Abstract
αA-Crystallin is a small heat shock protein that functions as a molecular chaperone and a lens structural protein. The single point mutation R49C in αA-crystallin causes hereditary human cataracts. We have previously investigated the in vivo properties of this mutant in a gene knock-in mouse model. Remarkably, homozygous mice carrying the αA-R49C mutant show nearly complete lens opacity concurrent with small lenses and small eyes. Here we have investigated the 90° light scattering, viscosity, refractive index and bis-ANS fluorescence of lens proteins isolated from the αA-R49C mouse lenses and find that the concentration of total water-soluble proteins showed a pronounced decrease in αA-R49C homozygous lenses. Light scattering measurements on proteins separated by gel permeation chromatography showed a small amount of high molecular weight aggregated material in the void volume which still remains soluble in AA-R49C homozygous lens homogenates. Increased binding of β-and γ-crystallin to the α-crystallin fraction was observed in αA-R49C heterozygous and homozygous lenses but not in wild type. Quantitative analysis with the hydrophobic fluorescence probe bis-ANS showed a pronounced increase in fluorescence yield upon binding to α-crystallin in αA-R49C lenses as compared with the wild type protein. These results suggest that the decrease in solubility of the αA-R49C mutant protein was due to an increase in its hydrophobicity and supra-aggregation of αA-crystallin that leads to cataract formation. Our study further shows that analysis of mutant proteins from the knock-in mouse model is an effective way to understand the mechanism of protein insolubilization in hereditary cataracts.
Keywords: Small heat shock protein, mutation, cataract, mechanism, hydrophobicity
Small heat shock proteins are defined by their 15–30 kDa subunit mass and a highly conserved “α-crystallin” domain of about 90 amino acids in their C-terminal region (1, 2). These proteins have recently received major attention due to their involvement in diverse human pathologies including neurological diseases and hereditary cataracts (3–6). Two members of this family, αA and αB-crystallin accumulate to very high concentrations in lens fiber cells and are present in a 3:1 stoichiometry (7). Both αA and αB-crystallin function as molecular chaperones and prevent non-specific aggregation of denaturing proteins in vitro (8, 9). αA-crystallin is expressed in only a small number of tissues outside the lens, whereas αB-crystallin is widely distributed and is upregulated under a variety of stress conditions (5). Targeted gene deletion studies of αA-crystallin, αB-crystallin or both proteins in mice have provided significant information about their roles in preserving lens transparency, growth and development (3, 10–13).
The transparency of the human eye lens depends on the properties of the crystallins which are expressed at very high concentrations in lens fiber cells. The subunit mass of αA and αB-crystallin is about 20 kDa and their amino acid sequences are 57% identical. αA-crystallin comprises nearly 20% of the total water soluble protein in newborn human lenses (14, 15). While the α-crystallins form oligomeric aggregates varying in molecular mass from 600 kDa to 1.2 MDa in lens fiber cells, these cells also express an abundance of another class of crystallins, βγ. The β-crystallins form smaller oligomers (60–180 kDa), whereas γ-crystallins are monomeric polypeptides with ~ 20 kDa molecular mass (16, 17). Aggregation and insolubilization of crystallins is considered a major cause of age-related and hereditary human cataract formation (18, 19). Age-related modifications in α-crystallin have been shown to increase the formation of water-soluble high molecular weight protein aggregates which eventually become insoluble (20, 21). The increase in protein aggregate size is directly responsible for an increase in light scattering and lens opacification. A decrease in protein solubility has also been reported with several cataract-causing point mutations in γ-crystallins (22–24).
In recent years, a number of point mutations have been discovered in the genes for αA and αB-crystallin that cause hereditary human cataract (6). Point mutations in αA-crystallin (R116C, R49C and G98R) have been shown to cause human hereditary cataract, and the R120G and D140N mutations in αB-crystallin causes desmin related myopathy and/or hereditary human cataracts (4, 25–28). The mechanisms that lead to cataract formation as a result of these mutations are not fully understood. Among these mutations, only the R49C mutation in αA-crystallin lies outside the conserved α-crystallin domain of the small heat shock proteins. The N-terminal region has been shown to be important in oligomeric assembly of α-crystallin (29–31). Increased exposure of the N-terminal region in a small heat shock protein Hsp16.5 correlates with enhanced substrate binding of destabilized substrates, suggesting that this region is important in determining the solubility of the α-crystallin oligomeric complex (32, 33). However, the mechanism by which α-crystallin becomes insoluble is poorly understood.
The size, morphology and temporal process of lens opacification in human patients with the arginine 49 to cysteine (αA-R49C) mutation are unknown at present. The effects of the R49C mutation in αA-crystallin has been investigated in cultured cells, and is known to enhance lens epithelial cell death, cause the mis-localization of αA-crystallin from the cytoplasm into the cellular nucleus, and diminish the resistance of cells to stress conditions (26). To understand how this point mutation in the αA-crystallin affects the lens in vivo, our laboratory recently generated a knock-in mouse model for hereditary human cataract caused by the R49C mutation in αA-crystallin (34). The advantage of this mouse model over in vitro expression of the mutant protein is that it replicates the heterozygosity of the human pathology in vivo. This model also allows testing gene dosing effects by analyses of lens changes in heterozygous and homozygous mice. We reported that αA-R49C heterozygosity causes protein insolubility and lens opacities that were apparent at an early postnatal age. αA-R49C homozygosity enhances protein insolubility and lens cell death in vivo leading to a small eye, small lens and severe cataracts at birth (34). The mechanism by which αA-R49C becomes insoluble is not known. This report investigated the consequence of the mutation on protein insolubility by measuring light scattering, refractive index and viscosity of proteins separated by gel permeation chromatography, in conjunction with analysis of hydrophobicity using the fluorescent probe bis-ANS. We demonstrate here that protein insolubility in αA-R49C lenses is likely to be the result of an increase in hydrophobicity of α-crystallin, and an increase in binding of β- and γ-crystallins to α-crystallin in the mutant lenses.
MATERIALS AND METHODS
Animals and lenses
Knock-in mice expressing the single point mutation C to T in codon 49 of the mouse αA-crystallin gene (cryaa) resulting in the substitution of arginine 49 to cysteine (R49C) were generated by methods described previously (34). Briefly, a knock-in plasmid was generated by cloning the 5’ and 3’ arms of the mouse αA-crystallin gene, followed by mutation and homologous recombination in mouse embryonic stem cells, which were implanted into mice to generate the αA-R49C knock-in mice. Heterozygous αA-R49C mice expressed one copy of the mutant gene while the second copy was wild type. Homozygous mice containing two copies of the mutant gene were generated by inter-breeding of heterozygous mice. Wild type littermates were used as controls. Mice used in this study were on 129Sv strain back-crossed with C57BL6 strain for several generations. PCR-based screening was routinely used to genotype the animals. Mice were maintained at Washington University by the Division of Comparative Medicine by trained veterinary staff. All protocols and procedures involving animals were approved by the Animal Studies Committee. Mice were euthanized using CO2 inhalation and lenses were dissected from 3 to 6 months old mice.
Preparation of samples for gel chromatography
For each chromatographic run, four mouse lenses (3 to 6 months old) were used to isolate water soluble proteins from wild type and αA-R49C heterozygous mouse eyes, but because of their smaller size, 12 lenses were used from αA-R49C homozygous mice. Lenses were homogenized in 50 mM Tris.Cl, pH 7.4 containing 100 mM NaCl, 1 mM DTT and 1 mM EDTA, and the water-soluble protein fraction was separated by centrifugation at 15,000 g. Proteins were injected at 1 mg/ml for different chromatographic runs.
Analytical chromatography and analysis
HPLC gel filtration chromatography (GPC) was performed using a Spectraseries P200 pump (Thermo Separation Products, Waltham MA) with a MetaChem degasser, (Torrance, CA) equipped with triple detectors (Viscotek Corp., Houston, TX) that measured refractive index (RI), right angle laser light scattering and viscosity, supplemented with an additional UV Waters 490E detector set at 280 nm (Waters Corporation, Milford, MA). Two columns were connected in series, an A4000PWXL and a G4000PWXL (Tosoh Biosep, Montgomery Ville, PA). Viscotek Trisec software (Viscotek Corp., Houston, TX) was used to calculate RI area, weight-averaged molecular weight, intrinsic viscosity, and radius of gyration. Samples were injected at various volumes and concentrations. The flow rate employed was 0.8 ml/min and the column buffer was 50 mM Tris, 100 mM NaCl, 1 mM EDTA, 1 mM DTT, 0.1% NaN3, pH 7.5. Samples from the column were collected for further analysis as follows: The amount of protein present in the wild type αA-crystallin, αA-R49C heterozygous and αA-R49C homozygous lens samples were calculated using refractive index (RI) area. Samples of each condition were then run using approximately equal amounts of total protein. Column fractions of each condition were collected at one minute intervals (800 µl/tube).
