Abstract
The extraocular muscles (EOMs) are a distinct muscle group that displays an array of unique contractile, structural, and regenerative properties. They also have differential sensitivity to certain diseases and are enigmatically spared in Duchenne muscular dystrophy (DMD). The EOMs are so distinct from other skeletal muscles that the term “allotype” has been coined to highlight EOM group-specific properties. We hypothesized that increased and distinct stem cells may underlie the continual myogenesis noted in EOM. The side population (SP) stem cells were isolated and studied. EOMs had 15× higher SP cell content compared with limb muscles. Expression profiling revealed 348 transcripts that define the EOM-SP transcriptome. Over 92% of transcripts were SP specific, because they were absent in previous whole muscle microarray studies. Cultured EOM-SP cells revealed superior in vitro proliferative capacity. Finally, assays of the committed progenitors or satellite cells performed on myofibers isolated from EOM and limb muscles independently validated the increased proliferative capacity of these muscles. We suggest a model in which unique EOM stem cells contribute to the continual myogenesis noted in EOM and consistent with a role for their sparing in DMD. We believe the greater numbers of stem cells, their unique transcriptome, the greater proliferative capacity of EOM stem cells, and the greater number of satellite cells also offer clues for novel cell-based therapeutic strategies.
Keywords: side population, microarrays, Duchenne muscular dystrophy
skeletal muscles comprise ∼40% of the total adult human body mass and are responsible for a number of functions including body support, force generation, and movement (20). To accomplish a diverse range of functions, specialized groups of muscles exist, exhibiting distinct properties (57). While the exact mechanism(s) pertaining to the origin of this diversity remains unclear, two main hypotheses have been proposed. The first emphasizes the instructive role played by environmental cues on a set of naive and noncommitted muscle precursors (reviewed in Ref. 51), while the second focuses on the role of lineage directives inherited by differentiating muscle cells from specialized progenitors in phenotypically distinct “allotypes” (25, 26) of muscles such as the extraocular muscles (EOMs) and limb muscles.
Indeed, the EOM allotype epitomizes functional diversity, because these specialized muscles are required to provide a variety of voluntary, saccadic, and reflex eye movements. They exhibit developmental, anatomic, metabolic, molecular, and functional properties that are at great variance from other (e.g., limb) skeletal muscles (5). Embryologically, the EOMs develop from prechordal mesoderm rather than somites (52) and are innervated by cranial nerves rather than spinal cord motoneurons. The EOM can be stimulated to twitch at extremely high frequencies, in contrast to limb muscle, in which similar frequencies of stimulation would lead to fusion and generation of tetanic contractions (4). Adult EOM can be multiply innervated and exhibit en grappe synapses, a pattern that is found in fetal rather than adult mammalian limb muscle (49). The expression pattern of myosin II isoforms in EOM is different from that seen in limb muscles; adult EOMs express an EOM-specific isoform (MYH13), continue to express the embryonic isoform MYH3, and can coexpress multiple myosin isoforms, in contrast to limb muscle (58, 59). EOM differs from limb muscle at the level of the transcriptome (15, 16, 47, 53) and the proteome (17, 18) as well. Both transcriptome-based analysis (15, 16) and cell labeling studies (40–42) have suggested that adult, uninjured EOMs have ongoing myogenesis/regeneration, in contrast to limb muscle, in which significant expression of regeneration markers or myonuclear addition would only be noted in the context of regeneration after injury or disease. But perhaps the diversity is most vividly exemplified by the differential response to diseases such as the congenital cranial dysinnervation disorders (12) and Duchenne muscular dystrophy (DMD).
DMD is the most common fatal X-linked neuromuscular disease. It is caused by mutations in the gene encoding dystrophin and affects 1 in 3,500 male newborns (24, 35). DMD is a progressive disease that presents clinically during the first decade of life, and patients become wheelchair dependent by their teens. Classical signs and symptoms include weakness and widespread muscle wasting, prominent calf enlargement, spinal deformities, and respiratory problems. Patients usually die in their thirties of respiratory and/or cardiac failure (11). While widespread muscle necrosis is pathognomonic of DMD, enigmatically, EOM function and histology are preserved in DMD patients (29, 32).
Several hypotheses have been proposed to explain EOM sparing in DMD; however, the exact mechanism(s) of sparing remain unclear (cf. discussion). On the basis of cell labeling (40–42) and expression profiling (15, 16) studies, we and others have proposed the hypothesis that the EOM may have a greater myogenic/regeneration capacity; however, how the greater myogenic capacity can be achieved has yet to be addressed. Adult skeletal muscle has postpartum growth and regenerative capacity due to the presence of muscle progenitors, including committed progenitors or satellite cells (SCs) that are capable of forming muscle (39, 75) as well as uncommitted progenitors or stem cells that are capable of forming a number of tissue types including muscle. A variety of cell populations, such as side population (SP) cells (22), pericytes (8), CD133+ progenitor cells (1), and mesoangioblasts (61), have all been suggested to form the muscle stem cell pool(s). However, it remains an open question whether the stem cell content, type, and myogenic capacity of EOMs are different compared with limb muscles.
To address this question, we quantified and characterized one stem cell population present in muscle, the SP cells, using a variety of cellular and molecular methods. In this study, we tested the hypothesis that an increased number of stem cells present in EOM may explain the continuous regeneration and subsequent sparing of these muscles in DMD.
MATERIALS AND METHODS
Animals.
Twelve- to sixteen-week-old C57BL/10ScSnJ, C57BL/10ScSn-Dmd mdx/J (Jackson Laboratory, Bar Harbor, ME), and C57BL/6-Tg(ACTB-EGFP)1 Osb/J GFP (gift from Dr. Alan Flake, Children's Hospital of Philadelphia) mice were used for SP experiments. Animal experiments were approved by the University of Pennsylvania Institutional Animal Care and Use Committee and conformed to the relevant regulatory standards.
SP cell preparations.
SP cells were fractionated from EOM and tibialis anterior (TA) muscles (n = 5 mice) by flow cytometry as previously described (44). Briefly, TA, quadriceps, and EOM were dissected, weighed, minced, and placed in skeletal muscle medium (SKM) [Ham's F-10 with 20% FBS in 0.5% penicillin-streptomycin (pen/strep)] at 4°C until digestion (all reagents from GIBCO-Invitrogen, Grand Island, NY, unless specified). Enzymatic digestion was performed with 1.2 U/ml dispase and 5 mg/ml collagenase IV (Worthington, Lakewood, NJ) for 45 min at 37°C and then quenched with SKM. Cell suspensions were filtered through 70-μm and 40-μm cell strainers, centrifuged (514 g) at 4°C, and resuspended in 3 ml of medium.
