Abstract
Formation of receptor complexes between μ-opioid and α2A-adrenergic receptors has been demonstrated in transfected cells. The functional significance and underlying mechanisms of such receptor interactions remain to be determined in neuronal systems. We examined functional interactions between endogenous μ and α2A receptors in mouse dorsal root ganglion neurons. Acute application of the μ agonist [d-Ala2,N-MePhe4, Gly-ol5]enkephalin (DAMGO) or the α2 agonist clonidine inhibited voltage-gated Ca2+ currents in these neurons. Prolonged treatment with either DAMGO or clonidine induced a mutual cross-desensitization between μ and α2A receptor-mediated current inhibition. The cross-desensitization was closely associated with simultaneous internalization of μ and α2A receptors. Morphine, a μ agonist triggering little μ receptor endocytosis, induced neither cross-desensitization nor internalization of α2A receptors. Furthermore, inhibition of p38 MAPK prevented the cross-desensitization as well as cointernalization of μ and α2A receptors. Changes in receptor trafficking profiles suggested that p38 MAPK activity was required for initiating μ receptor internalization and maintaining possible μ-α2A association during their cointernalization. Finally, the μ-α2A cross-desensitization was absent in dorsal root ganglion neurons lacking β-arrestin 2. These findings demonstrated p38 MAPK- and β-arrestin 2-dependent cross-regulation between neuronal μ and α2A receptors. By promoting receptor cross-desensitization and cointernalization, such functional interactions may serve as negative feedback mechanisms triggered by prolonged agonist exposure to modulate the signaling of functionally related G protein-coupled receptors.
G protein-coupled receptors (GPCRs)2 interact with each other through formation of receptor complexes, including homo- or heterodimers and possibly higher order oligomers (1–4). Heterooligomerization of GPCRs has been shown to enable cross-regulation between different receptor systems, resulting in various changes in receptor binding, signaling, and trafficking. For example, dimerization of μ-opioid and NK1 (neurokinin type 1) receptors in HEK-293 cells promotes agonist-induced cross-phosphorylation and cointernalization of the two receptors, whereas receptor binding and functional coupling are relatively unaffected (5). A similar pattern has been observed in cells expressing heterodimers of δ-opioid and β2-adrenergic receptors (6). Formation of μ- and δ-opioid receptor complexes alters receptor properties, leading to synergistic enhancement of receptor binding and signaling by μ and δ ligands (7). The μ-δ heterodimer may form as early as in the endoplasmic reticulum during receptor processing and allows co-trafficking of the two receptors (8). Controversial evidence exists, however, for agonist-induced, separate endocytosis of μ and δ receptors (9). Although these studies and many others have underscored the dynamic nature and divergent roles of receptor heterodimerization in GPCR modulation, the molecular basis and regulatory mechanisms for such interactions remain to be elucidated. In particular, most studies addressing this issue have been conducted in heterologous cells or in systems where receptors are overexpressed, which may lead to interactions nonexistent with endogenously expressed receptors. Further studies are necessary to identify and characterize interactions between naturally existing GPCRs in primary neurons.
We examined interactions between endogenous μ-opioid and α2A-adrenergic receptors in mouse dorsal root ganglion (DRG) neurons. Both μ and α2A receptors are coupled to Gi and Go proteins and induce similar cellular responses, such as inhibition of voltage-gated Ca2+ channels and activation of inwardly rectifying potassium channels. These cellular effects can lead to presynaptic inhibition of neurotransmitter release or hyperpolarization of postsynaptic neurons. Both are crucial mechanisms for opioid and adrenergic modulation of nociception. A functional synergy between the two systems has been demonstrated in vivo, evidenced by potentiation of morphine analgesia (10, 11) and alleviation of opiate withdrawal (12) by the α2-adrenergic agonist clonidine. Studies in transgenic mice lacking functional α2A receptors further indicate that the α2A receptor is the principal subtype mediating α2 agonist-induced analgesia at the spinal level (13) and responsible for the synergistic potentiation of morphine analgesia (14). The exact mechanisms for this adrenergic-opioid synergy, however, remain unclear. Recently, the μ-α2A receptor heterodimers have been detected in HEK-293 cells co-expressing both receptors (15, 16). It is of great interest to further explore whether heterodimerization of μ and α2A receptors serves as a novel mechanism coordinating the function of both receptors in pain-processing pathways. Here we report that endogenous μ and α2A receptors interact in DRG sensory neurons via p38 MAPK- and β-arrestin 2-dependent mechanisms, which promote agonist-selective cross-regulation of receptor signaling and internalization.
EXPERIMENTAL PROCEDURES
DRG Cultures—Primary DRG cultures were prepared as described previously (17). Briefly, the ganglia were collected from postnatal day 0–3 pups of C57 BL/6 mice, enzymatically dissociated for 30 min with minimal essential medium containing 0.25% trypsin at 37 °C, and triturated with fire-polished Pasteur pipettes. Dissociated neurons were plated onto glass coverslips coated with poly-l-ornithine and laminin. The cultures were maintained at 37 °C in 5% CO2; fed with serum-free Neurobasal-A medium supplemented with B-27, l-glutamine, 2.5s nerve growth factor (0.1 μg/ml; Invitrogen), and 5-fluoxy-d-uridine (0.1 mg/ml; Sigma); and studied after 2–5 days in vitro.
DRG cultures were also prepared using postnatal day 0–3 mice lacking α2A adrenergic receptors (α2A-/-) or β-arrestin 2 (β-arr2-/-) and their respective wild-type (+/+) controls. The α2A-/- (stock number 004367; Jackson Laboratory) (18) and β-arr2-/- lines (19) have both been fully back-crossed to the C57 BL/6 background (10 generations). The +/+ mice used in the same experiments with the knockouts were within two generations of heterozygous mating.
Electrophysiological Recordings—The voltage-gated Ca2+ currents were recorded from DRG neurons with 15–30-μm diameters under whole-cell voltage clamp conditions, as described (17). Cells were perfused with an external solution containing 10 mm CaCl2, 130 mm tetraethylammonium chloride, 5 mm HEPES, 25 mm d-glucose, and 0.25 μm tetrodotoxin at pH 7.35. The patch electrode was filled with an internal solution composed of 105 mm CsCl, 40 mm HEPES, 5 mm d-glucose, 2.5 mm MgCl2, 10 mm EGTA, 2 mm Mg-ATP, and 0.5 mm GTP at pH 7.2. Ca2+ currents were evoked every 10 s by 40-ms voltage steps from -80 to +10 mV using an Axopatch 200A patch clamp amplifier. Capacitance and series resistance were corrected with the compensation circuitry on the amplifier. Series resistance was compensated by 80–90%. Leak currents were subtracted using a P/6 protocol. Recorded signals were acquired and analyzed using Axon pCLAMP version 8.0 software (Axon Instruments). The amplitude of peak Ca2+ currents was determined using the peak detect feature of the software.
Drug Application and Desensitization Protocols—The μ and α2 receptor ligands (Sigma) were prepared as stock solutions in water, diluted with external solution to the final concentration for acute bath application, or added into culture medium for pretreatment. Various kinase inhibitors (Sigma) were dissolved in DMSO and diluted with culture medium for pretreatment with a final DMSO concentration of 0.1%. Cells pretreated with the medium containing 0.1% DMSO served as vehicle controls in these experiments. In additional control experiments, DAMGO- or clonidine-induced current inhibition was compared between untreated cells and cells pretreated with 0.1% DMSO for 4 h. No significant differences were observed between the two treatments (data not shown).