Molecular mass standards
Protein standards with known molecular mass were used to standardize the column and the detectors. Identical conditions were then used for separating lens crystallins. Molecular weight protein standards were obtained from Amersham Biosciences (GE Healthcare, Piscataway, NJ). The high molecular weight protein standards were dextran blue (2,000 kDa), thyroglobulin (669 kDa), catalase (232 kDa), aldolase (158 kDa). Low molecular weight protein standards were bovine serum albumin (67 kDa), carbonic anhydrase (35 kDa), chymotrypsin (25 kDa) and ribonuclease A (13.7 kDa).
Molecular weight determination from light scattering and refractive index
Using static light scattering as the analytical tool we determined the change in weight average molecular weight of water-soluble α-crystallin isolated from wild type, αA-R49C heterozygous and homozygous mutant mouse lenses. In this modality, the frequency of the scattered light is the same as that of the incoming light, and samples scatterers in the spatial domain as the light beam encounters their refractive index fluctuations along its optical pathway (18). The viscosity measurement is used to calculate the radius of gyration of the α-crystallin aggregate. Light scattering data was collected at a range of protein concentrations. Viscotek Trisec software (Viscotek Corp., Houston, Texas) was used to calculate refractive index (RI) area, weight-averaged molecular weight, intrinsic viscosity, and radius of gyration. The software uses an angular correction calculation to obtain molecular weights. This method has been adapted from Trisec GPC software handbook (Viscotek Corp., Houston, TX).
Fluorescence measurements
Fluorescence measurements were made with a Perkin-Elmer LS50 spectrofluorometer with a FLWinlab data station. The concentration of the protein used was 0.1 mg/ml (approximately 0.125 µM). Protein concentration was determined using A0.1% = 0.742. The excitation and emission slits were set to 5 nm. Samples with 4,4'-dianilino-1, 1'-binaphthalene-5,5'-disulfonic acid (bis-ANS) were excited at 360 nm, and fluorescence emission was measured between 400 and 600 nm in a cuvette with 1 cm pathlength at a scan rate of 100 nm/min. Bis-ANS (1 to 5 µM) was either scanned alone in buffer or added to the α-crystallin fraction from the gel permeation chromatography column. All measurements were made at 25°C.
Thermal stability
Time-dependent changes in light scattering of water-soluble protein fractions from wild type, heterozygous and homozygous lenses were measured by heating to 65°C. Light scattering was measured in a spectrofluorometer set at excitation and emission wavelengths of 360 nm. Temperature-induced stability of protein solutions was also measured by heating to different temperatures (25, 37, 42, 53 and 65°C) for 30 minutes and measuring light scattering intensity at 360 nm (9, 28).
Immunoblot analysis of gel permeation chromatography fractions
Mouse lens crystallin fractions, harvested from gel permeation chromatography columns, were mixed with 30 µl of SDS-PAGE sample buffer (5 ×) containing 0.02 M Tris.Cl, pH 6.8, 4% β-mercaptoethanol, 4 mg/ml bromophenol blue, 5% SDS and 60% glycerol. A 15 µl aliquot was analyzed by SDS-PAGE using 15% gels. Proteins were transferred to PVDF membranes and probed with primary antibodies to αA-crystallin (a monoclonal antibody to bovine αA-crystallin used at 1:40 dilution) (10), and polyclonal antibodies to total bovine α-crystallin fraction (35), total β-crystallin (36) and total γ-crystallin fraction used at 1:2000 dilution (35). The secondary antibodies were HRP-labeled anti-mouse or HRP-labeled anti-rabbit IgGs. Blots were incubated with luminol reagent (Santa Cruz Biotechnology) and exposed to Kodak film to visualize the protein bands. In these analyses crystallins isolated by gel permeation chromatography from human lenses were used as controls.
RESULTS
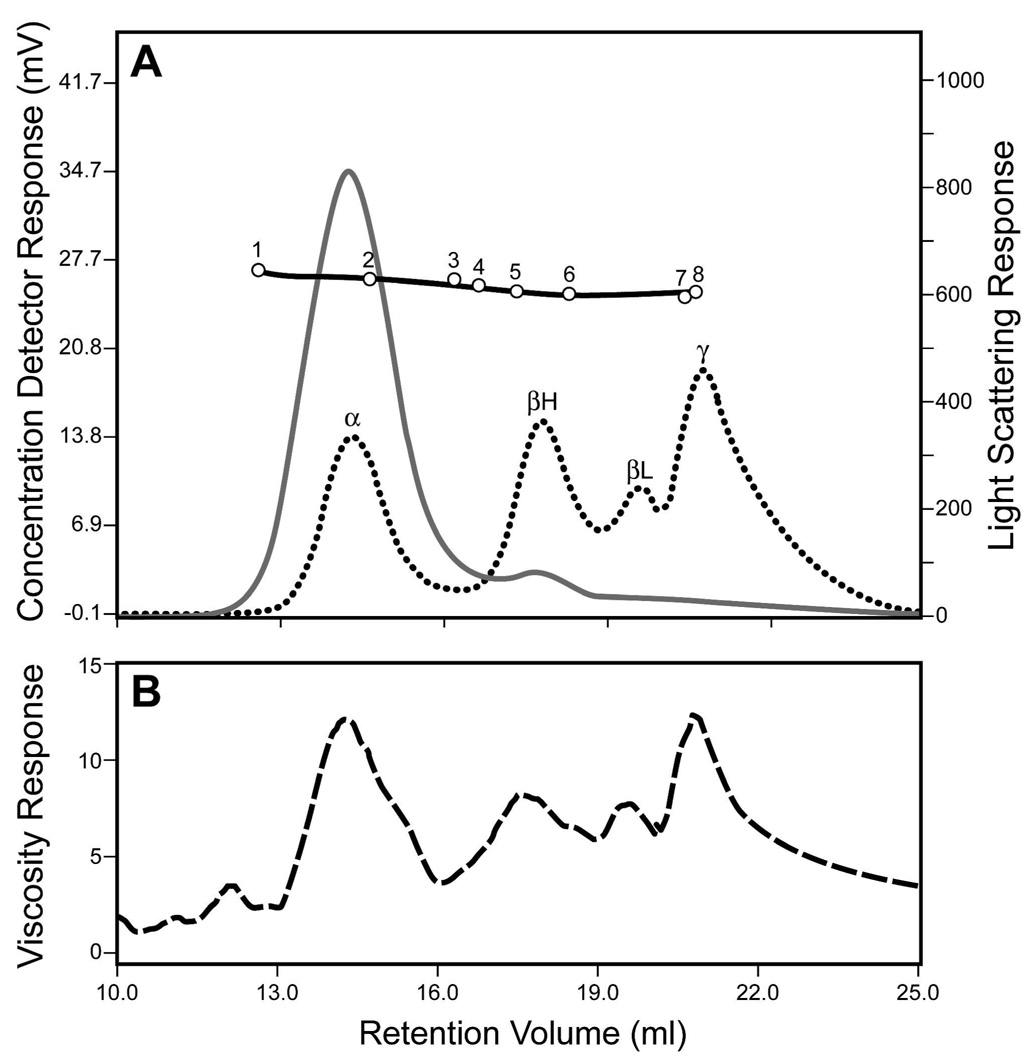
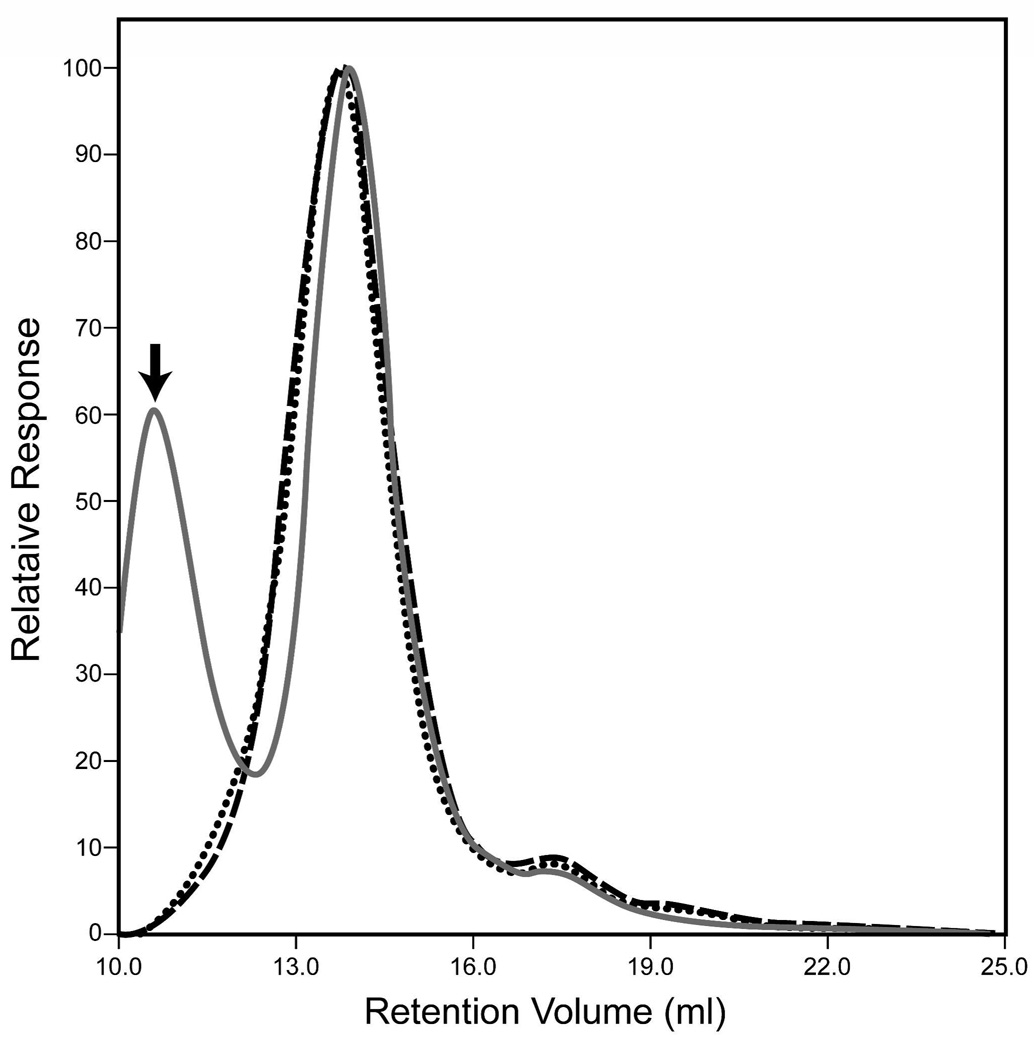
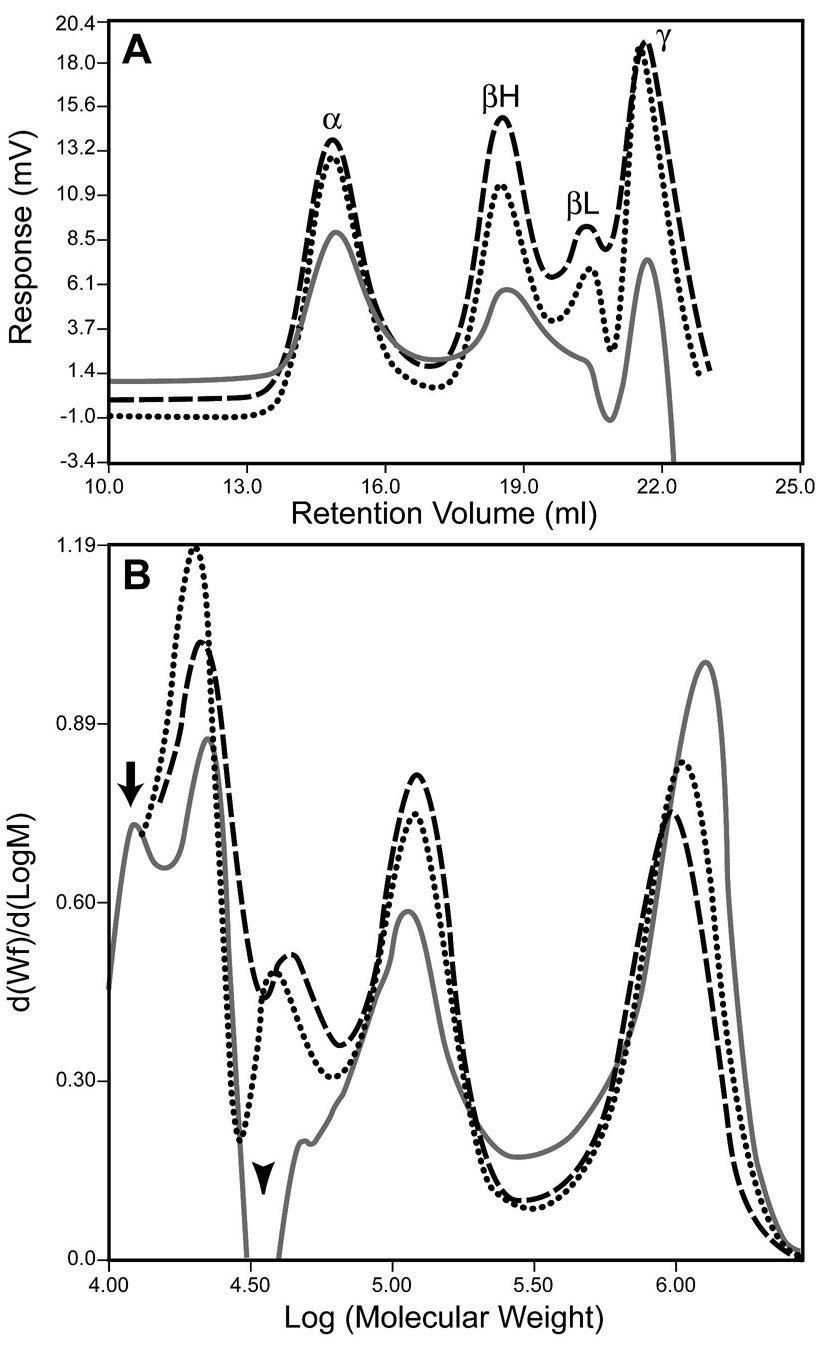
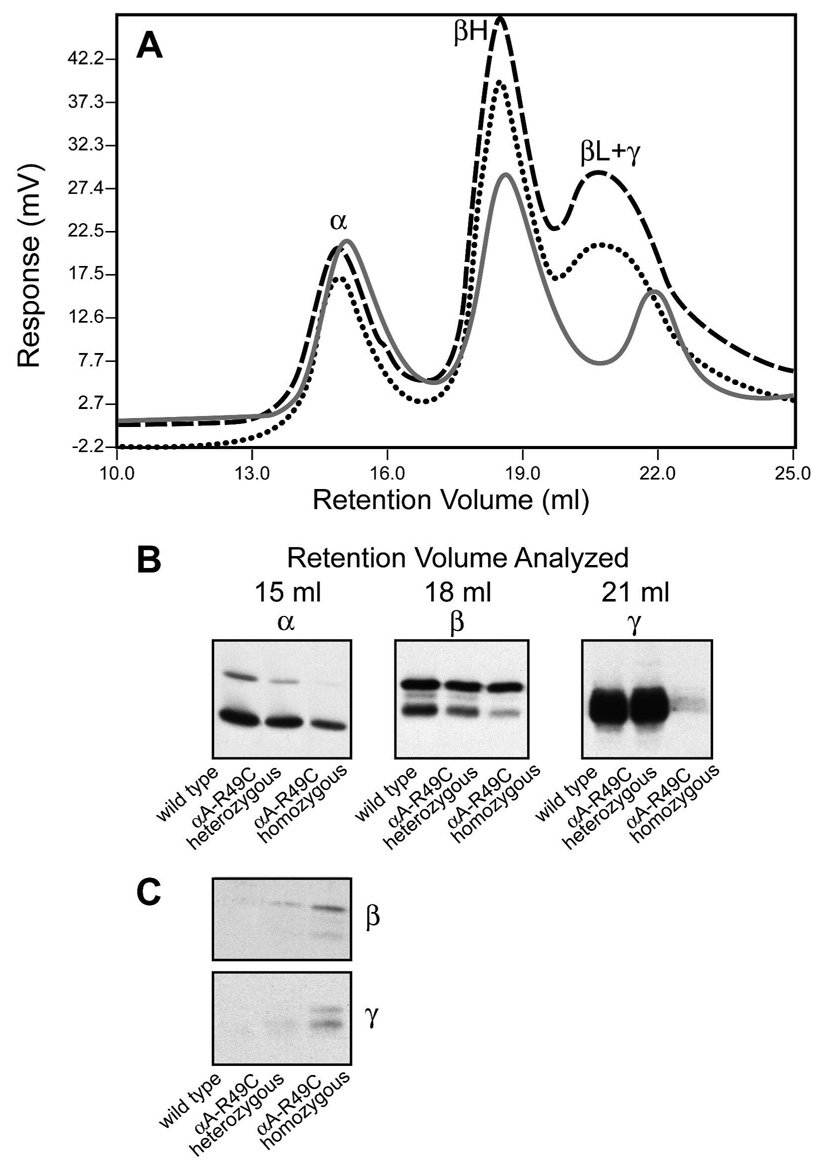
