Abstract
The pineal gland plays an essential role in vertebrate chronobiology by converting time into a hormonal signal, melatonin, which is always elevated at night. Here we have analyzed the rodent pineal transcriptome using Affymetrix GeneChip® technology to obtain a more complete description of pineal cell biology. The effort revealed that 604 genes (1,268 probe sets) with Entrez Gene identifiers are differentially expressed greater than 2-fold between midnight and mid-day (false discovery rate <0.20). Expression is greater at night in ∼70%. These findings were supported by the results of radiochemical in situ hybridization histology and quantitative real time-PCR studies. We also found that the regulatory mechanism controlling the night/day changes in the expression of most genes involves norepinephrine-cyclic AMP signaling. Comparison of the pineal gene expression profile with that in other tissues identified 334 genes (496 probe sets) that are expressed greater than 8-fold higher in the pineal gland relative to other tissues. Of these genes, 17% are expressed at similar levels in the retina, consistent with a common evolutionary origin of these tissues. Functional categorization of the highly expressed and/or night/day differentially expressed genes identified clusters that are markers of specialized functions, including the immune/inflammation response, melatonin synthesis, photodetection, thyroid hormone signaling, and diverse aspects of cellular signaling and cell biology. These studies produce a paradigm shift in our understanding of the 24-h dynamics of the pineal gland from one focused on melatonin synthesis to one including many cellular processes.
A defining feature of the pineal gland is a 24-h rhythm in melatonin synthesis. Melatonin provides vertebrates with a circulating signal of time and is essential for optimal integration of physiological functions with environmental lighting on a daily and seasonal basis (1–4).
The melatonin rhythm in mammals is driven by a circadian clock located in the suprachiasmatic nucleus (SCN),13 which is hard-wired to the pineal gland by a polysynaptic pathway that courses through central and peripheral neuronal structures. The pineal gland is innervated by projections from the superior cervical ganglia (SCG) in the form of a dense network of catecholamine-containing sympathetic fibers. Activation of the SCN → pineal pathway occurs at night and results in the release of norepinephrine (NE) from the sympathetic fibers into the pineal perivascular space (5). NE activates the pinealocyte through adrenergic receptors (5, 6). The best studied mechanism involves coincident “AND” gate activation of α1b- and β1-adrenergic receptors, which maximally stimulates adenylate cyclase, thereby elevating cAMP (7–13). Activation of α1b-adrenergic receptors alone elevates intracellular calcium and phospholipid signaling (1, 14–16). cAMP is believed to mediate the effects of NE on melatonin production to a large part by activating cAMP-dependent protein kinase. In rodents, this induces expression of Aanat, the penultimate enzyme in melatonin synthesis (17). Induction occurs through phosphorylation of cAMP-response element-binding protein (CREB) bound to cAMP-response elements (CREs) in the Aanat gene. A similar NE/cAMP mechanism also controls expression of Adra1b, Atp7b, Crem,14 Dio2, Fosl2, Id1, Dusp1, Mat2a, Nr4a1, Slc15a1, Pde4b2, Ptch1, and Rorb (18–27). In addition, a NE/cAMP mechanism decreases expression of Hs3st2 (28). Although it is likely that some of the effects of cAMP involve CREs, it is also likely that cAMP influences pineal gene expression through epigenetic mechanisms that alter chromatin structure, e.g. histone phosphorylation (29, 30), thereby having the potential of altering the expression of many genes and broadly promoting transcription by factors other than CREB. Whereas there is abundant evidence that the SCN/SCG/NE/cAMP system controls rhythmic gene expression in the pineal gland, it is also possible that other regulatory mechanisms exist, involving release of other transmitters, and additional second messengers (e.g. cGMP, Ca2+, and phospholipids).
The increased abundance of some of these night/day differentially expressed genes and of other genes in the pineal gland is determined in part by members of the OTX2/CRX family of homeodomain proteins, which play a similar role in the retina (31–34). These factors bind to photoreceptor conserved elements and closely related sequences. In addition, Pax6 and Otx2 are essential for development of both tissues (35–37). This developmental similarity is consistent with the common evolutionary origin of the pineal gland and retina from a primitive photodetector (38). Examples of OTX2/CRX-controlled genes expressed in both tissues include Aanat, Asmt, Sag, and Grk1 (20, 39–46). The first two encode proteins dedicated to melatonin synthesis; the latter two encode proteins associated with phototransduction in the retina. It is not clear whether the proteins encoded by these phototransduction genes play parallel roles in NE/cAMP signal transduction in the pinealocyte or if they are functionally vestigial in the context of the pinealocyte. Although OTX2 and CRX are of central importance in these tissues, it appears that other transcription factors and regulatory cascades are involved. For example, the importance of E-boxes in determining tissue-specific expression of Aanat is evident from several studies (42, 47), and NeuroD1 may also play a role in determining pineal gland-specific expression patterns (48).
Whereas in both the pineal gland and retina, photoreceptor conserved elements control developmental expression of the same gene, different mechanisms can operate in each tissue to control rhythmicity. For example, in the case of Aanat, CREs mediate cAMP control of 24-h rhythms in the pineal gland (49, 50). In the retina, however, E-box elements mediate circadian clock control of the 24-h rhythm in Aanat expression (51).
In addition to the accepted SCN/SCG/NE/cAMP pathway, reports in the literature have claimed that a circadian clock regulates daily changes in the expression of some genes in the mammalian pineal gland (52), as in the submammalian pineal gland (53, 54). The physiological impact of this remains unknown.
Here we have expanded our understanding of the transcriptional regulation and physiology of the pineal gland by employing Affymetrix GeneChip® technology, including a microarray that interrogates more than 13,663 genes that have been assigned Entrez Gene identifiers.15 Previous studies of this nature in the rat have identified 39 night/day differentially expressed genes (26); a more recent study identified 35 such genes with Entrez Gene identifiers (59 probe sets) (55). Our study had three specific goals. The first goal was to produce a comprehensive listing of genes that are differentially expressed on a night/day basis. The second goal was to identify the highly enriched genes that define pineal function, independent of whether they are tonically or night/day differentially expressed; this was done by comparing gene expression in the pineal gland to median expression among other tissues. The third goal was to determine the scope of the NE/cAMP regulatory cascade; this approach utilized an in vitro organ culture system. In addition to establishing the importance of this cascade, the organ culture studies identified sets of genes that were spontaneously up- or down-regulated more than 10-fold during culture in defined medium, providing evidence of the existence of unknown regulatory mechanisms. An unexpected discovery was that the pineal transcriptome includes a large number of immune/inflammation response-associated genes.
The findings of this study are of value to investigators interested in the pineal gland, chronobiology, neuroendocrinology, and immunology and to those who study specific genes that are night/day differentially and/or highly expressed in the pineal gland.
EXPERIMENTAL PROCEDURES
Animals
Three microarray experiments were done (experiments A, B, and C). For microarray experiments A and B (Cardiff University), Sprague-Dawley rats (2–3 months old) were maintained in standard laboratory conditions in a 14:10 light-dark (LD) cycle (lights on, 05:00 h). Animals were killed at mid-day (ZT7) or midnight (ZT19) by cervical dislocation, and pineal glands were rapidly dissected, placed in tubes on solid CO2, and stored at –80 °C. For microarray experiment C (NICHD, National Institutes of Health), for the time series analysis of gene expression by qRT-PCR (Fig. 4), and for organ culture experiments, Sprague-Dawley rats (2–3 months old, female) were housed for 2 weeks in LD 14:10 lighting cycles, killed by CO2 asphyxiation, and decapitated; pineal glands were rapidly dissected and either placed in tubes on solid CO2 and stored at –80 °C or were prepared for organ culture. Other tissues were also removed, and 10-mg samples were frozen and stored in a similar manner. For the qRT-PCR experiment (Fig. 4), tissues were collected at ZT1, -7, -13.5, -15, -16, -17.5, -19, and -22, placed in tubes on solid CO2, and stored at –80 °C. Glands for organ culture experiments were obtained at ZT4–6 and placed in culture within 60 min. For radiochemical in situ hybridization histology studies (University of Copenhagen), Sprague-Dawley and Wistar rats (Charles River, Germany) were housed for 2 weeks in a controlled lighting environment (LD 12:12). Animals were killed by decapitation at ZT6 and ZT18; their brains were removed, immediately placed in solid CO2, and stored at –80 °C until sectioned.
FIGURE 4.
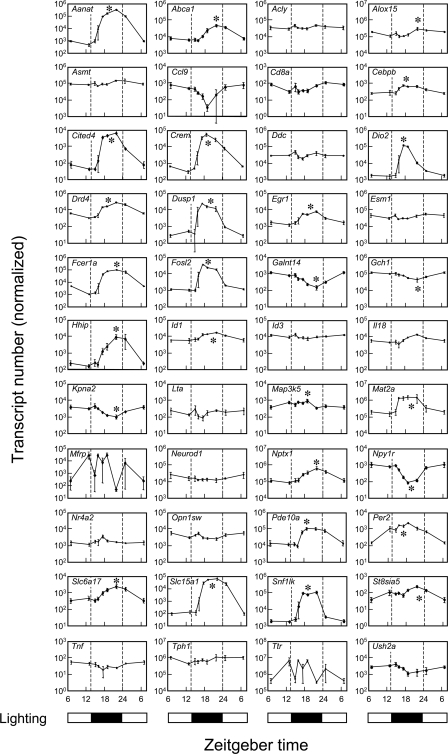
qRT-PCR analysis of transcripts that are night/day differentially expressed or have high rEx values or both. The lighting cycle is represented at the bottom of each column. Transcripts are identified by gene symbol. Each value is the mean ± S.E. of three determinations. Values were normalized to Actb, Gapdh, Hrpt1, and Rnr1. A single asterisk identifies statistically significant rhythmic patterns of gene expression (p < 0.01) based on log transformed raw values analyzed by one-way analysis of variance in JMP. For technical details, see “Experimental Procedures” and the supplemental material.
Animal use and care protocols were approved by local ethical review, and they were in accordance with National Institutes of Health guidelines, United Kingdom Home Office Regulations, and Health Sciences Animal Policy European Union Directive 86/609/EEC (approved by the Danish Council for Animal Experiments).
For organ culture, rat pineal glands were cultured in BGJb medium as described previously (56) and detailed in the supplemental material. Glands were incubated (1 gland/well) with fresh media containing NE (1 μm), dibutyryl cAMP (Bt2cAMP; 0.5 or 1 mm), or forskolin (10 μm) (Sigma). Following a 6-h treatment, glands were placed in microtubes on solid CO2.
To confirm that the glands were activated by the drugs, melatonin production in the culture media was measured by tandem mass spectroscopy as described (57), with an internal d4-melatonin standard. The amount of melatonin produced (nanomoles/gland/6 h; means ± S.E.) for the control, NE-treated, Bt2cAMP-treated, and forskolin-treated groups was (number of samples) 1.4 ± 0.1 (9); 20.3 ± 1.1 (9); 9.9 ± 0.9 (9); and 15.0 ± 1.2 (9), respectively.
Microarray
For the analysis of pineal glands in experiments A and B, two sets of six pooled samples of four rat pineal glands each were prepared (three night and three day). In experiment C, four pools, each containing three glands, were prepared for each time point; as part of this experiment, single retinas and 10-mg samples of the cerebellum, neocortex, hypothalamus, liver, and heart were also obtained. Glands were also obtained from organ culture experiments in which each treatment group was comprised of three pools, each containing four glands. Total RNA was isolated, labeled and used to interrogate Affymetrix GeneChips® as detailed in the supplemental material.
Microarray Data Sets
The microarray data presented here are derived from the experiments described below (A, B, and C) in conjunction with a published tissue profiling effort (Genomics Institute of the Novartis Research Foundation (GNF), Entrez Gene Expression Omnibus (GEO), dataset GDS589 (58)).
Microarray experiment A (Cardiff University) used the Affymetrix RG_U34A microarray (8,799 probe sets, 4,996 genes). Results from microarray experiment A were compared with data from the GNF data base, which had been generated using the same microarray. Expression data for the following 23 Sprague-Dawley tissues and isolated cells were used (number of samples per tissue is in parentheses): neocortex (39), cerebellum (17), striatum (13), hippocampus (3), hypothalamus (2), pituitary (2), amygdala (10), nucleus accumbens (6), locus ceruleus (2), dorsal raphe (2), ventral tegmental area (2), pineal gland (2), dorsal root ganglion (2), cornea (2), heart (2), intestine (4), kidney (2), spleen (2), thymus (2), bone marrow (2), muscle (2), Sertoli cells (10), and endothelial cells (2).
Microarray experiment B (Cardiff University) used the RAE230A microarray (15,923 probe sets, 10,174 genes). Microarray experiment C (NICHD, National Institutes of Health) used the Rat230_2 microarray (31,099 probe sets, 13,663 genes); this experiment included pineal glands and other tissues (retina, neocortex, cerebellum, hypothalamus, heart, and liver) obtained at mid-day and midnight, and glands obtained from organ culture.
