic cell cytoplasms were lassoed and mean fluorescence intensities plotted for the two markers. A cell in the middle of the transitional zone (defined as the closest cell to the stem cell with GS-II >30 and GIF >30) was designated cell position 0. The weighted average of nine nearest neighbors was used as a graphical curve fitting and smoothing method. At least 15 total units were measured from each group shown (≥3 mice/group).
Transfection and gene expression analysis
1 × 106 AGS or HGC-27 cells were transfected with 1.5 μg pmaxGFP plus 3 μg Mist1 cDNA or 3 ug empty vector using a Nucleofector (Amaxa, Gaithersburg, MD), program B-023, and cell line transfection solution V. Cells were sorted into GFP− or GFP+ populations using a FACSVantage (Becton-Dickinson, San Jose, CA) at 24 hours post-transfection, then replated for an additional 24 hours before harvesting.
For Western blot analysis, cells were lysed in RIPA buffer (Sigma, St. Louis, MO). Proteins were separated on a NuPAGE 4-12% polyacrylamide gel (Invitrogen) and transferred to PVDF. Primary antibodies used for blotting were rabbit anti-Mist1 (see above) and mouse anti-β-actin (A4316, Sigma). Secondary antibodies used were IRDye 800 conjugated donkey anti-rabbit IgG (Rockland, Gilbertsville, PA) and Alexa Fluor 680 conjugated goat anti-mouse IgG (Invitrogen).
Quantitative PCR was performed as described above, using the following human gene-specific primers. Mist1: forward CGG ATG CAC AAG CTA AAT AAC G, reverse CCG TCA GCG ATT TGA TGT AGT TC; CD2ap: forward TTC GAG GAA TTG GAT TTG GAG AC, reverse TCC ACA CTC TGA GTT TTG GGT; E-cadherin: forward CGA GAG CTA CAC GTT CAC GG, reverse GGC CTT TTG ACT GTA ATC ACA CC, based on sequences from PrimerBank (Wang and Seed, 2003).
Results
Zymogenic progenitor cells reside in a niche comprising almost exclusively parietal cells
Mucous neck cells (NCs) are the progenitors of mature, terminally-differentiated zymogenic cells (ZCs) (Suzuki et al., 1983; Karam and Leblond, 1993b; Ramsey et al., 2007). Neither the niche in which NCs reside nor the cellular or molecular mechanisms that initiate the transition of NC to ZC has been characterized. Based on the hypothesis that the NC microenvironment would influence this NC to ZC transition, we undertook a careful examination of the cellular composition of the NC/progenitor niche.
We found that NCs in the neck zone were largely confined to the gastric unit lumen and were surrounded by parietal cells (PCs). Specifically, PCs dominated the interface of the gastric epithelium with the basement membrane (BM) (Fig. 1B, C and E). Analysis of multiple transmission electron microscopy (tEM) images (Fig. 1C, Fig. S1, and data not shown) revealed that NCs make contact with the BM only by thin cellular processes or pedestals, with the bulk of the NC volume oriented towards the lumen. NCs make extensive contact with PCs at their basolateral surfaces and contact each other near their apical membranes (Fig. 1B, C and E).
To examine neck cell contacts with the BM more closely, we imaged whole gastric units in three dimensions by multiphoton excitation microscopy. Sequential optical slices were captured and rendered into three-dimensional reconstructions of gastric unit neck zones (Supplemental Movie). All NCs observed contacted the BM by thin processes visible in only one or two adjacent optical slices (Fig 1G). In all units examined, the same pattern was seen: PCs lined the BM, and NCs oriented the majority of their cytoplasm towards the gastric unit lumen. Thus, our histological approaches show the neck zone to be a pseudostratified epithelium. Furthermore, our data indicate that NCs would have limited exposure to differentiation-regulating signals present at the BM (e.g. bound to the extracellular matrix) or emanating from capillary blood flow adjacent to the BM.
In contrast, differentiated ZCs, which reside in the base zone, were organized as a monolayer, exhibiting a columnar or pyramidal morphology and maintaining extensive BM contact (Fig. 1B, D, and F). In stark contrast to the neck zone, PCs were far less common in the base, resulting in only occasional ZC-PC contact.
The correlation of the NC phenotype—but not the ZC phenotype—with extensive PC contact suggests that PCs may regulate the NC to ZC transition. Consistent with such a role, PCs are known to secrete morphogens such as Sonic hedgehog (van den Brink et al., 2001), Tgfα (Beauchamp et al., 1989), and Parathyroid hormone like hormone (Pthlh) (Jain and Samuelson, 2007) and are thought to be critical for maintaining gastric homeostasis. Interestingly, however, while they are thought to be required for terminal ZC differentiation, their role in instructing NCs has not been studied (Sharp et al., 1995; van den Brink et al., 2001; Mills et al., 2002; Stepan et al., 2004; Faller and Kirchner, 2005; Nomura et al., 2005; Shiotani et al., 2005; Stepan et al., 2005).
PCs are critical for normal zymogenic progenitor and differentiated ZC development
To determine whether PCs are required for the development of NCs and the zymogenic lineage as a whole, we examined the effect of genetic ablation of PCs. We employed a well-established mouse model of PC ablation, tox176 (Li et al., 1996; Mills et al., 2002), in which the attenuated diphtheria toxin A is expressed under the control of the H+/K+ ATPase β subunit promoter, targeting its production specifically to PCs. Parietal cells in these mice undergo apoptosis at an early, pre-parietal cell stage, preventing the development of mature PCs. Studies of the tox176 mouse have indicated that mature ZCs do not form in the absence of PCs, but the effects on NCs and their transition to ZCs have not been extensively characterized.
To determine whether the absence of PCs affected zymogenic lineage patterning, we examined tox176 gastric units using lineage- and cell-specific markers. We used an antibody against gastric intrinsic factor (GIF) to label ZCs specifically and the NC-specific lectin GS-II to identify NCs in wild-type and tox176 mice (Fig 2A). H+/K+ ATPase staining confirmed that no intact PCs were present in tox176 stomachs. In wild-type gastric units, the vast majority of zymogenic lineage cells (i.e., NCs and/or ZCs) labeled strongly with either GS-II or anti-GIF (but not both). In contrast, we observed clear defects in the zymogenic lineage of tox176 mice. Most of these cells labeled with both markers, regardless of position (Fig. 2A, B). The staining pattern of the NC-specific factor, Trefoil factor 2 (Tff2), was identical to that of GS-II (data not shown). In addition, the average GS-II and anti-GIF staining intensity was consistently and reproducibly lower in tox176 gastric cells than in wild-type (note distribution of points in Fig. 2B upper panel vs. lower panel). Moreover, tEM analysis revealed that GS-II+GIF+ double-positive cells in the bases of tox176 gastric units were ultrastructurally abnormal (Fig. 2C). These cells exhibited a less compact and less organized rough endoplasmic reticulum (rER) relative to wild-type ZCs; they also contained fewer granules than wild-type ZCs (data not shown). Finally, tox176 zymogenic lineage cells largely failed to express the mature ZC marker Mist1 (Fig. 2D).
Figure 2. PCs are required for normal differentiation of both NCs and ZCs.
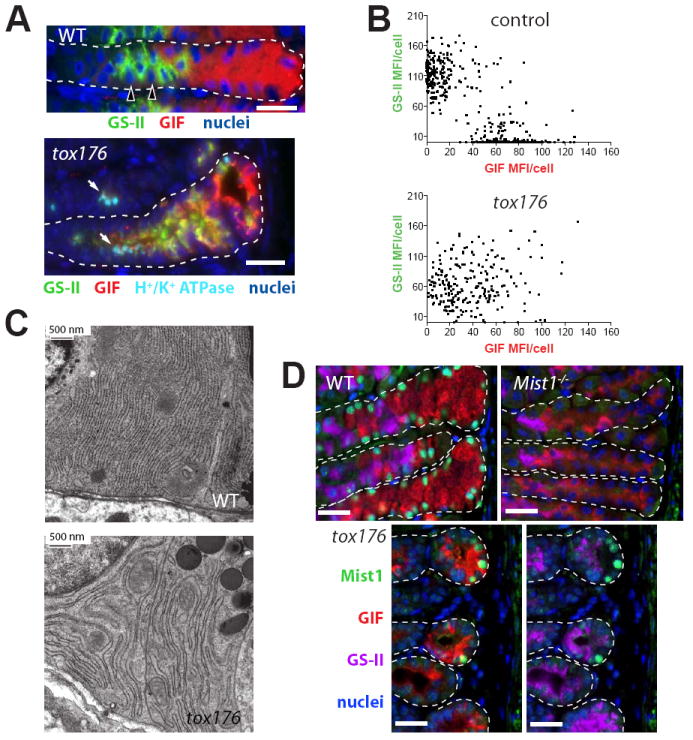
A, wild-type (WT) and tox176 stomachs immunostained with the NC-specific lectin Griffonia simplicifolia II (GS-II, green); anti-gastric intrinsic factor (GIF), a marker for murine ZCs (red); anti-H+/K+ ATPase (PC-specific; teal), and bisbenzimide (blue). Note that most cells in the tox176 unit are positive for both neck and zymogenic cell markers. The small foci of teal stain in tox176 (arrows) are remnants of apoptotic PCs; the PC channel is not shown in WT mice, so NC-basement membrane interactions are more easily seen (arrowheads indicate two PC nuclei). Scale bar = 20 μM. B, scatter plots of quantified GS-II and GIF mean fluorescence intensity (MFI) of individual cells in control (top) and tox176 (bottom) stomachs. While data points cluster at the axes in control stomachs, tox176 data points yield a diffuse pattern and reveal a lower average MFI in both channels. C, tEM images of rER in WT ZCs (top) and abnormal zymogenic lineage base zone cells from a tox176 stomach. Note the less organized, more distended rER in the tox176 cells. D, Mist1 expression in the gastric unit bases of WT, Mist1−/−, and tox176 stomachs (most of the neck zone is not shown). Stomach sections were stained with anti-Mist1 (green), GS-II (purple), anti-GIF (red), and bisbenzimide (blue). While all GIF+ cells in WT gastric units stained with anti-Mist1, only occasional Mist1+ cells were seen at the very base of tox176 units (note this field was selected to illustrate units with Mist1+ cells, and therefore overrepresents Mist1+ cell frequency). In the tox176 units, GIF and GS-II staining channels are shown separately. The Mist1−/− stomach is shown as an antibody specificity control. Scale bars = 20 μM.