The αA-R49C mutant is associated with cataract. We have generated knock-in mice expressing the αA-R49C heterozygosity of human patients as well as αA-R49C homozygous mice, and analyzed their lens proteins. To determine the effect of the mutation on the molecular mass of the α-crystallin aggregate, gel permeation chromatography of the water-soluble proteins from wild type, αA-R49C heterozygous and homozygous mouse lenses was performed. Figure 1 shows the chromatography profile for wild type water soluble lens proteins measured by viscosity, light scattering and refractive index. α, β-heavy (βH), β-light (βL) and γ-crystallin lens protein fractions showed an excellent separation based upon the differences in refractive index of these proteins. α-crystallin is the major contributor to light scattering. The viscosity curve parallels the refractive index profile of the proteins, and further shows a small peak in the protein fractions eluting in the void volume of the column. Table 1 shows the effect of αA-R49C mutation on protein concentration of the water-soluble lens fraction. The protein concentration of the water-soluble fraction of the αA-R49C knock-in mouse lens proteins decreased by 20% in heterozygous lenses and by 84% in homozygous lenses compared with wild type proteins (6.0 mg/ml for wild type, 4.8 mg/ml for heterozygous and 1 mg/ml for homozygous mouse lenses). The average of at least four pooled lenses from two different preparations for each genotype is shown. The significantly lower degree of insolubilization in heterozygous knock-in mouse lenses that express both the wild type and mutant αA-crystallin, suggests that presence of wild type αA-crystallin protects against protein insolubility. Furthermore, comparison of the light scattering profiles showed that αA-R49C homozygous lenses demonstrate a peak in the void volume corresponding to aggregates of proteins of ≥2 MDa molecular mass (Figure 2). Analysis of the water-soluble proteins of αA-R49C homozygous lenses by gel permeation chromatography showed a loss of the βL fraction from the soluble phase of the αA-R49C homozygous lenses (Figure 3A). Figure 3B shows a plot of the molecular masses of protein fractions obtained from the gel permeation chromatography column. A new low molecular weight 15 kDa protein (Figure 3B, arrow) and loss of a ~60 kDa protein (Figure 3B, arrowhead) was observed in the homozygous lenses. The weight average molecular masses of the major peaks were not significantly different among the three genotypes, but the greatly reduced protein concentration (Table 1) in the homozygous lenses suggests that the formation of water-insoluble high molecular weight aggregated proteins is the major effect of the mutation. Table 2 shows the weight average molecular weight, viscosity and radius of gyration of the major protein peaks obtained by gel permeation chromatography of the water-soluble lens proteins. The UV chromatogram of the water-soluble lens proteins also clearly demonstrated a loss of the βL and γ-crystallin peak in αA-R49C homozygous lenses (Figure 4A). A comparison of the results in Figure 3A and Figure 4A suggests that the refractive index detector separated the βL- and γ-crystallins much better than the UV detector. Identity of the protein peaks fractions separated by gel permeation chromatography was confirmed by immunoblot analysis with antibodies specific to bovine α, β and γ-crystallins (Figure 4B). Surprisingly, the amount of the low molecular weight material in αA-R49C homozygous lenses was much less than expected based on the refractive index (Figure 3A) and UV absorption (280 nm) profiles (Figure 4B), suggesting that the epitope interacting with the γ-crystallin antibody was drastically reduced in these lenses. The immunoblot analysis with a αA-crystallin-specific antibody (Figure 4B) clearly demonstrated that a small amount of αA-crystallin remained soluble in the αA-R49C homozygous lenses. αA-crystallin is known to bind partially unfolded, denaturing substrates in its role as a chaperone. To determine the effect of the αA-R49C mutation on the binding of β- and γ-crystallins to the α-crystallin fraction, we next analyzed the α-crystallin fractions from wild type, heterozygous and homozygous lenses with antibodies to β- and γ-crystallin. Immunoblot analysis indicated a stable binding of β- and γ-crystallin to the α-crystallin fraction from the αA-R49C mutant lenses (Figure 4C). In contrast, β- and γ-crystallin were not associated with α-crystallin from wild type lenses. This is a clear demonstration that α-crystallin is associated with β- and γ-crystallin in the mutant lenses.
Figure 1. Gel permeation profile of lens proteins from wild type mouse lenses.
(A) Light scattering (solid gray line) and refractive index (dotted black line) measurements on proteins eluting from the column. The column was calibrated using molecular weight markers 1, dextran blue (2 MDa); 2, thyroglobulin (669 kDa); 3, ferritin (440 kDa); 4, catalase (232 kDa); 5, aldolase (156 kDa); 6, bovine serum albumin (67 kDa); 7, chymotrypsin (25 kDa) and 8, ribonuclease A (12.5 kDa). (B) The dashed line indicates the viscosity of proteins eluted from the column. The first peak is α-crystallin (αA + αB), the second is β-heavy (βH) crystallin; the third peak is βL (β-light) crystallin; and the fourth is γ-crystallin.