Red blood corpuscle/ cells were lysed with NH4Cl, and the remaining cells were resuspended at 106 cells/ml in PBS with 0.5% BSA (Sigma). Verapamil (100 μM, Sigma) was added to one aliquot of cells and incubated 5 min at 37°C before 5 μg/ml of Hoechst 33342 (Sigma) was added to all samples and incubated 60 min at 37°C. Propidium iodide (PI, Sigma; 2 μg/ml) was added to the samples before fluorescence-activated cell sorting (FACS) with a BD LSRII cell sorter (BD Biosciences, San Jose, CA). Flow cytometries were analyzed with BD CellQuest Pro version 5.2 (BD Biosciences). Details of SP cell preparation and gating strategy and yields are provided in Supplemental Fig. S1 and Supplemental Table S1, respectively.1 The amount of SP cells per gram of dry tissue was calculated with the following equation: SP cell number per gram = number of mononuclear cells per gram × (%viability/100) × (%SP cells/100).
Sca-1 and CD45 expression.
Cells were incubated with 2 μg/106 cells of primary or isotype control MAb (BD Biosciences Pharmingen) for 15 min immediately after Hoechst staining, then cells were washed and resuspended in 0.5 ml of cold PBS-BSA, and PI was added before FACS analysis.
SP cell microarray hybridization and analysis.
SP cell preparations were washed, pelleted, and resuspended in 20 μl of PBS and stored at −80°C until processing for microarray analysis according to manufacturer's instructions and previously described methods from our laboratory (6, 15). Total RNA was isolated with the RNeasy micro kit (Qiagen, Valencia, CA). The purity, concentration, and integrity of RNA were determined with a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE) and an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). RNAs were subjected to linear amplification with the GeneChip two-cycle target-labeling protocol (Affymetrix, Santa Clara, CA).
Six independently separated EOM and six TA SP cell preparations were used for microarray analysis with Affymetrix Mouse 430 version 2.0 GeneChip arrays. One EOM and two TA SP cell GeneChip hybridizations failed to meet quality control standards and were discarded from further analysis. Raw intensities for each probe set were stored in electronic formats with GeneChip Operating System version 1.1, and expression summaries were calculated with Microarray Suite version 5.0 (MAS5) algorithms. All data were normalized by GC-RMA algorithm with Genespring version 7.3.1 software (Agilent) and filtered separately to obtain at least one present call for each transcript in any of nine conditions by using MAS5 output. Statistical analysis was applied on 28,987 of 45,101 probe sets that passed the filtering. Differentially expressed genes were identified by applying the two-class unpaired data settings in Significant Microarray Analysis version 2.21 software at the level of 0% false discovery rate (FDR). Verification of the accuracy of the analysis of relationships between the samples was done by principal component analysis (PCA) and visualization of the relative expression level of each transcript with Pearson correlation-based hierarchical clustering of samples using Genespring version 7.3.1 software (Agilent).
Validation of gene expression by quantitative PCR.
Quantitative PCR (qPCR) was performed as previously described (6, 15) in order to validate differential expression of Dspg3, Fbln1, Mmp23, Cd36, and Utrn on three independent EOM and TA SP cell preparations. GAPDH expression was used for normalization. RNA from each sample was reverse transcribed into cDNA with oligo(dT) primers and amplified with an ABI 7900HT real-time PCR system (Applied Biosystems, Foster City, CA) and gene-specific primers (Idaho Technology, Salt Lake City, UT). Sequence Detection Software (ABI, Version 2.2) was used for analysis and the ΔΔCt method (where Ct is threshold cycle) for computing gene expression levels as described previously (37). Primer sequences and PCR conditions are provided in Supplemental Table S2.
In vitro muscle formation assays.
SP cells were cultured in vitro to form myotubes with standard methods. Briefly, equal numbers of EOM and TA SP cells [from green fluorescent protein (GFP) mice] were resuspended in Rubinstein complete medium [69% DMEM, 17.3% M199, 9.7% horse serum, 0.97% chick embryo extract (SLI, Bolney, UK), 1.04% glutamine, 1.04% pen/strep, 1.04% Fungizone] and cultured alone or over a mdx myoblast feeding layer on glass-bottomed 35-mm petri dishes or eight-well culture chamber slides coated with Matrigel (BD Biosciences, Bedford, MA). The feeder layer (2.7 × 104/cm2 myoblasts) was plated 1 h before SP cells were plated. GFP+ cells and myotubes were counted with a Nikon Eclipse TE 2000-U at 48–72 h and 13–18 days after plating. Myotube formation was documented on 2-wk-old cultures stained with Hoechst 33342.
Statistical analysis.
GraphPad Prism version 4.0, (GraphPad Software, San Diego, CA) was used for statistical analysis, and a P value <0.05 was considered significant.
All primary microarray data are MIAME compliant, have been deposited in NCBI's Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/), and are accessible through GEO Series accession number GSE9294.
RESULTS
EOM contains a 15.7× greater number of SP cells per gram than limb muscle.
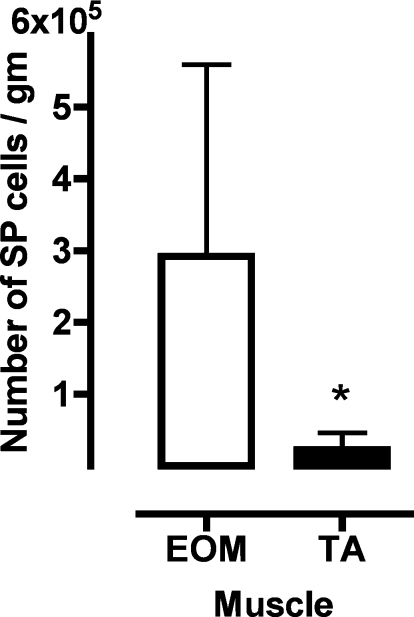
We evaluated the uncommitted stem cell compartment by quantifying the numbers of SP cells based on their ability to exclude Hoechst 33342 (21, 22, 44). This strategy allows the purification of living SP cells from a muscle mononuclear cell pool by FACS-based fractionation, without having to expose the cells to culture conditions. As shown in Fig. 1, we found that EOM (291,929 ± 267,346) contains 15 times more SP cells per gram compared with TA (22,799 ± 23,282; n = 7; P < 0.05). Over 95% of EOM-SP (96.62 ± 4.02) and TA-SP (97.06 ± 2.26) were CD45− (n = 13).