During recording, the external solution was continuously applied at 2 ml/min through a 0.5-ml recording chamber carrying the culture coverslip. After establishing a stable base line, the μ or α2 agonist was applied for up to 1 min to observe the maximal change in Ca2+ currents. Agonist-induced current inhibition was measured as the maximal reduction in the peak current amplitude during drug perfusion and expressed as percentage changes from the base-line level. The voltage dependence of agonist effect was assessed using a prepulse facilitation (PPF) protocol consisting of two normal test pulses (P1 and P2) and in between a strong depolarizing prepulse (-80 to +80 mV, 40 ms) delivered 10 ms before P2. The PPF was expressed by the amplitude ratio of currents activated by the two test pulses (P2/P1). To induce chronic desensitization, DRG cultures were pretreated with either μ or α2 agonist for 4 h. After extensive washing, whole-cell recording was performed in pretreated cells, and the acute inhibitory effect of μ or α2 agonist on Ca2+ currents was measured during a brief perfusion (0.5–1 min). The extent of desensitization was determined by the percentage reduction of the agonist effect in pretreated neurons relative to untreated neurons.
Immunocytochemical Analysis and Fluorescence Confocal Microscopy—Cellular distribution of μ and α2A receptors in DRG cultures was determined by immunocytochemical double labeling. After drug treatment, the cultures were fixed with 4% paraformaldehyde for 10 min, washed in phosphate-buffered saline (PBS), permeabilized, and preblocked with PBS containing 10% normal donkey serum and 0.1% Triton X-100 for 2 h at room temperature. The cultures were then incubated overnight at 4 °C with the primary antibodies against the C-terminal sequence of the μ receptor (20) or of the α2A receptor (21) (sc-1478 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); ab45871 (Abcam)). After washing, the cells were further incubated for 2 h with Alexa Fluor 488-labeled donkey anti-rabbit IgG for visualization of μ receptors and with biotinylated anti-goat IgG (Calbiochem) and Cy3-conjugated streptavidin (Calbiochem) to detect α2A receptors. Control samples were prepared in the absence of the primary or secondary antibodies. The specificity of the anti-α2A antibody was further confirmed by the absence of α2A receptor labeling in neurons from the α2A-/- mice (see Fig. 4B). The images of neurons with receptor labeling were acquired using a Leica TCS-SP confocal laser-scanning microscope and processed either as a single scan or as maximum intensity projections of multiple scans taken at successive 1-μm depths.
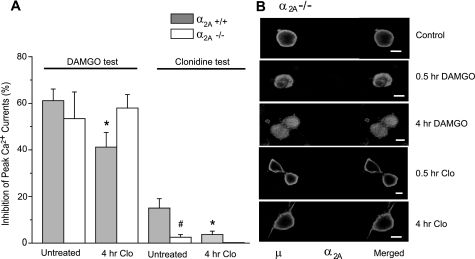
FIGURE 4.
Clonidine does not induce μ receptor internalization and cross-desensitization in α2A-/- neurons. A, DRG cultures derived from the α2A-/- and α2A+/+ mice were pretreated with 10 μm clonidine for 4 h and tested with DAMGO or clonidine. No cross-desensitization to DAMGO was observed in α2A-/- neurons. Also note the lack of responses to clonidine in α2A-/- neurons. n = 4–8 for each group; *, p < 0.05 compared with untreated neurons of the same genotype. #, p < 0.05 compared with cells with different genotype but receiving the same treatment. B, confocal microscopic images of α2A-/- neurons processed for double labeling of μ and α2A receptors. Note the absence of α2A receptor labeling and the inability of clonidine to induce μ receptor endocytosis in these neurons. Scale bars, 10 μm.
Flow Cytometric Measurement of Surface μ Receptors—Internalization of μ receptors was further accessed in living neurons by quantifying cell surface receptors with flow cytometry as described previously (22). DRG cultures were treated with clonidine, DAMGO, morphine, or control medium for 4 h at 37 °C. Monensin (10 μm) was added during the treatment to block recycling of internalized receptors in all experiments. DRG cells were then harvested in PBS containing 2 mm EDTA, spun at 300 × g for 5 min, and resuspended with PBS containing 1% normal goat serum and 0.1% NaN3. Cell surface μ receptors were labeled at 4 °C for 30 min with a polyclonal antibody against the third extracellular loop of the receptor (Chemicon International) (23) and visualized with Alexa Fluor 488-labeled donkey anti-rabbit IgG at 4 °C for another 30 min. Cell surface immunofluorescence was measured with a FACScan flow cytometer at 5,000–10,000 cells/sample. Data were acquired and analyzed using Cell Quest version 3.0.1 (BD Bioscience). The loss of cell surface μ receptors after agonist exposure was quantified by the reduction in the proportion of cells expressing detectable surface μ receptors (μ-positive cells) and in the density of surface receptors of μ-positive cells reflected by their mean fluorescence intensity. Nonspecific background fluorescence was determined in control samples processed without the primary antibody and was subtracted to obtain mean relative fluorescence intensity of experimental samples.
Measurement of Phospho-p38 MAPK—The cellular level of phospho-p38 MAPK was measured with flow cytometry (24), using a polyclonal antibody recognizing p38 MAPK dually phosphorylated at Thr180 and Tyr182 (Cell Signaling Technology) (25). After drug treatment, DRG cells were harvested from culture plates with PBS buffer containing 4 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, 0.1 mm calyculin A, 1 μg/ml leupeptin, and protease inhibitor mixture tablet (Sigma) (26). The cells were fixed in 2% formaldehyde for 10 min at room temperature, permeabilized in ice-cold 90% methanol for 30 min, incubated with the primary antibody for 60 min at room temperature, and labeled with an Alexa Fluor 488-conjugated second antibody for another 60 min. In between these steps, the cells were washed, centrifuged at 450 × g for 5 min, and resuspended with fresh PBS. After the final wash, the fluorescence signal of the cells was analyzed with a FACScan flow cytometer at 5,000 cells/sample, and the data were processed with CellQuest software.
Statistical Analysis—All data are presented as means ± S.E. One-way analysis of variance was applied for overall statistical significance across multiple group means, followed by the Bonferroni post hoc test for pairwise comparisons. Statistical significance was defined as p < 0.05.
RESULTS
α2-Adrenergic Agonist-induced Cross-desensitization to μ-Opioids—Brief bath application of norepinephrine (NE) at 10 μm induced acute inhibition of voltage-gated Ca2+ currents in DRG neurons (Fig. 1A). This effect was significantly attenuated by a selective α2 antagonist yohimbine, confirming involvement of α2 receptors in NE action (27.2 ± 5.0% current inhibition without yohimbine versus 9.7 ± 1.6% with 10 μm yohimbine, n = 12 and 8, p < 0.05). Acute application of a selective α2 agonist, clonidine (10 μm), induced similar reductions in Ca2+ currents (20.0 ± 4.2%, n = 12) reversible by co-application of yohimbine (5.7 ± 1.6%, n = 10, p < 0.01 compared with clonidine alone). The effect of NE or clonidine was substantially reduced in neurons pretreated with the same agonist for 4 h (6.4 ± 1.7%, n = 9 for NE; 7.6 ± 1.1%, n = 8 for clonidine, p < 0.01 compared with the effects in untreated neurons), indicating development of homologous desensitization to α2 receptor-mediated effect. As expected, this desensitization was blocked by yohimbine added during NE or clonidine pretreatment (Fig. 1A).
FIGURE 1.