Table 1.
Protein concentration of water-soluble crystallins isolated from wild type, αA-R49C heterozygous and αA-R49C homozygous mouse lenses*.
| Lens genotype | Protein concentration mg/ml |
|---|---|
| Wild type | 6.0 |
| αA-R49C heterozygous | 4.8 |
| αA-R49C homozygous | 1.0 |
Lens water-soluble proteins were prepared by homogenizing mouse lenses in 1 ml Tris.Cl buffer pH 7.5 containing 100 mM NaCl, 1 mM DTT, 1 mM EDTA, 0.1%NaN3 and centrifugation at 15,000 g as described in Materials and Methods. Four lenses were used for wild type and αA-R49C heterozygous mice. Because of the significantly smaller size of the lenses of the αA-R49C homozygous mice twelve lenses were used. Note the pronounced decrease in concentration of the soluble proteins in the αA-R49C homozygous lenses.
Figure 2. Abundance of a high molecular weight soluble protein species in αA-R49C homozygous lenses detected by light scattering.
Wild type (dashed line); αA-R49C heterozygous (dotted line); αA-R49C homozygous lenses (solid gray line). Note the protein peak in the void volume of the gel permeation chromatography column in αA-R49C homozygous lens proteins but not in wild type or αA-R49C heterozygous proteins.
Figure 3. Refractive index and differential molecular weight distribution of crystallins isolated from wild type and αA-R49C mutant lenses.
(A) Refractive index profile of crystallins separated by gel permeation chromatography. Wild type (dashed line); αA-R49C heterozygous (dotted line); αA-R49C homozygous lenses (solid gray line). Note the dramatic decrease in βL-crystallin in the soluble fraction of αA-R49C homozygous lenses. (B) Differential molecular weight distribution. Wild type (dashed line); αA-R49C heterozygous (dotted line); αA-R49C homozygous lenses (solid gray line). Note the diminution of βL-crystallin (60 kDa) in the αA-R49C homozygous lenses (arrowhead). Also note the increase in a 15 kDa protein in the αA-R49C homozygous lenses (arrow).
Table 2.
The effect of αA-R49C mutation on molecular weight, intrinsic viscosity and radius of gyration of major crystallin peaks.
| α-Crystallin Fraction | Molecular weight (kDa) | Intrinsic viscosity (dl/g) | Radius of gyration Rg (nm) |
|---|---|---|---|
| Wild type lens | 1,004 | 0.065 | 13.29 |
| αA-R49C heterozygous lens | 1,061 | 0.063 | 13.22 |
| αA-R49C Homozygous lens | 1,150 | 0.077 | 14.28 |
| β-heavy Crystallin Fraction | |||
| Wild type lens | 105.2 | 0.044 | 5.32 |
| αA-R49C heterozygous lens | 114.5 | 0.046 | 5.54 |
| αA-R49C Homozygous lens | 123.0 | 0.052 | 5.85 |
| β-light Crystallin Fraction | |||
| Wild type lens | 63.1 | 0.057 | 5.04 |
| αA-R49C heterozygous lens | 65.0 | 0.043 | 4.64 |
| αA-R49C Homozygous lens | N.D. | N.D. | N.D. |
| γ-Crystallin Fraction | |||
| Wild type lens | 22.6 | 0.0418 | 3.24 |
| αA-R49C heterozygous lens | 19.2 | 0.044 | 3.11 |
| αA-R49C Homozygous lens | 19.8 | 0.038 | 2.80 |
Figure 4. UV absorbance and immunoblot analysis of lens protein fractions from wild type and αA-R49C mutant lenses.
Wild type lenses (dashed line); αA-R49C heterozygous lenses (dotted line); αA-R49C homozygous lenses (solid gray line). (A) UV absorbance at 280 nm; (B) Immunoblots represent the peak fractions of α-crystallin, βH-crystallin and βL- +γ-crystallin obtained from chromatographic separation of wild type, αA-R49C heterozygous and αA-R49C homozygous lens water-soluble proteins, respectively. Immunoblot analysis of the chromatography peak fractions with antibodies to αA-crystallin, total β-crystallin and total γ-crystallin was performed. Note the distinct loss of immunoreactivity to γ-crystallin antibody with peak 4 (γ-crystallin) in the αA-R49C homozygous lens proteins. Note also that the concentration of the water-soluble α-crystallin fraction (αA + αB) was the highest in the wild type, and decreased gradually in the αA-R49C heterozygous and homozygous lenses. (C) Immunoblot analysis of the α-crystallin fraction of wild type, αA-R49C heterozygous and αA-R49C homozygous lenses with antibodies to β- and γ-crystallin. Note the gradual increase in β- and γ-crystallin immunoreactivity in the heterozygous and homozygous α-crystallin fractions. Note also that β- and γ-crystallin immunoreactivity was absent in the wild type α-crystallin fraction.
