Abstract
Studies on transglutaminases usually focus on the polymerization of protein substrates by intermolecular Nɛ(γ-glutamyl)lysine bridges, without considering the possibility that the monomeric protein units, themselves, could also become crosslinked internally. Both types of crosslinks are produced in the reaction of fibrinogen with red cell transglutaminase. We isolated the transglutaminase-modified, mostly monomeric form (92–96%) of fibrinogen with a Nɛ(γ-glutamyl)lysine content of ≈1.6 moles/mole of fibrinogen. The preparation was fully clottable by thrombin, but the rates of release of fibrinopeptides and clotting times were delayed compared with control. Hybrid Aα⋅γ type of crosslinking, the hallmark of the reaction of the transglutaminase with fibrinogen, occurred by bridging the Aα(408–421) chain segment of the protein to that of γ(392–406). Rotary shadowed electron microscope images showed many monomers to be bent, and the crosslinks seemed to bind the otherwise flexible αC domain closer to the backbone of fibrinogen.
After its assembly into a clot, fibrin is crosslinked by coagulation factor XIIIa ca. 10× faster than fibrinogen in solution (1, 2). Moreover, the conversion of the factor XIII zymogen to XIIIa is tightly controlled by the rate of fibrin formation (3–5); hence, in the clotting of plasma, the parent fibrinogen molecule does not normally participate to an appreciable extent in the crosslinking reaction. Fibrinogen, however, is a significant substrate for tissue transglutaminases (TG) (EC 2.3.2.13), which could be released from red cells at wound sites or in thrombi, from dying endothelial cells in atherosclerotic plaques, or from spreading tumor cells. These TG react with fibrinogen quite differently from factor XIIIa. The latter functions as a processive enzyme, moving along the preassembled fibrin filaments in the gel phase without being released into the clot liquor and thus without much effect on fibrinogen. It reacts rapidly with the γ chain sites and much more slowly on reaching the α chain sites of the protein (6), giving rise to homologous intermolecular γ⋅γ and αn chain combinations between the fibrin units (7). Under forced conditions, however, similar γ⋅γ and Aαn crosslinking can also be achieved with fibrinogen. In contrast, TG reacts quite readily with fibrinogen in the soluble phase, generating Aαp⋅γq type of crosslinked, intermolecular chain combinations (8, 9) as well as Aα⋅γ crosslinking within the monomeric fibrinogen units: [AαBβγ]2, themselves (10). We have now investigated in greater detail this latter mode of crosslinking of fibrinogen and identified regions of the molecule that are involved in the reactions of its Aα and γ chains with the red cell TG.
Materials and Methods
Isolation of Internally Crosslinked Fibrinogen.
Human erythrocyte transglutaminase was purified by affinity chromatography with a fibronectin fragment as fixed ligand, according to procedures described previously (11, 12). Human fibrinogen was prepared, and crosslinking with TG was carried out essentially as described in the earlier protocol (10), but on a somewhat larger scale, so that ≈85 mg of fibrinogen was allowed to react at a concentration of 0.57 μM with 0.0625 μM TG for 1 hr (37°C) in a buffer (434 ml) of 20 mM imidazole-HCl (pH 7.5), containing 150 mM NaCl and 5 mM CaCl2. The reaction was stopped by addition of 23 ml of 0.1 M EDTA. After ammonium sulfate precipitation, the crosslinked fibrinogen was fractionated by gel filtration on Sepharose 4B (2.6 × 96 cm), and the last peak on the elution profile, found to comprise predominantly monomeric fibrinogen, was used for detailed analysis. Nɛ(γ-glutamyl)lysine crosslink contents were measured after sequential, total degradation by a series of proteases, as previously (10).
Gel Electrophoresis.
Gel electrophoresis was performed in SDS (0.1%)/agarose (2%) under nonreducing conditions (13), as well as in SDS (2%)/polyacrylamide according to Laemmli (14) after reduction (40 mM DTT, 2% SDS, and 6 M urea, 37°C, 30 min). Proteins were stained with 0.025% Coomassie brilliant R (Sigma) in 10% acetic acid and 25% isopropanol, and the gels were scanned in an Ultrascan XL Laser Densitometer (Bromma, Sweden).
Electron Microscopy.