Together, our data indicated that PCs are required not only for terminal ZC differentiation as expected, but unexpectedly, they are also important for regulation of early zymogenic progenitor cell differentiation. In the absence of PCs and PC-derived signals, the vast majority of tox176 zymogenic lineage cells adopt characteristics of both NCs and ZCs. Neither NC nor ZCs develop properly, indicating that PC-derived factors influence lineage fate choices during early NC development.
Loss of PCs leads to increased progenitor proliferation and proliferation of zymogenic lineage cells expressing GIF
In other models of PC dysfunction or loss, the gastric mucosa exhibits increased proliferation. We examined the proliferation status of tox176 stomachs, and found an ~8-fold increase in BrdU+ cells compared to wild-type stomachs (Fig 3A,C)(see also (Syder et al., 1999)). In wild-type units, the proliferating cells were largely seen in or near the isthmus—the normal proliferative zone—of the units, luminal to the GSII+ and GIF+ cells. However, in tox176 units, there was also a dramatic increase in the number of GIF+ cells in the base that were also BrdU+ (Fig. 3B). Normally, GIF+ cells are post-mitotic, and, indeed, GIF+BrdU+ cells were almost never observed in wild-type units (Fig. 3B, C). The appearance of GIF+ proliferating cells in the bases of gastric units is further indication of the aberrant progression of zymogenic lineage differentiation in the absence of PCs. The presence of GIF+BrdU+ cells and GSII+ cells in the bases of gastric units are characteristic features of pseudopyloric metaplasia, also known as spasmolytic polypeptide expressing metaplasia (SPEM; see, e.g., (Nozaki et al., 2008)).
The absence of PCs leads to achlorhydria in tox176 mice (Li et al., 1996; Syder et al., 2004) and in other models of PC loss (Sharp et al., 1995; Nomura et al., 2005). Loss of acid secretion can lead to hypergastrinemia, which has been postulated to be the cause of the hyperproliferation seen in some models (Nomura et al., 2005). Therefore, we measured Gastrin mRNA levels from tox176 mice and nontransgenic littermate controls (Fig. 3D). No elevation in mRNA levels was seen in transgenic mice, however, indicating that hypergastrinemia is unlikely to be the cause of the steady-state hyperproliferation observed in these mice.
Mist1 and Cd2ap cooperate to maintain distinct neck and base zone niches
Our findings from the tox176 model suggested that PCs are an important component of the progenitor cell niche, promoting proper NC development and actively maintaining NCs in an undifferentiated state. Once NCs migrate to the end of this PC-rich niche and into the base, they would be expected to escape PC-elaborated factors, causing them to differentiate into ZCs (Fig. 4).
Figure 4. Model for results expected when PCs expand out of the neck (progenitor) zone and into the base (differentiated) cell zone.
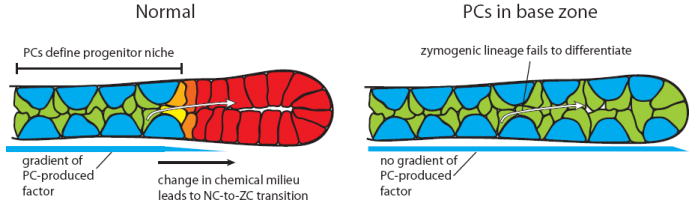
It has been assumed that PCs establish a chemical gradient or work by cell-cell contact to control terminal differentiation of the zymogenic lineage (left). If PCs were to be extended into the base zone (right), zymogenic lineage cells would be unable to escape this gradient and would fail to differentiate.
To test this hypothesis, we developed a mouse model in which PCs were redistributed throughout the gastric unit gland, leading to contact between PCs and zymogenic lineage cells in both the neck and base zones. Our previous work demonstrated that a deficiency in Mist1 resulted in a small increase in PCs in the base (VG Weis and JC Mills, unpublished observations), likely secondary to ZC cytoskeletal and cell shape defects (Ramsey et al., 2007). To identify other factors that would affect PC localization, we took a candidate gene approach and evaluated expression of the cytoskeletal adaptor CD2-associated protein (Cd2ap) in the gastric unit. This multifunctional scaffold protein has been implicated in gastric epithelial migration and in cell shape and motility of other cell types (Dustin et al., 1998; Hutchings et al., 2003; Lehtonen et al., 2004; Welsch et al., 2005; Bruck et al., 2006; Johnson et al., 2008). We found that Cd2ap was highly expressed at the luminal membrane in ZCs, but only at low levels in NCs (Fig. 5). Therefore, we examined Cd2ap−/− stomachs, but found no gross morphologic abnormalities in ZCs or elsewhere in the gastric unit (Fig. 5A).
Figure 5. Cd2ap protein expression increases as NCs transition into ZCs.
Stomach sections were stained with anti-Cd2ap (green), GS-II (blue), and anti-GIF. Note the strong luminal expression of Cd2ap in ZCs. The Cd2ap−/− section is shown as an antibody specificity control. Scale bars = 20 μM.
Interestingly, immunofluorescence analysis indicated a trend toward increased apical Cd2ap expression in Mist1−/− ZCs relative to wild-type ZCs (data not shown). We considered that Mist1 and Cd2ap might regulate overlapping ZC cytoskeletal pathways, and that the increase in Cd2ap expression in Mist1−/− mice might partially compensate for missing cytoskeletal mechanisms normally coordinated by Mist1. Therefore, we hypothesized that a deficiency in both Mist1 and Cd2ap would disable ZC cytoskeletal organization more dramatically than deficiency in Mist1 alone, thereby leading to disrupted base zone architecture and PC mislocalization.
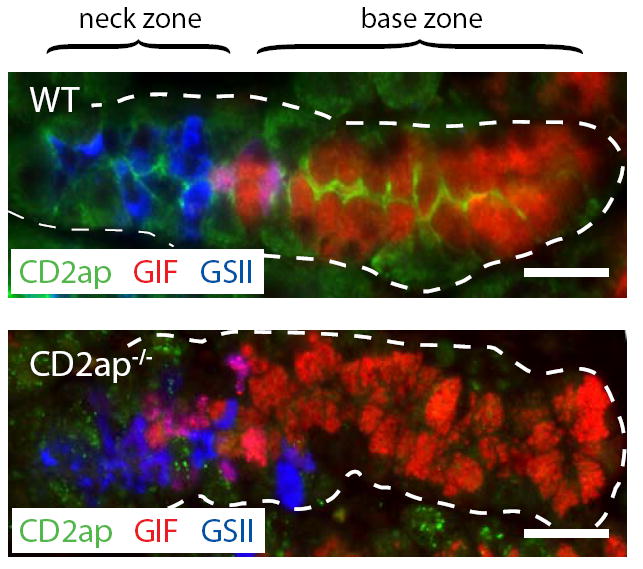
This proved to be true, as Mist1−/−Cd2ap−/− stomachs exhibited profoundly disrupted base zones. In fact, PCs were 2-3 times more common in Mist1−/−Cd2ap−/− base zones than in those of wild-type or Mist1−/− mice (Fig. 6A-C). Moreover, 20% of Mist1−/−Cd2ap−/− base zones exhibited PC density that approximated that of the average neck zone (Fig. 6D). This unusual base zone architecture and its similarity to the architecture of the neck are demonstrated by tEM (Fig. 6E and F). Fig. 6F also highlights the unusual shape of many of the presumably zymogenic lineage cells in the bases of Mist1−/−Cd2ap−/− units: like NCs, these cells made very little contact with the BM, and were instead squeezed between PCs.
Figure 6. In the Mist1−/− background, Cd2ap deficiency results in mislocalized PCs and disorganized base zones.