Fig. 1.
Extraocular muscle (EOM) contains 15 times more side population (SP) cells than tibialis anterior (TA). Total mononuclear cells from EOM and TA were labeled with Hoechst 33342 in the presence and absence of verapamil. SP cell population was selected with a third log cutoff between main population and SP in the density plots (see Supplemental Fig. S1). Values are mean ± SD numbers of SP cells per gram of tissue in EOM compared with TA; n = 7. *P < 0.05.
Expression profiling of EOM vs. limb muscle SP cells.
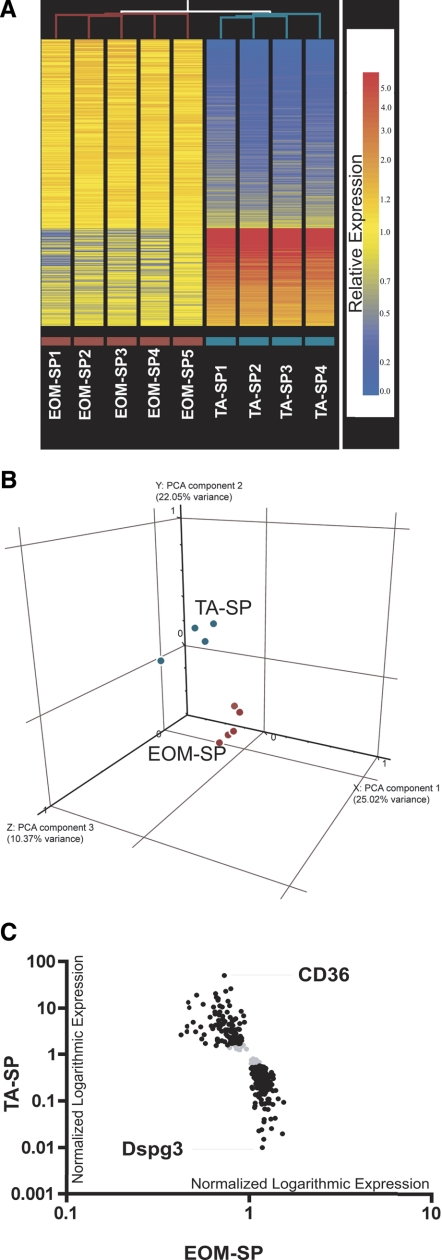
To identify the expression profile of SP cells fractionated from EOM versus limb muscle we performed microarray-based screening using Affymetrix Mouse Genome 430 2.0 Array GeneChips. We performed hierarchical clustering of nine samples to determine overall similarities within and between the EOM-SP and TA-SP samples. Branch-length analysis of data (Fig. 2A, top, red and cyan lines) demonstrated that the five EOM-SP samples were more similar to each other than to TA-SP samples, and, vice versa, the four TA-SP samples were more similar to each other than to EOM-SP. Further analysis of the profiling with a FDR cutoff set at a highly stringent 0% revealed 348 differentially expressed transcripts (0.77% of 45,101 evaluated transcripts) in the profile, of which 229 were upregulated in EOM-SP and 119 upregulated in TA-SP. Figure 2A, bottom, shows the heat map representation of hierarchical clustering of the entire profile. The complete list of 348 transcripts is provided in Supplemental Table S3.
Fig. 2.
Microarray heat map, principal component analysis (PCA), and scatter graph. A: hierarchical clustering analysis of all EOM-SP and TA-SP samples. Unsupervised clustering in Genespring 7.2 version was used to analyze similarities among replicate samples across sorted EOM-SP vs. TA-SP cells. Similarities between samples are indicated by the length of the lines that are at top of each sample column. Relative expression levels of 348 statistically significant transcripts are shown as horizontal colored bars. Red color refers to higher and blue color refers to lower expression as shown on scale bar. Replicate sample names with purple and cyan color codes are indicated at bottom. B: PCA: 3-dimensional plot showing distribution of EOM-SP (red) and TA-SP (cyan) transcripts. The 3 axes correspond to the components with the highest variance between the groups. C: microarray scatter graph of genes differentially expressed between EOM-SP and TA-SP. Black, normalized expression values of 313 transcripts (205 upregulated in EOM and 108 in TA) on a logarithmic scale after a 2-fold cutoff between EOM-SP and TA-SP. Gray, transcripts that did not pass the cutoff. The names of the highest-expressed transcripts in EOM (Dspg3) and TA (CD36) are shown.
To evaluate the overall relationship between the multivariate profiles of SP cells isolated from EOM and TA, we performed a PCA (62). Figure 2B shows the distribution of EOM-SP and TA-SP chip data in three-dimensional space, where the three axes correspond to the three components with highest variability in our data set. As can be seen in Fig. 2B, EOM-SP chips (red) and TA-SP chips (cyan) cluster as groups on different coordinates, demonstrating that the biggest variance is due to the tissue from which the SP cells were isolated.
To identify and graphically depict all the genes that were differentially expressed at greater than twofold levels, we performed a scatter graph analysis after imposing the fold change cutoff. Even with this limitation, a majority of transcripts (313 of 348 or 89.9%) were found to be differentially expressed: 205 upregulated in EOM-SP and 108 upregulated in TA-SP, as shown in Fig. 2C, which shows the two transcripts (Dspg3 and Cd36) found to be differentially expressed with the greatest magnitude of difference in the profile.
To validate the transcriptome analysis we performed qPCR on five transcripts (3 upregulated in EOM-SP and 2 upregulated in TA-SP) on RNA extracted from three independent EOM-SP and TA-SP preparations. As shown in Table 1, qPCR validated differential expression of Dspg3, Fbln1, Mmp23, Cd36, and Utrn in SP cells fractionated from EOM and limb muscle. Furthermore, as shown in Table 1, the magnitude of change was consistent with levels of differential expression revealed by microarray analysis.
Table 1.
Validation of expression profiling
| Affy ID | Gene Symbol | Microarray Fold | qPCR Fold | qPCR P Value |
|---|---|---|---|---|
| 1421114_a_at | Dspg3 | 338.4 | 259.3 | 3.99916E-07 |
| 1439688_at | Fbln1 | 13.7 | 5.0 | 1.64621E-05 |
| 1417282_at | Mmp23 | 7.6 | 2.3 | 1.95926E-06 |
| 1450884_at | Cd36 | −69.9 | −26.0 | 3.09794E-06 |
| 1452222_at | Utrn | −2.7 | −9.0 | 1.50034E-07 |
Five of 348 differentially expressed transcripts revealed by the microarray were independently validated by quantitative PCR (qPCR). Fold change of expression in the microarray and in the qPCR are shown.