Rotary-shadowed samples were prepared for transmission electron microscopy by spraying a dilute solution of the protein (≈20 μg/ml) in a volatile buffer (either 0.05 M ammonium formate at pH 7.4 or 0.125% acetic acid at pH 3.5) and 30% glycerol onto freshly cleaved mica and shadowing with tungsten in a vacuum evaporator (Denton Vacuum, Cherry Hill, NJ) (15–17). All experiments were repeated several times, and micrographs were taken of randomly selected areas to ensure that the results were reproducible and representative. The specimens were examined in a Philips 400 electron microscope (Philips Electronic Instruments, Mahwah, NJ) operating at 80 kV.
Reaction of the TG-Treated Fibrinogen with Thrombin.
Reaction of the TG-treated fibrinogen with thrombin was evaluated both in regard to the release of fibrinopeptides and clot development. Fibrinopeptide A (FPA) and B (FPB) were measured by HPLC (18) by testing mixtures of 0.5 mg/ml of the TG-treated fibrinogen (in comparison to the control protein) after incubation [37°C; 2, 6, 10, 30, and 60 min; 0.3 ml in 50 mM Tris⋅HCl (pH 7.5) and 0.15 M NaCl] with human α-thrombin (0.1 NIH units/ml; a gift of J.W. Fenton III, New York State Department of Health, Albany, NY). Synthetic FPA and FPB (Sigma) were used for calibration.
Clotting of the TG-treated fibrinogen was assayed by the rate of development of turbidity at 37°C in a Beckman DU-64 Spectrophotometer [at 600 nm, in a set of six semimicro polystyrene cuvettes (Bio-Rad), using a Beckman Kinetic Soft-pack module program with measurements taken at 30-sec intervals]. The 0.5-ml clotting mixtures contained various concentrations of the TG-modified protein or the control fibrinogen (0.25, 0.5, 0.75, and 1 mg/ml) and human α-thrombin (1.2 NIH units/ml) in a buffer of 10 mM sodium phosphate (pH 6.5) and 0.15 M NaCl.
Isolation and Sequencing of Crosslinked Fragments.
The transglutaminase-modified monomeric form of fibrinogen (63 mg), collected in the last peak from the Sepharose column, was reduced and carboxymethylated (19). Partial digestion was carried out (37°C, 20 hr) with l-1-tosylamido-2-phenylethyl chloromethyl ketone/trypsin (1:50 wt/wt; Worthington) in 0.1 M ammonium bicarbonate (65 ml). The digest was concentrated by lyophylization (to 5 ml), which was followed by several runs of HPLC (Beckman, Ultrasphere octyl) at room temperature (280 min) with a 0–45% gradient of acetonitrile in 0.1% trifluoroacetic acid (TFA). Combined fractions of the eluates were analyzed for Nɛ(γ-glutamyl)lysine contents (10), and two pools that were particularly rich in isopeptide content (I ≈ 29%; II ≈ 22% of total) were further purified. Processing of pool I required various acetonitrile/0.1% TFA gradients: 8.8–22.8% (180 min); 13.1–17.7% (120 min), and a 14% isocratic separation on C8 columns followed by 25% of the same on a C18 column. Fragment A was obtained from a repeat run on C8 by isocratic separation with 14% acetonotrile/0.1% TFA. The presence of isopeptides was monitored throughout the process of purification. The pool II material was fractionated on C8 with different gradients of acetonitrile in 0.1% TFA: 14.2–27.5% (180 min), 16–19.7% (90 min), and 17.5% for isocratic separation. The last fractionation yielded two isopeptide containing pools (IIb and IIc). The first of these (IIb) was passed through a C18 column with stepwise application of increasing concentrations of acetonitrile in 0.1% TFA (29%, 20 min; 31%, 20 min; 35%, 15 min; and 80%, 3 min). The second pool (IIc) was purified on a C18 column by using a stepwise elution procedure (31%, 20 min; 35%, 20 min; and 80%, 3 min). Final processing of pools IIb and IIc by isocratic separation in 19% acetononitrile/0.1% TFA on a C8 column yielded fragments B and C. Amino acid sequences of fragments A, B, and C were determined by using an Applied Biosystems Procise Sequencer.