Analysis of Microarray Results
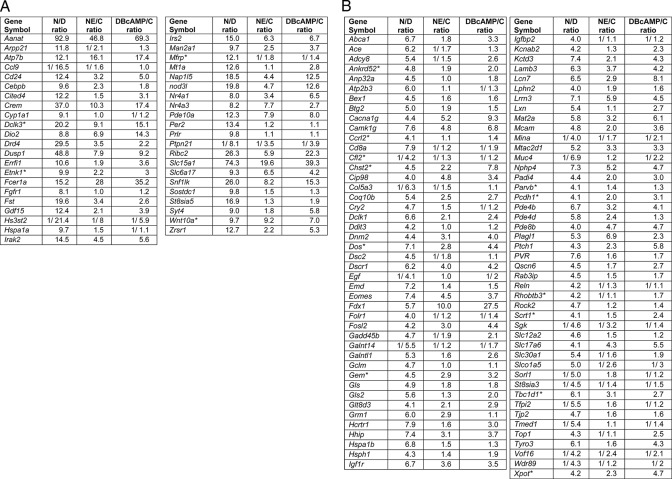
Night/Day Differences in Gene Expression—Affymetrix MAS5 Signal and Present Call values were stored in the NIH-LIMS, a data base for storage and retrieval of microarray data. The microarray data are available at the Entrez Gene Expression Omnibus, National Center for Biotechnology Information (59), and are accessible through GEO series accession number GSE12344 (ncbi.nlm.nih.gov), and at sne.nichd.nih.gov. Data were statistically analyzed using the MSCL Analyst Toolbox (P. J. Munson, J. J. Barb, abs.cit.nih.gov) and the JMP statistical software package (SAS, Inc., Cary, NC). Affymetrix signal values were incremented by a value of 0.1× microarray median value, then normalized to 1.1× microarray median values, and finally decimal log-transformed. This transformation is termed “Lmed” and has the desirable effect of reducing the influence of very small expression values. One-way, two-level analysis of variance testing differences between night and day were performed on the transformed data, and significance (p values, or false discovery rate (FDR) (60)) was reported (see supplemental Table S3). Night-day log fold changes were computed as the difference between the night and day Lmed values; in experiment C, NE/control and Bt2cAMP/control log fold change values were calculated similarly. Expression ratios are reported as linear values; values less than one are reported using the 1/X convention in which X = the night/day ratio, i.e. a night/day ratio of 0.01 is displayed as 1/100. Table 1 details the expression ratios (night/day, NE/control, Bt2cAMP/control) of all genes with a Entrez Gene identifier and with a night/day ratio greater than 4 or less than ¼. The supplemental Table S3 presents the expression ratios of all probe sets with a night/day ratio greater than 2 or less than ½.
TABLE 1.
Differential expression of genes in the pineal gland
Expression ratios listed under night/day (N/D) are the highest values obtained in experiments A, B or C (see “Experimental Procedures”). Expression ratios listed under NE/C (NE/control) and DBcAMP/C (Bt2cAMP/control) are from experiment C. Decreases in expression are indicated by the 1/X convention, indicating that expression was lower by a factor of X. Section A includes genes that exhibited a >8 or <1/8 fold night/day difference in expression; section B includes genes with a 4–8-fold or 1/4 to 1/8 night/day difference in expression. The supplemental Table S3 (sne.nichd.nih.gov) is an expanded version of this table, and includes genes with a 2–4-fold or 1/2 to 1/4 -fold night/day difference in expression, gene titles, probe set numbers, and Entrez Gene identifiers.
* Predicted gene is indicated.
Expression of Genes in the Pineal Gland Relative to Other Tissues—Gene expression in one tissue relative to expression in other tissues was defined as the relative tissue expression (rEx) value, which was calculated as the ratio of maximum expression (the highest of day or night) to the median expression of that gene in other tissues. In experiment A, median values were calculated from 23 Sprague-Dawley tissues in the GNF data base (see above) plus the day and night pineal gland values generated in experiment A. In experiment C, the median values were calculated from the average expression levels in each of seven tissues (see above). These averages were based on single mid-day and midnight values, except in the case of the pineal gland for which four mid-day and four midnight values were used. The larger of two rEx values obtained using the two experiments is presented in Table 3, which contains genes with rEx values greater than 8. The supplemental Table S4 includes rEx values from both experiments for probe sets with rEx values greater than 2.
TABLE 3.
Genes highly expressed in the pineal gland relative to other tissues
Genes were identified from data generated in experiments A and C. Gene titles, Entrez Gene identifiers, and individual rEx values obtained in each experiment are presented in supplemental Table S4 (sne.nichd.nih.gov); supplemental Table S4 also contains genes with rEx values 4–8. For details regarding calculation of rEx values see “Experimental Procedures.”
| Pineal rEx | Gene symbol |
|---|---|
| >16 | A2m, Aanat, Abca1, Abhd14b, Adra1b, Adrb1, Aipl1, Alox15, Arhgap24, Arr3, Asl, Asmt, Atp7b, Ca3, Cabp1, Cacna1f, Camk1g, Ccl9, Cd1d1, Cd24, Cdh22, Chga, Chrna3, Chrnb4, Cnga1, Cngb1, Cntrob,aCol8a1,aCplx3, Cpt1b, Crem, Crocc,aCrtac1, Crx, Ctsc, Cyp1b1, Dclk3,aDdc, Defb24, Drd4, Dusp1, Efemp1, Egflam, Esm1, Eya2, Fcer1a, Fdx1, Fkbp4, Fkbp5, Frmpd1,aFst, Fzd4, Gch, Gdf15, Gem,aGnat2,aGnb3, Grk1, Guca1a,aHs3st2, Hspa1a, Hspa1b, Hspb1, Igfbp6, Impg1, Impg2, Irak2, Irs1, Isl2, Ka15, Kcne2, Kcnh6, Kcnj14, Krt1-19, Lamp3, Lgals1, Lgals3, Lhx4,aLix1,aLpl, Lrrc21, M6prbp1, Map4k1,aMat2a, Mcam, Me2,aMiox, Mitf, Morn1, Mpp3, Mpp4, Mtac2d1, Mx2, Ncaph, Neurod1, Nphp4, Nphs1, Nptx1, Opn1sw, Osap, Otx2, Padi4, Pax4, Pax6, Pcbd1, Pcdh21, Pdc, Pde4b, Pla2g5, Plscr1, Rbp3, Rds, Ribc2, Rom1, Rorb,aRxrg, Sag, Scn7a, Serping1, Slc12a5, Slc15a1, Slc17a6, Slc24a1, Slc30a1, Slc39a4,aSlc6a6, Snap25, Snf1lk, Sorl1, Spink4, Stk22s1, Sv2b, Tm7sf2, Tph1, Ttr, Tulp1,aUnc119, Vof16 |
| 8-16 | Accn4, Acsl1, Acvr1, Adam2, Ak3l1, Als2cr4,aAmpd2, Anp32e, Anpep, Atp1b2, Atp6v1c2, Baiap2l1, Bmp6, Bzrp, Cacna1h, Ccdc125, Ccl2, Ccl6, Ccnd2, Cd63, Cd74, Cd8a, Cebpb, Cfd, Cflar, Chst2aCip98, Col15a1, Col1a1, Cr16, Crcp, Cyp1a1, Dcn, Depdc7, Dhrs8, Dnajc12, Dnm2, Dnm3, Dpt,aDsc2, Dscr1, Epb4.1, Errfi1, Etnk1,aExoc5, F5, Farp2,aFosl2, Foxd1, Frmd4b, G0s2, Gabrr1, Gale, Galnt4, Galntl1, Gla, Gls, Gmds, Gnas, Grm1, Hcn1, Hk2, Hsd3b7, Hspb6, Id1, Ifitm3, Igfbp2, Igsf1, Igsf4a, Il13ra2, Il17re, Irf7, Itgb2, Kctd14,aKctd3, Kit, Klhl4, Lad1,aLama2,aLamb1,aLmbr1l, Lmod1,aLnx1,aLox, Loxl1, Lrrc8e, Lum, Lxn, Mad2l2, Mak, Mak10, Man2a1, Mapk6, Msrb2, Msx1, Mt1a, Muc4, Mylk,aMyo5b, Nacad, Nr4a1, Nradd, Nrap,aNup107, Oasl1, Orai1, Pcbp3, Pde10a, Pde6b,aPdp2, Pgam2, Pgm1, Pid1, Pik3r3, Pla2g1b, Pla2r1,aPlcd1, Plcd4, Postn,aPqlc1, Prkar2b, Prkca, Prtg, Psph, Ptgis, Ptms, Ptprn, PVR, Qscn6, Rab3c, Rarres1, Rasgrf2, Rax, Rere, Resp18, Rnase1, Rreb1,aRT1-Aw2, Rtbnd,aSall1,aSema3a, Slc12a2, Slc19a2, Slc1a5, Slc25a10, Slc47a1, Slc4a2, Slc7a6,aSlco4a1, Sod3, Spint2, Svop, Tagln2, Tcn2, Tex14,aTimp1, Tmepai,aTnfrsf9, Ugdh, Wnt10a,aZmat2, Zrsr1 |
Predicted gene is indicated.
Comparison of Results across Different Microarray Platforms—Results from the three platforms were compared using the following mapping algorithm. The RG_U34A probe sets were first mapped to the Rat230_2 microarray using the Affymetrix ortholog map (available online). Exact matches for probe sets on the RAE230A microarray are found on the Rat230_2 microarray. In cases where a probe set maps to several probe sets on another microarray, each mapped probe set pair is considered as an independent pair, resulting in multiple pairs, each sharing a common probe set. Probe sets from the different microarrays were annotated using Affymetrix annotation files (available online) dated November 5, 2007. Each probe set was mapped to an Entrez Gene identifier. In cases of discrepant gene identification for the probe set pair, the annotations from the most recent microarray type were used. In cases where probe sets identify two or more genes, the gene symbol of the first gene on the annotation file is listed, unless otherwise indicated. Summaries over multiple probe sets mapping to the same gene were calculated by taking the maximum observed ratios for night/day. The rEx values were calculated in a similar manner.
Radiochemical in Situ Hybridization Histology
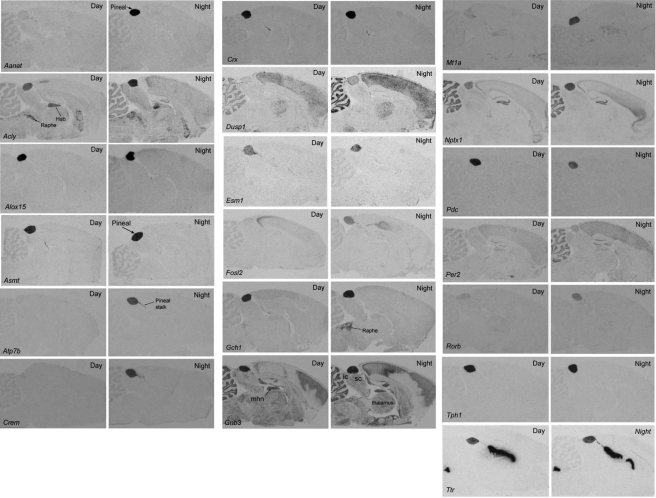
Sagittal sections of frozen rat brains were analyzed by in situ hybridization histology as described previously (34, 61) and detailed in the supplemental material. Sections were hybridized with 35S-labeled 38-mer oligonucleotide probes (supplemental Table S1). The sections were exposed to x-ray film or dipped into an LM-1® emulsion (Amersham Biosciences). The in situ hybridization images presented in Fig. 2 are available at high resolution on line at sne.nichd.nih.gov.
FIGURE 2.
Radiochemical in situ hybridization images. Each panel contains autoradiographs prepared from sections of rat brains through the pineal gland. The sections on the left are from animals killed during the day and those on the right are from animals killed during the night. The sections were incubated with antisense probes identified in the bottom left-hand corner of the Day image. Probes are detailed in supplemental Table S1. The results of quantitation of the signal strength of the pineal labeling appear in Table 5. For further details see “Experimental Procedures.” Hab, habenula; ic, inferior colliculus; mhn, medial habenular nucleus; Raphe, dorsal raphe nucleus; sc, superior colliculus. These figures are available in high resolution at sne.nichd.nih.gov.
qRT-PCR
For data in Fig. 4 and Table 6, pools of glands were used for each time point or treatment group. cDNA was synthesized from DNase-treated total RNA, and qRT-PCR results were quantitated and normalized (62) as detailed in the supplemental material. The primers used are described in supplemental Table S2.
TABLE 6.
Effects of NE, Bt2cAMP, or forskolin on transcript abundance in cultured pineal glands
Glands were cultured for 48 h prior to the initiation of the indicated treatment. Treatment duration was 6 h. Transcript abundance was measured by qRT-PCR and normalized as described under “Experimental Procedures.” Each value is based on results obtained from three pools of three glands and given as a treatment/control ratio. A more extensive version of this table, including absolute values of the mean ± S.E., is available in supplemental Table S6.
| Gene symbol | NE/control | Bt2cAMP/control | Forskolin/control |
|---|---|---|---|
| Aanat | 143.7a | 284.8a | 96.2a |
| Abca1 | 2.43a | 4.21a | 2.26a |
| Acly | 1/1.27 | 1.34 | 1.52 |
| Actbb | 1/1.20 | 1/1.17 | 1/1.22 |
| Alox15 | 1.14 | 1.62 | 1.59 |
| Asmt | 1.11 | 1.31 | 1.80 |
| Ccl9 | 1/3.27 | 1/3.50 | 1/10.49 |
| Cd8a | 1/1.30 | 2.45 | 3.78 |
| Cebpb | 2.10 | 4.78a | 2.40 |
| Cited4 | 50.1a | 84.1a | 49.1a |
| Crem | 31.6a | 32.5a | 27.6a |
| Ddc | 1/1.29 | 1.01 | 1/1.07 |
| Dio2 | 3.92a | 10.8a | 11.0a |
| Drd4 | 14.0a | 41.9a | 20.1a |
| Dusp1 | 13.7a | 18.7a | 5.86a |
| Egr1 | 1.31 | 1/1.03 | 1/1.61 |
| Esm1 | 1/1.09 | 3.32a | 2.42a |
| Fcer1a | 3.92a | 10.8a | 11.0a |
| Fosl2 | 3.09a | 4.74a | 2.91a |
| Galnt14 | 1/3.79a | 1/2.16a | 1/3.01a |
| Gapdhb | 1.00 | 1.32 | 1.00 |
| Gch1 | 1.25 | 1.18 | 1/1.01 |
| Hhip | 16.9a | 36.5a | 59.0a |
| Id1 | 2.36 | 2.98a | 1.97 |
| Id3 | 1.05 | 1.17 | 1/1.26 |
| Il18 | 1.36 | 1.56a | 1.46 |
| Kpna2 | 1/1.46 | 1.20 | 1.23 |
| Lta | 1.36 | 2.06 | 1.82 |
| Map3k5 | 1.20 | 2.45a | 2.17 |
| Mat2a | 9.12a | 11.0a | 6.78a |
| Mfrp | 1/9.71 | 1.46 | 1/6.67 |
| Neurod1 | 1.11 | 1.13 | 1.27 |
| Npy1r | 1/3.81a | 1/2.69a | 1/2.01 |
| Nr4a2 | 1.76 | 5.07a | 2.42 |
| Opn1sw | 1.39 | 1.48 | 1/1.03 |
| Per2 | 1.10 | 1.35 | 1.49 |
| Rnr1b | 1.23 | 1/1.08 | 1.69 |
| Slc6a17 | 4.40a | 11.3a | 10.8a |
| Slc15a1 | 479.3a | 472.2a | 174.7a |
| Snf1lk | 10.7a | 11.8a | 7.48a |
| St8sia5 | 1.64 | 2.27a | 1.78 |
| Tnf | 1/1.03 | 1/4.68a | 1/1.48 |
| Tph1 | 1/1.05 | 1.07 | 1/1.21 |
| Ttr | 1/1.29 | 1.16 | 1/1.33 |
| Ush2a | 1/2.50a | 1/1.53a | 1/2.38a |
p < 0.01.
