Abstract
T cell-mediated autoimmune diseases such as type 1 diabetes (T1D) are believed to be the result in part of inefficient negative selection of self-specific thymocytes. However, the events regulating thymic negative selection are not fully understood. In the current study, we demonstrate that nonobese diabetic (NOD) mice lacking expression of the Mer tyrosine kinase (MerTK) have reduced inflammation of the pancreatic islets and fail to develop diabetes. Furthermore, NOD mice deficient in MerTK expression (Mer−/−) exhibit a reduced frequency of β cell-specific T cells independent of immunoregulatory effectors. The establishment of bone marrow chimeric mice demonstrated that the block in β cell autoimmunity required hematopoietic-derived cells lacking MerTK expression. Notably, fetal thymic organ cultures and self-peptide administration showed increased thymic negative selection in Mer−/− mice. Finally, thymic dendritic cells (DC) prepared from Mer−/− mice exhibited an increased capacity to induce thymocyte apoptosis in a peptide-specific manner in vitro. These findings provide evidence for a unique mechanism involving MerTK-mediated regulation of thymocyte negative selection and thymic DC, and suggest a role for MerTK in contributing to β cell autoimmunity.
Keywords: dendritic cells, receptor tyrosine kinase, type 1 diabetes
The breakdown of central and/or peripheral tolerance (1–7) can result in the development of T cell mediated autoimmune diseases such as type 1 diabetes (T1D), multiple sclerosis, and rheumatoid arthritis (8–11). The failure in T cell tolerance is generally influenced by a number of genes, most of which have yet to be identified (12). In the nonobese diabetic (NOD) mouse, a spontaneous model for T1D, both inefficient negative selection and dysregulation of peripheral tolerance mechanisms contribute to the development and activation, respectively, of pathogenic T cells that target the insulin producing β cells found in the islets of Langerhans (13). Inefficient negative selection in NOD mice is partly the result of the peptide binding properties of the MHC class II molecule IAg7, and the relative insensitivity of NOD CD4+CD8+ double-positive (DP) thymocytes to apoptosis-inducing events (9, 14, 15). One intriguing possibility is that the efficiency of negative selection is also regulated by the activation and/or functional state of medullary thymic epithelial cells (mTEC) and resident dendritic cells (DC). For instance events that regulate the avidity between DP thymocytes and mTEC and/or thymic DC may influence the efficiency of negative selection. This is likely similar to mechanisms in the periphery.
The activation and maturation status of peripheral DC is critical for determining the outcome of a T cell response (16). Immature DC found under homeostatic conditions are characterized by low levels of MHC and T cell costimulatory molecules such as CD40, CD80, and CD86, and typically mediate clonal anergy/deletion of naïve T cells (17). However, upon activation and subsequent maturation, DC upregulate MHC and costimulatory molecules and secrete proinflammatory cytokines to promote robust expansion and effector T cell differentiation (17).
Our group and others demonstrated that the Mer tyrosine kinase (MerTK) negatively regulates antigen presenting cells (APC) such as DC and macrophages (Mφ) (18–21). MerTK belongs to a family of receptor tyrosine kinases (RTKs) consisting of Axl and Tyro3 (22, 23). Mice lacking expression of all 3 RTKs have hyperactive APC in the periphery, resulting in systemic autoimmunity characterized by T cell infiltrates in several tissues (24). In addition to DC and Mφ, MerTK is expressed by NK T cells, NK cells, epithelia and endothelia cell types, and B cells, but not T cells (25, 26). MerTK is necessary for efficient clearance of apoptotic cells (AC) by certain subsets of Mφ and retinal pigment epithelial cells (27–29). Recently, we demonstrated that MerTK is critical for transducing signals that mediate the inhibitory effect of AC on immature DC (18). The binding of AC by immature DC blocks subsequent activation and establishes a stable “tolergenic” DC phenotype. Interestingly, the gene encoding MerTK is found in a T1D genetic susceptibility locus in mice. Mertk is located in the 95% confidence interval of Idd13 on chromosome (Chr.) 2 (30). Idd13 consists of at least 2 subloci, one of which contains the β2 microglobulin gene (30). Currently, it is not known whether MerTK contributes to T1D susceptibility.
To assess the role of MerTK in the development of T1D, the MerTK “kinase-dead” mutation (MertkKD) was introgressed onto the NOD genetic background. Our findings support a key role for MerTK in immunoregulation of thymic negative selection. NOD mice lacking MerTK expression (Mer−/−) were protected from diabetes because of enhanced negative selection and the depletion of pathogenic β cell-specific T precursors. These findings identify a novel mechanism of thymic immune homeostasis and suggest a role for MerTK in contributing to T1D.
Results
MerTK-Deficient NOD Mice Remain Diabetes Free.