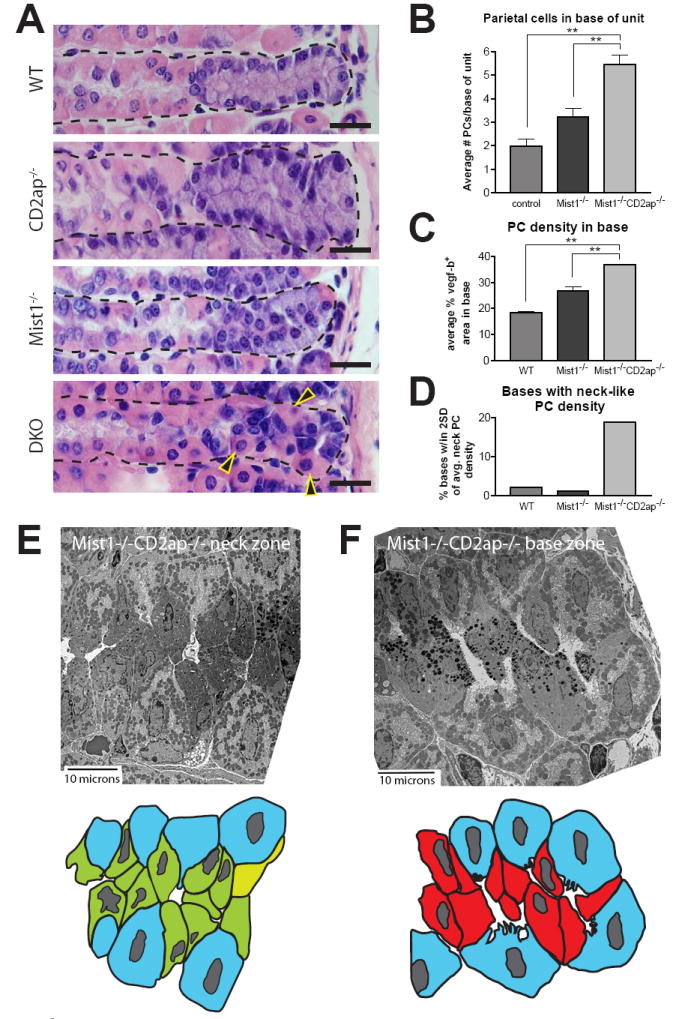
A, H&E stained sections of the genotypes indicated. Note the abundance of PCs (arrowheads) and extreme disorganization in the base of the Mist1−/−Cd2ap−/− unit. Scale bars = 20 μM. B, quantification of base zone PCs. C, base zone PC density calculations (see Methods). Both measures of PC presence in the base show 2-3 times the PC density in Mist1−/−Cd2ap−/− vs. WT bases. D, the fraction of units with base PC density approaching the density seen in the neck zone. Quantification compiled from 4 Mist1−/−Cd2ap−/−, 3 Mist1−/−, and 3 WT mice. E, tEMs and cartoon traces of Mist1−/−Cd2ap−/− progenitor and base zones. Note the similarity in architecture between the two zones.
Because it appeared that Mist1 and Cd2ap may interact or mediate overlapping pathways to regulate gastric unit architecture, we considered that Mist1, as a transcription factor, might modulate expression of Cd2ap. We examined this possibility by overexpressing Mist1 in two different human gastric epithelial cell lines, AGS (which has low levels of endogenous Mist1 mRNA) and HGC-27 (which does not normally express Mist1). We found that forced expression of high levels of Mist1 had no effect on expression of Cd2ap as measured by quantitative PCR in either cell line (Supplemental Figure 2). Rather than a direct interaction between the two genes, our data supports our hypothesis that Mist1 and Cd2ap act via parallel but overlapping pathways to maintain ZC shape and base zone architecture (see below).
ZC differentiation occurs in Mist1−/−Cd2ap−/− mice despite continued contact with PCs in the base
The presence of many PCs in the base zones of Mist1−/−Cd2ap−/− gastric units allowed us to test the hypothesis that PCs instruct NCs to remain progenitors and not differentiate into ZCs (Fig. 4). We took two approaches to test this model. First, we examined gastric unit patterning using lineage- and cell-specific markers as in Fig. 2A. Surprisingly, despite the presence of abundant PCs in Mist1−/−Cd2ap−/− gastric unit bases, the zymogenic lineage cells in the base were GIF+ and GS-II− (and Tff2−, data not shown) as in wild-type and Mist1−/− stomachs (Fig. 7A). Quantification of GS-II and anti-GIF fluorescence intensities relative to cell position clearly demonstrated that GS-II reactivity ended and GIF expression commenced at the start of the base zone in Mist1−/−Cd2ap−/− gastric units, as in wild-type and Mist1−/− units (Fig. 7B). Second, we examined Mist1−/−Cd2ap−/− gastric unit bases ultrastructurally. Despite distorted cell shape and cell-cell junctions (see also below), zymogenic lineage cells at the base of Mist1−/−Cd2ap−/− units exhibited abundant, well-organized rER like their wild-type and Mist1−/− counterparts (Fig. 7C). Zymogenic granules in Mist1−/−Cd2ap−/− cells resembled the small granules seen in Mist1−/− ZCs; no additional granule defect was seen. Therefore, these Mist1−/−Cd2ap−/− base zone cells were phenotypically ZCs in terms of lineage marker expression and features of their secretory apparatus.
Figure 7. Despite continued PC contact, ZCs develop in Mist1−/−Cd2ap−/− stomachs.
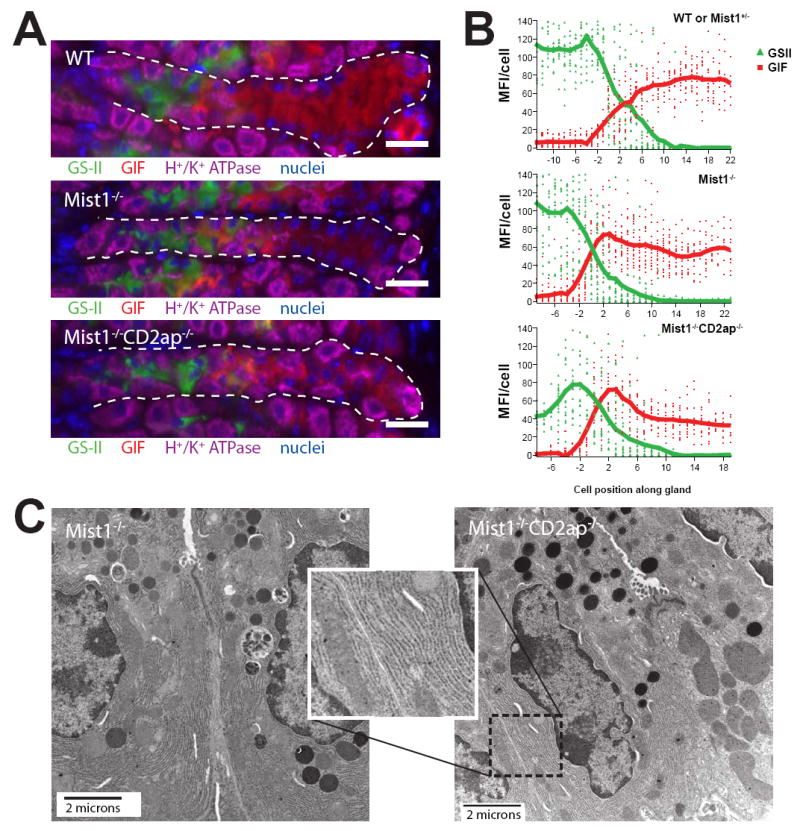
A, stomach sections immunostained with GS-II (green), anti-GIF (red), anti-H+/K+ ATPase (purple), and bisbenzimide (blue). Note that GS-II is shut off and GIF is turned on in the base zone of all genotypes, including Mist1−/−Cd2ap−/−. Scale bar = 20 μM. B, mean fluorescence values (GS-II, green; GIF, red) of individual cells along the length of ~15 glands (neck and base) from multiple mice were compiled to yield the trend lines shown (see Methods). Negative cell positions correspond to the neck zone; positive cell positions, to the base. C, tEM of Mist1−/− and Mist1−/−Cd2ap−/− ZCs. Note the abundant rER in cells of both genotypes and in the inset.
Taken together, these results demonstrate that while PCs are required for normal ZC and progenitor cell development, they are insufficient for the creation of a progenitor cell niche. Despite abundant PCs in the bases of Mist1−/−Cd2ap−/− units, including abundant ZC-PC contact and scant ZC contact with the BM, these cells faithfully downregulate GS-II-reactive markers and upregulate GIF at a defined point at the start of the base zone. Therefore, PCs do not simply serve as a sequestering or nurturing niche to maintain NCs in an undifferentiated state. Instead, whatever factors they elaborate to regulate zymogenic differentiation must work either systemically (i.e., the position of PCs is not particularly important) and/or indirectly through other cells and additional signals.
Loss of Cd2ap in a Mist1-deficient background leads to disrupted E-cadherin localization in ZCs and collapse of the pattern of regular cell-cell junctions and orientation
Our results demonstrated that disrupting the cytoskeletal maturation of ZCs led to ectopic localization of PCs into the base. These basal PCs intermingled with ZCs that, while morphologically abnormal, remained differentiated as assessed by their expression of normal ZC differentiation markers and lack of expression of NC markers. We next sought to understand the structural and cytoskeletal changes in the Mist1−/−Cd2ap−/− base that allowed PCs to successfully compete for this space.