Results and Discussion
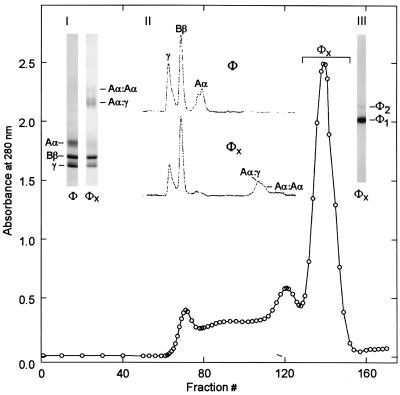
Human fibrinogen was reacted with the human red cell TG, and the monomerically crosslinked fibrinogen (marked φx in Fig. 1) was separated from the polymeric forms of the protein by gel filtration as described (10). The Nɛ(γ-glutamyl)lysine isopeptide content of the material was 1.6 moles per 340,000 g (i.e., 1 mole) of fibrinogen. SDS/PAGE analysis, carried out under reducing conditions (inset I in Fig. 1) showed that, apart from a weak band of homologous Aα⋅Aα pairing, the Aα⋅γ hybrid was the major crosslinked chain type in the preparation. As in our earlier work (10), of the three constituent chains of fibrinogen, the β chains remained unaffected by the reaction with TG. However, as shown by inset II in Fig. 1, virtually the entire band representing the monomeric forms of intact Aα chains (Mr ≈ 67,000) and ≈70% of the original γ chains (Mr ≈ 46,000) disappeared; there was no evidence for γ dimers. SDS electrophoresis in agarose, under nonreducing conditions, indicated the presence of a small amount (4–8%) of dimeric fibrinogen (marked φ2 in inset III of Fig. 1; estimating its percentage depends on whether allowance is made for a possibly two-fold difference in Coomassie blue binding by the dimer). Taken together, the analytical data showed that the preparation was predominantly an internally crosslinked fibrinogen product in which most of the Aα and γ chains within the [AαBβγ]2 structure of the protein were fused by Nɛ(γ-glutamyl)lysine bridges. This, in turn, suggests close contacts between the TG-reactive Gln and Lys side chains of the two separate chains of the protein that participate in this enzyme-driven, “zero distance” crosslinking event.
Figure 1.
Isolation of a predominantly intramolecularly crosslinked form of fibrinogen, after its reaction with red cell transglutaminase. (Main graph) Gel filtration of transglutaminase-treated fibrinogen. (Inset I) SDS/PAGE profile for the control fibrinogen (φ), obtained under reducing conditions, was compared with that of the material collected in fractions 129–152, marked φx. (Inset II) Densitometric scans of the gels shown in I. (Inset III) Nonreducing SDS/agarose electrophoresis of the φx pool shows it to be a predominantly monomeric derivative of fibrinogen (φ1), comprising a small percentage of crosslinked dimers (φ2).
Electronmicroscope Studies.
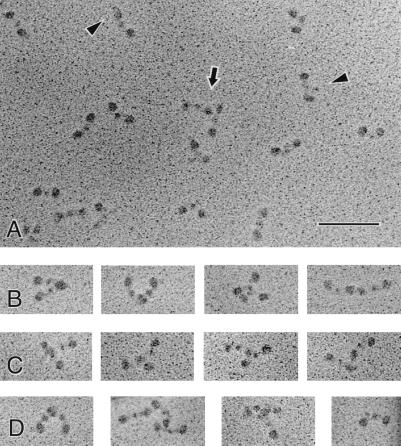
When rotary-shadowed with tungsten and examined by transmission electronmicroscopy, samples from fractions that eluted earlier than the φx material from the Sepharose column in Fig. 1 contained many large aggregates of fibrinogen. In contrast, samples from the φx peak were primarily monomers with some small oligomers, mostly dimers. Analysis of the percentage of molecules in oligomers was difficult because molecules may coincidentally be lying near each other on the grid even though they are not interacting. Counting of many molecules in a sparsely populated field showed 8.5% of them to be dimers, with the rest being monomeric. Occasionally, trimers were observed. In each case, samples were prepared at both pH 7.4 and in dilute acetic acid. At acid pH, in normal fibrinogen, the αC domains are known to dissociate from the central region of the molecule so that they can be visualized, but there is no unfolding of domains of the molecule (20), and there are usually two αC domains visible away from the backbone for each fibrinogen (21).
Monomers in the φx preparation had the trinodular shape characteristic of fibrinogen, but many molecules appeared to be slightly bent (Fig. 2A). In addition, few αC domains could be seen, and almost no molecules had both αC domains visible. Where they were visible, they were often closer to the ends of the molecules or other parts of the backbone (Fig. 2A), rather than in their typical position farther from the backbone. This result can be accounted for by the presence of intramolecular crosslinks that tie the αC domains to the molecular backbone.
Figure 2.