Bioinformatic analysis of EOM and limb muscle SP cell transcriptomes.
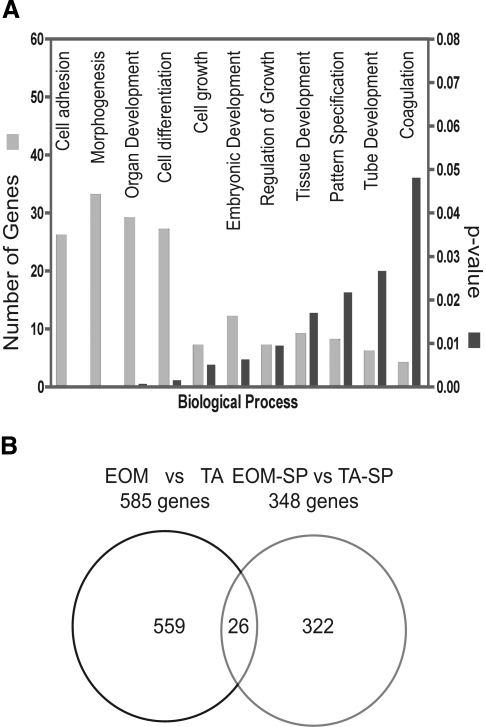
We used different bioinformatic strategies to further analyze the function of the genes that were found to be differentially expressed in the EOM and limb muscle SP cell transcriptomes. The bioinformatic program DAVID (version 2.1) was used to functionally cluster the entire list of differentially expressed genes. Interestingly, DAVID-based analysis led to clustering of 93 genes (from the 348 transcripts submitted), and the largest number of genes were clustered in biological processes that are associated with muscle formation/development, such as cell adhesion, morphogenesis, organ development, cell differentiation, cell growth, and regulation of growth and tissue development (Fig. 3A and Supplemental Table S4). To address whether the differences in gene expression noted among the SP cell transcriptomes were due to the previously reported differences between EOM and limb muscle transcriptomes (15, 16, 47, 53) rather than SP cells, we compared the previous “EOM vs. limb muscle” significant gene list with the new list from the EOM-SP vs. limb-SP comparison; we converted the original rat transcripts into their mouse orthologs (total number 585), and then we intersected the 348 transcripts from the SP cell microarray. We observed that of the 348 differentially expressed genes (i.e., upregulated in EOM-SP and upregulated in TA-SP) in the SP microarray, 322 genes were SP specific and only 26 genes were present in the previous (whole muscle) microarray comparison (Fig. 3B, Supplemental Table S5), demonstrating that the vast majority of genes identified in this study reflected differences in the transcriptomes of EOM-SP and TA-SP cells rather than of the tissue from which they were fractionated.
Fig. 3.
Biological process (DAVID) and Venn diagram. A: biological processes of genes differentially expressed between EOM-SP vs. TA-SP. With DAVID 2.1, 93 of 322 genes that are available in DAVID database are grouped based on Gene Ontology (GO; http://www.geneontology.org). Each biological process is ranked based on its P value, indicating its overexpression. B: Venn diagram representing stem cell-specific (EOM-SP vs. TA-SP cells) differentially expressed transcripts against EOM vs. limb muscles (EOM vs. TA) differentially expressed transcripts with 26-transcript overlap (92% concordance, 8% discordance in expression levels).
Differences in myogenic potential of SP cells fractionated from EOM and limb muscles.
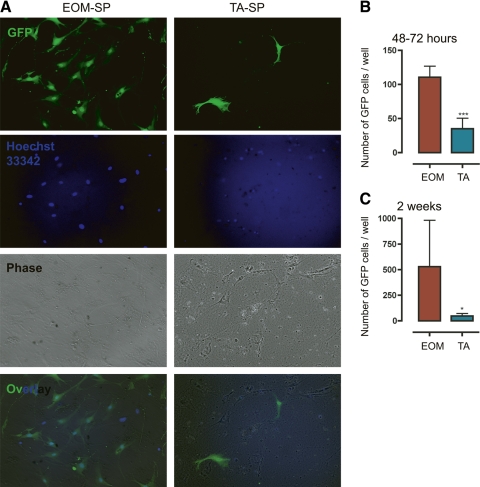
To ensure that the SP cells had myogenic potential and were capable of forming myotubes in vitro, we fractionated EOM-SP and TA-SP from GFP+ mice. Equal numbers of these were plated on a myoblast feeding layer made from mdx mice, and we evaluated them after 13 days in culture. To determine the relative myogenic potential in vitro, EOM-SP and TA-SP were cultured in parallel and the total number of GFP+ cells cultured per well was evaluated at two time points: early (48–72 h) and late (2 wk). We found that both EOM-SP and TA-SP were able to fuse and form multinucleated myotubes in vitro as shown in Supplemental Fig. S3. (In one case, fusion of EOM-SP-derived GFP+ cells to twitching myotubes was also observed, see Supplemental Movie S1; also see Supplemental Figs. S4–S6 for more examples of cultured EOM-SP and TA-SP cells.) As shown in Fig. 4B, at the early time point the number of GFP+ EOM-SP cells was higher than TA-SP (110.5 ± 16.3 vs. 35.0 ± 15.6; n = 2, P < 0.0001). Similarly, at the late time point (Fig. 4, A and C) the number of GFP+ EOM-SP cells was higher than TA-SP (528.3 ± 453.7 vs. 47 ± 25.36; n = 3, P < 0.05). As independent validation of the increased proliferative capacity of these muscles we have included Supplemental Fig. S2, which shows SC counts performed on myofibers isolated from EOM and limb muscles.
Fig. 4.
EOM-SP cells proliferate better in vitro than TA-SP cells. A: representative culture of EOM and TA green fluorescent protein-positive (GFP+) SP cells after 2 wk of culture over a myoblast feeding layer. Equal numbers of GFP+ SP cells were plated on a mdx-myoblast feeding layer. After 18 days, cells were counted. Left: EOM-SP. Right: TA-SP. From top to bottom: GFP, Hoechst, phase, and overlaid (Ov) images. B: a higher number of EOM-SP cells than TA-SP cells was present in culture 48–72 h after sorting. Cells were counted with a fluorescence microscope. Means ± SD are shown; n = 2. ***P < 0.0001. C: differences in the number of EOM and TA GFP+ cells in cultures increased over time. After 13–18 days, cells were counted with a fluorescence microscope. Means ± SD are shown; n = 3. *P < 0.05.