To determine the effect of MerTK deficiency on the progression of spontaneous T1D, NOD mice with the MerTKKD null mutation were established (18). Briefly, C57BL/6.MerTKKD were bred with NOD mice, and the MertkKD gene introgressed 11 generations onto the NOD genetic background (18). The original MerTKKD founder mouse was generated using a 129/Ola embryonic stem cell line. Microsatellite analyses mapped MertkKD within a 17-cM span of 129/Ola-derived Chr. 2 DNA (18). Both 129/Ola and NOD mice share the diabetogenic allele of B2m (B2ma) that is contained within the congenic interval. This allele sharing was confirmed via restriction fragment length polymorphism (Fig. S1). Wild-type (Mer+/+), heterozygous (Mer+/−), and homozygous (Mer−/−) female littermates were monitored for diabetes. The time of onset and frequency of diabetes were similar between Mer+/+ and Mer+/− female littermates (Fig. 1A). Strikingly, none of the Mer−/− female littermates developed diabetes up to 52 weeks of age (Fig. 1A).
Fig. 1.
Diabetes and insulitis are blocked in Mer−/− mice. (A) Diabetes incidence in Mer+/+, Mer+/−, and Mer−/− female mice. *, P < 0.001 versus Mer+/+ and Mer+/− mice. (B) Severity of insulitis was determined in H&E stained pancreatic sections (5 female mice/group) and scored for the percentage of islets with no visible insulitis (black), peri-insulitis (white), or intra-insulitis (gray). †, P = 0.004; ‡, P = 0.003; §, P = 0.016; and ¶, P = 0.003. (C) The percentage of CD19+ B cells, CD4+ T cells, and CD8+ T cells determined in islets from 12-wk-old female Mer+/− (n = 14) and Mer−/− (n = 7) mice by flow cytometry. (D) Diabetes incidence in groups of 5 NOD-129.Chr2−/−, NOD-129.Chr2+/−, and NOD-129.Chr2+/+ female littermates.
Lack of diabetes correlated with a reduced frequency of cell infiltration of the islets in Mer−/− versus Mer+/− female littermates. The majority of islets in 12 (63 ± 18% versus 33 ± 15%) and 17-wk-old (65 ± 12% versus 25 ± 8%) Mer−/− but not Mer+/− female mice were free of insulitis (Fig. 1B). Furthermore, the composition of the islet infiltrate differed markedly between Mer+/− and Mer−/− mice. Insulitis in 12-wk-old Mer+/− islets consisted mostly of T cells with 43 ± 13% CD4+ and 22 ± 5% CD8+, with B cells making up to 34 ± 10% of the infiltrating lymphocytes (Fig. 1C). In contrast, Mer−/− islets were mostly infiltrated with B cells (51 ± 14%) and CD8+ T cells (37 ± 11%), while CD4+ T cells (11 ± 4%) were reduced in the infiltrate (Fig. 1C). These data indicate that insulitis is altered and overt diabetes prevented in Mer−/− female mice.
Because Mertk is found within the Idd13 interval it was important to rule out that diabetes resistance in Mer−/− mice was independent of MertkKD and the result of an unidentified gene found in the 17-cM region of 129/Ola Chr. 2. NOD mice were established using a speed congenic approach which contained a 44-cM span of wild-type 129/Ola Chr. 2 including Idd13 and Mertk (Table S1). No significant difference in the time of onset or frequency of diabetes was observed between female wild-type NOD mice (NOD-129.Chr2−/−) compared to a fifth backcross generation of NOD female littermates heterozygous (NOD-129.Chr2+/−) or homozygous (NOD-129.Chr2+/+) for the 129/Ola Chr. 2 segment (Fig. 1D). Therefore the lack of diabetes in Mer−/− mice was MertkKD dependent.
β Cell-Specific T Cell Reactivity Is Reduced in Mer−/− Mice Independent of Peripheral Immunoregulation.
To further investigate the autoimmune status of Mer−/− mice, the frequency and nature of β cell-specific CD4+ and CD8+ T cells infiltrating the islets was examined. CD4+ and CD8+ T cells prepared from the islets of 12-wk-old Mer+/− female mice exhibited a typical type 1 effector T cell profile characterized by a high frequency of IFNγ secreting T cells in response to a panel of β cell autoantigens (Fig. 2A) and no detectable IL-4 or IL-10 secreting T cells (data not shown). In contrast, in Mer−/− female littermates the frequency of islet-infiltrating CD4+ and CD8+ T cells secreting IFNγ in response to the panel of β cell autoantigens was significantly reduced (Fig. 2A). Similar to Mer+/− mice, however, no increase in IL-4 or IL-10 secreting β cell-specific CD4+ or CD8+ T cells was detected via ELISPOT in the islets, pancreatic lymph nodes (PLN), or spleen of Mer−/− mice (data not shown). Staining with H2Kd tetramers complexed with the diabetogenic NRP-V7 mimetic peptide demonstrated a reduced frequency of NRP-V7-specific CD8+ T cells in the islet infiltrates of 12-wk-old Mer−/− (2.3%) compared to Mer+/− (9.4%) female littermates (Fig. 2B).