Electron micrographs of TG-modified molecules from the φx pool in Fig. 1. (A) A field of molecules, showing the variety of types of images that are present in a crosslinked sample, treated with dilute acetic acid so that the αC domains can be visualized. Most molecules are monomeric, although one dimer is present in the center (arrow). Monomers have the trinodular shape characteristic of fibrinogen, but many molecules appear to be slightly bent. In contrast to images of control fibrinogen in acetic acid, there are few αC domains visible, and almost no molecules have both αC domains visible. Also, some αC domains that are visible are closer to the ends of the molecules or other parts of the backbone (arrowheads), rather than their typical position farther from the backbone. (Bar = 100 nm.) (B–D) A gallery of images illustrating some typical oligomers observed. There appears to be a great deal of flexibility in the linkage between dimers, because a wide variety of different orientations of the molecules is present. Many molecules lie side-by-side with the ends of the two molecules near each other and a variable angle between their backbones. A few are end-to-end but oriented more linearly, like fibrin(ogen) crosslinked by Factor XIIIa, but in this case there is sometimes a larger gap between the ends. Some dimers are oriented end-to-center or have an end adjoining the connector between the center and end of the other molecule. (D, far right) Another example of a molecule with its αC domain close to the molecular backbone.
The arrangement of the two molecules making up the dimers was highly variable, suggesting that there is a great deal of flexibility in the connection between the two monomers. Fig. 2 B–D shows a gallery of images of the oligomers observed. Many molecules lay side-by-side with the ends of the two molecules near each other and a variable angle between their backbones. A few were end-to-end but oriented more linearly, like fibrin(ogen) crosslinked by Factor XIIIa (15, 22), but in this case there was at times a larger gap between the ends. Some dimers were oriented end-to-center or had an end adjoining the connector between the center and end of the other molecule.
Fig. 3 is a schematic representation of the images seen by rotary-shadowed electronmicroscopy in the TG-modified φx sample. The main backbone of fibrinogen is blue while the αC domains are yellow and appear to be tied by attachments (red) to the distal ends of the molecules.
Figure 3.
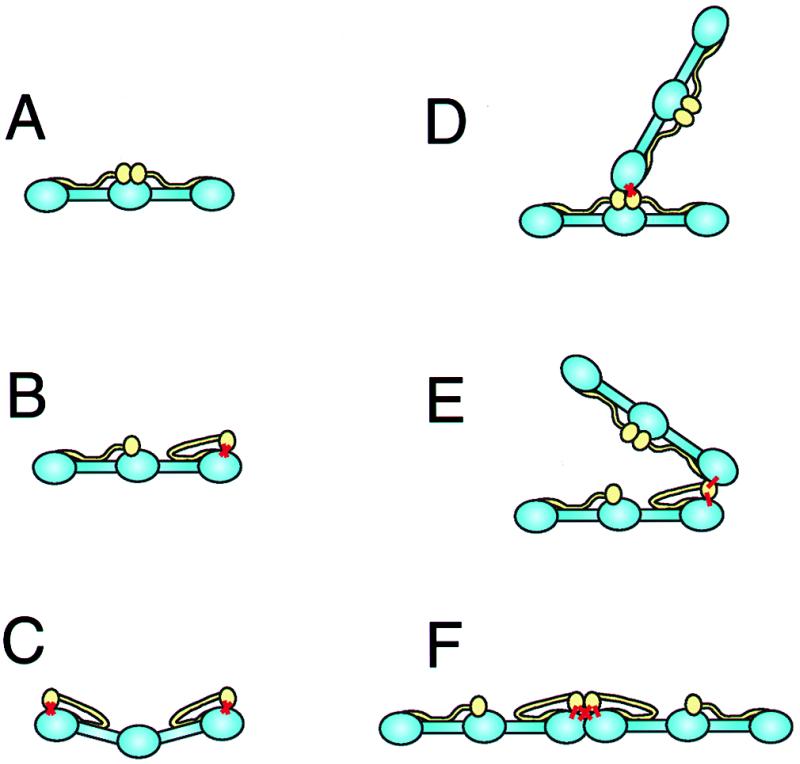
Schematic representation of intramolecular and intermolecular crosslinking of fibrinogen by TG, based on the appearance of molecules in the electron micrographs of Fig. 2. The main backbone of the fibrinogen molecules are blue while the αC domains are yellow. (A) Control fibrinogen. The αC domains are in the central region of the molecule. (B) Fibrinogen with one αC domain crosslinked intramolecularly to one γ chain. The sites of the crosslinks are indicated by thick red lines. The αC domain has dissociated from the central domain and is attached to the end of the molecule, where the C-terminal γ chain is located. (C) Fibrinogen with each αC domain crosslinked intramolecularly to one γ chain. The molecule is slightly bent, as observed in the electron micrographs, because of the strains introduced. (D) Fibrinogen with one αC domain crosslinked intermolecularly to the γ chain of another molecule. In this case, neither molecule has an intramolecular Aα:γ crosslink, so that the two molecules are oriented end-to-center. (E) Fibrinogen with one αC domain crosslinked intermolecularly to the γ chain of another molecule. In this case, one molecule has an intramolecular Aα:γ crosslink, so that the two molecules are oriented end-to-end. There is a great deal of flexibility in these junctions, allowing the two molecules to be oriented in a variety of ways. (F) Fibrinogen with one αC domain crosslinked intermolecularly to the γ chain of another molecule. In this case, both molecules have intramolecular Aα:γ crosslinks, so that the two molecules are oriented end-to-end. In addition, the molecules are constrained to a linear arrangement because of two intermolecular Aα:γ crosslinks.