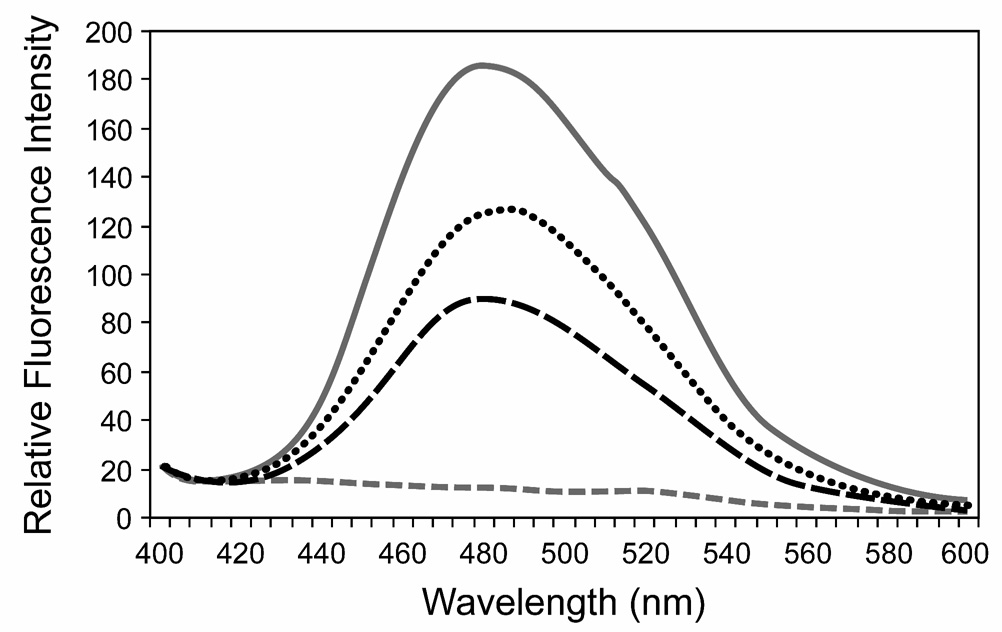
The difference in solubility of the mutant αA-crystallin in native state from those of wild type may be caused by local changes in protein conformation of the mutant αA-crystallin that expose hydrophobic patches as a result of point mutations. To test this possibility, we compared the surface hydrophobicity of the wild type and mutant αA-crystallin. The surface hydrophobicity was determined by bis-ANS fluorescence. Bis-ANS, a hydrophobic probe is non-fluorescent in aqueous solution and becomes fluorescent when bound to the hydrophobic residues on the surface of a molecule (37, 38). Figure 5 shows that the bis-ANS fluorescence increased in direct proportion with the increase in αA-R49C expression in the α-crystallin fractions from wild type, αA-R49C heterozygous and αA-R49C homozygous mouse lenses. Fluorescence intensity of bis-ANS in buffer increased eight-fold in the presence of α-crystallin from wild type lenses. The bis-ANS fluorescence further increased 1.5 and 2.2-fold over wild type in the presence of α-crystallin isolated from αA-R49C heterozygous and αA-R49C homozygous lenses, respectively.
Figure 5. Bis-ANS fluorescence in wild type and αA-R49C mutant lenses.
Fluorescence of bis-ANS (5 µM) was measured using an excitation wavelength of 360 nm. The emission was measured from 400 to 600 nm. Emission spectrum of bis-ANS in buffer (dashed gray line); 0.1 mg/ml α-crystallin fraction from wild type mouse lenses (black dashed line); α-crystallin fraction from αA-R49C heterozygous mouse lenses (dotted line); and α-crystallin fraction from αA-R49C homozygous mouse lenses (solid gray line). Note the fluorescence emission maximum of bis-ANS at 480 nm upon binding to α-crystallin. Note also the prominent enhancement in fluorescence intensity of bis-ANS bound to α-crystallin fraction isolated from αA-R49C mutant lenses.
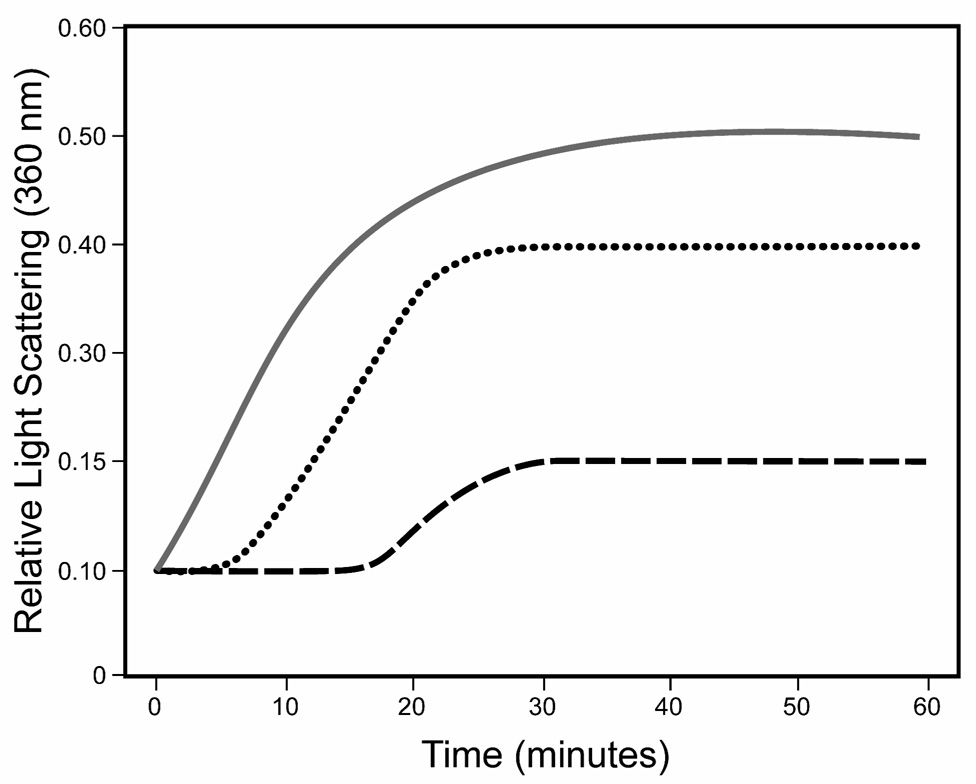
Previous studies showed that the thermodynamic stability of αA and αB-crystallin can be determined by measuring temperature-induced unfolding and insolubility (28, 39, 40). Therefore we next compared the thermal stability of the α-crystallin fraction from wild type and mutant lenses. The mutant protein was susceptible to heat-induced aggregation at 65°C within 10 minutes, whereas the wild type protein showed much lower light scattering (Figure 6). The mutant protein formed light scattering aggregates above 42°C, whereas the wild type protein remained clear and stable in solution up to 53°C. This suggested that the mutant protein is sensitive to temperatures above 42°C. The wild type protein remained in solution at 42°C but the mutant protein became insoluble and precipitated (data not shown).
Figure 6. Thermal stability of lens proteins from wild type and αA-R49C mutant lenses.
The thermal stability of α-crystallin fraction was determined by time-dependent change in light scattering as monitored at 360 nm at 65°C. Wild type (dashed line); αA-R49C heterozygous (dotted line); αA-R49C homozygous (solid gray line). By 10 minutes of incubation α-crystallin fraction of the αA-R49C lenses began to aggregate and precipitate out of solution. In contrast, wild type α-crystallin showed significantly lower light scattering.
DISCUSSION
The lenses of hereditary cataract patients in the family described by Mackay et al (26) with the αA-R49C mutation develop a ‘nuclear’ cataract at an early age. These patients have one chromosomal copy of normal αA-crystallin and one copy of the mutant allele that produces the αA-R49C protein. This chromosomal arrangement implies that in lens cells of these patients, a αA-crystallin molecule consists of complexes containing an equal amount of normal and mutant αA-crystallin subunits. To gain a better understanding of the effect of the mutation in vivo, we have created a knock-in mouse model to genetically recapitulate this cataract-causing mutation in αA-crystallin using a novel embryonic stem cell-based genetic approach (34). The αA-R49C heterozygous mice develop anterior and posterior lens changes at eye opening (three weeks old), and at 4 months, these mice also develop a nuclear opacity. The αA-R49C heterozygous mice have been interbred to generate αA-R49C homozygous mice. The homozygous mice not only develop cataracts, but also demonstrate a small eye phenotype not observed in the heterozygous mice or human patients which are heterozygous for the mutation, although micro-ophthalmia has been reported in another cataract causing mutation in αA-crystallin, αA-R116C (4).