Fig. 2.
β cell-specific T cells are reduced in Mer−/− islets. (A) The frequency of islet infiltrating β cell-specific CD3+ T cells was determined by ELISPOT. Data represent 5 mice/group. *, P = 0.011; †, P < 0.001; and ‡, P = 0.042. (B) Flow cytometric analysis of NRPV7-specific CD8+ T cells from islets of Mer+/− and Mer−/− mice. Islets were pooled from 5 mice/group. (C) Diabetes incidence in Mer+/− and Mer−/− mice after transfer of BDC CD4+ T cells. Flow cytometric determination of the absolute number (D) and frequency (E) of CD4+ CD25+ FoxP3+ T cells in PLN of Mer+/− and Mer−/− mice. Results are representative of at least 3 experiments.
The above findings indicated that the lack of diabetes in Mer−/− mice correlated with a reduced frequency of type 1 β cell-specific T effectors independent of immunoregulatory T cells. To confirm the latter, adoptive transfer experiments were performed. Initially, diabetes was monitored in 4-wk-old female Mer+/− or Mer−/− mice receiving 5 × 106 diabetogenic CD4+ T cells expressing the BDC2.5 clonotypic TCR. All Mer+/− and Mer−/− recipients developed diabetes within 12 days posttransfer (Fig. 2C). Additionally, CD4+ BDC T cells were mixed with splenocytes from either 8-wk-old Mer+/− or Mer−/− mice and then transferred into NOD.scid mice. All recipient mice developed diabetes within 4–6 days posttransfer regardless of whether Mer+/− or Mer−/− splenocytes were used (data not shown). No difference was detected in the number (Fig. 2D) or frequency (Fig. 2E) of FoxP3-expressing CD4+CD25+ immunoregulatory T cells residing in the PLN of 4-wk-old Mer+/− and Mer−/− female mice. These results demonstrate that Mer−/− mice have reduced β cell-specific pathogenic T cell reactivity independent of enhanced peripheral immunoregulation.
Prevention of T1D in Mer−/− Mice Is Mediated by Bone Marrow-Derived Cells.
MerTK is expressed by bone marrow (bm)-derived cells, and epithelial and endothelial cell types (22, 23). To determine the contribution of these cell types in preventing T1D in Mer−/− mice, bm chimeric NOD mice were established. Initially, 8-wk-old NOD or Mer−/− female mice were lethally irradiated and reconstituted with bm prepared from NOD mice expressing a transgene encoding green fluorescent protein (NOD.GFP). Reconstitution with donor bm typically resulted in ≥80% of splenic CD4+ T cells expressing GFP as measured by flow cytometry (Fig. S2). Both NOD and Mer−/− recipients developed diabetes at a similar time of onset and frequency (Fig. 3A), indicating that nonhematopoietic cells in Mer−/− recipients were insufficient to block the development of pathogenic effectors from donor NOD.GFP bm. Next, 8-wk-old NOD female mice were irradiated and reconstituted with NOD.GFP or Mer−/−.GFP bm. The majority of NOD.GFP bm recipients developed diabetes (Fig. 3B). In contrast, all Mer−/−.GFP bm recipients remained diabetes free (Fig. 3B). Lack of diabetes in Mer−/−.GFP bm recipients correlated with a reduced frequency of islet infiltrating H2Kd-NRPV7+ CD8+T cells relative to NOD.GFP bm recipients 8 wk posttransfer (Fig. 3C). These data demonstrate that reduced β cell autoimmunity in Mer−/− mice is mediated by bm-derived cells.
Fig. 3.
Diabetes inhibition in Mer−/− mice is mediated by bm cells. (A) Diabetes incidence in recipient Mer+/+ and Mer−/− mice reconstituted with NOD.GFP bm. (B) Diabetes incidence in recipient NOD mice reconstituted with Mer+/+.GFP or Mer−/−.GFP bm. *, P < 0.001. (C) Flow cytometric analysis of NRPV7-specific CD8+ T cells from islets of recipient NOD mice reconstituted with Mer+/+.GFP or Mer−/−.GFP bm. †, P < 0.001. Results are representative of at least 2 experiments.
Thymic Negative Selection in Mer−/− Mice Is Enhanced.