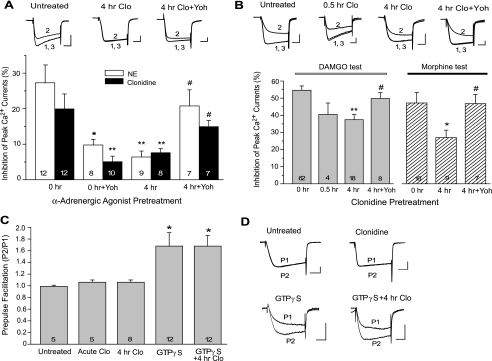
The α2-adrenergic agonist-induced cross-desensitization to μ-opioids in DRG neurons. A and B, DRG cultures were pretreated with NE or the selective α2 agonist clonidine at 10 μm for 4 h and then tested with the same agonist (A) or with the μ-opioid agonist DAMGO or morphine at 1 μm (B). Inhibition of whole-cell Ca2+ currents by α2 or μ agonists was significantly reduced in clonidine-pretreated cells, suggesting development of homologous α2 receptor desensitization and cross desensitization to μ-opioids. Both forms of desensitization were prevented by co-treatment with 10 μm yohimbine (Yoh), a selective α2 antagonist. The number of cells in each group is displayed at the bottom of the column.*, p < 0.05; **, p < 0.01 compared with untreated neurons (0 h). #, p < 0.05 compared with neurons pretreated with clonidine alone (4 h). The top panels are representative current recordings collected before (1), during (2), and after (3) acute test with clondine (A) or DAMGO (B). Cells were pretreated as labeled. Calibration was 1 nA, 10 ms. C, PPF in clonidine- or GTPγS-treated cells. D, representative recordings of PPF. Ca2+ currents elicited by two test pulses, P1 and P2, were superimposed. P2 was preceded by a 40-ms depolarizing prepulse (-80 to +80 mV; data not shown). Calibration was 0.5 nA, 10 ms.
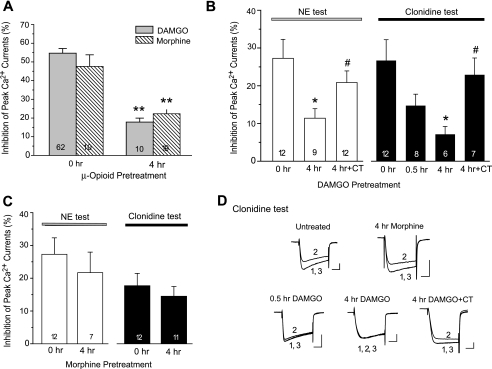
We then determined the influence of prolonged α2 agonist exposure on μ-opioid-induced Ca2+ current inhibition. In DRG neurons pretreated with 10 μm clonidine for 0.5 or 4 h, the subsequent DAMGO application (1 μm, 1 min) reduced Ca2+ currents by 40.5 ± 6.7% (n = 4) and 37.4 ± 3.0% (n = 18), respectively. The latter was significantly smaller than the responses in untreated cells (0 h, 54.7 ± 2.5%, n = 62, p < 0.01), indicative of a cross-desensitization to DAMGO. Similarly, morphine application (1 μm, 1 min) reduced Ca2+ currents by 47.6 ± 6.2% in control cells (0 h, n = 10) but by only 27.2 ± 4.4% in cells pretreated with clonidine for 4 h (n = 9, p < 0.05). Thus, prolonged NE or clonidine exposure heterologously reduced the effect of subsequently applied μ-opioid agonists. This cross-desensitization was prevented by yohimbine co-treatment (Fig. 1B).
Inhibition of neuronal Ca2+ channels by GPCRs is mediated by both voltage-dependent and voltage-independent mechanisms. The voltage-dependent inhibition requires direct binding of G-protein βγ subunits (Gβγ) to the Ca2+ channel, a process reversible by strong depolarization. To determine whether this direct Gβγ-channel interaction was affected by clonidine, we examined voltage dependence of clonidine action using the PPF protocol (Fig. 1, C and D). In cells acutely perfused with clonidine (10 μm, 1 min), the depolarizing prepulse produced little relief of inhibition, with a P2/P1 ratio similar to that under basal conditions (1.06 ± 0.04 versus 0.99 ± 0.02, n = 5 for each group, p > 0.05). Clonidine pretreatment for 4 h was also without effect on the P2/P1 ratio subsequently tested either in control perfusion medium (0.97 ± 0.02, n = 8) or during acute clonidine test (1.06 ± 0.03, n = 8). These results suggested that at the concentration we used, the effect of clonidine was primarily mediated by voltage-independent mechanisms. We then measured PPF induced by intracellular application of 0.1 mm GTPγS that can directly activate G proteins and release Gβγ subunits. GTPγS-induced PPF was not significantly different between untreated control cells and clonidine-pretreated cells (1.68 ± 0.23 versus 1.68 ± 0.18 at 8 min after the application, n = 12 for each group, p > 0.05) (Fig. 1, C and D). This confirmed that clonidine-induced desensitization was not associated with a diminished cell capacity for voltage-dependent Gβγ-Ca2+ channel interactions.
DAMGO-induced Cross-desensitization to α2A Adrenergic Responses—As we previously reported (17, 22), application of 1 μm DAMGO or morphine strongly inhibited Ca2+ currents in DRG neurons, but their effects were desensitized dramatically following a 4-h pretreatment (Fig. 2A). The inhibitory effect of 10 μm NE or clonidine on Ca2+ currents also significantly decreased in DAMGO-treated cells (11.4 ± 2.5%, n = 9 for NE; 5.3 ± 1.6%, n = 6 for clonidine, p < 0.05 for both as compared with untreated cells). This cross-desensitization was prevented by a selective μ receptor antagonist, Cys2, Tyr3, Arg5, Pen7-amide (CTAP), added during DAMGO pretreatment (Fig. 2B). Interestingly, the effect of NE or clonidine was not significantly reduced in morphine-pretreated cells (21.7 ± 6.3%, n = 7 for NE; 16.3 ± 3.3%, n = 11 for clonidine, p > 0.05 for both as compared with untreated cells), despite development of homologous morphine desensitization in these cells (Fig. 2A). Thus, the cross-desensitization to clonidine was induced by prolonged treatment with DAMGO but not morphine.
FIGURE 2.
The μ agonist-induced cross desensitization to α2-adrenergic responses in DRG neurons. A, DRG cultures were pretreated with either DAMGO or morphine at 1 μm for 4 h and tested with the same agonist after washing. Both μ agonists induced homologous μ receptor desensitization. B and C, cultures were pretreated with a μ agonist and tested with 10 μm NE or clonidine. DAMGO but not morphine induced cross-desensitization to the effect of α2 agonists. The selective μ antagonist CTAP (CT; 1 μm) applied with DAMGO blocked induction of cross-desensitization. *, p < 0.05; **, p < 0.01 compared with untreated neurons (0 h). #, p < 0.05 compared with neurons pretreated with DAMGO alone (4 h). D, representative current recordings collected before (1), during (2), and after (3) acute clonidine test. Cells were pretreated as labeled. Calibration was 0.5 nA, 10 ms.
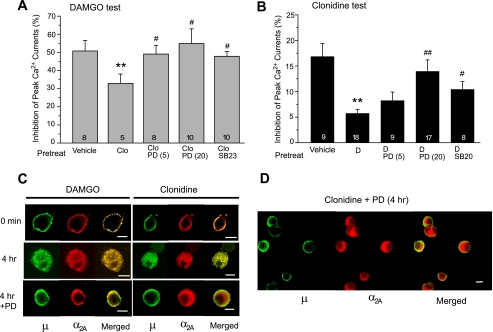
Since clonidine is relatively nonselective to different subtypes of the α2 receptors, we examined the role of α2A receptors in the acute and chronic effects of clonidine. Acute application of 10 μm clonidine induced negligible current inhibition in DRG neurons derived from the α2A-/- mice (2.5 ± 1.1%, n = 6, p > 0.05). In contrast to the wild-type neurons, the α2A-/- neurons also displayed no cross-desensitization to DAMGO following clonidine pretreatment (Fig. 4A). These results suggested that in our preparation, the α2A receptor was the major subtype responsible for clonidine-induced Ca2+ current inhibition and its cross-desensitization to DAMGO.