The lenses of αA-R49C homozygous mice allowed us to assess the effect on protein insolubility in vivo in the absence of any normal αA-crystallin subunits in this study. We demonstrate that the in vivo effect of αA-R49C is to make a large proportion of the soluble crystallins insoluble in the homozygous knock-in lenses. Most small heat shock proteins assemble into polydisperse and dynamic oligomers and their heterogeneity is enhanced by substrate binding. Investigators have shown that both αA and αB-crystallin form oligomers of different sizes and shapes (41). The oligomeric assembly of mutant αA-crystallin indicates that oligomers larger than wild type protein are formed (40). The cataract causing R116C mutant of αA-crystallin forms water-soluble oligomers with weight average molecular mass twice as high as wild type protein (42). Similarly, the G98R αA-crystallin mutant protein forms highly polydisperse aggregates with average molecular mass five-fold higher than the normal protein (40). Likewise, cataract-causing mutations in αB-crystallin also give rise to water-soluble aggregates with average masses 1.5 to 2-fold higher than wild type protein (28, 43). Our results with the α-crystallin fraction isolated from the in vivo knock-in lenses showed that the radius of gyration (Rg) of the water-soluble α-crystallin aggregate increased from a value of 13.3 nm in wild type lenses to 14.3 nm in homozygous lenses, respectively. These values are based on intrinsic viscosity measurements, which is generally low for compact proteins. Our software performed the Rg calculation but did not have the hydrodynamic radius (Rh) calculating capability. Rg values are 30 to 40% higher than Rh values. For example, the published Rh of wild type lens α-crystallin is 8 to 9 nm (40). Nonetheless, Rg values reported here is a reliable and useful parameter to assess changes in protein aggregation.
The molecular mass determinations reported here are based on light scattering measurements. The water-soluble α-crystallin molecular mass increased slightly from 1,004 kDa for wild type to 1,061 kDa and 1,150 for heterozygous and homozygous lenses, respectively. In contrast to previous studies however, our results show that the major effect in vivo of the αA-R49C mutation in the homozygous lenses was a drastic reduction in overall protein solubility. These results show that when αA-R49C starts to become a higher molecular weight species, it just becomes water-insoluble. The high molecular weight peak eluting in the void volume of the light scattering profile shows a small amount of high molecular weight aggregated material in αA-R49C homozygous lenses which is still water-soluble. The presence of this high molecular weight soluble protein aggregate in the αA-R49C homozygous lenses provides direct evidence for the formation of an unstable, intermediate species in the homozygous lenses.
The finding that disruption of the αA-crystallin N-terminal arginine 49 residue profoundly affects the solubility agrees with the paradigm that sequence divergence in the N-terminal domain of small heat shock proteins is a primary mechanism for regulating the oligomer solubility and degree of order (29, 44, 45). The N-terminal domain of small heat shock proteins is known to be important in recognition of substrates (33, 46). Investigators have suggested that perturbation in the N-terminal region is transmitted to the C-terminal region of α-crystallin domain leading to an expansion of the structure of Hsp16.5 (32). Previous studies on substrate binding to mutant αA-crystallin indicated an expansion of the oligomeric assembly, and were attributed to greater substrate binding, and increase in insolubility of substrate-chaperone complex. These mutants also displayed higher affinity to their substrates (32). In our αA-R49C heterozygous and homozygous mutant lenses but not the wild type, the α-crystallin fraction separated by gel permeation chromatography had increasing amounts of stably bound β- and γ-crystallins; two proteins highly expressed in lens fiber cells. These results indicate that as αA-crystallin starts to bind β- and γ-crystallin, it begins to become insoluble, which corresponds with the phenotype of the cataractous lens. The stably bound β- and γ-crystallin identified in the α-crystallin fraction are assumed to be in an extensively unfolded state and are recognized by the chaperone protein (32). These interactions are likely to be crucial in determining the ultimate fate of the α-crystallin oligomer. The data presented in this study can be interpreted by the model that the aggregate size of mutant αA-crystallin increases to produce a water-soluble high molecular weight intermediate species that precipitates out of solution. As lens crystallins exist at a high protein concentration in lens fiber cells, the above mechanism could be an important factor underlying the human disease phenotype.
The results of the present study showed that a significant proportion of the αA-crystallin was insoluble in αA-R49C homozygous lenses. To understand the mechanism of insolubilization by the αA-R49C mutation, the hydrophobicity of α-crystallin fraction isolated by gel permeation chromatography was measured (Figure 5). Bis-ANS is a sensitive probe that has been shown to bind to a hydrophobic region of the protein (47). Investigators have shown that increasing the temperature increases the binding of bis-ANS to α-crystallin due to the exposure of hydrophobic sites on the protein (48, 49). It has also been found that denaturation and refolding of α-crystallin enhances the binding of bis-ANS to the protein due to an increase in exposure of hydrophobic sites (47, 48). The gradual enhancement in fluorescence yield of bis-ANS from wild type to heterozygous to homozygous α-crystallin fraction indicates that hydrophobicity of the protein increases by the αA-R49C mutation. Interestingly, a hydrophobic sequence from residues 41 to 58 in the related protein αB-crystallin has been shown to be selective for interactions with fully unfolded substrates (46). Similarly, studies on αB-crystallin have shown that the D140N mutation associated with hereditary cataract leads to an increase in hydrophobicity and partial unfolding of the protein (28). In addition, with aging, α-crystallin undergoes partial unfolding and increased aggregation (14, 50). Solvent exposure of hydrophobic interfaces has been shown to cause a loss of protein solubility (51, 52). A delicate balance between the positions of hydrophobic and charged amino acid can also contribute to aggregation behavior of proteins (53). Although increased hydrophobicity of proteins may not always correlate with protein insolubility, it is likely to be one of the factors contributing to protein insolubility of the αA-R49C mutant lenses. The results of the present study suggest that the αA-R49C mutation exposes hydrophobic sites and leads to higher bis-ANS binding. The adverse effect of exposure of these hydrophobic patches in mutant αA-R49C is that the proteins aggregate at high concentrations present in the lens fiber cells in vivo. The bis-ANS fluorescence enhancement uncovers a change in protein aggregation dynamic of the α-crystallin fraction of αA-R49C mutant lenses.
The most interesting finding in the present study is that a minor change in molecular weight of the α-crystallin fraction causes a substantial increase in the amount of β- and γ-crystallin associated with the water-soluble fraction of homozygous lenses (Figure 4C). The reduction in βL-crystallin and the disappearance of γ-crystallin from the soluble fraction of αA-R49C homozygous lenses (Figure 4A and Figure B) may be explained by the enhanced hydrophobicity of the mutant α-crystallin in the αA-R49C homozygous lenses. βL-crystallin disappeared from the proteins separated by gel permeation chromatography, suggesting that it became insoluble. In 3 week old knock-in mouse lenses, the interaction between αA-crystallin and β-crystallin was found to increase in heterozygous lenses by co-immunoprecipitation analysis (34). The loss of the βL-crystallin in homozygous lenses in gel permeation chromatography suggests that the interaction between βL-crystallin and αA-R49C may not be sufficiently strong to persist during gel permeation chromatography. Alternatively, α-crystallin may be partly responsible for keeping the βL-crystallin in the soluble phase, and when αA-crystallin is mutated and becomes insoluble, the solubility of β-crystallin is also diminished. Furthermore, the appearance of the lower molecular weight peak and the dramatic reduction of immunoreactivity of γ-crystallin from homozygous lenses suggest a change in the γ-crystallin epitope reacting with the antibody. Since the γ-crystallin fraction was readily detected in the soluble fraction of both wild type and heterozygous lenses with the γ-crystallin antibody, future studies will examine whether the presence of wild type α-crystallin inhibits the loss of this low molecular weight γ-crystallin.
The results of our study with αA-R49C heterozygous lens homogenates suggest that the presence of wild type αA-crystallin likely plays a role in buffering the impact of the αA-R49C mutant protein by diluting its deleterious effect on the long term protein stability. The complete loss of functional αA-crystallin in homozygous lenses enhances the substrate binding to unfolding states leading to increased insolubilization. These results clearly demonstrate that the biological effect of the αA-R49C mutation is to increase the aggregation of the water-soluble α-crystallin, ultimately causing insolubilization and lens opacification.
The αA-R49C mutation lies outside the conserved α-crystallin domain. Investigators have examined the properties of several arginine mutants in the C-terminal domain of αA-crystallin in vitro (42, 54, 55). The N-terminal mutant αA-crystallin with the arginine 49 to alanine (R49A) mutation had a molar mass significantly higher than the wild type protein (56). The αA-R49C mutant protein isolated from the heterozygous and homozygous lenses were slightly higher in radius of gyration. While most of the α-crystallin from homozygous knock-in lenses becomes water-insoluble, a small amount of αA-crystallin was detectable in the soluble fraction. As the lens fiber cells express αA- and αB-crystallin in 3:1 stoichiometry, the fact that even in the absence of any wild type αA-crystallin in αA-R49C homozygous lenses, a small amount of αA-crystallin does remain water-soluble indicates a protective role of αB-crystallin. Other studies indicate that the αA-crystallin mutation R54H, which also lies outside the conserved α-crystallin domain produces high molecular mass aggregates and recessive cataracts in mice (57). It is also well established that the molecular mass of α-crystallin increases with aging and cataract, and a water-soluble high molecular weight α-crystallin has been reported in aged human lenses (50, 58). It should be noted that the present study characterizes only the water-soluble proteins of the lens, which is not one and the same as lens transparency (14). A clear adult human lens has a significant amount of water-insoluble material. Because of the differences in water-solubility observed in the present study (Table 1), the percentage of total lens proteins being analyzed in the water-soluble protein fraction probably represents a significant proportion (80–90%, based on studies in the literature) of the total lens protein in wild type lenses, but a markedly smaller fraction in the αA-R49C homozygous lenses, with heterozygous lenses being intermediate between these two values.