Data indicates genes used for normalization.
Functional Analysis
The programs used to identify clusters of genes associated with discrete functions were DAVID Bioinformatics Resources 2007 (david.abcc.ncifcrf.gov/), ModuleMiner (63) and Affymetrix NetAffx. The results of these analyses and common knowledge were used to generate Table 7.
TABLE 7.
Functional grouping of genes that are night/day differentially expressed (N/D >2 or <1/2) or at high levels (rEx >4) in the pineal gland
All genes appear in Tables 1 and 3 and, supplemental Tables S3 and S4.
| Functional group | Gene symbol |
|---|---|
| Specialized processes | |
| Immune response/inflammation | Abhd2,aAnd, Ahcy, Alms1,aArHgef9, Bbs7, Bcar1, Btg2, C3, Ccl2, Ccl6, Ccl7, Ccl9, Ccrl2,aCd1d1, Cd47, Cd74, Cd8a, Crcp, Ctsc, Ctss, Defb24, Dscr1, Fcer1a, Fras1,aGdf15, Gem,aHivep1, Hivep2, Icsbp1, Ifi35, IfiTm1,aIfnar1,aIgsf4a, Igsf9,aIgha, II13ra2, II17re, Il18, Il1rl1l, Ilk, Impdh2, Inhbb, Irak2, Irf7, Ler3, Litaf, Lrrc8, Lta4h, Mal2, Mdk, Mina, Mmd2, Mox2, Mx2, Oit1,aOptn, Pcna, Plscr1, Pvr, Pvrl2, RT1-A1, RT1-A2, RT1-A3, RT1-Aw2, RT1-Bb, RT1-Da, Sct2, Sema3a, Serping1, Slfn3, Stch, Stip1, Tfp12, Tpm4, Ush2a, Vof16 |
| Melatonin synthesis | Aanat, Acly, Asmt, Ddc, Gch1, Gchfr, Mat2a, Pcbd1, Tph1 |
| Photodetection | Genes linked to photodetection in the pineal gland are considered to be highly expressed in both the pineal gland and retina; they are listed in Table 4 and supplemental Table S5 |
| T3/RA signaling | Dio2, Hr, Rbp3, Rdh12,aRorb,aRxrg, Thrb, Ttr |
| Nonspecialized processes | |
| Adhesion | Cdh22, Celsr32, Cml5, Cntn4, Dsc2, Eva,aGja12,aGlycam1, Grn, Hnt, Mcam, Mfap4, Mpp4, Muc4, Nell2, Parvb, Pcdh21, Prph2, Pvr, Scarb2, Sdc4, Spon1, Ssx21p |
| Cell cycle/cell death | Acom1, Acvr1, Aprin, Bag1,aGiklk, Casp7, Ccnd2, Cdc25a, Cdc5l, Cdk5, Cdkn1b, Cdkn1c, Cflar, Ches1,aCommd5, Csnk2a2, Ddit3, Dnm1, Dnm2, Elmo3, Faim, Gos2, Gadd45a, Gadd45b, Igf1r, Igfbpl1,aJag1, Junb, Mad2l2, Mak10, Ntf3, Pafah1b1, Pard3, Pdia3, Plagl1, Ptgs2, Qscn6, Rarres1, Rgc32, Fhob, Slc31a1, Strn3, Tacc3, Vegfc |
| Cytoskeleton | Ap1g1, Baiapw, Bbs4,aCatna1, Clasp2, Clta, Col14a1,aCol3a1, Col4a3, Col8a1,aCope,aCpg2, Dnch1, Dncl2b,aDncl2b,aEmilin1,aEmls, Fgd2,alnb,aFlnc,aFmod, Fni, Fscn2,aHdac11,aKa15, Kif1b, Kif22, Kif2c, Krt1-18, Krt1-19, Krt25, Lad1,aLama2,aLamb1-1,aLap1b, Lcp1, Lix1, Lmod1, Lumk, Mapt, Marcks, Mfap5,aMgp, Mrgl19, Mtap2, Mylip,aNrap,aPgea1, Rpl3, Sas, Selpl,aSdo3, Spna2, Tctex1, Thbs4, Tmem16a,aTmem22, Tpm4, Tuba4, Tubb5, Unc119, Vil2, Vim |
| DNA modification | Adprt, Blm,aBnc2,aCntn1, Commd1,aCtps,aHerc3,aHmgb2, Kpna2, Mcm4, Pcna, Prc1,aPrim1, Ptms, Rere, Thap4, Tlk1,aTop1, Tspyl4, Zdhhc22, Zfp143, Zfp162, Zfp238, Zfp36l1, Zhx1, Znf444, Zswim5,a |
| Endothelium | Esm1, Vegfb, Vegfc, Vwf |
| Growth | Efemt1, Egf, Egfr, Egfr1, Fgf1, Fgfr1, Gadd45g, Gdf15, Gfer, Grb2, Igf1r, Igfbp2, Igfbp6, Pdgfrl, Pgf, Tgfb1, Tgfbi, Vegfb, Vegfc |
| Signaling | Calcium: Atp2b3, Cabp1, Cacna1f, Cacna1g, Calm1, Camk1g, Camk2b, Cip98, Dcamkl1, Dcamkl3 |
| Cyclic nucleotide: Adcy8, Akap11, Cnga1, Cngb1, Creb3, Guca1a, Gucy1a3, Hcn1, Pde4b, Pde4d, Pde6b, Pde8b, Pde10a, Prkar2b, Prkca | |
| G-protein: Arf3, Arr3, Arl2bp, Arl6ip5, Gem, Gna12, Gnaq, Gnas, Gnat2, Gnaz, Gnb1, Gnb3, Gng11, Grk1, Pdc1, Rgs2, Rgs4, Rgs7, Rgs9, Rgs17, Sag1, Tyro3 | |
| Membrane receptors/ligands: Acvr1, Adra1b, Adrb1, Agtrap, Bmp6, Chrna3, Chrnb1, Chrnb4, Crcp, Drd1a, Drd4, Ece1, Ednrb, Egf, Egfr, Fgf, Fgfr1, Fst, Fzd4, Grip2, Grm1, Grm2, Hcrtr1, Htr2c, Igf1r ,Igfbp2, Igfbp3, Igfbp5, Igfbp6, Lepr, Nog, Opn1sw, Prlr, Sort1, Vipr2 | |
| Lipid/ Phospholipid/cholesterol: Abca1,Alox15, Cyp27a1, Ephx1, Inpp5e, Itpr1, Lta4h, Ltb4dh, Pa2g1b, Pik3r3, Pla2g5, Plcb1, Plcd4, Ptgds, Ptgis | |
| MAP kinase: Dusp1, Errfi1, Map3k5, Map3k6, Map4k1, Mapk14, Mapk6 | |
| Protein phosphorylation, serine/threonine: Calm1, Camk1g, Camk2b, Cdk5, Cdkn1b, Crkas, Dcamkl1, Enh, Fez1, Gsk3b, Nell2, Pak2, Prkar2b, Prkca, Prkcdbp, Prkce, Prkcl1, Rock2, Sik2, Snrk, Stk2, Stk39 | |
| Protein phosphorylation, tyrosine: Crkas, Efna5, Jak1, Kit, Ntrk2, Ntrk3, Ptp2E, Ptp4a1, Ptpn16, Ptprj, Ptprr, Ptp-Td14, Tyro3 | |
| RNA modification | Ankrd24,aBfsp1, Bop1, Bzw2, Eif2ak4,aEif2c2, Eif3s9, Eif4g2, Ell2, Hdac5, Polr2d,aQtrt1, Rnase1, Rnase2,aRpat1, Sfpq, Xpota |
| Small molecule biology | Metal homeostasis: Atp7b, Chordc1, Mt1a, Mt2, Slc30a1 Slc39a4 |
| Ion homeostasis: Atp1a1, Atp1b1, Atp1b2, Atp2a2, Atp2b1, Cacna1h, Cacnb2, Clcn3, Cnga1, Cngb1, Hcn1, Kcnab2, Kcne2, Kcnh6, Kcnj14, Kctd3, Scn7a, Slc12a2, Slc12a5, Slc17a6, Slc24a1 | |
| Solute transport: Slc2a1, Slc2a4, Slc3a1, Slc4a2, Slc4a4, Slc6a6, Slc7a1, Slc7a7, Slc12a2, Slc12a5, Slc14a1, Slc15a1, Slc16a1, Slc16a6, Slc21a1, Slc21a7, Slc22a1, Slc25a10, Slc29a1, Slc30a1, Slc34a1 | |
| Transcription factors | Arntl, Bhlhb3, Cebpb, Crem, Cbx5, Cry2, Crx, Datf1, Eya2, Fosl2, Foxd1, Hdac5, Homer1, Homer2, Hr, Isl2, Jun, Junb, Mitf, Msx1, Neurod1, Nr1d2, Nr1h4, Nr2f6, Nr4a1, Nr4a3, Otx2, Pax4, Pax6, Per2, Ptch1, Rax, Rorb, Rxrg, Thrb |
| Vesicle biology | Cadps, Chga, Chgb, Clta, Cltb, Dnm1, Dnm2, Dnm3, Lphn2, Ptprn, Scg2, Scg3, Snap23, Snap25, Sny2, Stx3, Sv2b, Syt4 |
Predicted gene is indicated.
Detection of cis-Regulatory Elements
Computational detection of enriched cis-regulatory elements (position weight matrices (PWMs)) within microarray-derived gene sets was conducted using ModuleMiner (63).
RESULTS
Microarray Analysis
A Large Number of Genes Exhibit Night/Day Differences in Expression in the Pineal Gland—The results of analysis of night/day differences in gene expression using the RG_U34A, the RAE230A, and Rat230_2 microarrays are presented in Table 1 and supplemental Table S3. To examine the degree of agreement of data obtained by these microarrays, we compared results from the RG_U34A and Rat230_2 microarray; the latter and the RAE230A microarray contain the same probe sets, representing 10,156 Entrez Gene identifiers, and did not require comparative analysis. Comparison of the expression levels of the 4,459 genes (6,392 probe sets) present on both the RG_U34A and Rat230_2 microarrays revealed the results were in excellent agreement (r = 0.44) (supplemental Fig. S1).
Expression of 604 genes (1,268 probe sets) exhibits a significantly greater than 2-fold change on a night/day basis (FDR <0.20; Table 1 and supplemental Table S3). Approximately 2,000 additional genes exhibit a smaller but significant night/day change in expression (FDR < 0.20). These findings increase by more than 50-fold the known number of genes differentially expressed in the pineal gland. Among the 604 genes with a greater than 2-fold difference in expression, 72% increase in expression at night and 28% decrease. A scatter plot of the night versus day expression (supplemental Fig. S2A) provides an indication of the range of night/day differences; this plot used the largest night/day difference observed with any microarray type. The amplitude of these changes varied from a downward 20-fold to an upward ∼100-fold change. A set of 142 genes (209 probe sets) changes greater than 4-fold (Table 1 and supplemental Table S3). Among the genes listed in Table 1 are those previously reported to be night/day differentially expressed (see Introduction). Although the results obtained with the different microarrays are in excellent overall agreement, there are differences in the absolute magnitude of the night/day changes, which may reflect different probe set design, biological variation, technical differences, or a combination.
NE/cAMP Signaling Plays a Dominant Role in the Control of Night/Day Changes in Gene Expression—As noted in the Introduction, 13 genes were previously known to be differentially expressed on a night/day basis in the pineal gland and to be controlled by NE/cAMP signaling (1, 4). To determine whether additional genes exhibiting night/day differences in expression are also controlled by NE/cAMP signaling, we used a well established organ culture method in which glands are incubated for 48 h, during which time nerve endings disintegrate. After 48 h glands are treated with NE. A 6-h treatment period was selected to approximate the time period between lights off and midnight sampling in the in vivo experiments; a dose of 1 μm NE was selected because it is known to selectively activate α-adrenergic and β-adrenergic receptors in this system (6, 10, 12, 13, 56). Gene expression was studied using the Rat230_2 microarray (experiment C).
Approximately 98% of the probe sets that exhibited increased expression at night also exhibited increased expression following NE treatment, and 85% of the probe sets that exhibited decreased expression at night also exhibited decreased expression following NE treatment (supplemental Fig. S2B, Table 1, and supplemental Table S3). This finding supports the conclusion that night/day differences in gene expression in the pineal gland are due to a large degree to the release of NE from nerve terminals in the pineal gland (4).