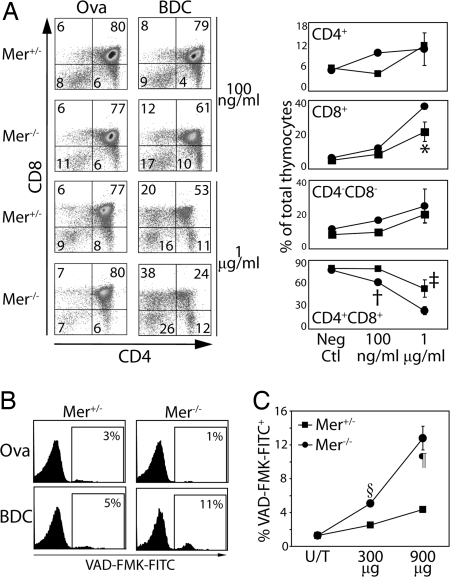
Reduced pathogenic T effectors in the absence of enhanced immunoregulation suggested that self-tolerance in Mer−/− mice was established via increased thymic negative selection. To test this hypothesis, fetal thymic organ cultures (FTOC) were established from Mertk+/− and Mertk−/− day 13 NOD embryo donors expressing the BDC2.5 clonotypic TCR, and cultured for 6 days with the BDC2.5 mimetic peptide (mBDC) or a control ovalbumin peptide (Ova323–339). In Ova323–339-treated FTOC, no difference was detected in the frequency of DP, CD4−CD8− double negative (DN), CD4+ single-positive (SP), and CD8+ SP thymocytes prepared from Mer+/−.BDC and Mer−/−.BDC thymic lobes (Fig. 4A). In contrast, the frequency of DP thymocytes was significantly reduced and a compensatory increase in CD8+ SP thymocytes detected in Mer−/−.BDC versus Mer+/−.BDC FTOC treated with mBDC (Fig. 4A). Depletion of DP thymocytes in mBDC-treated FTOC correlated with an increased frequency of apoptotic DP in Mer−/−.BDC versus Mer+/−.BDC thymic lobes (Fig. 4B).
Fig. 4.
Thymic negative selection is enhanced in Mer−/− mice. (A) Representative staining of thymocyte subsets in FTOC prepared from Mer+/−.BDC and Mer−/−.BDC fetal donors. (Right) Graphical representation of thymocyte subsets in Mer+/−.BDC (square) and Mer−/−.BDC (circle) FTOC. At least 3 thymi were used per group. *, P = 0.045; †, P = 0.023; and ‡, P = 0.038 (B) Flow cytometric analysis of VAD-FMK-FITC+ DP thymocytes in Mer+/− or Mer−/− mice injected i.v. with 900 μg of Ova323–339 or mBDC. (C) Graphical representation of data from 4 mice/group injected with mBDC. §, P < 0.001; ¶, P = 0.001. Results are representative of at least 3 experiments.
To determine whether enhanced negative selection could also be detected in vivo, 4-wk-old Mer+/−.BDC and Mer−/−.BDC female mice received a single injection of 300 or 900 μg of mBDC and 6 h later apoptosis within DP thymocytes measured. Treatment with mBDC induced increased apoptosis of DP thymocytes in Mer−/−.BDC versus Mer+/−.BDC mice (Fig. 4C). Induction of apoptosis was peptide specific because mBDC treatment of wild-type NOD mice did not induce increased apoptosis of DP thymocytes relative to an untreated group (data not shown).
Mer−/− Thymic DC Mediate Enhanced Thymic Negative Selection in Vitro.
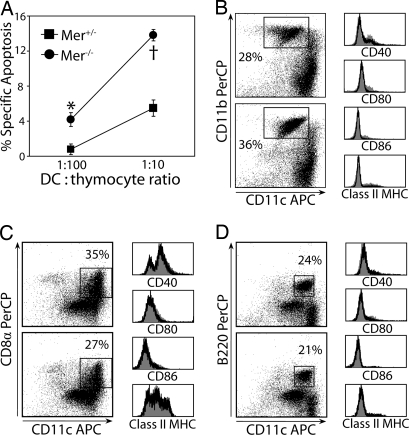
mTEC and DC mediate thymocyte negative selection. Because bm-derived cells were required to prevent T1D in Mer−/− mice (Fig. 3), DC likely promoted the enhanced thymic negative selection in Mer−/− mice. To test this possibility, an in vitro negative selection assay was used. Thymic DCs prepared from Mer+/− or Mer−/− mice were pulsed with mBDC or Ova322–339, cocultured with NOD.BDC thymocytes, and apoptosis was measured in DP thymocytes. Apoptosis of DP thymocytes was increased in cultures of mBDC-pulsed thymic DC isolated from Mer−/− versus Mer+/− mice (Fig. 5A). Only minor differences in the frequency of CD11c+CD11b+, CD11c+CD8α+, and plasmacytoid DC were detected in thymic DC prepared from Mer−/− or Mer+/− mice (Fig. 5 B−D). Furthermore, no difference in surface expression of CD40, CD80, CD86, and class II MHC (Fig. 5 B−D) or PD-L1 and OX40L (data not shown) among the 3 major thymic DC subsets (e.g., CD11c+CD11b+, CD11c+CD8α+, and CD11c+B220+) was detected between Mer+/+, Mer+/−, and Mer−/− mice. These findings demonstrate that thymic DCs lacking MerTK expression exhibit an enhanced capacity to induce thymocyte negative selection despite no marked change in thymic DC subsets or phenotypic markers for DC activation and maturation.