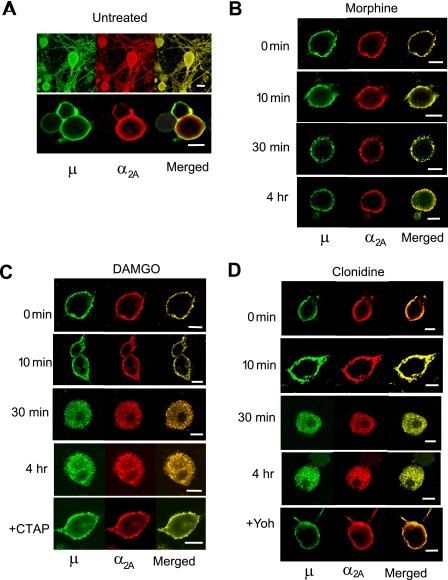
Agonist-induced Cointernalization of μ and α2A Receptors— Next we determined whether the mutual cross-desensitization between μ and α2A receptors was associated with changes in agonist-induced receptor internalization. Immunohistochemical double labeling and confocal microscopy showed that a substantial portion of μ and α2A receptors were colocalized on the cell membrane throughout the cell body and processes of cultured DRG neurons under basal conditions (Fig. 3A). Incubation with DAMGO for 30 min to 4 h induced simultaneous internalization of both receptors, which was blocked by co-incubation with the μ antagonist CTAP (Fig. 3C). Clonidine also elicited internalization of both μ and α2A receptors, reversible by the α2 antagonist yohimbine (Fig. 3D). In contrast, morphine treatment up to 4 h failed to internalize either μ or α2A receptors (Fig. 3B). Furthermore, clonidine did not induce μ receptor endocytosis in α2A-/- neurons (Fig. 4B). Thus, similar to the cross-desensitization, the cointernalization was agonist-selective and dependent upon the presence of functional μ and α2A receptors.
FIGURE 3.
Confocal microscopic images of DRG neurons showing colocalization and cointernalization of μ and α2A receptors. Cultured neurons were fixed, permeabilized, and labeled with polyclonal antibodies against native μ and α2A receptors. The μ receptor was visualized with Alexa Fluor 488-labeled anti-rabbit IgG (green), and the α2A receptor was detected with biotinylated anti-goat IgG and Cy3-conjugated streptavidin (red). A, in untreated neurons, μ and α2A receptors were colocalized in plasma membrane throughout cell bodies and processes as shown by multiple scan images (top). Single scan images (bottom) reveal a substantial but not complete overlapping of μ and α2A receptor localization in cell membrane. C and D, treatment with DAMGO (1 μm) or clonidine (10 μm) induced simultaneous internalization of μ and α2A receptors, which was blocked by the selective μ antagonist CTAP or the α2 antagonist yohimbine (Yoh), respectively. B, morphine (1 μm) failed to trigger internalization of either μ or α2A receptors. All experiments were repeated at least three times, and representative samples are shown here. Scale bars, 10 μm.
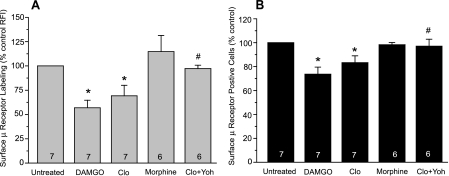
Fluorescence flow cytometry was then conducted in living DRG neurons to quantify agonist-induced μ receptor internalization. Surface μ receptors were detected in 77.7 ± 3% of control cells. DAMGO treatment for 4 h reduced the portion of cells expressing detectable levels of surface μ receptors (μ-positive cells) by 26.3 ± 5.8% (p < 0.05) relative to the controls (Fig. 5B). Furthermore, the intensity of surface μ receptor labeling in the μ-positive cells decreased by 43.1 ± 7.8% (p < 0.05) following DAMGO treatment (Fig. 5A). These changes confirmed loss of surface μ receptors due to DAMGO-induced receptor internalization. Consistent with previous reports in heterologous cells (20, 27), morphine was much less effective in triggering μ receptor internalization, causing little change in surface μ receptor expression after a 4-h treatment. Importantly, clonidine pretreatment reduced the portion of μ-positive cells by 16.7 ± 5.7% and the level of surface μ receptors by 30.8 ± 10.9% (p < 0.05 for both compared with the controls), and both effects were blocked by yohimbine (Fig. 5).
FIGURE 5.
Flow cytometric analysis of μ receptor internalization induced by μ or α2 agonists. DRG cultures were treated with DAMGO (1 μm), morphine (1 μm), or clonidine (10 μm) for 4 h. Cell surface μ receptors were labeled with an antibody recognizing the third extracellular loop of the μ receptor. Agonist-induced μ receptor internalization was quantified by percentage changes in the mean relative fluorescence intensity (RFI) of surface μ receptor labeling (A) and in the proportion of the cells expressing a detectable level of surface μ receptors (μ-positive cells) (B). Both DAMGO and clonidine significantly reduced surface μ receptor labeling, whereas morphine failed to do so. The effect of clonidine was blocked by co-treatment with yohimbine (Clo + Yoh). *, p < 0.05 compared with untreated cells. #, p < 0.05 compared with cells treated with clonidine alone (Clo).
Blockade of μ-α2A Cross-desensitization and Cointernalization by p38 MAPK Inhibitors—To explore the signaling mechanisms underlying μ-α2A interactions, we examined potential involvement of several opioid-activated protein kinases in the cross-desensitization. DRG neurons were pretreated with clonidine (10 μm, 4 h) in the presence of a selective kinase inhibitor, washed, and tested for acute DAMGO responses. As shown in Fig. 6A, co-treatment with a selective p38 MAPK inhibitor PD169316 (26) attenuated clonidine-induced cross desensitization. Acute DAMGO responses were significantly greater in cells co-treated with 5 or 20 μm PD169316 (49.0 ± 4.8% (n = 8) or 54.9 ± 8.1% (n = 10)) compared with cells treated with clonidine alone (37.4 ± 3.0%, n = 18, p < 0.05 for both comparisons). Another selective p38 MAPK inhibitor, SB239063 (28), also significantly reduced the cross-desensitization when co-applied with clonidine. In contrast, inhibitors of several other protein kinases failed to prevent clonidine-induced cross-desensitization, which included the phosphoinositide 3-kinase inhibitor LY294002 (10 μm), the extracellular signal-regulated kinase (ERK) inhibitor PD98059 (10 μm), the protein kinase A inhibitor rpCAMP (1 mm), and the protein kinase C inhibitor Go6987 (0.1 μm). Acute DAMGO responses in cells co-treated with these inhibitors was 41.7 ± 2.7% (n = 14), 38.5 ± 6.3% (n = 7), 31.5 ± 4.5% (n = 7), and 36.8 ± 6.6% (n = 6), respectively, not significantly different from that in cells treated with clonidine alone.