Reactions of the TG-Modified Fibrinogen with Thrombin.
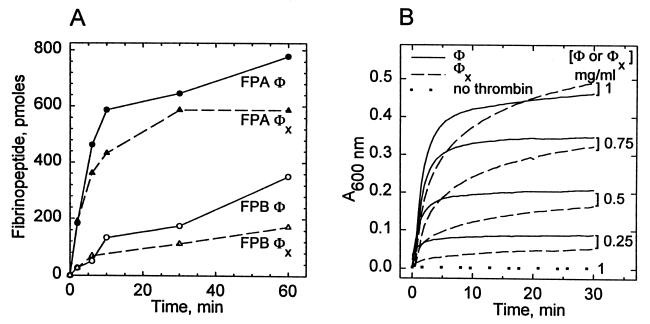
From the point of view of biological function, the single defining attribute of the fibrinogen molecule is its conversion to a clot-forming substrate through limited proteolysis by thrombin. The exposure of the new N-terminal Gly end groups, which accompanies the release of fibrinopeptide moieties, is a prerequisite for the assembly of the fibrin network (23–27). Thus, it was of interest to examine whether the TG-treated φx derivative would be cleaved by thrombin and whether it would then be able to form a clot. The data in Fig. 4A demonstrate that, although somewhat slower, the thrombin-catalyzed release of fibrinopeptides with the φx material occurs in the same order as with the control: i.e., FPA release preceding that of FPB.
Figure 4.
Prior reaction of fibrinogen with TG causes a delay in the rates of release of fibrinopeptides A and B (FPA and FPB) by thrombin (A) and also longer clotting times (B). Rates for the TG-treated φx material (see Fig. 1) were compared with those for the control fibrinogen (φ). Clotting with thrombin was monitored by development of turbidity (at 600 nm) at various concentrations of φ and φx (0.25, 0.5, 0.75, and 1 mg/ml).
As measured by the development of turbidity, the rate of clotting of the TG-treated fibrinogen with thrombin was also reduced in comparison to control over the entire range of fibrinogen concentration (0.25–1 mg/ml) examined (Fig. 4B). However, final values of clot turbidities did not seem to differ significantly, suggesting that perhaps the TG-modified φx protein—although it reacts more sluggishly with thrombin than the virgin φ—would still be able to form regular protofibrillar assemblies and fibers after the release of fibrinopeptides.
Flanking Sequences and Spatial Localization of Crosslinks in Fibrinogen Introduced by Transglutaminase.