Fig. 5.
Mer−/− thymic DCs mediate enhanced thymic negative selection. (A) The frequency of VAD-FMK-FITC+ DP thymocytes after coculture with mBDC pulsed Mer+/− or Mer−/− thymic DC as determined by flow cytometry. *, P = 0.029; †, P = 0.002. (B–D) Flow cytometric analysis of sorted CD11c+ thymic DC pooled from 10 thymi of 4-wk-old Mer+/− and Mer−/− mice and stained for CD11c, CD40, CD80, CD86, and (B) CD11b, (C) CD8α, and (D) B220. Results are representative of at least 2 experiments.
Discussion
The events that influence the efficacy of thymic negative selection remain ill defined. The current study identifies a novel mechanism regulating negative selection of self-specific thymocytes and the development of T cell-mediated autoimmunity. Three key observations were made in this study.
First, MerTK influences thymocyte selection. Mer−/− mice exhibited increased negative selection of β cell-specific thymocytes as determined by FTOC (Fig. 4). Depletion of DP thymocytes was increased ≈2-fold in mBDC-treated Mer−/−.BDC FTOC (Fig. 4A). A similar result was obtained in vivo when apoptosis of DP thymocytes was measured in Mer−/−.BDC mice injected with mBDC (Fig. 4C). Notably, no difference in the frequency of the respective thymocyte subsets was detected in control FTOC prepared from Mer−/−.BDC and NOD.BDC embryos, demonstrating that thymocyte development per se was not aberrantly affected by the MerTK deficiency. Indeed, equivalent T cell responses are induced in Mer−/− and NOD mice immunized with a foreign antigen (data not shown). Therefore, the lack of MerTK expression selectively affected the repertoire of autoreactive T precursors.
Enhanced negative selection of β cell-specific thymocytes effectively blocked the diabetogenic response in Mer−/− mice. The pool of peripheral pathogenic T effectors was reduced in Mer−/− mice (Fig. 2) thereby altering the progression of insulitis both quantitatively and qualitatively. This reduction in pathogenic T cells limited β cell destruction (Fig. 1) independent of immunoregulatory T cells (Fig. 2). The latter is consistent with our earlier observation that a greater frequency of adoptively transferred BDC2.5 CD4+ T cells develop into pathogenic type 1 effectors in Mer−/− compared to NOD recipients in which β cell injury is induced by streptozotocin treatment (18). In addition to the islets, NOD mice exhibit T cell-mediated autoimmunity specific for other tissues. Histological analysis demonstrated reduced infiltration of the salivary glands and large intestine in 12-wk-old Mer−/− versus wild-type NOD female mice (Fig. S3), indicating that the effect of MerTK deficiency on negative selection is not limited to β cell-specific thymocytes. Presumably, salivary gland and gut antigens are expressed in the thymus and are presented more efficiently in Mer−/− mice. Our findings are in marked contrast to results for mice lacking MerTK, Axl, and Tyro3 expression (24). In the triple knockout mice that were on a heterogeneous genetic background, T cells were found infiltrating several peripheral tissues. It is possible that the deficiency of Axl and Tyro3 may alter the effects of the MertkKD mutation on thymocyte selection. Alternatively, self-antigens targeted by T cells in the triple knockout mice may not be presented in the thymus, which may also explain why C57BL/6 mice lacking MerTK expression develop lupus-like autoimmunity with age (27).