Cd2ap is known to regulate subcellular localization of the key cell-cell adherens junction component E-cadherin (Mustonen et al., 2005; Johnson et al., 2008). Loss of Cd2ap in gastric mucosal cells in vitro leads to mislocalized E-cadherin and impaired motility of epithelial cells within the monolayer (Mustonen et al., 2005). In Drosophila, loss of the ortholog of Cd2ap, cindr, in pupil epithelial cells leads to irregular deposition of E-cadherin and inability of the Cd2ap-deficient cells to exclude other cells from their niche (Johnson et al., 2008).
To test whether similar abnormalities in E-cadherin occurred in Mist1−/−Cd2ap−/− ZCs, we took two approaches. First, we examined E-cadherin expression by immunofluorescence in wild-type, Mist1−/−, and Mist1−/−Cd2ap−/− gastric unit bases (Fig 8). In wild-type stomachs, we found that E-cadherin was localized to the lateral and apical borders of ZCs, on each side extending the full height of the ZC, perpendicular to the basement membrane and to the lumen. Mist1−/− gastric units exhibited a similar E-cadherin expression pattern, although ZCs were shorter on average. In Mist1−/−Cd2ap−/− units, however, E-cadherin expression appeared haphazard. Lateral E-cadherin staining often extended farther towards the lumen on one side of the ZC than the other, and rarely to the full height of the ZC, leading to an irregular apical epithelial surface and, thus, a tortuous lumen.
Figure 8. Loss of Cd2ap in Mist1−/− mice leads to irregular distribution of E-cadherin.
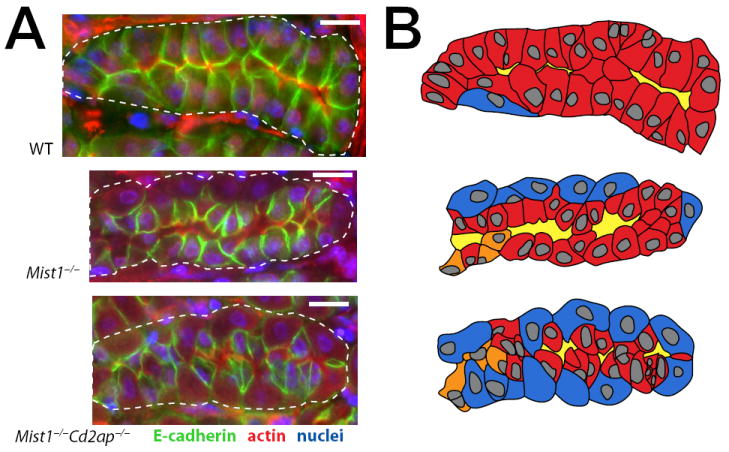
A, WT, Mist1−/−, and Mist1−/−Cd2ap−/− gastric unit bases immunostained with E-cadherin (green), actin (red), anti-GIF (channel not shown in panel), and bisbenzimide (blue). Note in the WT base, E-cadherin reveals that ZCs form ordered, junctional complexes with other ZCs. E-cadherin staining is perpendicular to the membrane. Because the cells are the same height, the lumen is regular and parallel to the basement membrane. In the Mist1−/− base, E-cadherin junctions are still perpendicular to the membrane, and ZCs mostly form junctional complexes with other ZCs. ZCs are of relatively consistent height. E-cadherin immunostaining tends to be more intense, although the cells are shorter. The shorter cells define a broader caliber lumen. Note, that more PCs are also in the base relative to WT. In the Mist1−/−Cd2ap−/− base, E-cadherin immunostaining shows that ZCs form junctional complexes with other ZCs much less frequently, and E-cadherin is haphazardly arranged with respect to the basement membrane. Cell height is difficult to determine due to the lack of consistent orientation of E-cadherin staining with respect to the baement membrane. E-cadherin staining intensity tends to be less intense. Due to irregular cell-cell interactions, the lumen is tortuous. Scale bars=15 μm. B, For clarification of base architecture, cartoons of the units in A are depicted. The ZCs (red) were distinguished by GIF staining from PCs (blue) and NC-ZC transitional cells (orange). The lumen is marked in yellow.
Next, we examined ZCs and their cell-cell junctions by EM. While wild-type ZCs formed tight and adherens junctions near the apex of the cell (Fig. 9A), junctional complexes in Mist1−/−Cd2ap−/− ZCs were positioned at variable distances from the BM, often well short of the full height of the ZC (Fig. 9B, C). This aberrant placement of junctional complexes yielded asymmetrical ZCs, deep clefts between cells, and unusual cytoplasmic protrusions into the lumen, giving rise to an irregular lumen (Fig. 9B, C). This contrasted strongly with the smooth luminal surface and strict planar polarity seen in wild-type ZC monolayers (Fig 9A). Given the key role of E-cadherin in establishing adherens junctions (Johnson et al., 1986; Classen et al., 2005; Kane et al., 2005), the Mist1−/−Cd2ap−/− phenotype is likely reflective of improper E-cadherin localization or function and resultant misplaced junctional complexes.
Figure 9. Mislocalization of junctional complexes leads to markedly irregular lumens in Mist1−/−Cd2ap−/− mice.
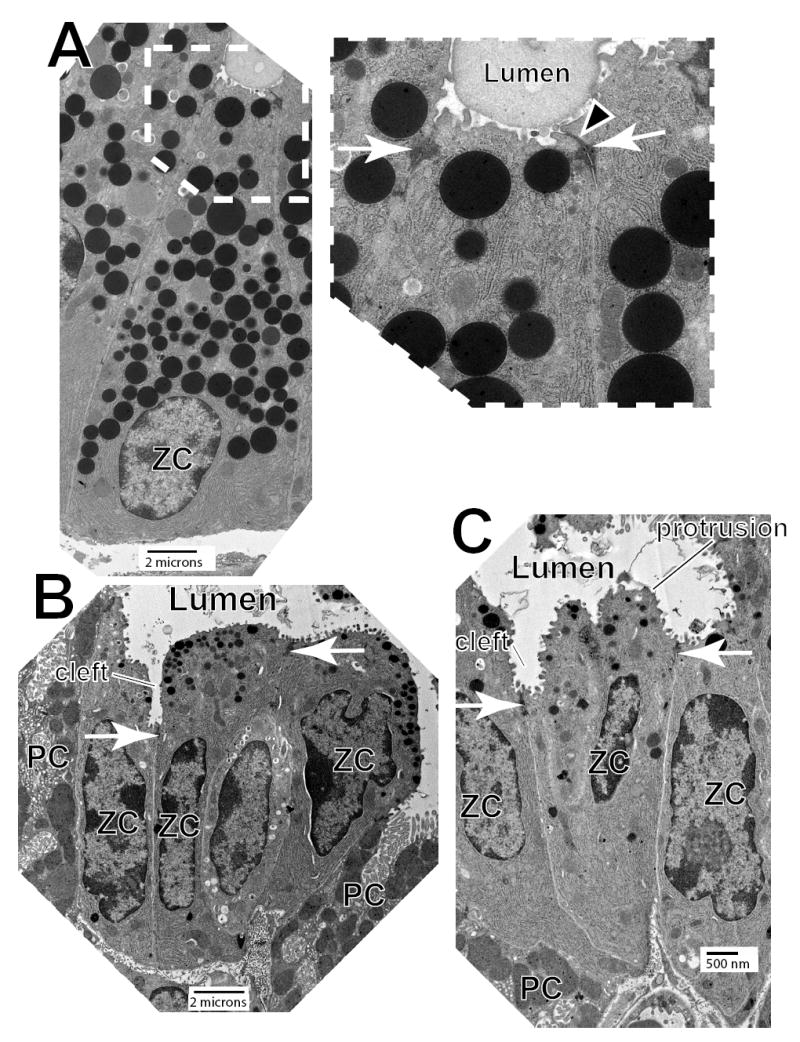
A, Transmission EM of a typical WT ZC at the very base of the gastric unit. Note that the cell has a pyramidal shape (typical of ZCs bending around the cul de sac of the unit). inset- higher magnification view of the apical lumen and junctional complexes. Tight (arrowhead) and adherens (arrows) junctions are near the apex of each ZC, and the luminal plasma membrane is smooth and regular. B,C Mist1−/−Cd2ap−/− ZCs have ultrastructurally normal adherens junctions (arrows), but the junctions are haphazardly localized with respect to the basement membrane. The apical plasma membrane is irregular, and its luminal surface is characterized by frequent protrusions between junctions and clefts at junctions. Note frequent parietal cells (“PC”) intercalating between ZCs and in between ZCs and the basement membrane.