DISCUSSION
In adults, stem cells are known to reside in tissues undergoing rapid turnover such as the hematopoietic system, gut lining, and epidermis. Adult skeletal muscle is not associated with rapid cell turnover compared with tissues such as the gut; however, it has a limited ability to respond to physiological stimuli such as increased workload and pathophysiological insults such as trauma, disease, or toxins. SCs are critical for the ability to regenerate and repair muscle; however, uncommitted stem cell progenitors such as SP cells are also capable of considerable myogenesis.
While previous studies have compared uncommitted SP cells with main population cells (3, 46, 68) and SP cells from different tissues and/or organs (22, 45, 56), no previous study has compared the content of SP cells between different skeletal muscle groups. In this study we found that the number of SP cells per gram in EOM is 15 times the number in limb muscle. Transcriptome analysis using Affymetrix GeneChips revealed that 348 transcripts were found differentially expressed in EOM-SP vs. TA-SP cells out of 45,101 evaluated transcripts (which include >34,000 well-characterized mouse genes). Thus the vast majority (∼92%) of transcripts identified in this study reflected transcriptome level differences between SP cells obtained from different tissues rather than from the tissue from which they were isolated (15, 16, 53). However, a small fraction (∼8% or 26 of 348) of differentially expressed transcripts could potentially be ascribed to differences in EOM and limb muscle transcriptomes per se. This group included three forms of Gst (Gst m4, Mgst 1, and Gst a4) and two isoforms of UDP (33Galt1 and Galntl1) that were upregulated in EOM-SP cells. The presence of high levels of chemoprotective enzymes such as Gst and Udp offers an advantage in overcoming oxidative stress; resistance to such stress has been described in other stem cells (2, 63). Additionally, the bicoid class of homeodomain transcription factor Pitx-2 was found to be upregulated. Pitx-2 is an upstream activator of the myogenic regulatory factors Myf5 and MyoD that plays an important role in EOM and craniofacial muscle development. Indeed, mutations of Pitx-2 lead to Axenfeld-Rieger syndrome associated with craniofacial malformation and ocular and EOM dysgenesis (65).
Bioinformatic analysis of the transcriptomes revealed a number of interesting differences between the EOM and limb muscle stem cell transcriptomes. Genes found upregulated in the EOM transcriptome included Tgfbi, Fn1, Aebp1, Dpt, Tro, Alcam, and Col14a1 (23, 34, 69), which have been shown to be expressed by stem cells in different tissues. Biological process clustering with DAVID revealed differential expression of a group of 26 cell adhesion molecules, including the endothelial markers CD36 (10), Scarb1 (74), CD93 (76), Vwf (67), Myh9 (27), and Nrp2 (13), which were all downregulated in EOM-SP. Interestingly, CD36 (14), Scarb1(74), and CD93(76) have also been associated with fatty acid transmembrane transport and clearance of apoptotic cells in different populations of stem cells. The genes Nrp2, Kitl (9), Itga (73), and Dll1 (33) were downregulated in EOM-SP cells and have been previously associated with a proliferative stimulus for stem cells. Additionally, two tumor suppressor genes, Jup (71) and Stim2 (50, 60), were downregulated in the EOM-SP cells. Consistent with the increasingly important role ascribed to the interaction between stem cells and extracellular matrix in formation and maintenance of stem cell niches (64), as well as the role played by Tcf4, a downstream effector of Wnt-β-catenin signaling pathway in chicken limb bud muscle pattern formation (30), we found Dspg3, a small leucine-rich repeat protein greatly upregulated in EOM-SP.
Analysis with KEGG (http://www.genome.ad.jp/kegg/pathway.html) allowed identification of a number of candidate pathways related to Notch, Vegf, Follistatin, and BMP signaling as differentially regulated in the SP transcriptomes. Transcriptional evidence of Notch pathway inhibition in EOM-SP comes from the downregulation of DLL1, Notch 1, Nrarp, and Evi1 and upregulation of the inhibitor HDAC4. Previous studies (48, 66) have demonstrated that Notch inhibits muscle differentiation and blockade of Notch signaling either by g-secretase inhibition or by Numb overexpression causes myotube hypertrophy by recruiting reserve cells that do not normally fuse (33). Vegf, VEGFR1, and Cdc42 were found to be downregulated in EOM for reasons that are unclear since the EOM are highly vascular; however, this may reflect heterogeneity of different stem cell populations in muscle. FST, Fstl-1, Tmeff2, and HDAC4 (7, 19, 28, 36, 43) were upregulated, suggesting mechanisms by which EOM could have more efficient myogenesis. Bmp1, Bmpr1b, Htra1, Twsg1, and Rgmb genes were upregulated in EOM-SP cells and are interesting in the context of FST and myogenesis since the metalloprotease Bmp1 is known to cleave the myostatin propeptide and activate latent myostatin (72). Consistent with the transcriptome-level differences identified here, it has been reported that SP cells fractionated from skin and muscle differ in their capacity to restore dystrophin, suggesting that inherent differences exist even among the same stem cell populations isolated from different tissues (45). The limited numbers of surviving cells in these reports provide an important impetus toward efforts to identify and study myogenic progenitors from different tissue sources to increase the likelihood of making these approaches therapeutically effective in DMD. The differential expression of these genes may reflect mechanisms enabling EOM-SP cells to undertake the more efficient myogenesis previously noted in EOM (15, 16, 40–42). We found that EOM-SPs were able to proliferate more efficiently compared with TA-SPs, after equal numbers of them were cultured up to 2 wk in vitro, despite identical matrix/culture conditions. Together, these findings support a role of lineage directives inherited by differentiating muscle cells from specialized progenitors in phenotypically distinct “allotypes” such as the EOM.