The second key observation made in this study is that increased negative selection in Mer−/− mice is the result of an enhanced capacity of thymic DC to delete thymocytes (Fig. 5A). This conclusion is supported by results demonstrating that diabetes is prevented in bm chimeric mice in which hematopoietic-derived cells lacked MerTK expression (Fig. 3). Furthermore, mTEC and cortical TEC do not express MerTK in wild-type NOD mice (C.J.K. and R.T., unpublished results). Although a role for thymic Mφ's, which also express MerTK cannot be ruled out, it is generally believed that the primary function of Mφ is to clear apoptotic cells in the thymus with little if any participation in thymocyte negative selection. We and others have demonstrated a critical role for MerTK in negatively regulating DC activation and maturation (18–20, 24). MerTK is required to induce AC-mediated inhibition of immature DC from peripheral tissues by blocking the activation of the NF-κB pathway (18, 20). NF-κB is an important transcription factor regulating expression of a number of genes required for DC activation, maturation, and effector function, including genes encoding MHC classes I and II, costimulatory molecules, and cytokines (17). Somewhat surprisingly the increased capacity of Mer−/− thymic DC to mediate DP thymocyte apoptosis did not correspond with phenotypic changes typically associated with DC activation or maturation (Fig. 5). For instance, elevated expression of MHC and/or costimulatory molecules would be expected to enhance the stimulatory function of the DC, and therefore increase thymocyte negative selection. Initial microarray experiments suggest that the mRNA expression profiles of Mer−/− versus NOD thymic DC differ, including genes associated with cell membrane structure and/or cell-to-cell interactions. These proteins may affect the avidity of the interaction between DC and thymocytes, and the efficacy of negative selection. On the basis of our findings, we propose that MerTK provides “tonic” signaling that regulates the capacity of thymic DC to stimulate thymocytes and induce apoptotic signals that drive negative selection. It is noteworthy that levels of expression of tissue-specific antigens in the thymus have also been linked with the development of autoreactive T precursors. For instance, reduced thymic expression of insulin is believed to limit negative selection of insulin-specific thymocytes (12). Therefore, the efficiency of negative selection is likely to be in part influenced by levels of tissue-specific antigens expressed in the thymus, coupled with the stimulatory capacity of thymic DC, which is regulated by MerTK.
Third, this study provides evidence supporting a role for MerTK in T1D. The fact that Mertk resides within the 95% confidence interval of Idd13 suggests that MerTK polymorphisms may contribute to the progression of T1D. Importantly, Mer−/− mice express the diabetogenic isoform of the B2m gene located in Idd13, and NOD female mice homozygous for a 44-cM segment of 129/Ola Chr. 2 DNA spanning Idd13 developed diabetes at a similar time of onset and frequency as wild-type NOD littermates (Fig. 1D). The latter directly demonstrates that the lack of diabetes in Mer−/− mice is in fact the result of the KD mutation introduced into Mertk. Distinct expression profiles and/or isoforms of MerTK may reduce the efficacy of thymic DC to mediate negative selection, resulting in an increased frequency of β cell-specific T precursors residing in the periphery. As alluded to above, work by our group demonstrated that MerTK also affects the APC function of peripheral DC (18). Here, it was found that PLN DCs lacking MerTK expression readily promote the differentiation of type 1 effectors in vivo if a sufficient frequency of diabetogenic T precursors is present. Therefore, MerTK may contribute to T1D by regulating the development of pathogenic T precursors both in the thymus and periphery.
In summary, data are provided demonstrating a unique mechanism in which MerTK regulates the efficiency of DC to mediate thymic negative selection thereby influencing the development of autoreactive T cells. In addition to T1D, MerTK may also play a role in shaping the repertoire of pathogenic T cells in other autoimmune diseases.
Methods
Mice.
NOD/LtJ “NOD,” NOD.Cg-Tg(TcraBDC2.5)1Doi Tg(TcrbBDC2.5)2Doi “NOD.BDC,” 129P2/OlaHsd “129/Ola,” and NOD.GFP mice were maintained and bred under specific pathogen-free conditions. The establishment and genotyping of the 11th backcross generation of Mer−/− mice have been described (18). NOD-129.Chr2+/+ mice were established by introgressing 129/Ola Chr. 2 DNA for 5 generations onto the NOD genetic background. Microsatellite analysis was performed to delineate a 44-cM segment of 129/Ola Chr. 2 containing Idd13. Mice were diagnosed as diabetic following 2 consecutive readings of >250 mg/dL blood glucose. The Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill approved our use of mice.
Isolation and Analyses of Islet-Infiltrating Cells.
Pancreata were perfused with collagenase P (Roche Diagnostics), digested at 37 °C, and islets enriched by Ficoll gradient and then handpicked. T cells were isolated from dissociated islets with anti-CD3 microbeads (Miltenyi Biotec). ELISPOT was carried out as described (31) with the following modifications. Irradiated splenocytes (5 × 106/well) and purified islet-infiltrating T cells (1 × 104/well) were cocultured with 10 μg/mL peptide for 48 h. Islet histology was carried out as described (32). Briefly pancreata were formalin fixed, embedded in paraffin, and sections cut 100 μm apart, and stained with hematoxylin and eosin (H&E).
Flow Cytometry.
The following monoclonal antibodies used for staining were purchased from BD Biosciences: anti-CD11c-APC, -CD4-APC, -CD4-PE, -CD8-PerCP, -CD3-FITC, and -CD19-PE. FoxP3-expressing CD4+CD25+ T cells were identified using a mouse regulatory T cell staining kit (eBioscience). H2Kd MHC class I tetramers were prepared as described (32). Islet-infiltrating T cells were stained with anti-CD3-FITC, -CD8-PerCP, and PE-labeled tetramer loaded with either influenza nucleoprotein (NP) or NRP-V7. Stained cells were analyzed on a FACScan or FACScalibur (BD Biosciences) using Summit software (Cytomation).