The disorganization in the base of Mist1−/−Cd2ap−/− mice resembles what occurs in other systems when E-cadherin localization is defective. Without dynamic, coordinated E-cadherin distribution, migrating epithelial cells that are attempting to establish monolayers have weak and/or irregular cell-cell interactions, causing: 1) irregular lumens (due to inconsistent height of cells along their apical surface) and 2) failure to exclude other cells from intermingling within the monolayer (Johnson et al., 1986; Bertet et al., 2004; Classen et al., 2005; Kane et al., 2005). Because Cd2ap regulates dynamic localization of E-cadherin (Lehtonen et al., 2004; Mustonen et al., 2005; Johnson et al., 2008), our data suggest that Cd2ap deficiency causes aberrant organization of Mist1−/−Cd2ap−/− bases via mislocalization of E-cadherin. Therefore, while strong E-cadherin networks, dynamically regulated by Cd2ap, may help wild-type ZCs form and fortify a monolayer able to exclude PCs (Fig. 10), Mist1−/−Cd2ap−/− ZCs fail to do so. Interestingly, Mist1 overexpression in gastric epithelial cell lines did not affect E-cadherin message levels (Supplemental Figure 2B and 2C), indicating that Mist1 is unlikely to exert direct transcriptional control over E-cadherin expression. Therefore, while CD2ap may act directly on E-cadherin to regulate cell-cell junctions, Mist1 may act indirectly to regulate E-cadherin and/or may regulate other cytoskeletal pathways which work in parallel to coordinate strong ZC-ZC interactions that exclude PCs from the base.
Figure 10. “Push-back” model illlustrating why PCs mostly localize to the progenitor niche.
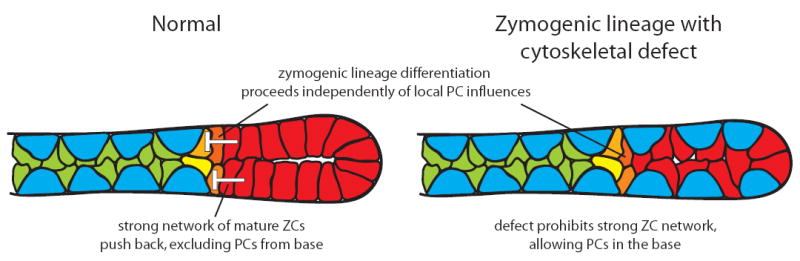
left - In normal WT gastric units, as the mucus-secreting NCs (green) in the progenitor zone transition into mature, enzyme-secreting ZCs (red), they develop strong cytoskeletal networks and adhesions to neighboring ZCs, thus establishing planar polarity. This epithelial monolayer that ZCs thus form forcibly excludes PCs (ZC-derived force depicted by white t-bars in the figure). right - Our results have shown that disrupting cell-cell adhesions in ZCs leads to failure of ZCs to exclude PCs from the base; however, neither the increased proximity of PCs to ZCs nor the ZC cytoskeletal defects affect the downregulation of NC differentiation markers and upregulation of ZC differentiation markers. Thus, the timing or location of the transition from mucus secretion to digestive-enzyme secretion is not affected by the position of PCs. We conclude that PCs are normally located in the progenitor region of the gastric unit not to establish a distinct progenitor niche to direct NC-ZC differentiation (as modeled in Fig. 4); rather, PCs simply accumulate there because differentiated ZCs exclude them from the base.
Discussion
Here, we have demonstrated that the gastric epithelial zymogenic progenitor niche contains abundant PCs. These PCs occupy the vast majority of the space along the basement membrane, while the zymogenic progenitors largely line the lumen. This pseudostratified architecture contrasts with the relatively PC-free, simple epithelial structure of the base zone where differentiated ZCs reside. We have shown that PCs are required for normal zymogenic lineage differentiation, because when PCs are genetically ablated from gastric units, nearly all ZCs and their progenitor NCs inappropriately co-express both NC and ZC markers. However, in Mist1−/−Cd2ap−/− mice, which have gastric units where PCs are abundant in both the neck and the base, the zymogenic lineage still exhibits the normal patterning of immature (NC) markers in the neck zone and mature (ZC) markers in the base. This surprising finding indicates that, even though PCs define the distinctive architecture of the NC niche and are required to support normal NC development, they are not sufficient to maintain the lineage at the NC stage. Furthermore, although the ZCs in Mist1−/−Cd2ap−/− mice express the correct markers, the base zones of these mice exhibit profound changes in ZC morphology and aberrant organization of the key cytoskeletal protein E-cadherin, suggesting that the reason PCs are present primarily in the progenitor zone in wild-type mice is that ZCs normally reorganize their cytoskeleton as they differentiate to form a strong, polarized epithelium that excludes PCs.
Abnormal zymogenic lineage differentiation correlates with stomach cancer
Maintaining normal zymogenic lineage differentiation is clearly critical for gastric homeostasis, as metaplasias in humans predispose to the development of cancer (Correa, 1992; Houghton et al., 2004; Cai et al., 2005; Faller and Kirchner, 2005; Merchant, 2005). Alterations in zymogenic lineage development have most often been linked with the development of cancers that exhibit the more common intestinal-type histology, but have also been implicated as the direct antecedent for diffuse, signet ring type gastric carcinomas (Humar et al., 2007) in which early carcinoma cells phenocopy normal NCs. Interestingly, the most common hereditary gastric carcinomas are of the diffuse-type, NC-like variety, and the heritable mutation that predisposes patients toward carcinogenesis is in the gene for E-cadherin (Charlton et al., 2004; Lynch et al., 2005). Early in the course of hereditary gastric carcinoma syndromes, NCs apparently misregulate E-Cadherin and intercalate through the basement membrane. They then accumulate in the interstitial tissue as relatively innocuous cell collections that are easily missed on routine H& E analysis. Eventually, these cells acquire additional mutations that allow them to invade deeper into the mucosa and then metastasize (Humar et al., 2007).
The importance of PCs in ZC differentiation
Several lines of evidence support the idea that proper patterning of the entire zymogenic lineage, from early progenitor to mature ZC, is fundamentally dependent upon PCs. Earlier reports of mice lacking PCs (Sharp et al., 1995; Li et al., 1996; Spicer et al., 2000; Mills et al., 2002) showed that the presence of PCs correlated with the presence of ultrastructurally mature ZCs; however, the transition of NCs to ZCs along the entire unit was neither extensively examined nor quantified. Additionally, the loss of PCs in humans correlates with metaplasia (aberrant differentiation) and atrophy (loss) of ZCs. One common pattern of metaplasia, spasmolytic peptide expressing metaplasia (SPEM) or pseudopyloric metaplasia, is characterized by the co-expression of NC and ZC markers in zymogenic lineage cells in the base of the gastric unit (Ikeda et al., 2005; Leys et al., 2006). In a mouse model of SPEM, drug-induced elimination of PCs results in loss of ZC-specific ultrastructural features (especially stacked lamellar rER) and the rapid expression of NC-specific genes by mature ZCs within hours (Nozaki et al., 2008). The tox176 mice described in the current study exhibit a chronic form of SPEM, with basal cells expressing Tff2 while co-expressing ZC markers.
Our current results confirm that PCs are required for the development and/or maintenance of ZCs and, additionally, show for the first time that PCs are required for the normal NC phenotype. The known role of PCs as morphogen secretors (van den Brink et al., 2001; Mills et al., 2002; Lees et al., 2005; Shiotani et al., 2005; Stepan et al., 2005), coupled with their location in the progenitor niche but not in the base, led us to hypothesize that it was migration of ZCs away from the neck—and therefore from the secreted or cell-surface mediators elaborated by PCs—that induced the downregulation of NC markers and increased expression of ZC markers. In this operating model (Fig. 4), PCs secrete locally acting cues (e.g., Sonic hedgehog) that directly inhibit the transition from NC to ZC. Additionally, normal NC maturation, which depends upon PCs, would be a prerequisite for the development of ZCs. Thus, PC ablation would lead to loss of phenotypic NCs followed by loss of ZCs.
However, our results from Mist1−/−Cd2ap−/− mice show that increased contact of PCs with cells in the base does not affect the NC transition into ZCs. Thus, PCs must act indirectly and/or must not provide direct positional instructions to NCs about the timing of the transition to ZCs. The simplest cellular model to explain our data would be that PCs act indirectly to delay expression of ZC genes in NCs but also to maintain terminal maturation of ZCs once they form. In the absence of PCs, expression of NC genes and ZC genes would be more fluid; cells can express both at the same time or either at low levels without complete commitment to the ZC phenotype. Indeed, because ZC and NC marker expression changes occur within hours to days following acute PC loss (Nozaki et al., 2008), it appears that existing ZCs and NCs can change their differentiation state. Therefore, the development of metaplasia following loss of PCs may be a relatively dynamic process, suggesting that metaplastic cells may represent a completely reversible state of the zymogenic lineage rather than a parallel, aberrant differentiation pathway originating at the stem cell. If so, repopulation by PCs or therapeutic replacement of key PC-derived factor(s) may restore these abnormal cells to their appropriate zymogenic lineage state.
Potential molecular mechanisms underlying PC regulation of zymogenic differentiation
In addition to directly PC-derived factors, other PC-dependent indirect signaling mechanisms can also influence gastric unit differentiation. As an example, it is understood that lack of PCs leads to hypochlorhydria, which in turn leads to hypergastrinemia secondary to G cell hyperplasia in the antrum (Sharp et al., 1995; Nomura et al., 2005; Zavros et al., 2005; Jain and Samuelson, 2006; Nam et al., 2007). The effects of PC absence on NC and ZC gene expression may be mediated in part through Gastrin or through histamine released by endocrine ECL cells within the corpus gastric units themselves (Takaishi et al., 2005; Chen et al., 2006). We assessed levels of Gastrin in the antra of hypochlorhydric germ-free tox176 mice but, unexpectedly, observed no increase relative to wild-type littermates, suggesting that sustained hypergastrinemia is not required for the chronically increased proliferation and aberrant differentiation (SPEM) in tox176 mice.