FIGURE 6.
p38 MAPK activity is required for μ-α2A cross-desensitization and cointernalization. A and B, DRG cultures were pretreated with 1 μm DAMGO (D) or 10 μm clonidine (Clo) for 4 h in the presence or absence of a selective p38 inhibitor and examined for the cross-desensitization. Clonidine-induced cross-desensitization to DAMGO was completely prevented by 5 or 20 μm PD169316 (PD; 5 and 20), or by 20 μm SB239063 (SB23). Similarly, DAMGO-induced cross-desensitization to clonidine was dose-dependently reduced by PD169316 and significantly attenuated by another p38 inhibitor SB203580 (SB20; 20 μm). **, p < 0.01 compared with vehicle controls pretreated with medium containing 0.1% DMSO. #, p < 0.05; ##, p < 0.01 compared with cells pretreated with clonidine or DAMGO alone. C and D, PD169316 (20 μm) blocked DAMGO-induced cointernalization of μ and α2A receptors. The p38 inhibitor did not block clonidine-induced α2A receptor internalization but prevented cointernalization of μ receptors, leading to dissociation of the two receptors. Immunocytochemical labeling of μ and α2A receptors was performed as described in the legend to Fig. 3. Scale bars, 10 μm.
In a separate set of experiments, DRG neurons were pretreated with DAMGO (1 μm, 4 h) in the presence of one of the kinase inhibitors and tested for acute responses to clonidine. Blocking p38 MAPK activity with PD169316 (5 and 20 μm) during pretreatment attenuated DAMGO-induced cross-desensitization in a dose-dependent manner (Fig. 6B). The responses to subsequent clonidine test were significantly greater in cells co-treated with 20 μm PD169316 compared with those treated with DAMGO alone (13.9 ± 2.4% versus 5.7 ± 0.8%, n = 17 and 18, p < 0.01). Co-treatment with another specific p38 inhibitor SB203580 (20 μm) (29) also significantly increased clonidine responses (Fig. 6B). Again, the inhibitors for phosphoinositide 3-kinase, ERK, protein kinase A, and protein kinase C were unable to prevent the cross-desensitization. Clonidine-induced current inhibition ranged from 4.0 to 7.5% in cells co-treated with one of these inhibitors, indistinguishable from those treated with DAMGO alone. None of the inhibitors examined above elicited significant changes in basal Ca2+ currents when applied acutely in the absence of μ or α2 agonist (2.2–4.9% current inhibition, n = 4–6, p > 0.05). In addition, pretreatment with PD169316 (20 μm) alone for 4 h did not affect the acute effect of DAMGO or clonidine (49.0 ± 4.1 and 19.8 ± 3.9%, n = 11 and 9, p > 0.05 compared with vehicle-treated controls). Thus, blockade of the cross-desensitization by p38 MAPK inhibitors was not due to any direct effect of the inhibitors per se on Ca2+ currents or to interference of acute DAMGO or clonidine action. Together, these results suggested that p38 MAPK activity was necessary for the μ-α2 interaction leading to the mutual cross-desensitization.
Since the cross-desensitization was closely associated with cointernalization of μ and α2A receptors, we investigated whether reversal of the cross-desensitization by PD169316 was accompanied by blockade of receptor cointernalization. The p38 MAPK inhibitor was found to completely block internalization of both μ and α2A receptors in DAMGO-treated cells (Fig. 6C). Interestingly, PD169316 selectively blocked internalization of μ but not α2A receptors in clonidine-treated cells. This effect led to separation of the two receptors during clonidine treatment, with α2A receptors being internalized but μ receptors remaining on the plasma membrane of DRG neurons (Fig. 6, C and D). These results suggested that activation of p38 MAPK was necessary for μ receptor endocytosis as well as for supporting cointernalization of μ and α2A receptors.
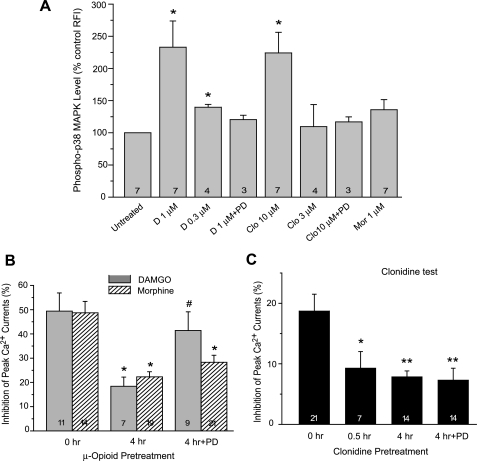
Activation of p38 MAPK by DAMGO and Clonidine, but Not by Morphine—To obtain direct biochemical evidence for p38 activation by μ and α2A agonists, we measured p38 MAPK phosphorylation in DRG neurons. In a time course analysis, DAMGO treatment (1 μm) induced rapid and sustained activation of p38 MAPK, increasing the phospho-p38 MAPK level to 200, 207, 378, and 336% of the control level after 5, 15, 30, and 60 min of exposure, respectively. When comparing the peak effect of each agonist after a 30-min exposure, DAMGO (0.3 and 1 μm) or clonidine (3 and 10 μm) induced dose-dependent increases in phospho-p38 MAPK levels, and their effects were blocked by the p38 inhibitor PD169316 (Fig. 7A). Morphine (1 μm) produced a small and insignificant increase in p38 MAPK phosphorylation (136 ± 16%, p > 0.05 compared with vehicle controls).
FIGURE 7.
Agonist-selective activation of p38 MAPK and its differential roles in homologous μ and α2A receptor desensitization. A, a 30-min treatment with DAMGO (1 μm) or clonidine (10 μm), but not morphine (1 μm), induced significant increases in the level of phospho-p38 MAPK in DRG neurons. B and C, DRG neurons were pretreated and tested with the same agonist to measure the homologous μ or α2A receptor desensitization. Co-treatment with the p38 inhibitor PD169316 (20 μm) prevented DAMGO desensitization but not morphine or clonidine desensitization. *, p < 0.05; **, p < 0.01 compared with untreated neurons (0 h). #, p < 0.05 compared with neurons pretreated with the same agonist in the absence of the p38 inhibitor (4 h).
In agreement with differential activation of p38 MAPK by the two opioid agonists, PD169316 selectively blocked DAMGO- but not morphine-induced homologous desensitization (Fig. 7B). Acute DAMGO responses in cells co-treated with PD169316 (41.4 ± 7.7%, n = 9) were significantly greater than those pretreated with DAMGO alone (18.4 ± 3.8%, n = 7, p < 0.05) and comparable with untreated controls (49.4 ± 7.5%, n = 11, p > 0.05). In contrast, morphine-induced current inhibition was similar between cells treated with morphine alone and those co-treated with PD169316 (22.3 ± 2.1% versus 28.3 ± 2.9%, n = 19 and 21, p > 0.05). Furthermore, although it blocked DAMGO-induced cross-desensitization to clonidine, PD169316 did not prevent homologously induced clonidine desensitization (Fig. 7C). This indicated that p38 MAPK activity was also differentially involved in the homologous versus heterologous desensitization of α2A receptors.
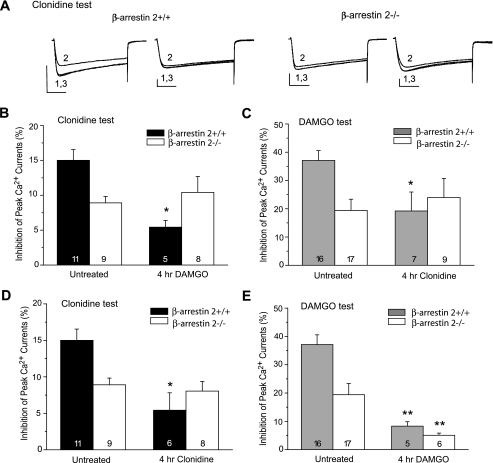
The Essential Role of β-Arrestin 2 in μ-α2A Cross-desensitization— β-Arrestins are key adaptor proteins involved in receptor endocytosis, intracellular sorting, and MAPK regulation. We determined involvement of β-arrestin 2 in μ and α2A receptor desensitization using DRG neurons from β-arr2-/- mice and their wild-type controls (Fig. 8). As expected, the 4-h DAMGO or clonidine treatment induced both homologous and cross-desensitization in β-arr2+/+ neurons. Similar to our previous observations (30), acute responses to DAMGO were smaller in untreated β-arr2-/- neurons compared with their wild-type counterparts. This, however, did not affect development of homologous DAMGO desensitization in β-arr2-/- neurons (Fig. 8E). Importantly, the cross-desensitization was completely absent in β-arr2-/- neurons following either DAMGO or clonidine treatment (Fig. 8, A–C). Thus, similar to p38 MAPK, β-arrestin 2 was required for the receptor interactions leading to the mutual cross-desensitization. Different from the effect of p38 inhibition, however, genetic deletion of β-arr2 abolished clonidine- but not DAMGO-induced homologous desensitization (Fig. 8, D and E).