In summary, using a novel gene knock-in mouse model for human hereditary cataract, the results of this study provide key insights into the mechanism of protein insolubilization by a point mutation in αA-crystallin causing hereditary human cataracts, show clear evidence that β- and γ-crystallin stably bind α-crystallin in mutant lenses, reveal an increase in surface hydrophobicity of the mutant protein, and demonstrate the existence of a water-soluble high molecular weight protein intermediate in the cataractous lenses. These data suggest that AAR49C has a tendency to become unstable and bind more unfolded substrate proteins. Expression of both wild type and mutant αA-crystallin in the heterozygous lenses reduces the formation of proteins containing exposed hydrophobic regions, reducing their transition into high molecular weight water-insoluble species. Although the molecular weight of the water-soluble α-crystallin fraction was not very different among the three different genotypes studied, the binding of β- and γ-crystallins was different, because of the differences in hydrophobicity of α-crystallin. Further studies of α-crystallin with neutron scattering in solution are in progress to determine the molecular structure of the αA-R49C protein.
Acknowledgments
This work is supported by the NIH grant EY05681 to U.P.A., Core Grant EY02687, an unrestricted grant to the Department of Ophthalmology and Visual Sciences at Washington University from RPB, and the VA Merit Review Grant to N.R.
Abbreviations
- αA-R49C
arginine to cysteine mutation in AA-crystallin
- bis-ANS
1,1’-bi(4-anilino)naphthalene-5,5’-disulfonic acid
- DTT
dithiothreitol
- HRP
horseradish peroxidase
- IgG
immunoglobulin
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis.
REFERENCES
- 1.Van Montfort R, Slingsby C, Vierling E. Structure and function of the small heat shock protein/alpha-crystallin family of molecular chaperones. Adv Protein Chem. 2001;59:105–156. doi: 10.1016/s0065-3233(01)59004-x. [DOI] [PubMed] [Google Scholar]
- 2.de Jong WW, Caspers GJ, Leunissen JA. Genealogy of the alpha-crystallin--small heat-shock protein superfamily. Int J Biol Macromol. 1998;22:151–162. doi: 10.1016/s0141-8130(98)00013-0. [DOI] [PubMed] [Google Scholar]
- 3.Andley UP. Crystallins in the eye: Function and pathology. Prog Retin Eye Res. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Litt M, Kramer P, LaMorticella DM, Murphey W, Lovrien EW, Weleber RG. Autosomal dominant congenital cataract associated with a missense mutation in the human alpha crystallin gene CRYAA. Hum Mol Genet. 1998;7:471–474. doi: 10.1093/hmg/7.3.471. [DOI] [PubMed] [Google Scholar]
- 5.Sax CM, Piatigorsky J. Expression of the alpha-crystallin/small heat-shock protein/molecular chaperone genes in the lens and other tissues. Adv Enzymol Relat Areas Mol Biol. 1994;69:155–201. doi: 10.1002/9780470123157.ch5. [DOI] [PubMed] [Google Scholar]
- 6.Andley UP. Crystallins and hereditary cataracts: molecular mechanisms and potential for therapy. Expert Rev Mol Med. 2006;8:1–19. doi: 10.1017/S1462399406000111. [DOI] [PubMed] [Google Scholar]
- 7.Kappe G, Franck E, Verschuure P, Boelens WC, Leunissen JA, de Jong WW. The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1-10. Cell Stress Chaperones. 2003;8:53–61. doi: 10.1379/1466-1268(2003)8<53:thgecs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun TX, Das BK, Liang JJ. Conformational and functional differences between recombinant human lens alphaA- and alphaB-crystallin. J Biol Chem. 1997;272:6220–6225. doi: 10.1074/jbc.272.10.6220. [DOI] [PubMed] [Google Scholar]
- 10.Andley UP, Song Z, Wawrousek EF, Bassnett S. The molecular chaperone alphaA-crystallin enhances lens epithelial cell growth and resistance to UVA stress. J Biol Chem. 1998;273:31252–31261. doi: 10.1074/jbc.273.47.31252. [DOI] [PubMed] [Google Scholar]
- 11.Andley UP, Song Z, Wawrousek EF, Brady JP, Bassnett S, Fleming TP. Lens epithelial cells derived from alphaB-crystallin knockout mice demonstrate hyperproliferation and genomic instability. Faseb J. 2001;15:221–229. doi: 10.1096/fj.00-0296com. [DOI] [PubMed] [Google Scholar]
- 12.Brady JP, Garland D, Duglas-Tabor Y, Robison WG, Jr, Groome A, Wawrousek EF. Targeted disruption of the mouse alpha α-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein alpha B-crystallin. Proc Natl Acad Sci U S A. 1997;94:884–889. doi: 10.1073/pnas.94.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brady JP, Garland DL, Green DE, Tamm ER, Giblin FJ, Wawrousek EF. AlphaB-crystallin in lens development and muscle integrity: a gene knockout approach. Invest Ophthalmol Vis Sci. 2001;42:2924–2934. [PubMed] [Google Scholar]
- 14.Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Hejtmancik F, Piatigorsky J. Lens proteins and their molecular biology. second ed. Vol. 2. Philadelphia: WB Saunders; 2000. [Google Scholar]
- 16.Lampi KJ, Shih M, Ueda Y, Shearer TR, David LL. Lens proteomics: analysis of rat crystallin sequences and two-dimensional electrophoresis map. Invest Ophthalmol Vis Sci. 2002;43:216–224. [PubMed] [Google Scholar]
- 17.Jaenicke R, Slingsby C. Lens crystallins and their microbial homologs: structure, stability, and function. Crit Rev Biochem Mol Biol. 2001;36:435–499. doi: 10.1080/20014091074237. [DOI] [PubMed] [Google Scholar]
- 18.Bettelheim FA. Light scattering in lens research: an essay on accomplishments and promises. Exp Eye Res. 2004;79:747–752. doi: 10.1016/j.exer.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Ponce A, Sorensen C, Takemoto L. Role of short-range protein interactions in lens opacifications. Mol Vis. 2006;12:879–884. [PubMed] [Google Scholar]
- 20.Benedek GB, Chylack LT, Jr, Libondi T, Magnante P, Pennett M. Quantitative detection of the molecular changes associated with early cataractogenesis in the living human lens using quasielastic light scattering. Curr Eye Res. 1987;6:1421–1432. doi: 10.3109/02713688709044506. [DOI] [PubMed] [Google Scholar]
- 21.Thurston GM, Hayden DL, Burrows P, Clark JI, Taret VG, Kandel J, Courogen M, Peetermans JA, Bowen MS, Miller D, Sullivan KM, Storb R, Stern H, Benedek GB. Quasielastic light scattering study of the living human lens as a function of age. Curr Eye Res. 1997;16:197–207. doi: 10.1076/ceyr.16.3.197.15410. [DOI] [PubMed] [Google Scholar]
- 22.Pande A, Annunziata O, Asherie N, Ogun O, Benedek GB, Pande J. Decrease in protein solubility and cataract formation caused by the Pro23 to Thr mutation in human gamma D-crystallin. Biochemistry. 2005;44:2491–2500. doi: 10.1021/bi0479611. [DOI] [PubMed] [Google Scholar]
- 23.Pande A, Pande J, Asherie N, Lomakin A, Ogun O, King J, Benedek GB. Crystal cataracts: human genetic cataract caused by protein crystallization. Proc Natl Acad Sci U S A. 2001;98:6116–6120. doi: 10.1073/pnas.101124798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pande A, Pande J, Asherie N, Lomakin A, Ogun O, King JA, Lubsen NH, Walton D, Benedek GB. Molecular basis of a progressive juvenile-onset hereditary cataract. Proc Natl Acad Sci U S A. 2000;97:1993–1998. doi: 10.1073/pnas.040554397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, Faure A, Chateau D, Chapon F, Tome F, Dupret JM, Paulin D, Fardeau M. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20:92–95. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- 26.Mackay DS, Andley UP, Shiels A. Cell death triggered by a novel mutation in the alphaA-crystallin gene underlies autosomal dominant cataract linked to chromosome 21q. Eur J Hum Genet. 2003;11:784–793. doi: 10.1038/sj.ejhg.5201046. [DOI] [PubMed] [Google Scholar]
- 27.Santhiya ST, Soker T, Klopp N, Illig T, Prakash MV, Selvaraj B, Gopinath PM, Graw J. Identification of a novel, putative cataract-causing allele in CRYAA (G98R) in an Indian family. Mol Vis. 2006;12:768–773. [PubMed] [Google Scholar]