Several alternative models are worth consideration. For example, NCs may be influenced by factors associated with blood and/or basement membrane, and PCs simply form a barrier that restricts access to those factors. We do not favor that model, because cells in the base of the Mist1−/−Cd2ap−/− units also have only limited access to the basement membrane and blood, yet still differentiate away from the NC fate. Also, PCs in the base may be qualitatively different from those in the neck, and only those in the neck niche may be able to instruct NCs. We think this is unlikely, because it complicates the model considerably, implying that PCs have a mechanism for sensing when they are basal and then must dramatically change their elaboration of factors at that point. We have performed functional genomic assays of PCs from different regions of the normal gastric unit and have labeled PCs in all the genotypes described in the current manuscript with a panel of markers using immunofluorescence. Though we see some evidence of small changes in levels of expression of certain genes, as has been reported (Karam et al., 1997), we, like others, have not seen evidence of qualitative or sudden changes in gene expression between PCs in the neck and those in the base.
Two other potential confounders of the tox176 results should be addressed. In the PC ablation experiments in the current study, the tox176 mice were raised under gnotobiotic (germ-free) conditions, thereby minimizing the effects of a non-acid-secreting stomach. We observe the same differentiation aberrations in conventionally raised, PC-ablated stomachs, but interpretation is complicated by substantial infiltration of inflammatory cells in such mice, which does not occur in young adult gnotobiotic tox176 mice (Syder et al., 1999). Also, it could be argued that the tox176 transgene may be expressed in a progenitor that is common to PCs and NCs, thereby confounding the experiments. We think this unlikely, because the promoter driving tox176 expression in these mice has been characterized in multiple reports (including those where the promoter drives Cre endonuclease expression on a Rosa26 reporter background) to be specific to cells committed exclusively to the PC fate (Syder et al., 2004; Lopez-Diaz et al., 2006).
New insight into functional significance of Cd2ap
Our results shed new light on the function of Cd2ap. There is robust literature on the in vivo importance of Cd2ap in kidney proximal tubule development and homeostasis (reviewed in (Wolf and Stahl, 2003)); however, despite relatively widespread Cd2ap expression and despite documented effects of loss of Cd2ap in non-renal tissue in vitro (Mustonen et al., 2005), the only other mammalian tissue with an in vivo phenotype following Cd2ap knockout is the testis (Grunkemeyer et al., 2005), where loss of Cd2ap disturbs spermatogonial development via effects on Sertoli cells, the non-dividing, cellular component of this niche.
Our results showing profound cell shape abnormalities in Mist1−/−Cd2ap−/− ZCs are also similar to those recently reported by Johnson et al. in Drosophila (Johnson et al., 2008). In that study, ablation of the fly ortholog for Cd2ap, cindr, leads to misshapen cells that fail to compete effectively for their niche during eye development and are crowded out by surrounding cells for basement membrane space. In flies and in gastric mucosal cells in vitro, (Mustonen et al., 2005), loss of Cd2ap leads to mislocalized E-cadherin. Restoration of E-cadherin in Drosophila rescues the Cd2ap loss of function phenotype. Thus, at least in certain situations, epithelial cells that must establish a niche with strong, ordered cell-cell junctions that exclude other cells use Cd2ap to coordinate the necessary dynamic changes in E-cadherin-based cell-cell adhesions. Our results here are the first to indicate that Cd2ap-regulated localization of E-cadherin is required for coordinated development of epithelial monolayers in vivo in mammals. Given that misregulated E-cadherin expression in zymogenic precursor cells is characteristic of familial gastric carcinoma syndromes (Humar et al., 2007), it would be interesting to determine whether there are alterations in Cd2ap expression in early cancers arising in such patients.
It should be stated that we cannot rule out a mechanism whereby Cd2ap does not affect E-cadherin localization directly to cause the phenotype we observe in the Mist1−/−Cd2ap−/− ZCs. For instance, loss of Cd2ap may cause abnormalities in other cytoskeletal elements which, in turn, cause misaligned ZC-ZC interactions. In this scenario, the abnormal E-cadherin localization would be secondary rather than causal. Formally proving that Cd2ap acts through E-cadherin in ZC maturation will be difficult, given the already marked allelic complexity of the Mist1−/−Cd2ap−/− mutants (null alleles at Cd2ap and Mist1 plus a transgenic podocyte-specific Cd2ap). Future experiments might focus directly on the role dynamic regulation of E-cadherin plays in ZC differentiation. For example, would ZC-specific disruption of other dynamic E-cadherin regulators, such as cdc42 or Src (Shen et al., 2008), in Mist1−/− or wild-type mice phenocopy the disordered ZC junctions and greatly increased numbers of intercalating PCs, characteristic of the Mist1−/−Cd2ap−/− ZCs?
In pancreas, the other major organ affected by absence of Mist1, loss of Cd2ap does not exacerbate the Mist1−/− phenotype (data not shown). Thus, Cd2ap and Mist1 may play a unique role in the gastric unit, where the continual conversion of NCs to ZCs involves migration and considerable cell architecture change. Indeed, given our data, the most likely explanation for the increase in base zone PCs in Mist1−/−Cd2ap−/− stomachs is a failure of ZCs to exclude PCs due to ZC structural deficiencies. In other words, as they migrate into the base, ZCs may normally upregulate Cd2ap and Mist1-dependent targets to form stable intercellular junctions and cytoskeletal networks that resist encroachment of PCs (Fig. 10). Because ZCs establish this monolayer of ZC-ZC adhesions (i.e. ZCs develop planar polarity as they develop from NCs), PCs normally are mostly restricted to the middle (progenitor) portion of gastric units. Thus, our results provide a potential molecular mechanism for the hallmark histological position of PCs and ZCs in mammalian gastric units.
Prospectus
Our current experiments shed light on the cellular and molecular mechanisms regulating zymogenic lineage patterning in the normal gastric unit. Importantly, however, the mechanisms that mediate PC-zymogenic lineage interactions are critical not only to help us understand a previously uncharacterized tissue stem cell niche but also because alterations in zymogenic differentiation are critical for development of gastric cancer. Overall, the zymogenic lineage, with its constant slow turnover, intriguing transdifferentiation from NC to ZC, and complex interplay with PCs is an excellent system in which to study how mammalian cell progenitor niches and migration work in an adult system with disease implications.
Supplementary Material
Neck cells extend thin processes between PCs to contact the basement membrane. tEM analysis of the gastric unit neck zone in cross section is shown at higher power than in Figure 1. Note how these images capture narrow areas of NC contact with the basement membrane (arrowheads). L, gastric unit lumen; NC, neck cell (labels are in NC nuclei); PC, parietal cell.
Mist1 does not regulate transcription of Cd2ap or Cin85. A, RT-PCR for Mist1 and Cd2ap transcripts. AGS cells express low endogenous levels of Mist1, while HGC cells do not express detectable levels. Transfection of Mist1 cDNA into both cell lines achieved high level overexpression. Cd2ap was expressed at detectable levels in both lines. B, Western blot analysis demonstrated overexpression of Mist1 protein in AGS transfectants. C, AGS or HGC cells were transfected with Mist1 plasmid or empty vector, and high expressors were isolated by flow cytometry (see Methods). (In data not shown, we have used this in vitro transfection system to identify multiple Mist1 targets by expression microarray analysis, and confirmed these changes by RT-PCR and chromatin immunoprecipitation.) Expression levels of the transcripts indicated were measured by quantitative real-time PCR on transfectant cDNAs. Note that the axis plots PCR cycles above background, so higher numbers mean more abundant amplicon (See Methods and legend to Fig. 3). Error bars represent standard deviation.
Multiphoton microscopy-generated three-dimensional rendering of gastric unit neck zones. The base zones, which begin at the bottom of the image, are cropped from this image. Green, GS-II; red, anti-H+/K+ ATPase; blue, anti-GIF.