FIGURE 8.
The crucial role of β-arrestin 2 in μ-α2A cross-desensitization. DRG neurons derived from the β-arrestin 2-/- and β-arrestin 2+/+ mice were pretreated with 1 μm DAMGO or 10 μm clonidine for 4 h and tested for the cross-desensitization and homologous desensitization. The μ-α2A cross-desensitization induced by DAMGO (B) or clonidine (C) was clearly seen in β-arrestin 2+/+ neurons but completely absent in β-arr2-/- neurons. Representative current recordings (A) were collected before (1), during (2), and after (3) acute clonidine test. Calibration was 2 nA, 20 ms. Clonidine-induced α2A receptor desensitization was also absent in β-arr2-/- neurons (D), but DAMGO-induced μ receptor desensitization remained intact in these neurons (E). *, p < 0.05; **, p < 0.01 compared with untreated neurons with the same genotype.
DISCUSSION
Cross-regulation of μ and α2A Receptor Signaling in Neurons— Although formation of μ and α2A receptor complexes has been convincingly demonstrated in cell lines and transfected neurons (15), functional significance and regulatory mechanisms for the μ-α2A interaction remain to be clarified. The initial report showed that heterodimerization of μ and α2A receptors in HEK293 cells enhanced receptor signaling in response to acute application of morphine or clonidine (15). A recent study, however, demonstrates a direct conformational cross-talk between μ and α2A receptors within the heterocomplex that allows rapid inaction of one receptor by the other with subsecond kinetics (31). In locus coeruleus neurons naturally expressing high levels of α2A and μ receptors, analysis of the effect of co-applied μ and α2A agonists reveals no functional interactions between the two receptors (32). These findings raised the questions as to whether and how naturally existing μ and α2A receptors in neurons could interact with each other to regulate cellular function. The present study demonstrated strong functional interactions between endogenous μ and α2A receptors in sensory neurons. Such interactions required p38 MAPK activity and β-arrestin 2 and promoted receptor co-trafficking and cross desensitization.
Desensitization of GPCR signaling involves modifications at the receptor level and in downstream signal transduction pathways. We previously reported that chronic homologous DAMGO desensitization in DRG neurons was partially mediated by phosphoinositide 3-kinase- and ERK-mediated changes in voltage-dependent Gβγ-Ca2+ channel interactions (17). In the present study, however, neither acute nor prolonged clonidine treatment altered PPF, a direct measure of voltage-dependent Gβγ effect on Ca2+ channels. The μ-α2A cross-desensitization was also unaffected by the selective inhibitors for phosphoinositide 3-kinase or ERK. These results suggest that modification of Gβγ-Ca2+ channel interactions by these two kinases did not play a significant role in the cross-desensitization. Another scenario for the cross-desensitization to occur at the post-receptor level would be desensitization of signaling pathways shared by both receptors. Our results did not support this possibility either. p38 MAPK and β-arrestin 2 differentially regulated homologous μ and α2A desensitization, suggesting that divergent rather than common signaling pathways were engaged by μ and α2A receptors.
Receptor Cointernalization Contributes to μ-α2A Cross-desensitization—An important feature of the cross-desensitization was its close association with agonist-induced cointernalization of μ and α2A receptors. It is well documented that both μ and α2A receptors undergo agonist-induced rapid endocytosis via clathrin coated-pits, a process regulated by receptor phosphorylation and binding with nonvisual β-arrestins (27, 33, 34). How this event regulates GPCR signaling, especially in the case of opioid desensitization, has been a subject of intense investigation (35–37). Several studies in primary neurons and AtT20 cells indicate that receptor internalization does not contribute to rapid desensitization of μ receptors coupled to voltage-gated Ca2+ channels (38, 39) or inwardly rectifying potassium channels (40) when measured on the time scale of several seconds to minutes. Evidence exists, however, that chronic opioid desensitization developed in hours can be greatly affected by μ receptor internalization and recycling (41). Continued internalization of μ receptors during prolonged agonist treatment can attenuate opioid responses via physical removal of the receptor from cell surface, but it also can promote receptor dephosphorylation and recycling. When rapid recycling occurs, the internalization is considered an important means for receptor resensitization, effectively reducing the extent of apparent desensitization (41, 42). Alternatively, if internalized receptors are trapped in endosomes, significant loss of surface receptors can occur, leading to enhancement of desensitization (43). Extended or repeated agonist exposure can also target internalized receptor to lysosomes for degradation, causing receptor down-regulation and more persistent signaling reduction (44, 45). Therefore, the functional consequence of receptor internalization may vary considerably in different model systems, depending upon the rate and extent of endocytosis as well as its coupling with distinct intracellular sorting pathways that determine the postendocytic fate of the receptor.
Receptor oligomerization has added a new dynamic to the complex relationship between internalization and desensitization. Studies in HEK293 cells show that formation of heterocomplexes between μ receptors and other GPCRs often alters receptor trafficking profiles. In many cases, co-expressed receptors are both internalized in response to a single selective agonist (5, 6, 8), suggesting that ligand-activated receptor may “drag” another receptor in the same complex to the endocytic pathway (46). Similarly, we demonstrated that μ and α2A receptors colocalized on the plasma membrane of untreated DRG neurons and underwent simultaneous internalization when either receptor was activated. These findings were in agreement with the presence of μ-α2A complexes. Nevertheless, a fraction of μ and α2A receptors were likely to exist as homodimers or monomers (4, 8), as indicated by the less than complete colocalization of the two receptors in DRG neurons and a relatively low percentage of μ receptors cointernalized by clonidine measured with flow cytometry.
Importantly, the propensity of μ and α2A agonists to promote cross-desensitization was closely related to their ability to induce receptor cointernalization. Exposure to DAMGO or clonidine induced both cointernalization and cross-desensitization, whereas morphine treatment resulted in neither. Furthermore, blocking μ-α2A cointernalization with p38 MAPK inhibitors effectively prevented the cross-desensitization. These findings strongly supported a crucial role of receptor cointernalization in μ-α2A cross desensitization. It is conceivable that a substantial portion of μ and α2A receptors coexist on cell surface as receptor complexes and internalize together via p38 MAPK-dependent mechanisms. During chronic agonist treatment, receptor cointernalization may be coupled with delayed recycling, which reduces cell surface receptors and attenuates signaling. Indeed, μ receptors are known to traffic through both early and late sorting endosomes in DRG neurons, two sorting pathways differing significantly in the rate of recycling (39). If interactions with α2A receptors promote μ receptors to traffic through the slower late sorting pathway, their recycling and resensitization could be significantly delayed.
p38 MAPK and β-Arrestin 2 Are Key Regulators of μ-α2A Cross-regulation—We observed strong activation of p38 MAPK by DAMGO and clonidine. The enhanced p38 MAPK activity may play two different roles in the cross-regulation of μ and α2A receptors. First, activation of p38 MAPK is known to facilitate μ receptor internalization by enhancing the function of endocytic machinery regulated by the small GTPase Rab5 (47, 48). By driving μ receptor endocytosis, p38 MAPK activity may trigger simultaneous internalization of those α2A receptors that are directly or indirectly associated with μ receptors. This scenario can well explain our findings that inhibition of p38 MAPK effectively blocked DAMGO-initiated internalization of both μ and α2A receptors. Second, our results indicated that p38 activity was not required for clonidine-initiated α2A receptor internalization but was necessary for co-trafficking of μ receptors with activated α2A receptors. Thus, activation of p38 MAPK may be essential for maintaining μ-α2A association during the cointernalization.