The φx preparation (see Fig. 1), comprising mostly the internally crosslinked form of fibrinogen, was partially digested with trypsin after reduction and carboxymethylation. The tryptic fragments were separated by HPLC, and batches of the collected fractions were further digested with proteases for monitoring Nɛ(γ-glutamyl)lysine content (10). Two pools, particularly rich in isopeptide (accounting for ≈29 and 22% of the crosslinks in the preparation) were further purified by a series of HPLC runs. As presented in Table 1, reliable sequence information could be obtained for three fractions (A, B, and C). The Edman degradation of fragment A yielded a double sequence, corresponding to stretches of amino acids (A1) from residues 408–421 in the Aα chain and (A2) 392–406 in the γ chain of fibrinogen. Lack of recovery of phenylthiohydantoin derivatives for Lys at positions 413 and 418 in the Aα-derived fragment (marked by X in Table 1) and for Gln 398 and 399 in the γ chain fragment (marked X′) was taken to indicate that these four residues were all involved in crossbridge formation. Fragment B yielded multiple sequences, some of them overlapping and seemingly all derived from the Aα chains of the protein. Stretches containing residues 349–364 in B3, 408–418 in B4, 414–424 in B5, and 419–424 in B6 could be reliably identified. The B4 and B5 sequences confirmed the participation of Lys 413 and Lys 418 in the TG-catalyzed crosslinking reaction, as was evident also from the pairing of peptides A1 and A2. In addition, the B6 sequence showed that Lys 421 of the Aα chain was also involved in crosslink formation. Clearly, the Aα chain 349–364 sequence in B3 is related to the predictable tryptic fragment of residues 349–406, in which Arg 406 is a cleavage site for trypsin. We suspect that, although not identified directly, Gln 366—the only Gln in the entire 349–406 segment—is a likely crosslinking partner of the three Aα Lys 413, 418, and 421 residues. The information, obtained from the analysis of fragment C, suggests that the same Gln 366 can also form a bridge by crosslinking with Lys 448 in peptide C8.
Table 1.
Nε(γ-glutamyl)lysine crosslinked sequences obtained from the tryptic digest of fibrinogen after reaction with TG
| A | |||||||||||||||||
| 1. | E | Y | H | T | E | X | L | V | T | S | X | G | D | K | |||
| Aα | 408 | 413 | 418 | 421 | |||||||||||||
| 2. | L | T | I | G | E | G | X′ | X′ | H | H | L | G | G | A | K | ||
| γ | 392 | 398 | 399 | 406 | |||||||||||||
| B | |||||||||||||||||
| 3. | G | S | A | G | H | W | T | S | E | S | S | V | S | G | S | T | |
| Aα | 349 | 364 | |||||||||||||||
| 4. | E | Y | H | T | E | X | L | V | T | S | K | ||||||
| Aα | 408 | 413 | 418 | ||||||||||||||
| 5. | L | V | T | S | X | G | D | K | E | L | R | ||||||
| Aα | 414 | 418 | 424 | ||||||||||||||
| 6. | G | D | X | E | L | R | |||||||||||
| Aα | 419 | 421 | 424 | ||||||||||||||
| C | |||||||||||||||||
| 7. | G | S | A | G | H | W | T | S | E | S | S | V | |||||
| Aα | 349 | 360 | |||||||||||||||
| 8. | T | V | T | X | T | V | I | G | P | D | G | H | K | ||||
| Aα | 445 | 448 | 457 |
Peptides A (pool I) and B and C (pool II) from HPLC were subjected to Edman degradation. The numbers below the amino acids represent positions in the sequences of the Aα and -γ chains of fibrinogen. X, no PTH derivative of a known amino acid appeared at these lysine positions in the sequences. X′, no PTH derivative of a known amino acid appeared at these glutamine positions in the sequence. Numbering of residues corresponds to that of Henschen et al. (28).
It is interesting that both vicinal Gln residues, i.e., Gln 398 and 399 (see Table 1, A1), near the C termini of the γ chains can participate in forming hybrid crosslinks with the Aα chains in fibrinogen under the influence of TG. As far as the factor XIIIa-catalyzed reaction with fibrin is concerned, the exact mode of participation of these residues is still unresolved. When probed with glycineethylester or dansylcadaverine, designed to block the acceptor crosslinking sites of the protein (29), both Glns became modified (30, 31). However, one report (30) claimed that only Gln 398 was involved in generating the crosslinked γ·γ chain pairs whereas the other study (31) suggested that Gln 399 was the predominant residue in the homologous crosslinking of γ chains. In the reaction of fibrinogen with red cell TG, γ Gln 398 and 399 were shown to be linked to Aα Lys 413 and 418. This allows for the possibility that, in the hybrid Aα⋅γ crosslinking of the chains of fibrinogen, Aα Lys 413⋅γ Gln 398, Aα Lys 418⋅γ Gln 398⋅Aα Lys 413⋅γ Gln 399 and/or Aα Lys 418⋅γ Gln 399 pairing could occur.
The reactivity of Aα Gln 366 to TG (Table 1, B3 and C7) parallels the reaction of this site with factor XIIIa (32). Apparently, the Aα Gln 366 residue is able to form crosslinks with several partners in the Aα chains of fibrinogen: i.e., Aα Lys 413, 418, 421, and 448 (see Table 1, B5, B4, B6, and C8).