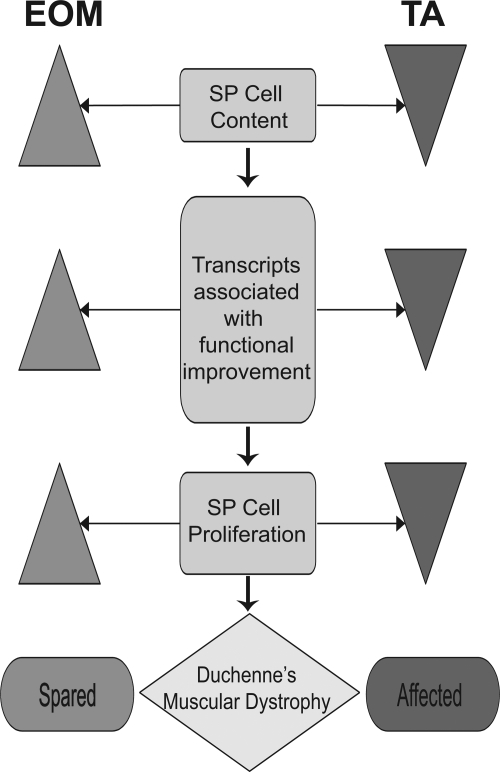
From a disease perspective, several hypotheses have been proposed to explain the enigmatic EOM sparing in DMD, including smaller surface-to-volume ratio resulting in less mechanical stress (31), higher capacity to regulate intracellular calcium in EOM than other skeletal muscles (32), higher antioxidant capacity in EOM (55), overexpression of dystrophin-related protein (DRP)/utrophin (38, 54), increased elasticity due to differential expression of M bands (70), and increased myogenesis (15, 16, 40–42). However, independent tests of these hypotheses either have not been performed or have been equivocal, e.g., DRP/utrophin upregulation where utrophin upregulation has not been confirmed at the protein or mRNA level (15, 16, 32, 53). Our identification in EOM of a unique stem cell content with greater numbers of SP cells with a specific expression profile coupled with the greater capacity of these cells for in vitro myogenesis provides a model (Fig. 5) and support for the hypothesis that an increased stem cell content may underlie the efficient and continual myogenesis and therefore may contribute to sparing of adult EOM in DMD.
Fig. 5.
Model for the role of stem cells in EOM sparing in Duchenne muscular dystrophy (DMD). Flow chart summarizes findings from stem cell quantitation, microarray, and in vitro studies. Left: EOM. Right: TA. We believe that an increased and unique stem cell content underlies the continuous myogenesis observed in normal EOM muscle and may explain the EOM sparing in DMD.
GRANTS
This work was supported by National Institutes of Health Grants EY-013862 and AR-051696 to T. S. Khurana.
Supplementary Material
Acknowledgments
We thank Drs. J. Moore, E. Holzbaur, A. Bhandoola, J Tobias, and D. Baldwin at the University of Pennsylvania, J. Hayden and F. Keeney at the Wistar Institute, as well as Drs. L. M. Kunkel, E. Gussoni, and F. Montanaro at Harvard Medical School and the Children's Hospital Boston for advice and help and Profs. T. Partridge and C. A. Collins at the Imperial College, London, UK for teaching us the single myofiber cultures and satellite cell assays.
Address for reprint requests and other correspondence: T. S. Khurana, A-601 Richards Bldg., 3700 Hamilton Walk, Philadelphia, PA 19104-6085 (e-mail: tsk@mail.med.upenn.edu; http://www.med.upenn.edu/pmi/members/khurana.shtml).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Alessandri G, Pagano S, Bez A, Benetti A, Pozzi S, Iannolo G, Baronio M, Invernici G, Caruso A, Muneretto C, Bisleri G, Parati E. Isolation and culture of human muscle-derived stem cells able to differentiate into myogenic and neurogenic cell lineages. Lancet 364: 1872–1883, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong L, Saretzki G, Peters H, Wappler I, Evans J, Hole N, von Zglinicki T, Lako M. Overexpression of telomerase confers growth advantage, stress resistance, and enhanced differentiation of ESCs toward the hematopoietic lineage. Stem Cells 23: 516–529, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Asakura A, Rudnicki MA. Side population cells from diverse adult tissues are capable of in vitro hematopoietic differentiation. Exp Hematol 30: 1339–1345, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Asmussen G, Gaunitz U. Mechanical properties of the isolated inferior oblique muscle of the rabbit. Pflügers Arch 392: 183–190, 1981. [DOI] [PubMed] [Google Scholar]
- 5.Bron A, Tripathi R, Tripathi B. The extraocular muscles and ocular movements. In: Wolff's Anatomy of the Eye and the Orbit (8th ed.). London: Chapman & Hall Medical, 1997, chapt. 4, p. 107–177.
- 6.Budak MT, Bogdanovich S, Wiesen MH, Lozynska O, Khurana TS, Rubinstein NA. Layer-specific differences of gene expression in extraocular muscles identified by laser-capture microscopy. Physiol Genomics 20: 55–65, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet 38: 228–233, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol 9: 255–267, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Domen J, Weissman IL. Hematopoietic stem cells need two signals to prevent apoptosis; BCL-2 can provide one of these, Kitl/c-Kit signaling the other. J Exp Med 192: 1707–1718, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dube JJ, Bhatt BA, Dedousis N, Bonen A, O'Doherty RM. Leptin, skeletal muscle lipids, and lipid-induced insulin resistance. Am J Physiol Regul Integr Comp Physiol 293: R642–R650, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Engel A, Ozawa E. Dystrophinopathies. In: Myology, edited by Engel A, Franzini-Armstrong C. New York: McGraw-Hill, 2004, chapt. 34, p. 961–1025.
- 12.Engle EC Genetic basis of congenital strabismus. Arch Ophthalmol 125: 189–195, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Favier B, Alam A, Barron P, Bonnin J, Laboudie P, Fons P, Mandron M, Herault JP, Neufeld G, Savi P, Herbert JM, Bono F. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood 108: 1243–1250, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Febbraio M, Silverstein RL. CD36: implications in cardiovascular disease. Int J Biochem Cell Biol 39: 2012–2030, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer MD, Budak MT, Bakay M, Gorospe JR, Kjellgren D, Pedrosa-Domellof F, Hoffman EP, Khurana TS. Definition of the unique human extraocular muscle allotype by expression profiling. Physiol Genomics 22: 283–291, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Fischer MD, Gorospe JR, Felder E, Bogdanovich S, Pedrosa-Domellof F, Ahima RS, Rubinstein NA, Hoffman EP, Khurana TS. Expression profiling reveals metabolic and structural components of extraocular muscles. Physiol Genomics 9: 71–84, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Fraterman S, Zeiger U, Khurana TS, Rubinstein NA, Wilm M. Combination of peptide OFFGEL fractionation and label-free quantitation facilitated proteomics profiling of extraocular muscle. Proteomics 7: 3404–3416, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Fraterman S, Zeiger U, Khurana TS, Wilm M, Rubinstein NA. Quantitative proteomics profiling of sarcomere associated proteins in limb and extraocular muscle allotypes. Mol Cell Proteomics 6: 728–737, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Glenisson W, Castronovo V, Waltregny D. Histone deacetylase 4 is required for TGFbeta1-induced myofibroblastic differentiation. Biochim Biophys Acta 1773: 1572–1582, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Goldman Y, Dantzig J. Muscle: anatomy, physiology, biochemistry. In: Kelley's Textbook of Rheumatology (7th ed.), edited by Harris E, Budd R, Firestein G, Genovese M, Sergent J, Ruddy S, Sledge C. Philadelphia, PA: Elsevier, 2005, chapt. 5.