Another key regulator of the μ-α2A cross-modulation identified in this study was β-arrestin 2. Deletion of β-arrestin 2 in DRG neurons prevented μ-α2A cross-desensitization, an effect similar to that of p38 MAPK inhibitors. Studies have shown that β-arrestins act as scaffold proteins to regulate spatial distribution and activity of MAPK cascades (49) and that activation of p38 MAPK by GPCRs requires the presence of β-arrestin isoforms (50–52). Thus, β-arrestin 2 may regulate the cross-desensitization via its control over p38 MAPK. Such a serial pathway could well explain the requirement of both molecules for the cross-desensitization. Our data also showed that β-arrestin and p38 MAPK clearly differed in regulating the homologous desensitization. β-arrestin 2 but not p38 MAPK was required for clonidine-induced α2A desensitization, and their roles were reversed for DAMGO-induced μ desensitization. One possibility is that homologous desensitization was primarily mediated by endocytosis of μ or α2A homodimers and monomers, which have distinct requirement for p38 MAPK and β-arrestins as compared with μ-α2A heterodimers.
In untreated β-arr2-/- neurons, we observed less current inhibition by DAMGO or clonidine compared with the wild-type controls. The reduced μ agonist effect was reported previously in these neurons and explained by decreased constitutive internalization and recycling of μ receptors that are constitutively coupled with Ca2+ channels. Such receptors remain on the cell membrane, reducing the pool of receptors available for ligand activation (30). A similar reduction in constitutive recycling of α2A receptors may be responsible for decreased clonidine action in β-arr2-/- neurons. Such changes, however, would not account for the lack of the cross-desensitization in β-arr2-/- neurons, since DAMGO-induced μ desensitization remained intact in these neurons.
The nonvisual β-arrestins (1 and 2) are multifunctional proteins regulating diverse cellular functions in addition to their best-recognized roles in initiating GPCR internalization. Heterodimerization of μ receptors with other GPCRs can alter receptor interaction with β-arrestin 2, leading to delayed recycling of cointernalized receptors (5) or a shift in the activation pattern of ERK pathways (53). Therefore, the absence of μ-α2A cross-desensitization in β-arr2-/- neurons could be the result of a lack of cointernalization, altered recycling, or activation of specific signaling cascades independent of internalization. A possible model congruent with our data and these recent findings is β-arrestin 2-dependent formation of a macromolecular signaling complex. The complex contains both μ and α2A receptors, either present as heterooligomers or indirectly associated with each other through binding with β-arrestins. Receptor activation leads to recruitment of specific signaling pathways, such as p38 MAPK, to the complex in an agonist-selective manner, which in turn modulates receptor trafficking and desensitization. Lack of β-arrestin 2 prevents the formation of the complex or destabilizes it, thus disrupting μ-α2A cross-regulation.
In summary, functional interactions between neuronal μ and α2A receptors can lead to mutual cross-desensitization and receptor cointernalization. p38 MAPK and β-arrestin 2 serve as two key regulators of such interactions. These findings provide new insight into the complex relationship between opioid and adrenergic systems in the pain processing pathways and functional significance of GPCR signaling complexes.
Acknowledgments
We thank Dr. David J. Jentsch for providing the α2A-/- and α2A+/+ mice and Dr. Robert J. Lefkowitz for β-arr2-/- and β-arr2+/+ mice.
This work was supported, in whole or in part, by National Institutes of Health Grant P50DA05010. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: GPCR, G protein-coupled receptor; DRG, dorsal root ganglion; DAMGO, [d-Ala2,N-MePhe4,Gly-ol5]enkephalin; Gβγ, G protein βγ subunits; MAPK, mitogen-activated protein kinase; PPF, prepulse facilitation; β-arr2, β-arrestin 2; PBS, phosphate-buffered saline; NE, norepinephrine; GTPγS, guanosine 5′-3-O-(thio)triphosphate; CTAP, Cys2, Tyr3, Arg5, Pen7-amide; ERK, extracellular signal-regulated kinase.
References
- 1.Bouvier, M. (2001) Nat. Rev. Neurosci. 2 274-286 [DOI] [PubMed] [Google Scholar]
- 2.Marshall, F. H. (2001) Curr. Opin. Pharmacol. 1 40-44 [DOI] [PubMed] [Google Scholar]
- 3.Devi, L. A. (2001) Trends. Pharmacol. Sci. 22 532-537 [DOI] [PubMed] [Google Scholar]
- 4.Gurevich, V. V., and Gurevich, E. V. (2008) Trends Neurosci. 31 74-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfeiffer, M., Kirscht, S., Stumm, R., Koch, T., Wu, D., Laugsch, M., Schroder, H., Hollt, V., and Schulz, S. (2003) J. Biol. Chem. 278 51630-51637 [DOI] [PubMed] [Google Scholar]
- 6.Jordan, B. A., Trapaidze, N., Gomes, I., Nivarthi, R., and Devi, L. A. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 343-348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomes, I., Jordan, B. A., Gupta, A., Trapaidze, N., Nagy, V., and Devi, L. A. (2000) J. Neurosci. 20 RC110, 1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasbi, A., Nguyen, T., Fan, T., Cheng, R., Rashid, A., Alijaniaram, M., Rasenick, M. M., O'Dowd, B. F., and George, S. R. (2007) Biochemistry 46 12997-13009 [DOI] [PubMed] [Google Scholar]
- 9.Law, P. Y., Erickson-Herbrandson, L. J., Zha, Q. Q., Solberg, J., Chu, J., Sarre, A., and Loh, H. H. (2005) J. Biol. Chem. 280 11152-11164 [DOI] [PubMed] [Google Scholar]
- 10.Ossipov, M. H., Harris, S., Lloyd, P., and Messineo, E. (1990) J. Pharmacol. Exp. Ther. 255 1107-1116 [PubMed] [Google Scholar]
- 11.Fairbanks, C. A., and Wilcox, G. L. (1999) J. Pharmacol. Exp. Ther. 288 1107-1116 [PubMed] [Google Scholar]
- 12.Maldonado, R. (1997) Neurosci. Biobehav. Rev. 21 91-104 [DOI] [PubMed] [Google Scholar]