NE activates adenylate cyclase and elevates intracellular cAMP levels in the pineal gland. Here it was found that most effects of NE were mimicked by treatment with 0.5 mm Bt2cAMP (supplemental Fig. S2C, Table 1, and supplemental Table S3). 95% of the probe sets that exhibited decreased expression following NE treatment also exhibited decreased expression following Bt2cAMP treatment. The finding that Bt2cAMP treatment broadly mimics the effects of NE on gene expression provides evidence that cAMP is the primary second messenger mediating NE control of gene expression in this tissue.
Although it is apparent that NE or Bt2cAMP treatments change gene expression in a pattern similar to the changes seen on a night/day basis, there are striking exceptions, i.e. genes that exhibit marked night/day changes in expression that exhibit 10-fold lower response to NE treatment. These include Ccl9, Cd8a, Cyp1a1, Drd4, Mfrp, Per2, Prlr, Slco1a5, and several genes that do not have gene symbols (Table 1).
Organ Culture Has Marked Effects on Expression of a Minor Component of Genes—To determine whether changes in gene expression are induced by organ culture itself, we compared the day values from the in vivo study to control values from the organ culture study (experiment C). The normalized expression levels of more than 95% of the genes were unchanged after organ culture. However, marked changes occurred in 5% of the genes, and most notable were the greater than 10-fold decreases in gene expression that occurred in 51 genes (102 probe sets; Table 2), including 11 genes (24 probe sets) that decrease greater than 30-fold, and the greater than 10-fold increase in expression of 13 genes (20 probes sets; Table 2). Some of the genes that exhibit a greater than 30-fold decrease in expression are hemoglobin genes, suggesting that in some cases expression of a gene is low because blood cells that express these genes are present in the pineal gland when removed for in vivo experiments but are lost from the pineal gland during culture.
TABLE 2.
Genes expressed 10-fold higher or lower following organ culture
Genes were identified from data generated in experiment C. Unidentified genes are not included in the table. For further details see “Experimental Procedures.”
| Gene expression following organ culture | Gene symbol |
|---|---|
| Higher (>30-fold) | Mmp3, Spp1 |
| Higher (10-30-fold) | Akr1b8, Ccl2, Ccl20, Fcgr3, Fxyd2, Fzd1, Gpnmb, Hmox1, Igfbp3, S100a4, Wnt2 |
| Lower (10-30-fold) | Alas2, Arr3, Asmt, Atp1a2, Atp2b3, Car14,aCcl9, Cd74, Cd8a, Cdkn1c, Cirbp, ClicLIC6, Cln6,aCml5, Cyp1a1, Enpp2, Folr1, Gjb6, Gpc3, Grk1, Grm1, Guca1a,aIl17re, Kcne2, Mdk, Mfap5,aMpp4, Nphs1, Nrxn3, Ogn,aPtgds, Reep6, RT1-Da, Sag, Slc24a1, Slco1c1, Spink4, Vtn, Vwf, Wasl |
| Lower (>30-fold) | Cox8h, Defb24, Drd4, Hba-a1, Hbb, Igfbp2, Mfrp,aOpn1sw, Prlr, Sostdc1, St8sia5 |
Predicted gene is indicated.
Among the nine non-hemoglobin genes that exhibited the largest decrease (>30-fold) in expression during culture in control glands, six were also highly rhythmic, suggesting day levels seen in vivo may reflect physiological regulation by NE and/or another factor. The decrease in expression during culture may reflect the absence of a factor that is necessary for NE stimulation of these genes.
Highly Expressed Genes That Characterize the Pineal Gland—Highly expressed genes were identified by determining the ratio of expression in the pineal gland relative to the median expression among other tissues (see “Experimental Procedures”), yielding rEx values. This was done using data obtained in experiment A (RG_U34A microarray) and in experiment C (Rat230_2 microarray); the median expression values were based on 23 (58) and 7 tissues, respectively. In both cases, brain tissues comprise approximately half of the tissues sampled. The calculated tissue medians are given in supplemental Table S4.
This effort identified 996 genes (1,654 probe sets) with rEx values of 4 to ∼300. One hundred fifty six genes (255 probe sets) had rEx values greater than 16 (Table 3; supplemental Table S4).
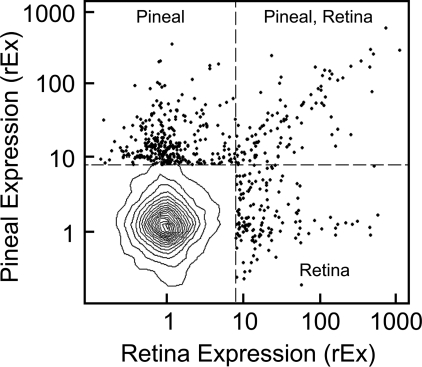
The rEx values for the pineal gland were compared with those of six other tissues in an effort to identify other tissues strongly expressing the same genes. Only in the case of the retina was there a striking similarity in the genes with high rEx values, consistent with evidence that both tissues evolved from a common ancestral photodetector (64). Approximately 17% of the highly expressed genes (rEx > 8) in the pineal gland are also expressed in the retina at similar levels (Fig. 1; Table 4, and supplemental Table S5). Among these highly expressed genes are those that encode signal transduction proteins (e.g. Sag, Pdc, and Grk1) and genes encoding developmental and regulatory transcription factors (e.g. Otx2, Crx, Pax6, and Neurod1).
FIGURE 1.
Pineal rEx versus retina rEx. This figure demonstrates that a subset of genes is predominantly expressed in the pineal gland or retina or both. Data are based on results obtained in experiment C. The probe sets for the entire microarray are represented by 5% density contours (95% of probe sets are within the outermost contour line). Only symbols representing probe sets with rEx values greater than 8 for either the pineal gland or retina (∼1% of 31,099 probe sets on the Rat230_2 microarray) are shown; the remaining probe sets are not highly expressed in either tissue relative to other tissues. The plotted probe sets fall into three sectors as follows: Pineal, in which 218 genes (256 probe sets) are highly expressed primarily in the pineal gland; Pineal, Retina, in which 55 genes (63 probe sets) are highly expressed at similar levels in both the pineal gland and retina; and Retina, in which 93 genes (109 probe sets) are highly expressed primarily in the retina. The genes represented by these probe sets are listed in Table 4; supplemental Table S5 contains a detailed description of these genes and other unannotated probe sets. An interactive version of this figure that identifies each symbol is available at sne.nichd.nih.gov.
TABLE 4.
Genes highly expressed in the pineal gland and/or the retina
Genes were identified from data generated in experiment C and represent the symbols shown in the corresponding sectors in Fig. 1. The supplemental Table S5 (sne.nichd.nih.gov) contains the gene titles, Entrez Gene identifiers, and the rEx values for individual genes. The supplemental Table S5 also includes Entrez Gene identifiers for unidentified sequences (i.e. expressed sequence tags) not included here. For details regarding the calculation of rEx values see “Experimental Procedures.”
| Group | Gene symbol |
|---|---|
| Pineal | A2m, Aanat, Abca1, Abhd14b, Accn4, Acvr1, Adam2, Adra1b, Adrb1, Alox15, Als2cr4,aAmpd2, Anp32e, Arhgap24, Asl, Asmt, Atp6v1c2, Atp7b, Baiap2l1, Bzrp, Ca3, Cabp1, Cacna1h, Camk1g, Ccdc125, Ccl2, Ccl6, Ccl9, Ccnd2, Cd1d1, Cd24, Cd63, Cd8a, Cfd, Cflar, Chga, Chrna3, Chrnb4, Chst2,aCip98, Cntrob,aCol15a1, Col8a1,aCrcp, Crem, Crtac1, Ctsc, Cyp1a1, Dclk3,aDcn, Ddc, Defb24, Depdc7, Dhrs8, Dnajc12, Dpt,aDsc2, Dscr1, Esm1, Etnk1,aEya2, F5, Farp2,aFcer1a, Fdx1, Fkbp5, Fosl2, Frmd4b, Fzd4, G0s2, Gale, Galnt4, Galntl1, Gch, Gdf15, Gem,aGla, Gls, Gmds, Gnas, Hs3st2, Hsd3b7, Hspb1, Ifitm3, Igfbp6, Igsf1, Il13ra2, Il17re, Irak2, Irf7, Irs1, Isl2, Itgb2, Ka15, Kcne2, Kctd14,aKctd3, Kit, Klhl4, Krt1-19, Lad1,aLama2,aLamb1,aLgals1, Lgals3, Lhx4,aLix1,aLmbr1l, Lmod1,aLnx1,aLpl, Lrrc8e, Lum, Lxn, M6prbp1, Mad2l2, Mak10, Map4k1,aMat2a, Mcam, Me2,aMiox, Mitf, Morn1, Mpp3, Msrb2, Mt1a, Mtac2d1, Muc4, Mx2, Mylk,aMyo5b, Nacad, Ncaph, Nphp4, Nphs1, Nptx1, Nradd, Nrap,aNup107, Oasl1, Padi4, Pcbd1, Pde10a, Pde4b, Pgam2, Pgm1, Pid1, Pik3r3, Pla2g1b, Pla2g5, Plcd4, Plscr1, Postn,aPqlc1, Prkar2b, Prtg, Psph, Ptgis, Ptprn, PVR, Qscn6, Rarres1, Rasgrf2, Rere, Resp18, Ribc2, Rnase1, Rorb,aRreb1,aRxrg, Sall1,aScn7a, Sema3a, Serping1, Slc15a1, Slc17a6, Slc19a2, Slc1a5, Slc25a10, Slc39a4,aSlc47a1, Slc7a6,aSnf1lk, Sod3, Sorl1, Spink4, Tagln2, Tex14,aTm7sf2, Tnfrsf9, Ugdh, Vof16, Wnt10a,aZmat2, Zrsr1 |
| Pineal, retina | Aipl1, Arr3, Cacna1f, Cnga1, Cngb1, Cplx3, Crocc,aCrx, Drd4, Egflam, Frmpd1,aGabrr1, Gnat2,aGnb3, Grk1, Guca1a,aHspa1b, Impg1, Impg2, Kcnh6, Kcnj14, Lamp3, Lrrc21, Mak, Mpp4, Neurod1, Opn1sw, Osap, Otx2, Pax4, Pcbp3, Pcdh21, Pdc, Pde6b,aPla2r1,aRax, Rbp3, Rds, Rom1, Rtbnd,aSag, Slc24a1, Slc6a6, Slco4a1, Stk22s1, Tulp1,aUnc119 |
| Retina | Abca4,aAgpat3,aArf4l,aC1ql2, Calb2, Capn3, Cart, Cav, Cbln2, Cds1, Chrna6, Cirbp, Col11a1, Col2a1, Col5a3, Col9a1, Cryaa, Cryba1, Cryba2, Cryba4, Crybb1, Crybb2, Crybb3, Cryga, Crygb, Crygd, Cryge, Dapl1, Dkk3, Elovl4,aFscn2,aGlra2, Gnat1,aGnb1, Gpsm2, Gsg1, Hk2, Hmx1,aIgsf9,aIsl1, Kcnb1, Mab21l1, Mdm1, Msh5, Ng23, Opn1mw, Opn4, Pax6, Pcdh8, Pcp2, Pde6a,aPde6h, Ppap2c, Prom1, Rcvrn, Rdh11, Rdh12,aReep6, Rfrp, Rgr,aRho, Rhpn1,aRp1h, Six6,aSmarcd1,aSncg, St6galnac2, Stx3, Susd3,aSynpr, Tbc1d12,aTbx2,aTcfap2a,aTcfap2b,aTmem136, Txnl6,aVip, Vipr2, Wdr89 |
Predicted gene is indicated.
Genes with high rEx values in the pineal gland but not the retina include genes encoding enzymes required for melatonin synthesis (e.g. Tph1, Gch1, Ddc, Aanat, Asmt, and Mat2a). Among those with high rEx values in the retina but not the pineal gland are Rho and Opn1mw, which encode photosensitive G-protein-coupled receptors. A surprising observation was that expression of Opn1sw, which encodes another G-protein-coupled light receptor, is 4-fold greater in the pineal gland; this is surprising because the mammalian pineal gland is not generally regarded as being directly photosensitive.
Radiochemical in Situ Hybridization Histological Analysis Confirms Gene Profiling Results
Radiochemical in situ hybridization analysis of sagittal brain sections (Fig. 2) was used to confirm night/day differences in gene expression and to obtain a detailed anatomical analysis of areas of expression of the genes with a high rEx value from the microarray analysis. Quantitation of the radiochemical labeling in the pineal gland revealed the results of histological studies were in excellent agreement with the microarray results, as regards both the degree of night/day differences in expression and rEx values (Table 5).
TABLE 5.