Analysis of the electron microscope images of the crosslinked molecules allows the spatial localization of the Aα⋅γ crosslinks. For φx molecules in dilute acetic acid, most of the αC domains are not visible because they are linked to the backbone, and many of the αC domains that are visible are close to the ends of the molecules. Because the glutamines acting as acceptors in the γ chain are near the ends of the molecule, these results imply that the lysine residues in the α chains that are crosslinked are likely to be in or near the globular part of the αC domains (Fig. 3B) (20, 21, 33). Such a location for the αC domains requires their dissociation from their normal position near the center of the molecule, consistent with the proposed dynamic equilibrium of the αC domains (20, 21). However, it appears that this linkage may put the molecules under some strain because many molecules appear to be bent (Fig. 3C).
The crosslinking sites can also be identified in the Aα⋅γ dimers. The configuration of the dimers will depend on whether there are intramolecular crosslinks. Dimers with an end (γ)-to-center (Aα) orientation would arise from molecules without intramolecular crosslinks (Fig. 3D). A molecule with an intramolecular Aα⋅γ crosslink would have one of its αC domains near the molecular end (Fig. 3B). If one of the donor lysines on this Aα chain were then crosslinked to the γ chain of another molecule, the dimer would be end-to-end (Fig. 3E). The orientation of these two molecules could vary, depending on what other crosslinks were present (Fig. 3 E and F). Because there are two donors and two acceptors in each half-molecule, several combinations of intra- and intermolecular crosslinks are possible. Similarly, trimers would be generated by the sharing of donor-acceptor crosslinks among three molecules.
The wide variety of configurations of dimeric crosslinked molecules suggests that these connections are very flexible. Such flexibility could arise from flexibility of the chain attaching the globular portion of the αC domains to the rest of the molecule (20, 21) and the known flexibility of the C-terminal segments of the γ chains (34–36).
Acknowledgments
This work was supported by grants from the National Institutes of Health [Grants HL-02212 to L.L., HL-30954 to J.W.W., and GM-3086 (T.J.L.)].
Abbreviations
- TG
transglutaminase
- FPA
fibrinopeptide A
- FPB
fibrinopeptide B
- TFA
trifluoroacetic acid
References
- 1.Samokhin G P, Lorand L. J Biol Chem. 1995;270:21827–21832. doi: 10.1074/jbc.270.37.21827. [DOI] [PubMed] [Google Scholar]
- 2.Lorand L, Parameswaran K N, Murthy S N P. Proc Natl Acad Sci USA. 1998;95:537–541. doi: 10.1073/pnas.95.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Credo R B, Curtis C G, Lorand L. Biochemistry. 1981;20:3770–3778. doi: 10.1021/bi00516a016. [DOI] [PubMed] [Google Scholar]
- 4.Lewis S D, Janus T J, Lorand L, Shafer J A. Biochemistry. 1985;24:6772–6777. doi: 10.1021/bi00345a007. [DOI] [PubMed] [Google Scholar]
- 5.Naski M G, Lorand L, Shafer J. Biochemistry. 1991;30:934–941. doi: 10.1021/bi00218a008. [DOI] [PubMed] [Google Scholar]
- 6.Lorand L, Chenoweth D. Proc Natl Acad Sci USA. 1969;63:1247–1252. doi: 10.1073/pnas.63.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz M L, Pizzo S V, Hill R L, McKee P A. J Biol Chem. 1973;248:1395–1407. [PubMed] [Google Scholar]
- 8.Murthy S N P, Lorand L. Proc Natl Acad Sci USA. 1990;87:9679–9682. doi: 10.1073/pnas.87.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shainoff J R, Urbanic D A, DiBello P M. J Biol Chem. 1991;266:6429–6437. [PubMed] [Google Scholar]