- 28.Liu Y, Zhang X, Luo L, Wu M, Zeng R, Cheng G, Hu B, Liu B, Liang JJ, Shang F. A Novel {alpha}B-Crystallin Mutation Associated with Autosomal Dominant Congenital Lamellar Cataract. Invest Ophthalmol Vis Sci. 2006;47:1069–1075. doi: 10.1167/iovs.05-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basha E, Friedrich KL, Vierling E. The N-terminal arm of small heat shock proteins is important for both chaperone activity and substrate specificity. J Biol Chem. 2006;281:39943–39952. doi: 10.1074/jbc.M607677200. [DOI] [PubMed] [Google Scholar]
- 30.Giese KC, Basha E, Catague BY, Vierling E. Evidence for an essential function of the N terminus of a small heat shock protein in vivo, independent of in vitro chaperone activity. Proc Natl Acad Sci U S A. 2005;102:18896–18901. doi: 10.1073/pnas.0506169103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghosh JG, Estrada MR, Houck SA, Clark JI. The function of the beta3 interactive domain in the small heat shock protein and molecular chaperone, human alphaB crystallin. Cell Stress Chaperones. 2006;11:187–197. doi: 10.1379/CSC-186.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koteiche HA, McHaourab HS. Mechanism of a hereditary cataract phenotype. Mutations in alphaA-crystallin activate substrate binding. J Biol Chem. 2006;281:14273–14279. doi: 10.1074/jbc.M512938200. [DOI] [PubMed] [Google Scholar]
- 33.Shi J, Koteiche HA, McHaourab HS, Stewart PL. Cryoelectron microscopy and EPR analysis of engineered symmetric and polydisperse Hsp16.5 assemblies reveals determinants of polydispersity and substrate binding. J Biol Chem. 2006;281:40420–40428. doi: 10.1074/jbc.M608322200. [DOI] [PubMed] [Google Scholar]
- 34.Xi JH, Bai F, Gross J, Townsend RR, Menko AS, Andley UP. Mechanism of small heat shock protein function in vivo: A knockin mouse model demonstrates that the R49C mutation in alpha α-crystallin enhances protein insolubility and cell death. J Biol Chem. 2007;283:5801–5814. doi: 10.1074/jbc.M708704200. [DOI] [PubMed] [Google Scholar]
- 35.Andley UP, Mathur S, Griest TA, Petrash JM. Cloning, expression, and chaperone-like activity of human alphaA-crystallin. J Biol Chem. 1996;271:31973–31980. doi: 10.1074/jbc.271.50.31973. [DOI] [PubMed] [Google Scholar]
- 36.Fleming TP, Song Z, Andley UP. Expression of growth control and differentiation genes in human lens epithelial cells with extended life span. Invest Ophthalmol Vis Sci. 1998;39:1387–1398. [PubMed] [Google Scholar]
- 37.Liang JJ, Fu L. Decreased subunit exchange of heat-treated lens alpha A-crystallin. Biochem Biophys Res Commun. 2002;293:7–12. doi: 10.1016/S0006-291X(02)00176-6. [DOI] [PubMed] [Google Scholar]
- 38.Liang JJ, Sun TX, Akhtar NJ. Heat-induced conformational change of human lens recombinant alphaA- and alphaB-crystallins. Mol Vis. 2000;6:10–14. [PubMed] [Google Scholar]
- 39.Sun TX, Akhtar NJ, Liang JJ. Thermodynamic stability of human lens recombinant alphaA- and alphaB-crystallins. J Biol Chem. 1999;274:34067–34071. doi: 10.1074/jbc.274.48.34067. [DOI] [PubMed] [Google Scholar]
- 40.Murugesan R, Santhoshkumar P, Sharma KK. Cataract-causing alphaAG98R mutant shows substrate-dependent chaperone activity. Mol Vis. 2007;13:2301–2309. [PubMed] [Google Scholar]
- 41.Haley DA, Bova MP, Huang QL, McHaourab HS, Stewart PL. Small heat-shock protein structures reveal a continuum from symmetric to variable assemblies. J Mol Biol. 2000;298:261–272. doi: 10.1006/jmbi.2000.3657. [DOI] [PubMed] [Google Scholar]
- 42.Shroff NP, Cherian-Shaw M, Bera S, Abraham EC. Mutation of R116C results in highly oligomerized alpha α-crystallin with modified structure and defective chaperone-like function. Biochemistry. 2000;39:1420–1426. doi: 10.1021/bi991656b. [DOI] [PubMed] [Google Scholar]
- 43.Bova MP, Yaron O, Huang Q, Ding L, Haley DA, Stewart PL, Horwitz J. Mutation R120G in alphaB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function. Proc Natl Acad Sci U S A. 1999;96:6137–6142. doi: 10.1073/pnas.96.11.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bova MP, McHaourab HS, Han Y, Fung BK. Subunit exchange of small heat shock proteins. Analysis of oligomer formation of alphaA-crystallin and Hsp27 by fluorescence resonance energy transfer and site-directed truncations. J Biol Chem. 2000;275:1035–1042. doi: 10.1074/jbc.275.2.1035. [DOI] [PubMed] [Google Scholar]
- 45.Berengian AR, Parfenova M, McHaourab HS. Site-directed spin labeling study of subunit interactions in the alpha-crystallin domain of small heat-shock proteins. Comparison of the oligomer symmetry in alphaA-crystallin, HSP 27, and HSP 16.3. J Biol Chem. 1999;274:6305–6314. doi: 10.1074/jbc.274.10.6305. [DOI] [PubMed] [Google Scholar]
- 46.Ghosh JG, Shenoy AK, Jr, Clark JI. N- and C-Terminal motifs in human alphaB crystallin play an important role in the recognition, selection, and solubilization of substrates. Biochemistry. 2006;45:13847–13854. doi: 10.1021/bi061471m. [DOI] [PubMed] [Google Scholar]
- 47.Sharma KK, Kaur H, Kumar GS, Kester K. Interaction of 1,1'-bi(4-anilino)naphthalene-5,5'-disulfonic acid with alpha-crystallin. J Biol Chem. 1998;273:8965–8970. doi: 10.1074/jbc.273.15.8965. [DOI] [PubMed] [Google Scholar]
- 48.Das BK, Liang JJ. Detection and characterization of alpha-crystallin intermediate with maximal chaperone-like activity. Biochem Biophys Res Commun. 1997;236:370–374. doi: 10.1006/bbrc.1997.6950. [DOI] [PubMed] [Google Scholar]
- 49.Das KP, Surewicz WK. Temperature-induced exposure of hydrophobic surfaces and its effect on the chaperone activity of alpha-crystallin. FEBS Lett. 1995;369:321–325. doi: 10.1016/0014-5793(95)00775-5. [DOI] [PubMed] [Google Scholar]
- 50.Liang JN. Fluorescence study of the effects of aging and diabetes mellitus on human lens alpha-crystallin. Curr Eye Res. 1987;6:351–355. doi: 10.3109/02713688709025187. [DOI] [PubMed] [Google Scholar]
- 51.Norledge BV, Mayr EM, Glockshuber R, Bateman OA, Slingsby C, Jaenicke R, Driessen HP. The X-ray structures of two mutant crystallin domains shed light on the evolution of multi-domain proteins. Nat Struct Biol. 1996;3:267–274. doi: 10.1038/nsb0396-267. [DOI] [PubMed] [Google Scholar]
- 52.Pande A, Zhang J, Shekhtman A, Pande J. Invest Ophthalmol Vis Sci Supplement. 2008. p. 284. [Google Scholar]
- 53.Rivers RC, Kumita JR, Tartaglia GG, Dedmon MM, Pawar A, Vendruscolo M, Dobson CM, Christodoulou J. Molecular determinants of the aggregation behavior of alpha- and beta-synuclein. Protein Sci. 2008;17:887–898. doi: 10.1110/ps.073181508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andley UP, Patel HC, Xi JH. The R116C mutation in alpha α-crystallin diminishes its protective ability against stress-induced lens epithelial cell apoptosis. J Biol Chem. 2002;277:10178–10186. doi: 10.1074/jbc.M109211200. [DOI] [PubMed] [Google Scholar]
- 55.Brown Z, Ponce A, Lampi K, Hancock L, Takemoto L. Differential binding of mutant (R116C) and wildtype alphaA crystallin to actin. Curr Eye Res. 2007;32:1051–1054. doi: 10.1080/02713680701769989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Biswas A, Miller A, Oya-Ito T, Santhoshkumar P, Bhat M, Nagaraj RH. Effect of site-directed mutagenesis of methylglyoxal-modifiable arginine residues on the structure and chaperone function of human alphaA-crystallin. Biochemistry. 2006;45:4569–4577. doi: 10.1021/bi052574s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xia CH, Liu H, Chang B, Cheng C, Cheung D, Wang M, Huang Q, Horwitz J, Gong X. Arginine 54 and Tyrosine 118 residues of {alpha}A-crystallin are crucial for lens formation and transparency. Invest Ophthalmol Vis Sci. 2006;47:3004–3010. doi: 10.1167/iovs.06-0178. [DOI] [PubMed] [Google Scholar]
- 58.Liang JN, Andley UP, Chylack LT., Jr Spectroscopic studies on human lens crystallins. Biochim Biophys Acta. 1985;832:197–203. doi: 10.1016/0167-4838(85)90332-2. [DOI] [PubMed] [Google Scholar]