Quantitative analysis of the in situ hybridization signal in the pineal gland
The antisense probes used appear in supplemental Table S1. Quantitation was done as described under “Experimental Procedures”; results are given as the mean ± S.E.
|
Gene symbol
|
Microarray
|
In situ hybridization
|
|||
|---|---|---|---|---|---|
| Night/daya | rExb | Day | Night | Night/day | |
| dpm/mg tissue | dpm/mg tissue | ||||
| Aanat | 92.9 | 196.0 | 13.4 ± 1.1 | 3059.2 ± 183.5 | 227.8c |
| Acly | 1.3 | 6.9 | 386.8 ± 63.0 | 722.0 ± 148.5 | 1.9c |
| Alox15 | 1.7 | 324.0 | 924.5 ± 64.2 | 927.0 ± 79.7 | 1.0 |
| Asmt | 1.3 | 198.0 | 1737.5 ± 107.8 | 2156.4 ± 170.8 | 1.2c |
| Atp7b | 12.1 | 17.9 | 4.8 ± 0.9 | 424.8 ± 55.3 | 87.6c |
| Crem | 37.0 | 167.7 | 40.2 ± 1.6 | 1277.4 ± 240.9 | 31.8c |
| Crx | 2.2 | 151.1 | 1277.2 ± 79.0 | 1411.0 ± 333.7 | 1.1 |
| Dusp1 | 48.8 | 85.8 | 9.0 ± 0.8 | 26.5 ± 7.4 | 3.0c |
| Esm1 | 1.9 | 198.7 | 134.5 ± 16.7 | 196.7 ± 20.5 | 1.5c |
| Fosl2 | 4.2 | 8.1 | 15.8 ± 1.1 | 79.9 ± 26.8 | 5.1c |
| Gch1 | 1.3 | 236.4 | 980.8 ± 67.1 | 965.9 ± 82.7 | 1.0 |
| Gnb3 | 1.0 | 101.8 | 3262.5 ± 309.1 | 3989.8 ± 228.1 | 1.2c |
| Mt1a | 12.6 | 12.2 | 90.8 ± 14.4 | 1031.3 ± 247.1 | 11.4c |
| Nptx1 | 3.5 | 48.9 | 86.5 ± 5.2 | 543.1 ± 24.7 | 6.3c |
| Pdc | 1/2.7 | 359.4 | 566.7 ± 53.4 | 170.9 ± 18.7 | 1/3.3c |
| Per2 | 13.4 | 5.1 | 19.5 ± 2.5 | 37.2 ± 1.8 | 1.9c |
| Rorb | 3.4 | 32.9 | 34.7 ± 4.7 | 170.8 ± 32.8 | 4.9c |
| Tph1 | 1.2 | 259.4 | 3092.3 ± 16.7 | 3049.5 ± 36.0 | 1.0 |
| Ttr | 1.5 | 65.1 | 1476.9 ± 138.7 | 2362.0 ± 291.8 | 1.6c |
The rEx values provided by microarray analysis were also confirmed by the results of in situ hybridization; genes with high rEx values were found by in situ hybridization to be expressed highly or exclusively in the pineal gland relative to other brain regions on the section (Fig. 2). In some cases, however, genes with high pineal rEx values are also strongly and selectively expressed in other brain regions. For example, Acly is highly expressed in the habenular nucleus and in the mesencephalic raphe complex; Dusp1 is highly expressed in the cortex, cerebellum, and thalamus; Gnb3 is expressed at moderate levels in many brain regions; Nptx1 is expressed in the cerebellum and dentate gyrus; and Ttr is very strongly expressed in the choroid plexus (Fig. 2). In addition, the results of in situ hybridization provide examples of nocturnally elevated extra pineal expression of specific genes, including Per2 in the cerebellum and cortex and Fosl2 in the cortex, and this was consistently observed upon repeated examination of brain sections from all animals. Although these findings require further in-depth investigation, this is beyond the scope of this study.
In sections that contained the pineal stalk or deep pineal gland, both structures were labeled with the same density and pattern as the superficial pineal gland. An example of this is found in the Crx panel (see Fig. 2). However, because the deep pineal gland was absent from most sections because of its small size and to differences in the plane of section, this was not seen on a regular basis.
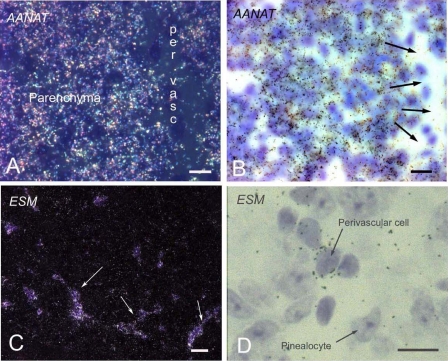
It is highly likely that most of these genes are expressed in the dominant cell type in the pineal gland, the pinealocyte (5), as occurs with Aanat (Fig. 3, A and B). However, it is also clear that some of the expressed genes with high rEx values may be expressed in non-pinealocytes, as demonstrated by the endothelial cell marker Esm1, which has a high rEx value (supplemental Table S4) and is expressed in a pattern consistent with localization in cells confined to the perivascular spaces of the gland, where endothelial cells occur (Fig. 3, C and D).
FIGURE 3.
Staining of pinealocytes and endothelial cells in the pineal gland. A, image presenting Aanat-labeling of pinealocytes. Dark field photomicrograph of radiochemical in situ hybridization of a part of the superficial pineal gland of the rat with an antisense probe binding to mRNA encoding AANAT. The section was dipped in a photographic emulsion and developed after exposure; the grains are seen using dark field visualization as white dots in the emulsion. Dense labeling is seen above the pineal parenchyma with no labeling of the perivascular space (per vasc). B, same section shown in A in transmitted light. Arrows indicate the perivascular space, which is not labeled in A. Cresyl violet counterstaining is used. C, labeling of the perivascular spaces; endothelial labeling image. Dark field photomicrograph of radiochemical in situ hybridization of the superficial pineal with an antisense probe binding to mRNA encoding Esm1. Note the labeling of the perivascular spaces (arrows). D, high power photomicrograph taken in transmitted light of part of the superficial pineal showing Esm1 labeling of the perivascular cells and only few grains above the pinealocytes. Cresyl violet counterstaining. Bars, A, B and D = 20 μm. C = 50 μm. The probes used are described in supplemental Table S1.
qRT-PCR Analysis Confirms Results of Gene Profiling
qRT-PCR was used to confirm and extend in vitro and in vivo results obtained from microarrays. This effort included genes known to exhibit large night/day differences in the pineal gland, which serve as references (e.g. Aanat, Fosl2, Crem, and Dio2), in addition to genes that did not exhibit a night/day difference; 44 genes were examined. qRT-PCR confirmed rhythmic expression that had initially been indicated by microarray analysis (Fig. 4), providing evidence that the two-point night/day sampling strategy is a productive and reliable approach, as a first step toward the identification of night/day rhythmically expressed genes. In most cases, the amplitude of the rhythm was greater using qRT-PCR. The use of multiple time points in the qRT-PCR study provided a more complete profiling of the pattern of transcript abundance. Comparison of these patterns reveals differences in the timing of the peak in transcript abundance, consistent with previous reports (65, 66).
In some cases there was a comparatively large variation at transition times between low and high expression (e.g. Fosl2 and Cited4), which may reflect individual variation (Fig. 4). In the case of Ttr and Mfrp, such variation does not appear to be associated with a distinct 24-h pattern of expression; the similar pattern of variation might reflect a common biological link (i.e. regulation) not shared by the other genes examined.
qRT-PCR was also used to confirm the microarray results from organ culture studies. The results confirm the microarray results, providing additional support for the conclusion that night/day differentially expressed genes are controlled by a NE/cAMP mechanism (Table 6). The effects of forskolin were also examined; forskolin increases adenylate cyclase activity (67, 68), resulting in an increase in cAMP. It was found to mimic the effects of Bt2cAMP and NE, providing further support for the conclusion that cAMP mediates the effects of NE.
Detection of cis-Regulatory Elements
Computational detection of enriched cis-regulatory elements (PWMs) obtained via submission of gene groups to ModuleMiner (63) showed that multiple, diverse PWMs were enriched in the submitted groups (see supplemental Tables S7 and S8). Individual gene groups were found to be associated with distinct collections of high scoring PWMs. Notable enrichments of PWM families in particular gene groups included CREB (rhythmic > 8, <1/8), SP1 (rhythmic > 8), Oct1 (retina), and Hox (Crx) (pineal and retina, pineal). None of these were enriched in the control group, which included genes with low rEx values (<1.5) and night/day expression differences between ½ and 2; this group was, however, enriched in the Pax4 PWM. Also notable was the relative absence of PWMs of regulatory transcription factor (TF) sites compared with basal transcriptional sites in the pineal group.
DISCUSSION
The results of these studies provide the most comprehensive profile of the pineal transcriptome available, contributing to a more meaningful understanding of tissue function and providing new evidence of metabolic pathways and functional capacities of the pineal gland that have been overlooked or unrecognized. These advances include a greater than 10-fold increase in the number of genes known to be night/day differentially expressed in the pineal gland; in addition, this study has revealed that night/day differential expression is regulated by NE/cAMP signaling. Moreover, this effort has identified for the first time a large set of highly expressed genes that may or may not be differentially expressed on a night/day basis. The lists of night/day differentially expressed genes and highly expressed genes provide a valuable new data base for future studies of the pineal gland.
Gene Expression Considered in Light of the Cellular Composition of the Pineal Gland
Discussion of the results of microarray studies is appropriately preceded by consideration of the organization and composition of the tissue. Pinealocytes are the dominant cell in the rodent pineal gland (∼95%) and are recognized to function as a melatonin factory (5). Interstitial cells are located between pinealocytes and generally resemble brain fibrillary astrocytes. In addition, the mammalian pineal gland contains a dense vasculature composed of endothelial cells and pericytes of the capillaries in fairly large perivascular spaces (Fig. 3), with a ground substance consisting of acid mucopolysaccharides. Some arterioles are also present, which adds the smooth muscle cell to the list of minor cellular components of the pineal gland. In addition, minor populations of phagocytes are located in the pineal perivascular spaces and in the parenchyma of the gland (69). Finally, T-cells and other members of the immune family of cells can be transiently present in the gland or located in a cluster just outside the gland (5).
This cellular complexity makes it clear that some of the highly or night/day differentially expressed genes may be located in cells other than the pinealocyte. This possibility is made clear by the example of Esm1, an endothelial cell marker that is known to be highly expressed in the pineal gland (70, 71). Comparison of the patterns of expression of Esm1 and of pinealocyte markers as revealed by in situ hybridization histology makes it clear that Esm1 is not expressed in pinealocytes and is likely to mark endothelial cells. Previous studies with another gene, Id1, have provided a reason of a different nature that also argues for the importance of consideration of the cellular localization of transcripts. These studies found that expression of Id1 follows a daily rhythm (26) and is expressed at levels that are ∼8-fold higher than in other tissues. Id1 is expressed in the pineal gland at very high levels in a small population of glial-like cells and at lower levels in pinealocytes (72). Similarly, in evaluating the conditional changes in gene expression (night/day, NE, Bt2cAMP), it is clear that genes may be differentially expressed in non-pinealocyte sites and that evidence from histological studies or gene profiling of purified cell preparations or both is required to establish the site of expression of a gene of interest to provide the cellular context in which a gene is expressed.
Large Number of Genes That Exhibit Daily Changes in Expression in the Pineal Gland
An unexpected finding from this effort was the large number of genes that exhibit night/day differences in expression; more than 600 genes are differentially expressed on a night/day basis more than 2-fold, with ∼70% increasing at night. The far greater number of night/day differentially expressed genes seen in this study as compared with the less than 40 genes seen in previous studies (26, 55) may reflect several factors, including the larger number of probe sets interrogated by the RAE230A microarray (15,923 probe sets, 10,174 genes) and the Rat230_2 microarray (31,099 probe sets, 13,663 genes), as compared with the two platforms used in the previous studies, including the Affymetrix RG_U34A microarray (8,799 probe sets, 4,996 genes) and the Atlas Rat 1.2 cDNA expression array (1,176 genes). Other factors that may have contributed to the differences are the larger number of replicates in this study, which is based on a total of 10 pools of night and of day glands; previous studies used less. In addition, technical differences and differences in statistical analyses may have contributed to the number of genes detected.
A striking feature of the global change in gene expression is that many genes are differentially expressed on a night/day basis with a greater than 10-fold amplitude. This characteristic is consistent with the dedicated role that the pineal gland has in time-keeping.
It is likely that the number of night/day differentially expressed genes will grow in the future for several reasons. One is that the two-point sampling (mid-day versus midnight) used here may not have revealed daily rhythms in expression that peak closer to dawn or dusk, which might be revealed by more frequent sampling. For example, recent studies have revealed such a rhythm in expression of Pax4 (73). In addition, the night/day differentially expressed genes identified in Table 1 only include those that have been assigned Entrez Gene identifiers and exceed a 2-fold night/day threshold. Approximately 500 of these have not been assigned Entrez Gene identifiers, and although these may represent noncoding RNAs of unrecognized genes, some may represent unidentified genes. Accordingly, the complete annotation of the rat genome will further expand the number of genes that are expressed differentially on a 24-h basis in the pineal gland, as will more frequent time sampling. Furthermore, the precise nature of the transcripts encoded by genes cannot be reliably predicted from the results of microarray studies, because the microarray probes are based on 3′ sequence; this may not detect differential splicing and/or the actions of alternative promoters, which can markedly alter the expressed transcript, as seen with Crem, Slc15a1, Atp7b, and Pde4b (21, 23, 24, 74).
It is important to note that changes in mRNA may be large and unequivocal, but one cannot reliably predict that such changes will be translated into changes in protein. Such a relationship is seen in the pineal gland in the case of Mat2a, Slc15a1, Pde4b, and Fcer1a (22, 23, 75, 76); in these cases, the transcripts and encoded proteins appear to have similar stability. However, the daily changes in gene expression in other cases may be only slowly translated into changes in proteins over a period of days or weeks, not hours, thereby providing an integrated measure of prior levels of activity, i.e. changes in the duration of the periods of expression of some genes may lead to very gradual changes in the level of the encoded protein as has been shown to be the case in the pineal gland with the Crem splice variant Icer (24, 77) and the adrenergic receptor Adra1b (19). Likewise, post-translational regulation can cause very rapid changes in protein, without the encoding mRNA changing as seen with Aanat (78, 79).
Adrenergic/cAMP Signaling Plays a Dominant Role in Controlling the Global Changes in Pineal Gene Expression
As indicated in the Introduction, previous studies of night/day differentially expressed genes have provided convincing evidence that NE controls cAMP accumulation, which in turn controls gene expression. These studies have established clearly that NE is released at night in the dark and that when release is blocked, night/day differences in gene expression are blocked. Moreover, it has been shown repeatedly that the night/day differences in gene expression can be mimicked in organ or cell culture by treatment with NE, which elevates cyclic AMP in this tissue. Furthermore, it has been established that in all cases, the effects of NE on gene expression are mimicked by elevation of cAMP or by cAMP protagonists. The results of this study markedly expand the list of adrenergically regulated genes and in doing so demonstrate that a single regulatory signal can have a profound effect on the transcriptome of one tissue.