- 10.Murthy S N P, Wilson J, Guy S L, Lorand L. Proc Natl Acad Sci USA. 1991;88:10601–10604. doi: 10.1073/pnas.88.23.10601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radek J T, Jeong J-M, Murthy S N P, Ingham K C, Lorand L. Proc Natl Acad Sci USA. 1993;90:3152–3156. doi: 10.1073/pnas.90.8.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parameswaran K N, Cheng X-F, Chen E C, Velasco P T, Wilson J H, Lorand L. J Biol Chem. 1997;272:10311–10317. doi: 10.1074/jbc.272.15.10311. [DOI] [PubMed] [Google Scholar]
- 13.Moroi M, Inoue N, Yamasaki M. Biochim Biophys Acta. 1975;379:217–226. doi: 10.1016/0005-2795(75)90025-2. [DOI] [PubMed] [Google Scholar]
- 14.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Fowler W E, Erickson H P. J Mol Biol. 1979;134:241–249. doi: 10.1016/0022-2836(79)90034-2. [DOI] [PubMed] [Google Scholar]
- 16.Veklich Y, Ang E K, Lorand L, Weisel J W. Proc Natl Acad Sci USA. 1998;95:1438–1442. doi: 10.1073/pnas.95.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisel J W, Stauffacher C V, Bullitt E, Cohen C. Science. 1985;230:1388–1391. doi: 10.1126/science.4071058. [DOI] [PubMed] [Google Scholar]
- 18.Ng A S, Lewis S D, Shafer J A. Methods Enzymol. 1993;222:341–358. doi: 10.1016/0076-6879(93)22023-9. [DOI] [PubMed] [Google Scholar]
- 19.Gurd F R N. Methods Enzymol. 1967;11:532–541. [Google Scholar]
- 20.Gorkun O V, Veklich Y I, Medved L V, Henschen A H, Weisel J W. Biochemistry. 1994;33:6986–6997. doi: 10.1021/bi00188a031. [DOI] [PubMed] [Google Scholar]
- 21.Veklich Y I, Gorkun O V, Medved L V, Nieuwenhuizen W, Weisel J W. J Biol Chem. 1993;268:13577–13585. [PubMed] [Google Scholar]
- 22.Weisel J W, Francis C W, Nagaswami C, Marder V J. J Biol Chem. 1993;268:26618–26624. [PubMed] [Google Scholar]
- 23.Bailey K, Bettelheim F R, Lorand L, Middlebrook W R. Nature (London) 1951;107:233. doi: 10.1038/167233a0. [DOI] [PubMed] [Google Scholar]
- 24.Lorand L, Middlebrook W R. Biochem J. 1952;52:196–199. doi: 10.1042/bj0520196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorand L. Nature (London) 1951;167:992–994. doi: 10.1038/167992a0. [DOI] [PubMed] [Google Scholar]
- 26.Lorand L. Biochem J. 1952;52:200–203. doi: 10.1042/bj0520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laudano A P, Doolittle R F. Biochemistry. 1980;19:1013–1019. doi: 10.1021/bi00546a028. [DOI] [PubMed] [Google Scholar]
- 28.Henschen A, Lottspeich F, Kehl M, Southan C. Ann N Y Acad Sci. 1983;408:28–43. doi: 10.1111/j.1749-6632.1983.tb23232.x. [DOI] [PubMed] [Google Scholar]
- 29.Lorand L, Rule N G, Ong H H, Furlanetto R, Jacobsen A, Downey J, Oner N, Bruner-Lorand J. Biochemistry. 1968;7:1214–1223. doi: 10.1021/bi00843a043. [DOI] [PubMed] [Google Scholar]
- 30.Chen R, Doolittle R F. Biochemistry. 1971;10:4486–4491. doi: 10.1021/bi00800a021. [DOI] [PubMed] [Google Scholar]
- 31.Purves L, Purves M, Brandt W. Biochemistry. 1987;26:4640–4646. doi: 10.1021/bi00389a008. [DOI] [PubMed] [Google Scholar]
- 32.Fretto L J, Ferguson E W, Steinman H M, McKee P A. J Biol Chem. 1978;253:2184–2195. [PubMed] [Google Scholar]
- 33.Medved L V, Gorkun O V, Privalov P L. FEBS Lett. 1983;160:291–295. doi: 10.1016/0014-5793(83)80985-5. [DOI] [PubMed] [Google Scholar]
- 34.Yee V C, Pratt K P, Cote H C, Trong I L, Chung D W, Davie E W, Stenkamp R E, Teller D C. Structure (London) 1997;5:125–138. doi: 10.1016/s0969-2126(97)00171-8. [DOI] [PubMed] [Google Scholar]
- 35.Spraggon G, Everse S J, Doolittle R F. Nature (London) 1997;389:455–462. doi: 10.1038/38947. [DOI] [PubMed] [Google Scholar]
- 36.Mosesson M W, Siebenlist K R, Meh D A, Wall J S, Hainfeld J F. Proc Natl Acad Sci USA. 1998;95:10511–10516. doi: 10.1073/pnas.95.18.10511. [DOI] [PMC free article] [PubMed] [Google Scholar]