- 13.Lakhlani, P. P., MacMillan, L. B., Guo, T. Z., McCool, B. A., Lovinger, D. M., Maze, M., and Limbird, L. E. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 9950-9955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone, L. S., MacMillan, L. B., Kitto, K. F., Limbird, L. E., and Wilcox, G. L. (1997) J. Neurosci. 17 7157-7165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jordan, B. A., Gomes, I., Rios, C., Filipovska, J., and Devi, L. A. (2003) Mol. Pharmacol. 64 1317-1324 [DOI] [PubMed] [Google Scholar]
- 16.Zhang, Y. Q., and Limbird, L. E. (2004) Biochem. Soc. Trans. 32 856-860 [DOI] [PubMed] [Google Scholar]
- 17.Tan, M., Groszer, M., Tan, A. M., Pandya, A., Liu, X., and Xie, C. W. (2003) J. Neurosci. 23 10292-10301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hein, L., Limbird, L. E., Eglen, R. M., and Kobilka, B. K. (1999) Ann. N. Y. Acad. Sci. 881 265-271 [DOI] [PubMed] [Google Scholar]
- 19.Bohn, L. M., Lefkowitz, R. J., Gainetdinov, R. R., Peppel, K., Caron, M. G., and Lin, F. T. (1999) Science 286 2495-2498 [DOI] [PubMed] [Google Scholar]
- 20.Keith, D. E., Anton, B., Murray, S. R., Zaki, P. A., Chu, P. C., Lissin, D. V., Monteillet-Agius, G., Stewart, P. L., Evans, C. J., and von Zastrow, M. (1998) Mol. Pharmacol. 53 377-384 [PubMed] [Google Scholar]
- 21.Nasser, Y., Ho, W., and Sharkey, K. A. (2006) J. Comp. Neurol. 495 529-553 [DOI] [PubMed] [Google Scholar]
- 22.Walwyn, W. M., Keith, D. E., Jr., Wei, W., Tan, A. M., Xie, C. W., Evans, C. J., Kieffer, B. L., and Maidment, N. T. (2004) Neuroscience 123 111-121 [DOI] [PubMed] [Google Scholar]
- 23.Williams, J. P., Thompson, J. P., McDonald, J., Barnes, T. A., Cote, T., Rowbotham, D. J., and Lambert, D. G. (2007) Anesth. Analg. 105 998-1005 [DOI] [PubMed] [Google Scholar]
- 24.Perez, O. D., and Nolan, G. P. (2002) Nat. Biotechnol. 20 155-162 [DOI] [PubMed] [Google Scholar]
- 25.Uddin, S., Lekmine, F., Sharma, N., Majchrzak, B., Mayer, I., Young, P. R., Bokoch, G. M., Fish, E. N., and Platanias, L. C. (2000) J. Biol. Chem. 275 27634-27640 [DOI] [PubMed] [Google Scholar]
- 26.Kummer, J. L., Rao, P. K., and Heidenreich, K. A. (1997) J. Biol. Chem. 272 20490-20494 [DOI] [PubMed] [Google Scholar]
- 27.Whistler, J. L., and von Zastrow, M. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 9914-9919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Underwood, D. C., Osborn, R. R., Bochnowicz, S., Webb, E. F., Rieman, D. J., Lee, J. C., Romanic, A. M., Adams, J. L., Hay, D. W., and Griswold, D. E. (2000) Am. J. Physiol. 279 L895-L902 [DOI] [PubMed] [Google Scholar]
- 29.Cuenda, A., Rouse, J., Doza, Y. N., Meier, R., Cohen, P., Gallagher, T. F., Young, P. R., and Lee, J. C. (1995) FEBS Lett. 364 229-233 [DOI] [PubMed] [Google Scholar]
- 30.Walwyn, W., Evans, C. J., and Hales, T. G. (2007) J. Neurosci. 27 5092-5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vilardaga, J. P., Nikolaev, V. O., Lorenz, K., Ferrandon, S., Zhuang, Z., and Lohse, M. J. (2008) Nat. Chem. Biol. 4 126-131 [DOI] [PubMed] [Google Scholar]
- 32.Stone, L. S., and Wilcox, G. L. (2004) Neurosci. Lett. 361 265-268 [DOI] [PubMed] [Google Scholar]
- 33.Zhang, J., Ferguson, S. S., Barak, L. S., Bodduluri, S. R., Laporte, S. A., Law, P. Y., and Caron, M. G. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 7157-7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeGraff, J. L., Gagnon, A. W., Benovic, J. L., and Orsini, M. J. (1999) J. Biol. Chem. 274 11253-11259 [DOI] [PubMed] [Google Scholar]
- 35.Connor, M., Osborne, P. B., and Christie, M. J. (2004) Br. J. Pharmacol. 143 685-696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marie, N., Aguila, B., and Allouche, S. (2006) Cell. Signal. 18 1815-1833 [DOI] [PubMed] [Google Scholar]
- 37.Martini, L., and Whistler, J. L. (2007) Curr. Opin. Neurobiol. 17 556-564 [DOI] [PubMed] [Google Scholar]
- 38.Borgland, S. L., Connor, M., Osborne, P. B., Furness, J. B., and Christie, M. J. (2003) J. Biol. Chem. 278 18776-18784 [DOI] [PubMed] [Google Scholar]
- 39.Walwyn, W. M., Wei, W., Xie, C. W., Chiu, K., Kieffer, B. L., Evans, C. J., and Maidment, N. T. (2006) Neuroscience 142 493-503 [DOI] [PubMed] [Google Scholar]
- 40.Arttamangkul, S., Torrecilla, M., Kobayashi, K., Okano, H., and Williams, J. T. (2006) J. Neurosci. 26 4118-4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu, Y., Law, P. Y., and Loh, H. H. (2003) J. Biol. Chem. 278 36733-36739 [DOI] [PubMed] [Google Scholar]
- 42.Koch, T., Schulz, S., Schroder, H., Wolf, R., Raulf, E., and Hollt, V. (1998) J. Biol. Chem. 273 13652-13657 [DOI] [PubMed] [Google Scholar]
- 43.Law, P. Y., Erickson, L. J., El-Kouhen, R., Dicker, L., Solberg, J., Wang, W., Miller, E., Burd, A. L., and Loh, H. H. (2000) Mol. Pharmacol. 58 388-398 [DOI] [PubMed] [Google Scholar]
- 44.Afify, E. A., Law, P. Y., Riedl, M., Elde, R., and Loh, H. H. (1998) Brain Res. Mol. Brain Res. 54 24-34 [DOI] [PubMed] [Google Scholar]
- 45.Tsao, P. I., and von Zastrow, M. (2000) J. Biol. Chem. 275 11130-11140 [DOI] [PubMed] [Google Scholar]
- 46.He, L., Fong, J., von Zastrow, M., and Whistler, J. L. (2002) Cell 108 271-282 [DOI] [PubMed] [Google Scholar]
- 47.Mace, G., Miaczynska, M., Zerial, M., and Nebreda, A. R. (2005) EMBO J. 24 3235-3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cavalli, V., Vilbois, F., Corti, M., Marcote, M. J., Tamura, K., Karin, M., Arkinstall, S., and Gruenberg, J. (2001) Mol. Cell 7 421-432 [DOI] [PubMed] [Google Scholar]
- 49.McDonald, P. H., Chow, C. W., Miller, W. E., Laporte, S. A., Field, M. E., Lin, F. T., Davis, R. J., and Lefkowitz, R. J. (2000) Science 290 1574-1577 [DOI] [PubMed] [Google Scholar]
- 50.Sun, Y., Cheng, Z., Ma, L., and Pei, G. (2002) J. Biol. Chem. 277 49212-49219 [DOI] [PubMed] [Google Scholar]
- 51.McLaughlin, N. J., Banerjee, A., Kelher, M. R., Gamboni-Robertson, F., Hamiel, C., Sheppard, F. R., Moore, E. E., and Silliman, C. C. (2006) J. Immunol. 176 7039-7050 [DOI] [PubMed] [Google Scholar]
- 52.Bruchas, M. R., Macey, T. A., Lowe, J. D., and Chavkin, C. (2006) J. Biol. Chem. 281 18081-18089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rozenfeld, R., and Devi, L. A. (2007) FASEB J. 21 2455-2465 [DOI] [PMC free article] [PubMed] [Google Scholar]