A previous study did not find a correlation between night/day differentially expressed genes and those induced by NE treatment (26, 55, 80). It is likely that this difference is because of the short NE treatment period (1 h) used in the previous study (80). The current set of experiments used a 6-h treatment period, which more closely reflects the period animals were in the dark in the in vivo studies.
As discussed above, the number of genes controlled by this signaling cascade is likely to increase because of annotation issues and also because only a single 6-h treatment period was studied, which might not detect changes that occur rapidly and transiently or those that are slow to develop. In addition, another reason that gene expression might not increase in some cases is the artificial conditions of organ culture, including use of a defined minimal medium. The absence of hormones and other factors critical for expression of some genes might preclude a response to NE or Bt2cAMP. Accordingly, it is possible that future investigations will find that NE induction of some genes may require one or more coregulating factors that are absent from the current organ culture medium. As a result, the number of genes regulated by NE would increase.
The effects of cAMP, as addressed in the Introduction, are likely to be mediated by cAMP-dependent protein kinase and reflect either a specific pCREB/CRE interaction or more general epigenetic mechanisms, including regulation by histone H3 phosphorylation and acetylation. Epigenetic modulation of chromatin organization could influence access of transcription factors to regulatory elements in genes. In this case, cAMP can be seen as a transcriptional regulator acting through epigenetic mechanisms that do not involve pCREB/CRE interactions.
The finding that cAMP suppresses expression of some genes may be explained by the induction of inhibitory transcription factors, as recently discussed (81), and also along epigenetic lines, because cAMP may act to block access to a regulatory element by altering chromatin structure.
The finding that most of the genes that are differentially expressed on a night/day basis are also regulated by NE provides evidence for concluding that these genes are expressed on a circadian basis, i.e. a daily rhythm will be seen in constant darkness and does not require light/dark transitions. This conclusion is supported by the fact that the release of NE into the pineal extracellular space is controlled by the SCN. As discussed in the Introduction, the SCN/SCG/NE/cAMP regulatory system has been found to regulate a small number of genes in the pineal gland based on classical biochemical and physiological evidence. The results of this study provide reason to conclude that all genes found in this study to be controlled by NE and Bt2cAMP are physiologically controlled by the endogenous circadian oscillator in the SCN and that their rhythms can be correctly described as circadian in nature.
Genes That Are Spontaneously Up- or Down-regulated in Organ Culture
The relative level of expression of most genes compared with total gene expression does not change remarkably after pineal glands are placed in culture. However, we discovered a subset of genes that exhibited greater than 10-fold positive and negative changes during culture. Decreased gene expression may reflect the absence from organ culture of a regulatory factor or hormone normally present in the circulation or of a transmitter (e.g. dopamine or neuroactive peptides) that is normally released from the sympathetic nerve endings. Gene expression may also change in response to the culture environment (95% O2 and defined medium) or because physiologically relevant local control mechanisms do not function in vitro. For example, these changes could reflect the absence of interactions between pinealocytes and the vasculature.
It is of special interest to note that there was a marked decrease in the expression of one of the more important genes in the melatonin synthesis pathway, Asmt, which encodes the last enzyme in melatonin synthesis. This has not been reported previously. Studies with rodents have revealed that expression of this enzyme can be regulated by adrenergic mechanisms (82, 83). Other studies of this gene in Y79 cells, a human retinoblastoma-derived line, indicate that expression of the gene is controlled by 9-cis-retinoic acid (84). However, we have not been able to prevent the decrease in Asmt expression in the cultured rat pineal gland by treatment with 9-cis-retinoic acid, NE, or Bt2cAMP.16 Accordingly, the factors controlling expression of this gene remain unknown.
Studies with retinoblastoma cells have also found that 9-cis-retinoic acid regulates expression of Crx and a set of genes expressed in cones (85), pointing to the need for further studies on 9-cis-retinoic acid and the role it plays in pineal biology. Similarly, expression of the opsin gene Opn1sw falls more than 50-fold in culture; its expression is controlled by T3 signaling (86). Accordingly, it is possible that treatment of pineal glands with T3 and retinoic acid, which are known to act in concert through heterodimeric receptor complexes, may prevent some of the spontaneous large changes in gene expression that occur during culture and that media used for pineal organ cultures should be supplemented accordingly.
As mentioned under “Results,” it is also likely that the disappearance of some genes reflects the loss of blood cells normally present in the vasculature, as is probably the case for Hba-a1 and Hbb, which are expressed in the red blood cells.
Functional Implications
Functional clustering (see under “Experimental Procedures”) of the genes selected for inclusion in Table 7 (rhythmically expressed, highly pineal enriched, or both) places these genes into two broad functional categories. These are as follows: first, groups of genes that participate in specialized functions; and second, genes that have a nonspecialized, more common role in cell biology.
Specialized Functional Gene Groups
Two of these gene groups are predictable from previous knowledge (melatonin production and phototransduction), whereas two other groups (immune response and T3/retinoic acid signaling) are less predictable.
Melatonin Production—Most predictable are those genes that code for proteins involved in melatonin production, including both enzymes that function directly in the melatonin synthesis pathway and cofactors required for these enzymes. It is also very likely that many of the genes encoding proteins dedicated to protein phosphorylation and to cAMP and calcium signaling are involved in the control of melatonin synthesis, in part through regulation of Aanat expression and processing of AANAT protein.
Phototransduction—Gene expression similarities between the pinealocyte and the retina have been documented before, but they were limited to less than 20 genes. This study greatly extends the number of genes known to be highly expressed primarily in pineal/retina to over 55 genes (63 probe sets). A common expression pattern reflects well documented evolutionary relationships between the pineal gland and retina (54, 87, 88); but by revealing the real extent of pineal gland/retina coexpression, we have freshly questioned the functional relevance of phototransduction-related genes in the mammalian pineal gland (see below). Highly expressed pineal gland/retina genes also include transcription factors (Otx2, Crx, Neurod1, and Pax6) that likely drive the common transcriptional outputs in these tissues. The work done here also led to the finding that both tissues express high levels of Pax4, an ortholog of Pax6 (89). Importantly, adult expression of these transcription factor genes implies roles in maintenance of cellular phenotype, in addition to roles during development.
It is of interest to note that the expression of one opsin gene in the pineal gland is higher than that in the retina; Opn1sw is expressed 4-fold higher in the pineal gland as compared with the retina. In contrast, Opn1mw and Rho are expressed at 18- or >400-fold higher in the retina, respectively. This and the finding that the pineal gland expresses many genes involved in phototransduction provide genetic evidence that this tissue might detect light; however, there is no evidence that the adult pineal gland has this capacity. Photodetection by neonatal pineal gland has been reported (90–92); however, the underlying mechanism involved has not been elucidated, and it is not clear whether this involves the phototransduction system that operates in the retina or another mechanism. Pineal Opn1sw may be functionally vestigial in the adult pinealocyte as regards detection of light. However, it is also possible that it plays a passive role in signal transduction that does not involve detection of light; for example, it might influence signal transduction by binding to receptors and other proteins involved in adrenergic signaling.
Immune/Inflammation Response—Our identification of a large cluster of immune/inflammation-associated genes expressed in the rat pineal gland reflects similar findings in an avian species (88) and is therefore of interest. A potential, immune-related, functional specialization for the pineal gland is indicated by the presence of perivascular phagocytes that act as antigen-presenting cells (69), and also by the strong expression of Fcera1 (a receptor dedicated to IgE signaling), and a related gene in the pineal gland (76). Further study of these genes may lead to a better understanding of the role of the pineal gland in the immune response.
T3/Retinoic Acid Signaling—Our finding of high levels of transcripts associated with T3/retinoic acid signaling suggests a potential functional specialization related to this signaling pathway. Previously there has been a report of effects of T3 on melatonin synthesis and a substantial body of evidence indicating that Dio2 is night/day differentially expressed in this tissue (93–95). Together with reports of effects of T3 on retinal function (86, 96–99), the accumulated evidence argues for future studies that involve specific functional interventions of this signaling pathway in both the pineal gland and retina.
Nonspecialized Functional Gene Groups
Cellular Signaling—A functional cluster of “cellular signaling” genes derived from the genes of Table 7 is consistent, at least in part, with the evidence of adrenergic control of pineal function, which involves a broad range of signal transduction-related proteins. As indicated above, many of these are likely involved in regulating melatonin production. The high rEx of Drd4 and known rhythmic pattern of expression (26) point to a related role for dopamine in pineal function because dopamine is colocalized in pineal nerve processes as a precursor of NE; as such, it is likely to be released with NE. The role of dopamine in the pineal gland remains to be fully established. It is of interest that Drd4 expression was not elevated by NE or Bt2cAMP in organ culture. This raises the question of how expression of this gene is regulated in vivo.
This study has also highlighted groups of genes that have not received significant attention. These include genes related to prostaglandin synthesis and the lipoxygenase 15 pathway, which leads to production of hepoxilins and related compounds (100–106). The regulation and function of these pathways and their cellular sources and targets requires further investigation. Other less well studied genes in a pineal context included the receptors for prolactin, acetylcholine, GABA, glutamate, and interleukin, suggesting roles in pineal gland signaling. Acetylcholine receptor expression is consistent with anatomical evidence (5) that reveals the presence of nonsympathetic, probably parasympathetic, nerve fibers in the pineal gland of the rat and with biochemical studies that have shown the presence of both muscarinic (107) and nicotinic (108) cholinergic receptors. Furthermore, it has been shown that a cholinergic input to the rat pineal gland causes the release of glutamate, which has been reported to act via glutamate receptors on the pinealocyte membrane to inhibit melatonin synthesis (109, 110).
Genes Dedicated to Small Molecule Biology—Included among this cluster of genes is the taurine transporter Slc6a6, perhaps explaining the high concentration of taurine in the pineal gland (111); this gene is also highly expressed in the retina, another tissue with a high concentration of taurine (112). Genes that regulate metal homeostasis are also clustered here. Zn2+ is essential for the synthesis of melatonin, because it is required by Gch to generate biopterin (113, 114), the cofactor for Tph1, the first enzyme in melatonin synthesis. Another essential role of metals of special relevance to pineal physiology is Sn+, which is essential for T3 signaling because it is required by Dio2 (115).
Cell:Cell and Cell:Extracellular Matrix Contacts—Genes encoding proteins involved in cell:cell and cell:extracellular matrix contacts represent another group of genes that have not been well studied in the context of the pineal gland. This includes several members of the cadherin family, which form homophilic Ca2+-dependent associations. Knowledge of the site of expression of these genes may be of practical utility in identifying, purifying and immobilizing populations of cells recovered from the pineal gland.
Circadian Clock Genes—Generally absent from the list of strongly rhythmic or highly expressed (Table 7) genes are circadian clock genes, exceptions being Per2 and Rorb. Expression levels of Arntl, Clock, Per1, Per2, Per3, Cry1, Cry2, and Arnt are less than 4-fold the median tissue level of expression. Reports in the literature have established that these genes are expressed in the pineal gland, some following a 24-h pattern (116–119). Their absence from the lists of highly expressed or highly rhythmic genes may reflect a relatively unimportant role that the circadian clock system plays in the mammalian pinealocyte in generating daily rhythms. The strong rhythm of Per2 may reflect a related role in biological time-keeping, in that it might influence the dynamics (intensity, duration) of the responses of genes with E-boxes, which includes Aanat (120, 121).
cis-Regulatory Elements
A question raised by our finding of large groups of pineal gland-specific and night/day differentially expressed genes is whether these groups of genes share common transcriptional regulatory DNA sequences. This question was addressed using ModuleMiner (63) using multiple groups of genes as inputs. In general, the results of this effort did not provide an indication that pineal specificity or night/day differences can be explained by the presence of a few dominant regulatory elements. This may relate to inherent limitations of this analysis, which include the analysis of only 10 kb 5′ to the transcription start site and filtering that eliminates genes that do not exhibit human-mouse conservation of regulatory elements. Conversely, it is possible that rhythmic or relatively high expression of genes in the pineal gland reflects multiple parallel/hierarchical regulatory cascades of such complexity that they would be impenetrable to this mode of analysis. However, some of the results of this analysis (see supplemental Table S7) are worthy of further consideration.
Enrichment of a CREB family PWM in the group of highly rhythmic genes (rhythmic >8, <1/8) is consistent with known, cAMP-driven, mechanisms of gene regulation in the pineal gland, as discussed in the Introduction. The NE/cAMP cascade is evident from the results of the experiments presented here, in which gene expression was broadly enhanced by treatment with Bt2cAMP, a stable cAMP analog; with forskolin, an activator of adenylate cyclase; or with NE, which elevates cAMP in the pineal gland. Conversely, the absence of a similar enrichment in less highly rhythmic genes suggests the presence of alternative mechanisms. The enrichment of SP1 PWMs in moderately rhythmic and highly rhythmic groups is interesting in this context and may warrant further investigation because a previous study has indicated a role for SP1 in pineal gene rhythmicity (122).