- 21.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 183: 1797–1806, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 401: 390–394, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Hirata H, Murakami Y, Miyamoto Y, Tosaka M, Inoue K, Nagahashi A, Jakt LM, Asahara T, Iwata H, Sawa Y, Kawamata S. ALCAM (CD166) is a surface marker for early murine cardiomyocytes. Cells Tissues Organs 184: 172–180, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman EP, Brown RH Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51: 919–928, 1987. [DOI] [PubMed] [Google Scholar]
- 25.Hoh JF, Hughes S. Myogenic and neurogenic regulation of myosin gene expression in cat jaw-closing muscles regenerating in fast and slow limb muscle beds. J Muscle Res Cell Motil 9: 59–72, 1988. [DOI] [PubMed] [Google Scholar]
- 26.Hoh JF, Hughes S, Chow C, Hale PT, Fitzsimons RB. Immunocytochemical and electrophoretic analyses of changes in myosin gene expression in cat posterior temporalis muscle during postnatal development. J Muscle Res Cell Motil 9: 48–58, 1988. [DOI] [PubMed] [Google Scholar]
- 27.Huang Y, Shi H, Zhou H, Song X, Yuan S, Luo Y. The angiogenic function of nucleolin is mediated by vascular endothelial growth factor and nonmuscle myosin. Blood 107: 3564–3571, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Iezzi S, Di Padova M, Serra C, Caretti G, Simone C, Maklan E, Minetti G, Zhao P, Hoffman EP, Puri PL, Sartorelli V. Deacetylase inhibitors increase muscle cell size by promoting myoblast recruitment and fusion through induction of follistatin. Dev Cell 6: 673–684, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Kaminski HJ, al-Hakim M, Leigh RJ, Katirji MB, Ruff RL. Extraocular muscles are spared in advanced Duchenne dystrophy. Ann Neurol 32: 586–588, 1992. [DOI] [PubMed] [Google Scholar]
- 30.Kardon G, Harfe BD, Tabin CJ. A Tcf4-positive mesodermal population provides a prepattern for vertebrate limb muscle patterning. Dev Cell 5: 937–944, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Karpati G, Carpenter S. Small-caliber skeletal muscle fibers do not suffer deleterious consequences of dystrophic gene expression. Am J Med Genet 25: 653–658, 1986. [DOI] [PubMed] [Google Scholar]
- 32.Khurana TS, Prendergast RA, Alameddine HS, Tome FM, Fardeau M, Arahata K, Sugita H, Kunkel LM. Absence of extraocular muscle pathology in Duchenne's muscular dystrophy: role for calcium homeostasis in extraocular muscle sparing. J Exp Med 182: 467–475, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitzmann M, Bonnieu A, Duret C, Vernus B, Barro M, Laoudj-Chenivesse D, Verdi JM, Carnac G. Inhibition of Notch signaling induces myotube hypertrophy by recruiting a subpopulation of reserve cells. J Cell Physiol 208: 538–548, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Klein G, Kibler C, Schermutzki F, Brown J, Muller CA, Timpl R. Cell binding properties of collagen type XIV for human hematopoietic cells. Matrix Biol 16: 307–317, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 50: 509–517, 1987. [DOI] [PubMed] [Google Scholar]
- 36.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA 98: 9306–9311, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-Delta DeltaCT method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Matsumura K, Ervasti JM, Ohlendieck K, Kahl SD, Campbell KP. Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature 360: 588–591, 1992. [DOI] [PubMed] [Google Scholar]
- 39.Mauro A Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9: 493–495, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McLoon LK, Wirtschafter J. Activated satellite cells are present in uninjured extraocular muscles of mature mice. Trans Am Ophthalmol Soc 100: 119–123, 2002. [PMC free article] [PubMed] [Google Scholar]
- 41.McLoon LK, Wirtschafter J. Activated satellite cells in extraocular muscles of normal adult monkeys and humans. Invest Ophthalmol Vis Sci 44: 1927–1932, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLoon LK, Wirtschafter JD. Continuous myonuclear addition to single extraocular myofibers in uninjured adult rabbits. Muscle Nerve 25: 348–358, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Minetti GC, Colussi C, Adami R, Serra C, Mozzetta C, Parente V, Fortuni S, Straino S, Sampaolesi M, Di Padova M, Illi B, Gallinari P, Steinkuhler C, Capogrossi MC, Sartorelli V, Bottinelli R, Gaetano C, Puri PL. Functional and morphological recovery of dystrophic muscles in mice treated with deacetylase inhibitors. Nat Med 12: 1147–1150, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Montanaro F, Liadaki K, Schienda J, Flint A, Gussoni E, Kunkel LM. Demystifying SP cell purification: viability, yield, and phenotype are defined by isolation parameters. Exp Cell Res 298: 144–154, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Montanaro F, Liadaki K, Volinski J, Flint A, Kunkel LM. Skeletal muscle engraftment potential of adult mouse skin side population cells. Proc Natl Acad Sci USA 100: 9336–9341, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muskiewicz KR, Frank NY, Flint AF, Gussoni E. Myogenic potential of muscle side and main population cells after intravenous injection into sub-lethally irradiated mdx mice. J Histochem Cytochem 53: 861–873, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Niemann CU, Krag TO, Khurana TS. Identification of genes that are differentially expressed in extraocular and limb muscle. J Neurol Sci 179: 76–84, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Nofziger D, Miyamoto A, Lyons KM, Weinmaster G. Notch signaling imposes two distinct blocks in the differentiation of C2C12 myoblasts. Development 126: 1689–1702, 1999. [DOI] [PubMed] [Google Scholar]
- 49.Oda K The relationship between motor endplate size and muscle fiber diameter in different muscle groups of the rat. Jpn J Physiol 35: 1091–1095, 1985. [DOI] [PubMed] [Google Scholar]
- 50.Peel SE, Liu B, Hall IP. A key role for STIM1 in store operated calcium channel activation in airway smooth muscle. Respir Res 7: 119, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pette D Historical perspectives: plasticity of mammalian skeletal muscle. J Appl Physiol 90: 1119–1124, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Pollard H Memoirs: observations on the development of the head in Gobius capito. J Cell Sci s2–35: 335–352, 1894. [Google Scholar]
- 53.Porter JD, Khanna S, Kaminski HJ, Rao JS, Merriam AP, Richmonds CR, Leahy P, Li J, Andrade FH. Extraocular muscle is defined by a fundamentally distinct gene expression profile. Proc Natl Acad Sci USA 98: 12062–12067, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Porter JD, Rafael JA, Ragusa RJ, Brueckner JK, Trickett JI, Davies KE. The sparing of extraocular muscle in dystrophinopathy is lost in mice lacking utrophin and dystrophin. J Cell Sci 111: 1801–1811, 1998. [DOI] [PubMed] [Google Scholar]
- 55.Ragusa RJ, Chow CK, St Clair DK, Porter JD. Extraocular, limb and diaphragm muscle group-specific antioxidant enzyme activity patterns in control and mdx mice. J Neurol Sci 139: 180–186, 1996. [PubMed] [Google Scholar]
- 56.Rochon C, Frouin V, Bortoli S, Giraud-Triboult K, Duverger V, Vaigot P, Petat C, Fouchet P, Lassalle B, Alibert O, Gidrol X, Pietu G. Comparison of gene expression pattern in SP cell populations from four tissues to define common “stemness functions”. Exp Cell Res 312: 2074–2082, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Rubinstein N, Kelly A. The diversity of muscle fiber types and its origin during development. In: Myology (3rd ed.), edited by Engel A, Franzini-Armstrong C. New York: McGraw-Hill, 2004, chapt. 4, p. 87–103.