Adoptive Transfer of Diabetogenic BDC CD4+ T cells.
CD4+ T cells were isolated from the spleens of 10- to 12-wk-old female NOD.BDC using a BD IMag CD4 T lymphocyte enrichment set (BD Biosciences). BDC CD4+ T cells were injected i.p. (5 × 106 cells) into 4-wk-old Mer+/− or Mer−/− recipients. Alternatively, BDC CD4+ T cells were cotransferred with Mer+/− or Mer−/− splenocytes (5.0 × 106) into NOD.scid recipients.
Bm Chimera Mice.
Bm was isolated from NOD.GFP or Mer−/−. GFP mice. CD4+ and CD8+ T cells were depleted with GK1.5 and HO2.2, respectively, and rabbit serum complement. Bm cells (2 × 106) were transferred i.v. into irradiated (1000 gy) 8-wk-old Mer+/− or Mer−/− mice. Chimerism was confirmed by the presence of GFP+ lymphocytes in peripheral blood.
FTOC.
Mer+/−.BDC male and female mice were intercrossed and timed pregnancies established. Thymic lobes were removed from E13 embryos (in addition to fetal liver for genotyping), separated, and placed on Millicell culture plate insert discs (Millipore). Millicell discs were overlaid on complete RPMI medium 1640 (10% FCS, L-glutamine, Na-pyruvate, nonessential amino acids, penicillin/streptomycin and β-ME). After 3 days, medium containing either Ova322–339 or mBDC (RTRPLWVRME) was replaced daily for 4 days. Thymic lobes were dissociated and stained with VAD-FMK-FITC, anti-CD4-APC, -CD8-PerCP, and -Vβ4-PE (clonotypic TCR β chain of the BDC TCR).
In Vivo Thymic Selection.
Four-wk-old female Mer+/−.BDC or Mer−/−.BDC mice were injected i.v. with 300 or 900 μg of Ova322–339 or mBDC in PBS. Thymi were removed 8 h later and dissociated. Thymocytes were stained with anti-CD4-APC, -CD8-PerCP, -Vβ4-PE, and VAD-FMK-FITC.
In Vitro Negative Selection by Thymic DC.
Thymi were isolated from 4-wk-old Mer+/− or Mer−/− mice, physically disrupted, and then digested for 1 h at 37 °C in complete RPMI medium 1640 containing collagenase D (Roche Diagnostics) and DNase I (Sigma-Aldrich). DCs were pulsed with Ova322–339 or mBDC and isolated by anti-CD11c magnetic microbeads (Miltenyi), stained with anti-CD11c-APC, and sorted with a MoFlo cell sorter (Dako). The resulting populations were routinely >95% CD11c+ DC. Purified peptide-pulsed DCs were cocultured with freshly isolated NOD.BDC thymocytes (5 × 105) for 6 h. Thymocytes were then stained with PE-anti-CD4, PerCP-anti-CD8, and VAD-FMK-FITC.
Statistical Analyses.
Statistical analyses were performed using GraphPad Prism software version 4. Diabetes frequencies were compared by Kaplan-Meir log rank test. All other data were compared by unpaired Student's t test. Findings were considered significant with P values ≤0.05.
Supplementary Material
Acknowledgments.
This work was supported by the Juvenile Diabetes Research Foundation (JDRF) Grant 1–2005-984 and National Institute of Allergy and Infectious Diseases (NIAID) Grant AI066075 (R.T.); JDRF Grant 3–2001-860 and NIAID Grant AI056374 (C.E.M.); and NIAID Grant AI50736 (G.M.). R.R.F. is supported by a National Institutes of Health (NIH) fellowship; C.J.K. is supported by NIH Training Grant 5T32 AI07273.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900683106/DCSupplemental.