Surprisingly, we did not observe widespread enrichment of Hox family PWMs across the tissue-specific (pineal and retina) groups of genes; this is surprising because the Crx and Otx transcription factors are known to regulate pineal gland- and retina-specific gene expression, as discussed in the Introduction (31, 32, 123). Our results show the Crx PWM to be enriched only in the combined submission of pineal only and pineal/retina groups. This finding may indicate a more limited (pineal gland- and retina-related) role for Hox family-related mechanisms than is currently believed. With respect to the retina, our analysis has revealed strong enrichment of another PWM family (Oct1) that is recognized as one PWM family/transcription factor that is associated with the visual perception GO term (GO:0007601; Matbase, Genomatix). With respect to the pineal gland, an intriguing finding of the ModuleMiner analysis was the relative absence of conserved regulatory transcription factor sites in the “pineal only” group. This is an interesting result in which ModuleMiner detects only a few enriched sequences, and these are primarily either core promoter elements such as initiator and cap or CEBP, all of which are among the most common sites found in eukaryotic promoters (124). This finding taken together with the Pineal only and Pineal/retina result is suggestive of common cis-regulatory rules for pineal/retinal tissues but the absence of a pineal only mechanism, indicating either a novel cis or trans (e.g. microRNA) level of control not detected by this analysis or that the pineal gland-specific cascade is controlled by multiple unrelated mechanisms.
Another surprising finding of the ModuleMiner analysis was the enrichment of Pax4 sites within the control group of pineal genes, genes expressed in this tissue that are neither rhythmic nor highly expressed relative to other tissues. Given the high relative expression of Pax4 in the rat pineal gland (73), this may point to a negative mode of regulation in which the “control” gene group is suppressed via a Pax4-related mechanism. In this respect, previous studies have provided evidence of repressive actions of Pax4 (125).
Final Statement
The results of these studies provide investigators with a rich and comprehensive genetic profiling of the rodent pineal gland and should provide a sound foundation for future investigations of this tissue focused on the factors controlling developmental and rhythmic gene expression. The evidence provided here also provides a basis for future research on the role of T3/retinoic acid signaling in the pineal gland and on the role of this tissue in the immune/inflammation response. The finding that most genes that exhibit daily changes in expression are regulated by NE/cAMP signaling raises intriguing questions regarding the mechanisms involved, especially the role of epigenetic events. Analysis of the factors regulating these large changes in gene expression and expression of genes at relatively high levels in the pineal gland may be of interest to investigators studying these genes in other tissues, especially the retina. The indication from the results of analysis of cis-regulatory elements that transcript abundance in the pineal gland involves PWMs, which have not previously appeared in the pineal literature, opens new doors to the analysis of the molecular control of gene expression in this tissue.
Supplementary Material
Acknowledgments
The support of John Walker and Andrew Su (Novartis Research Foundation) in facilitating the use of data in the Novartis Research Foundation Data Base is greatly appreciated, as is the expert histological assistance of Ursula Rentzmann (University of Copenhagen).
Note Added in Proof—The Sertoli cell data used for calculating median expression levels in experiment A were obtained from Ref. 126.
This work was supported, in whole or in part, by the National Institutes of Health (NICHD, Intramural Research Program to M. J. B., S. L. C., J. K., Q. S., P. G., F. M., S. G., J. L. W., and D. C. K.; Center for Information Technology to Z. G. R. and P. J. M.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Procedures, Tables S1–S8, and Figs. S1 and S2.
Footnotes
The abbreviations used are: SCN, suprachiasmatic nucleus; Bt2cAMP, dibutyryl cAMP; CREB, cAMP-response element-binding protein; pCREB, phosphor-CREB; CRE, cAMP response element; cGMP, cyclic guanosine monophosphate; FDR, false discovery rate; qRT-PCR, quantitative real time-PCR; PWM, position weight matrices; SCG, superior cervical ganglia; NE, norepinephrine; ZT, Zeitgeber time; LD, light-dark; T3, thyroid hormone.
One transcript isoform of Crem, termed Icer, is known to be highly rhythmic in the rat pineal gland. Therefore, throughout the text, when Crem is mentioned in the context of the pineal gland, the term refers to the Icer isoform.
Where numbers of genes in various categories are given, this refers to probe sets that have been annotated with Entrez Gene identifiers by Affymetrix as of November 5, 2007, and updated manually as of June 15, 2008. The gene symbols that are used have been taken from Entrez Gene; associated Gene titles and Entrez Gene identifiers are given in supplemental Tables S3, S4, and S5. Gene symbols beginning with LOC, RGD, or MGC are not included in the tables in the text; they are included in the supplemental tables.
D. C. Klein, J. L. Weller, and F. G. Amaral, unpublished studies.
References
- 1.Maronde, E., and Stehle, J. H. (2007) Trends Endocrinol. Metab. 18 142–149 [DOI] [PubMed] [Google Scholar]
- 2.Arendt, J. (1994) Melatonin and the Mammalian Pineal Gland, 1st Ed., pp. 1–331, Chapman & Hall, New York
- 3.Lincoln, G. A. (2006) Chronobiol. Int. 23 301–306 [DOI] [PubMed] [Google Scholar]
- 4.Klein, D. C. (1985) CIBA Found. Symp. 117 38–56 [DOI] [PubMed] [Google Scholar]
- 5.Moller, M., and Baeres, F. M. (2002) Cell Tissue Res. 309 139–150 [DOI] [PubMed] [Google Scholar]
- 6.Klein, D. C., Sugden, D., and Weller, J. L. (1983) Proc. Natl. Acad. Sci. U. S. A. 80 599–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugden, L. A., Sugden, D., and Klein, D. C. (1987) J. Biol. Chem. 262 741–745 [PubMed] [Google Scholar]
- 8.Auerbach, D. A., Klein, D. C., Woodard, C., and Aurbach, G. D. (1981) Endocrinology 108 559–567 [DOI] [PubMed] [Google Scholar]
- 9.Chik, C. L., Ho, A. K., and Klein, D. C. (1988) Endocrinology 122 702–708 [DOI] [PubMed] [Google Scholar]
- 10.Sugden, D., and Klein, D. C. (1984) Endocrinology 114 435–440 [DOI] [PubMed] [Google Scholar]
- 11.Sugden, A. L., Sugden, D., and Klein, D. C. (1986) J. Biol. Chem. 261 11608–11612 [PubMed] [Google Scholar]
- 12.Sugden, D., Anwar, N., and Klein, D. C. (1996) Br. J. Pharmacol. 118 1246–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanecek, J., Sugden, D., Weller, J., and Klein, D. C. (1985) Endocrinology 116 2167–2173 [DOI] [PubMed] [Google Scholar]
- 14.Ho, A. K., and Klein, D. C. (1987) J. Biol. Chem. 262 11764–11770 [PubMed] [Google Scholar]
- 15.Ho, A. K., and Klein, D. C. (1987) J. Neurochem. 48 1033–1038 [DOI] [PubMed] [Google Scholar]
- 16.Ho, A. K., Chik, C. L., and Klein, D. C. (1988) J. Pineal Res. 5 553–564 [DOI] [PubMed] [Google Scholar]
- 17.Klein, D. C. (2007) J. Biol. Chem. 282 4233–4237 [DOI] [PubMed] [Google Scholar]
- 18.Baler, R., Coon, S. L., and Klein, D. C. (1996) Biochem. Biophys. Res. Commun. 220 975–978 [DOI] [PubMed] [Google Scholar]
- 19.Coon, S. L., McCune, S. K., Sugden, D., and Klein, D. C. (1997) Mol. Pharmacol. 51 551–557 [DOI] [PubMed] [Google Scholar]
- 20.Li, X., Chen, S., Wang, Q., Zack, D. J., Snyder, S. H., and Borjigin, J. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 1876–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borjigin, J., Payne, A. S., Deng, J., Li, X., Wang, M. M., Ovodenko, B., Gitlin, J. D., and Snyder, S. H. (1999) J. Neurosci. 19 1018–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, J. S., Bailey, M. J., Ho, A. K., Moller, M., Gaildrat, P., and Klein, D. C. (2007) Endocrinology 148 1475–1485 [DOI] [PubMed] [Google Scholar]
- 23.Gaildrat, P., Moller, M., Mukda, S., Humphries, A., Carter, D. A., Ganapathy, V., and Klein, D. C. (2005) J. Biol. Chem. 280 16851–16860 [DOI] [PubMed] [Google Scholar]
- 24.Stehle, J. H., Foulkes, N. S., Molina, C. A., Simonneaux, V., Pevet, P., and Sassone-Corsi, P. (1993) Nature 365 314–320 [DOI] [PubMed] [Google Scholar]
- 25.Baler, R., and Klein, D. C. (1995) J. Biol. Chem. 270 27319–27325 [DOI] [PubMed] [Google Scholar]
- 26.Humphries, A., Klein, D., Baler, R., and Carter, D. A. (2002) J. Neuroendocrinol. 14 101–108 [DOI] [PubMed] [Google Scholar]
- 27.Borjigin, J., Deng, J., Wang, M. M., Li, X., Blackshaw, S., and Snyder, S. H. (1999) J. Biol. Chem. 274 35012–35015 [DOI] [PubMed] [Google Scholar]
- 28.Borjigin, J., Deng, J., Sun, X., De Jesus, M., Liu, T., and Wang, M. M. (2003) J. Biol. Chem. 278 16315–16319 [DOI] [PubMed] [Google Scholar]
- 29.Chik, C. L., Arnason, T. G., Dukewich, W. G., Price, D. M., Ranger, A., and Ho, A. K. (2007) Endocrinology 148 1465–1472 [DOI] [PubMed] [Google Scholar]
- 30.Ho, A. K., Price, D. M., Dukewich, W. G., Steinberg, N., Arnason, T. G., and Chik, C. L. (2007) Endocrinology 148 4592–4600 [DOI] [PubMed] [Google Scholar]
- 31.Furukawa, A., Koike, C., Lippincott, P., Cepko, C. L., and Furukawa, T. (2002) J. Neurosci. 22 1640–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furukawa, T., Morrow, E. M., Li, T., Davis, F. C., and Cepko, C. L. (1999) Nat. Genet. 23 466–470 [DOI] [PubMed] [Google Scholar]
- 33.Appelbaum, L., and Gothilf, Y. (2006) Mol. Cell. Endocrinol. 252 27–33 [DOI] [PubMed] [Google Scholar]
- 34.Rath, M. F., Munoz, E., Ganguly, S., Morin, F., Shi, Q., Klein, D. C., and Moller, M. (2006) J. Neurochem. 97 556–566 [DOI] [PubMed] [Google Scholar]
- 35.Estivill-Torrus, G., Vitalis, T., Fernandez-Llebrez, P., and Price, D. J. (2001) Mech. Dev. 109 215–224 [DOI] [PubMed] [Google Scholar]
- 36.Gehring, W. J. (2002) Int. J. Dev. Biol. 46 65–73 [PubMed] [Google Scholar]
- 37.Nishida, A., Furukawa, A., Koike, C., Tano, Y., Aizawa, S., Matsuo, I., and Furukawa, T. (2003) Nat. Neurosci. 6 1255–1263 [DOI] [PubMed] [Google Scholar]
- 38.Klein, D. C. (2006) Chronobiol. Int. 23 5–20 [DOI] [PubMed] [Google Scholar]
- 39.Dinet, V., Girard-Naud, N., Voisin, P., and Bernard, M. (2006) Exp. Eye Res. 83 276–290 [DOI] [PubMed] [Google Scholar]
- 40.Kimura, A., Singh, D., Wawrousek, E. F., Kikuchi, M., Nakamura, M., and Shinohara, T. (2000) J. Biol. Chem. 275 1152–1160 [DOI] [PubMed] [Google Scholar]
- 41.Appelbaum, L., Anzulovich, A., Baler, R., and Gothilf, Y. (2005) J. Biol. Chem. 280 11544–11551 [DOI] [PubMed] [Google Scholar]
- 42.Appelbaum, L., Toyama, R., Dawid, I. B., Klein, D. C., Baler, R., and Gothilf, Y. (2004) Mol. Endocrinol. 18 1210–1221 [DOI] [PubMed] [Google Scholar]
- 43.Bernard, M., Dinet, V., and Voisin, P. (2001) J. Neurochem. 79 248–257 [DOI] [PubMed] [Google Scholar]
- 44.Gothilf, Y., Toyama, R., Coon, S. L., Du, S. J., Dawid, I. B., and Klein, D. C. (2002) Dev. Dyn. 225 241–249 [DOI] [PubMed] [Google Scholar]
- 45.Mani, S. S., Besharse, J. C., and Knox, B. E. (1999) J. Biol. Chem. 274 15590–15597 [DOI] [PubMed] [Google Scholar]
- 46.Young, J. E., Kasperek, E. M., Vogt, T. M., Lis, A., and Khani, S. C. (2007) Genomics 90 236–248 [DOI] [PubMed] [Google Scholar]
- 47.Gothilf, Y., Coon, S. L., Toyama, R., Chitnis, A., Namboodiri, M. A., and Klein, D. C. (1999) Endocrinology 140 4895–4903 [DOI] [PubMed] [Google Scholar]