Acknowledgments
We thank the Washington University Digestive Diseases Research Core Center Murine Models Core, supported by grant #P30 DK52574. Further grant support: J.C.M. (K08 DK066062, R01 DK079798) and A.S.S. (R01 DK06642804, R01 DK05836608). Thanks also to Karen Green for EM assistance and to Drs. Indira Mysorekar and Jim Skeath for thoughtful review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Beauchamp RD, Barnard JA, McCutchen CM, Cherner JA, Coffey RJ., Jr Localization of transforming growth factor alpha and its receptor in gastric mucosal cells. Implications for a regulatory role in acid secretion and mucosal renewal. J Clin Invest. 1989;84:1017–23. doi: 10.1172/JCI114223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–71. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- Blostein R, Zhang R, Gottardi CJ, Caplan MJ. Functional properties of an H,K-ATPase/Na,K-ATPase chimera. J Biol Chem. 1993;268:10654–8. [PubMed] [Google Scholar]
- Brown SL, Riehl TE, Walker MR, Geske MJ, Doherty JM, Stenson WF, Stappenbeck TS. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest. 2007;117:258–69. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck S, Huber TB, Ingham RJ, Kim K, Niederstrasser H, Allen PM, Pawson T, Cooper JA, Shaw AS. Identification of a novel inhibitory actin-capping protein binding motif in CD2-associated protein. J Biol Chem. 2006;281:19196–203. doi: 10.1074/jbc.M600166200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Carlson J, Stoicov C, Li H, Wang TC, Houghton J. Helicobacter felis eradication restores normal architecture and inhibits gastric cancer progression in C57BL/6 mice. Gastroenterology. 2005;128:1937–52. doi: 10.1053/j.gastro.2005.02.066. [DOI] [PubMed] [Google Scholar]
- Charlton A, Blair V, Shaw D, Parry S, Guilford P, Martin IG. Hereditary diffuse gastric cancer: predominance of multiple foci of signet ring cell carcinoma in distal stomach and transitional zone. Gut. 2004;53:814–20. doi: 10.1136/gut.2002.010447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Aihara T, Zhao CM, Hakanson R, Okabe S. Differentiation of the gastric mucosa. I. Role of histamine in control of function and integrity of oxyntic mucosa: understanding gastric physiology through disruption of targeted genes. Am J Physiol Gastrointest Liver Physiol. 2006;291:G539–44. doi: 10.1152/ajpgi.00178.2006. [DOI] [PubMed] [Google Scholar]
- Classen AK, Anderson KI, Marois E, Eaton S. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell. 2005;9:805–17. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–40. [PubMed] [Google Scholar]
- Dustin ML, Olszowy MW, Holdorf AD, Li J, Bromley S, Desai N, Widder P, Rosenberger F, van der Merwe PA, Allen PM, Shaw AS. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell. 1998;94:667–77. doi: 10.1016/s0092-8674(00)81608-6. [DOI] [PubMed] [Google Scholar]
- Faller G, Kirchner T. Immunological and morphogenic basis of gastric mucosa atrophy and metaplasia. Virchows Arch. 2005;446:1–9. doi: 10.1007/s00428-004-1157-3. [DOI] [PubMed] [Google Scholar]
- Grunkemeyer JA, Kwoh C, Huber TB, Shaw AS. CD2-associated protein (CD2AP) expression in podocytes rescues lethality of CD2AP deficiency. J Biol Chem. 2005;280:29677–81. doi: 10.1074/jbc.M504004200. [DOI] [PubMed] [Google Scholar]
- Hanby AM, Poulsom R, Playford RJ, Wright NA. The mucous neck cell in the human gastric corpus: a distinctive, functional cell lineage. J Pathol. 1999;187:331–7. doi: 10.1002/(SICI)1096-9896(199902)187:3<331::AID-PATH241>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Mills JC, Roth KA, Stappenbeck TS, Wong MH, Gordon JI. Combining gnotobiotic mouse models with functional genomics to define the impact of the microflora on host physiology. Methods Microbiol. 2002;31:559–589. [Google Scholar]
- Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–71. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- Huh WJ, Pan XO, Mysorekar IU, Mills JC. Location, allocation, relocation: isolating adult tissue stem cells in three dimensions. Curr Opin Biotechnol. 2006;17:511–7. doi: 10.1016/j.copbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Humar B, Fukuzawa R, Blair V, Dunbier A, More H, Charlton A, Yang HK, Kim WH, Reeve AE, Martin I, Guilford P. Destabilized adhesion in the gastric proliferative zone and c-Src kinase activation mark the development of early diffuse gastric cancer. Cancer Res. 2007;67:2480–9. doi: 10.1158/0008-5472.CAN-06-3021. [DOI] [PubMed] [Google Scholar]
- Hutchings NJ, Clarkson N, Chalkley R, Barclay AN, Brown MH. Linking the T cell surface protein CD2 to the actin-capping protein CAPZ via CMS and CIN85. J Biol Chem. 2003;278:22396–403. doi: 10.1074/jbc.M302540200. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Nishikura K, Watanabe H, Watanabe G, Ajioka Y, Hatakeyama K. Histopathological differences in the development of small intestinal metaplasia between antrum and body of stomach. Pathol Res Pract. 2005;201:487–96. doi: 10.1016/j.prp.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Jain RN, Al-Menhali AA, Keeley TM, Ren J, El-Zaatari M, Chen X, Merchant JL, Ross TS, Chew CS, Samuelson LC. Hip1r is expressed in gastric parietal cells and is required for tubulovesicle formation and cell survival in mice. J Clin Invest. 2008;118:2459–70. doi: 10.1172/JCI33569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RN, Samuelson LC. Differentiation of the gastric mucosa. II. Role of gastrin in gastric epithelial cell proliferation and maturation. Am J Physiol Gastrointest Liver Physiol. 2006;291:G762–5. doi: 10.1152/ajpgi.00172.2006. [DOI] [PubMed] [Google Scholar]
- Jain RN, Samuelson LC. Transcriptional profiling of gastrin-regulated genes in mouse stomach. Physiol Genomics. 2007;29:1–12. doi: 10.1152/physiolgenomics.00176.2006. [DOI] [PubMed] [Google Scholar]
- Johnson CL, Kowalik AS, Rajakumar N, Pin CL. Mist1 is necessary for the establishment of granule organization in serous exocrine cells of the gastrointestinal tract. Mech Dev. 2004;121:261–72. doi: 10.1016/j.mod.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Maro B, Takeichi M. The role of cell adhesion in the synchronization and orientation of polarization in 8-cell mouse blastomeres. J Embryol Exp Morphol. 1986;93:239–55. [PubMed] [Google Scholar]
- Johnson RI, Seppa MJ, Cagan RL. The Drosophila CD2AP/CIN85 orthologue Cindr regulates junctions and cytoskeleton dynamics during tissue patterning. J Cell Biol. 2008;180:1191–204. doi: 10.1083/jcb.200706108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane DA, McFarland KN, Warga RM. Mutations in half baked/E-cadherin block cell behaviors that are necessary for teleost epiboly. Development. 2005;132:1105–16. doi: 10.1242/dev.01668. [DOI] [PubMed] [Google Scholar]
- Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec. 1993a;236:259–79. doi: 10.1002/ar.1092360202. [DOI] [PubMed] [Google Scholar]
- Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec. 1993b;236:297–313. doi: 10.1002/ar.1092360204. [DOI] [PubMed] [Google Scholar]
- Karam SM, Yao X, Forte JG. Functional heterogeneity of parietal cells along the pit-gland axis. Am J Physiol. 1997;272:G161–71. doi: 10.1152/ajpgi.1997.272.1.G161. [DOI] [PubMed] [Google Scholar]
- Lees C, Howie S, Sartor RB, Satsangi J. The hedgehog signalling pathway in the gastrointestinal tract: implications for development, homeostasis, and disease. Gastroenterology. 2005;129:1696–710. doi: 10.1053/j.gastro.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Lehtonen S, Lehtonen E, Kudlicka K, Holthofer H, Farquhar MG. Nephrin forms a complex with adherens junction proteins and CASK in podocytes and in Madin-Darby canine kidney cells expressing nephrin. Am J Pathol. 2004;165:923–36. doi: 10.1016/S0002-9440(10)63354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys CM, Nomura S, Rudzinski E, Kaminishi M, Montgomery E, Washington MK, Goldenring JR. Expression of Pdx-1 in human gastric metaplasia and gastric adenocarcinoma. Hum Pathol. 2006;37:1162–8. doi: 10.1016/j.humpath.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Li Q, Karam SM, Gordon JI. Diphtheria toxin-mediated ablation of parietal cells in the stomach of transgenic mice. J Biol Chem. 1996;271:3671–6. [PubMed] [Google Scholar]
- Lopez-Diaz L, Hinkle KL, Jain RN, Zavros Y, Brunkan CS, Keeley T, Eaton KA, Merchant JL, Chew CS, Samuelson LC. Parietal cell hyperstimulation and autoimmune gastritis in cholera toxin transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2006;290:G970–9. doi: 10.1152/ajpgi.00461.2005. [DOI] [PubMed] [Google Scholar]
- Lynch HT, Grady W, Suriano G, Huntsman D. Gastric cancer: new genetic developments. J Surg Oncol. 2005;90:114–33. doi: 10.1002/jso.20214. discussion 133. [DOI] [PubMed] [Google Scholar]
- Merchant JL. Inflammation, atrophy, gastric cancer: connecting the molecular dots. Gastroenterology. 2005;129:1079–82. doi: 10.1053/j.gastro.2005.07.038. [DOI] [PubMed] [Google Scholar]