References
- 1.Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: A large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–840. doi: 10.1016/s1074-7613(00)00080-7. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Pechhold K, Koczwara K. Immunomodulation of autoimmune diabetes by dendritic cells. Curr Diab Rep. 2008;8:107–113. doi: 10.1007/s11892-008-0020-3. [DOI] [PubMed] [Google Scholar]
- 4.Brocker T, Riedinger M, Karjalainen K. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J Exp Med. 1997;185:541–550. doi: 10.1084/jem.185.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delaney JR, Sykulev Y, Eisen HN, Tonegawa S. Differences in the level of expression of class I major histocompatibility complex proteins on thymic epithelial and dendritic cells influence the decision of immature thymocytes between positive and negative selection. Proc Natl Acad Sci USA. 1998;95:5235–5240. doi: 10.1073/pnas.95.9.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashton-Rickardt P, et al. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994;76:651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 7.Anderson G, Moore N, Owen J, Jenkinson E. Cellular interactions in thymocyte development. Annu Rev Immunol. 1996;14:73–99. doi: 10.1146/annurev.immunol.14.1.73. [DOI] [PubMed] [Google Scholar]
- 8.Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–297. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 9.Kanagawa O, Martin S, Vaupel B, Carrasco-Marin E, Unanue E. Autoreactivity of T cells from nonobese diabetic mice: An I-Ag7-dependent reaction. Proc Natl Acad Sci USA. 1998;95:1721–1724. doi: 10.1073/pnas.95.4.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jurewicz A, Biddison WE, Antel JP. MHC class I-restricted lysis of human oligodendrocytes by myelin basic protein peptide-specific CD8 T lymphocytes. J Immunol. 1998;160:3056–3059. [PubMed] [Google Scholar]
- 11.Panayi GS, Lanchbury JS, Kingsley GH. The importance of the T cell in initiating and maintaining the chronic synovitis of rheumatoid arthritis. Arthritis Rheum. 1992;35:729–735. doi: 10.1002/art.1780350702. [DOI] [PubMed] [Google Scholar]
- 12.Maier LM, Wicker LS. Genetic susceptibility to type 1 diabetes. Curr Opin Immunol. 2005;17:601–608. doi: 10.1016/j.coi.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Anderson MS, Bluestone JA. The NOD mouse: A model of immune dysregulation. Ann Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 14.Zucchelli S, et al. Defective central tolerance induction in NOD mice: Genomics and genetics. Immunity. 2005;22:385–396. doi: 10.1016/j.immuni.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Liston A, et al. Generalized resistance to thymic deletion in the NOD mouse: a polygenic trait characterized by defective induction of Bim. Immunity. 2004;21:817–830. doi: 10.1016/j.immuni.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Schuler G, Steinman RM. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985;161:526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallet MA, Sen P, Tisch R. Immunoregulation of dendritic cells. Clin Med Res. 2005;3:166–175. doi: 10.3121/cmr.3.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallet MA, et al. MerTK is required for apoptotic cell-induced T cell tolerance. J Exp Med. 2008;205:219–232. doi: 10.1084/jem.20062293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 20.Sen P, et al. Apoptotic cells induce Mer tyrosine kinase-dependent blockade of NF-kappaB activation in dendritic cells. Blood. 2007;109:653–660. doi: 10.1182/blood-2006-04-017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Todt JC, Hu B, Curtis JL. The receptor tyrosine kinase MerTK activates phospholipase C gamma2 during recognition of apoptotic thymocytes by murine macrophages. J Leukoc Biol. 2004;75:705–713. doi: 10.1189/jlb.0903439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: Biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 25.Behrens EM, et al. The mer receptor tyrosine kinase: Expression and function suggest a role in innate immunity. Eur J Immunol. 2003;33:2160–2167. doi: 10.1002/eji.200324076. [DOI] [PubMed] [Google Scholar]
- 26.Shao WH, Eisenberg RA, Cohen PL. The Mer receptor tyrosine kinase is required for the loss of B cell tolerance in the chronic graft-versus-host disease model of systemic lupus erythematosus. J Immunol. 2008;180:7728–7735. doi: 10.4049/jimmunol.180.11.7728. [DOI] [PubMed] [Google Scholar]
- 27.Cohen PL, et al. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196:135–140. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott RS, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 29.Feng W, Yasumura D, Matthes MT, LaVail MM, Vollrath D. Mertk triggers uptake of photoreceptor outer segments during phagocytosis by cultured retinal pigment epithelial cells. J Biol Chem. 2002;277:17016–17022. doi: 10.1074/jbc.M107876200. [DOI] [PubMed] [Google Scholar]
- 30.Serreze DV, et al. Subcongenic analysis of the Idd13 locus in NOD/Lt mice: Evidence for several susceptibility genes including a possible diabetogenic role for beta 2-microglobulin. J Immunol. 1998;160:1472–1478. [PubMed] [Google Scholar]
- 31.Pop SM, et al. The type and frequency of immunoregulatory CD4+ T cells governs the efficacy of antigen-specific immunotherapy in nonobese diabetic mice. Diabetes. 2007;56:1395–1402. doi: 10.2337/db06-0543. [DOI] [PubMed] [Google Scholar]
- 32.Wong CP, Li L, Frelinger JA, Tisch R. Early autoimmune destruction of islet grafts is associated with a restricted repertoire of IGRP-specific CD8+ T cells in diabetic nonobese diabetic mice. J Immunol. 2006;176:1637–1644. doi: 10.4049/jimmunol.176.3.1637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.