- 58.Rubinstein NA, Hoh JF. The distribution of myosin heavy chain isoforms among rat extraocular muscle fiber types. Invest Ophthalmol Vis Sci 41: 3391–3398, 2000. [PubMed] [Google Scholar]
- 59.Rubinstein NA, Porter JD, Hoh JF. The development of longitudinal variation of myosin isoforms in the orbital fibers of extraocular muscles of rats. Invest Ophthalmol Vis Sci 45: 3067–3072, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Sabbioni S, Barbanti-Brodano G, Croce CM, Negrini M. GOK: a gene at 11p15 involved in rhabdomyosarcoma and rhabdoid tumor development. Cancer Res 57: 4493–4497, 1997. [PubMed] [Google Scholar]
- 61.Sampaolesi M, Blot S, D'Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthelemy I, Perani L, Mantero S, Guttinger M, Pansarasa O, Rinaldi C, Cusella De Angelis MG, Torrente Y, Bordignon C, Bottinelli R, Cossu G. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature 444: 574–579, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Sanguinetti G, Milo M, Rattray M, Lawrence ND. Accounting for probe-level noise in principal component analysis of microarray data. Bioinformatics 21: 3748–3754, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Saretzki G, Armstrong L, Leake A, Lako M, von Zglinicki T. Stress defense in murine embryonic stem cells is superior to that of various differentiated murine cells. Stem Cells 22: 962–971, 2004. [DOI] [PubMed] [Google Scholar]
- 64.Scadden DT The stem-cell niche as an entity of action. Nature 441: 1075–1079, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Semina EV, Reiter R, Leysens NJ, Alward WL, Small KW, Datson NA, Siegel-Bartelt J, Bierke-Nelson D, Bitoun P, Zabel BU, Carey JC, Murray JC. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet 14: 392–399, 1996. [DOI] [PubMed] [Google Scholar]
- 66.Shawber C, Nofziger D, Hsieh JJ, Lindsell C, Bogler O, Hayward D, Weinmaster G. Notch signaling inhibits muscle cell differentiation through a CBF1-independent pathway. Development 122: 3765–3773, 1996. [DOI] [PubMed] [Google Scholar]
- 67.Somani A, Nguyen J, Milbauer LC, Solovey A, Sajja S, Hebbel RP. The establishment of murine blood outgrowth endothelial cells and observations relevant to gene therapy. Transl Res 150: 30–39, 2007. [DOI] [PubMed] [Google Scholar]
- 68.Tamaki T, Akatsuka A, Okada Y, Matsuzaki Y, Okano H, Kimura M. Growth and differentiation potential of main- and side-population cells derived from murine skeletal muscle. Exp Cell Res 291: 83–90, 2003. [DOI] [PubMed] [Google Scholar]
- 69.Wagner W, Wein F, Roderburg C, Saffrich R, Faber A, Krause U, Schubert M, Benes V, Eckstein V, Maul H, Ho AD. Adhesion of hematopoietic progenitor cells to human mesenchymal stem cells as a model for cell-cell interaction. Exp Hematol 35: 314–325, 2007. [DOI] [PubMed] [Google Scholar]
- 70.Wiesen MH, Bogdanovich S, Agarkova I, Perriard JC, Khurana TS. Identification and characterization of layer-specific differences in extraocular muscle m-bands. Invest Ophthalmol Vis Sci 48: 1119–1127, 2007. [DOI] [PubMed] [Google Scholar]
- 71.Williamson L, Raess NA, Caldelari R, Zakher A, de Bruin A, Posthaus H, Bolli R, Hunziker T, Suter MM, Muller EJ. Pemphigus vulgaris identifies plakoglobin as key suppressor of c-Myc in the skin. EMBO J 25: 3298–3309, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolfman NM, McPherron AC, Pappano WN, Davies MV, Song K, Tomkinson KN, Wright JF, Zhao L, Sebald SM, Greenspan DS, Lee SJ. Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases. Proc Natl Acad Sci USA 100: 15842–15846, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xiao Q, Zeng L, Zhang Z, Hu Y, Xu Q. Stem cell-derived Sca-1+ progenitors differentiate into smooth muscle cells, which is mediated by collagen IV-integrin alpha1/beta1/alphav and PDGF receptor pathways. Am J Physiol Cell Physiol 292: C342–C352, 2007. [DOI] [PubMed] [Google Scholar]
- 74.Yeh YC, Hwang GY, Liu IP, Yang VC. Identification and expression of scavenger receptor SR-BI in endothelial cells and smooth muscle cells of rat aorta in vitro and in vivo. Atherosclerosis 161: 95–103, 2002. [DOI] [PubMed] [Google Scholar]
- 75.Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem 54: 1177–1191, 2006. [DOI] [PubMed] [Google Scholar]
- 76.Zhang M, Bohlson SS, Dy M, Tenner AJ. Modulated interaction of the ERM protein, moesin, with CD93. Immunology 115: 63–73, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.