- Mills JC, Andersson N, Hong CV, Stappenbeck TS, Gordon JI. Molecular characterization of mouse gastric epithelial progenitor cells. Proc Natl Acad Sci U S A. 2002;99:14819–24. doi: 10.1073/pnas.192574799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JC, Gordon JI. A new approach for filtering noise from high-density oligonucleotide microarray datasets. Nucleic Acids Res. 2001;29:E72–2. doi: 10.1093/nar/29.15.e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JC, Syder AJ, Hong CV, Guruge JL, Raaii F, Gordon JI. A molecular profile of the mouse gastric parietal cell with and without exposure to Helicobacter pylori. Proc Natl Acad Sci U S A. 2001;98:13687–92. doi: 10.1073/pnas.231332398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–5. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- Mustonen H, Lepisto A, Lehtonen S, Lehtonen E, Puolakkainen P, Kivilaakso E. CD2AP contributes to cell migration and adhesion in cultured gastric epithelium. Biochem Biophys Res Commun. 2005;332:426–32. doi: 10.1016/j.bbrc.2005.04.140. [DOI] [PubMed] [Google Scholar]
- Nam KT, Varro A, Coffey RJ, Goldenring JR. Potentiation of oxyntic atrophy-induced gastric metaplasia in amphiregulin-deficient mice. Gastroenterology. 2007;132:1804–19. doi: 10.1053/j.gastro.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Nomura S, Yamaguchi H, Ogawa M, Wang TC, Lee JR, Goldenring JR. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G362–75. doi: 10.1152/ajpgi.00160.2004. [DOI] [PubMed] [Google Scholar]
- Nozaki K, Ogawa M, Williams JA, Lafleur BJ, Ng V, Drapkin RI, Mills JC, Konieczny SF, Nomura S, Goldenring JR. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology. 2008;134:511–22. doi: 10.1053/j.gastro.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Pin CL, Bonvissuto AC, Konieczny SF. Mist1 expression is a common link among serous exocrine cells exhibiting regulated exocytosis. Anat Rec. 2000;259:157–67. doi: 10.1002/(SICI)1097-0185(20000601)259:2<157::AID-AR6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Pin CL, Rukstalis JM, Johnson C, Konieczny SF. The bHLH transcription factor Mist1 is required to maintain exocrine pancreas cell organization and acinar cell identity. J Cell Biol. 2001;155:519–30. doi: 10.1083/jcb.200105060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey VG, Doherty JM, Chen CC, Stappenbeck TS, Konieczny SF, Mills JC. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development. 2007;134:211–22. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Sharp R, Babyatsky MW, Takagi H, Tagerud S, Wang TC, Bockman DE, Brand SJ, Merlino G. Transforming growth factor alpha disrupts the normal program of cellular differentiation in the gastric mucosa of transgenic mice. Development. 1995;121:149–61. doi: 10.1242/dev.121.1.149. [DOI] [PubMed] [Google Scholar]
- Shen Y, Hirsch DS, Sasiela CA, Wu WJ. Cdc42 regulates E-cadherin ubiquitination and degradation through an epidermal growth factor receptor to Src-mediated pathway. J Biol Chem. 2008;283:5127–37. doi: 10.1074/jbc.M703300200. [DOI] [PubMed] [Google Scholar]
- Shiotani A, Iishi H, Uedo N, Ishiguro S, Tatsuta M, Nakae Y, Kumamoto M, Merchant JL. Evidence that loss of sonic hedgehog is an indicator of Helicobater pylori-induced atrophic gastritis progressing to gastric cancer. Am J Gastroenterol. 2005;100:581–7. doi: 10.1111/j.1572-0241.2005.41001.x. [DOI] [PubMed] [Google Scholar]
- Spicer Z, Miller ML, Andringa A, Riddle TM, Duffy JJ, Doetschman T, Shull GE. Stomachs of mice lacking the gastric H,K-ATPase alpha - subunit have achlorhydria, abnormal parietal cells, and ciliated metaplasia. J Biol Chem. 2000;275:21555–65. doi: 10.1074/jbc.M001558200. [DOI] [PubMed] [Google Scholar]
- Stepan V, Pausawasdi N, Ramamoorthy S, Todisco A. The Akt and MAPK signal-transduction pathways regulate growth factor actions in isolated gastric parietal cells. Gastroenterology. 2004;127:1150–61. doi: 10.1053/j.gastro.2004.06.059. [DOI] [PubMed] [Google Scholar]
- Stepan V, Ramamoorthy S, Nitsche H, Zavros Y, Merchant JL, Todisco A. Regulation and function of the sonic hedgehog signal transduction pathway in isolated gastric parietal cells. J Biol Chem. 2005;280:15700–8. doi: 10.1074/jbc.M413037200. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Tsuyama S, Murata F. Cells intermediate between mucous neck cells and chief cells in rat stomach. Cell Tissue Res. 1983;233:475–84. doi: 10.1007/BF00212218. [DOI] [PubMed] [Google Scholar]
- Syder AJ, Guruge JL, Li Q, Hu Y, Oleksiewicz CM, Lorenz RG, Karam SM, Falk PG, Gordon JI. Helicobacter pylori attaches to NeuAc alpha 2,3Gal beta 1,4 glycoconjugates produced in the stomach of transgenic mice lacking parietal cells. Mol Cell. 1999;3:263–74. doi: 10.1016/s1097-2765(00)80454-2. [DOI] [PubMed] [Google Scholar]
- Syder AJ, Karam SM, Mills JC, Ippolito JE, Ansari HR, Farook V, Gordon JI. A transgenic mouse model of metastatic carcinoma involving transdifferentiation of a gastric epithelial lineage progenitor to a neuroendocrine phenotype. Proc Natl Acad Sci U S A. 2004;101:4471–6. doi: 10.1073/pnas.0307983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi S, Cui G, Frederick DM, Carlson JE, Houghton J, Varro A, Dockray GJ, Ge Z, Whary MT, Rogers AB, Fox JG, Wang TC. Synergistic inhibitory effects of gastrin and histamine receptor antagonists on Helicobacter-induced gastric cancer. Gastroenterology. 2005;128:1965–83. doi: 10.1053/j.gastro.2005.03.027. [DOI] [PubMed] [Google Scholar]
- van den Brink GR, Hardwick JC, Tytgat GN, Brink MA, Ten Kate FJ, Van Deventer SJ, Peppelenbosch MP. Sonic hedgehog regulates gastric gland morphogenesis in man and mouse. Gastroenterology. 2001;121:317–28. doi: 10.1053/gast.2001.26261. [DOI] [PubMed] [Google Scholar]
- Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003;31:e154. doi: 10.1093/nar/gng154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch T, Endlich N, Gokce G, Doroshenko E, Simpson JC, Kriz W, Shaw AS, Endlich K. Association of CD2AP with dynamic actin on vesicles in podocytes. Am J Physiol Renal Physiol. 2005;289:F1134–43. doi: 10.1152/ajprenal.00178.2005. [DOI] [PubMed] [Google Scholar]
- Wolf G, Stahl RA. CD2-associated protein and glomerular disease. Lancet. 2003;362:1746–8. doi: 10.1016/S0140-6736(03)14856-8. [DOI] [PubMed] [Google Scholar]
- Xie T, Li L. Stem cells and their niche: an inseparable relationship. Development. 2007;134:2001–6. doi: 10.1242/dev.002022. [DOI] [PubMed] [Google Scholar]
- Yen TH, Wright NA. The gastrointestinal tract stem cell niche. Stem Cell Rev. 2006;2:203–12. doi: 10.1007/s12015-006-0048-1. [DOI] [PubMed] [Google Scholar]
- Zavros Y, Eaton KA, Kang W, Rathinavelu S, Katukuri V, Kao JY, Samuelson LC, Merchant JL. Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma. Oncogene. 2005;24:2354–66. doi: 10.1038/sj.onc.1208407. [DOI] [PubMed] [Google Scholar]
- Zinselmeyer BH, Lynch JN, Zhang X, Aoshi T, Miller MJ. Video-rate two-photon imaging of mouse footpad - a promising model for studying leukocyte recruitment dynamics during inflammation. Inflamm Res. 2008;57:93–6. doi: 10.1007/s00011-007-7195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neck cells extend thin processes between PCs to contact the basement membrane. tEM analysis of the gastric unit neck zone in cross section is shown at higher power than in Figure 1. Note how these images capture narrow areas of NC contact with the basement membrane (arrowheads). L, gastric unit lumen; NC, neck cell (labels are in NC nuclei); PC, parietal cell.
Mist1 does not regulate transcription of Cd2ap or Cin85. A, RT-PCR for Mist1 and Cd2ap transcripts. AGS cells express low endogenous levels of Mist1, while HGC cells do not express detectable levels. Transfection of Mist1 cDNA into both cell lines achieved high level overexpression. Cd2ap was expressed at detectable levels in both lines. B, Western blot analysis demonstrated overexpression of Mist1 protein in AGS transfectants. C, AGS or HGC cells were transfected with Mist1 plasmid or empty vector, and high expressors were isolated by flow cytometry (see Methods). (In data not shown, we have used this in vitro transfection system to identify multiple Mist1 targets by expression microarray analysis, and confirmed these changes by RT-PCR and chromatin immunoprecipitation.) Expression levels of the transcripts indicated were measured by quantitative real-time PCR on transfectant cDNAs. Note that the axis plots PCR cycles above background, so higher numbers mean more abundant amplicon (See Methods and legend to Fig. 3). Error bars represent standard deviation.
Multiphoton microscopy-generated three-dimensional rendering of gastric unit neck zones. The base zones, which begin at the bottom of the image, are cropped from this image. Green, GS-II; red, anti-H+/K+ ATPase; blue, anti-GIF.