- 48.Munoz, E. M., Bailey, M. J., Rath, M. F., Shi, Q., Morin, F., Coon, S. L., Moller, M., and Klein, D. C. (2007) J. Neurochem. 102 887–899 [DOI] [PubMed] [Google Scholar]
- 49.Roseboom, P. H., and Klein, D. C. (1995) Mol. Pharmacol. 47 439–449 [PubMed] [Google Scholar]
- 50.Baler, R., Covington, S., and Klein, D. C. (1997) J. Biol. Chem. 272 6979–6985 [DOI] [PubMed] [Google Scholar]
- 51.Tosini, G., Chaurasia, S. S., and Michael Iuvone, P. (2006) Chronobiol. Int. 23 381–391 [DOI] [PubMed] [Google Scholar]
- 52.Fukuhara, C., Liu, C., Ivanova, T. N., Chan, G. C., Storm, D. R., Iuvone, P. M., and Tosini, G. (2004) J. Neurosci. 24 1803–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iuvone, P. M., Bernard, M., Alonso-Gomez, A., Greve, P., Cassone, V. M., and Klein, D. C. (1997) Biol. Signals 6 217–224 [DOI] [PubMed] [Google Scholar]
- 54.Falcon, J., Gothilf, Y., Coon, S. L., Boeuf, G., and Klein, D. C. (2003) J. Neuroendocrinol. 15 378–382 [DOI] [PubMed] [Google Scholar]
- 55.Fukuhara, C., and Tosini, G. (2008) Neurosci. Res. 60 192–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parfitt, A., Weller, J. L., and Klein, D. C. (1976) Neuropharmacology 15 353–358 [DOI] [PubMed] [Google Scholar]
- 57.Yu, A. M., Idle, J. R., Byrd, L. G., Krausz, K. W., Kupfer, A., and Gonzalez, F. J. (2003) Pharmacogenetics 13 173–181 [DOI] [PubMed] [Google Scholar]
- 58.Walker, J. R., Su, A. I., Self, D. W., Hogenesch, J. B., Lapp, H., Maier, R., Hoyer, D., and Bilbe, G. (2004) Genome Res. 14 742–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edgar, R., Domrachev, M., and Lash, A. E. (2002) Nucleic Acids Res. 30 207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benjamini, Y., and Hochberg, Y. (1995) J. R. Stat. Soc. Ser. B 57 289–300 [Google Scholar]
- 61.Moller, M., Phansuwan-Pujito, P., Morgan, K. C., and Badiu, C. (1997) Cell Tissue Res. 288 279–284 [DOI] [PubMed] [Google Scholar]
- 62.Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A., and Speleman, F. (2002) Genome Biol. 3 research0034.1–0034.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Loo, P., Aerts, S., Thienpont, B., De Moor, B., Moreau, Y., and Marynen, P. (2008) Genome Biol. 9 R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klein, D. C. (2004) J. Biol. Rhythms 19 264–279 [DOI] [PubMed] [Google Scholar]
- 65.Ho, A. K., Terriff, D. L., Price, D. M., Wloka, M. T., and Chik, C. L. (2007) Endocrinology 148 743–751 [DOI] [PubMed] [Google Scholar]
- 66.Bai, L., Zimmer, S., Rickes, O., Rohleder, N., Holthues, H., Engel, L., Leube, R., and Spessert, R. (2008) Brain Res. 1203 89–96 [DOI] [PubMed] [Google Scholar]
- 67.Insel, P. A., and Ostrom, R. S. (2003) Cell. Mol. Neurobiol. 23 305–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seamon, K. B., and Daly, J. W. (1986) Adv. Cyclic Nucleotide Protein Phosphorylation Res. 20 1–150 [PubMed] [Google Scholar]
- 69.Moller, M., Rath, M. F., and Klein, D. C. (2006) Chronobiol. Int. 23 393–401 [DOI] [PubMed] [Google Scholar]
- 70.Wang, X., Brownstein, M. J., and Young, W. S., III (1996) Brain Res. Mol. Brain Res. 41 269–278 [DOI] [PubMed] [Google Scholar]
- 71.Wang, X., Brownstein, M. J., and Young, W. S. (1997) J. Neurosci. Methods 73 187–191 [DOI] [PubMed] [Google Scholar]
- 72.Kofler, B., Bulleyment, A., Humphries, A., and Carter, D. A. (2002) Histochem. J. 34 167–171 [DOI] [PubMed] [Google Scholar]
- 73.Rath, M., Bailey, M. J., Kim, J.-S., Ho, A. K., Gaildrat, P., Coon, S. L., Møller, M., and Klein, D. C. (2009) Endocrinology, in press [DOI] [PMC free article] [PubMed]
- 74.Kim, J. S., Bailey, M. J., Ho, A. K., Moller, M., Gaildrat, P., and Klein, D. C. (2007) Endocrinology 148 1475–1485 [DOI] [PubMed] [Google Scholar]
- 75.Kim, J. S., Coon, S. L., Blackshaw, S., Cepko, C. L., Moller, M., Mukda, S., Zhao, W. Q., Charlton, C. G., and Klein, D. C. (2005) J. Biol. Chem. 280 677–684 [DOI] [PubMed] [Google Scholar]
- 76.Ganguly, S., Grodzki, C., Sugden, D., Moller, M., Odom, S., Gaildrat, P., Gery, I., Siraganian, R. P., Rivera, J., and Klein, D. C. (2007) J. Biol. Chem. 282 32758–32764 [DOI] [PubMed] [Google Scholar]
- 77.Foulkes, N. S., Duval, G., and Sassone-Corsi, P. (1996) Nature 381 83–85 [DOI] [PubMed] [Google Scholar]
- 78.Roseboom, P. H., Coon, S. L., Baler, R., McCune, S. K., Weller, J. L., and Klein, D. C. (1996) Endocrinology 137 3033–3045 [DOI] [PubMed] [Google Scholar]
- 79.Klein, D. C., Coon, S. L., Roseboom, P. H., Weller, J. L., Bernard, M., Gastel, J. A., Zatz, M., Iuvone, P. M., Rodriguez, I. R., Begay, V., Falcon, J., Cahill, G. M., Cassone, V. M., and Baler, R. (1997) Recent Prog. Horm. Res. 52 307–358 [PubMed] [Google Scholar]
- 80.Fukuhara, C., Dirden, J. C., and Tosini, G. (2003) J. Pineal Res. 35 196–203 [DOI] [PubMed] [Google Scholar]
- 81.Amelio, A. L., Miraglia, L. J., Conkright, J. J., Mercer, B. A., Batalov, S., Cavett, V., Orth, A. P., Busby, J., Hogenesch, J. B., and Conkright, M. D. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 20314–20319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ceinos, R. M., Chansard, M., Revel, F., Calgari, C., Miguez, J. M., and Simonneaux, V. (2004) NeuroSignals 13 308–317 [DOI] [PubMed] [Google Scholar]
- 83.Ribelayga, C., Pevet, P., and Simonneaux, V. (1997) Brain Res. 777 247–250 [DOI] [PubMed] [Google Scholar]
- 84.Bernard, M., and Klein, D. C. (1996) J. Neurochem. 67 1032–1038 [DOI] [PubMed] [Google Scholar]
- 85.Li, A., Zhu, X., Brown, B., and Craft, C. M. (2003) Investig. Ophthalmol. Vis. Sci. 44 996–1007 [DOI] [PubMed] [Google Scholar]
- 86.Scheetz, T. E., Kim, K. Y., Swiderski, R. E., Philp, A. R., Braun, T. A., Knudtson, K. L., Dorrance, A. M., DiBona, G. F., Huang, J., Casavant, T. L., Sheffield, V. C., and Stone, E. M. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 14429–14434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Falcon, J., Marmillon, J. B., Claustrat, B., and Collin, J. P. (1989) J. Neurosci. 9 1943–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bernard, M., Iuvone, P. M., Cassone, V. M., Roseboom, P. H., Coon, S. L., and Klein, D. C. (1997) J. Neurochem. 68 213–224 [DOI] [PubMed] [Google Scholar]
- 89.Mansouri, A., Hallonet, M., and Gruss, P. (1996) Curr. Opin. Cell Biol. 8 851–857 [DOI] [PubMed] [Google Scholar]
- 90.Araki, M. (1992) Dev. Biol. 149 440–447 [DOI] [PubMed] [Google Scholar]
- 91.Araki, M., and Tokunaga, F. (1990) Cell Differ. Dev. 31 129–135 [DOI] [PubMed] [Google Scholar]
- 92.Tosini, G., Doyle, S., Geusz, M., and Menaker, M. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 11540–11544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nir, I., and Hirschmann, N. (1978) J. Neural Transm. 42 117–126 [DOI] [PubMed] [Google Scholar]
- 94.Tanaka, K., Murakami, M., and Greer, M. A. (1987) Endocrinology 121 74–77 [DOI] [PubMed] [Google Scholar]
- 95.Murakami, M., Greer, S. E., McAdams, S., and Greer, M. A. (1989) Life Sci. 44 425–429 [DOI] [PubMed] [Google Scholar]
- 96.Harpavat, S., and Cepko, C. L. (2003) Thyroid 13 1013–1019 [DOI] [PubMed] [Google Scholar]
- 97.Roberts, M. R., Srinivas, M., Forrest, D., Morreale de Escobar, G., and Reh, T. A. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 6218–6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Forrest, D., Reh, T. A., and Rusch, A. (2002) Curr. Opin. Neurobiol. 12 49–56 [DOI] [PubMed] [Google Scholar]
- 99.Ng, L., Hurley, J. B., Dierks, B., Srinivas, M., Salto, C., Vennstrom, B., Reh, T. A., and Forrest, D. (2001) Nat. Genet. 27 94–98 [DOI] [PubMed] [Google Scholar]
- 100.Pace-Asciak, C. R., Reynaud, D., and Demin, P. (1993) Biochem. Biophys. Res. Commun. 197 869–873 [DOI] [PubMed] [Google Scholar]
- 101.Pace-Asciak, C. R., Reynaud, D., and Demin, P. (1995) J. Lipid Mediat. Cell Signal. 12 307–311 [DOI] [PubMed] [Google Scholar]
- 102.Reynaud, D., Delton, I., Gharib, A., Sarda, N., Lagarde, M., and Pace-Asciak, C. R. (1994) J. Neurochem. 62 126–133 [DOI] [PubMed] [Google Scholar]
- 103.Reynaud, D., Demin, P., and Pace-Asciak, C. R. (1994) J. Biol. Chem. 269 23976–23980 [PubMed] [Google Scholar]
- 104.Reynaud, D., Demin, P. M., and Pace-Asciak, C. R. (1995) Adv. Prostaglandin Thromboxane Leukotriene Res. 23 183–185 [PubMed] [Google Scholar]
- 105.Reynaud, D., and Pace-Asciak, C. R. (1997) Biochim. Biophys. Acta 1346 305–316 [DOI] [PubMed] [Google Scholar]
- 106.Hada, T., Hagiya, H., Suzuki, H., Arakawa, T., Nakamura, M., Matsuda, S., Yoshimoto, T., Yamamoto, S., Azekawa, T., Morita, Y., Ishimura, K., and Kim, H.-Y. (1994) Biochim. Biophys. Acta 1211 221–228 [DOI] [PubMed] [Google Scholar]
- 107.Laitinen, J. T., Laitinen, K. S., and Kokkola, T. (1995) Cell. Mol. Neurobiol. 15 177–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yeh, J. J., Yasuda, R. P., Davila-Garcia, M. I., Xiao, Y., Ebert, S., Gupta, T., Kellar, K. J., and Wolfe, B. B. (2001) J. Neurochem. 77 336–346 [DOI] [PubMed] [Google Scholar]
- 109.Schomerus, C., and Korf, H. W. (1999) Reprod. Nutr. Dev. 39 305–314 [DOI] [PubMed] [Google Scholar]
- 110.Yamada, H., Ogura, A., Koizumi, S., Yamaguchi, A., and Moriyama, Y. (1998) J. Neurosci. 18 4946–4952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Klein, D. C., Wheler, G. H., and Weller, J. L. (1983) Prog. Clin. Biol. Res. 125 169–181 [PubMed] [Google Scholar]
- 112.Altshuler, D., Lo Turco, J. J., Rush, J., and Cepko, C. (1993) Development (Camb.) 119 1317–1328 [DOI] [PubMed] [Google Scholar]
- 113.Auerbach, G., Herrmann, A., Bracher, A., Bader, G., Gutlich, M., Fischer, M., Neukamm, M., Garrido-Franco, M., Richardson, J., Nar, H., Huber, R., and Bacher, A. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 13567–13572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ren, J., Kotaka, M., Lockyer, M., Lamb, H. K., Hawkins, A. R., and Stammers, D. K. (2005) J. Biol. Chem. 280 36912–36919 [DOI] [PubMed] [Google Scholar]
- 115.Kohrle, J. (2005) Thyroid 15 841–853 [DOI] [PubMed] [Google Scholar]
- 116.Namihira, M., Honma, S., Abe, H., Tanahashi, Y., Ikeda, M., and Honma, K. (1999) Neurosci. Lett. 267 69–72 [DOI] [PubMed] [Google Scholar]
- 117.Fukada, Y., and Okano, T. (2002) Mol. Neurobiol. 25 19–30 [DOI] [PubMed] [Google Scholar]
- 118.Karolczak, M., Burbach, G. J., Sties, G., Korf, H. W., and Stehle, J. H. (2004) Eur. J. Neurosci. 19 3382–3388 [DOI] [PubMed] [Google Scholar]
- 119.Simonneaux, V., Poirel, V. J., Garidou, M. L., Nguyen, D., Diaz-Rodriguez, E., and Pevet, P. (2004) Brain Res. Mol. Brain Res. 120 164–172 [DOI] [PubMed] [Google Scholar]
- 120.Humphries, A., Wells, T., Baler, R., Klein, D. C., and Carter, D. A. (2007) Mol. Cell. Endocrinol. 270 43–49 [DOI] [PubMed] [Google Scholar]
- 121.Chen, W., and Baler, R. (2000) Brain Res. Mol. Brain Res. 81 43–50 [DOI] [PubMed] [Google Scholar]
- 122.Davies, J. S., and Carter, D. A. (2006) Mol. Cell. Endocrinol. 252 19–26 [DOI] [PubMed] [Google Scholar]
- 123.Appelbaum, L., Vallone, D., Anzulovich, A., Ziv, L., Tom, M., Foulkes, N. S., and Gothilf, Y. (2006) J. Mol. Endocrinol. 36 337–347 [DOI] [PubMed] [Google Scholar]
- 124.Bajic, V. B., Choudhary, V., and Hock, C. K. (2004) In Silico Biol. 4 109–125 [PubMed] [Google Scholar]
- 125.Smith, S. B., Ee, H. C., Conners, J. R., and German, M. S. (1999) Mol. Cell. Biol. 19 8272–8280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McLean, D. J., Friel, P. J., Pouchnik, D., and Griswold, M. D. (2002) Mol. Endocrinol. 16 2780–2792 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.